Advancing Biosensing and Imaging with DNA-Templated Metal Nanoclusters: Synthesis, Applications, and Future Challenges—A Review
Abstract
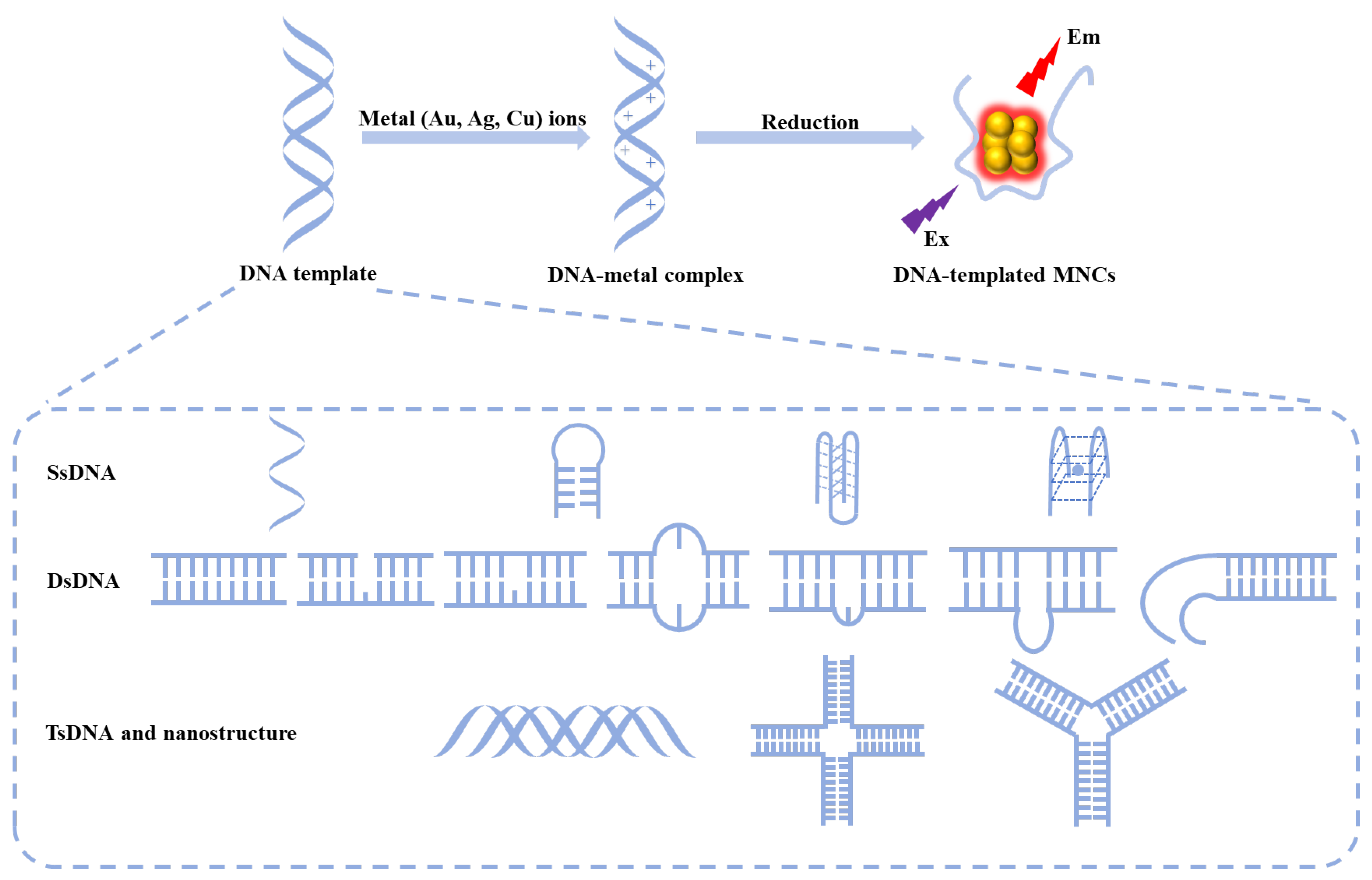
:1. Introduction
2. MNCs with Different DNA Sequences and Structural Variations
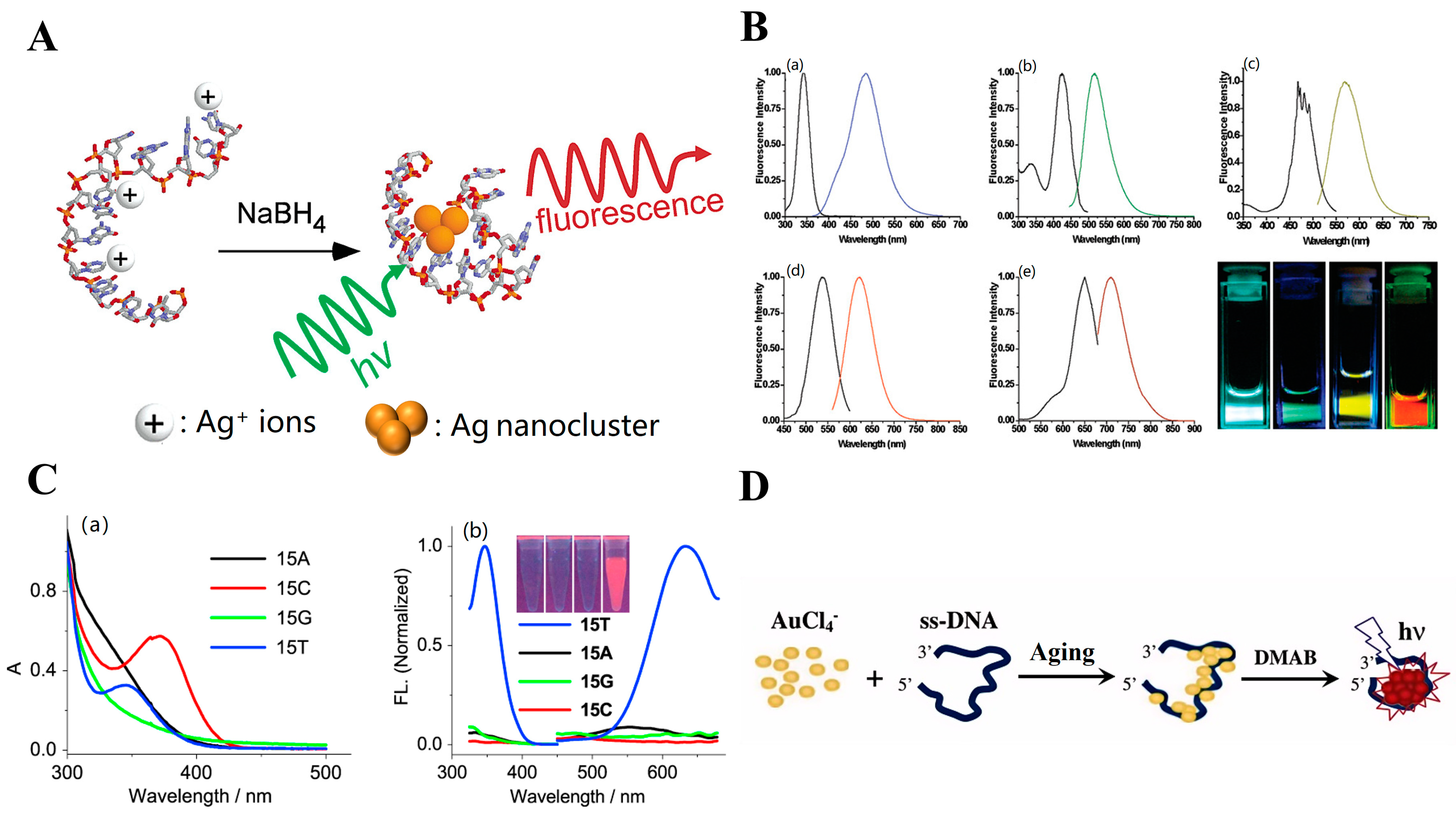
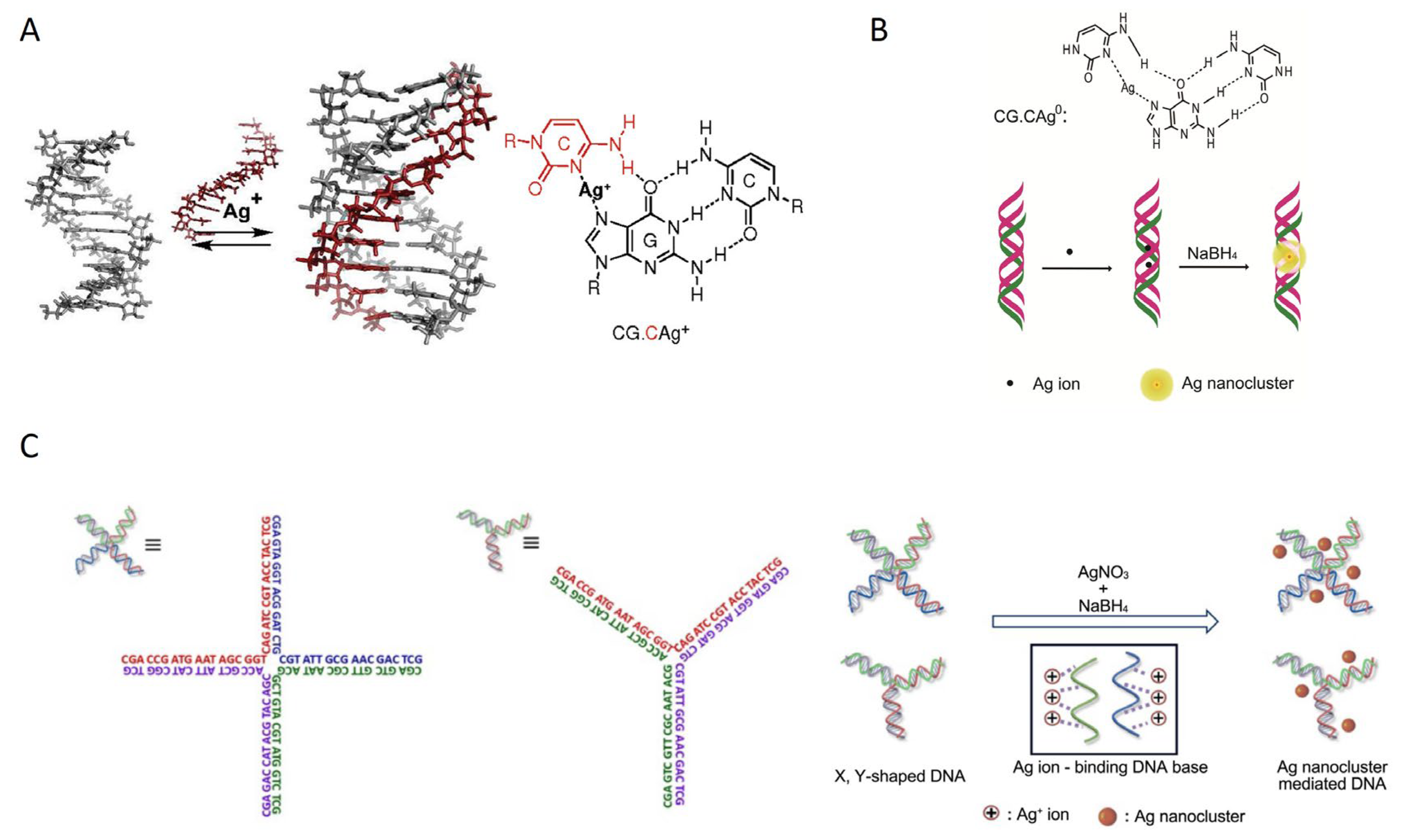
2.1. ssDNA Templates
| Template Type | DNA Sequence (5′-3′) | λex/λem (nm) | QY (%) | Metal | Ref. |
|---|---|---|---|---|---|
ssDNA | AG2TCGC2GC3 | 560/638 | - | Ag | [19] |
| C12 | 340/485, 440/525, 580/655 | - | Ag | [34] | |
| C12 | 650/700 | 17 | Ag | [35] | |
| C3T3A2C4 C3TCT2A2C3 C3T2A2TC4 C2TC2T2C2TC2 C3TA2CTC4 | -/485 -/520 -/572 -/620 -/705 | - | Ag | [20] | |
| T15 T20 T30 | 340/615 | - 3.1 (T20) 6.8 (T30) | Cu | [25,26] | |
| GAG2CGCTGC5AC2ATGAGC | 467 | - | Au | [29] | |
Hairpin Loop | CGCGC12CGCG | 560/615 | 42 | Ag | [36] |
| TATCCGTCnACGGATA (n = 5–9) | 420/636 | - | Au | [37] | |
| TGCCTATT5ACGGATA | 340/620 | - | Cu | [25] | |
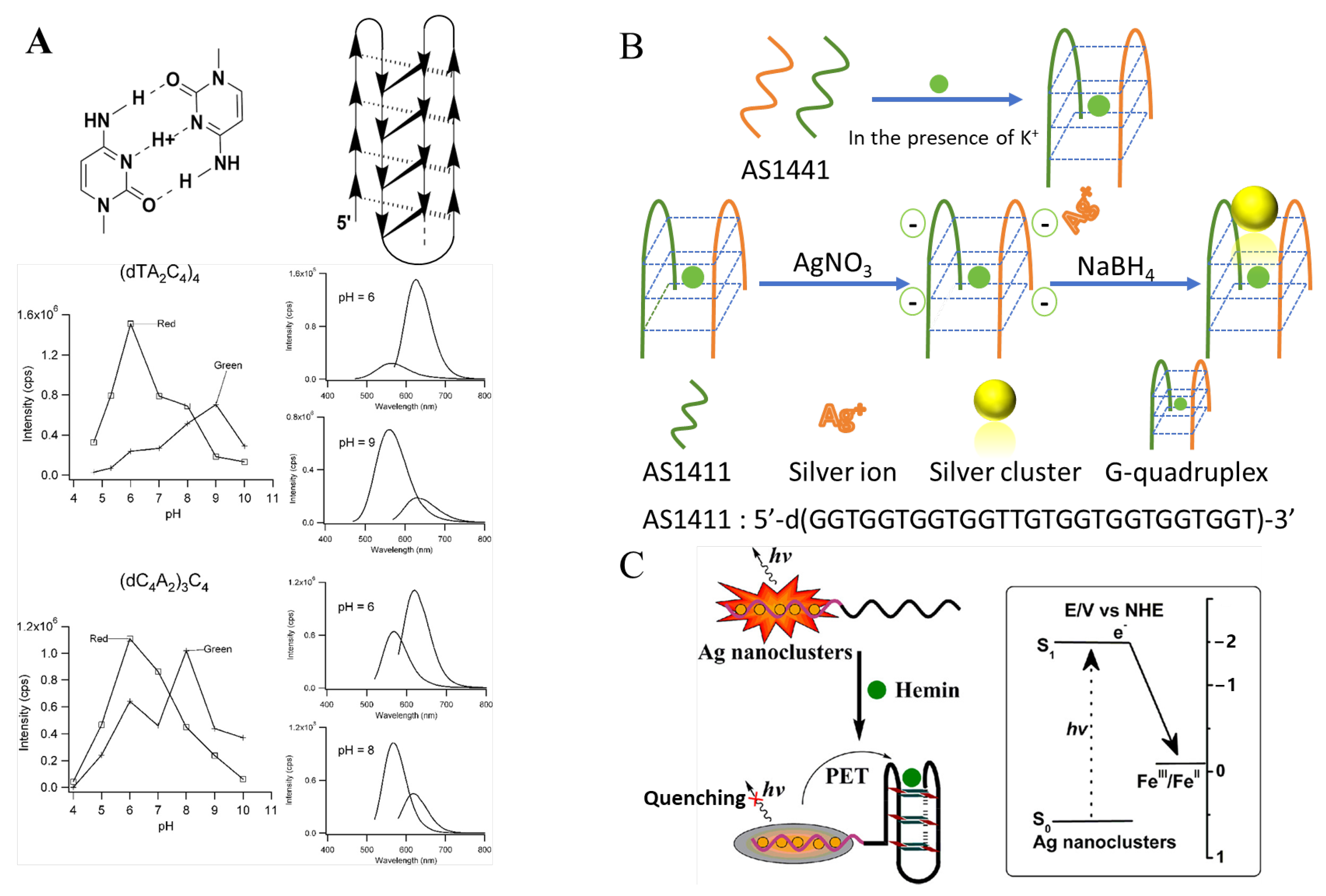
i-motif | (TA2C4)4 (C4A2)3C4 | 460/560 560/652 500/570 560/625 | - - | Ag | [38] |
G-Quadruplexes | (GGT)4TG(TGG)4 | 510/680 | - | Ag | [39] |
2.1.1. Primary Structure ssDNA
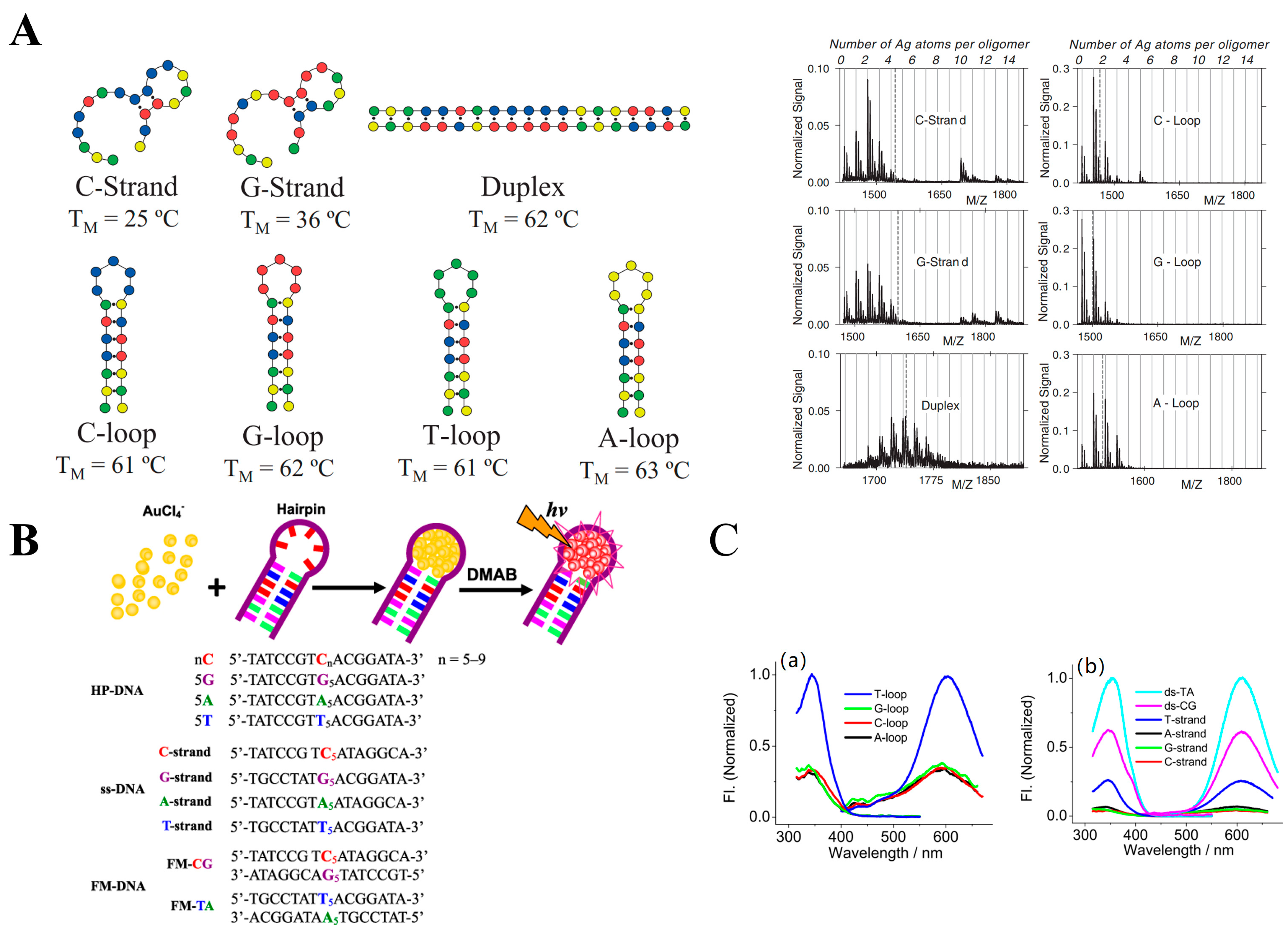
2.1.2. Hairpin Loop
2.1.3. i-Motifs and G-Quadruplexes as Template
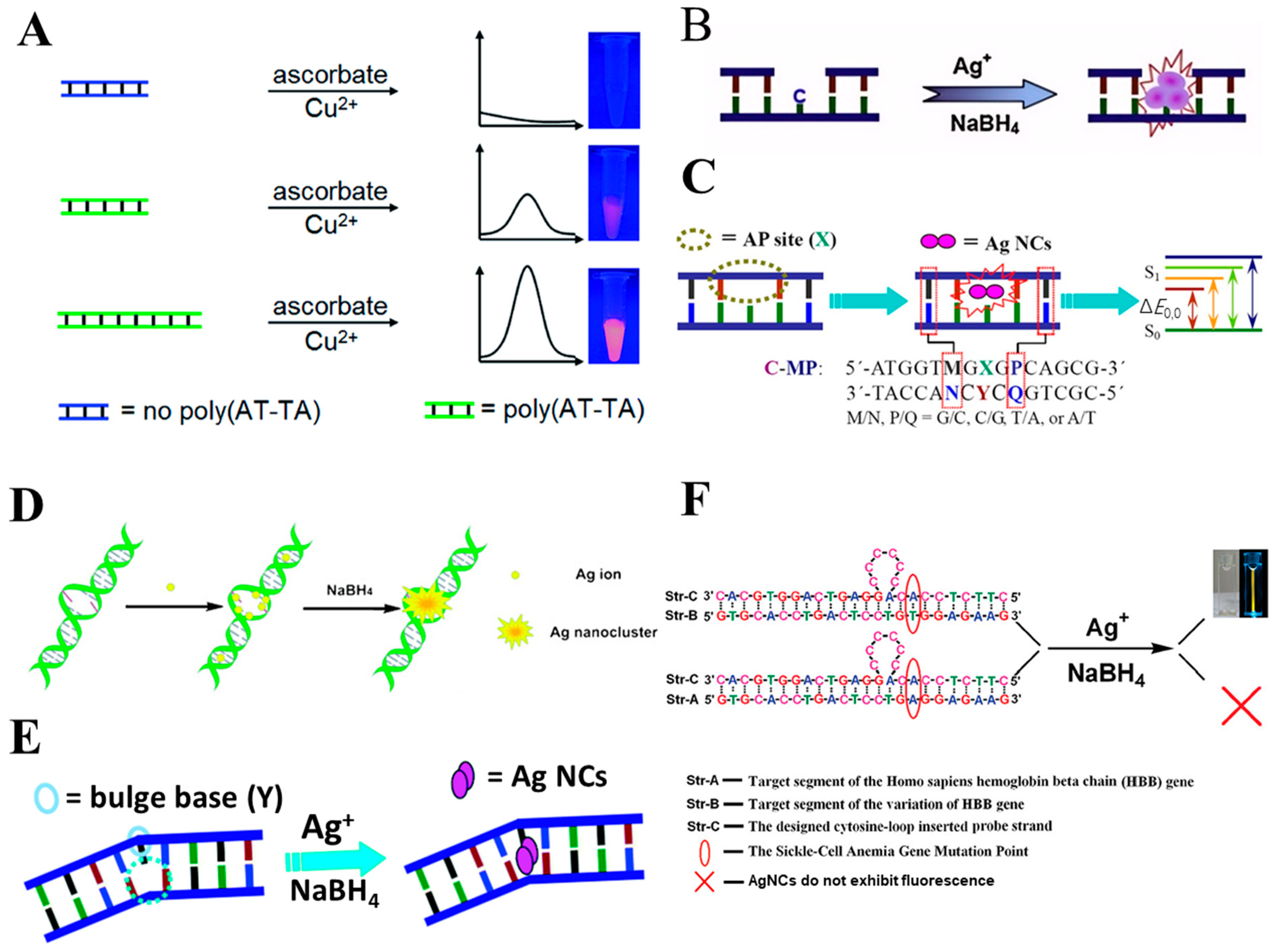
2.2. dsDNA Strand
| Template Type | DNA Sequence | λex/λem (nm) | QY (%) | Metal | Ref. |
|---|---|---|---|---|---|
Duplex | 5′-ATATATATATATATATATATAT-3′ 3′-TATATATATATATATATATATA-5′ | 340/590 | - | Cu | [69] |
Gap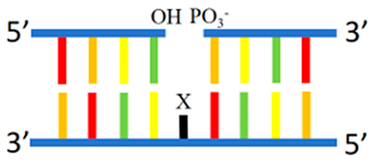 | 5′-GCTCATG2TG2 CGCAGCGCCTC-3′ 3′-CGAGTAC2AC2YGCGTCGCGGAG-5′ Y = C, A, G, or T | 560/643 (Y = C) | 47.2 | Ag | [58] |
| 5′-C2ACG2ATCTGA G3TGA3TAT2CTC-3′ 3′-G2TGC2TAGACTYC3ACT3ATA2GAG-5′ Y = C, A, G, or T | 585/665 (Y = C) | - | Ag | [58] | |
AP site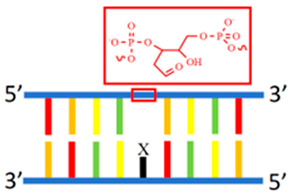 | 5′-ATGT2GGYGGTCATTYGGT2ATG-3′ 3′-TACA2CCCCCAGTCCCCCA2TAC-5′ | 588/670 | - | Ag | [60] |
| 5′-ATG2TGGYGGCAGCG-3′ 3′-TAC2ACCXCCGTCGC-5′ | 588/670 (X = C) | - | Ag | [59] | |
Mismatched site | 5′-C3TA2C3TA2C3TA2C3T-3′ 3′-G3AT2G3XT2G3AT2G3A-5′ X = A, T, G, or C | 520/570 (X = T) | 8.1 | Ag | [57] |
| 5′-GCATGTAC2CnG2A2GATCG-3′ 3′-CGTACATG2GnC2T2CTAGC-5′ n = 3, 4, 5 | 563/654 (n = 4) | - | Ag | [61] | |
Bulge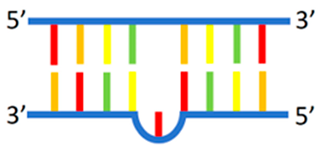 | 5′-ATGGTGG GGCAGCG-3′ 3′-TACCACCYCCGTCGC-5′ Y = bulge base (C, A, G, or T) | 589/652 (Y = C) | - | Ag | [62] |
| 5′-CGCTGCGYGCACCAT-3′ 3′-GCGACGCXCGTGGAT-5′ Y = T, C, A, or G, X = AP site | 565/624 (Y = T) | - | Ag | [63] | |
Loop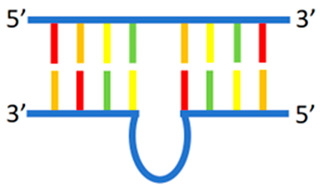 | 5′-GTGCAC2TGACTC2TGTG2AGA2G-3′ 3′-CACGTG2ACTGAG2CnCAC2TCT2C-5′ n = 4, 6, 8 | 520/572 (n = 6) | - | Ag | [64] |
| 5′-CT2CTC2AC6CAG2AGTCAG2TGCAC-3′ 3′-GA2GAG2AGTC2TCAGTC2ACGTGACT2GAT2GT-5′ | 575/635 | - | Ag | [65] | |
Nanocluster beacons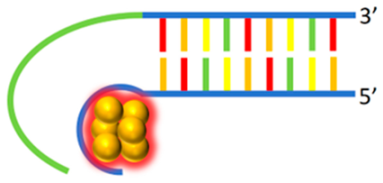 | 5′-CCCTTAATCCCCTGTAGCTAGACCAAA- ATCACCTAT-3′ 3′-CCCACTCCATCGAGATTTCAC GGGTGGGGTGGGGTGGGG-5′ | 580/636 | - | Ag | [66] |
| 5′-CCCTTAATCCCCTATTTCAAGCCGGAA ATAGCAATAAGAC-3′ 3′-GGGTCATCAAGATACAGCAAGAAG- ATAGGGTGGGGTGGGGTGGGG-5′ | 580/636 | - | Ag | [66] |
2.2.1. dsDNA Containing Duplex, Gap, Abasic, Mismatched Site, Bulge, and Loop as Template
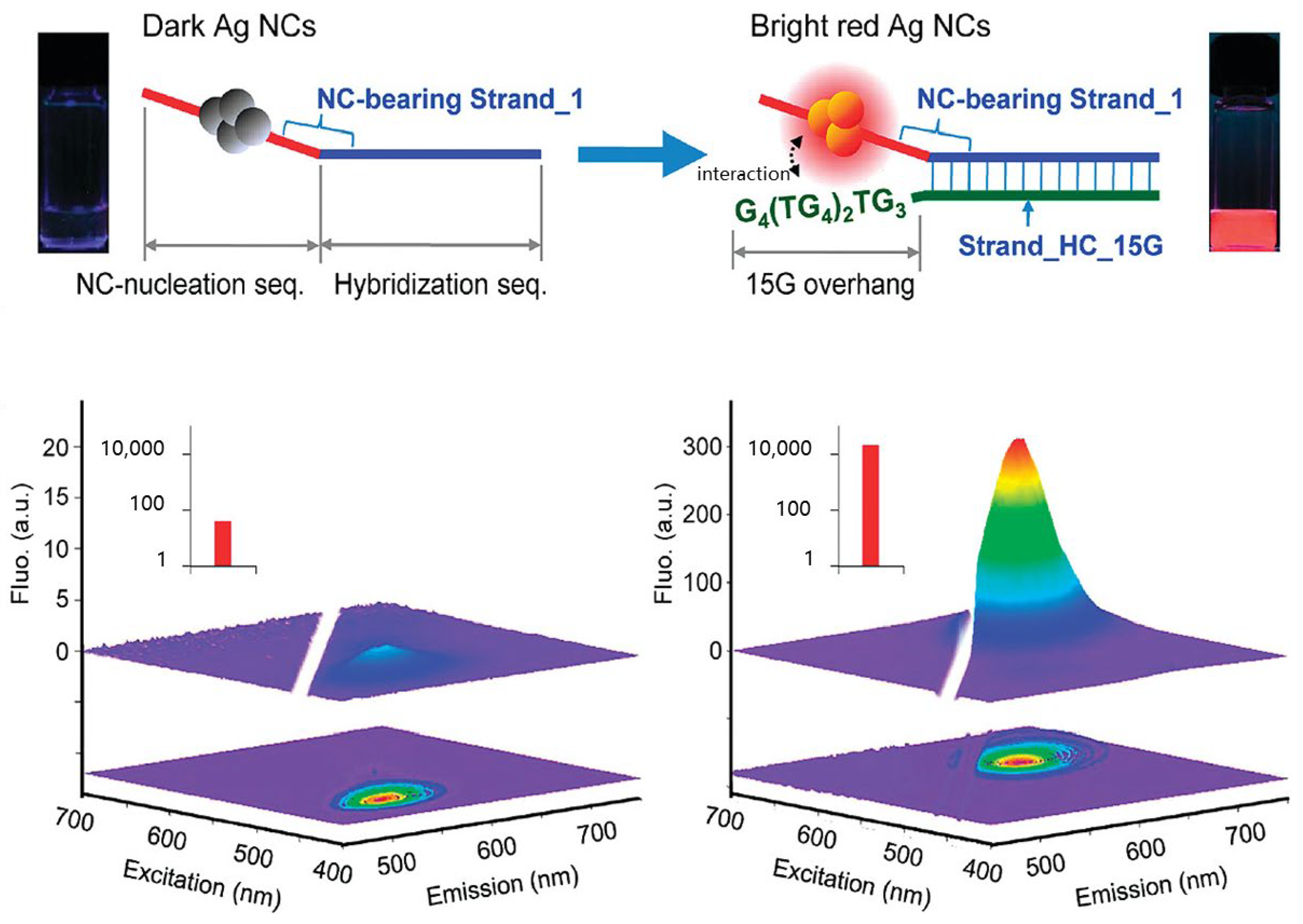
2.2.2. NanoCluster Beacons
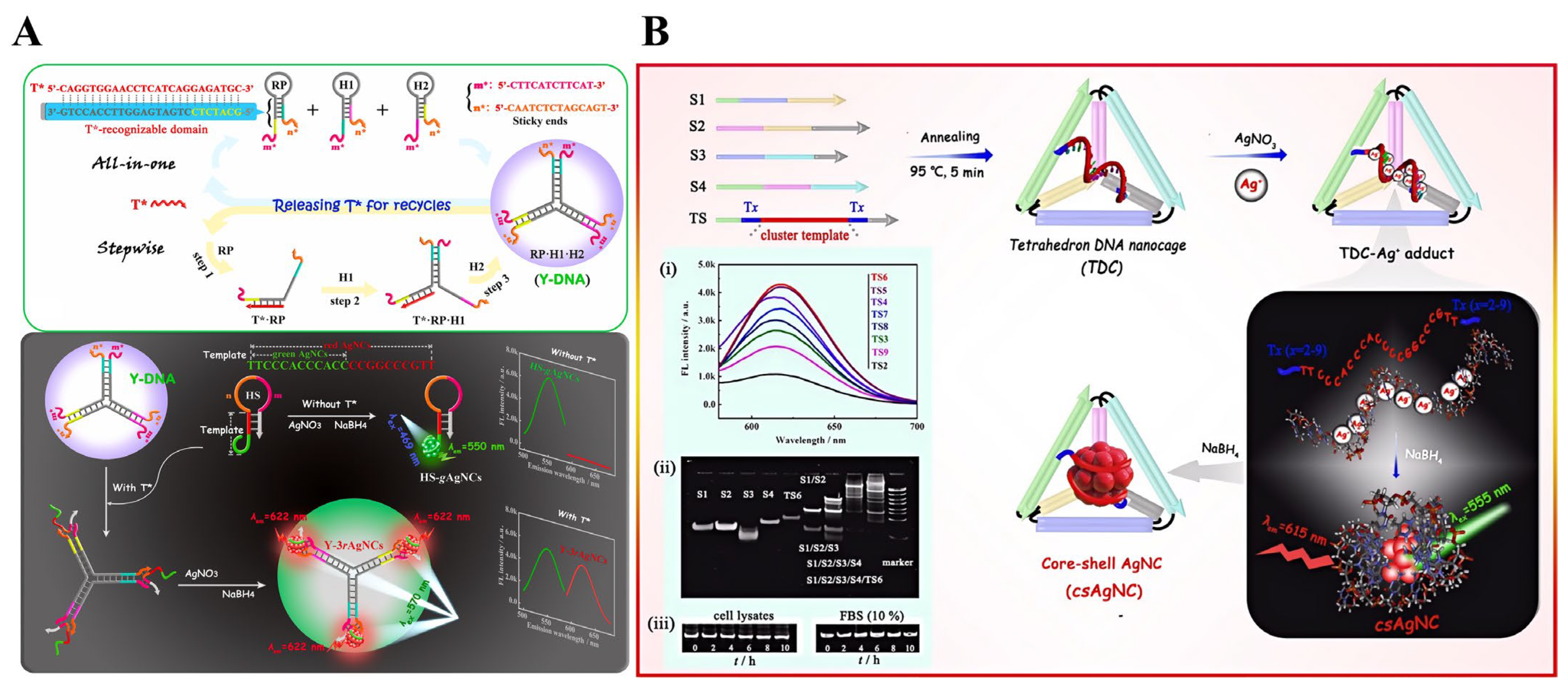
2.3. tsDNA and DNA Nanostructures
3. Applications of Structurally Diverse DNA-MNCs for Biosensing and Imaging
3.1. ssDNA-Templated MNCs
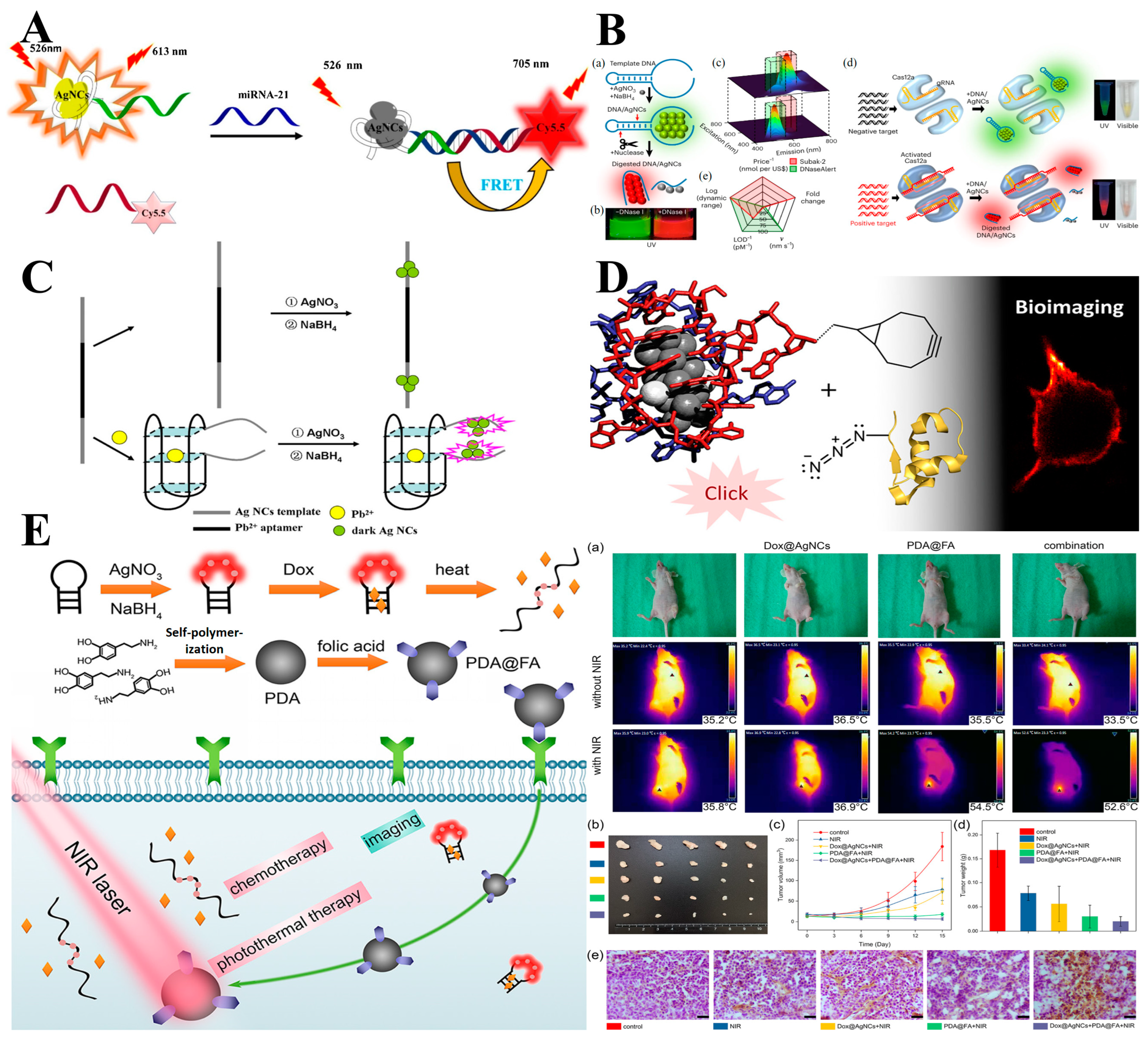
3.2. dsDNA-Templated MNCs
3.3. tsDNA and Nanostructure-Templated MNCs
4. Conclusions and Outlook
- Enhancing stability and quantum yield: Explore new DNA sequences and metal ion combinations to improve the stability and QYs of DNA-MNCs. This might involve designing DNA scaffolds to better stabilize and facilitate the formation of highly emissive NCs. The development of DNA-AuNCs should be an effective pathway to acquire stable DNA-MNCs due the chemical inertness of Au. To enhance the QYs of DNA-MNCs, the introduction of aggregation-induced emission (AIE) effect should be a desirable approach. Possible ligand molecule designs with restricted inter/inner molecular motion could significantly improve the fluorescence of DNA-MNCs.
- Development of NIR-II DNA-MNC emitters: Emitters in the second NIR window (NIR-II) are exceptionally rare, hindering their application in biosensing and therapy. Utilize machine learning and deep learning models to guide the synthesis of DNA-AgNCs with emissions in the NIR-II window. This would involve creating large databases of DNA sequences and their corresponding emission properties for model training. Developing predictive models could generate DNA sequences likely to produce NIR-II emitters, reducing the need for extensive experimental screening.
- Crystal growth innovation: The current scarcity of known crystal structures for DNA-MNCs poses significant challenges for their atomical-precision study. Thus, rational designs for functionalizing them with proper targeted labeling are difficult, due to the lack of exact structural information. Explore new techniques for growing DNA-MNCs suitable for X-ray crystallography, including the use of novel crystallization agents, temperature control, or alternative growth environments. Also, explore the use of microcrystal electron diffraction (microED) and other electron microscopy techniques, such as cryo-electron microscopy, to overcome the limitations of traditional X-ray diffraction, facilitating the structural determination of DNA-MNCs.
- Biocompatibility and toxicity studies: While in vitro cytotoxicity experiments of DNA-MNCs have shown good biocompatibility, their in vivo toxicity and organ metabolism are far more than understanding. Thus, it is encouraged to conduct comprehensive in vivo studies to evaluate the long-term toxicity and metabolism of DNA-MNCs. Pharmacokinetic and biodistribution studies would help us to understand the biological destination of DNA-MNCs and their behavior in biological systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yau, S.H.; Varnavski, O.; Goodson, T., III. An ultrafast look at Au nanoclusters. Acc. Chem. Res. 2013, 46, 1506–1516. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, W. Sub-nanometre sized metal clusters: From synthetic challenges to the unique property discoveries. Chem. Soc. Rev. 2012, 41, 3594–3623. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhu, M.; Wu, Z.; Jin, R. Quantum sized gold nanoclusters with atomic precision. Acc. Chem. Res. 2012, 45, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.-J.; Xu, K. Fluorescent metal nanoclusters: From synthesis to applications. TrAC Trends Anal. Chem. 2014, 58, 90–98. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Higaki, T.; Li, Y.; Zhao, S.; Li, Q.; Li, S.; Du, X.S.; Yang, S.; Chai, J.; Jin, R. Atomically tailored gold nanoclusters for catalytic application. Angew. Chem. 2019, 131, 8377–8388. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Zhou, X.; Khusbu, F.Y.; Ma, C. Recent advances in the bioanalytical and biomedical applications of DNA-templated silver nanoclusters. TrAC Trends Anal. Chem. 2020, 124, 115786. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, J.; Jiang, H.; Wang, X. Gold nanoclusters for theranostic applications. Coord. Chem. Rev. 2021, 431, 213689. [Google Scholar] [CrossRef]
- Xu, F.; Qing, T.; Qing, Z. DNA-coded metal nano-fluorophores: Preparation, properties and applications in biosensing and bioimaging. Nano Today 2021, 36, 101021. [Google Scholar] [CrossRef]
- Yang, M.; Xie, Y.; Zhu, L.; Li, X.; Xu, W. Functional Nucleic Acid Enzymes: Nucleic Acid-Based Catalytic Factories. ACS Catal. 2024, 14, 16392–16422. [Google Scholar] [CrossRef]
- Zhou, B.; Khan, I.M.; Ding, X.; Niazi, S.; Zhang, Y.; Wang, Z. Fluorescent DNA-Silver nanoclusters in food safety detection: From synthesis to application. Talanta 2024, 273, 125834. [Google Scholar] [CrossRef]
- Niazi, S.; Khan, I.M.; Akhtar, W.; ul Haq, F.; Pasha, I.; Khan, M.K.I.; Mohsin, A.; Ahmad, S.; Zhang, Y.; Wang, Z. Aptamer functionalized gold nanoclusters as an emerging nanoprobe in biosensing, diagnostic, catalysis and bioimaging. Talanta 2023, 268, 125270. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lu, M.; Wang, J.; Man, S.; Peng, W.; Ma, L. Recent advances of DNA-templated metal nanoclusters for food safety detection: From synthesis, applications, challenges, and beyond. J. Agric. Food Chem. 2024, 72, 5542–5554. [Google Scholar] [CrossRef] [PubMed]
- Duguid, J.; Bloomfield, V.A.; Benevides, J.; Thomas, G. Raman spectroscopy of DNA-metal complexes. I. Interactions and conformational effects of the divalent cations: Mg, Ca, Sr, Ba, Mn, Co, Ni, Cu, Pd, and Cd. Biophys. J. 1993, 65, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.T.; Zheng, J.; Hud, N.V.; Dickson, R.M. DNA-templated Ag nanocluster formation. J. Am. Chem. Soc. 2004, 126, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.I.; Choi, S.; Hsiang, J.-C.; Antoku, Y.; Vosch, T.; Bongiorno, A.; Tzeng, Y.-L.; Dickson, R.M. Oligonucleotide-stabilized Ag nanocluster fluorophores. J. Am. Chem. Soc. 2008, 130, 5038–5039. [Google Scholar] [CrossRef]
- Huard, D.J.; Demissie, A.; Kim, D.; Lewis, D.; Dickson, R.M.; Petty, J.T.; Lieberman, R.L. Atomic structure of a fluorescent Ag8 cluster templated by a multistranded DNA scaffold. J. Am. Chem. Soc. 2018, 141, 11465–11470. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, C.; Kanazawa, H.; Vosch, T.; Kondo, J. Crystal structure of a NIR-emitting DNA-stabilized Ag16 nanocluster. Angew. Chem. Int. Ed. 2019, 58, 17153–17157. [Google Scholar] [CrossRef] [PubMed]
- Gonzàlez-Rosell, A.; Malola, S.; Guha, R.; Arevalos, N.R.; Matus, M.F.; Goulet, M.E.; Haapaniemi, E.; Katz, B.B.; Vosch, T.; Kondo, J. Chloride ligands on DNA-stabilized silver nanoclusters. J. Am. Chem. Soc. 2023, 145, 10721–10729. [Google Scholar] [CrossRef]
- Rotaru, A.; Dutta, S.; Jentzsch, E.; Gothelf, K.; Mokhir, A. Selective dsDNA-templated formation of copper nanoparticles in solution. Angew. Chem. Int. Ed. 2010, 49, 5665–5667. [Google Scholar] [CrossRef]
- Liu, G.; Shao, Y.; Peng, J.; Dai, W.; Liu, L.; Xu, S.; Wu, F.; Wu, X. Highly thymine-dependent formation of fluorescent copper nanoparticles templated by ss-DNA. Nanotechnology 2013, 24, 345502. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; He, X.; He, D.; Wang, K.; Xu, F.; Qing, T.; Yang, X. Poly (thymine)-templated selective formation of fluorescent copper nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 9719–9722. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhu, M.; Yang, Z.; Song, X.; Yuan, X.; Zhang, Z.; Hu, W.; Xie, J. Molecule-like synthesis of ligand-protected metal nanoclusters. Nat. Rev. Mater. 2024. [Google Scholar] [CrossRef]
- Liu, G.; Shao, Y.; Ma, K.; Cui, Q.; Wu, F.; Xu, S. Synthesis of DNA-templated fluorescent gold nanoclusters. Gold Bull. 2012, 45, 69–74. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. DNA-templated fluorescent gold nanoclusters reduced by Good’s buffer: From blue-emitting seeds to red and near infrared emitters. Can. J. Chem. 2015, 93, 615–620. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, G.; Lin, R.; Pei, X.; Liu, F.; Li, N. Pre-Incubation of Auric Acid with DNA Is Unnecessary for the Formation of DNA-Templated Gold Nanoclusters. Chem.–Asian J. 2016, 11, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.M. Nucleic Acids in Chemistry and Biology; Royal Society of Chemistry: London, UK, 2006. [Google Scholar]
- Mastracco, P.; Copp, S.M. Beyond nature’s base pairs: Machine learning-enabled design of DNA-stabilized silver nanoclusters. Chem. Commun. 2023, 59, 10360–10375. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.M.; Johnsen, K.R.; Kiser, J.R.; Antoku, Y.; Dickson, R.M.; Petty, J.T. Ag nanocluster formation using a cytosine oligonucleotide template. J. Phys. Chem. C 2007, 111, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Vosch, T.; Antoku, Y.; Hsiang, J.-C.; Richards, C.I.; Gonzalez, J.I.; Dickson, R.M. Strongly emissive individual DNA-encapsulated Ag nanoclusters as single-molecule fluorophores. Proc. Natl. Acad. Sci. USA 2007, 104, 12616–12621. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yu, J.; Patel, S.A.; Tzeng, Y.-L.; Dickson, R.M. Tailoring silver nanodots for intracellular staining. Photochem. Photobiol. Sci. 2011, 10, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shao, Y.; Wu, F.; Xu, S.; Peng, J.; Liu, L. DNA-hosted fluorescent gold nanoclusters: Sequence-dependent formation. Nanotechnology 2012, 24, 015503. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, B.; Springer, K.; Buckman, J.G.; Story, S.P.; Abe, O.H.; Hasan, Z.W.; Prudowsky, Z.D.; Rudisill, S.E.; Degtyareva, N.N.; Petty, J.T. DNA templates for fluorescent silver clusters and i-motif folding. J. Phys. Chem. C 2009, 113, 19518–19524. [Google Scholar] [CrossRef]
- Ai, J.; Guo, W.; Li, B.; Li, T.; Li, D.; Wang, E. DNA G-quadruplex-templated formation of the fluorescent silver nanocluster and its application to bioimaging. Talanta 2012, 88, 450–455. [Google Scholar] [CrossRef]
- Sengupta, B.; Ritchie, C.M.; Buckman, J.G.; Johnsen, K.R.; Goodwin, P.M.; Petty, J.T. Base-directed formation of fluorescent silver clusters. J. Phys. Chem. C 2008, 112, 18776–18782. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Y.; Ma, X.; Xia, M.; Li, S.; Zhang, Y. Thiolated DNA-templated silver nanoclusters with strong fluorescence emission and a long shelf-life. Nanoscale 2018, 10, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Babanova, S.; Rocha, R.C.; Desireddy, A.; Artyushkova, K.; Boncella, A.E.; Atanassov, P.; Martinez, J.S. A Hybrid DNA-Templated Gold Nanocluster for Enhanced Enzymatic Reduction of Oxygen. J. Am. Chem. Soc. 2015, 137, 11678–11687. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Wu, Y.-T.; Tseng, W.-L. UV-light-induced improvement of fluorescence quantum yield of DNA-templated gold nanoclusters: Application to ratiometric fluorescent sensing of nucleic acids. ACS Appl. Mater. Interfaces 2015, 7, 23708–23716. [Google Scholar] [CrossRef] [PubMed]
- Matus, M.F.; Häkkinen, H. Understanding ligand-protected noble metal nanoclusters at work. Nat. Rev. Mater. 2023, 8, 372–389. [Google Scholar] [CrossRef]
- New, S.; Lee, S.; Su, X. DNA-templated silver nanoclusters: Structural correlation and fluorescence modulation. Nanoscale 2016, 8, 17729–17746. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.M.; Bogdanov, P.; Debord, M.; Singh, A.; Gwinn, E. Base motif recognition and design of DNA templates for fluorescent silver clusters by machine learning. Adv. Mater. 2014, 26, 5839–5845. [Google Scholar] [CrossRef]
- Copp, S.M.; Gorovits, A.; Swasey, S.M.; Gudibandi, S.; Bogdanov, P.; Gwinn, E.G. Fluorescence color by data-driven design of genomic silver clusters. ACS Nano 2018, 12, 8240–8247. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.M.; Swasey, S.M.; Gorovits, A.; Bogdanov, P.; Gwinn, E.G. General approach for machine learning-aided design of DNA-stabilized silver clusters. Chem. Mater. 2019, 32, 430–437. [Google Scholar] [CrossRef]
- Mastracco, P.; Gonzàlez-Rosell, A.; Evans, J.; Bogdanov, P.; Copp, S.M. Chemistry-informed machine learning enables discovery of DNA-stabilized silver nanoclusters with near-infrared fluorescence. ACS Nano 2022, 16, 16322–16331. [Google Scholar] [CrossRef] [PubMed]
- Copp, S.M.; Schultz, D.; Swasey, S.; Pavlovich, J.; Debord, M.; Chiu, A.; Olsson, K.; Gwinn, E. Magic numbers in DNA-stabilized fluorescent silver clusters lead to magic colors. J. Phys. Chem. Lett. 2014, 5, 959–963. [Google Scholar] [CrossRef]
- Gwinn, E.G.; O’Neill, P.; Guerrero, A.J.; Bouwmeester, D.; Fygenson, D.K. Sequence-Dependent fluorescence of DNA-Hosted silver nanoclusters. Adv. Mater. 2008, 20, 279–283. [Google Scholar] [CrossRef]
- Guéron, M.; Leroy, J.-L. The i-motif in nucleic acids. Curr. Opin. Struct. Biol. 2000, 10, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Day, H.A.; Huguin, C.; Waller, Z.A. Silver cations fold i-motif at neutral pH. Chem. Commun. 2013, 49, 7696–7698. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, L.; Fu, Y.; Sun, Y.; Zhang, J.; Zhang, R. Effects of polymorphic DNA on the fluorescent properties of silver nanoclusters. Photochem. Photobiol. Sci. 2013, 12, 1864–1872. [Google Scholar] [CrossRef]
- Huppert, J.L. Four-stranded nucleic acids: Structure, function and targeting of G-quadruplexes. Chem. Soc. Rev. 2008, 37, 1375–1384. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Guo, S.; Li, T.; Li, J.; Wang, E. Photoinduced electron transfer of DNA/Ag nanoclusters modulated by G-quadruplex/hemin complex for the construction of versatile biosensors. J. Am. Chem. Soc. 2013, 135, 2403–2406. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pu, F.; Hu, D.; Wang, C.; Ren, J.; Qu, X. Site-specific DNA-programmed growth of fluorescent and functional silver nanoclusters. Chem.–Eur. J. 2011, 17, 3774–3780. [Google Scholar] [CrossRef]
- Cui, Q.; Ma, K.; Shao, Y.; Xu, S.; Wu, F.; Liu, G.; Teramae, N.; Bao, H. Gap site-specific rapid formation of fluorescent silver nanoclusters for label-free DNA nucleobase recognition. Anal. Chim. Acta 2012, 724, 86–91. [Google Scholar] [CrossRef]
- Ma, K.; Cui, Q.; Liu, G.; Wu, F.; Xu, S.; Shao, Y. DNA abasic site-directed formation of fluorescent silver nanoclusters for selective nucleobase recognition. Nanotechnology 2011, 22, 305502. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Shao, Y.; Cui, Q.; Wu, F.; Xu, S.; Liu, G. Base-stacking-determined fluorescence emission of DNA abasic site-templated silver nanoclusters. Langmuir 2012, 28, 15313–15322. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Shao, Y.; Liu, L.; Zhang, L.; Fu, W.; Liu, H. Role of anion polarizability in fluorescence sensitization of DNA-templated silver nanoclusters. Nanotechnology 2014, 25, 235501. [Google Scholar] [CrossRef]
- Peng, J.; Shao, Y.; Liu, L.; Zhang, L.; Liu, H. Specific recognition of DNA bulge sites by in situ grown fluorescent Ag nanoclusters with high selectivity. Dalton Trans. 2014, 43, 1534–1541. [Google Scholar] [CrossRef]
- Peng, J.; Shao, Y.; Liu, L.; Zhang, L.; Liu, H.; Wang, Y. Ag nanoclusters as probes for turn-on fluorescence recognition of TpG dinucleotide with a high selectivity. Anal. Chim. Acta 2014, 850, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yuan, J.; Dong, Q.; Wang, E. Highly sequence-dependent formation of fluorescent silver nanoclusters in hybridized DNA duplexes for single nucleotide mutation identification. J. Am. Chem. Soc. 2010, 132, 932–934. [Google Scholar] [CrossRef]
- Guo, W.; Yuan, J.; Wang, E. Strand exchange reaction modulated fluorescence “off–on” switching of hybridized DNA duplex stabilized silver nanoclusters. Chem. Commun. 2011, 47, 10930–10932. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Sharma, J.; Han, J.J.; Martinez, J.S.; Werner, J.H. A DNA−silver nanocluster probe that fluoresces upon hybridization. Nano Lett. 2010, 10, 3106–3110. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Sharma, J.; Han, J.J.; Martinez, J.S.; Werner, J.H. A beacon of light. IEEE Nanotechnol. Mag. 2011, 5, 28–33. [Google Scholar] [CrossRef]
- Obliosca, J.M.; Babin, M.C.; Liu, C.; Liu, Y.-L.; Chen, Y.-A.; Batson, R.A.; Ganguly, M.; Petty, J.T.; Yeh, H.-C. A complementary palette of NanoCluster Beacons. ACS Nano 2014, 8, 10150–10160. [Google Scholar] [CrossRef] [PubMed]
- Qing, T.; Qing, Z.; Mao, Z.; He, X.; Xu, F.; Wen, L.; He, D.; Shi, H.; Wang, K. dsDNA-templated fluorescent copper nanoparticles: Poly (AT-TA)-dependent formation. RSC Adv. 2014, 4, 61092–61095. [Google Scholar] [CrossRef]
- Tyagi, S.; Marras, S.A.; Kramer, F.R. Wavelength-shifting molecular beacons. Nat. Biotechnol. 2000, 18, 1191–1196. [Google Scholar] [CrossRef]
- Ihara, T.; Ishii, T.; Araki, N.; Wilson, A.W.; Jyo, A. Silver ion unusually stabilizes the structure of a parallel-motif DNA triplex. J. Am. Chem. Soc. 2009, 131, 3826–3827. [Google Scholar] [CrossRef]
- Feng, L.; Huang, Z.; Ren, J.; Qu, X. Toward site-specific, homogeneous and highly stable fluorescent silver nanoclusters fabrication on triplex DNA scaffolds. Nucleic Acids Res. 2012, 40, e122. [Google Scholar] [CrossRef]
- Park, J.; Song, J.; Park, J.; Park, N.; Kim, S. Branched DNA-based synthesis of fluorescent silver nanocluster. Bull. Korean Chem. Soc. 2014, 35, 1105–1109. [Google Scholar] [CrossRef]
- Peng, M.; Fang, Z.; Na, N.; Ouyang, J. A versatile single-molecule counting-based platform by generation of fluorescent silver nanoclusters for sensitive detection of multiple nucleic acids. Nanoscale 2019, 11, 16606–16613. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Zhang, F.; Chen, C.; Cai, C. DNA-programming multicolor silver nanoclusters for sensitively simultaneous detection of two HIV DNAs. Sens. Actuators B Chem. 2019, 296, 126608. [Google Scholar] [CrossRef]
- Nasirian, V.; Shamsipur, M.; Molaabasi, F.; Mansouri, K.; Sarparast, M.; Salim, V.; Barati, A.; Kashanian, S. miRNA-21 rapid diagnosis by one-pot synthesis of highly luminescent red emissive silver nanoclusters/DNA. Sens. Actuators B Chem. 2020, 308, 127673. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Li, Y.; Liao, D.; Zhang, W.; Han, X.; Man, S. A ratiometric fluorescent biosensing platform for ultrasensitive detection of Salmonella typhimurium via CRISPR/Cas12a and silver nanoclusters. J. Hazard. Mater. 2023, 443, 130234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F.; Aizen, R.; Yehezkeli, O.; Willner, I. Graphene oxide/nucleic-acid-stabilized silver nanoclusters: Functional hybrid materials for optical aptamer sensing and multiplexed analysis of pathogenic DNAs. J. Am. Chem. Soc. 2013, 135, 11832–11839. [Google Scholar] [CrossRef]
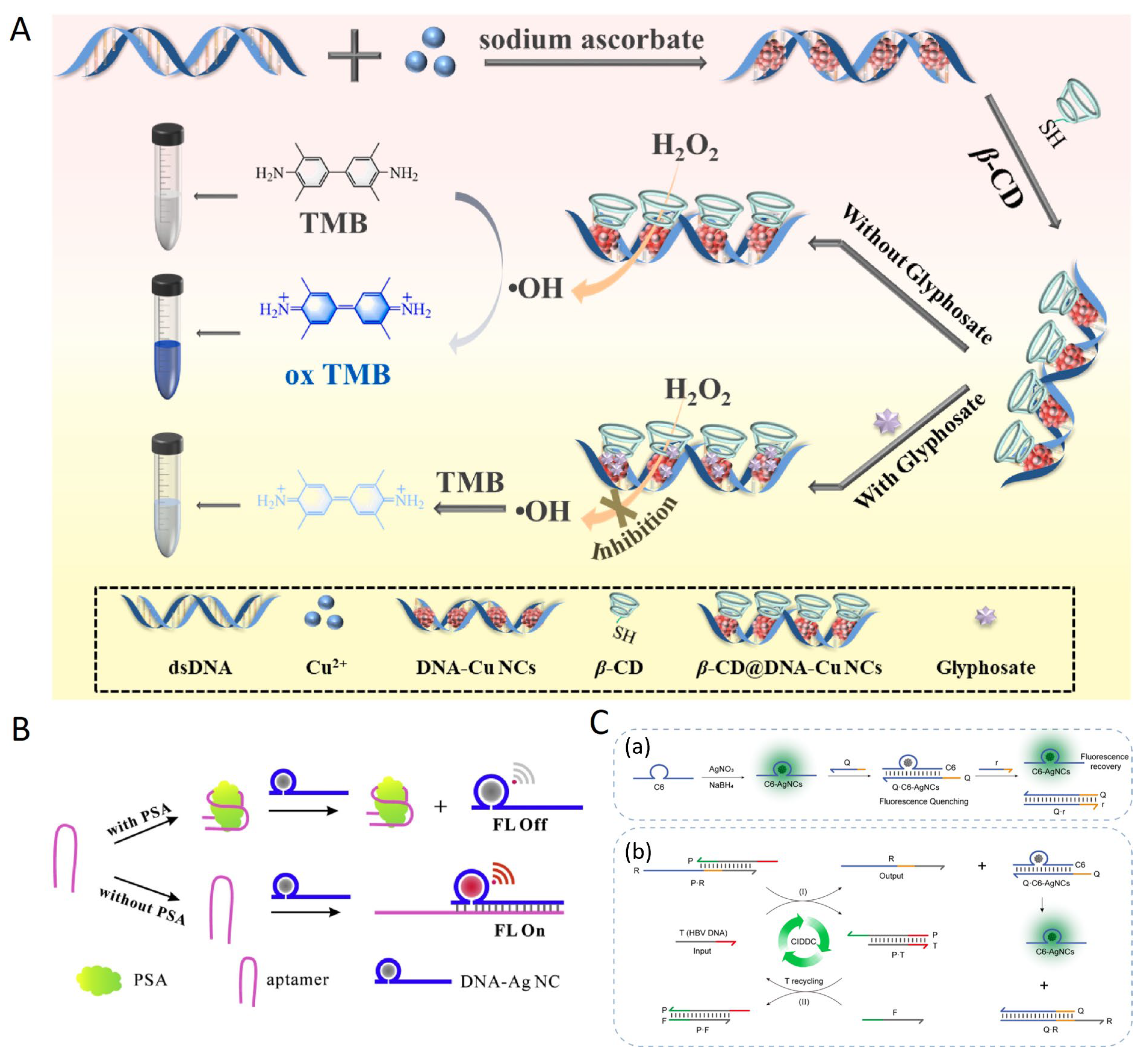
- Fang, B.-Y.; An, J.; Liu, B.; Zhao, Y.-D. Hybridization induced fluorescence enhanced DNA-Ag nanocluster/aptamer probe for detection of prostate-specific antigen. Colloids Surf. B Biointerfaces 2019, 175, 358–364. [Google Scholar] [CrossRef]
- Hong, S.; Walker, J.N.; Luong, A.T.; Mathews, J.; Shields, S.W.; Kuo, Y.-A.; Chen, Y.-I.; Nguyen, T.D.; He, Y.; Nguyen, A.-T. A non-FRET DNA reporter that changes fluorescence colour upon nuclease digestion. Nat. Nanotechnol. 2024, 19, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Liu, Y.-R.; Kong, R.-M.; Donovan, M.J.; Zhang, X.-B.; Tan, W.; Shen, G.-L.; Yu, R.-Q. Double-strand DNA-templated formation of copper nanoparticles as fluorescent probe for label free nuclease enzymedetection. Biosens. Bioelectron. 2013, 42, 31–35. [Google Scholar] [CrossRef]
- Ge, J.; Dong, Z.-Z.; Bai, D.-M.; Zhang, L.; Hu, Y.-L.; Ji, D.-Y.; Li, Z.-H. A novel label-free fluorescent molecular beacon for the detection of 3′–5′ exonuclease enzymatic activity using DNA-templated copper nanoclusters. New J. Chem. 2017, 41, 9718–9723. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, K.S.; Jung, Y.K.; Park, H.G. A label-free fluorescent assay for deoxyribonuclease I activity based on DNA-templated silver nanocluster/graphene oxide nanocomposite. Biosens. Bioelectron. 2017, 93, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Q.; Jin, Y.; Li, B. Fluorescent enzyme-linked immunoassay strategy based on enzyme-triggered in-situ synthesis of fluorescent copper nanoclusters. Sens. Actuators B Chem. 2019, 281, 28–33. [Google Scholar] [CrossRef]
- Peng, M.; Na, N.; Ouyang, J. A fluorescence light-up silver nanocluster beacon modulated by metal ions and its application in telomerase-activity detection. Chem.–Eur. J. 2019, 25, 3598–3605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Long, C.; Xu, X.; Qing, T.; Zhang, P.; Feng, B. High specific MNase assay for rapid identification of Staphylococcus aureus using AT-rich dsDNA substrate. Talanta 2019, 204, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X.; Long, C.; Xu, J.; Jiang, Z.; Feng, B.; Zhang, P.; Fei, J.; Qing, T. DNA/RNA chimera-templated copper nanoclusters for label-free detection of reverse transcription-associated ribonuclease H. Sens. Actuators B Chem. 2020, 316, 128072. [Google Scholar] [CrossRef]
- Huang, L.; Li, P.; Lin, C.; Wu, Y.; Chen, Z.; Fu, F. DNA-templated fluorescent silver nanoclusters on-off switch for specific and sensitive determination of organic mercury in seafood. Biosens. Bioelectron. 2021, 183, 113217. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Zhang, Y.; Wang, L.; Chen, Q. An upconversion biosensor based on DNA hybridization and DNA-templated silver nanoclusters for the determination of acrylamide. Biosens. Bioelectron. 2022, 215, 114581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, Y.; Qi, Z.; Lu, L.; Qian, Y. DNA-templated silver nanoclusters for fluorescence turn-on assay of acetylcholinesterase activity. Anal. Chem. 2013, 85, 8455–8461. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, H.-D.; Chen, Y.; Huang, K.-J.; Liu, Y.-M. A label-free and ultrasensitive fluorescent sensor for dopamine detection based on double-stranded DNA templated copper nanoparticles. Sens. Actuators B Chem. 2015, 220, 146–153. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, H.-D.; Chen, Y.; Liu, Y.-M. Inhibition of double-stranded DNA templated copper nanoparticles as label-free fluorescent sensors for L-histidine detection. New J. Chem. 2015, 39, 8896–8900. [Google Scholar] [CrossRef]
- Peng, J.; Ling, J.; Tan, Y.-Y.; Jing, C.-J.; Li, X.-J.; Chen, L.Q.; Cao, Q.-E. Poly (thymine)-templated copper nanoparticles as a fluorescence probe for highly selective and rapid detection of cysteine. Spectrosc. Lett. 2017, 50, 137–142. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, T.; Wang, S.; Ma, J.; Zhou, T.; Wang, F.; Wang, X.; Zhang, G. DNA-templated gold nanocluster as a novel fluorometric sensor for glutathione determination. J. Photochem. Photobiol. A Chem. 2019, 370, 89–93. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, C. Highly sensitive and selective detection of Pb2+ using a turn-on fluorescent aptamer DNA silver nanoclusters sensor. Talanta 2018, 182, 125–130. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Gao, X.; Lin, X.; Liu, Y.; Wang, S. A single fluorophore ratiometric nanosensor based on dual-emission DNA-templated silver nanoclusters for ultrasensitive and selective Pb2+ detection. Sens. Actuators B Chem. 2019, 282, 712–718. [Google Scholar] [CrossRef]
- Huang, X.-F.; Ren, B.-X.; Peng, C.-F.; Wei, X.-L.; Xie, Z.-J. Fluorescent sensing of mercury (II) and copper (II) ions based on DNA-templated Cu/Ag nanoclusters. Microchem. J. 2020, 158, 105214. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Liu, Z.; Yang, C.; Li, Y.; Ma, X.; Lv, T.; Sun, C. Label-free fluorescence sensing strategy based on functional nucleic acids via energy transfer between DNA-templated silver nanoclusters and gold nanorods. J. Agric. Food Chem. 2022, 70, 12220–12231. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, Z.; Li, J.; Li, T.; Dong, S. Fluorescent silver nanoclusters in hybridized DNA duplexes for the turn-on detection of Hg 2+ ions. Chem. Commun. 2011, 47, 11065–11067. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Nucleases: Diversity of structure, function and mechanism. Q. Rev. Biophys. 2011, 44, 1–93. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Shahat, A. Functionalized novel mesoporous adsorbent for selective lead (II) ions monitoring and removal from wastewater. Sens. Actuators B Chem. 2014, 203, 854–863. [Google Scholar] [CrossRef]
- Qing, Z.; Xu, J.; Hu, J.; Zheng, J.; He, L.; Zou, Z.; Yang, S.; Tan, W.; Yang, R. In situ amplification-based imaging of RNA in living cells. Angew. Chem. 2019, 131, 11698–11709. [Google Scholar] [CrossRef]
- Qing, Z.; Hu, J.; Xu, J.; Zou, Z.; Lei, Y.; Qing, T.; Yang, R. An intramolecular catalytic hairpin assembly on a DNA tetrahedron for mRNA imaging in living cells: Improving reaction kinetics and signal stability. Chem. Sci. 2020, 11, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Choi, S.; Richards, C.I.; Antoku, Y.; Dickson, R.M. Live cell surface labeling with fluorescent Ag nanocluster conjugates. Photochem. Photobiol. 2008, 84, 1435–1439. [Google Scholar] [CrossRef]
- Rück, V.; Mishra, N.K.; Sørensen, K.K.; Liisberg, M.B.; Sloth, A.B.; Cerretani, C.; Mollerup, C.B.; Kjaer, A.; Lou, C.; Jensen, K.J. Bioconjugation of a near-infrared DNA-stabilized silver nanocluster to peptides and human insulin by copper-free click chemistry. J. Am. Chem. Soc. 2023, 145, 16771–16777. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liisberg, M.B.; Nolt, G.L.; Fu, X.; Cerretani, C.; Li, L.; Johnson, L.A.; Vosch, T.; Richards, C.I. DNA-AgNC Loaded Liposomes for Measuring Cerebral Blood Flow Using Two-Photon Fluorescence Correlation Spectroscopy. ACS Nano 2023, 17, 12862–12874. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Kuo, C.L.; Nuthalapati, K.; Chiang, C.S.; Hwang, K.C. Nucleus-targeting gold nanoclusters for simultaneous in vivo fluorescence imaging, gene delivery, and NIR-light activated photodynamic therapy. Adv. Funct. Mater. 2015, 25, 5934–5945. [Google Scholar] [CrossRef]
- Chen, Y.; Montana, D.M.; Wei, H.; Cordero, J.M.; Schneider, M.; Le Guével, X.; Chen, O.; Bruns, O.T.; Bawendi, M.G. Shortwave infrared in vivo imaging with gold nanoclusters. Nano Lett. 2017, 17, 6330–6334. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qiao, Z.; Yu, Y.; Tang, J.; He, X.; Shi, H.; Ye, X.; Lei, Y.; Wang, K. In situ fluorescence activation of DNA–silver nanoclusters as a label-free and general strategy for cell nucleus imaging. Chem. Commun. 2018, 54, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Heo, G.S.; Zhao, Y.; Sultan, D.; Zhang, X.; Detering, L.; Luehmann, H.P.; Zhang, X.; Li, R.; Choksi, A.; Sharp, S. Assessment of copper nanoclusters for accurate in vivo tumor imaging and potential for translation. ACS Appl. Mater. Interfaces 2019, 11, 19669–19678. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Cai, W.; Feng, H.; Du, T.; Liu, W.; Jiang, H.; Pasquarelli, A.; Weizmann, Y.; Wang, X. In situ self-assembling Au-DNA complexes for targeted cancer bioimaging and inhibition. Proc. Natl. Acad. Sci. USA 2020, 117, 308–316. [Google Scholar] [CrossRef]
- Wu, J.; Li, N.; Yao, Y.; Tang, D.; Yang, D.; Ong’achwa Machuki, J.; Li, J.; Yu, Y.; Gao, F. DNA-stabilized silver nanoclusters for label-free fluorescence imaging of cell surface glycans and fluorescence guided photothermal therapy. Anal. Chem. 2018, 90, 14368–14375. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, M.; Ouyang, N.; Tang, Y.; Miao, P. Synergistic chemo-thermal therapy of cancer by DNA-templated silver nanoclusters and polydopamine nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 21653–21660. [Google Scholar] [CrossRef]
- Tai, S.; Qian, Z.; Ren, H.; Barimah, A.O.; Peng, C.; Wei, X. Highly selective and sensitive colorimetric detection for glyphosate based on β-CD@ DNA-CuNCs enzyme mimics. Anal. Chim. Acta 2022, 1222, 339992. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Yao, Q.; Yi, J.; Si, J.; Gao, Y.; Su, S.; Zhu, C. Leveraging Concentration Imbalance-Driven DNA Circuit as an Operational Amplifier to Enhance the Sensitivity of Hepatitis B Virus DNA Detection with Hybridization-Responsive DNA-Templated Silver Nanoclusters. JACS Au 2024, 4, 2323–2334. [Google Scholar] [CrossRef]
- Yang, C.; Deng, H.; He, J.; Zhang, X.; Gao, J.; Shang, X.; Zuo, S.; Yuan, R.; Xu, W. Amplifiable ratiometric fluorescence biosensing of nanosilver multiclusters populated in three-way-junction DNA branches. Biosens. Bioelectron. 2022, 199, 113871. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Luo, S.; Deng, H.; Yang, C.; Zhang, Y.; Li, M.; Yuan, R.; Xu, W. Fluorescent features and applicable biosensing of a core–shell Ag nanocluster shielded by a DNA tetrahedral nanocage. Anal. Chem. 2023, 95, 14805–14815. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Parvez, S.; Shu, T. Advancing Biosensing and Imaging with DNA-Templated Metal Nanoclusters: Synthesis, Applications, and Future Challenges—A Review. Chemosensors 2024, 12, 271. https://doi.org/10.3390/chemosensors12120271
Li J, Parvez S, Shu T. Advancing Biosensing and Imaging with DNA-Templated Metal Nanoclusters: Synthesis, Applications, and Future Challenges—A Review. Chemosensors. 2024; 12(12):271. https://doi.org/10.3390/chemosensors12120271
Chicago/Turabian StyleLi, Jiacheng, Sidra Parvez, and Tong Shu. 2024. "Advancing Biosensing and Imaging with DNA-Templated Metal Nanoclusters: Synthesis, Applications, and Future Challenges—A Review" Chemosensors 12, no. 12: 271. https://doi.org/10.3390/chemosensors12120271
APA StyleLi, J., Parvez, S., & Shu, T. (2024). Advancing Biosensing and Imaging with DNA-Templated Metal Nanoclusters: Synthesis, Applications, and Future Challenges—A Review. Chemosensors, 12(12), 271. https://doi.org/10.3390/chemosensors12120271






