A Simple Green Method for the Determination of Hydrogen Peroxide and Fe(III)/Fe(II) Species Based on Monitoring the Decolorization Process of Polymethine Dye Using an Optical Immersion Probe
Abstract
:1. Introduction
2. Experimental
2.1. Computational Details
2.2. Reagents and Equipment
2.3. Procedure for H2O2 Determination
2.4. Procedure for Fe(III)/Fe(II) Determination in the Presence of Hydrogen Peroxide
2.5. Procedure for Fe(III) Determination
2.6. Joint Determination of Fe(II) and Fe(III) Species
2.7. Real Sample Analysis
3. Results and Discussion
3.1. Computational Interpretation of the Sensing Mechanism
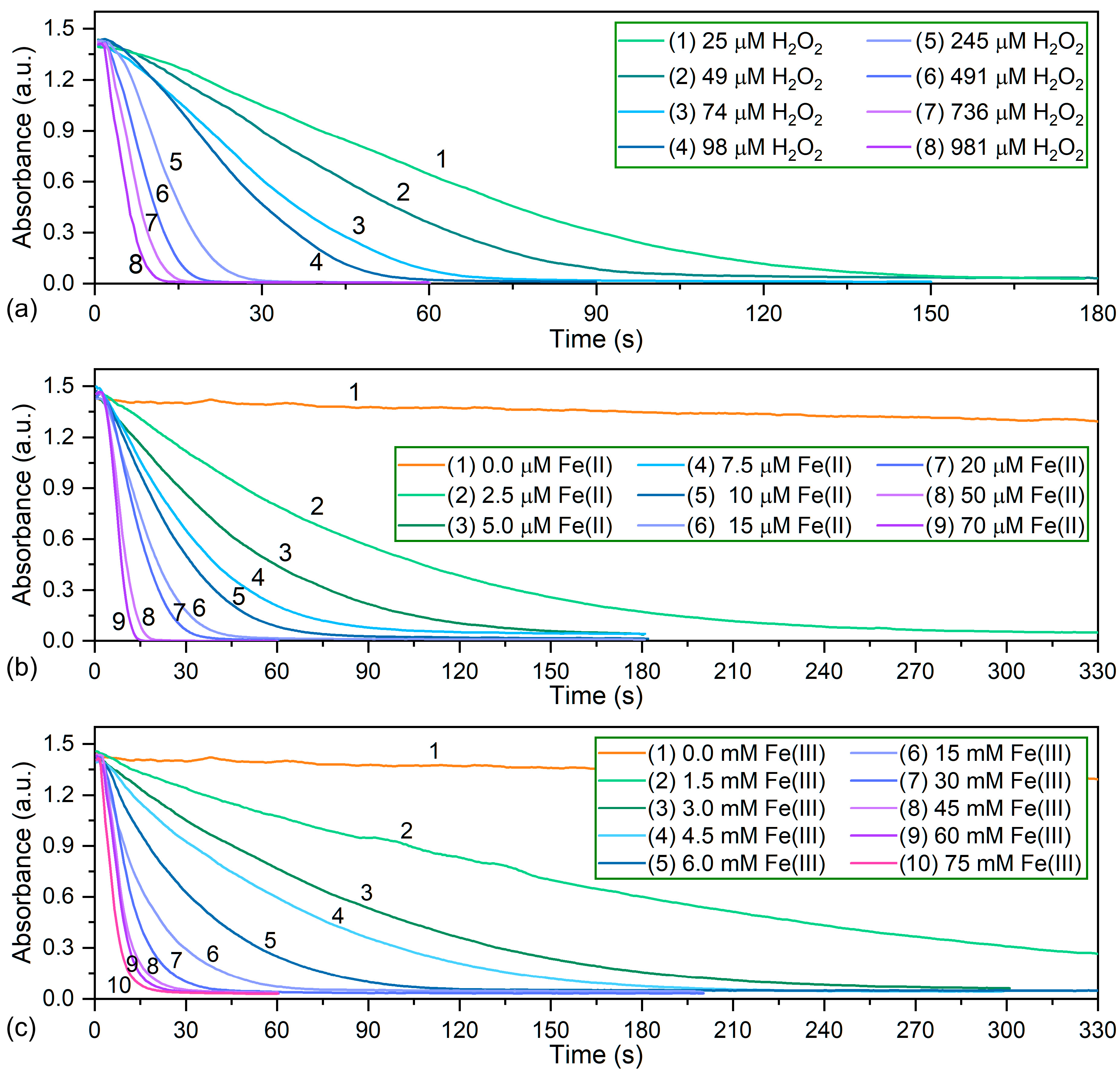
3.2. Optimization of Reaction Conditions
3.3. Interference Study
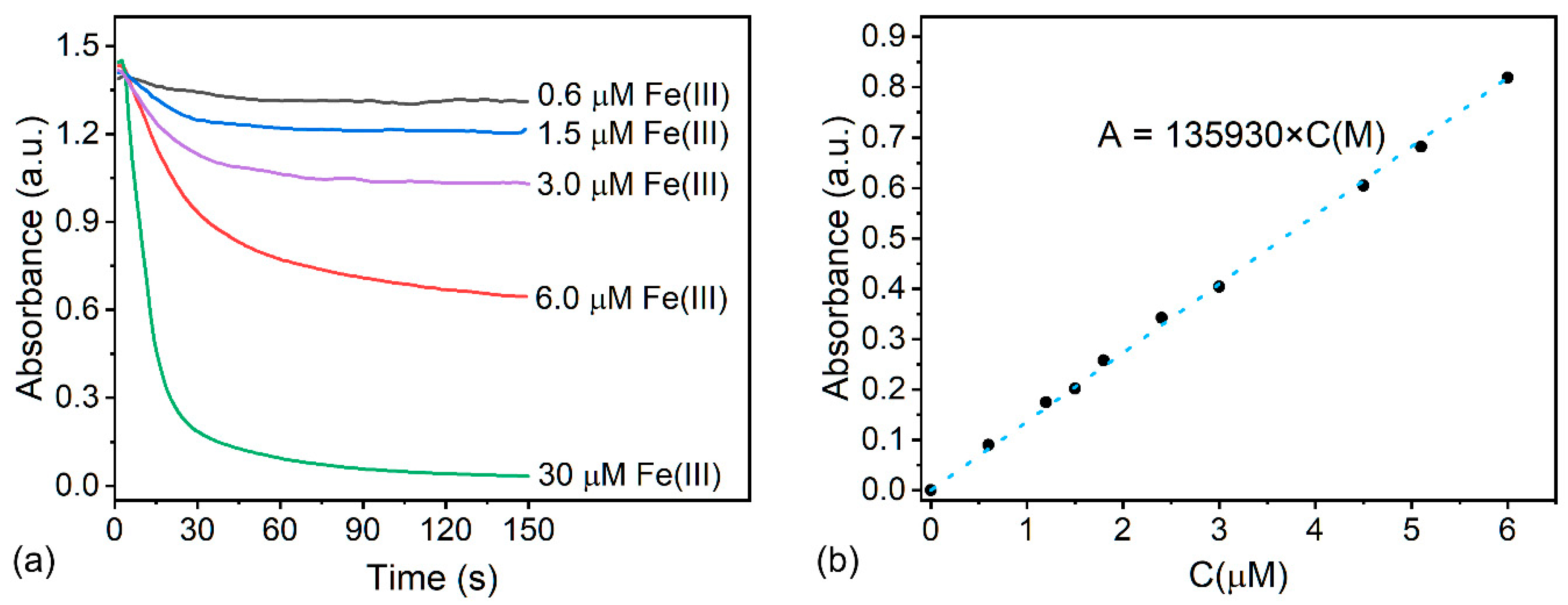
3.4. Analytical Parameters
3.5. Analysis of Real Samples
3.6. Comparison with Methods Proposed in the Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruben Vasquez-Medrano, R.; Prato-Garcia, D.; Vedrenne, M. Chapter 4—Ferrioxalate-Mediated Processes in Advanced Oxidation Processes for Waste Water Treatment Emerging Green Chemical Technology, 1st ed.; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 89–113. [Google Scholar]
- Tóth, J.; Bazel, Y. Development of a new kinetic spectrophotometric method for the determination of chromium with an optical probe. Appl. Spectrosc. 2019, 73, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Assiri, M.A.; Munir, F.; Waseem, M.T.; Irshad, H.; Rauf, W.; Shahzad, S.A. Restricted intramolecular vibrations assisted enhanced fluorescence emission response of probe: A new experimental and theoretical approach for the detection of hydrogen peroxide. Microchem. J. 2023, 193, 109220. [Google Scholar] [CrossRef]
- Shang, L.L.; Song, X.; Niu, C.B.; Lv, Q.Y.; Li, C.L.; Cui, H.F. Red fluorescent nanoprobe based on Ag@Au nanoparticles and graphene quantum dots for H2O2 determination and living cell imaging. Microchim. Acta 2021, 188, 291. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.N.; Jayeoye, T.J.; Eze, R.C. Construction, characterization and application of locust bean gum/ Phyllanthus reticulatus anthocyanin—based plasmonic silver nanocomposite for sensitive detection of ferrous ions. Environ. Res. 2023, 228, 115864. [Google Scholar] [CrossRef] [PubMed]
- Alraqa, S.Y.; Kaya, E.N.; Taşci, N.; Erbahar, D.; Durmuş, M. Pyrene substituted Phthalonitrile derivative as a fluorescent sensor for detection of Fe3+ ions in solutions. J. Fluoresc. 2022, 32, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Morgante, P.; Autschbach, J. Strategies to calculate Fukui functions and applications to radicals with SOMO–HOMO inversion. J. Chem. Theory Comput. 2023, 19, 3929–3942. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Bulat, F.A. Average local ionization energy: A review. J. Mol. Model. 2010, 16, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Hanson-Heine, M.W.D.; George, M.W.; Besley, N.A. A scaled CIS(D) based method for the calculation of valence and core electron ionization energies. J. Chem. Phys. 2019, 151, 034104. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, M.; Neese, F. Efficient implementation of the analytical second derivatives of Hartree-Fock and hybrid DFT energies within the framework of the conductor-like polarizable continuum model. J. Comput. Chem. 2019, 40, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An improved chain of spheres for exchange algorithm. J. Chem. Phys. 2021, 155, 104109. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system—Version 5.0, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Molec. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Gesevicius, D.; Neels, A.; Jenatsch, S.; Hack, E.; Viani, L.; Athanasopoulos, S.; Nüesch, F.; Heier, J. Increasing photovoltaic performance of an organic cationic chromophore by anion exchange. Adv. Sci. 2018, 5, 1700496. [Google Scholar] [CrossRef]
- Gesevicius, D.; Neels, A.; Yakunin, S.; Hack, E.; Kovalenko, M.V.; Nuesch, F.; Heier, J. Superweak coordinating anion as superstrong enhancer of cyanine organic semiconductor properties. Chem. Phys. Chem. 2018, 19, 3356–3363. [Google Scholar] [CrossRef]
- Xu, W.; Leary, E.; Sangtarash, S.; Jirasek, M.; González, M.T.; Christensen, K.E.; Abellán Vicente, L.; Agraït, N.; Higgins, S.J.; Nichols, R.J.; et al. A Peierls transition in long polymethine molecular wires: Evolution of molecular geometry and single-molecule conductance. J. Am. Chem. Soc. 2021, 143, 48, 20472–20481. [Google Scholar] [CrossRef]
- Pucci, R.; Angilella, G.G.N. Density functional theory, chemical reactivity, and the Fukui functions. Found. Chem. 2022, 24, 59–71. [Google Scholar] [CrossRef]
- Fizer, M.; Fizer, O. Theoretical study on charge distribution in cetylpyridinium cationic surfactant. J. Mol. Model. 2021, 27, 203. [Google Scholar] [CrossRef] [PubMed]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y.; Mariychuk, R. Benchmark of different charges for prediction of the partitioning coefficient through the hydrophilic/lipophilic index. J. Mol. Model. 2018, 24, 141. [Google Scholar] [CrossRef]
- Fizer, M.; Slivka, M.; Baumer, V.; Slivka, M.; Fizer, O. Alkylation of 2-oxo(thioxo)-thieno[2,3-d]pyrimidine-4-ones: Experimental and theoretical study. J. Mol. Struct. 2019, 1198, 126858. [Google Scholar] [CrossRef]
- Bruhn, G.; Davidson, E.R.; Mayer, I.; Clark, A.E. Löwdin population analysis with and without rotational invariance. Int. J. Quantum Chem. 2006, 106, 2065–2072. [Google Scholar] [CrossRef]
- De Proft, F.; Van Alsenoy, C.; Peeters, A.; Langenaeker, W.; Geerlings, P. Atomic charges, dipole moments, and Fukui functions using the Hirshfeld partitioning of the electron density. J. Comput. Chem. 2002, 23, 1198–1209. [Google Scholar] [CrossRef]
- Saha, S.; Roy, R.K.; Ayers, P.W. Are the Hirshfeld and Mulliken population analysis schemes consistent with chemical intuition? Int. J. Quantum Chem. 2008, 109, 1790–1806. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Cameron, A.D. Electrochemistry and electronic spectra of cyanine dye radicals in acetonitrile. J. Phys. Chem. 1993, 97, 4916–4925. [Google Scholar] [CrossRef]
- Zhang, H.; Masui, Y.; Masai, H.; Terao, J. Post-synthetic modification of near-infrared absorbing cyclodextrin-encapsulated cyanine dyes for controlling absorption wavelength, stability, and singlet oxygen generation. Bull. Chem. Soc. Jpn. 2024, 97, uoae055. [Google Scholar] [CrossRef]
- Lisovskaya, A.; Carmichael, I.; Harriman, A. Pulse radiolysis investigation of radicals derived from water-soluble cyanine dyes: Implications for super-resolution microscopy. J. Phys. Chem. A 2021, 125, 5779–5793. [Google Scholar] [CrossRef]
- Olson, A.S.; Jameson, A.J.; Kyasa, S.K.; Evans, B.W.; Dussault, P.H. Reductive cleavage of organic peroxides by iron salts and thiols. ACS Omega 2018, 3, 14054–14063. [Google Scholar] [CrossRef] [PubMed]
- Reiss, H.; Heller, A. The absolute potential of the standard hydrogen electrode: A new estimate. J. Phys. Chem. 1985, 89, 4207–4213. [Google Scholar] [CrossRef]
- Fornari, R.P.; de Silva, P. A computational protocol combining DFT and cheminformatics for prediction of pH-dependent redox potentials. Molecules 2021, 26, 3978. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.G.; Dixon, D.A. Absolute hydration free energy of the proton from first-principles electronic structure calculations. J. Phys. Chem. A 2001, 105, 11534–11540. [Google Scholar] [CrossRef]
- Kakalejčikova, S.; Bazel, Y.; Fizer, M. Extraction-less green spectrofluorimetric method for determination of mercury using an Astra Phloxine fluorophore: Comprehensive experimental and theoretical studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 310, 123946. [Google Scholar] [CrossRef]
- Meguellati, K.; Spichty, M.; Ladame, S. Reversible synthesis and characterization of dynamic imino analogues of trimethine and pentamethine cyanine dyes. Org. Lett. 2009, 11, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H. Über α.β-ungesättigte β-Anilino-carbonyl-Verbindungen als Modellsubstanzen für den Chromophor von C-Curarin-III (C-Fluorocurarin). Chem. Ber. 1959, 92, 1809–1817. [Google Scholar] [CrossRef]
- Pfoertner, K.; Bernauer, K. Über die sensibilisierte Photooxydation einiger cyclischer N-Aryl-enamine. 3. Mitteilung über Indole, Indolenine und Indoline. Helv. Chim. Acta 1968, 51, 1787–1794. [Google Scholar] [CrossRef]
- Tomita, M.; Ageo, S.; Yamamoto, R. On the absorption spectra of some indole compounds. Spectrochemical studies on alkaloids and related compounds. J. Pharm. Soc. Jpn. 1944, 64, 164–167. [Google Scholar]
- Toth, J.; Bazel, Y.; Balogh, I. A fully automated system with an optical immersion probe (OIP) for high-precision spectrophotometric measurements. Talanta 2021, 226, 122185. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE–Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Gul, U.; Kanwal, S.; Tabassum, S.; Gilani, M.A.; Rahim, A. Microwave-assisted synthesis of carbon dots as reductant and stabilizer for silver nanoparticles with enhanced-peroxidase like activity for colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2020, 187, 135. [Google Scholar] [CrossRef]
- Zou, J.; Cai, H.; Wang, D.; Xiao, J.; Zhou, Z.; Yuan, B. Spectrophotometric determination of trace hydrogen peroxide via the oxidative coloration of DPD using a Fenton system. Chemosphere 2019, 224, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, D.; Qiu, S.; Xiao, J.; Cai, H.; Zou, J. Multi-wavelength spectrophotometric determination of hydrogen peroxide in water byoxidative coloration of ABTS via Fenton reaction. Environ. Sci. Pollut. Res. 2019, 26, 27063–27072. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Cui, T.; Li, S.; Qian, Y.; Yue, Y.; Zhong, W.; Xu, B.; Yue, W. PtS2 nanosheets as a peroxidase-mimicking nanozyme for colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2021, 188, 174. [Google Scholar] [CrossRef]
- Yavuz, T.; Pelit, L. Sensitive determination of hydrogen peroxide in real water samples by high spin peroxo complex. Turk. J. Chem. 2020, 44, 435–447. [Google Scholar] [CrossRef]
- Lua, C.; Wang, Y.; Xu, B.; Zhang, W.; Xie, Y.; Chen, Y.; Wang, L.; Wang, X. A colorimetric and fluorescence dual-signal determination for iron (II) and H2O2 in food based on sulfur quantum dots. Food Chem. 2022, 366, 130613. [Google Scholar] [CrossRef]
- Khani, S.; Mohajer, F.; Ziarani, G.M.; Badiei, A.; Ghasemi, J.B. Using the extract of pomegranate peel as a natural indicator for colorimetric detection and simultaneous determination of Fe3+ and Fe2+ by partial least squares–artificial neural network. J. Chemom. 2023, 37, e3390. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Yang, C.; Zhu, H.; Li, Y.; Sun, T.; Zhou, C. A direct and rapid method for determination of total iron in environmental samples and hydrometallurgy using UV–Vis spectrophotometry. Microchem. J. 2022, 179, 107478. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, T.; Sun, T.; Wang, Y.; Zhou, C.; Zhu, H.; Li, Y. A simple, sensitive and selective spectrophotometric method for determining iron in water samples. Microchem. J. 2021, 165, 106154. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, G.; Tan, X.; Qian, X.; Zhang, T.; Gui, J.; Yang, L.; Xie, X. Nitrogen-doped carbon dots with high quantum yield for colorimetric and fluorometric detection of ferric ions and in a fluorescent ink. Microchim. Acta 2019, 186, 67. [Google Scholar] [CrossRef] [PubMed]
- Filipe João, A.; Squissato, A.L.; Fernandes, G.M.; Cardoso, R.M.; Batista, A.D.; Muñoz, R.A.A. Iron (III) determination in bioethanol fuel using a smartphone-based device. Microchem. J. 2019, 146, 1134–1139. [Google Scholar] [CrossRef]
- Lapanantnoppakhun, S.; Tengjaroensakul, U.; Mungkornasawakul, P.; Puangpila, C.; Kittiwachana, S.; Saengtempiam, J.; Hartwell, S.K. Green analytical chemistry experiment: Quantitative analysis of iron in supplement tablets with Vis spectrophotometry using tea extract as a chromogenic agent. J. Chem. Educ. 2019, 97, 207–214. [Google Scholar] [CrossRef]
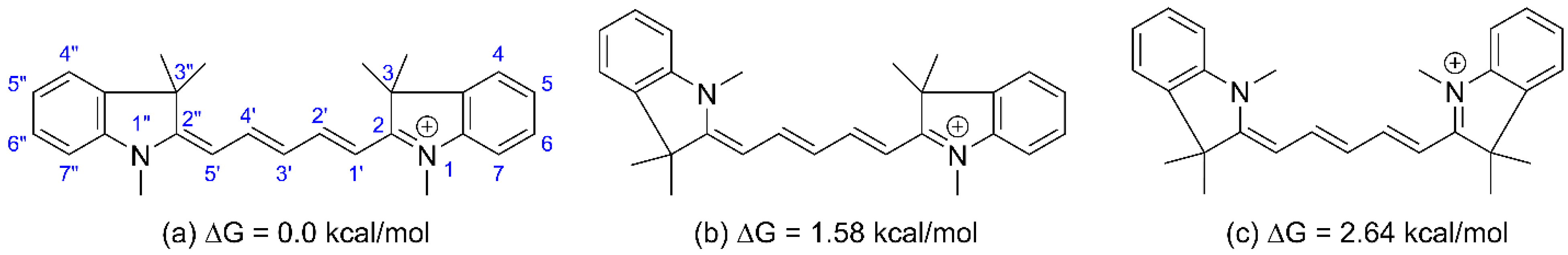
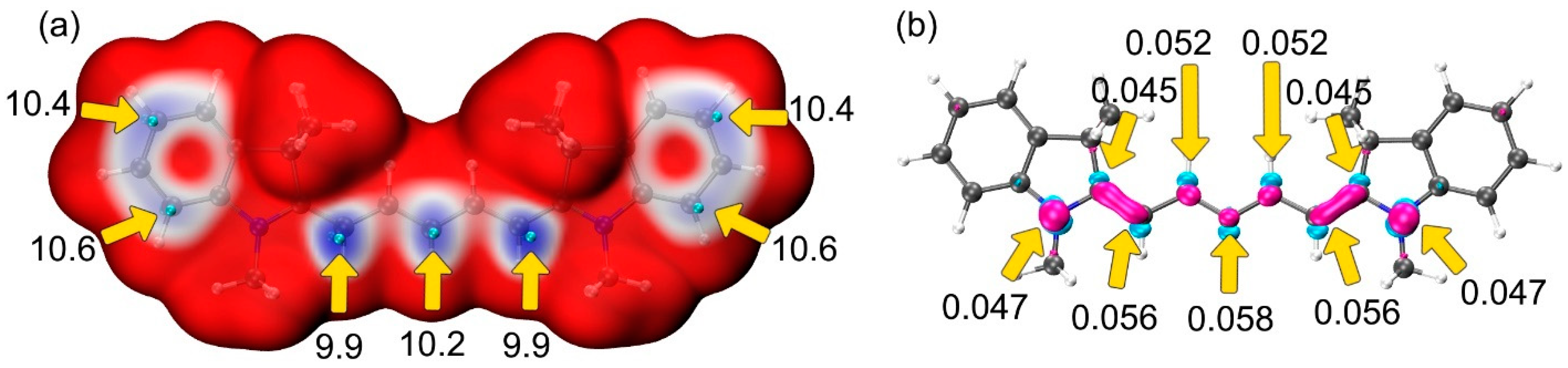
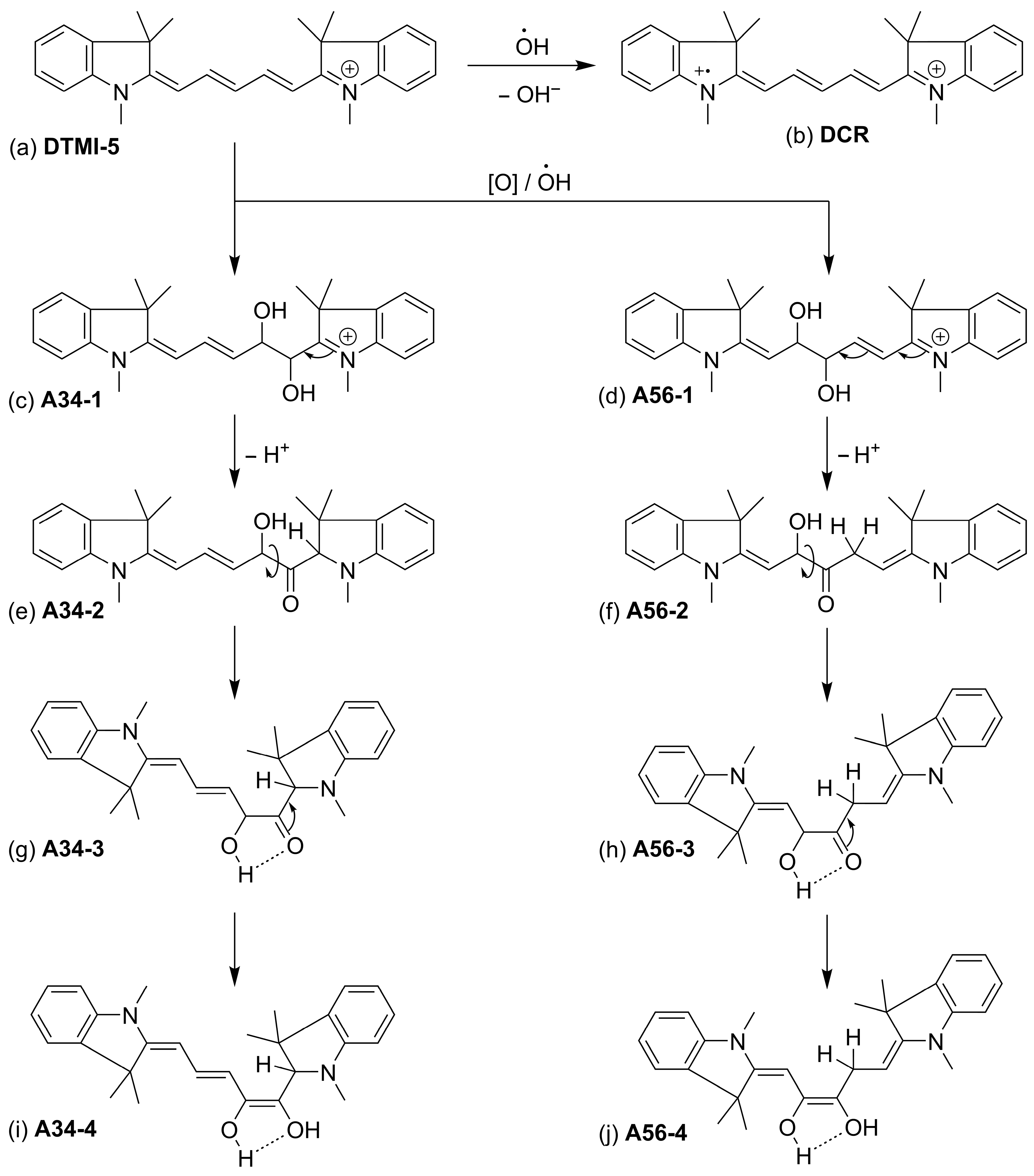
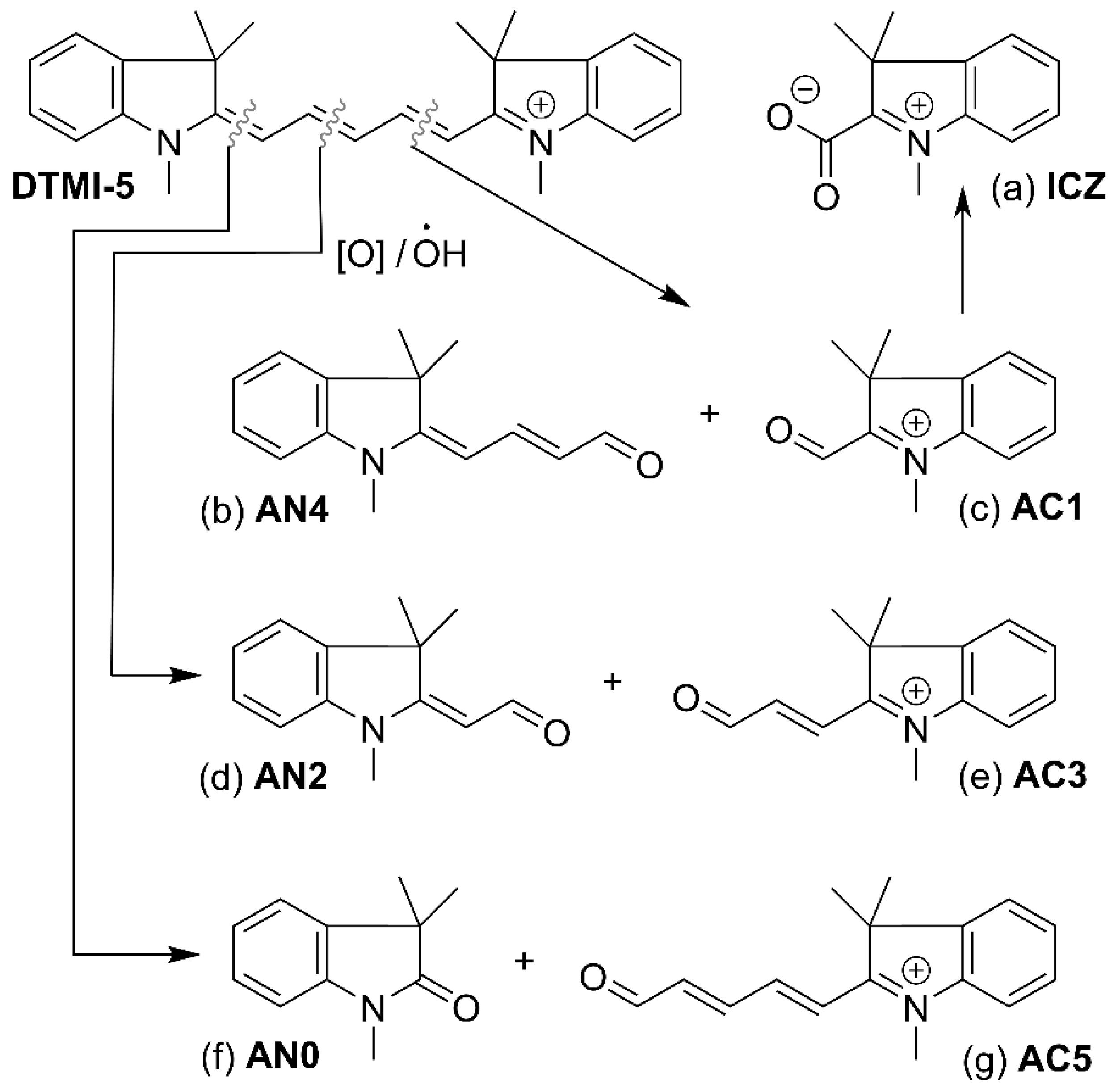
| Structure | U0, V | λmax, nm |
|---|---|---|
| A56-1 | +1.098 | 304.0 |
| A34-1 | +1.186 | 302.1 |
| A56-2 | +1.236 | 270.1 |
| A34-2 | +1.327 | 286.4 |
| A56-3 | +1.157 | 270.6 |
| A34-3 | +1.313 | 282.0 |
| A56-4 | +1.359 | 281.0 |
| A34-3 | +1.651 | 318.3 |
| DCR | +0.827 | 424.9 |
| ICZ | +0.335 | 237.0 |
| AN4 | +0.722 | 358.1 |
| AC1 | +0.722 | 302.9 |
| AN2 | +0.641 | 295.8 |
| AC3 | +0.641 | 316.4 |
| AN0 | +0.444 | 242.9 |
| AC5 | +0.444 | 337.4 |
| Added H2O2, % | Found H2O2, % | Recovery, % |
|---|---|---|
| – | 5.92 ± 1.15 | – |
| 9.07 | 15.9 ± 0.9 | 110.0 |
| 17.3 | 24.3 ± 2.5 | 106.4 |
| Sample | Added Fe(II), µM | Added Fe(III), µM | Found Fe(II), µM | Recovery, % | Found Fe(III), µM | Recovery, % |
|---|---|---|---|---|---|---|
| Model solution 1 | – | – | 5.11 ± 1.18 | 102.2 | 2.43 ± 0.32 | 101.2 |
| Model solution 2 | – | – | 16.8 ± 2.9 | 112.0 | 14.8 ± 0.7 | 98.7 |
| Model solution 3 | – | – | 14.4 ± 2.7 | 96.0 | 5.89 ± 0.55 | 103.3 |
| Tap water | – | – | ˂LOD | – | 0.592 ± 0.144 | – |
| – | 3.22 | ˂LOD | – | 3.93 ± 0.38 | 103.5 | |
| 8.91 | – | 9.46 ± 0.58 | 106.2 | 0.576 ± 0.097 | – | |
| Fertilizer 4 | – | – | 0.024 ± 0.003% | – | 0.017 ± 0.001% | – |
| – | 0.028% | – | – | 0.049 ± 0.002% | 112.8 | |
| 0.032% | – | 0.055 ± 0.007 | 96.9 | 0.018 ± 0.001% | – |
| Reagent | Sample | LOD, µM | Determination Range, µM | Ref. |
|---|---|---|---|---|
| H2O2 | ||||
| carbon silver nano-assembly | - | 0.009 | 0.1–100 | [43] |
| N,N-diethyl-p-phenylenediamine, Fe(II) | water | 0.05 | 0–12 | [44] |
| 2,2′-azino-bis(3-ethylbenzothiazoline-6 sulfonate, Fe(II) | water | 0.1 | 0–40 | [45] |
| PtS2 nanosheets, 3,3′,5,5′-tetramethylbenzidine | - | 0.33 | 1–100 | [46] |
| peroxo-Fe(III)-EDTA complex | water | 2.5 | 8.3–4080 | [47] |
| DTMI-5, Fe(II) | oxidation emulsion | 12.3 | 40–980 | This work |
| Fe(II) | ||||
| plasmonic Ag nanocomposite based on locust bean gum and Phyllanthus reticulatus anthocyanin | juice, ferrous fumarate, water | 0.38 | 0.1–100 | [5] |
| sulfur quantum dots | milk, honey, water | 0.54 | 1.25–500 | [48] |
| pomegranate peel extract | water | 140 µg/L | 1000–10,000 µg/L | [49] |
| DTMI-5, H2O2 | water, fertilizer | 0.88 | 3–70 | This work |
| Fe(III) | ||||
| nitroso R salt, NaI, cetyl trimethyl ammonium chloride | water, CRM, electrolyte, purification liquid | 0.045 (total iron) | 0.15–64 | [50] |
| 1-nitroso-2-naphthol-3,6-disulphonic acid disodium salt, hexadecyl trimethyl ammonium bromide | water | 0.067 | 0.2–95 | [51] |
| N-carbon dots | water | 0.28 | 1–21 | [52] |
| KSCN | bioethanol fuel | 6.18 mg/L | 0.5–10 mg/L | [53] |
| pomegranate peel extract | water | 530 µg/L | 1000–12,000 µg/L | [49] |
| tea extract | iron supplement samples | 2000 µg/L | ˂40,000 µg/L | [54] |
| DTMI-5 | water, fertilizer | 0.16 | 0.5–6 | This work |
| DTMI-5, H2O2 | water, fertilizer | 0.39 | 1.32–45 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skok, A.; Bazel, Y.; Fizer, M. A Simple Green Method for the Determination of Hydrogen Peroxide and Fe(III)/Fe(II) Species Based on Monitoring the Decolorization Process of Polymethine Dye Using an Optical Immersion Probe. Chemosensors 2024, 12, 270. https://doi.org/10.3390/chemosensors12120270
Skok A, Bazel Y, Fizer M. A Simple Green Method for the Determination of Hydrogen Peroxide and Fe(III)/Fe(II) Species Based on Monitoring the Decolorization Process of Polymethine Dye Using an Optical Immersion Probe. Chemosensors. 2024; 12(12):270. https://doi.org/10.3390/chemosensors12120270
Chicago/Turabian StyleSkok, Arina, Yaroslav Bazel, and Maksym Fizer. 2024. "A Simple Green Method for the Determination of Hydrogen Peroxide and Fe(III)/Fe(II) Species Based on Monitoring the Decolorization Process of Polymethine Dye Using an Optical Immersion Probe" Chemosensors 12, no. 12: 270. https://doi.org/10.3390/chemosensors12120270
APA StyleSkok, A., Bazel, Y., & Fizer, M. (2024). A Simple Green Method for the Determination of Hydrogen Peroxide and Fe(III)/Fe(II) Species Based on Monitoring the Decolorization Process of Polymethine Dye Using an Optical Immersion Probe. Chemosensors, 12(12), 270. https://doi.org/10.3390/chemosensors12120270






