A Practical Approach to Triclosan Detection: A Novel Y2O3@GCN-Modified Carbon Paste Electrode for Sensitive and Selective Detection in Environmental and Consumer Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Y2O3 Nanoparticles
2.2. Preparation of Graphitic Carbon Nitride (GCN) and Y2O3@GCN Nanocomposite
2.3. Preparation of Carbon Paste Electrode and Real-World Samples Analysis
3. Results and Discussion
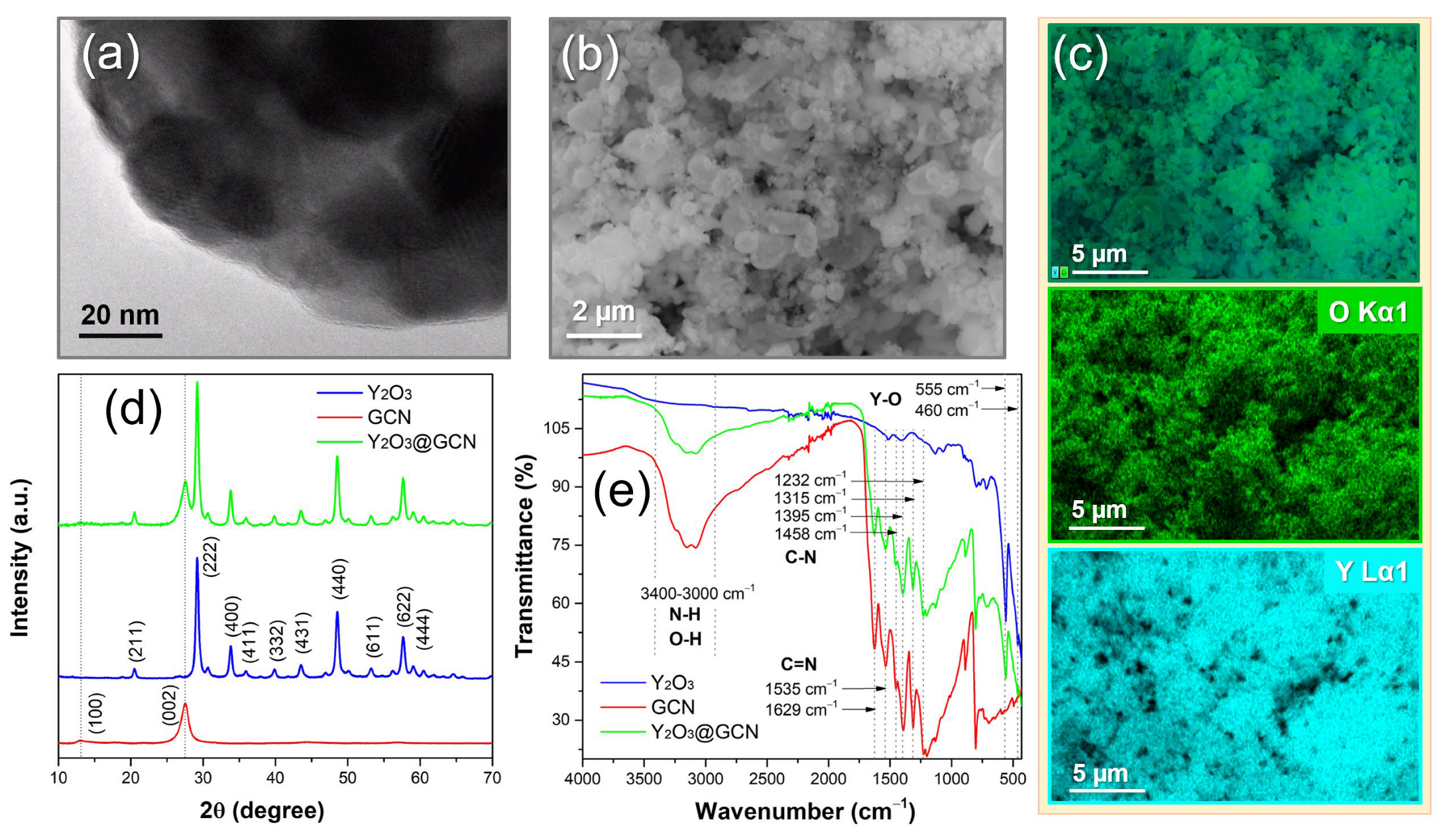
3.1. Structural and Morphological Characterization of Nanocomposite Materials
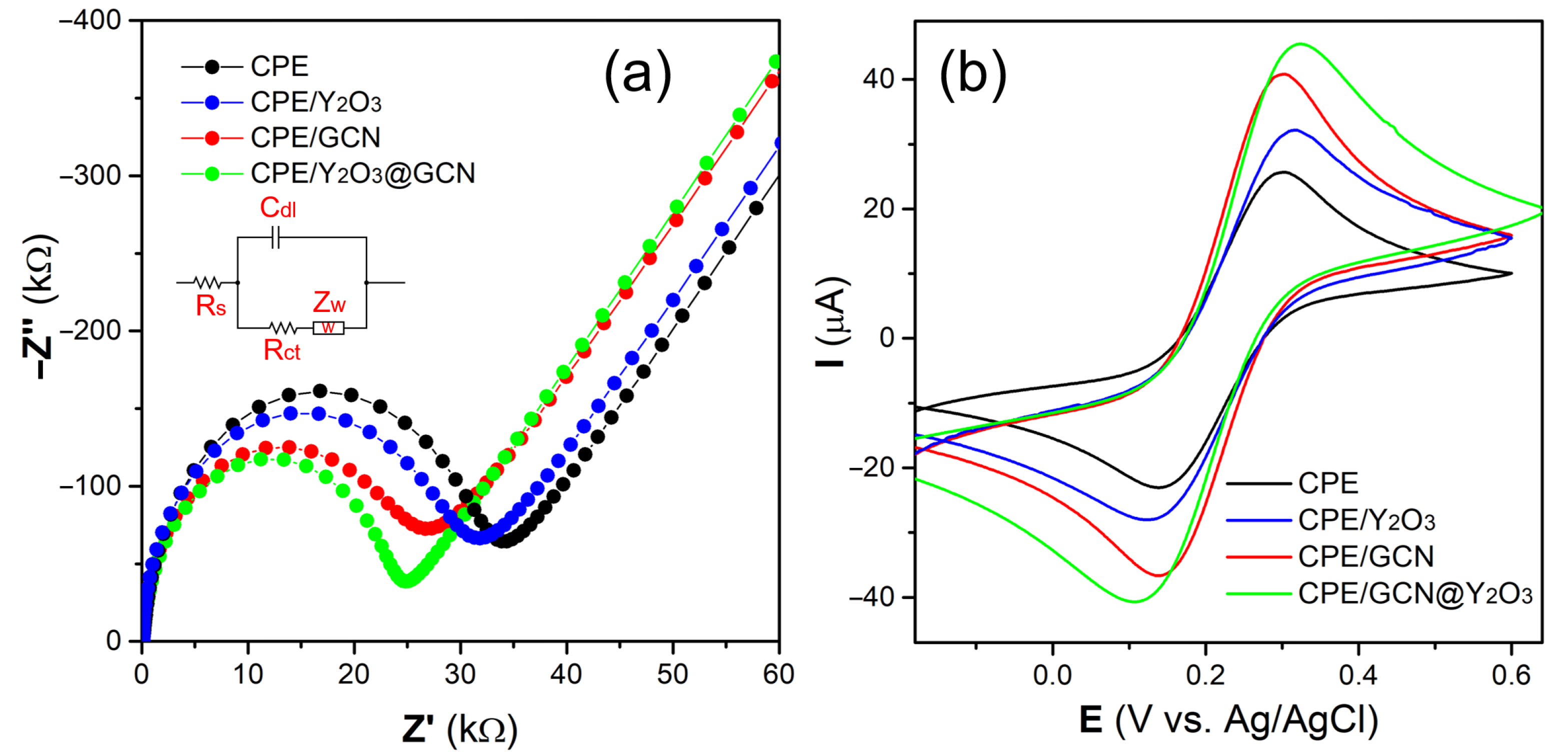
3.2. Electrochemical Characterization of the Nanocomposite
3.2.1. Electrochemical Impedance Spectroscopy and Cyclic Voltammetry Analysis
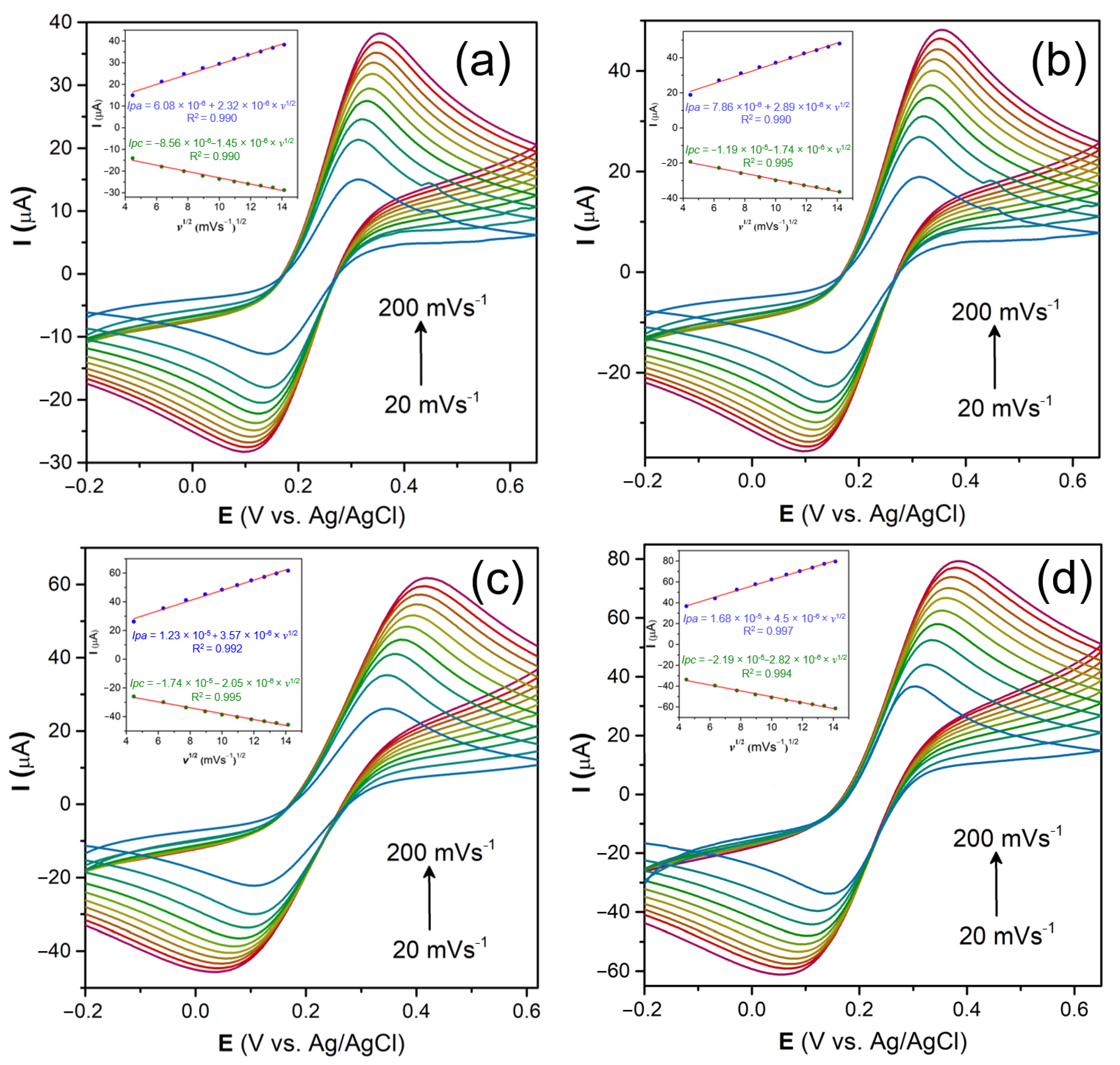
3.2.2. Scan Rate Measurements
3.3. Electrochemical Detection of TSC
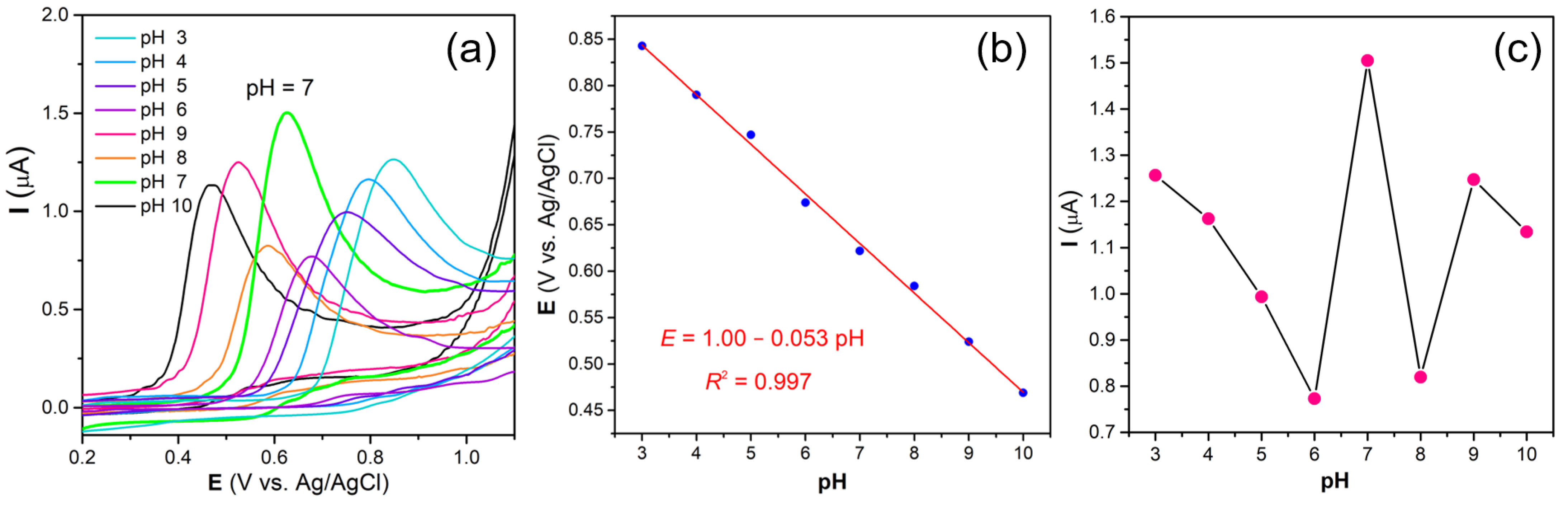
3.4. pH Optimization of Working Solutions
3.5. Determination of TSC via SWV Analysis
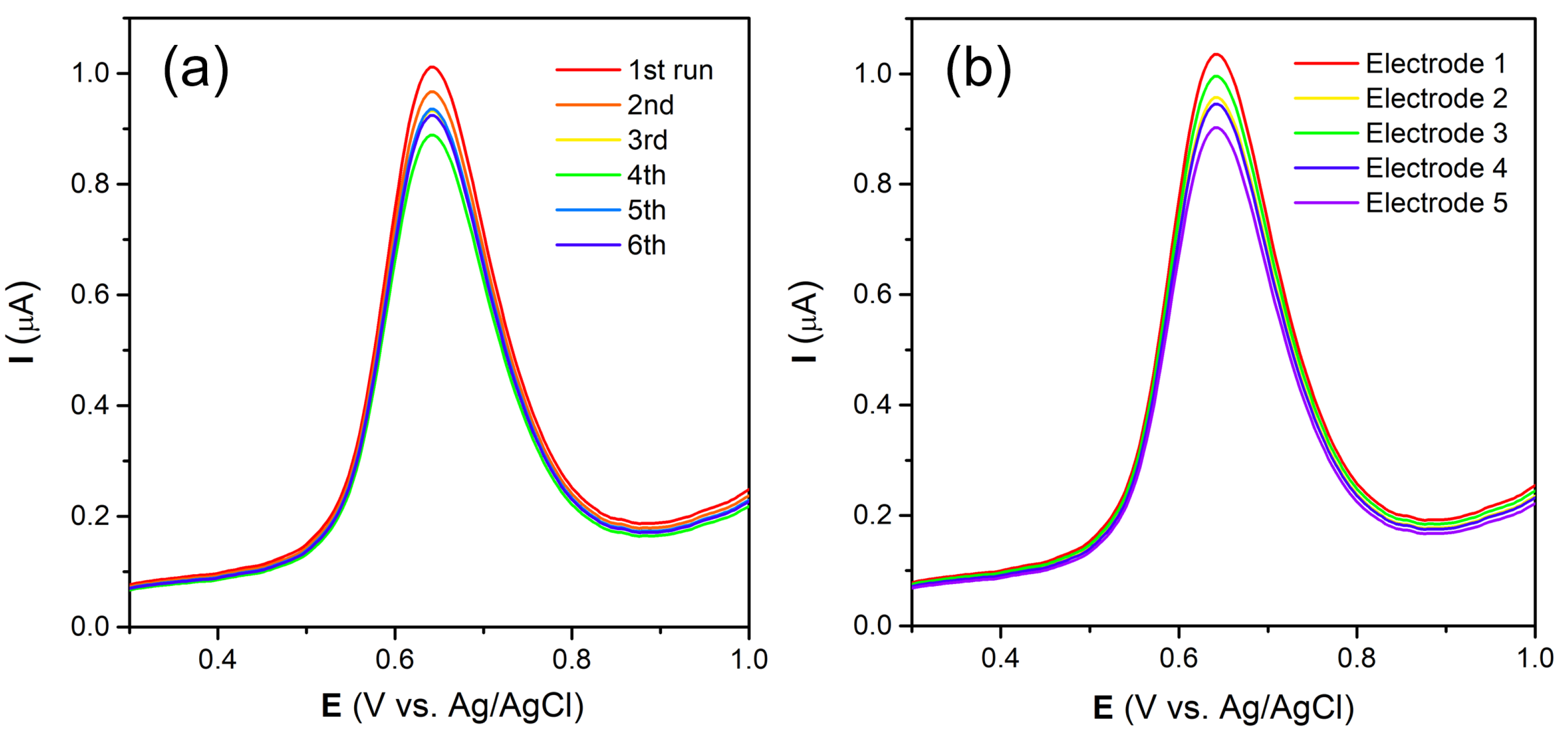
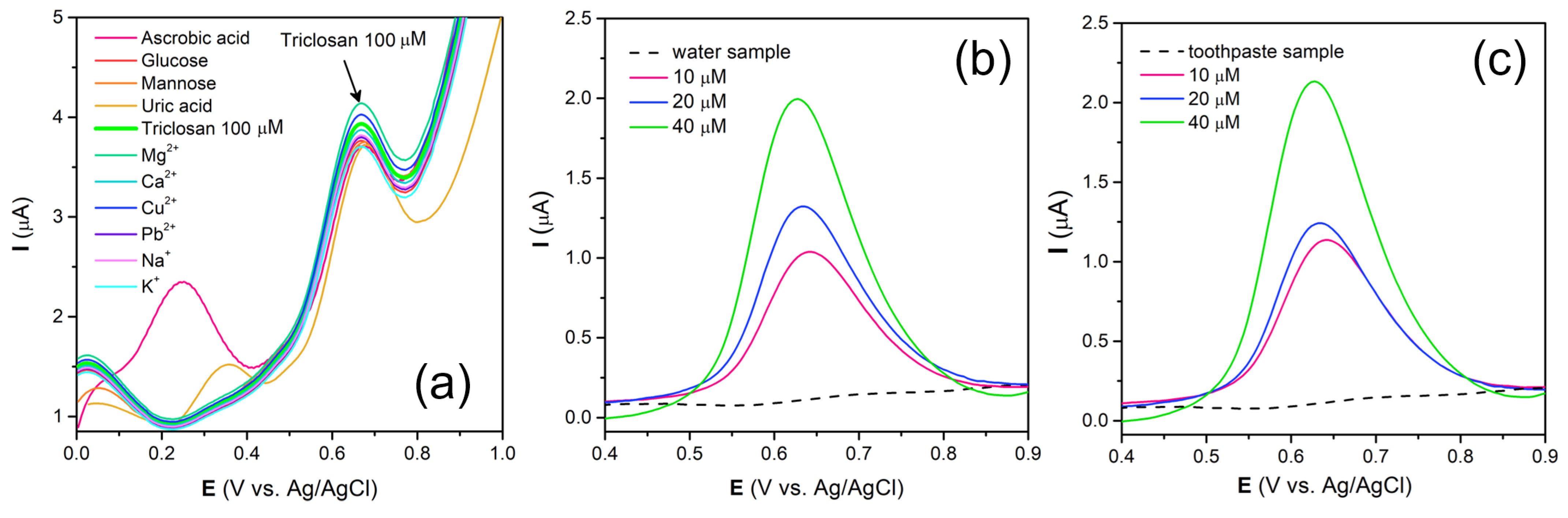
3.6. Interference and Practical Application Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdankhah, S.P.; Scheie, A.A.; Høiby, E.A.; Lunestad, B.-T.; Heir, E.; Fotland, T.Ø.; Naterstad, K.; Kruse, H. Triclosan and Antimicrobial Resistance in Bacteria: An Overview. Microb. Drug Resist. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Dann, A.B.; Hontela, A. Triclosan: Environmental Exposure, Toxicity and Mechanisms of Action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.; Müller, S.; Tixier, C.; Pillonel, L. Triclosan: Occurrence and Fate of a Widely Used Biocide in the Aquatic Environment: Field Measurements in Wastewater Treatment Plants, Surface Waters, and Lake Sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-L.; Stingley, R.L.; Beland, F.A.; Harrouk, W.; Lumpkins, D.L.; Howard, P. Occurrence, Efficacy, Metabolism, and Toxicity of Triclosan. J. Environ. Sci. Health Part C 2010, 28, 147–171. [Google Scholar] [CrossRef]
- Aranami, K.; Readman, J.W. Photolytic Degradation of Triclosan in Freshwater and Seawater. Chemosphere 2007, 66, 1052–1056. [Google Scholar] [CrossRef]
- Liu, T.; Wu, D. High-performance Liquid Chromatographic Determination of Triclosan and Triclocarban in Cosmetic Products. Intern. J. Cosmet. Sci 2012, 34, 489–494. [Google Scholar] [CrossRef]
- Tohidi, F.; Cai, Z. GC/MS Analysis of Triclosan and Its Degradation by-Products in Wastewater and Sludge Samples from Different Treatments. Environ. Sci. Pollut. Res. 2015, 22, 11387–11400. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Motia, S.; Tudor, I.A.; Ribeiro, P.A.; Raposo, M.; Bouchikhi, B.; El Bari, N. Electrochemical Sensor Based on Molecularly Imprinted Polymer for Sensitive Triclosan Detection in Wastewater and Mineral Water. Sci. Total Environ. 2019, 664, 647–658. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Sruthi, V.P.; Satija, J.; Senthilkumar, S.; Kim, Y.-J. Materials and Design Strategies for the Electrochemical Detection of Antineoplastic Drugs: Progress and Perspectives. Mater. Sci. Eng. R Rep. 2024, 161, 100840. [Google Scholar] [CrossRef]
- Hayat, A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Shah Syed, J.A.; Amin, M.A.; Ali, T.; Bashir, T.; Palamanit, A.; et al. Graphitic Carbon Nitride (g–C3N4)–Based Semiconductor as a Beneficial Candidate in Photocatalysis Diversity. Int. J. Hydrogen Energy 2022, 47, 5142–5191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Wang, L.; Xing, J. Graphitic Carbon Nitride Emitter: From Structural Modification to Optoelectronics Applications. Adv. Opt. Mater. 2023, 11, 2301547. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Khaneghah, M.H.; Sohouli, E. Graphitic Carbon Nitride: Synthesis and Characterization. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 573–590. ISBN 978-0-12-821996-6. [Google Scholar]
- Muhmood, T.; Ahmad, I.; Haider, Z.; Haider, S.K.; Shahzadi, N.; Aftab, A.; Ahmed, S.; Ahmad, F. Graphene-like Graphitic Carbon Nitride (g-C3N4) as a Semiconductor Photocatalyst: Properties, Classification, and Defects Engineering Approaches. Mater. Today Sustain. 2024, 25, 100633. [Google Scholar] [CrossRef]
- Cao, S.-M.; Chen, H.-B.; Dong, B.-X.; Zheng, Q.-H.; Ding, Y.-X.; Liu, M.-J.; Qian, S.-L.; Teng, Y.-L.; Li, Z.-W.; Liu, W.-L. Nitrogen-Rich Metal-Organic Framework Mediated Cu–N–C Composite Catalysts for the Electrochemical Reduction of CO2. J. Energy Chem. 2021, 54, 555–563. [Google Scholar] [CrossRef]
- Chen, J.; Huang, B.; Cao, R.; Li, L.; Tang, X.; Wu, B.; Wu, Y.; Hu, T.; Yuan, K.; Chen, Y. Steering Local Electronic Configuration of Fe–N–C-Based Coupling Catalysts via Ligand Engineering for Efficient Oxygen Electroreduction. Adv. Funct. Mater. 2023, 33, 2209315. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Wang, Z.-B.; Sui, X.-L.; Li, C.-Z.; Zhao, L.; Gu, D.-M. Nitrogen-Doped Carbon with Mesoporous Structure as High Surface Area Catalyst Support for Methanol Oxidation Reaction. RSC Adv. 2016, 6, 39310–39316. [Google Scholar] [CrossRef]
- Mijajlović, A.; Ognjanović, M.; Manojlović, D.; Vlahović, F.; Đurđić, S.; Stanković, V.; Stanković, D. Eu2O3@Cr2O3 Nanoparticles-Modified Carbon Paste Electrode for Efficient Electrochemical Sensing of Neurotransmitters Precursor L-DOPA. Biosensors 2023, 13, 201. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on G-C3N4-Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Ognjanović, M.; Nikolić, K.; Bošković, M.; Pastor, F.; Popov, N.; Marciuš, M.; Krehula, S.; Antić, B.; Stanković, D.M. Electrochemical Determination of Morphine in Urine Samples by Tailoring FeWO4/CPE Sensor. Biosensors 2022, 12, 932. [Google Scholar] [CrossRef]
- Đurđić, S.; Vlahović, F.; Ognjanović, M.; Gemeiner, P.; Sarakhman, O.; Stanković, V.; Mutić, J.; Stanković, D.; Švorc, Ľ. Nano-Size Cobalt-Doped Cerium Oxide Particles Embedded into Graphitic Carbon Nitride for Enhanced Electrochemical Sensing of Insecticide Fenitrothion in Environmental Samples: An Experimental Study with the Theoretical Elucidation of Redox Events. Sci. Total Environ. 2024, 909, 168483. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Mao, L.; Bao, T.; Wen, W.; Wang, S.; Gomathi, T.; Gnanasundaram, N.; Rebezov, M.; Shariati, M.A.; Chung, I.-M.; et al. Yttrium Oxide Nanoparticle Synthesis: An Overview of Methods of Preparation and Biomedical Applications. Appl. Sci. 2021, 11, 2172. [Google Scholar] [CrossRef]
- Naveed Ur Rehman, M.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Akbar, U.A.; Manzoor, S.; Hakeem, A.S.; Ashiq, M.N.; Iqbal, F. Facile Synthesis of Novel PANI Covered Y2O3–ZnO Nanocomposite: A Promising Electrode Material for Supercapacitor. Solid State Sci. 2022, 128, 106883. [Google Scholar] [CrossRef]
- Shyam Sunder, G.S.; Rohanifar, A.; Devasurendra, A.M.; Kirchhoff, J.R. Selective Determination of l-DOPA at a Graphene Oxide/Yttrium Oxide Modified Glassy Carbon Electrode. Electrochim. Acta 2019, 301, 192–199. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ahmed, A.H.M.T.; Bhattacharjee, I.; Firdoushi, S.; Biswas, D.; Mukherjee, S.; Mondal, B.; Bandyopadhyay, R.; Tudu, B. Electrochemical Detection of Indigo Carmine in Candies Using Y2O3 Nanoparticles Infused Graphite Electrode. J. Food Compos. Anal. 2024, 135, 106626. [Google Scholar] [CrossRef]
- Shashikumar, J.; Patil, B.; Swamy, B.E.K.; Nagabhushana, H.; Sharma, S.; Lalitha, P. Effect of RGO-Y2O3 and RGO-Y2O3:Cr3+ Nano Composite Sensor for Dopamine. Sci. Rep. 2021, 11, 9372. [Google Scholar] [CrossRef]
- Pathan, A.A.; Desai, K.R.; Bhasin, C.P. Synthesis of La2O3 Nanoparticles Using Glutaric Acid and Propylene Glycol for Future CMOS Applications. Int. J. Nanomater. Chem. 2017, 3, 21–25. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly Stable and Selective Ethanol Sensor Based on α-Fe2O3 Nanoparticles Prepared by Pechini Sol–Gel Method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Zaki, T.; Kabel, K.I.; Hassan, H. Using Modified Pechini Method to Synthesize α-Al2O3 Nanoparticles of High Surface Area. Ceram. Int. 2012, 38, 4861–4866. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ultrathin Graphitic Carbon Nitride Nanosheet: A Highly Efficient Fluorosensor for Rapid, Ultrasensitive Detection of Cu2+. Anal. Chem. 2013, 85, 5595–5599. [Google Scholar] [CrossRef]
- Khan, M.; Farah, H.; Iqbal, N.; Noor, T.; Amjad, M.Z.B.; Ejaz Bukhari, S.S. A TiO2 Composite with Graphitic Carbon Nitride as a Photocatalyst for Biodiesel Production from Waste Cooking Oil. RSC Adv. 2021, 11, 37575–37583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Qian, J.; Zhang, T.; Sun, B.; Han, Y. One-Step Synthesis and Characterization of Y2O3 Nanoparticles via Emulsion Detonation Method. Ceram. Int. 2024, 50, 27995–28003. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Wu, L.; Li, C.-F.; Hu, Z.-Y.; Liu, J.; Deng, Z.; Chen, L.-H.; Li, Y.; Su, B.-L. In-Situ Growing Mesoporous CuO/O-Doped g-C3N4 Nanospheres for Highly Enhanced Lithium Storage. ACS Appl. Mater. Interfaces 2019, 11, 32957–32968. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of G-C3N4 at Different Temperatures for Superior Visible/UV Photocatalytic Performance and Photoelectrochemical Sensing of MB Solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Wang, Z.; Ho, W.-K. Enhanced Visible Light Photocatalytic Activity and Oxidation Ability of Porous Graphene-like g-C3N4 Nanosheets via Thermal Exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Milović, M.; Jugović, D.; Vujković, M.; Kuzmanović, M.; Mraković, A.; Mitrić, M. Towards a Green and Cost-Effective Synthesis of Polyanionic Cathodes: Comparative Electrochemical Behaviour of LiFePO4/C, Li2FeP2O7/C and Li2FeSiO4/C Synthesized Using Methylcellulose Matrix. Bull. Mater. Sci. 2021, 44, 144. [Google Scholar] [CrossRef]
- Jugović, D.; Mitrić, M.; Milović, M.; Ivanovski, V.N.; Škapin, S.; Dojčinović, B.; Uskoković, D. Structural and Electrochemical Properties of the Li2FeP2O7/C Composite Prepared Using Soluble Methylcellulose. J. Alloys Compd. 2019, 786, 912–919. [Google Scholar] [CrossRef]
- Jugović, D.; Milović, M.; Ivanovski, V.N.; Škapin, S.; Barudžija, T.; Mitrić, M. Microsized Fayalite Fe2SiO4 as Anode Material: The Structure, Electrochemical Properties and Working Mechanism. J. Electroceram. 2021, 47, 31–41. [Google Scholar] [CrossRef]
- Malode, S.J.; Prabhu, K.; Pollet, B.G.; Kalanur, S.S.; Shetti, N.P. Preparation and Performance of WO3/rGO Modified Carbon Sensor for Enhanced Electrochemical Detection of Triclosan. Electrochim. Acta 2022, 429, 141010. [Google Scholar] [CrossRef]
- Sri, V.R.; Shwetharani, R.; Mohammed, J.; Mabkhoot, A.; Balakrishna, R.G.; Harraz, F.A. Review on Electrochemical Sensing of Triclosan Using Nanostructured Semiconductor Materials. ChemElectroChem 2022, 9, e202101664. [Google Scholar] [CrossRef]
- Knežević, S.; Ostojić, J.; Ognjanović, M.; Savić, S.; Kovačević, A.; Manojlović, D.; Stanković, V.; Stanković, D. The Environmentally Friendly Approaches Based on the Heterojunction Interface of the LaFeO3/Fe2O3@g-C3N4 Composite for the Disposable and Laboratory Sensing of Triclosan. Sci. Total Environ. 2023, 857, 159250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Q.-J.; Wang, L. Development and Characterization of an Amperometric Sensor for Triclosan Detection Based on Electropolymerized Molecularly Imprinted Polymer. Microchem. J. 2009, 91, 222–226. [Google Scholar] [CrossRef]
- Baranowska, I.; Bijak, K. Voltammetric Determination of Disinfectants at Multiwalled Carbon Nanotube Modified Glassy Carbon Electrode. J. Anal. Chem. 2013, 68, 891–895. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Z.; Wan, Q.; Liu, Y.; Yang, N. β-Cyclodextrin Functionalized Graphene Nano Platelets for Electrochemical Determination of Triclosan: β-Cyclodextrin Functionalized Graphene Nano Platelets. Phys. Status Solidi A 2014, 211, 2773–2777. [Google Scholar] [CrossRef]
- Libansky, M.; Zima, J.; Barek, J.; Dejmkova, H. Construction of an Electrochemical Cell System Based on Carbon Composite Film Electrodes and Its Application for Voltammetric Determination of Triclosan. Electroanalysis 2014, 26, 1920–1927. [Google Scholar] [CrossRef]
- Luyen, N.D.; Toan, T.T.T.; Trang, H.T.; Nguyen, V.T.; Son, L.V.T.; Thanh, T.S.; Thanh, N.M.; Quy, P.T.; Khieu, D.Q. Electrochemical Determination of Triclosan Using ZIF-11/Activated Carbon Derived from the Rice Husk Modified Electrode. J. Nanomater 2021, 2021, 8486962. [Google Scholar] [CrossRef]



| Electrode | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|
| o-PD-MIP/GCE | 0.2–3 | 0.8 | [43] |
| MWCNT/GC | 0.103–149 | 5.8 | [44] |
| b-CNT/GNPs/GC | 2.0–100 | 0.6 | [45] |
| CCF | 4.2–47 | 0.25 | [46] |
| LaFeO3/Fe2O3@g-C3N4/SPE | 0.3–7 | 0.09 | [42] |
| ZIF-11/activated carbon | 0.1–8 | 0.076 | [47] |
| Y2O3/GCN/CPE | 0.5–100 | 0.14 | This work |
| Sample | Found (µM) | Spiked (µM) | Found After Spiking (µM) | Recovery (%) |
|---|---|---|---|---|
| - | 10 | 11.84 | 118.44 | |
| Tap water | - | 20 | 21.64 | 108.18 |
| - | 40 | 42.33 | 105.82 | |
| - | 10 | 11.75 | 117.50 | |
| Toothpaste sample | - | 20 | 18.81 | 94.04 |
| - | 40 | 46.23 | 115.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mijajlović, A.; Ognjanović, M.; Stanković, V.; Mutić, T.; Đurđić, S.; Petković, B.B.; Stanković, D.M. A Practical Approach to Triclosan Detection: A Novel Y2O3@GCN-Modified Carbon Paste Electrode for Sensitive and Selective Detection in Environmental and Consumer Products. Chemosensors 2024, 12, 272. https://doi.org/10.3390/chemosensors12120272
Mijajlović A, Ognjanović M, Stanković V, Mutić T, Đurđić S, Petković BB, Stanković DM. A Practical Approach to Triclosan Detection: A Novel Y2O3@GCN-Modified Carbon Paste Electrode for Sensitive and Selective Detection in Environmental and Consumer Products. Chemosensors. 2024; 12(12):272. https://doi.org/10.3390/chemosensors12120272
Chicago/Turabian StyleMijajlović, Aleksandar, Miloš Ognjanović, Vesna Stanković, Tijana Mutić, Slađana Đurđić, Branka B. Petković, and Dalibor M. Stanković. 2024. "A Practical Approach to Triclosan Detection: A Novel Y2O3@GCN-Modified Carbon Paste Electrode for Sensitive and Selective Detection in Environmental and Consumer Products" Chemosensors 12, no. 12: 272. https://doi.org/10.3390/chemosensors12120272
APA StyleMijajlović, A., Ognjanović, M., Stanković, V., Mutić, T., Đurđić, S., Petković, B. B., & Stanković, D. M. (2024). A Practical Approach to Triclosan Detection: A Novel Y2O3@GCN-Modified Carbon Paste Electrode for Sensitive and Selective Detection in Environmental and Consumer Products. Chemosensors, 12(12), 272. https://doi.org/10.3390/chemosensors12120272






