Photoacoustic Imaging of pH-Sensitive Optical Sensors in Biological Tissues
Abstract
1. Introduction
2. Principles of Photoacoustic Imaging

3. Mechanisms of pH-Sensitive Sensors for Photoacoustic Imaging
4. Contrast-Enhanced Photoacoustic Imaging of pH-Sensitive Sensors
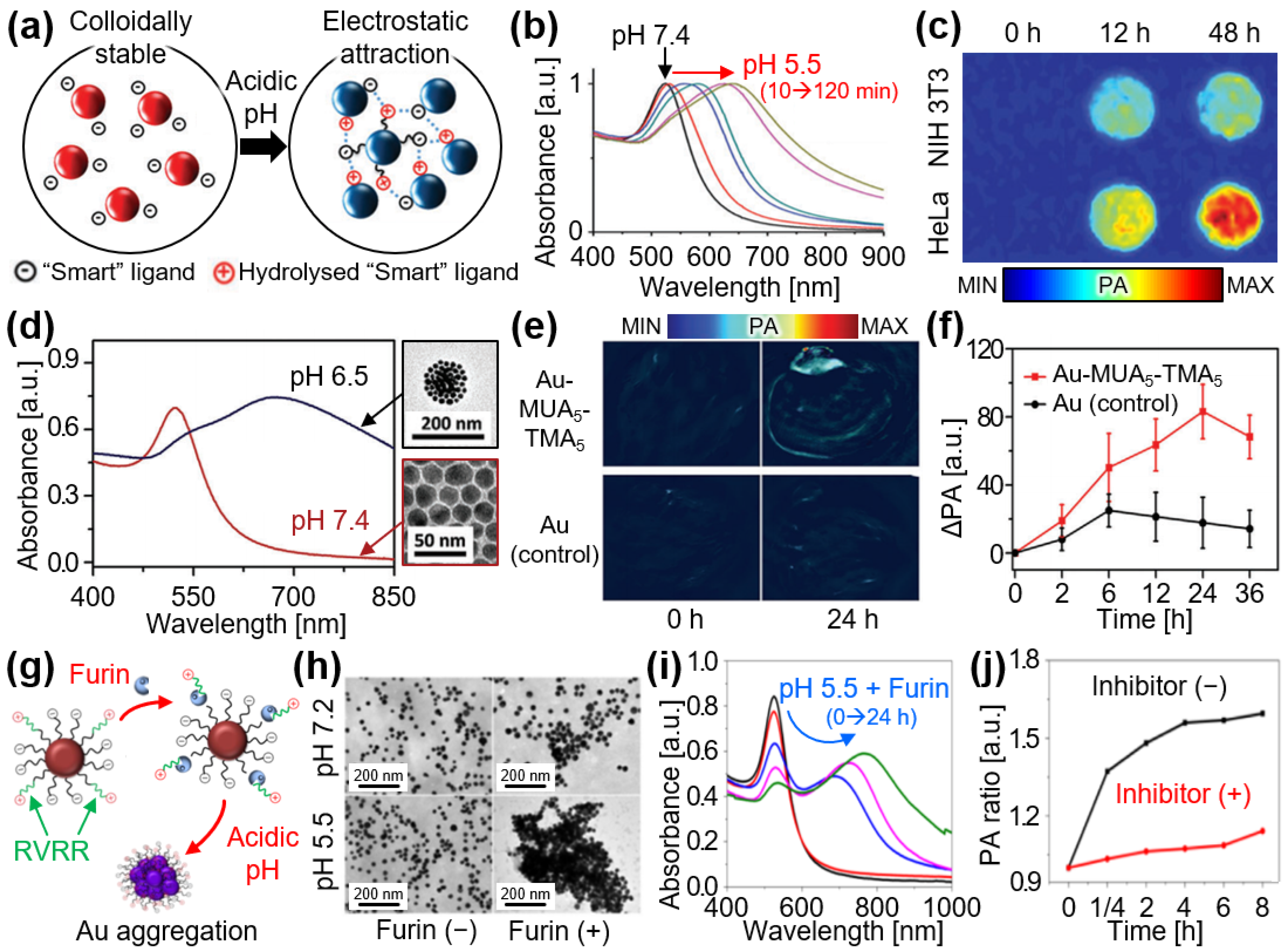
4.1. Aggregation
4.2. Separation
4.3. Protonation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L. Functional Photoacoustic Microscopy of Hemodynamics: A Review. Biomed. Eng. Lett. 2022, 12, 97–124. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic Imaging in Cancer Detection, Diagnosis, and Treatment Guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef]
- Zhao, Z.; Swartchick, C.B.; Chan, J. Targeted Contrast Agents and Activatable Probes for Photoacoustic Imaging of Cancer. Chem. Soc. Rev. 2022, 51, 829–868. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Cole, A.J.; Van de Sompel, D.; Gambhir, S.S. Gold Nanorods for Ovarian Cancer Detection with Photoacoustic Imaging and Resection Guidance via Raman Imaging in Living Mice. ACS Nano 2012, 6, 10366–10377. [Google Scholar] [CrossRef]
- Agarwal, A.; Huang, S.; O’Donnell, M.; Day, K.; Day, M.; Kotov, N.; Ashkenazi, S. Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging. J. Appl. Phys. 2007, 102, 064701. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. 2020, 132, 5943–5953. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 13, 306–311. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic Imaging in Biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Apriyanto, D.K.; Setiawan, A.; Widyaningrum, R. Therapeutic and Contrast Agents for Photoacoustic Imaging-Guided Photothermal Therapy: A Narrative Review. Nanotheranostics 2024, 8, 506. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Xavierselvan, M.; Cook, J.; Duong, J.; Diaz, N.; Homan, K.; Mallidi, S. Photoacoustic Nanodroplets for Oxygen Enhanced Photodynamic Therapy of Cancer. Photoacoustics 2022, 25, 100306. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic Tomography of Blood Oxygenation: A Mini Review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Kennedy, M.J.; Dixon, A.J.; Sun, N.; Cao, R.; Soetikno, B.T.; Chen, R.; Zhou, Q.; Shung, K.K.; Hossack, J.A. Simultaneous Photoacoustic Microscopy of Microvascular Anatomy, Oxygen Saturation, and Blood Flow. Opt. Lett. 2015, 40, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Sivaramakrishnan, M.; Stoica, G.; Wang, L.V. Imaging of Hemoglobin Oxygen Saturation Variations in Single Vessels In Vivo using Photoacoustic Microscopy. Appl. Phys. Lett. 2007, 90, 053901. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.H.; Park, B.; Seo, H.M.; Bang, C.H.; Park, G.S.; Park, Y.M.; Rhie, J.W.; Lee, J.H.; Kim, C. Multispectral Ex Vivo Photoacoustic Imaging of Cutaneous Melanoma for Better Selection of the Excision Margin. Br. J. Dermatol. 2018, 179, 780–782. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Chuah, S.Y.; Razansky, D.; Ho, C.J.H.; Malempati, P.; Dinish, U.; Bi, R.; Fu, C.Y.; Ford, S.J.; Lee, J.S.-S. Noninvasive Real-Time Characterization of Non-Melanoma Skin Cancers with Handheld Optoacoustic Probes. Photoacoustics 2017, 7, 20–26. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Yang, S.; Xing, D. Toward In Vivo Biopsy of Melanoma Based on Photoacoustic and Ultrasound Dual Imaging with an Integrated Detector. Biomed. Opt. Express 2016, 7, 279–286. [Google Scholar] [CrossRef]
- Langhout, G.C.; Grootendorst, D.J.; Nieweg, O.E.; Wouters, M.W.J.M.; van der Hage, J.A.; Jose, J.; van Boven, H.; Steenbergen, W.; Manohar, S.; Ruers, T.J.M. Detection of Melanoma Metastases in Resected Human Lymph Nodes by Noninvasive Multispectral Photoacoustic Imaging. Int. J. Biomed. Imaging 2014, 2014, 163652. [Google Scholar] [CrossRef]
- Karlas, A.; Kallmayer, M.; Bariotakis, M.; Fasoula, N.-A.; Liapis, E.; Hyafil, F.; Pelisek, J.; Wildgruber, M.; Eckstein, H.-H.; Ntziachristos, V. Multispectral Optoacoustic Tomography of Lipid and Hemoglobin Contrast in Human Carotid Atherosclerosis. Photoacoustics 2021, 23, 100283. [Google Scholar] [CrossRef]
- Cao, Y.; Kole, A.; Hui, J.; Zhang, Y.; Mai, J.; Alloosh, M.; Sturek, M.; Cheng, J.-X. Fast Assessment of Lipid Content in Arteries In Vivo by Intravascular Photoacoustic Tomography. Sci. Rep. 2018, 8, 2400. [Google Scholar] [CrossRef] [PubMed]
- Buma, T.; Conley, N.C.; Choi, S.W. Multispectral Photoacoustic Microscopy of Lipids using a Pulsed Supercontinuum Laser. Biomed. Opt. Express 2018, 9, 276–288. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast Agents for Molecular Photoacoustic Imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef]
- Kilian, H.I.; Ma, C.; Zhang, H.; Chen, M.; Nilam, A.; Quinn, B.; Tang, Y.; Xia, J.; Yao, J.; Lovell, J.F. Intraperitoneal Administration for Sustained Photoacoustic Contrast Agent Imaging. Photoacoustics 2022, 28, 100406. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-Color Photoacoustic Lymph Node Imaging using Nanoformulated Naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Kukačka, J.; Steinkamp, P.; de Wit, J.; Voskuil, F.; Hooghiemstra, W.; Bader, M.; Jüstel, D.; Ntziachristos, V.; van Dam, G. Multispectral Optoacoustic Tomography for In Vivo Detection of Lymph Node Metastases in Oral Cancer Patients using an EGFR-Targeted Contrast Agent and Intrinsic Tissue Contrast: A Proof-of-Concept Study. Photoacoustics 2022, 26, 100362. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Zhang, Y.; Zhu, J.; Wang, L. Five-Wavelength Optical-Resolution Photoacoustic Microscopy of Blood and Lymphatic Vessels. Adv. Photonics 2021, 3, 016002. [Google Scholar] [CrossRef]
- Forbrich, A.; Heinmiller, A.; Zemp, R.J. Photoacoustic Imaging of Lymphatic Pumping. J. Biomed. Opt. 2017, 22, 106003. [Google Scholar] [CrossRef]
- Nagaoka, R.; Tabata, T.; Yoshizawa, S.; Umemura, S.-i.; Saijo, Y. Visualization of Murine Lymph Vessels using Photoacoustic Imaging with Contrast Agents. Photoacoustics 2018, 9, 39–48. [Google Scholar] [CrossRef]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid-Conjugated Silica Nanoparticles. Adv. Funct. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, C.S.; Waltzer, W.C.; Liu, J.; Xie, H.; Yuan, Z.; Pan, Y. In vivo Bladder Imaging with Microelectromechanical-Systems-Based Endoscopic Spectral Domain Optical Coherence Tomography. J. Biomed. Opt. 2007, 12, 034009. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Park, B.; Ye, J.; Wang, X.; Liu, G.; Yang, X.; Jiang, Z.; Han, M.; Fan, Y.; Song, J. Surfactant-Stripped Semiconducting Polymer Micelles for Tumor Theranostics and Deep Tissue Imaging in the NIR-II Window. Small 2022, 18, 2104132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, J.; Wang, T.; Chen, X.; Huang, P. Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications. Theranostics 2016, 6, 2394. [Google Scholar] [CrossRef] [PubMed]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.C.; Kim, C.; Xia, J. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, 1902279. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Giammanco, G.; Hu, C.-H.; Bush, J.; Cordova, L.S.; Lawrence, D.J.; Moran, J.L.; Chitnis, P.V.; Veneziano, R. Size-Tunable ICG-based Contrast Agent Platform for Targeted Near-Infrared Photoacoustic Imaging. Photoacoustics 2022, 29, 100437. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and Bioactive Engineered Nanomaterials for Targeted Tumor Photothermal Therapy: A Review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef]
- Beztsinna, N.; Tsvetkova, Y.; Jose, J.; Rhourri-Frih, B. Photoacoustic Imaging of Tumor Targeting with Riboflavin-Functionalized Theranostic Nanocarriers. Int. J. Nanomed. 2017, 12, 3813. [Google Scholar] [CrossRef]
- He, L.; Nie, T.; Xia, X.; Liu, T.; Huang, Y.; Wang, X.; Chen, T. Designing Bioinspired 2D MoSe2 Nanosheet for Efficient Photothermal-Triggered Cancer Immunotherapy with Reprogramming Tumor-Associated Macrophages. Adv. Funct. Mater. 2019, 29, 1901240. [Google Scholar] [CrossRef]
- Lin, L.-S.; Yang, X.; Niu, G.; Song, J.; Yang, H.-H.; Chen, X. Dual-Enhanced Photothermal Conversion Properties of Reduced Graphene Oxide-Coated Gold Superparticles for Light-Triggered Acoustic and Thermal Theranostics. Nanoscale 2016, 8, 2116–2122. [Google Scholar] [CrossRef]
- Chen, Z.; Tu, Y.; Zhang, D.; Liu, C.; Zhou, Y.; Li, X.; Wu, X.; Liu, R. A Thermosensitive Nanoplatform for Photoacoustic Imaging and NIR Light Triggered Chemo-Photothermal Therapy. Biomater. Sci. 2020, 8, 4299–4307. [Google Scholar] [CrossRef]
- Lin, S.; Shah, A.; Hernández-Gil, J.; Stanziola, A.; Harriss, B.I.; Matsunaga, T.O.; Long, N.; Bamber, J.; Tang, M.-X. Optically and Acoustically Triggerable Sub-Micron Phase-Change Contrast Agents for Enhanced Photoacoustic and Ultrasound Imaging. Photoacoustics 2017, 6, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Sun, H.; Zhang, H.; He, L.; Qiu, L.; Lin, J.; Xia, H.; Zhang, Y.; Ji, S.; Shi, H. Quantitatively Visualizing Tumor-Related Protease Activity In Vivo using a Ratiometric Photoacoustic Probe. J. Am. Chem. Soc. 2019, 141, 3265–3273. [Google Scholar] [CrossRef]
- Olefir, I.; Tzoumas, S.; Restivo, C.; Mohajerani, P.; Xing, L.; Ntziachristos, V. Deep Learning-Based Spectral Unmixing for Optoacoustic Imaging of Tissue Oxygen Saturation. IEEE Trans. Med. Imaging 2020, 39, 3643–3654. [Google Scholar] [CrossRef]
- ANSI Z136.1; American National Standard for the Safe Use of Lasers. American National Standards Institute: Washington, DC, USA, 2022.
- Zhang, W.; Sun, X.; Huang, T.; Pan, X.; Sun, P.; Li, J.; Zhang, H.; Lu, X.; Fan, Q.; Huang, W. 1300 nm Absorption Two-Acceptor Semiconducting Polymer Nanoparticles for NIR-II Photoacoustic Imaging System Guided NIR-II Photothermal Therapy. Chem. Commun. 2019, 55, 9487–9490. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.M.; Park, J.; Cho, S.-W.; Han, S.; Ahn, J.; Cho, S.; Kim, C.; Kim, C.-S.; Kim, J. Transportable Multispectral Optical-Resolution Photoacoustic Microscopy using Stimulated Raman Scattering Spectrum. IEEE Trans. Instrum. Meas. 2024, 73, 4502309. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Park, S.; Cho, S.; Yoo, J.; Kim, C.; Kim, J. Ultrasound-Guided Breath-Compensation in Single-Element Photoacoustic Imaging for Three-Dimensional Whole-Body Images of Mice. Front. Phys. 2022, 10, 457. [Google Scholar] [CrossRef]
- Choi, S.; Yang, J.; Lee, S.Y.; Kim, J.; Lee, J.; Kim, W.J.; Lee, S.; Kim, C. Deep Learning Enhances Multiparametric Dynamic Volumetric Photoacoustic Computed Tomography In Vivo (DL-PACT). Adv. Sci. 2023, 10, 2202089. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Ahn, J.; Kim, Y.; Lim, J.; Park, J.; Kim, H.H.; Kim, W.J.; Kim, C. An Ultrasensitive and Broadband Transparent Ultrasound Transducer for Ultrasound and Photoacoustic Imaging In-Vivo. Nat. Commun. 2024, 15, 1444. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic Microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, S.; Knieling, F.; Clingman, B.; Bohndiek, S.; Wang, L.V.; Kim, C. Clinical Translation of Photoacoustic Imaging. Nat. Rev. Bioeng. 2024. [Google Scholar] [CrossRef]
- Kim, J.; Anthony, M.Y.; Kubelick, K.P.; Emelianov, S.Y. Gold Nanoparticles Conjugated with DNA Aptamer for Photoacoustic Detection of Human Matrix Metalloproteinase-9. Photoacoustics 2022, 25, 100307. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface Charge Switchable Nanoparticles based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. pH-Induced Aggregation of Gold Nanoparticles for Photothermal Cancer Therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Cheng, Y.; Cheng, Q. Photostability Enhancement of Silica-Coated Gold Nanostars for Photoacoustic Imaging Guided Photothermal Therapy. Photoacoustics 2021, 23, 100284. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, Stimuli-Sensitive Nanoparticulate Systems for Drug Delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Cao, Y.; Dumani, D.S.; Hallam, K.A.; Emelianov, S.Y.; Ran, H. Real-Time Monitoring of NIR-Triggered Drug Release from Phase-Changeable Nanodroplets by Photoacoustic/Ultrasound Imaging. Photoacoustics 2023, 30, 100474. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, Z.; Zheng, K.; Shi, D.; Su, G.; Ge, D.; Zhao, Q.; Fu, X.; Hou, Z. A Novel Self-Targeting Theranostic Nanoplatform for Photoacoustic Imaging-Monitored and Enhanced Chemo-Sonodynamic Therapy. J. Mater. Chem. B 2021, 9, 5547–5559. [Google Scholar] [CrossRef]
- Sarma, T.; Kim, G.; Sen, S.; Cha, W.-Y.; Duan, Z.; Moore, M.D.; Lynch, V.M.; Zhang, Z.; Kim, D.; Sessler, J.L. Proton-Coupled Redox Switching in an Annulated π-Extended Core-Modified Octaphyrin. J. Am. Chem. Soc. 2018, 140, 12111–12119. [Google Scholar] [CrossRef]
- Song, J.; Kim, J.; Hwang, S.; Jeon, M.; Jeong, S.; Kim, C.; Kim, S. “Smart” Gold Nanoparticles for Photoacoustic Imaging: An Imaging Contrast Agent Responsive to the Cancer Microenvironment and Signal Amplification via pH-Induced Aggregation. Chem. Commun. 2016, 52, 8287–8290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, L.; Wang, X.; Jia, Q.; Chen, Z.; Yang, Z.; Ji, R.; Tian, J.; Wang, Z. Acid-Induced In Vivo Assembly of Gold Nanoparticles for Enhanced Photoacoustic Imaging-Guided Photothermal Therapy of Tumors. Adv. Healthc. Mater. 2020, 9, 2000394. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, X.; Xu, J.; Sun, R.; Xia, H.; Ding, J.; Chin, Y.E.; Chai, Z.; Shi, H.; Gao, M. Furin Enzyme and pH Synergistically Triggered Aggregation of Gold Nanoparticles for Activated Photoacoustic Imaging and Photothermal Therapy of Tumors. Anal. Chem. 2021, 93, 9277–9285. [Google Scholar] [CrossRef]
- Li, S.; Lui, K.-H.; Tsoi, T.-H.; Lo, W.-S.; Li, X.; Hu, X.; Tai, W.C.-S.; Hung, C.H.-L.; Gu, Y.-J.; Wong, W.-T. pH-Responsive Targeted Gold Nanoparticles for In Vivo Photoacoustic Imaging of Tumor Microenvironments. Nanoscale Adv. 2019, 1, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lui, K.-H.; Li, X.; Fang, X.; Lo, W.-S.; Gu, Y.-J.; Wong, W.-T. pH-Triggered poly (ethylene glycol)–poly (lactic acid/glycolic acid)/croconaine Nanoparticles-Assisted Multiplexed Photoacoustic Imaging and Enhanced Photothermal Cancer Therapy. ACS Appl. Bio Mater. 2021, 4, 4152–4164. [Google Scholar] [CrossRef]
- Wu, J.; You, L.; Chaudhry, S.T.; He, J.; Cheng, J.-X.; Mei, J. Ambient Oxygen-Doped Conjugated Polymer for pH-Activatable Aggregation-Enhanced Photoacoustic Imaging in the Second Near-Infrared Window. Anal. Chem. 2021, 93, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zou, Y.; Liu, L.; Li, R.; Xue, F.; Yi, T. pH-Responsive Ag2S Nanodots Loaded with Heat Shock Protein 70 Inhibitor for Photoacoustic Imaging-Guided Photothermal Cancer Therapy. Acta Biomater. 2020, 115, 358–370. [Google Scholar] [CrossRef]
- Yang, Z.; Cheng, R.; Zhao, C.; Sun, N.; Luo, H.; Chen, Y.; Liu, Z.; Li, X.; Liu, J.; Tian, Z. Thermo-and pH-Dual Responsive Polymeric Micelles with Upper Critical Solution Temperature Behavior for Photoacoustic Imaging-Guided Synergistic Chemo-Photothermal Therapy Against Subcutaneous and Metastatic Breast Tumors. Theranostics 2018, 8, 4097. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Wang, E.; Yan, N.; Sheng, S.; Chen, J.; Lin, L.; Guo, Z.; Tian, H.; Chen, X. Effective Eradication of Tumors by Enhancing Photoacoustic-Imaging-Guided Combined Photothermal Therapy and Ultrasonic Therapy. Adv. Funct. Mater. 2021, 31, 2009314. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.; Lee, J.; Kang, Y.; Han, M.; Lee, J.; Kim, C.; Kim, W.J. Transcytosis-Inducing Multifunctional Albumin Nanomedicines with Deep Penetration Ability for Image-Guided Solid Tumor Treatment. Small 2023, 19, 2303668. [Google Scholar] [CrossRef]
- Tian, Q.; Li, Y.; Jiang, S.; An, L.; Lin, J.; Wu, H.; Huang, P.; Yang, S. Tumor pH-Responsive Albumin/Polyaniline Assemblies for Amplified Photoacoustic Imaging and Augmented Photothermal Therapy. Small 2019, 15, 1902926. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sedgwick, A.C.; Sen, S.; Ren, Y.; Sun, Q.; Chau, C.; Arambula, J.F.; Sarma, T.; Song, L.; Sessler, J.L. Expanded Porphyrins: Functional Photoacoustic Imaging Agents that Operate in the NIR-II Region. Chem. Sci. 2021, 12, 9916–9921. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.; Chen, J.; Zeng, J.; Cheng, Z.; Liu, Z. A Self-Assembled Albumin-Based Nanoprobe for In Vivo Ratiometric Photoacoustic pH Imaging. Adv. Mater. 2015, 27, 6820–6827. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Tang, W.; Fan, W.; Dai, Y.; Shen, Z.; Lin, L.; Cheng, S.; Liu, Y.; Niu, G. Stimuli-Responsive Nanotheranostics for Real-Time Monitoring Drug Release by Photoacoustic Imaging. Theranostics 2019, 9, 526. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, C.; Lv, Y.; Xiang, J.; Ma, G.; Li, C.; Hu, Y.; Guo, C.; Sun, H.; Cai, L. Preparation of Near-Infrared Photoacoustic Imaging and Photothermal Treatment Agent for Cancer using a Modifiable Acid-Triggered Molecular Platform. Analyst 2024, 149, 3064–3072. [Google Scholar] [CrossRef]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S.; et al. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef]
- Noltes, M.E.; Bader, M.; Metman, M.J.; Vonk, J.; Steinkamp, P.J.; Kukačka, J.; Westerlaan, H.E.; Dierckx, R.A.; van Hemel, B.M.; Brouwers, A.H. Towards In Vivo Characterization of Thyroid Nodules Suspicious for Malignancy using Multispectral Optoacoustic Tomography. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2736–2750. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A.; Shinchi, M.; Nakamura, A.; Wada, T.; Ito, K.; Asano, T.; Shinmoto, H.; Tsuda, H.; Ishihara, M. Pilot Study of Prostate Cancer Angiogenesis Imaging using a Photoacoustic Imaging System. Urology 2017, 108, 212–219. [Google Scholar] [CrossRef]
- Kukačka, J.; Metz, S.; Dehner, C.; Muckenhuber, A.; Paul-Yuan, K.; Karlas, A.; Fallenberg, E.M.; Rummeny, E.; Jüstel, D.; Ntziachristos, V. Image Processing Improvements Afford Second-Generation Handheld Optoacoustic Imaging of Breast Cancer Patients. Photoacoustics 2022, 26, 100343. [Google Scholar] [CrossRef]
- Ivankovic, I.; Merčep, E.; Schmedt, C.-G.; Deán-Ben, X.L.; Razansky, D. Real-Time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 45–50. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-Breath-Hold Photoacoustic Computed Tomography of the Breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef] [PubMed]
- Jeng, G.-S.; Li, M.-L.; Kim, M.; Yoon, S.J.; Pitre Jr, J.J.; Li, D.S.; Pelivanov, I.; O’Donnell, M. Real-Time Interleaved Spectroscopic Photoacoustic and Ultrasound (PAUS) Scanning with Simultaneous Fluence Compensation and Motion Correction. Nat. Commun. 2021, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Vercauteren, T.; Xia, W. Learning-based Sound Speed Estimation and Aberration Correction for Linear-Array Photoacoustic Imaging. Photoacoustics 2024, 38, 100621. [Google Scholar] [CrossRef]






| Base | pH-Sensitive Material | Size [nm] (pH) | [nm] (pH) | PA Imaging | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Tumor | Normal | Tumor | Configuration | [nm] | Application | |||
| Au | Citraconic amide | 10 (7.4) | - | 520 (7.4) | - | AR-PAM | 680 | HeLa breast tumor | [62] |
| Au | MUA-TMA | 15 (7.4) | 180 (6.5) | 524 (7.4) | 650–700 (6.5) | PACT | 808 | U87MG glioblastoma | [63] |
| Au | RVRR | 63 (7.2) | 327 (5.5) | 520 (7.2) | 700–900 (5.5) | PACT | 750 | HCT-116 colon tumor | [64] |
| Au | Citraconic amide | 16.4 (7.4) | ≥500 (5.8) | 550–600 (7.4) | 650 (5.8) | PACT | 680, 800 | U87MG glioblastoma | [65] |
| Croconaine | Croconaine | 185 (7.4) | 190 (6.5) | 630 (7.4) | 815 (6.5) | PACT | 770 | MDA-MB-231 breast tumor | [66] |
| PPE | Doped PPE | - | 4000 (6) | 1150 (7.4) | 1100 (6) | PACT | 1064 | PC-3 prostate tumor | [67] |
| pH-Sensitive Nanoparticles | Therapy | PA Imaging | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Base | pH-Sensitive Material | [nm] | Power [W/cm2] | Time [min] | Configuration | [nm] | Application | |
| Ag2S | PEG | 808 | 1.5 | 10 | PACT | 680 | HepG2 liver tumor | [68] |
| DOX + IR780 | mPEG-PAAV | 808 | 1.0 | 5 | PACT | 720 | 4T1 breast tumor | [69] |
| Au | PEG | 808 | 1.0 | 6 | PACT | 830 | MCF-7 breast tumor | [70] |
| mAlb | DMMA | 808 | 1.0 | 5 | AR-PAM | 830 | 4T1 breast tumor | [71] |
| Base | [nm] (pH) | PA Imaging | Ref. | |||
|---|---|---|---|---|---|---|
| Normal | Tumor | Configuration | Application | |||
| PANI | 570 (8) | 800 (3) | PACT | - | 4T1 breast tumor | [72] |
| Octaphyrin | 1200 (7.4) | 1200 (5) | PACT | 1200 | HepG2 liver tumor | [73] |
| BPOx, IR825 | 580 (7) | 620 (5.5) | PACT | 680, 825 | 4T1 breast tumor | [74] |
| PDI | 450 (7.4) | 500 (5) | PACT | 680, 825 | U87MG glioblastoma | [75] |
| Heptamethine cyanine dye | 680 (10) | 760 (4) | PACT | 680, 760 | MCF-7 breast tumor | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kye, H.; Jo, D.; Jeong, S.; Kim, C.; Kim, J. Photoacoustic Imaging of pH-Sensitive Optical Sensors in Biological Tissues. Chemosensors 2024, 12, 257. https://doi.org/10.3390/chemosensors12120257
Kye H, Jo D, Jeong S, Kim C, Kim J. Photoacoustic Imaging of pH-Sensitive Optical Sensors in Biological Tissues. Chemosensors. 2024; 12(12):257. https://doi.org/10.3390/chemosensors12120257
Chicago/Turabian StyleKye, Hyunjun, Dongyoung Jo, Sanghwa Jeong, Chulhong Kim, and Jeesu Kim. 2024. "Photoacoustic Imaging of pH-Sensitive Optical Sensors in Biological Tissues" Chemosensors 12, no. 12: 257. https://doi.org/10.3390/chemosensors12120257
APA StyleKye, H., Jo, D., Jeong, S., Kim, C., & Kim, J. (2024). Photoacoustic Imaging of pH-Sensitive Optical Sensors in Biological Tissues. Chemosensors, 12(12), 257. https://doi.org/10.3390/chemosensors12120257







