Recent Progress in Saliva-Based Sensors for Continuous Monitoring of Heavy Metal Levels Linked with Diabetes and Obesity
Abstract
1. Introduction
2. The Links Between Heavy Metals, Obesity and Type 2 Diabetes
- Oxidative Stress Induction—Toxic metals such as arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) stimulate the production of reactive oxygen species (ROS), including superoxide radicals, hydrogen peroxide, and nitric oxide, leading to oxidative stress [26].
- β-Cell Dysfunction—Oxidative stress induced by toxic metals impairs insulin gene expression in pancreatic β-cells, reducing insulin secretion, disrupting glucose uptake, and altering glucose regulation pathways, which may contribute to insulin resistance and T2D development [27].
- Essential Trace Metal Imbalance—Imbalanced levels of essential trace metals, whether due to deficiency or overexposure, disrupt pancreatic islet cell function, impairing glucose metabolism and insulin signaling. While normal levels of these metals enhance insulin sensitivity and action, imbalances increase the risk of diabetes [28].
- Antioxidant Effects of Essential Metals—Essential trace metals, such as zinc and copper, help counteract oxidative stress caused by toxic metals, protecting β-cells, maintaining insulin homeostasis, and reducing the risk of T2D through their antioxidant properties [29].
- Competition between Metals—Toxic metals compete with essential metals for absorption, transport, protein binding, and metabolism. Both toxic metals (e.g., As, Cd, Hg, Pb) and some essential metals (e.g., cobalt (Co), copper (Cu), chromium (Cr), nickel (Ni), selenium (Se)) act as metalloestrogens, disrupting endocrine pathways and increasing the risk of T2D [30].
- Effects on Body Weight and Lipid Metabolism—Heavy metals influence body weight and lipid metabolism. Lead exposure, for example, increases food intake, body weight, and insulin response, while mercury (Hg), manganese (Mn), and cobalt (Co) can disrupt adipose tissue metabolism, potentially exacerbating obesity-related diseases. Additionally, nickel allergies and low-nickel diets have been shown to affect weight management [31].
2.1. Mercury (Hg)
2.2. Cadmium (Cd)
2.3. Lead (Pb)
2.4. Arsenic (As)
2.5. Nickel (Ni)
2.6. Copper (Cu)
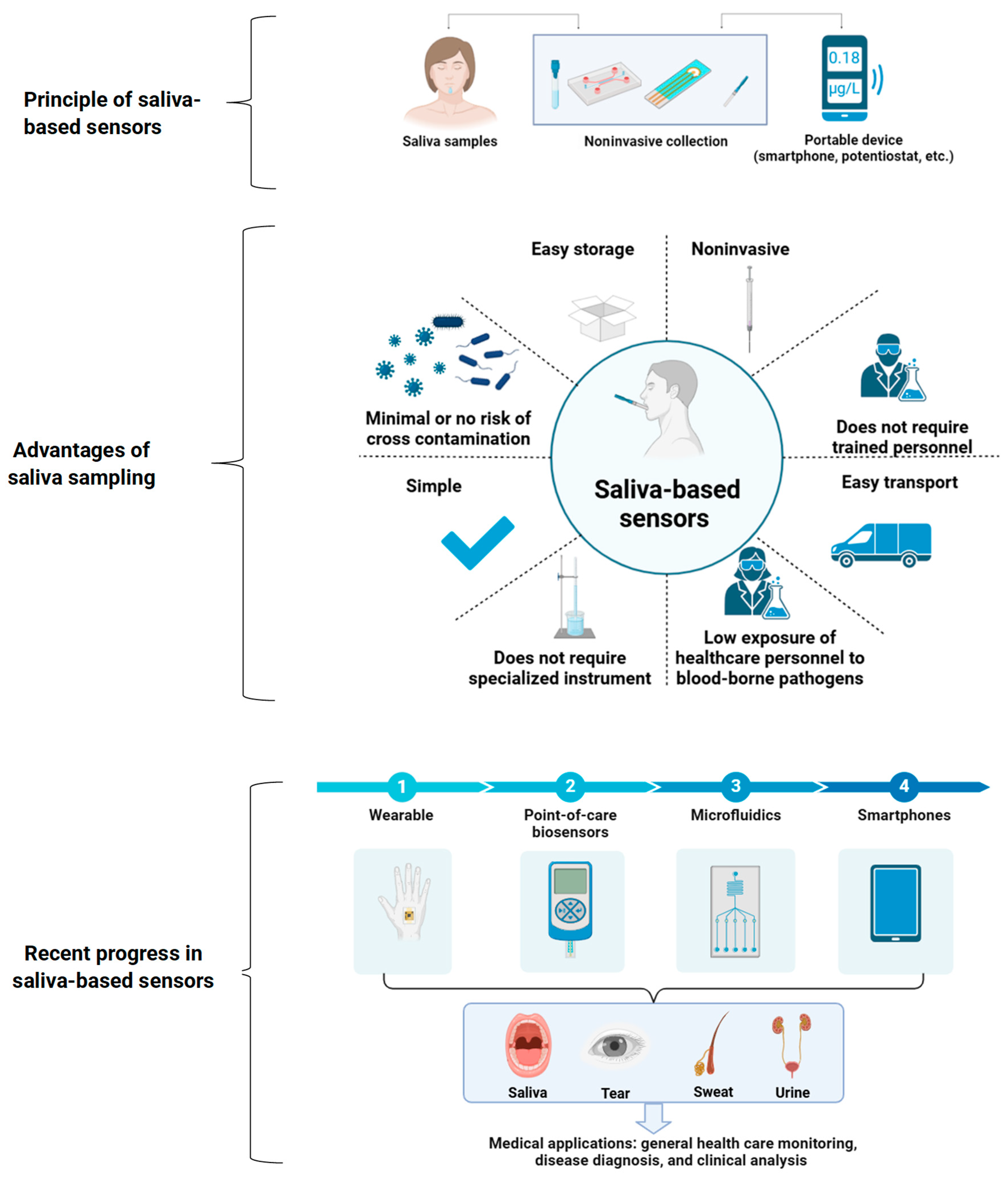
3. Saliva-Based Sensors: Principles, Advantages, and Recent Progress
3.1. Challenges in Traditional Saliva Biomarker Detection Methods
3.2. Principles of Saliva-Based Sensors
3.3. Advances in Nanomaterials for Saliva-Based Sensors
3.4. Sensor Design: Overcoming Challenges in Sensitivity and Reproducibility
3.5. Saliva-Based Wearable Sensors: Real-Time Monitoring and Integration with Intraoral Devices
3.6. Point-of-Care (POC) Saliva Sensors
3.7. Integration with Smartphones and Artificial Intelligence (AI)
3.8. Consensus
4. Mechanism of Electrochemical Sensors for Heavy Metal Detection
5. Challenges and Limitations of Saliva-Based Sensors
6. Clinical Applications and Potential Implications in Diabetes and Obesity Management
7. Future Perspectives
8. Conclusions
9. Additional Considerations
- Ethical and Regulatory Issues: The widespread use of saliva-based sensors in clinical practice requires careful attention to ethical issues, particularly regarding data privacy and patient consent. The continuous nature of monitoring may raise concerns about the handling and security of sensitive health data. Furthermore, these devices must undergo rigorous regulatory evaluation to ensure their safety and effectiveness, particularly in regard to compliance with standards set by regulatory agencies such as the FDA [161].
- Global Health Impact: The increasing prevalence of obesity and diabetes, especially in developing countries where environmental contamination may be more common, underscores the potential value of saliva-based sensors in public health. These sensors could provide an affordable and accessible tool for monitoring heavy metal exposure and metabolic health, particularly in regions with limited access to traditional healthcare facilities.
- Economic Considerations: Although initial costs for developing and implementing saliva-based sensors may be high, the long-term economic benefits of preventing or mitigating the onset of obesity and diabetes could outweigh these costs. By enabling early detection and intervention, these sensors could reduce the long-term healthcare costs associated with managing chronic complications such as cardiovascular disease, kidney failure, and neuropathy, which are commonly associated with diabetes and obesity [162].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Azevedo, M.J. The State of Health System(s) in Africa: Challenges and Opportunities. In Historical Perspectives on the State of Health and Health Systems in Africa; Springer: Berlin, Germany, 2017; pp. 1–73. [Google Scholar]
- Zheng, X.; Zhang, F.; Wang, K.; Zhang, W.; Li, Y.; Sun, Y.; Sun, X.; Li, C.; Dong, B.; Wang, L.; et al. Smart Biosensors and Intelligent Devices for Salivary Biomarker Detection. TrAC Trends Anal. Chem. 2021, 140, 116281. [Google Scholar] [CrossRef]
- Bohr, A.; Memarzadeh, K. The Rise of Artificial Intelligence in Healthcare Applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. [Google Scholar]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liao, Z.; Liu, W. Developments in Diagnostic Applications of Saliva in Human Organ Diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Varga, G. Physiology of the Salivary Glands. Surgery 2015, 33, 581–586. [Google Scholar] [CrossRef]
- Zürcher, C.; Humpel, C. Saliva: A Challenging Human Fluid to Diagnose Brain Disorders with a Focus on Alzheimer’s Disease. Neural. Regen. Res. 2023, 18, 2606–2610. [Google Scholar] [CrossRef]
- Basilicata, M.; Pieri, M.; Marrone, G.; Nicolai, E.; Di Lauro, M.; Paolino, V.; Tomassetti, F.; Vivarini, I.; Bollero, P.; Bernardini, S.; et al. Saliva as Biomarker for Oral and Chronic Degenerative Non-Communicable Diseases. Metabolites 2023, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Malon, R.S.P.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-Based Biosensors: Noninvasive Monitoring Tool for Clinical Diagnostics. Biomed. Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef]
- Jain, K.K. Role of Biomarkers in Health Care. In The Handbook of Biomarkers; Humana Press: Totowa, NJ, USA, 2010; pp. 115–188. [Google Scholar]
- Song, M.; Bai, H.; Zhang, P.; Zhou, X.; Ying, B. Promising Applications of Human-Derived Saliva Biomarker Testing in Clinical Diagnostics. Int. J. Oral Sci. 2023, 15, 2. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Casati, S.; Goldoni, R.; Thomaz, D.V.; Kehr, N.S.; Galimberti, D.; Del Fabbro, M.; Tartaglia, G.M. Salivary Biomarkers: Novel Noninvasive Tools to Diagnose Chronic Inflammation. Int. J. Oral Sci. 2023, 15, 27. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a Potential Non-Invasive Liquid Biopsy for Early and Easy Diagnosis/Prognosis of Head and Neck Cancer. Transl. Oncol. 2024, 40, 101827. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.; Crewther, B.; Cook, C.; Punyadeera, C.; Pandey, A.K. Sensing Methods for Stress Biomarker Detection in Human Saliva: A New Frontier for Wearable Electronics and Biosensing. Mater. Adv. 2024, 5, 5339–5350. [Google Scholar] [CrossRef]
- Zhou, C.; Cai, Z.; Jin, B.; Lin, H.; Xu, L.; Jin, Z. Saliva-Based Detection of SARS-CoV-2: A Bibliometric Analysis of Global Research. Mol. Cell. Biochem. 2024, 479, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tian, L.; Li, W.; Wang, K.; Yang, Q.; Lin, J.; Zhang, T.; Dong, B.; Wang, L. Recent Advances and Perspectives Regarding Paper-Based Sensors for Salivary Biomarker Detection. Chemosensors 2023, 11, 383. [Google Scholar] [CrossRef]
- Lin, C.-W.; Tsai, Y.-H.; Peng, Y.-S.; Yang, J.-T.; Lu, Y.-P.; Chen, M.-Y.; Tung, C.-W. A Novel Salivary Sensor with Integrated Au Electrodes and Conductivity Meters for Screening of Diabetes. Biosensors 2023, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzam, N.; Al-Azzam, S.; Khassawneh, B.; Araydah, M.; Karasneh, R.A.; Aldeyab, M.A. Factors Contributing to Poor COVID-19 Outcomes in Diabetic Patients: Findings from a Single-Center Cohort Study. PLoS ONE 2023, 18, e0290946. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Poudyal, S.; Subba, H.K.; Khatiwada, S. Metabolic Syndrome and Life Style Factors among Diabetes Patients Attending in a Teaching Hospital, Chitwan. PLoS ONE 2023, 18, e0286139. [Google Scholar] [CrossRef] [PubMed]
- Pasquel, F.J.; Lansang, M.C.; Dhatariya, K.; Umpierrez, G.E. Management of Diabetes and Hyperglycaemia in the Hospital. Lancet Diabetes Endocrinol. 2021, 9, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, Y.; Liu, S.; Liu, Q.; Xu, M.; Zhang, J.; Wei, Y.; Mo, X.; Lin, Y.; Tang, X.; et al. Associations between Multiple Heavy Metals Exposure and Glycated Hemoglobin in a Chinese Population. Chemosphere 2022, 287, 132159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, H.; Zhu, X.; Xiang, R.; Miao, Y.; Zhang, Y.; Song, Y.; Chen, J.; Zhang, L. Effects of Multi-metal Exposure on the Risk of Diabetes Mellitus among People Aged 40–75 Years in Rural Areas in Southwest China. J. Diabetes Investig. 2022, 13, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, R.; Dash, S. Obesity and Cardiovascular Disease: The Emerging Role of Inflammation. Front. Cardiovasc. Med. 2021, 8, 768119. [Google Scholar] [CrossRef]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult Obesity Complications: Challenges and Clinical Impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 204201882093495. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Aschner, M.; Ke, T.; Ferrer, B.; Zhou, J.-C.; Chang, J.-S.; Santamaría, A.; Chao, J.C.-J.; Aaseth, J.; Skalny, A.V. Adipotropic Effects of Heavy Metals and Their Potential Role in Obesity. Fac. Rev. 2021, 10, 32. [Google Scholar] [CrossRef]
- Ghorbani Nejad, B.; Raeisi, T.; Janmohammadi, P.; Mehravar, F.; Zarei, M.; Dehghani, A.; Bahrampour, N.; Darijani, M.H.; Ahmadipour, F.; Mohajeri, M.; et al. Mercury Exposure and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 7640227. [Google Scholar] [CrossRef]
- Zheng, T. Current Understanding of the Relationship between Metal Exposures and Risk of Type 2 Diabetes. Curr. Res. Diabetes Obes. J. 2018, 7, 555710. [Google Scholar] [CrossRef]
- Ramírez-Cruz, A.; Rios-Lugo, M.J.; Soto-Sánchez, J.; Juárez-Pérez, C.A.; Cabello-López, A.; Jiménez-Ramírez, C.; Chang-Rueda, C.; Cruz, M.; Hernández-Mendoza, H.; Vazquez-Moreno, M. Overweight, Obesity, Hypertriglyceridemia, and Insulin Resistance Are Positively Associated with High Serum Copper Levels in Mexican Adults. Metabolites 2024, 14, 282. [Google Scholar] [CrossRef]
- Szablewski, L. Changes in Cells Associated with Insulin Resistance. Int. J. Mol. Sci. 2024, 25, 2397. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy Metals, Islet Function and Diabetes Development. Islets 2009, 1, 169–176. [Google Scholar] [CrossRef]
- Alonso, M.L.; Montaña, F.P.; Miranda, M.; Castillo, C.; Hernández, J.; Benedito, J.L. Interactions between Toxic (As, Cd, Hg and Pb) and Nutritional Essential (Ca, Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) Elements in the Tissues of Cattle from NW Spain. BioMetals 2004, 17, 389–397. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Millán-Martínez, M.; Domínguez-Riscart, J.; Lechuga-Sancho, A.M.; González-Domínguez, R. Metal Homeostasis and Exposure in Distinct Phenotypic Subtypes of Insulin Resistance among Children with Obesity. Nutrients 2023, 15, 2347. [Google Scholar] [CrossRef] [PubMed]
- Cuciureanu, M.; Caratașu, C.-C.; Gabrielian, L.; Frăsinariu, O.E.; Checheriță, L.E.; Trandafir, L.M.; Stanciu, G.D.; Szilagyi, A.; Pogonea, I.; Bordeianu, G.; et al. 360-Degree Perspectives on Obesity. Medicina 2023, 59, 1119. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Amerikanou, C.; Kleftaki, S.-A.; Karavoltsos, S.; Tagkouli, D.; Sakellari, A.; Valsamidou, E.; Gioxari, A.; Kalogeropoulos, N.; Kaliora, A.C. Vanadium, Cobalt, Zinc, and Rubidium Are Associated with Markers of Inflammation and Oxidative Stress in a Greek Population with Obesity. Front. Endocrinol. 2023, 14, 1265310. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lv, Y.; Liu, X.; Wang, G. Association between Heavy Metal Mercury in Body Fluids and Tissues and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Ann. Transl. Med. 2023, 11, 114. [Google Scholar] [CrossRef]
- Tian, H.; Guo, X.; Wang, X.; He, Z.; Sun, R.; Ge, S.; Zhang, Z. Chromium Picolinate Supplementation for Overweight or Obese Adults. Cochrane Database Syst. Rev. 2013, 2013, CD010063. [Google Scholar] [CrossRef] [PubMed]
- Khodavirdipour, A.; Haddadi, F.; Keshavarzi, S. Chromium Supplementation; Negotiation with Diabetes Mellitus, Hyperlipidemia and Depression. J. Diabetes Metab. Disord. 2020, 19, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Lee, I.; Lee, A.; Park, H.; Kim, M.J.; Kim, S.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Lead, Mercury, and Cadmium Exposures Are Associated with Obesity but Not with Diabetes Mellitus: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Res. 2022, 204, 111888. [Google Scholar] [CrossRef]
- Wang, G.; DiBari, J.; Bind, E.; Steffens, A.M.; Mukherjee, J.; Bartell, T.R.; Bellinger, D.C.; Hong, X.; Ji, Y.; Wang, M.-C.; et al. In Utero Exposure to Mercury and Childhood Overweight or Obesity: Counteracting Effect of Maternal Folate Status. BMC Med. 2019, 17, 216. [Google Scholar] [CrossRef]
- Cho, K.Y. Association of Blood Mercury Levels with the Risks of Overweight and High Waist-to-Height Ratio in Children and Adolescents: Data from the Korean National Health and Nutrition Examination Survey. Children 2021, 8, 1087. [Google Scholar] [CrossRef]
- Tsai, T.-L.; Kuo, C.-C.; Pan, W.-H.; Wu, T.-N.; Lin, P.; Wang, S.-L. Type 2 Diabetes Occurrence and Mercury Exposure—From the National Nutrition and Health Survey in Taiwan. Environ. Int. 2019, 126, 260–267. [Google Scholar] [CrossRef]
- Jeon, J.; Morris, J.S.; Park, K. Toenail Mercury Levels Positively Correlate with Obesity and Abdominal Obesity among Korean Adults. J. Trace Elem. Med. Biol. 2021, 64, 126678. [Google Scholar] [CrossRef]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef] [PubMed]
- Stahr, S.; Chiang, T.; Bauer, M.A.; Runnells, G.A.; Rogers, L.J.; Do, H.V.; Kadlubar, S.A.; Su, L.J. Low-Level Environmental Heavy Metals Are Associated with Obesity Among Postmenopausal Women in a Southern State. Expo. Health 2021, 13, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Bae, M.-J.; Kim, M.-J.; Oh, S.S.; Park, K.S.; Lee, C.J.; Park, S.; Koh, S.-B.; Cho, J.; Kim, C. Heavy Metal Exposure Linked to Metabolic Syndrome in Korean Male Firefighters: FRESH Cohort Cross-Sectional Analysis. Sci. Rep. 2023, 13, 14016. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Jin, M.H.; Kang, J.-H.; Lee, S., II; Lee, S.; Kim, S.-H.; Oh, S.Y. Relationship between Heavy Metal Exposure and Type 2 Diabetes: A Large-Scale Retrospective Cohort Study Using Occupational Health Examinations. BMJ Open 2021, 11, e039541. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mendoza, H.; Rios-Lugo, M.J.; Álvarez-Loredo, H.E.; Romero-Guzmán, E.T.; Gaytán-Hernández, D.; Martínez-Navarro, I.; Juárez-Flores, B.I.; Chang-Rueda, C. Serum Lead Levels and Its Association with Overweight and Obesity. J. Trace Elem. Med. Biol. 2022, 72, 126984. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, J.; Guo, P.; Wang, J.; Wang, P.; Liu, L.; Wu, M.; Wang, P.; Liu, N. Association between Environmental Lead/Cadmium Co-Exposure in Drinking Water and Soil and Type 2 Diabetes Mellitus/Obesity in Southern China. Front. Public Health 2022, 10, 941922. [Google Scholar] [CrossRef]
- Rangel-Moreno, K.; Gamboa-Loira, B.; López-Carrillo, L.; Cebrián, M.E. Prevalence of Type 2 Diabetes Mellitus in Relation to Arsenic Exposure and Metabolism in Mexican Women. Environ. Res. 2022, 210, 112948. [Google Scholar] [CrossRef] [PubMed]
- Brown, C. Arsenic Exposure Linked to Diabetes Risk in Canada. Can. Med. Assoc. J. 2015, 187, E438–E439. [Google Scholar] [CrossRef]
- Wen, W.-L.; Wang, C.-W.; Wu, D.-W.; Chen, S.-C.; Hung, C.-H.; Kuo, C.-H. Associations of Heavy Metals with Metabolic Syndrome and Anthropometric Indices. Nutrients 2020, 12, 2666. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ji, S.; Sun, Q.; Zhao, F.; Li, Z.; Zhang, M.; Li, Y.; Zheng, L.; Song, H.; Zhang, W.; et al. Association of Urinary Nickel Levels with Diabetes and Fasting Blood Glucose Levels: A Nationwide Chinese Population-Based Study. Ecotoxicol. Environ. Saf. 2023, 252, 114601. [Google Scholar] [CrossRef]
- Titcomb, T.J.; Liu, B.; Lehmler, H.; Snetselaar, L.G.; Bao, W. Environmental Nickel Exposure and Diabetes in a Nationally Representative Sample of US Adults. Expo. Health 2021, 13, 697–704. [Google Scholar] [CrossRef]
- Watanabe, M.; Masieri, S.; Costantini, D.; Tozzi, R.; De Giorgi, F.; Gangitano, E.; Tuccinardi, D.; Poggiogalle, E.; Mariani, S.; Basciani, S.; et al. Overweight and Obese Patients with Nickel Allergy Have a Worse Metabolic Profile Compared to Weight Matched Non-Allergic Individuals. PLoS ONE 2018, 13, e0202683. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.-N.; Wolf, R.M.; Ralle, M.; Dev, S.; Pierson, H.; Askin, F.; Steele, K.E.; Magnuson, T.H.; Schweitzer, M.A.; et al. Obesity Is Associated with Copper Elevation in Serum and Tissues. Metallomics 2019, 11, 1363–1371. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Shan, R.; Wang, C. Associations between Dietary Copper Intake, General Obesity and Abdominal Obesity Risk: A Nationwide Cohort Study in China. Front. Nutr. 2022, 9, 1009721. [Google Scholar] [CrossRef]
- Vazquez-Moreno, M.; Sandoval-Castillo, M.; Rios-Lugo, M.J.; Klünder-Klünder, M.; Cruz, M.; Martínez-Navarro, I.; Romero-Guzmán, E.T.; Victoria-Campos, C.I.; Vilchis-Gil, J.; Hernández-Mendoza, H. Overweight and Obesity Are Positively Associated with Serum Copper Levels in Mexican Schoolchildren. Biol. Trace Elem. Res. 2023, 201, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-G.; Wang, J.; Jin, Y. Ultrasensitive Determination of Mercury in Human Saliva by Atomic Fluorescence Spectrometry Based on Solidified Floating Organic Drop Microextraction. Microchim. Acta 2012, 177, 153–158. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Skalnaya, M.G.; Popova, E.V.; Sinitskii, A.I.; Nemereshina, O.N.; Gatiatulina, E.R.; Nikonorov, A.A.; Skalny, A.V. Mercury and Metabolic Syndrome: A Review of Experimental and Clinical Observations. BioMetals 2015, 28, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Fabricio, G.; Malta, A.; Chango, A.; De Freitas Mathias, P.C. Environmental Contaminants and Pancreatic Beta-Cells. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 257–263. [Google Scholar] [CrossRef]
- Shin, Y.-Y.; Ryu, I.-K.; Park, M.-J.; Kim, S.-H. The Association of Total Blood Mercury Levels and Overweight among Korean Adolescents: Analysis of the Korean National Health and Nutrition Examination Survey (KNHANES) 2010–2013. Korean J. Pediatr. 2018, 61, 121–128. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nukavarapu, S.P.; Jala, V.R. Effects of Heavy Metals on Gut Barrier Integrity and Gut Microbiota. Microbiota Host 2023, 2, e230015. [Google Scholar] [CrossRef]
- Buha, A.; Đukić-Ćosić, D.; Ćurčić, M.; Bulat, Z.; Antonijević, B.; Moulis, J.-M.; Goumenou, M.; Wallace, D. Emerging Links between Cadmium Exposure and Insulin Resistance: Human, Animal, and Cell Study Data. Toxics 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Is Environmental Cadmium Exposure Causally Related to Diabetes and Obesity? Cells 2023, 13, 83. [Google Scholar] [CrossRef]
- Filippini, T.; Wise, L.A.; Vinceti, M. Cadmium Exposure and Risk of Diabetes and Prediabetes: A Systematic Review and Dose-Response Meta-Analysis. Environ. Int. 2022, 158, 106920. [Google Scholar] [CrossRef]
- Xiao, L.; Li, W.; Zhu, C.; Yang, S.; Zhou, M.; Wang, B.; Wang, X.; Wang, D.; Ma, J.; Zhou, Y.; et al. Cadmium Exposure, Fasting Blood Glucose Changes, and Type 2 Diabetes Mellitus: A Longitudinal Prospective Study in China. Environ. Res. 2021, 192, 110259. [Google Scholar] [CrossRef]
- Park, S.S.; Skaar, D.A.; Jirtle, R.L.; Hoyo, C. Epigenetics, Obesity and Early-Life Cadmium or Lead Exposure. Epigenomics 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Nguyen, J.; Patel, A.; Gensburg, A.; Bokhari, R.; Lamar, P.; Edwards, J. Diabetogenic and Obesogenic Effects of Cadmium in Db/Db Mice and Rats at a Clinically Relevant Level of Exposure. Toxics 2022, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Hoyo, C.; Mattingly, C.J.; Luo, Y.; Tzeng, J.-Y.; Murphy, S.K.; Buchwalter, D.B.; Planchart, A. Cadmium Exposure Increases the Risk of Juvenile Obesity: A Human and Zebrafish Comparative Study. Int. J. Obes. 2018, 42, 1285–1295. [Google Scholar] [CrossRef]
- Tyrrell, J.B.; Hafida, S.; Stemmer, P.; Adhami, A.; Leff, T. Lead (Pb) Exposure Promotes Diabetes in Obese Rodents. J. Trace Elem. Med. Biol. 2017, 39, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Leff, T.; Stemmer, P.; Tyrrell, J.; Jog, R. Diabetes and Exposure to Environmental Lead (Pb). Toxics 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, C.; Nie, X.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; et al. Blood Lead Level and Its Association with Body Mass Index and Obesity in China—Results from SPECT-China Study. Sci. Rep. 2015, 5, 18299. [Google Scholar] [CrossRef]
- Pánico, P.; Velasco, M.; Salazar, A.M.; Picones, A.; Ortiz-Huidobro, R.I.; Guerrero-Palomo, G.; Salgado-Bernabé, M.E.; Ostrosky-Wegman, P.; Hiriart, M. Is Arsenic Exposure a Risk Factor for Metabolic Syndrome? A Review of the Potential Mechanisms. Front. Endocrinol. 2022, 13, 878280. [Google Scholar] [CrossRef]
- Kulshrestha, A. Arsenic-Induced Abnormalities in Glucose Metabolism: Biochemical Basis and Potential Therapeutic and Nutritional Interventions. World J. Transl. Med. 2014, 3, 96–111. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic Exposure Perturbs the Gut Microbiome and Its Metabolic Profile in Mice: An Integrated Metagenomics and Metabolomics Analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mondal, V.; Hosen, Z.; Hossen, F.; Siddique, A.E.; Tony, S.R.; Islam, Z.; Islam, S.; Hossain, S.; Islam, K.; Sarker, K.; et al. Arsenic Exposure-Related Hyperglycemia Is Linked to Insulin Resistance with Concomitant Reduction of Skeletal Muscle Mass. Environ. Int. 2020, 143, 105890. [Google Scholar] [CrossRef] [PubMed]
- Castriota, F.; Rieswijk, L.; Dahlberg, S.; La Merrill, M.A.; Steinmaus, C.; Smith, M.T.; Wang, J.-C. A State-of-the-Science Review of Arsenic’s Effects on Glucose Homeostasis in Experimental Models. Environ. Health Perspect. 2020, 128, 16001. [Google Scholar] [CrossRef]
- Shakya, A.; Dodson, M.; Artiola, J.F.; Ramirez-Andreotta, M.; Root, R.A.; Ding, X.; Chorover, J.; Maier, R.M. Arsenic in Drinking Water and Diabetes. Water 2023, 15, 1751. [Google Scholar] [CrossRef]
- Xia, W.; Guo, X.; Xie, P.; Feng, L.; Wu, B.; Gao, J.; Ma, S.; Liu, H.; Sun, C.; Qu, G.; et al. Associations of Nickel Exposure with Diabetes: Evidence from Observational Studies. Environ. Sci. Pollut. Res. 2023, 30, 100233–100247. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Yao, Z.; Zhang, H.; Wang, Z.; Lei, J.; Guo, H. The Relationship between Plasma Nickel Concentrations and Type 2 Diabetes Mellitus Risk: A Protective Effect within a Specific Range. J. Trace Elem. Med. Biol. 2024, 82, 127362. [Google Scholar] [CrossRef] [PubMed]
- Eljazzar, S.; Abu-Hijleh, H.; Alkhatib, D.; Sokary, S.; Ismail, S.; Al-Jayyousi, G.F.; Tayyem, R. The Role of Copper Intake in the Development and Management of Type 2 Diabetes: A Systematic Review. Nutrients 2023, 15, 1655. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H.; Kawashima, Y.; Yanai, H. Serum Zn/Cu Ratio Is Associated with Renal Function, Glycemic Control, and Metabolic Parameters in Japanese Patients with and without Type 2 Diabetes: A Cross-Sectional Study. Front. Endocrinol. 2016, 7, 147. [Google Scholar] [CrossRef]
- Ruan, S.; Guo, X.; Ren, Y.; Cao, G.; Xing, H.; Zhang, X. Nanomedicines Based on Trace Elements for Intervention of Diabetes Mellitus. Biomed. Pharmacother. 2023, 168, 115684. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Rucker, R.B.; Commisso, J.F.; Keen, C.L. Diabetes and Dietary Copper Alter 67Cu Metabolism and Oxidant Defense in the Rat. J. Nutr. Biochem. 2005, 16, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites 2022, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Shakeeb, N.; Varkey, P.; Ajit, A. Human Saliva as a Diagnostic Specimen for Early Detection of Inflammatory Biomarkers by Real-Time RT-PCR. Inflammation 2021, 44, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.-L.; Dong, H.-L.; Liu, M.-M.; Wu, P.-L.; Cao, Y.; Zhang, Y.; Gao, F.-G.; Zhu, H.-Y. The Potential Roles of Salivary Biomarkers in Neurodegenerative Diseases. Neurobiol. Dis. 2024, 193, 106442. [Google Scholar] [CrossRef] [PubMed]
- Hajjo, R.; Sabbah, D.A.; Al Bawab, A.Q. Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers. Diagnostics 2022, 12, 1742. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Samara, M.; Ampadi Ramachandran, R.; Gosh, S.; George, H.; Wang, R.; Pesavento, R.P.; Mathew, M.T. A Review on Saliva-Based Health Diagnostics: Biomarker Selection and Future Directions. Biomed. Mater. Devices 2024, 2, 121–138. [Google Scholar] [CrossRef]
- Leake, S.L.; Pagni, M.; Falquet, L.; Taroni, F.; Greub, G. The Salivary Microbiome for Differentiating Individuals: Proof of Principle. Microbes Infect. 2016, 18, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, K.D.; Charbonneau, M.-E.; O’Riordan, M.X.D. Bacterial Metabolism Shapes the Host–Pathogen Interface. Microbiol. Spectr. 2016, 4, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Bakulski, K.M.; Goodrich, J.M.; Peterson, K.E.; Marazita, M.L.; Foxman, B. Low Levels of Salivary Metals, Oral Microbiome Composition and Dental Decay. Sci. Rep. 2020, 10, 14640. [Google Scholar] [CrossRef]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary Biomarkers Detection: Analytical and Immunological Methods Overview. TrAC Trends Anal. Chem. 2019, 113, 301–316. [Google Scholar] [CrossRef]
- Abrao Nemeir, I.; Saab, J.; Hleihel, W.; Errachid, A.; Jafferzic-Renault, N.; Zine, N. The Advent of Salivary Breast Cancer Biomarker Detection Using Affinity Sensors. Sensors 2019, 19, 2373. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva Diagnostics—Current Views and Directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and Their Widespread Impact on Human Health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jyotsna; Stanley Abraham, L.; Hanumant Singh, R.; Panda, R.C.; Senthilvelan, T. Biomedical Applications of Carbon-Based Nanomaterials. In Nanomaterials and Their Biomedical Applications; Springer: Berlin, Germany, 2021; pp. 157–174. [Google Scholar]
- Ehtesabi, H. Application of Carbon Nanomaterials in Human Virus Detection. J. Sci. Adv. Mater. Devices 2020, 5, 436–450. [Google Scholar] [CrossRef]
- Ehtesabi, H. Carbon Nanomaterials for Salivary-Based Biosensors: A Review. Mater. Today Chem. 2020, 17, 100342. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-Based Biosensor and Their Applications: A Review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef] [PubMed]
- Zucolotto, V. Specialty Grand Challenges in Biosensors. Front. Sens. 2020, 1, 3. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.-H. Recent Advances in Graphene-Based Nanobiosensors for Salivary Biomarker Detection. Biosens. Bioelectron. 2021, 171, 112723. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.G.; Silva, A.P.; Nunes-Pereira, J. Current On-Skin Flexible Sensors, Materials, Manufacturing Approaches, and Study Trends for Health Monitoring: A Review. ACS Sens. 2024, 9, 1104–1133. [Google Scholar] [CrossRef]
- Alemdar, S.; Pekel Bayramgil, N.; Karakuş, S. Applications of Cutting-Edge Biosensors in Healthcare and Biomedical Research. In New Advances in Biosensing; Karakuş, S., Ed.; IntechOpen: London, UK, 2024. [Google Scholar]
- Liu, Z.; Cascioli, V.; McCarthy, P.W. Healthcare Monitoring Using Low-Cost Sensors to Supplement and Replace Human Sensation: Does It Have Potential to Increase Independent Living and Prevent Disease? Sensors 2023, 23, 2139. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G. Current Advances in Biosensor Design and Fabrication. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–25. [Google Scholar]
- Feng, L.; Song, S.; Li, H.; He, R.; Chen, S.; Wang, J.; Zhao, G.; Zhao, X. Nano-Biosensors Based on Noble Metal and Semiconductor Materials: Emerging Trends and Future Prospects. Metals 2023, 13, 792. [Google Scholar] [CrossRef]
- El-Warrak, L.; Nunes, M.; Luna, G.; Barbosa, C.E.; Lyra, A.; Argôlo, M.; Lima, Y.; Salazar, H.; de Souza, J.M. Towards the Future of Public Health: Roadmapping Trends and Scenarios in the Post-COVID Healthcare Era. Healthcare 2023, 11, 3118. [Google Scholar] [CrossRef]
- Alanzi, T.M.; Alzahrani, W.; Almoraikhi, M.; Algannas, A.; Alghamdi, M.; Alzahrani, L.; Abutaleb, R.; Ba Dughaish, R.; Alotibi, N.; Alkhalifah, S.; et al. Adoption of Wearable Insulin Biosensors for Diabetes Management: A Cross-Sectional Study. Cureus 2023, 15, e50782. [Google Scholar] [CrossRef] [PubMed]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. Biomed. Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- Lee, J.; Garon, E.; Wong, D. Salivary Diagnostics. Orthod. Craniofac. Res. 2009, 12, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping Healthcare with Wearable Biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Haji Mohammadi, M.; Mulder, S.; Khashayar, P.; Kalbasi, A.; Azimzadeh, M.; Aref, A.R. Saliva Lab-on-a-Chip Biosensors: Recent Novel Ideas and Applications in Disease Detection. Microchem. J. 2021, 168, 106506. [Google Scholar] [CrossRef]
- Jung, C.; Kim, M.-G. Direct Use of a Saliva-Collected Cotton Swab in Lateral Flow Immunoassay for the Detection of Cotinine. Biosensors 2022, 12, 214. [Google Scholar] [CrossRef]
- Timpel, J.; Klinghammer, S.; Riemenschneider, L.; Ibarlucea, B.; Cuniberti, G.; Hannig, C.; Sterzenbach, T. Sensors for in Situ Monitoring of Oral and Dental Health Parameters in Saliva. Clin. Oral Investig. 2023, 27, 5719–5736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.-H.; Nguyen, C.M.; Huynh, M.A.; Vu, H.H.; Nguyen, T.-K.; Nguyen, N.-T. Field Effect Transistor Based Wearable Biosensors for Healthcare Monitoring. J. Nanobiotechnol. 2023, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Norris, H.L.; Friedman, J.; Chen, Z.; Puri, S.; Wilding, G.; Edgerton, M. Salivary Metals, Age, and Gender Correlate with Cultivable Oral Candida Carriage Levels. J. Oral Microbiol. 2018, 10, 1447216. [Google Scholar] [CrossRef]
- Mani, V.; Beduk, T.; Khushaim, W.; Ceylan, A.E.; Timur, S.; Wolfbeis, O.S.; Salama, K.N. Electrochemical Sensors Targeting Salivary Biomarkers: A Comprehensive Review. TrAC Trends Anal. Chem. 2021, 135, 116164. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Omidfar, K.; Ahmadi, A.; Syedmoradi, L.; Khoshfetrat, S.M.; Larijani, B. Point-of-Care Biosensors in Medicine: A Brief Overview of Our Achievements in This Field Based on the Conducted Research in EMRI (Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences) over the Past Fourteen Years. J. Diabetes Metab. Disord. 2020, 23, 1455–1459. [Google Scholar] [CrossRef]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef]
- Filippidou, M.-K.; Chatzandroulis, S. Microfluidic Devices for Heavy Metal Ions Detection: A Review. Micromachines 2023, 14, 1520. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent Progress in Biomedical Sensors Based on Conducting Polymer Hydrogels. ACS Appl. Bio. Mater. 2023, 6, 1720–1741. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Lee, N.Y. Recent Progress in Smartphone-Based Techniques for Food Safety and the Detection of Heavy Metal Ions in Environmental Water. Chemosphere 2021, 275, 130096. [Google Scholar] [CrossRef]
- Lan, Y.; He, B.; Tan, C.S.; Ming, D. Applications of Smartphone-Based Aptasensor for Diverse Targets Detection. Biosensors 2022, 12, 477. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, V. Ultra-Portable, Smartphone-Based Spectrometer for Heavy Metal Concentration Measurement in Drinking Water Samples. Appl. Water Sci. 2021, 11, 177. [Google Scholar] [CrossRef]
- Pungjunun, K.; Yakoh, A.; Chaiyo, S.; Siangproh, W.; Praphairaksit, N.; Chailapakul, O. Smartphone-Based Electrochemical Analysis Integrated with NFC System for the Voltammetric Detection of Heavy Metals Using a Screen-Printed Graphene Electrode. Microchim. Acta 2022, 189, 191. [Google Scholar] [CrossRef]
- Zhang, A.-L. Smartphone-Based Colorimetric Detection of Heavy Metal Copper (II) Ion by Help of Surface Acoustic Wave. Ferroelectrics 2021, 583, 41–50. [Google Scholar] [CrossRef]
- Yadav, N.; Maurya, B.M.; Chettri, D.; Pooja; Pulwani, C.; Jajula, M.; Kanda, S.S.; Babu, H.W.S.; Elangovan, A.; Velusamy, P.; et al. Artificial Intelligence in Heavy Metals Detection: Methodological and Ethical Challenges. Hyg. Environ. Health Adv. 2023, 7, 100071. [Google Scholar] [CrossRef]
- Zheng, P.; Li, M.; Jurevic, R.; Cushing, S.K.; Liu, Y.; Wu, N. A Gold Nanohole Array Based Surface-Enhanced Raman Scattering Biosensor for Detection of Silver(i) and Mercury(Ii) in Human Saliva. Nanoscale 2015, 7, 11005–11012. [Google Scholar] [CrossRef]
- Ilea, A.; Andrei, V.; Feurdean, C.; Băbțan, A.-M.; Petrescu, N.; Câmpian, R.; Boșca, A.; Ciui, B.; Tertiș, M.; Săndulescu, R.; et al. Saliva, a Magic Biofluid Available for Multilevel Assessment and a Mirror of General Health—A Systematic Review. Biosensors 2019, 9, 27. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, Y.; Na, M.; Hwang, S.-J.; Yoon, Y. Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors 2024, 24, 431. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, A.; Hakim Shah, A.; Ur Rahman, A.; Ur Rahman, F.; Ali, S.; Ur Rehman, A.; Ullah, R.; Ullah, I.; Fayaz, M.; Shi, K. Electrochemical Techniques for the Detection of Heavy Metals. In Heavy Metals—Recent Advances; Almayyahi, B.A., Ed.; IntechOpen: London, UK, 2023. [Google Scholar]
- Anchidin-Norocel, L.; Gutt, G.; Tătăranu, E.; Amariei, S. Electrochemical Sensors and Biosensors: Effective Tools for Detecting Heavy Metals in Water and Food with Possible Implications for Children’s Health. Int. J. Electrochem. Sci. 2024, 19, 100643. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Hianik, T. Electrochemical Detection of Heavy Metal Ions Based on Nanocomposite Materials. J. Compos. Sci. 2023, 7, 473. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, Q.; Long, T.; He, X.; Luo, Z.; Gu, R.; Wang, W.; Xiang, P. The Innovative and Accurate Detection of Heavy Metals in Foods: A Critical Review on Electrochemical Sensors. Food Control 2023, 150, 109743. [Google Scholar] [CrossRef]
- Zeadally, S.; Siddiqui, F.; Baig, Z.; Ibrahim, A. Smart Healthcare. PSU Res. Rev. 2019, 4, 149–168. [Google Scholar] [CrossRef]
- Jaime, F.J.; Muñoz, A.; Rodríguez-Gómez, F.; Jerez-Calero, A. Strengthening Privacy and Data Security in Biomedical Microelectromechanical Systems by IoT Communication Security and Protection in Smart Healthcare. Sensors 2023, 23, 8944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Wang, H. Bioreceptors as the Key Components for Electrochemical Biosensing in Medicine. Cell Rep. Phys. Sci. 2024, 5, 101801. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Cheng, Y.; Huang, W.; Xiang, L. Electrochemical Biosensors Based on Saliva Electrolytes for Rapid Detection and Diagnosis. J. Mater. Chem. B 2023, 11, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Pradeep Kumar Agrawal, J.B. Application of Mass Spectrometry for the Discovery of Biomarker in Human Saliva: Quantification of Heavy Metals in Saliva of Smokers and Non-Smokers Using ICP-MS; University of Greenwich: London, UK, 2010. [Google Scholar]
- Bhowmick, S.; Kundu, A.K.; Adhikari, J.; Chatterjee, D.; Iglesias, M.; Nriagu, J.; Guha Mazumder, D.N.; Shomar, B.; Chatterjee, D. Assessment of Toxic Metals in Groundwater and Saliva in an Arsenic Affected Area of West Bengal, India: A Pilot Scale Study. Environ. Res. 2015, 142, 328–336. [Google Scholar] [CrossRef]
- Topal, S.; Şaylan, M.; Zaman, B.T.; Bakırdere, S. Determination of Trace Cadmium in Saliva Samples Using Spray Assisted Droplet Formation-Liquid Phase Microextraction Prior to the Measurement by Slotted Quartz Tube-Flame Atomic Absorption Spectrophotometry. J. Trace Elem. Med. Biol. 2021, 68, 126859. [Google Scholar] [CrossRef]
- Thaweboon, S.; Thaweboon, B.; Veerapradist, W. Lead in Saliva and Its Relationship to Blood in the Residents of Klity Village in Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36, 1576. [Google Scholar]
- Kumar, V.; Kumari, N.; Ealla, K.K.R.; Gour, S.; Srivastava, H.; Rallabhandi, S. Comparative Analysis of Trace Elements in the Saliva and Serum of Patients with Oral Submucous Fibrosis and Squamous Cell Carcinoma. Mol. Clin. Oncol. 2024, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.; Golasik, M.; Piekoszewski, W.; Walas, S.; Napierala, M.; Wyganowska-Swiatkowska, M.; Kurhanska-Flisykowska, A.; Wozniak, A.; Florek, E. Essential and Toxic Metals in Oral Fluid–a Potential Role in the Diagnosis of Periodontal Diseases. Biol. Trace Elem. Res. 2016, 173, 275–282. [Google Scholar] [CrossRef]
- Heavy Metal Test Kit. Available online: https://vitalityplus.au/Product/Heavy-Metal-Test-Kit/ (accessed on 4 November 2024).
- Chuanyi Mark Lu, MD, PhD, Get Tested. Available online: https://www.testing.com/tests/heavy-metals/ (accessed on 4 November 2024).
- Available online: https://www.chemsee.com/Product/Total-Heavy-Metal-Detection-Kit-Low-1-Test/ (accessed on 4 November 2024).
- Moritz Jaax, What Is Heavy Metal Poisoning—Why Does It Happen? Available online: https://www.cerascreen.co.uk/blogs/health-portal/heavy-metal-poisoning?srsltid=AfmBOopthboXgMhQrMyP6vlcAfbHInh9hUktSJRhHhEAYPRZVQHcfbHr (accessed on 4 November 2024).
- Chauhan, S.; Dahiya, D.; Sharma, V.; Khan, N.; Chaurasia, D.; Nadda, A.K.; Varjani, S.; Pandey, A.; Bhargava, P.C. Advances from Conventional to Real Time Detection of Heavy Metal(Loid)s for Water Monitoring: An Overview of Biosensing Applications. Chemosphere 2022, 307, 136124. [Google Scholar] [CrossRef]
- Available online: https://www.instrumentstrade.com/sjb-801-portable-heavy-metal-ion-detector-for-water-quality-testing_p1171.html (accessed on 4 November 2024).
- Available online: https://biodevicetech.com/Materials/157603883747601.Pdf (accessed on 4 November 2024).
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-Invasive Mouthguard Biosensor for Continuous Salivary Monitoring of Metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Martins-Lopes, P.; Fernandes, J.R. Biosensors as Diagnostic Tools in Clinical Applications. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188726. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Saaem, I.; Wu, P.C.; Brown, A.S. Personalized Diagnostics and Biosensors: A Review of the Biology and Technology Needed for Personalized Medicine. Crit. Rev. Biotechnol. 2014, 34, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-Associated Metabolic Pathways Affected by Heavy Metals and Metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
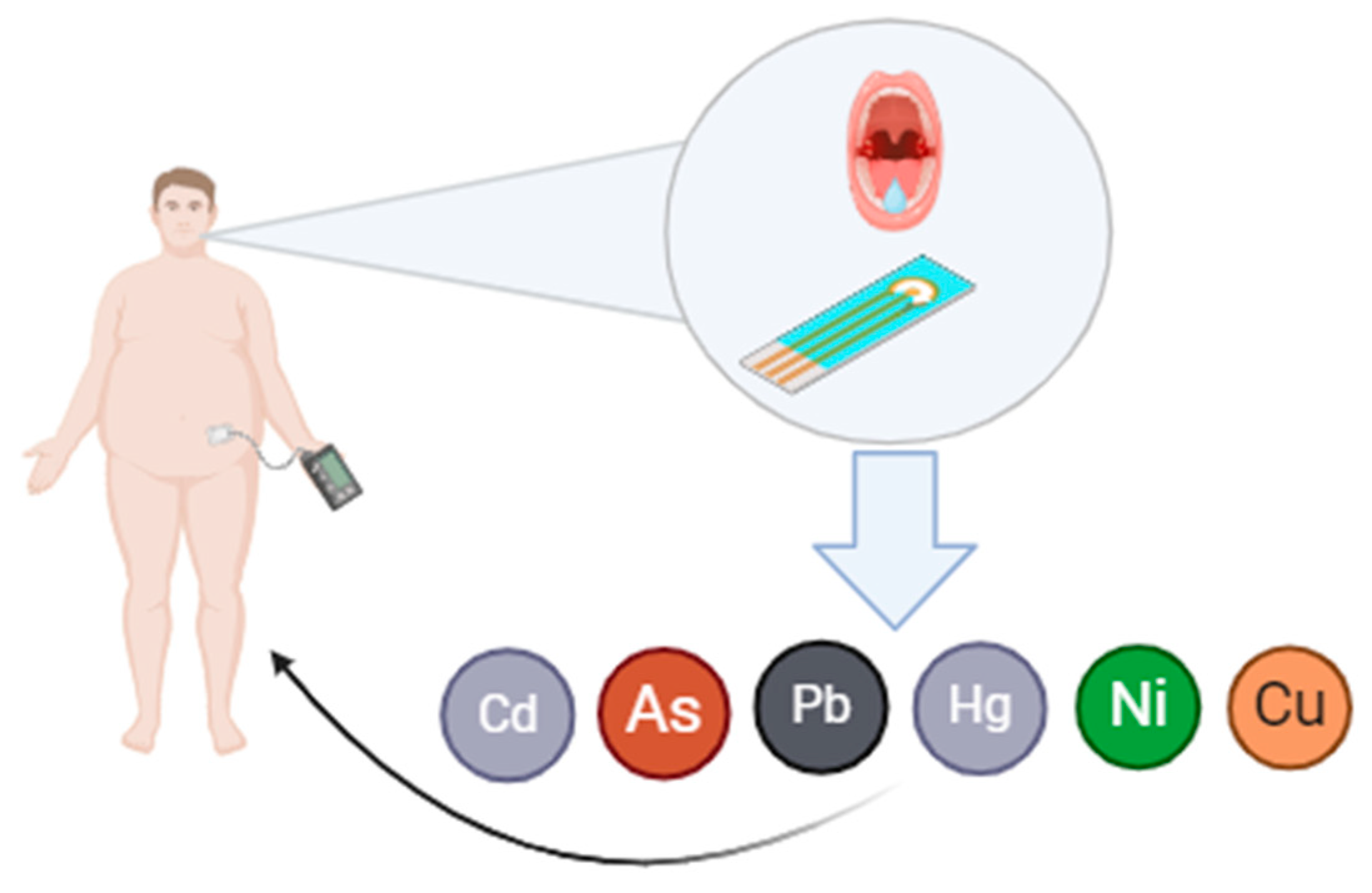
| Heavy Metal | Study Population | Location | Study Period | Diabetes (T2DM) Association | Obesity Association | MetS Association | Reference |
|---|---|---|---|---|---|---|---|
| Hg | 3787 adults | Republic of Korea | 2015–2017 | No | ↑ | - | [38] |
| 1442 mother–child pairs | US | - | - | ↑ | - | [39] | |
| 327 (10–18 years) | Republic of Korea | 2010–2013 | - | ↑ | - | [40] | |
| 646 adults | Taiwan | 2005–2008 | ↑ | - | - | [41] | |
| 495 adults | Republic of Korea | - | - | ↑ | - | [42] | |
| Cd | 177 adults | Thailand | 2020–2021 | ↑ | - | - | [43] |
| 270 adults | Southern State | - | - | ↑ | - | [44] | |
| 965 adults | Republic of Korea | - | - | - | ↑ | [45] | |
| 200 adults | Republic of Korea | 2002–2018 | No | - | - | [46] | |
| Pb | 3787 adults | Republic of Korea | 2015–2017 | - | ↑ | - | [38] |
| 177 adults | Thailand | 2020–2021 | ↑ | - | - | [43] | |
| 85 adults | Mexico | - | - | ↑ | - | [47] | |
| 1035 adults | Republic of Korea | 2002–2018 | No | - | - | [46] | |
| 1274 adults | Southern China | - | - | ↑ | - | [48] | |
| 965 adults | Republic of Korea | - | - | - | No | [45] | |
| As | 270 adults | Southern State | - | - | ↑ | - | [44] |
| 227 adults | Mexico | 2007–2011 | ↑ | - | - | [49] | |
| 3517 adults | Canada | 2012–2013 | ↑ | - | - | [50] | |
| Ni | 2444 adults | Taiwan | 2016–2018 | - | - | ↑ | [51] |
| 10,890 adults | China | 2017–2018 | ↑ | - | - | [52] | |
| 1585 adults | U.S. | 2017–2018 | ↑ | - | - | [53] | |
| 1128 adults | Italy | 2010–2016 | ↑ | - | - | [54] | |
| Cu | 2444 adults | Taiwan | 2016–2018 | - | - | ↑ | [51] |
| 117 adults | U.S. | 2013–2015 | - | ↑ | - | [55] | |
| 13,282 adults | China | 1997–2011 | - | ↑* | - | [56] | |
| 191 schoolchildren | Mexico | - | - | ↑ | - | [57] |
| Type of Sensor/Traducer | Metal Ions | Limit of Detection (LOD) | Reference |
|---|---|---|---|
| SERS | Ag (I) | 0.17 nM | [136,137] |
| SERS | Hg (II) | 2.3 pM | [136,137] |
| Microfluidic device (colorimetric) | Cu (II), Ni (II), Cr (VI) | 0.29 ppm, 0.33 ppm, 0.35 ppm | [138] |
| Microfluidic device (colorimetric) | Hg (II) | - | [138] |
| Naked eyes/UV-Vis spec. | Sb (III), Hg (II), Pb (II) | 33.7 nM, 6.34 nM, 2.38 nM | [138] |
| Naked eyes | Hg (II) | 0.5 mM | [138] |
| Voltametric sensor | Hg (II) | 0.15 nM | [138] |
| TFs-based sensors | Cu (II) | 10 nM | [138] |
| TFs-based sensors | As (III), As (V) | 10 µg/L | [138] |
| Sampling Protocol | Elements | Equipment | Reference |
|---|---|---|---|
| Subjects refrained from any food consumption or at least 1 h after any food consumption. | Cr, Co, Ni, Zn, As, Cd, In, La, Hg, Pb | ICP-MS | [147] |
| Fasted >/= 1 h, oral rinse with Milli-Q water, collected saliva into 15 mL bottles over 5 min, and stored the samples in a salt–ice mixture at −20 °C until analysis. | As, Mn, Ni, Cr, Pb, Se, Zn | ICP-MS, HG-AAS | [148] |
| Saliva samples were collected in the morning before breakfast, with volunteers rinsing their mouths with sterilized distilled water, and then 7.0–8.0 mL of saliva was collected, filtered, vortexed, centrifuged, and stored at –18 °C before analysis, with daily preparation and dilution using ultrapure water. | Cd | SQT-FAAS | [149] |
| Subjects refrained from eating, drinking, smoking, and oral hygiene for 2 h before morning saliva collection. | Pb | AAS | [150] |
| Prior to the collection of saliva, patients were not allowed to eat or drink for 2 h. | Cu, Zn, Se, Mo | ICP-OES | [151] |
| Unstimulated oral fluid (3 mL) was collected after rinsing with deionized water and centrifuged. Blood (8 mL) was drawn from a forearm vein into tubes without anticoagulant. All samples were stored at −80 °C. | Ca, Cd, Cu, Fe, Mg, Mn, Pb, Zn | ICP-MS | [152] |
| Device for Heavy Metal Detection | Heavy Metals | Method | Limit of Detection | Time of Analyses | Cost | Reference |
|---|---|---|---|---|---|---|
| Heavy metals test for water, urine and saliva | Pb, Cu, Zn, Cd, Cr, Hg | Colorimetric | semi-quantitative ppm–ppb level | - | USD 39.95- | [153] |
| Heavy metals test for urine/hair | Al, Pb, As, Cd, Cr, Co, Ni, Hg, Zn | Colorimetric | - | - | EUR 14,900/199 | [154] |
| THMLOW-01 detection kit for heavy metals and trace arsenic | As, Cd, Cu, Pb, Hg, Tl, Zn | Colorimetric | - | 15–120 s | USD 25.95 | [155] |
| Heavy metal test for urine | Al, As, Pb Cd, Cr, Co, Ni, Hg, Zn. | Colorimetric | - | - | GBP 89 | [156] |
| ChemSee generic heavy metal detector | Pb, Hg, Cd, Co, Ni, Zn | Colorimetric | - | 15–60 s | USD 9.95–87.90 | [155] |
| AppliTrace | Cd, Pb, Zn, Cu, As | Anodic stripping voltammetry | 1 μg/L | 40 min | - | [157] |
| uMED | Cd, Zn, Pb | Square wave anodic stripping voltammetry | 4 μg/L | - | USD 25 | [157] |
| Portable heavy metal ion detector INE-SJB-801 | Cd, Zn, As, Hg | Anodic stripping voltammetry | (0–100) μg/L | - | USD 11,800.00 | [158] |
| DEP-Chip | Cd, Zn, As, Pb, Cu | Differential pulse voltammetry | 2.6 μg/L 14.4 μg/L 4.0 μg/L 5.0 μg/L 15.5 μg/L | 5 min | <USD 1 | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anchidin-Norocel, L.; Savage, W.K.; Nemțoi, A.; Dimian, M.; Cobuz, C. Recent Progress in Saliva-Based Sensors for Continuous Monitoring of Heavy Metal Levels Linked with Diabetes and Obesity. Chemosensors 2024, 12, 269. https://doi.org/10.3390/chemosensors12120269
Anchidin-Norocel L, Savage WK, Nemțoi A, Dimian M, Cobuz C. Recent Progress in Saliva-Based Sensors for Continuous Monitoring of Heavy Metal Levels Linked with Diabetes and Obesity. Chemosensors. 2024; 12(12):269. https://doi.org/10.3390/chemosensors12120269
Chicago/Turabian StyleAnchidin-Norocel, Liliana, Wesley K. Savage, Alexandru Nemțoi, Mihai Dimian, and Claudiu Cobuz. 2024. "Recent Progress in Saliva-Based Sensors for Continuous Monitoring of Heavy Metal Levels Linked with Diabetes and Obesity" Chemosensors 12, no. 12: 269. https://doi.org/10.3390/chemosensors12120269
APA StyleAnchidin-Norocel, L., Savage, W. K., Nemțoi, A., Dimian, M., & Cobuz, C. (2024). Recent Progress in Saliva-Based Sensors for Continuous Monitoring of Heavy Metal Levels Linked with Diabetes and Obesity. Chemosensors, 12(12), 269. https://doi.org/10.3390/chemosensors12120269





