Investigation of Long-Term Performance of a Proposed Cost-Effective HCl Non-Dispersive Infrared Analyzer at Real Stationary Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Development of HCl Analyzer
2.2.1. Negligible-Interference Bandpass Filter
2.2.2. Improvement of the Zero-Drift Issue
2.2.3. Investigation of Optimal Path Length
2.3. Performance of Proposed HCl NDIR Analyzer at Laboratory-Scale Inspection
2.4. Long-Term Field Trials of the Proposed HCl NDIR Analyzer
3. Results and Discussion
3.1. Development of HCl Analyzer
3.1.1. Negligible-Interference Bandpass Filter
3.1.2. Improvement of the Zero-Drift Issue
3.1.3. Investigation of Optimal Path Length
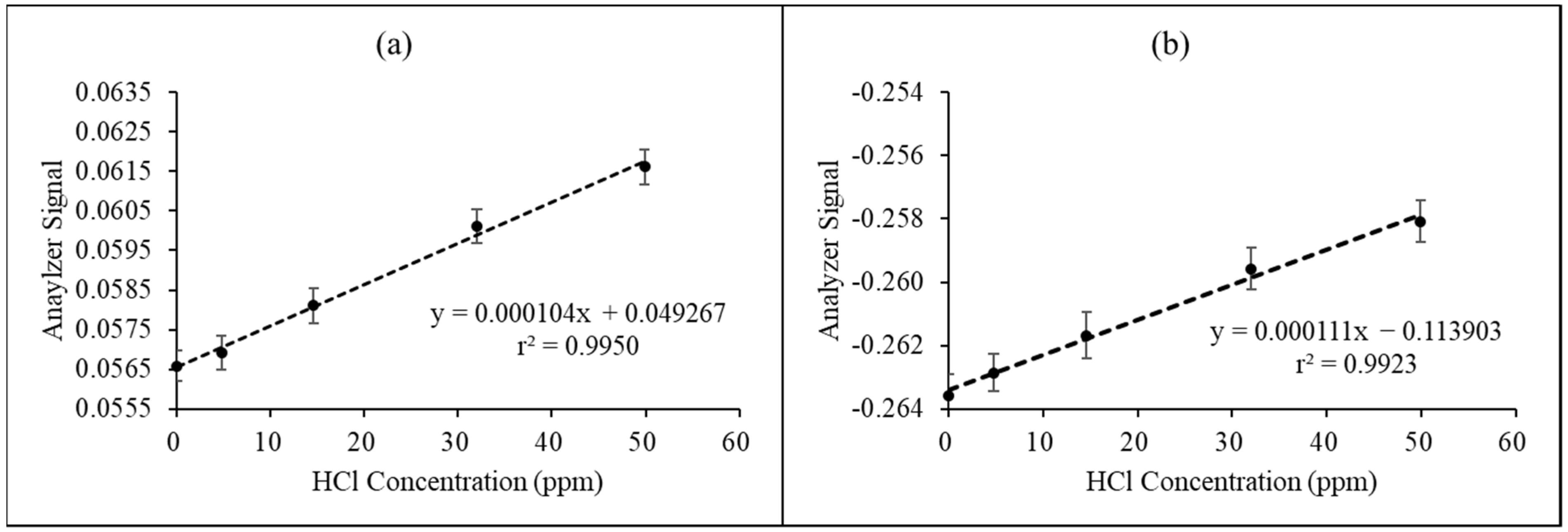
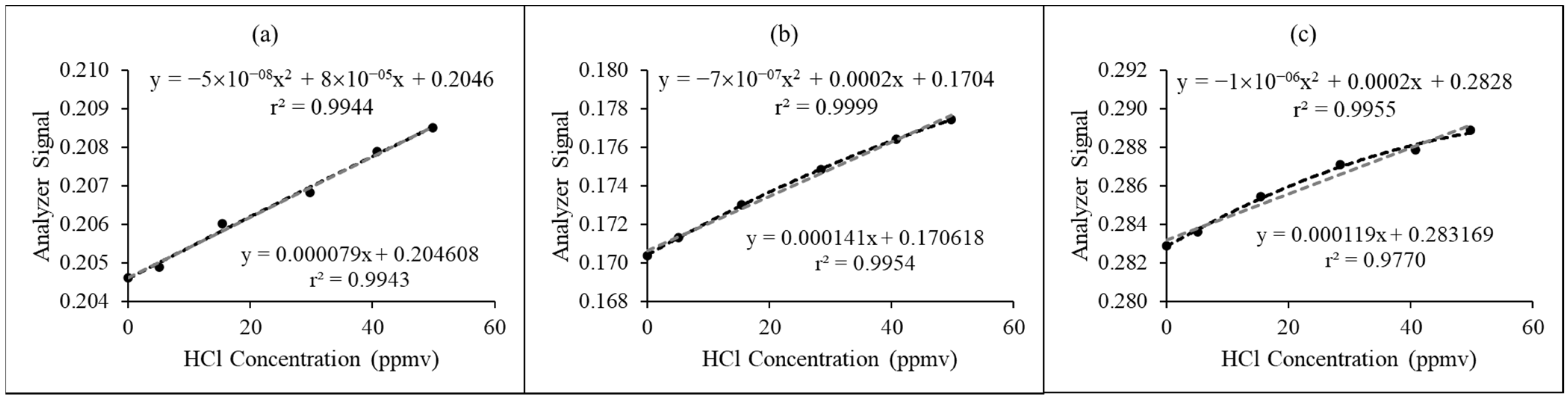
3.2. Performance of Proposed HCl NDIR Analyzer at Laboratory-Scale Inspection
3.3. Long-Term Field Trials of the Proposed HCl NDIR Analyzer
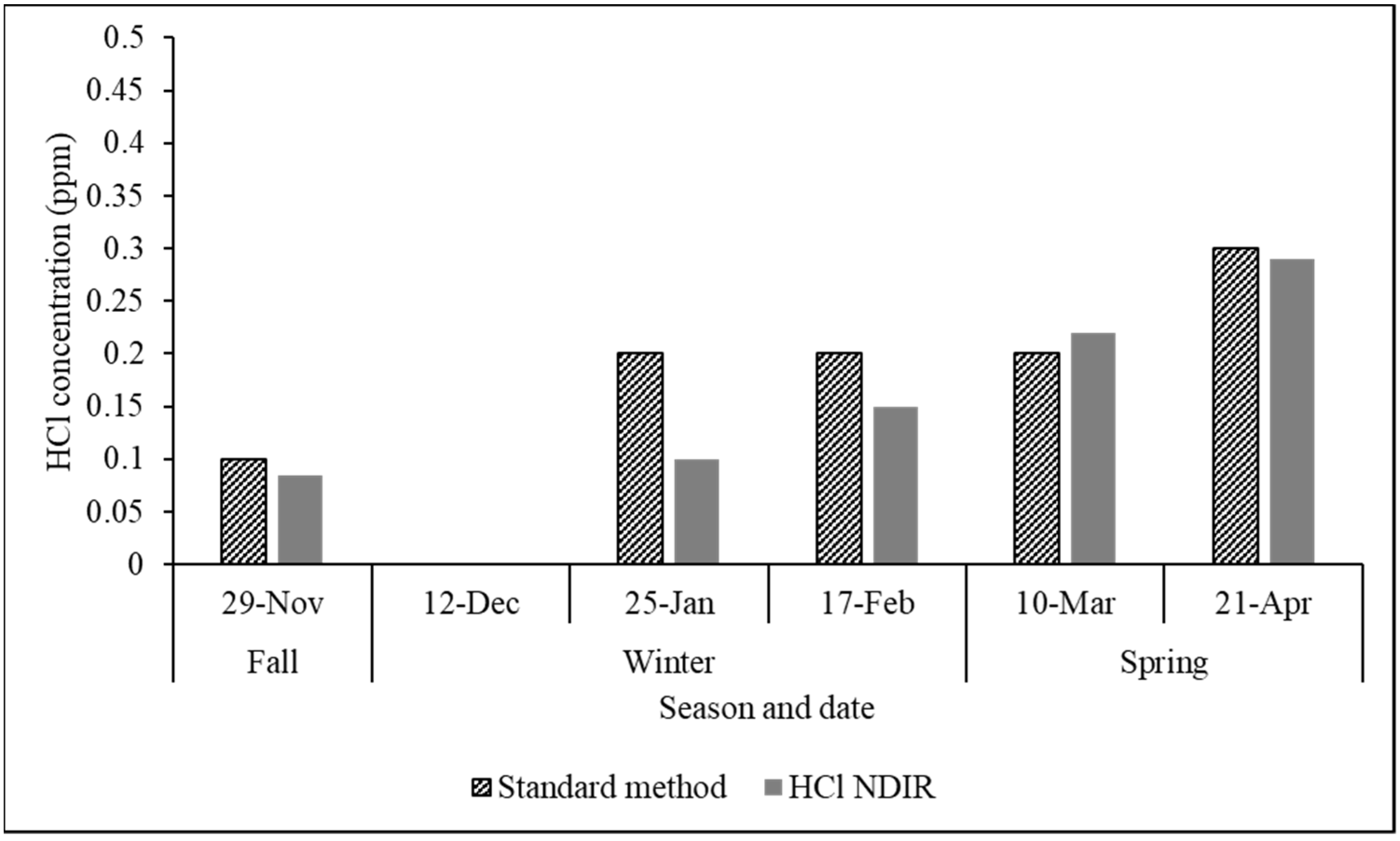
3.3.1. Long-Term Field Trials of the Proposed Analyzer at a Metalworking Factory
3.3.2. Long-Term Field Trials of the Proposed Analyzer at a Cement Factory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US National Library of Medicine. Hydrochloric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrochloric-acid (accessed on 16 November 2023).
- Pohanish, R.P. Sittig’s Handbook of Toxic and Hazardous Chemicals and Carcinogens; William Andrew: Norwich, NY, USA, 2017; ISBN 978-0-323-38968-6. [Google Scholar]
- Fu, X.; Wang, T.; Wang, S.; Zhang, L.; Cai, S.; Xing, J.; Hao, J. Anthropogenic Emissions of Hydrogen Chloride and Fine Particulate Chloride in China. Environ. Sci. Technol. 2018, 52, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.N.; Dinh, T.V.; Lee, J.H.; Park, B.G.; Choi, I.Y.; Lee, S.W.; Kim, I.Y.; Kim, J.C. Effects of Humidity Pretreatment Devices on the Loss of Hcl Gas Emitted from Industrial Stacks. Atmosphere 2022, 13, 33. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Chi, H.H.; Meng, X.; Li, Y.; Levendis, Y.A. Hydrogen Chloride Emissions from Combustion of Raw and Torrefied Biomass. Fuel 2017, 200, 37–46. [Google Scholar] [CrossRef]
- Rokni, E.; Ren, X.; Panahi, A.; Levendis, Y.A. Emissions of SO2, NOx, CO2, and HCl from Co-Firing of Coals with Raw and Torrefied Biomass Fuels. Fuel 2018, 211, 363–374. [Google Scholar] [CrossRef]
- Crisp, T.A.; Lerner, B.M.; Williams, E.J.; Quinn, P.K.; Bates, T.S.; Bertram, T.H. Observations of Gas Phase Hydrochloric Acid in the Polluted Marine Boundary Layer. J. Geophys. Res. 2014, 119, 6897–6915. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, H.; Yun, X.; Zhong, Q.; Henderson, B.H.; Wang, X.; Shi, L.; Gunthe, S.S.; Huey, L.G.; Tao, S.; et al. Global Emissions of Hydrogen Chloride and Particulate Chloride from Continental Sources. Environ. Sci. Technol. 2022, 56, 3894–3904. [Google Scholar] [CrossRef] [PubMed]
- Dene, C.; Pisano, J.T.; Durbin, T.D.; Bumiller, K.; Crabbe, K.; Muzio, L.J. Laboratory Testing of a Continuous Emissions Monitor for Hydrochloric Acid. J. Air Waste Manag. Assoc. 2014, 64, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Mackay, K.L.; Chanda, A.; Mackay, G.; Pisano, J.T.; Durbin, T.D.; Crabbe, K.; Smith, T. Measurement of Hydrogen Chloride in Coal-Fired Power Plant Emissions Using Tunable Diode Laser Spectrometry. J. Appl. Spectrosc. 2016, 83, 627–633. [Google Scholar] [CrossRef]
- Zhang, X.; Schreifels, J. Continuous Emission Monitoring Systems at Power Plants in China: Improving SO2 Emission Measurement. Energy Policy 2011, 39, 7432–7438. [Google Scholar] [CrossRef]
- Ahmed, N.N.; Kale, G.M.; Kumar, R.V.; Fray, D.J. Development and Testing of HCl Gas Sensor for Flue Gas Monitoring. Solid State Ion. 1996, 86–88, 1013–1016. [Google Scholar] [CrossRef]
- Dene, C.E.; Pisano, J.T.; Durbin, T.D.; Bumiller, K.; Crabbe, K.; Muzio, L.J. Laboratory Investigation of Three Distinct Emissions Monitors for Hydrochloric Acid. J. Air Waste Manag. Assoc. 2016, 66, 1191–1201. [Google Scholar] [CrossRef]
- Kim, J.G.; Bai, B.C. A Chemical Safety Assessment of Lyocell-Based Activated Carbon Fiber with a High Surface Area through the Evaluation of HCl Gas Adsorption and Electrochemical Properties. Separations 2024, 11, 79. [Google Scholar] [CrossRef]
- Bandgar, S.S.; Hingangavkar, G.M.; Kore, E.K.; Selvaraj, M.; Mulik, R.N.; Patil, V.B. Excellence Evaluation of Samarium Doped Mn-Zn Ferrite Nanocrystals for Room Temperature HCl Detection. Mater. Chem. Phys. 2024, 314, 128861. [Google Scholar] [CrossRef]
- Oh, H.J.; Park, Y.K.; Bae, J.H.; Lim, J.; Lee, S.J.; Kim, D.-k.; Jang, S.J.; Yeang, B.J.; Lee, H.D.; Lim, S.J.; et al. Durable and Washable Sheath/Core-Type Polyamide 6-RhYK/PP Colorimetric Bicomponent Fiber as Reversible Hydrogen Chloride (HCl) Gas Sensor. Sens. Actuators B Chem. 2024, 407, 135455. [Google Scholar] [CrossRef]
- Shokr, E.K.; Elkot, S.A.; Kamel, M.S.; Ali, H.M. Sol-Gel Spin-Coated V2O3 Thin Films for Optical HCl and Iodine Gas Sensing. Opt. Mater. 2024, 151, 115263. [Google Scholar] [CrossRef]
- Markina, D.I.; Anoshkin, S.S.; Masharin, M.A.; Khubezhov, S.A.; Tzibizov, I.; Dolgintsev, D.; Terterov, I.N.; Makarov, S.V.; Pushkarev, A.P. Perovskite Nanowire Laser for Hydrogen Chloride Gas Sensing. ACS Nano 2023, 17, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, C.; Mayer, P.; Bechtold, T.; Pham, T. Multifunctional Polypyrrole-Based Textile Sensors for Integration into Personal Protection Equipment. Sensors 2024, 24, 1387. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiang, Y.; Zhan, H.; Zhou, Y.; Kang, J. DFT Investigation of Transition Metal-Doped Graphene for the Adsorption of HCl Gas. Diam. Relat. Mater. 2023, 136, 109995. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, G.; Wang, C.; Wang, Y.F.; Xing, Y.H.; Bai, F.Y. Smart Stimulation Response of a Pyrene-Based Lanthanide(III) MOF: Fluorescence Enhancement to HX (F and Cl) or R-COOH and Artificial Applicable Film on HCl Vapor Sensing. ACS Appl. Mater. Interfaces 2023, 15, 50275–50289. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Wang, Z.; Yan, J.; Chen, Y. Ultra-Trace Chlorinated Gases Optical Sensor with Moisture-Resistant Based on Structural-Customizable UiO-66 3D Photonic Crystals. Sens. Actuators B Chem. 2023, 393, 134219. [Google Scholar] [CrossRef]
- Qiao, S.; Sampaolo, A.; Patimisco, P.; Spagnolo, V.; Ma, Y. Ultra-Highly Sensitive HCl-LITES Sensor Based on a Low-Frequency Quartz Tuning Fork and a Fiber-Coupled Multi-Pass Cell. Photoacoustics 2022, 27, 100381. [Google Scholar] [CrossRef]
- Li, M.; Tang, J.; Luo, Y.; Yang, J.; Liu, J.; Peng, J.; Fang, Y. Imine Bond-Based Fluorescent Nanofilms toward High-Performance Detection and Efficient Removal of HCl and NH3. Anal. Chem. 2023, 95, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent Advances in Biosensors for Diagnosis and Detection of Sepsis: A Comprehensive Review. Biosens. Bioelectron. 2019, 124–125, 205–215. [Google Scholar] [CrossRef]
- Owojaiye, G.; Sun, Y. Focal Design Issues Affecting the Deployment of Wireless Sensor Networks for Pipeline Monitoring. Ad Hoc Netw. 2013, 11, 1237–1253. [Google Scholar] [CrossRef]
- Goodfellow, H.D.; Wang, Y. Industrial Ventilation Design Guidebook; Academic Press: Cambridge, MA, USA, 2021; ISBN 978-0-12-816673-4. [Google Scholar]
- Li, K.; Wang, B.; Yuan, M.; Yang, Z.; Yu, C.; Zheng, W. CO Detection System Based on TDLAS Using a 4.625 Μm Interband Cascaded Laser. Int. J. Environ. Res. Public Health 2022, 19, 12828. [Google Scholar] [CrossRef]
- Weng, W.; Larsson, J.; Bood, J.; Aldén, M.; Li, Z. Quantitative Hydrogen Chloride Detection in Combustion Environments Using Tunable Diode Laser Absorption Spectroscopy with Comprehensive Investigation of Hot Water Interference. Appl. Spectrosc. 2022, 76, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yao, S.; Ren, W.; Lu, Z.; Li, Z. TDLAS Monitoring of Carbon Dioxide with Temperature Compensation in Power Plant Exhausts. Appl. Sci. 2019, 9, 442. [Google Scholar] [CrossRef]
- Dello Russo, S.; Zifarelli, A.; Sampaolo, A.; Giglio, M.; Wei, T.; Wu, H.; Dong, L.; Patimisco, P.; Tittel, F.K.; Spagnolo, V. Quartz Tuning Forks Employed as Photodetectors in TDLAS Sensors. In Proceedings of the Photonic Instrumentation Engineering VIII, Online, 6–11 March 2021; Soskind, Y., Busse, L.E., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 17. [Google Scholar]
- Dinh, T.V.; Lee, J.Y.; Ahn, J.W.; Kim, J.C. Development of a Wide-Range Non-Dispersive Infrared Analyzer for the Continuous Measurement of CO2 in Indoor Environments. Atmosphere 2020, 11, 1024. [Google Scholar] [CrossRef]
- Institute Of Clean Air Companies. White Paper: Monitoring of Hcl; Institute of Clean Air Companies, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Singh, M.K.; Singh, A. Characterization of Polymers and Fibres; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128239865. [Google Scholar]
- Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A. Fourier Transform Infrared (FTIR) Spectroscopy. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar]
- Bacsik, Z.; Mink, J.; Keresztury, G. FTIR Spectroscopy of the Atmosphere. I. Principles and Methods. Appl. Spectrosc. Rev. 2004, 39, 295–363. [Google Scholar] [CrossRef]
- Griffiths, P.R.; de Haseth, J.A. Fourier Transform Infrared Spectrometry; Wiley: Hoboken, NJ, USA, 2007; ISBN 9780471194040. [Google Scholar]
- Nguyen, T. Applications of Fourier Transform Infrared Spectroscopy in Surface and Interface Studies. Prog. Org. Coat. 1985, 13, 1–34. [Google Scholar] [CrossRef]
- El Fels, L.; Zamama, M.; Hafidi, M. Advantages and Limitations of Using FTIR Spectroscopy for Assessing the Maturity of Sewage Sludge and Olive Oil Waste Co-Composts. In Biodegradation and Bioremediation of Polluted Systems—New Advances and Technologies; InTech: London, UK, 2015. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Liang, J.G.; Jiang, Y.; Wu, J.K.; Wang, C.; von Gratowski, S.; Gu, X.; Pan, L. Multiplex-Gas Detection Based on Non-Dispersive Infrared Technique: A Review. Sens. Actuators A Phys. 2023, 356, 114318. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Ci, G.; Zhao, X.; Lv, J.; Liang, J.; Ming, A.; Wei, F.; Mao, C. Development of High-Precision NO2 Gas Sensor Based on Non-Dispersive Infrared Technology. Sensors 2024, 24, 4146. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K. Non-Dispersive Infrared Gas Sensing Technology: A Review. IEEE Sens. J. 2022, 22, 6–15. [Google Scholar] [CrossRef]
- Xu, M.; Peng, B.; Zhu, X.; Guo, Y. Multi-Gas Detection System Based on Non-Dispersive Infrared (NDIR) Spectral Technology. Sensors 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, W.; Lin, Y.; Xu, Y.; Tao, J. Development of a Compact NDIR CO2 Gas Sensor for a Portable Gas Analyzer. Micromachines 2024, 15, 1203. [Google Scholar] [CrossRef]
- Yu, K.; Yang, X.; Wang, Y.; Zhang, P.; Zhang, L.; Tan, Q. A Temperature-Compensated CO2 Detection System Based on Non-Dispersive Infrared Spectral Technology. Rev. Sci. Instrum. 2024, 95, 085002. [Google Scholar] [CrossRef]
- Su, M.; Chen, Y.; Li, Q.; Wei, Y.; Liu, J.; Chang, Z.; Liu, X.; Zhang, A. Temperature Compensation Model for Non-Dispersive Infrared CO2 Gas Sensor Based on WOA-BP Algorithm. Front. Energy Res. 2024, 12, 1407630. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, S.; Wu, D.; Han, Z. Highly-Efficient Gas Sensing Using Dielectric-Based Narrow-Band Thermal Emitters Operating in the Mid-Infrared. Infrared Phys. Technol. 2024, 137, 105132. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Duan, Z.; Li, J.; Yuan, Z.; Tai, H. Designing an Optical Gas Chamber with Stepped Structure for Non-Dispersive Infrared Methane Gas Sensor. Sens. Actuators A Phys. 2024, 367, 115052. [Google Scholar] [CrossRef]
- Liang, J.; Wang, L.; Wu, L.; Zhang, J.; Wang, Z.; Zhang, T.; Zhang, Y.; Li, G. Non-Dispersive Infrared SF6 Sensor with Temperature Compensation Using ISSA_BP Neural Network. Sens. Actuators A Phys. 2024, 373, 115434. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Ming, A.; Fang, Y.; Fang, S.; Bi, S.; Chen, J.; Xu, R.; Wei, F.; Mao, C. Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries. Energies 2023, 16, 3620. [Google Scholar] [CrossRef]
- ES 01906.1; HCl-Automated Measuring Method in Stack. National Institute of Environmental Research. Available online: https://www.law.go.kr/LSW//flDownload.do?flSeq=141390927 (accessed on 4 December 2024).
- Korea Ministry of Environment. Enforcement Decree of the Special Act on the Improvement of Air Environment in Air Management Areas. Available online: https://www.law.go.kr/LSW//lsInfoP.do?lsId=013722&ancYnChk=0#0000 (accessed on 4 December 2024).
- United States Environmental Protection Agency. Performance Specification 18-Performance Specifications and Test Procedures For Gaseous Hydrogen Chloride (Hci) Continuous Emission Monitoring Systems at Stationary Sources. Available online: https://www.epa.gov/emc/performance-specification-18-gaseous-hydrogen-chloride (accessed on 4 December 2024).
- ISO 10849:2022; International Organization for Standardization. Available online: https://www.iso.org/standard/79892.html?browse=ics (accessed on 4 December 2024).
- ISO 7935:2024; International Organization for Standardization. Available online: https://www.iso.org/standard/83052.html?browse=ics (accessed on 4 December 2024).
- Dinh, T.V.; Choi, I.Y.; Park, B.G.; Lee, J.H.; Kim, I.Y.; Gil, H.N.; Lee, S.W.; Kim, J.C. Development of a Negligible Zero-Drift Ndir Analyzer for Measuring Nh3 Emitted from an Urban Household Solid Waste Incinerator. Atmosphere 2021, 12, 858. [Google Scholar] [CrossRef]
- Martinez-Anton, J.C.; Silva-Lopez, M. Improvements in NDIR Gas Detection within the Same Optical Chamber. In Proceedings of the Optical Design and Engineering IV, Marseille, France, 6–8 September 2011; SPIE: Bellingham, WA, USA, 2011; Volume 8167, p. 816723. [Google Scholar]
- Dinh, T.V.; Ahn, J.W.; Choi, I.Y.; Song, K.Y.; Chung, C.H.; Kim, J.C. Limitations of Gas Filter Correlation: A Case Study on Carbon Monoxide Non-Dispersive Infrared Analyzer. Sens. Actuators B Chem. 2017, 243, 684–689. [Google Scholar] [CrossRef]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A Review on Non-Dispersive Infrared Gas Sensors: Improvement of Sensor Detection Limit and Interference Correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Besson, J.P.; Schilt, S.; Thévenaz, L. Sub-Ppm Multi-Gas Photoacoustic Sensor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 899–904. [Google Scholar] [CrossRef]
- Bednyakova, A.; Erushin, E.; Miroshnichenko, I.; Kostyukova, N.; Boyko, A.; Redyuk, A. Enhancing Long-Term Stability of Photoacoustic Gas Sensor Using an Extremum-Seeking Control Algorithm. Infrared Phys. Technol. 2023, 133, 104821. [Google Scholar] [CrossRef]
- Halfacre, J.W.; Stewart, J.; Herndon, S.C.; Roscioli, J.R.; Dyroff, C.; Yacovitch, T.I.; Flynn, M.; Andrews, S.J.; Brown, S.S.; Veres, P.R.; et al. Using Tunable Infrared Laser Direct Absorption Spectroscopy for Ambient Hydrogen Chloride Detection: HCl-TILDAS. Atmos. Meas. Tech. 2023, 16, 1407–1429. [Google Scholar] [CrossRef]
- Furlani, T.C.; Ye, R.X.; Stewart, J.; Crilley, L.R.; Edwards, P.M.; Kahan, T.F.; Young, C.J. Development and Validation of a New in Situ Technique to Measure Total Gaseous Chlorine in Air. Atmos. Meas. Tech. 2023, 16, 181–193. [Google Scholar] [CrossRef]
- Harvard-Smithsonian Center for Astrophysics. The HITRAN Database. Available online: https://hitran.org/ (accessed on 30 April 2024).
- Deckwer, W.-D. On The Mechanism Of Heat Transfer In Bubble Column Reactors. Chem. Eng. Sci. 1980, 35, 1341–1346. [Google Scholar] [CrossRef]
- Dinh, T.V.; Ahn, J.W.; Choi, I.Y.; Kim, J.C. A Novel Bandpass Filter for the Analysis of Carbon Monoxide Using a Non-Dispersive Infrared Technique. Atmosphere 2018, 9, 495. [Google Scholar] [CrossRef]
- Massart, D.L.; Dijkstra, A. Leonard Kaufman Evaluation and Optimization of Laboratory Methods and Analytical Procedures, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1978; Volume 1, ISBN 978-0-444-41743-5. [Google Scholar]
- Wong, J.Y.; Schell, M. Zero Drift NDIR Gas Sensors. Sens. Rev. 2011, 31, 70–77. [Google Scholar] [CrossRef]
- Dinh, T.V.; Kim, D.J.; Ahn, J.W.; Choi, I.Y.; Lee, J.Y.; Son, Y.S.; Kim, J.C. A Potential Approach to Compensate the Gas Interference for the Analysis of NO by a Non-Dispersive Infrared Technique. Anal. Chem. 2020, 92, 12152–12159. [Google Scholar] [CrossRef] [PubMed]
- Korea Environment Corporation. CleanSYS. Available online: https://cleansys.or.kr/index.do (accessed on 21 October 2024).
- James, A. Jahnke Continuous Emission Monitoring, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2022; ISBN 978-1-119-43402-3. [Google Scholar]
- Dinh, T.V.; Kim, J.C. Moisture Removal Techniques for a Continuous Emission Monitoring System: A Review. Atmosphere 2021, 12, 61. [Google Scholar] [CrossRef]
- ES 01305.1e; Hydrogen Chloride in Flue Gas—Ion Chromatography. National Institute of Environmental Research. Available online: https://www.law.go.kr/LSW//flDownload.do?flSeq=141390927 (accessed on 21 October 2024).
- ES 01902.1a; Installation Method of Automated Measuring Instrument. National Institute of Environmental Research. Available online: https://www.law.go.kr/LSW//flDownload.do?flSeq=141390927 (accessed on 21 October 2024).
- ES 01114.c; Methods for Sampling of Flue Gas. National Institute of Environmental Research. Available online: https://www.law.go.kr/LSW//flDownload.do?flSeq=141390927 (accessed on 21 October 2024).
- Kang, Y.; Aye, L.; Ngo, T.D.; Zhou, J. Performance Evaluation of Low-Cost Air Quality Sensors: A Review. Sci. Total Environ. 2022, 818, 151769. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.R.; Malings, C.; Pandis, S.N.; Presto, A.A.; McNeill, V.F.; Westervelt, D.M.; Beekmann, M.; Subramanian, R. From Low-Cost Sensors to High-Quality Data: A Summary of Challenges and Best Practices for Effectively Calibrating Low-Cost Particulate Matter Mass Sensors. J. Aerosol. Sci. 2021, 158, 105833. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. Public Notice on Type Approval, Quality Inspection, Etc. of Environmental Measuring Devices. Available online: https://www.law.go.kr/LSW//flDownload.do?flSeq=135645335 (accessed on 7 November 2024).
- Ning, H.; Tang, R.; Li, C.; Gu, X.; Gong, Z.; Zhu, C.; Li, J.; Wang, K.; Yu, J. Recent advances in process and materials for dry desulfurization of industrial flue gas: An overview. Sep. Purif. Technol. 2025, 353, 128425. [Google Scholar] [CrossRef]
- Sun, Y.W.; Liu, C.; Chan, K.L.; Xie, P.H.; Liu, W.Q.; Zeng, Y.; Wang, S.M.; Huang, S.H.; Chen, J.; Wang, Y.P.; et al. Stack Emission Monitoring Using Non-Dispersive Infrared Spectroscopy with an Optimized Nonlinear Absorption Cross Interference Correction Algorithm. Atmos. Meas. Tech. 2013, 6, 1993–2005. [Google Scholar] [CrossRef]
- S.FAC, Inc. TMS300-FTIR. Available online: https://www.s-fac.co.kr/kr/product/product_view.php?idx=40 (accessed on 9 November 2024).
- ENVEA. MIR-9000. Available online: https://www.envea.global/s/process/gas-monitors-p/ch4/mir-9000/ (accessed on 9 November 2024).
- Gasmet. CX4000. Available online: https://www.gasmet.com/products/category/emission-monitoring-systems/cx4000/ (accessed on 9 November 2024).
- Thermo. Fisher Scientific 15i. Available online: https://www.thermofisher.com/order/catalog/product/15I (accessed on 9 November 2024).
- Protea Ltd. AtmofFIR CEM. Available online: https://www.protea.ltd.uk/atmosfir (accessed on 9 November 2024).
- EcoChem. MCS. Available online: https://www.ecochem.biz/products/stack-monitoring-systems/mc3-based-cems/ (accessed on 9 November 2024).
- Sick. MCS200HW. Available online: https://www.sick.com/kr/ko/catalog/products/analyzers/gas-analyzers/mcs200hw/system-mcs200hw/p/p679083 (accessed on 9 November 2024).
- HORIBA. TX-100. Available online: https://www.horiba.com/kor/process-and-environmental/products/detail/action/show/Product/%EA%B0%80%EB%B3%80-%EB%A0%88%EC%9D%B4%EC%A0%80-%EA%B0%80%EC%8A%A4-%EB%B6%84%EC%84%9D%EA%B8%B0-tx-100-142/ (accessed on 9 November 2024).
- Neo Monitors. LaserGasTM II SP. Available online: https://neomonitors.com/product/lasergas-sp/ (accessed on 9 November 2024).
- ENVEA. LAS 5000XD. Available online: https://www.envea.global/s/process/gas-monitors-p/co/las-5000xd/ (accessed on 9 November 2024).
- METTLER TOLEDO. GPro 500. Available online: https://www.mt.com/in/en/home/products/Process-Analytics/gas-analyzer/Tunable-Diode-Laser-TDL/hydrogen-chloride-GPro-500.html (accessed on 9 November 2024).
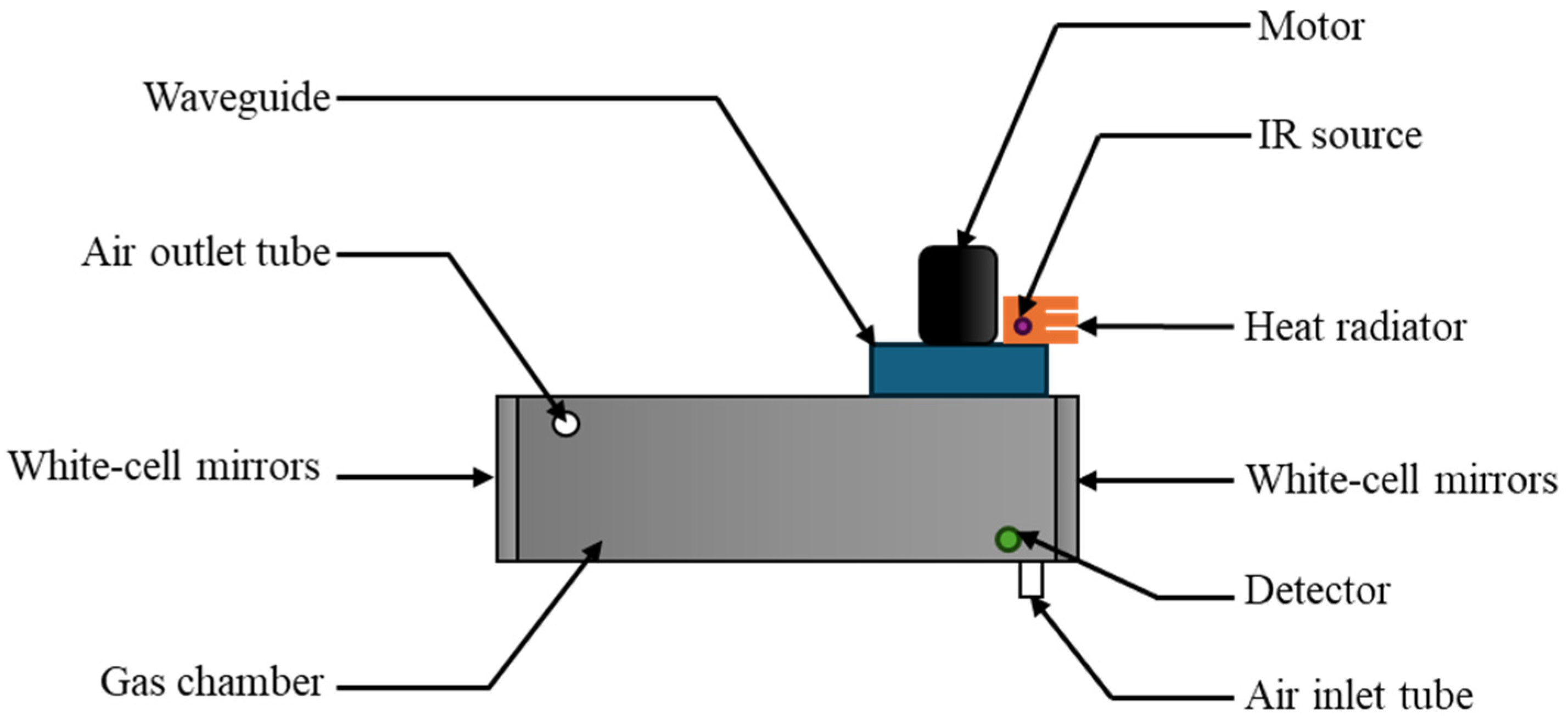
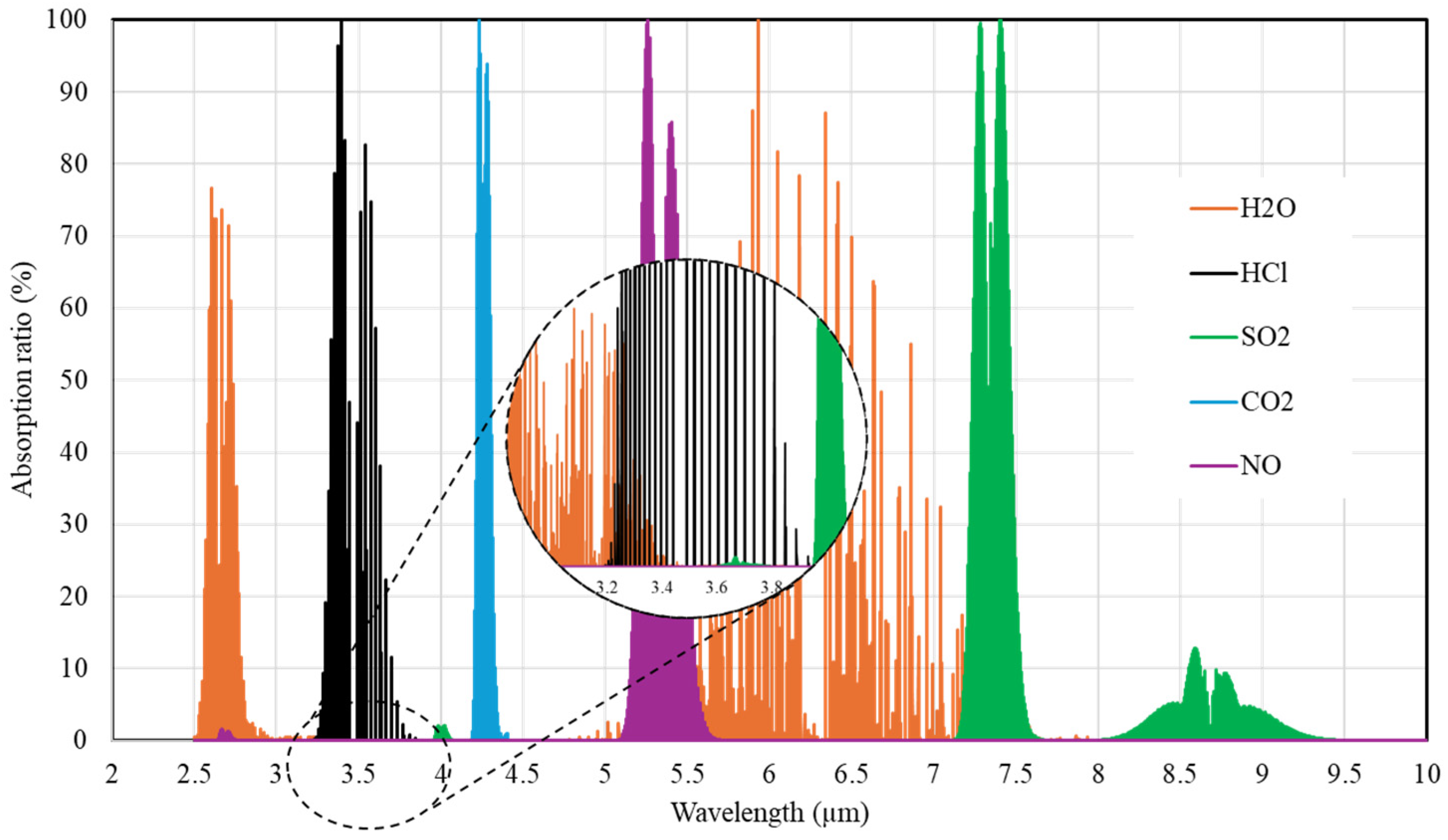


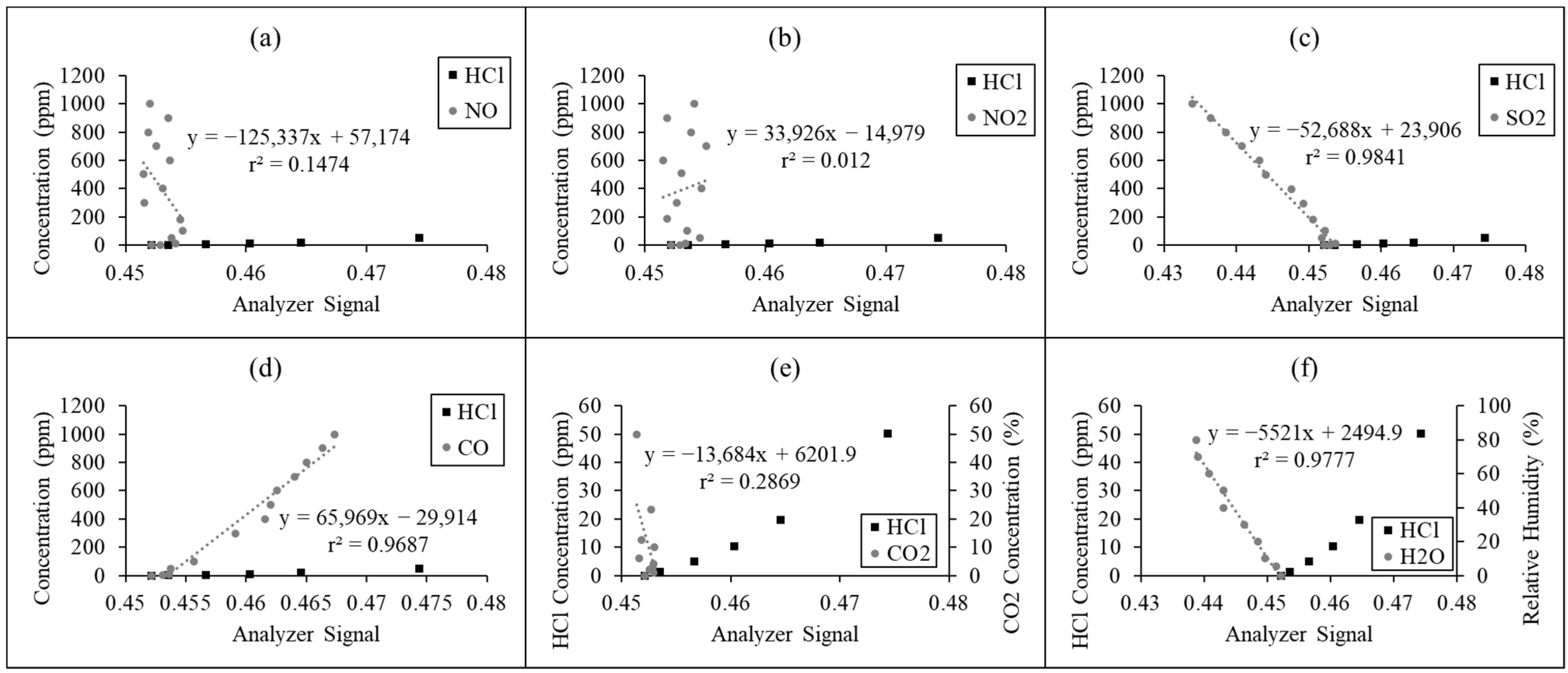
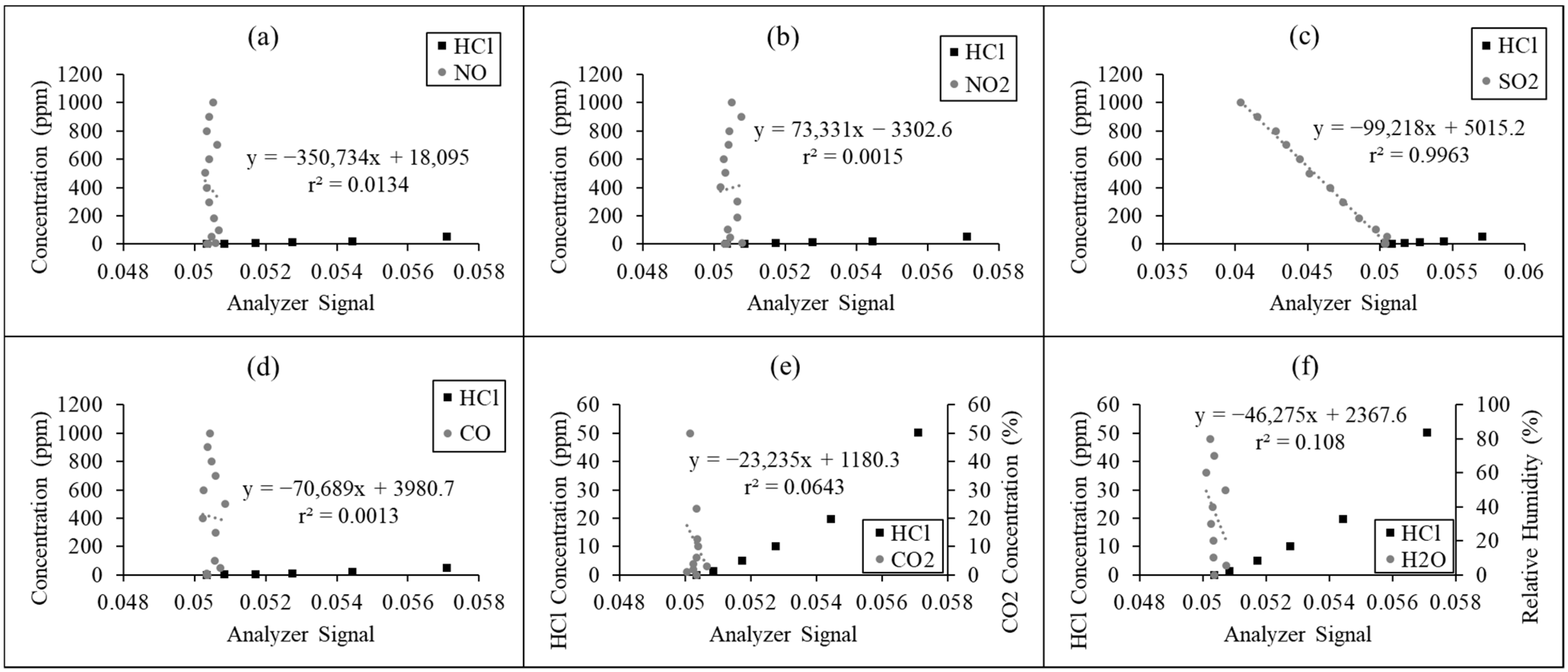



| No. | Technique | Operating Range | Limit of Detection | Application | Ref. |
|---|---|---|---|---|---|
| 1 | Electrochemical sensor: lyocell-based activated carbon fibers with a high surface area for electrochemical detector | 0~20 ppm | - | Environmental and industrial gas management | [14] |
| 2 | Optical sensor: colorimetric sheath/core-type polyamide 6 (PA6)-RhYK/polypropylene bicomponent fiber | 1~100 ppm | 1 ppm | Industrial sites and daily life | [16] |
| 3 | Optical sensor: samarium-doped Smx: Mn0.8Zn0.2Fe2-xO4 (SMZFO) (x = 0.0–0.1) nanocrystals | 10~100 ppm | 10 ppm | Workplace safety | [15] |
| 4 | Optical sensor: sol–gel spin-coated V2O3 thin films | 0~32 ppm | - | Workplace safety | [17] |
| 5 | Optical sensor: thin, homogeneous polypyrrole layers on flexible textile polyamide fabrics | 20~100 ppm | 20 ppm | Personal protection | [19] |
| 6 | Optical sensor: Mn-doped graphene | - | - | Industry and safety | [20] |
| 7 | Optical sensor: tunable perovskite nanowire laser based on a CsPbBr3 nanowire integrated with a nanostructured Al2O3 substrate | 5~500 ppm | 1.12 ppm | Workplace in chemical industry | [18] |
| 8 | Optical sensor: UiO-66 three-dimensional photonic crystals | 0~2.5 ppm | 10.9 ppb | Atmosphere | [22] |
| 9 | Optical sensor: fluorescent nanofilms based on imine linkage using 4,4′-[4,4′-Biphenyldiylbis (oxy)] dianiline (DAPODP) and 4,4′,4″,4‴-(ethene-1,1,2,2-tetrayl) tetrabenzaldehyde (TPE-CHO) | 0~96 ppm | 150 ppb | Industrial workplace | [24] |
| 10 | Optical sensor: 3D Ln-Metal–organic frameworks based on the H4TBAPy ligand [H4TBAPy = (1,3,6,8-tetrakis (p-benzoic acid) pyrene)] | 0~80 ppm | 0.18 ppm | Workplace in chemical industry | [21] |
| 11 | Optical sensor: thermoelastic spectroscopy based on a custom low-frequency quartz tuning fork | 0~500 ppm | 0.148 ppm | Chemical processing | [23] |
| 12 | Optical sensor: multi-gas photoacoustic sensor | 0~25 ppm | 24 ppb | - | [61] |
| Compound | Concentration (ppm, %-CO2, %RH–H2O) |
|---|---|
| HCl | 0, 5, 15, 30, 40, 50 |
| NO | 0, 1, 10, 50, 100, 180, 300, 400, 500, 600, 700, 800, 900, 1000 |
| NO2 | 0, 1, 10, 50, 100, 180, 300, 400, 500, 600, 700, 800, 900, 1000 |
| SO2 | 0, 1, 10, 50, 100, 180, 300, 400, 500, 600, 700, 800, 900, 1000 |
| CO | 0, 1, 10, 50, 100, 180, 300, 400, 500, 600, 700, 800, 900, 1000 |
| CO2 | 0, 1, 2, 3, 4, 6, 10, 12, 23, 50 |
| H2O | 0, 5, 10, 20, 30, 40, 50, 60, 70, 80 |
| Mixture | HCl (ppm) | NH3 (ppm) | CO (ppm) | NO (ppm) | NO2 (ppm) | CO2 (%) | SO2 (ppm) | H2O (%RH) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 10 | 1 | 10 |
| 2 | 1 | 5 | 25 | 20 | 2.5 | 15 | 25 | 20 |
| 3 | 1 | 10 | 50 | 45 | 5 | 20 | 50 | 40 |
| 4 | 5 | 1 | 1 | 1 | 1 | 10 | 1 | 10 |
| 5 | 5 | 5 | 25 | 20 | 2.5 | 15 | 25 | 20 |
| 6 | 5 | 10 | 50 | 45 | 5 | 20 | 50 | 40 |
| 7 | 10 | 1 | 1 | 1 | 1 | 10 | 1 | 10 |
| 8 | 10 | 5 | 25 | 20 | 2.5 | 15 | 25 | 20 |
| 9 | 10 | 10 | 50 | 45 | 5 | 20 | 50 | 40 |
| No. | City | Min (ppm) | Max (ppm) | Emission Standard |
|---|---|---|---|---|
| 1 | Sangju | 0.0 | 0.2 | 16 |
| 2 | Seoul | 0.3 | 4.2 | 16 |
| 3 | Jangheung | 2.1 | 5.6 | 16 |
| 4 | Muan | 0.1 | 4.1 | 24 |
| 5 | Yangsan | 0.06 | 0.058 | 20 |
| 6 | Gyeongju | 0.0 | 0 | 16 |
| 7 | Pocheon | 0.0 | 11 | 24 |
| 8 | Seosan | 0.0 | 1.6 | 30 |
| 9 | Bucheon | 0.0 | 2.3 | 16 |
| 10 | Gochang | 0.0 | 1.1 | 24 |
| No. | Type | Manufacturer | Model | Price (USD) | Limit of Detection | 24 h Zero Drift | 24 h Span Drift | Reproductivity | Linearity | Response Time | Range | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | (%) | (%) | (%) | (%) | (s) | (ppm) | ||||||
| 1 | FTIR | S.Fac Inc. (Daejeon, Republic of Korea) | TMS300-FTIR | >56,000 | 0.31 | 2 | - | 2 | - | ~100 | [82] | |
| 2 | NDIR-GFC | Envea (Poissy, France) | MIR-9000 | >40,000 | - | 2 | 1 | 2 | 1 | - | ~15/5000 | [83] |
| 3 | FTIR | Gasmet (Vantaa, Finland) | CX4000 | >70,000 | - | 2 | - | - | 2 | 120 | - | [84] |
| 4 | Thermo Scientific (Waltham, MA, USA) | 15i (HCP-PKI) | >30,000 | 0.2 | 0.2 ppm | 2 | - | 2 | 120 | ~5000 | [85] | |
| 5 | FTIR | Protea Ltd. (Middlewich, Cheshire, UK) | AtmosFIR CEM | - | 0.2 | - | - | 1 | 2 | 120 | ~100 | [86] |
| 6 | EcoChem (League, TX, USA) | MC3 | >47,000 | 1% of scale | - | - | - | - | 10 | ~100 | [87] | |
| 7 | SICK (Waldkirch, Breisgau, Germany) | MCS200HW | >100,000 | 2% of scale | 3 | 3 | - | - | 200 | ~1840 | [88] | |
| 8 | TDLAS | HORIBA (Kyoto, Japan) | TX-100 | >59,000 | - | 2 | 2 | 1 | 1 | 2 | ~500 | [89] |
| 9 | TDLAS | Neo Monitors AS (Skedsmokorset, Norway) | LaserGas-II SP | - | 0.05 | - | - | 1 | - | 2 | - | [90] |
| 10 | TDLAS | Envea (Poissy, France) | LAS 5000XD | - | 1% of scale | - | - | - | 1 | 1 | ~10, 5000 | [91] |
| 11 | TDLAS | METTLER TOLEDO (Columbus, OH, USA) | Gpro 500 | - | 0.6 | 2 | 2 | 0.25 | 1 | 4 | ~1% | [92] |
| 12 | NDIR | This study | - | 10,000 | 0.07 | 0.02 | 0.6 | 0.26 | 0.96 | 75 | 0~50 | - |
| Mix | STD | NDIR Analyzer | Ion Chromatography | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | RSD | RPD | Min | Max | Mean | RSD | RPD | ||
| 1 | 1.00 | 0.98 | 1.02 | 1.01 | 1.50 | 0.57 | 0.98 | 1.01 | 0.99 | 1.54 | 1.00 |
| 2 | 1.00 | 0.98 | 1.01 | 1.00 | 1.22 | 0.14 | 1.00 | 1.01 | 1.00 | 0.58 | 0.00 |
| 3 | 1.00 | 0.98 | 1.03 | 1.01 | 1.89 | 0.57 | 0.99 | 1.02 | 1.00 | 1.73 | 0.00 |
| 4 | 5.02 | 4.98 | 5.10 | 5.02 | 0.82 | 0.00 | 5.00 | 5.05 | 5.02 | 0.50 | 0.00 |
| 5 | 5.02 | 4.98 | 5.10 | 5.04 | 0.89 | 0.40 | 4.98 | 5.07 | 5.03 | 0.90 | 0.20 |
| 6 | 5.02 | 4.96 | 5.04 | 5.01 | 0.63 | 0.26 | 5.00 | 5.04 | 5.02 | 0.41 | 0.00 |
| 7 | 10.0 | 9.97 | 10.1 | 10.0 | 0.56 | 0.04 | 10.0 | 10.13 | 10.1 | 0.55 | 0.50 |
| 8 | 10.0 | 9.99 | 10.1 | 10.0 | 0.43 | 0.21 | 10.0 | 10.12 | 10.1 | 0.40 | 0.60 |
| 9 | 10.0 | 9.95 | 10.0 | 9.99 | 0.37 | 0.34 | 10.0 | 10.1 | 10.0 | 0.20 | 0.20 |
| No. | NDIR | Standard Method | RMSE | MNE (%) | MNB (%) | RPD (%) |
|---|---|---|---|---|---|---|
| 1 | 0.21 ± 0.16 | 0.24 ± 0.03 | 0.15 | 51.0 | −5.75 | 0.66 |
| 2 | 0.21 ± 0.16 | 0.22 ± 0.03 | 0.14 | 58.2 | −5.56 | 0.30 |
| 3 | 0.46 ± 0.08 | 0.39 ± 0.06 | 0.14 | 31.9 | 31.9 | 1.64 |
| 4 | 0.33 ± 0.01 | 0.49 ± 0.16 | 0.12 | 19.8 | −13.5 | 3.92 |
| 5 | 0.11 ± 0.14 | 0.24 ± 0.01 | 0.14 | 45.3 | −31.2 | 3.32 |
| 6 | 0.17 ± 0.15 | 0.22 ± 0.05 | 0.09 | 36.3 | 17.6 | 1.19 |
| 7 | 0.06 ± 0.02 | 0.29 ± 0.14 | 0.29 | 82.2 | −82.2 | 5.91 |
| 8 | 0.17 ± 0.10 | 0.23 ± 0.05 | 0.10 | 38.9 | −38.9 | 1.51 |
| 9 | 0.30 ± 0.09 | 0.28 ± 0.05 | 0.04 | 13.0 | −13.0 | 0.50 |
| 10 | 0.59 ± 0.20 | 0.57 ± 0.26 | 0.26 | 47.2 | 39.3 | 0.52 |
| 11 | 0.53 ± 0.20 | 0.40 ± 0.06 | 0.03 | 6.80 | 1.33 | 3.16 |
| 12 | 0.27 ± 0.26 | 0.35 ± 0.01 | 0.24 | 62.9 | −62.9 | 2.03 |
| No. | NDIR | Standard Method | RMSE | MNE (%) | MNB (%) | RPD (%) |
|---|---|---|---|---|---|---|
| 1 | 0.41 ± 0.19 | 0.39 ± 0.07 | 0.12 | 23.9 | 3.68 | 0.45 |
| 2 | 0.84 ± 0.25 | 0.36 ± 0.02 | 0.59 | 156 | 156 | 9.55 |
| 3 | 0.73 ± 0.18 | 0.34 ± 0.04 | 0.31 | 97.8 | 97.8 | 7.84 |
| 4 | 0.47 ± 0.15 | 0.32 ± 0.01 | 0.24 | 69.2 | 69.2 | 3.06 |
| 5 | 0.59 ± 0.65 | 0.51 ± 0.37 | 0.28 | 28.7 | 13.8 | 1.65 |
| 6 | 0.54 ± 0.66 | 0.89 ± 0.27 | 0.52 | 49.2 | −41.9 | 7.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.-G.; Dinh, T.-V.; Lee, S.-W.; Choi, I.-Y.; Cho, B.-C.; Baek, D.-H.; Kim, J.-C.; Kim, J.-C. Investigation of Long-Term Performance of a Proposed Cost-Effective HCl Non-Dispersive Infrared Analyzer at Real Stationary Sources. Chemosensors 2024, 12, 262. https://doi.org/10.3390/chemosensors12120262
Park B-G, Dinh T-V, Lee S-W, Choi I-Y, Cho B-C, Baek D-H, Kim J-C, Kim J-C. Investigation of Long-Term Performance of a Proposed Cost-Effective HCl Non-Dispersive Infrared Analyzer at Real Stationary Sources. Chemosensors. 2024; 12(12):262. https://doi.org/10.3390/chemosensors12120262
Chicago/Turabian StylePark, Byeong-Gyu, Trieu-Vuong Dinh, Sang-Woo Lee, In-Young Choi, Byung-Chan Cho, Da-Hyun Baek, Jong-Choon Kim, and Jo-Chun Kim. 2024. "Investigation of Long-Term Performance of a Proposed Cost-Effective HCl Non-Dispersive Infrared Analyzer at Real Stationary Sources" Chemosensors 12, no. 12: 262. https://doi.org/10.3390/chemosensors12120262
APA StylePark, B.-G., Dinh, T.-V., Lee, S.-W., Choi, I.-Y., Cho, B.-C., Baek, D.-H., Kim, J.-C., & Kim, J.-C. (2024). Investigation of Long-Term Performance of a Proposed Cost-Effective HCl Non-Dispersive Infrared Analyzer at Real Stationary Sources. Chemosensors, 12(12), 262. https://doi.org/10.3390/chemosensors12120262








