Conventional and Emerging Diagnostic Approaches for Differentiated Thyroid Carcinoma
Abstract
1. Introduction
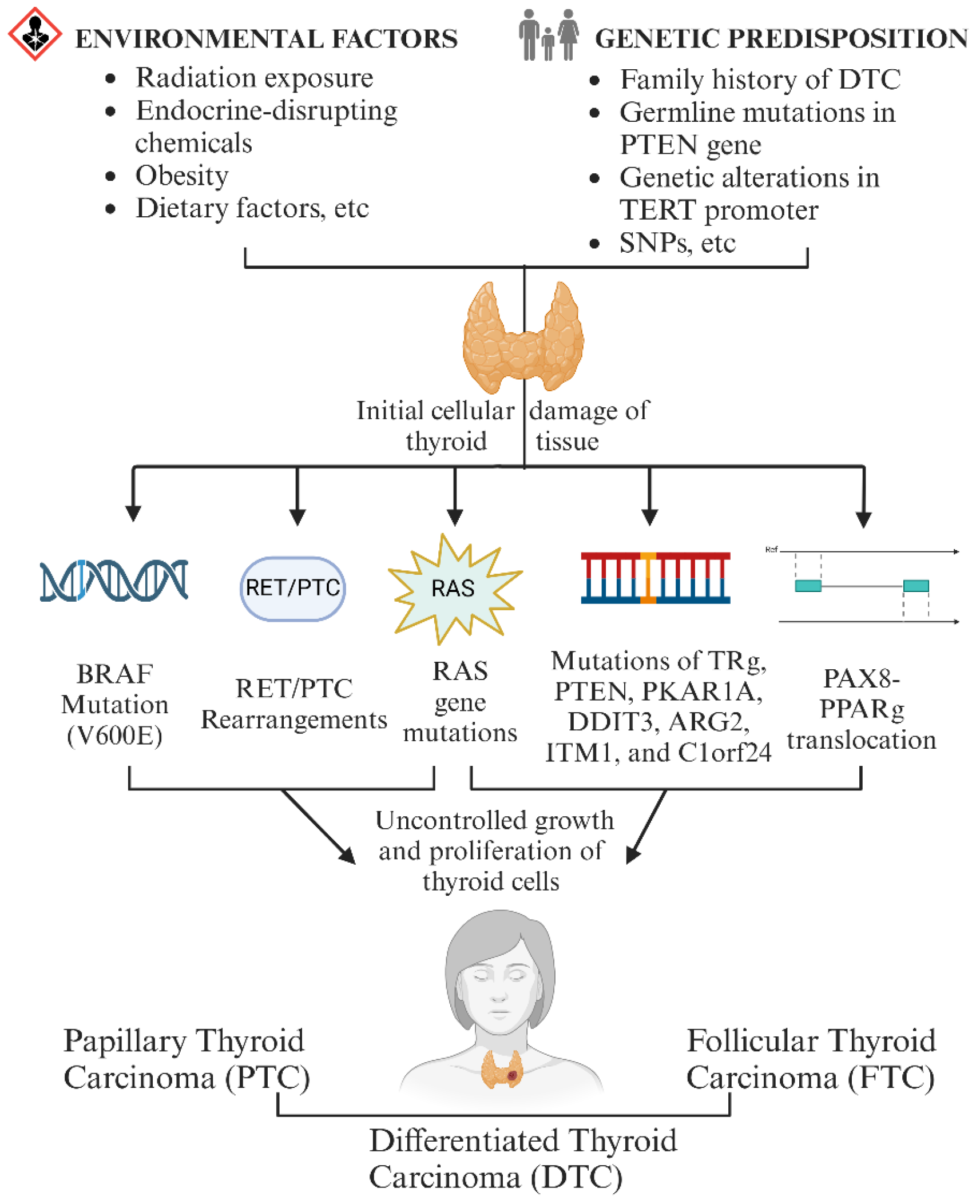
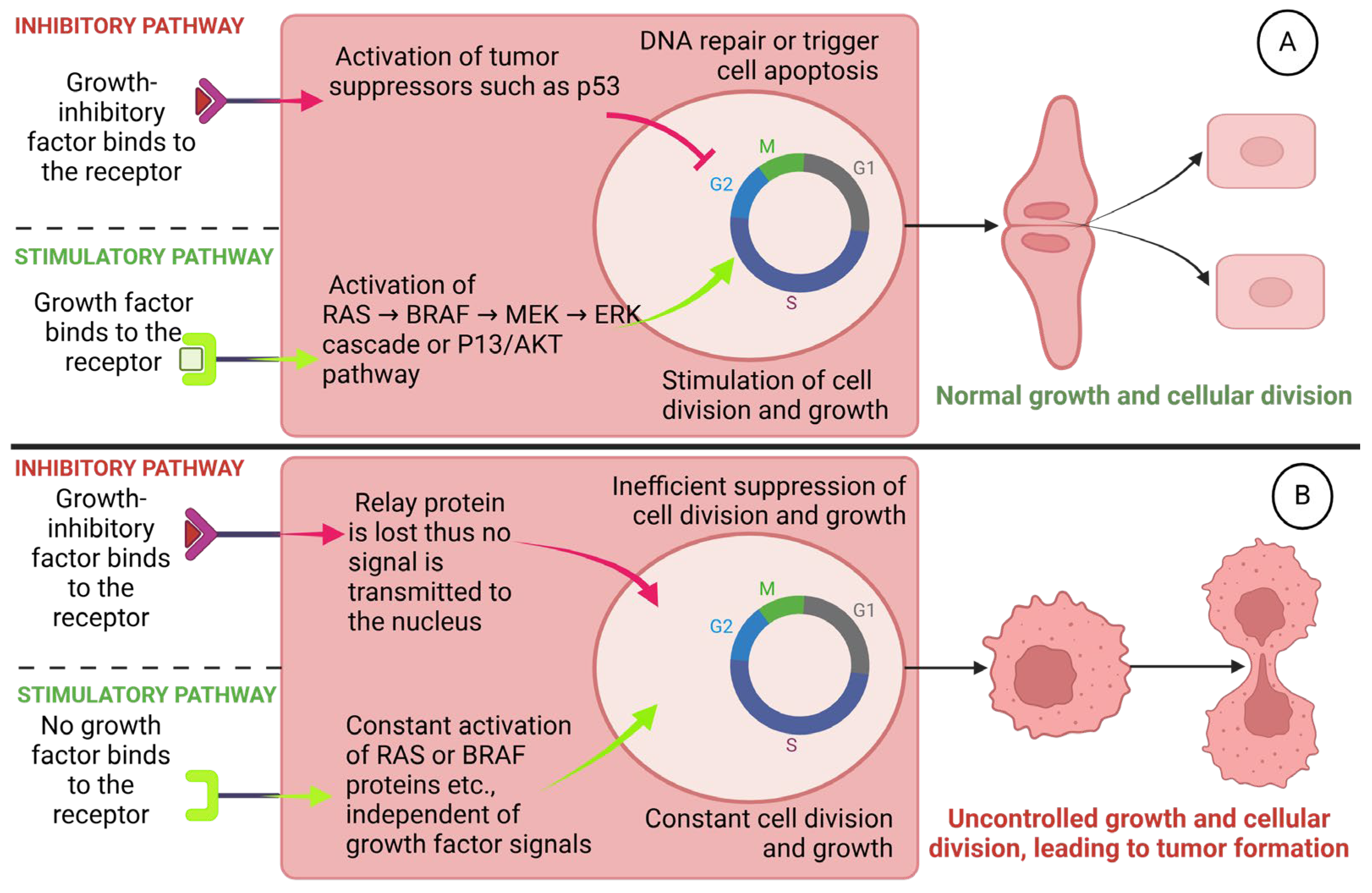
1.1. The Pathogenesis of DTC
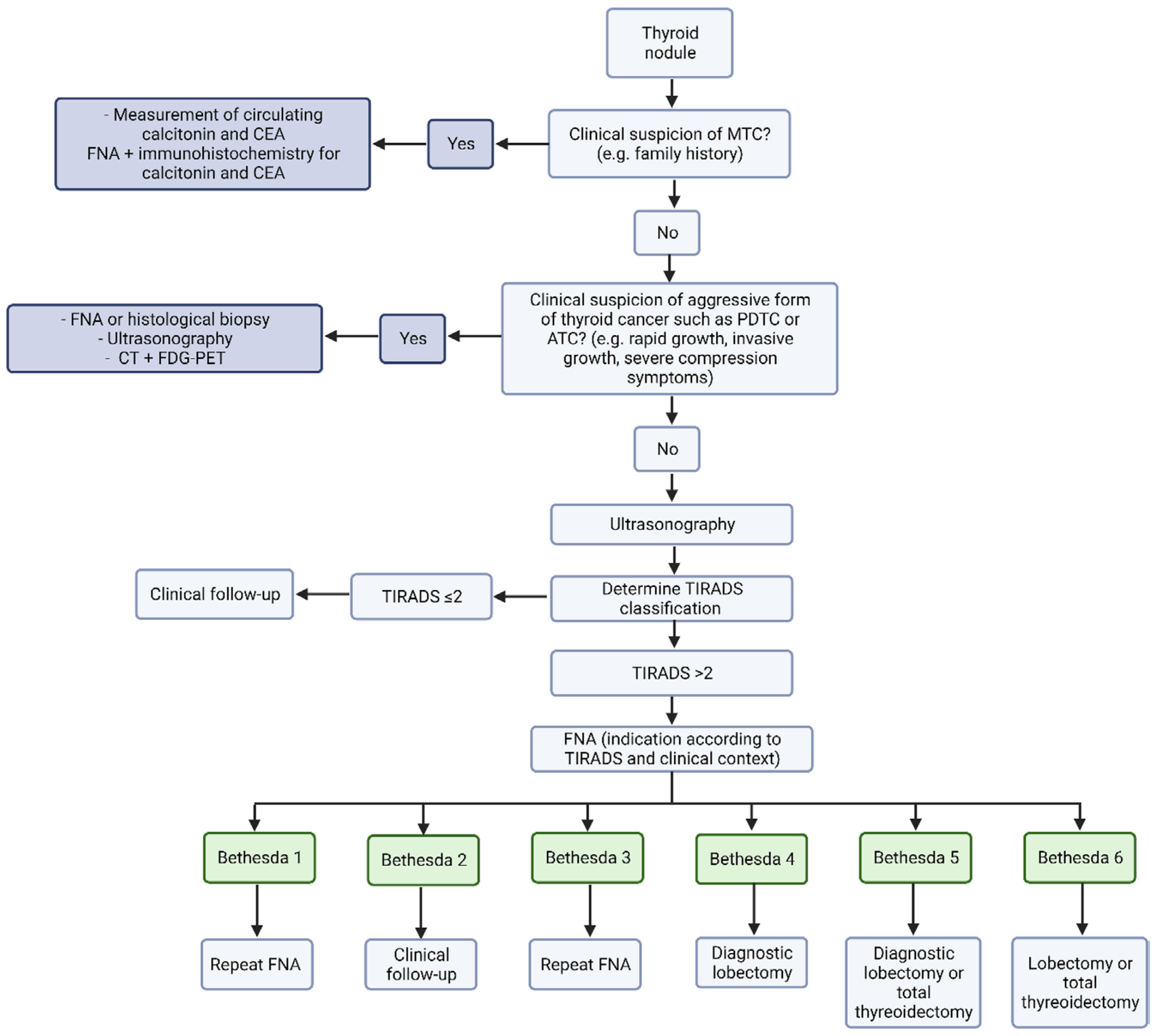
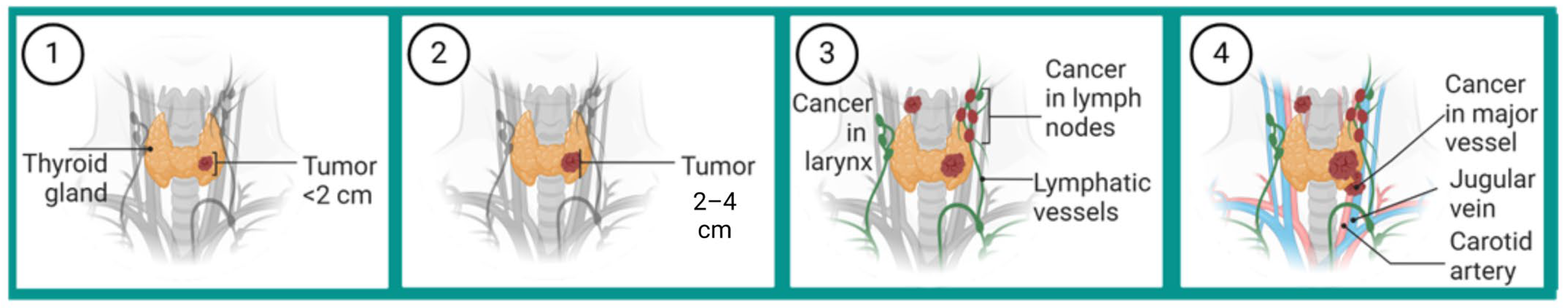
1.2. Clinical Presentation and Diagnostic Process
1.3. Histological Features
| Histological Features | PTC | FTC | References |
|---|---|---|---|
| Papillae formation | Present | Absent | [6,31] |
| Macroscopic changes | Circumscribed, solid, firm, and white in color, often cystic | Well-differentiated (follicular/colloidal differentiation) or poorly differentiated (solid growth, absence of follicles) | [6] |
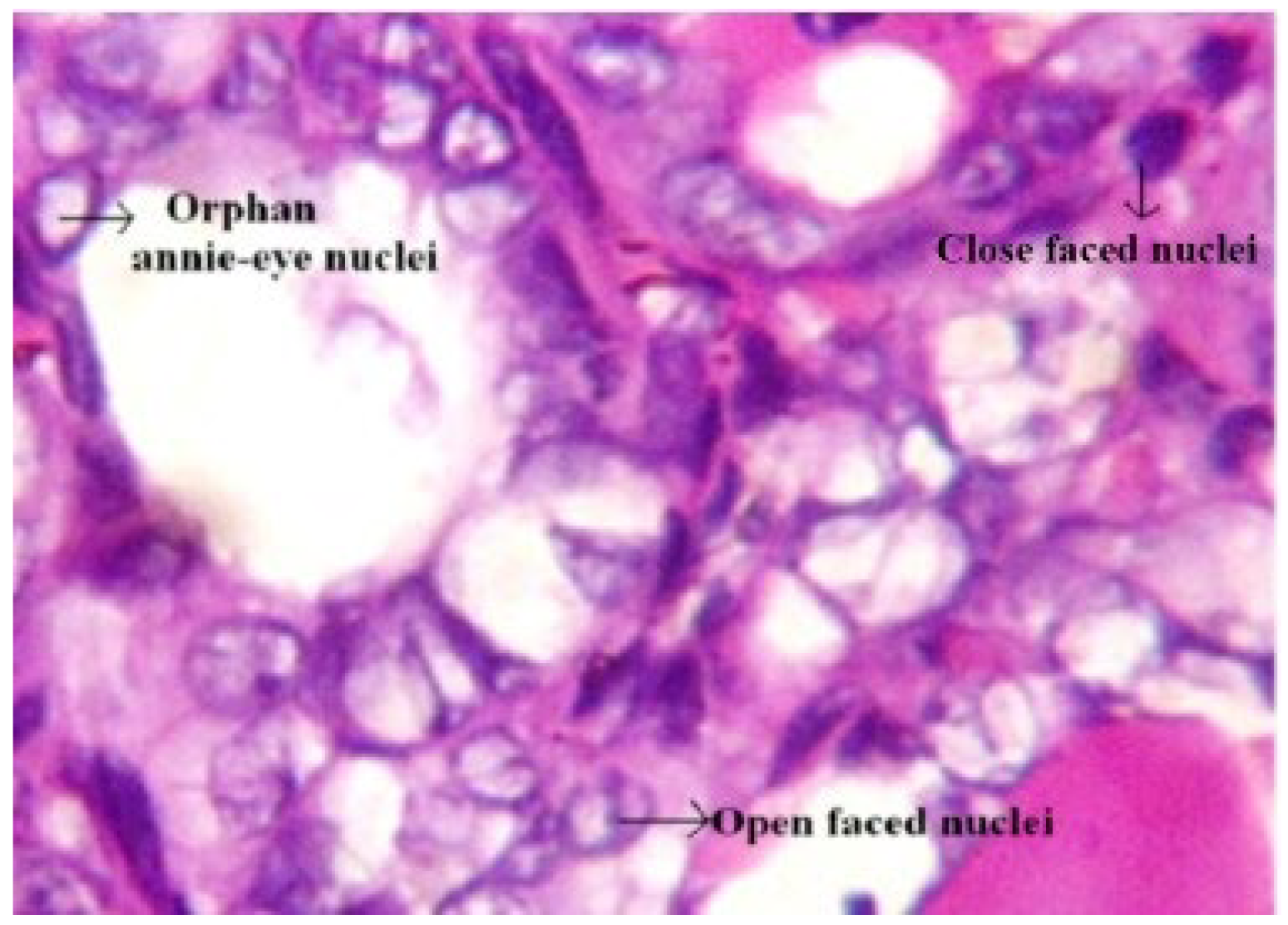
| Nuclear changes | Cuboidal form cells with nuclear “grooving” and cytoplasmic inclusions, “Orphan Annie Eye”. | Lack of PTC changes, meaning no inclusions, grooves, or “ground-glass” nuclei. | [6,32,33] |
| Growth pattern | Solid, extra-thyroid extension to adjacent tissues | Encapsulated, invasive | [6,32,33] |
| Psamoma bodies | Commonly present in 50% of cases | Rare | [6,33] |
| Vascular/Capsular invasion | Rare, focus on lymphatic spread | Common, defining feature | [34] |
| Architecture | Papillary architecture with ramifications | Micro-follicular architecture | [6,32,33] |
| Multifocality | In 18% to 85% of patients | Less common, usually unifocal | [6] |
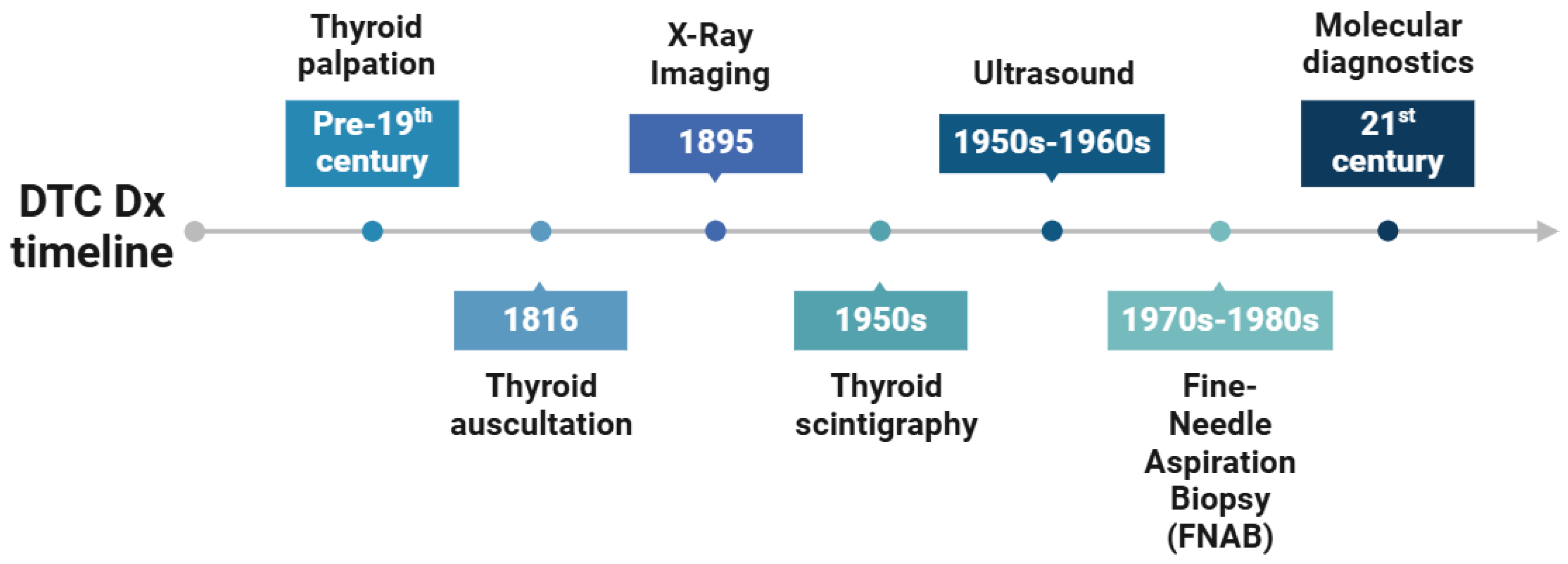
1.4. Historical Background and Evolution of DTC Diagnostics
1.4.1. Physical Examination and Palpation
1.4.2. Scintigraphy
1.4.3. Radioactive Iodine
2. Conventional DTC Diagnostic Methods
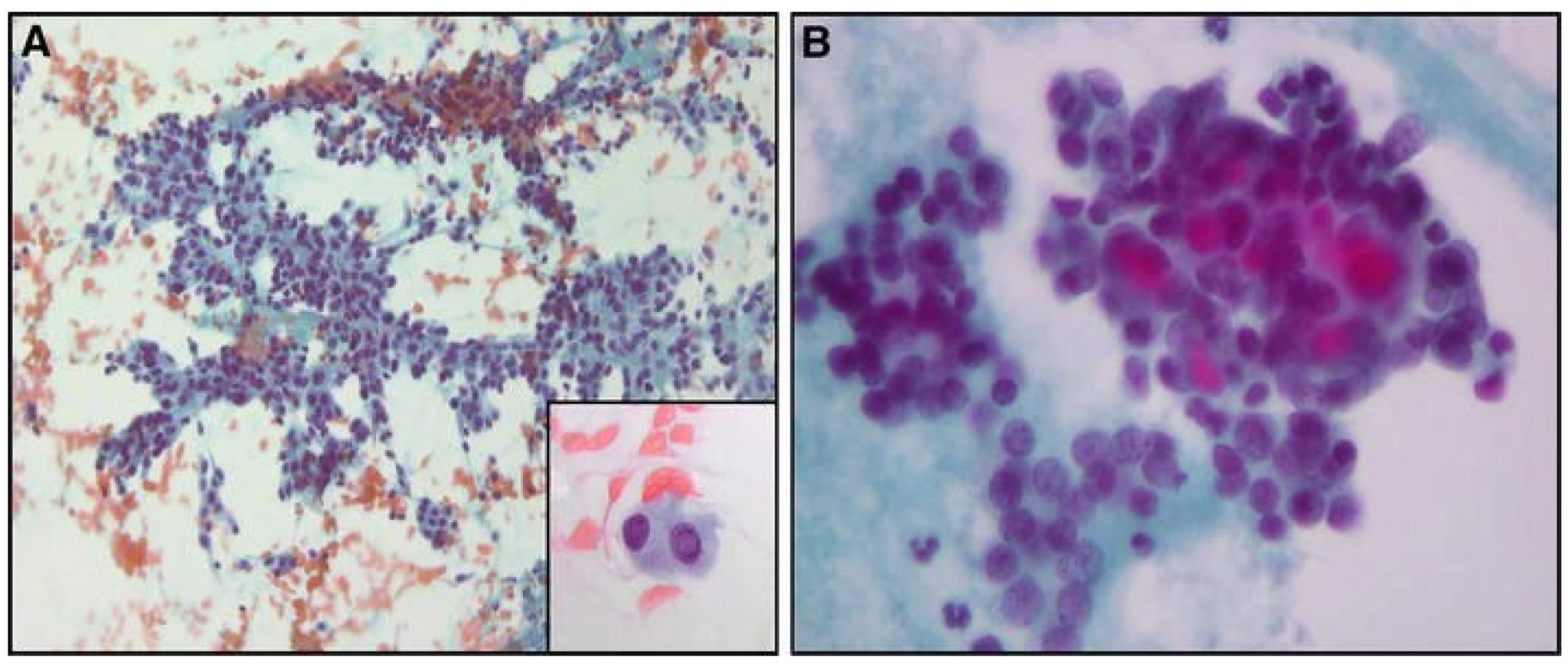
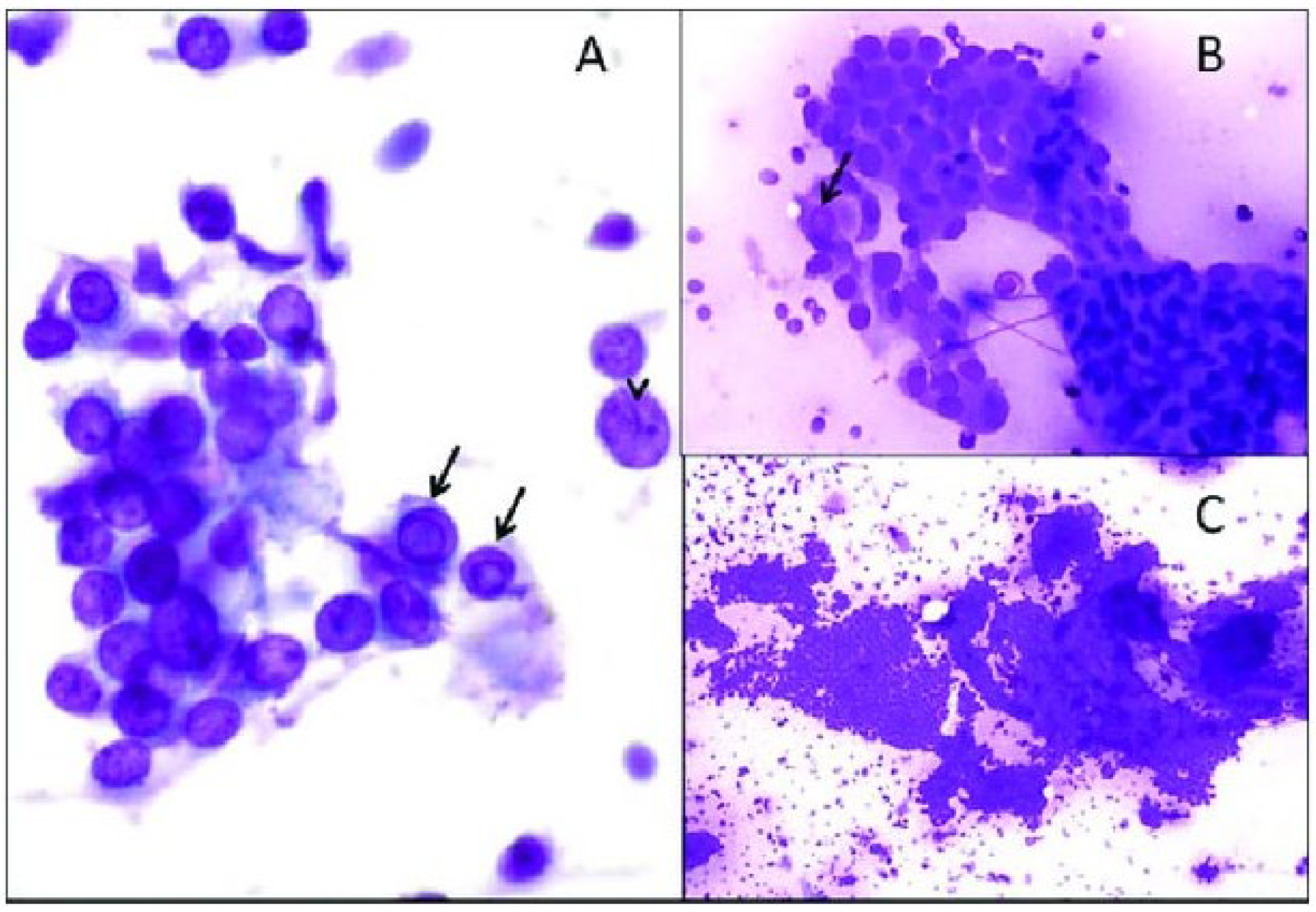
2.1. Fine Needle Aspiration Biopsy
| Aspect | Cytology | FNA-Tg | IHC | References |
|---|---|---|---|---|
| Purpose | Assess cell morphology. | Quantitate Tg levels. | Identify specific proteins (including Tg) in the cells. | [58,64] |
| Sample preparation | Direct smear on a microscope slide | Aspirate is centrifuged, and then the supernatant is analyzed | Fixation, embedding, sectioning of the sample, and antibody staining | [58,60,61] |
| Analysis method | Microscopy | ELISA/CLIA | Immunohistochemistry | [58,62] |
| Diagnostic use | Detect abnormal or cancerous cells | Detect metastasis of DTC and monitor recurrent DTC post-treatment | Provide additional diagnostic information | [57,64,65] |
2.1.1. Cytology
2.1.2. FNA-Thyroglobulin
2.1.3. Immunohistochemistry
2.2. Imaging Modalities
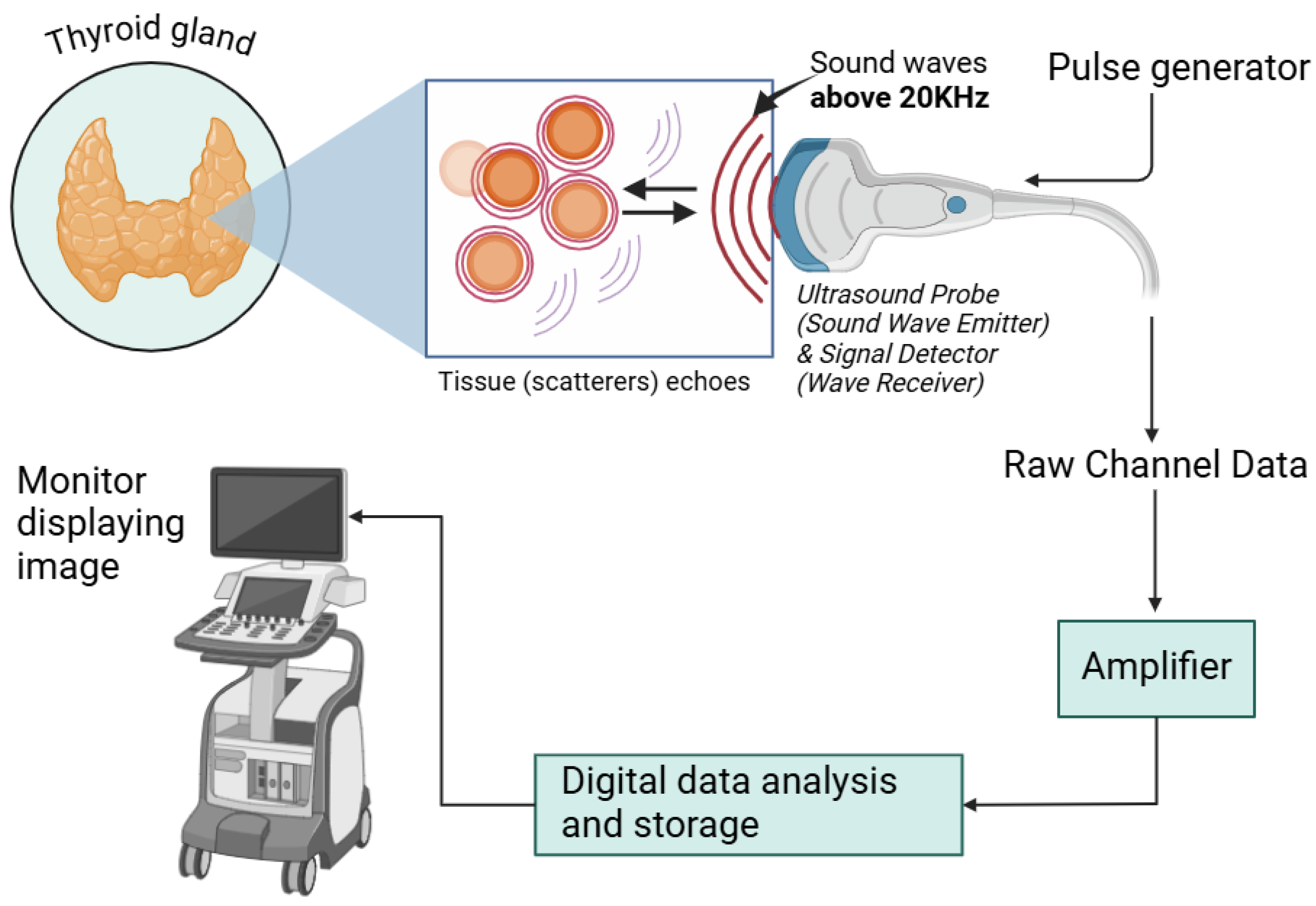
2.2.1. Ultrasound
2.2.2. Computed Tomography
2.2.3. Magnetic Resonance Imaging
2.2.4. Positron Emission Tomography
2.3. Molecular Testing and Biomarkers
2.3.1. Genetic Mutation Analysis
2.3.2. Genetic Expression and Profiling
2.3.3. Next Generation Sequencing
2.4. Liquid Chromatography-Mass Spectrometry
2.5. Thyroid Function Test
3. Recent Advancements in DTC Diagnostics
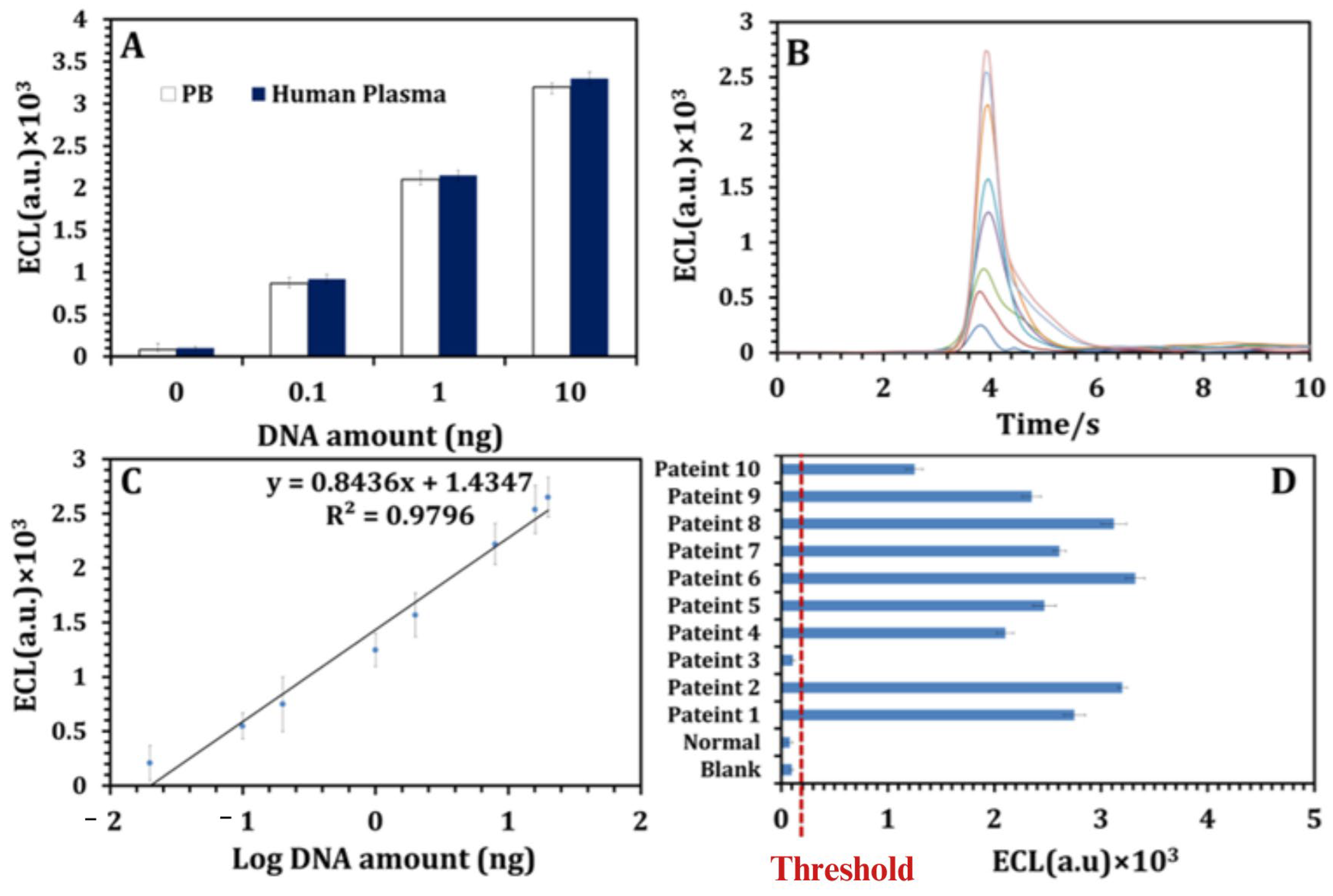
3.1. Liquid Biopsy
3.2. NanoString
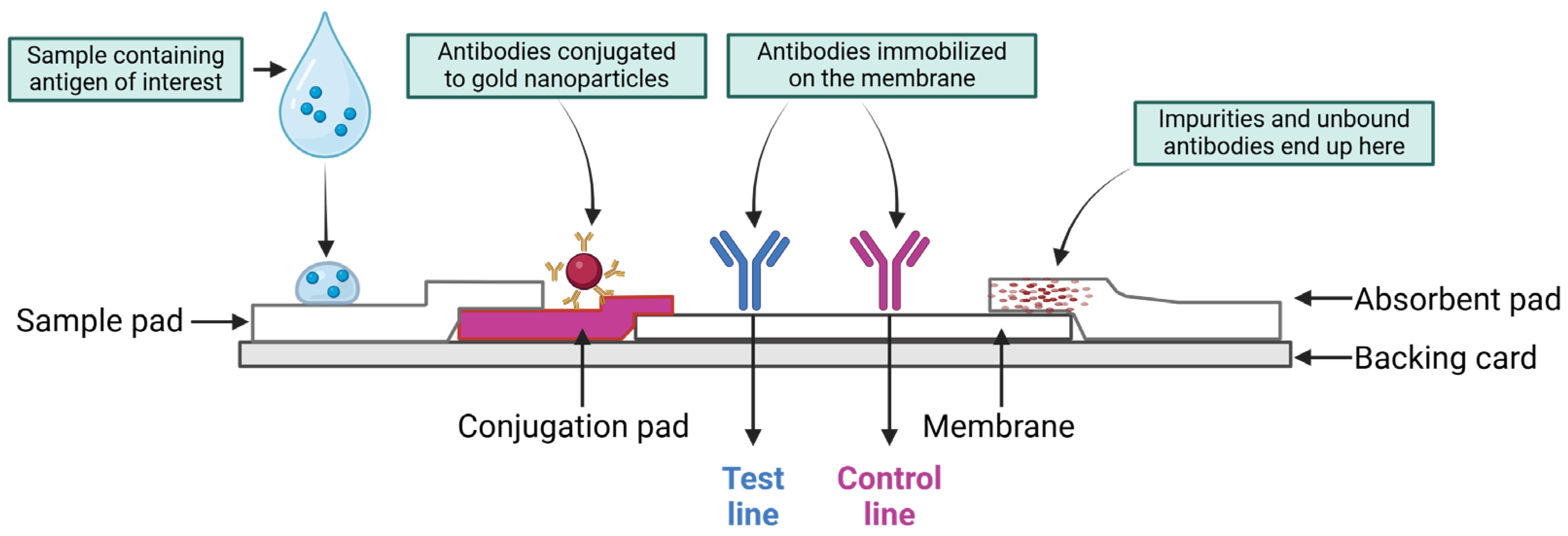
3.3. Lateral Flow Immunoassay
3.4. Elastography
4. Application of (Bio)sensors in Detecting DTC
5. Challenges in Clinical Implementation and Standardization
6. Future Perspectives: Emerging AI in DTC Diagnostics
7. The Impact of Diagnostic Methods on Patient Experience
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kent, W.D.; Hall, S.F.; Isotalo, P.A.; Houlden, R.L.; George, R.L.; Groome, P.A. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. Can. Med Assoc. J. 2007, 177, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018, 1553–1568. [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Zhang, D.; Long, J.; Gong, Y.; Ye, F.; Liu, S.; Li, Y. The global burden of thyroid cancer and its attributable risk factor in 195 countries and territories: A systematic analysis for the Global Burden of Disease Study. Cancer Med. 2021, 10, 4542–4554. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I.; Perrier, N.; Clayman, G.L. Thyroid cancer. In 60 Years of Survival Outcomes at the University of Texas MD Anderson Cancer Center; Springer: Berlin/Heidelberg, Germany, 2012; pp. 295–310. [Google Scholar]
- Arrangoiz, R.; Cordera, F.; Caba, D.; Moreno, E.; Luque-de-Leon, E.; Muñ, M. Thyroid cancer. J. Otolaryngol. Head Neck Surg. 2019, 8, 217–270. [Google Scholar] [CrossRef]
- van Houten, P.; Netea-Maier, R.T.; Smit, J.W. Differentiated thyroid carcinoma: An update. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101687. [Google Scholar] [CrossRef]
- Haselkorn, T.; Bernstein, L.; Preston-Martin, S.; Cozen, W.; Mack, W.J. Descriptive epidemiology of thyroid cancer in Los Angeles County, 1972–1995. Cancer Causes Control. 2000, 11, 163–170. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Kruger, E.; Toraih, E.A.; Hussein, M.H.; Shehata, S.A.; Waheed, A.; Fawzy, M.S.; Kandil, E. Thyroid carcinoma: A review for 25 years of environmental risk factors studies. Cancers 2022, 14, 6172. [Google Scholar] [CrossRef]
- Gordon, A.J.; Dublin, J.C.; Patel, E.; Papazian, M.; Chow, M.S.; Persky, M.J.; Jacobson, A.S.; Patel, K.N.; Suh, I.; Morris, L.G.; et al. American Thyroid Association guidelines and national trends in management of papillary thyroid carcinoma. Arch. Otolaryngol. Neck Surg. 2022, 148, 1156–1163. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Leboulleux, S. Current practice in patients with differentiated thyroid cancer. Nat. Rev. Endocrinol. 2021, 17, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Conti, G.O.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M.A.; Ferrante, M. Role of emerging environmental risk factors in thyroid cancer: A brief review. Int. J. Environ. Res. Public Health 2019, 16, 1185. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef]
- Weinberg, R.A. How cancer arises. Sci. Am. 1996, 275, 62–70. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Morris, L.F.; Haugen, B.R.; Shah, J.P.; Sosa, J.A.; Rohren, E.; Subramaniam, R.M.; Hunt, J.L.; Perrier, N.D. Thyroid–differentiated and anaplastic carcinoma. In AJCC Cancer Staging Manual; AJCC: Chicago, IL, USA, 2016; pp. 881–898. [Google Scholar]
- Zidane, M.; Cazier, J.-B.; Chevillard, S.; Ory, C.; Schlumberger, M.; Dupuy, C.; Deleuze, J.-F.; Boland, A.; Haddy, N.; Lesueur, F. Genetic susceptibility to radiation-related differentiated thyroid cancers: A systematic review of literature. Endocr.-Relat. Cancer 2019, 26, R583–R596. [Google Scholar] [CrossRef]
- An, S.-Y.; Kim, S.Y.; Oh, D.J.; Min, C.; Sim, S.; Choi, H.G. Obesity is positively related and tobacco smoking and alcohol consumption are negatively related to an increased risk of thyroid cancer. Sci. Rep. 2020, 10, 19279. [Google Scholar] [CrossRef]
- Abu Arar, Y.; Shilo, M.; Bilenko, N.; Friger, M.; Marsha, H.; Fisher, D.; Fraenkel, M.; Yoel, U. Are Higher Body Mass Index and Worse Metabolic Parameters Associated with More Aggressive Differentiated Thyroid Cancer? A Retrospective Cohort Study. Healthcare 2024, 12, 581. [Google Scholar] [CrossRef]
- Fiore, E.; Rago, T.; Provenzale, M.; Scutari, M.; Ugolini, C.; Basolo, F.; Di Coscio, G.; Berti, P.; Grasso, L.; Elisei, R. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: Thyroid autonomy may play a protective role. Endocr.-Relat. Cancer 2009, 16, 1251–1260. [Google Scholar] [CrossRef]
- Malaguarnera, R.; Vella, V.; Vigneri, R.; Frasca, F. p53 family proteins in thyroid cancer. Endocr.-Relat. Cancer 2007, 14, 43–60. [Google Scholar] [CrossRef]
- Trybek, T.; Kowalik, A.; Góźdź, S.; Kowalska, A. Telomeres and telomerase in oncogenesis. Oncol. Lett. 2020, 20, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F. PTEN: Multiple functions in human malignant tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Campennì, A.; Tuncel, M.; Petranović Ovčariček, P. Integrated Diagnostics of Thyroid Nodules. Cancers 2024, 16, 311. [Google Scholar] [CrossRef]
- Wu, H.H.; Swadley, M. The Bethesda system for reporting thyroid cytopathology: Into the clinic. Pathol. Lab. Med. Int. 2015, 7, 47–54. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Paone, G.; Ceriani, L.; Pusztaszeri, M. Cellular and molecular basis for thyroid cancer imaging in nuclear medicine. Clin. Transl. Imaging 2013, 1, 149–161. [Google Scholar] [CrossRef]
- Rosai, J. Papillary Thyroid Carcinoma: A Root-and-Branch Rethink; Oxford University Press: Oxford, UK, 2008; pp. 683–686. [Google Scholar]
- Jothi, J.A.A.; Rajam, V.M.A. Effective segmentation of orphan annie-eye nuclei from papillary thyroid carcinoma histopathology images using a probabilistic model and region-based active contour. Biomed. Signal Process. Control. 2016, 30, 149–161. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Critical prognostic parameters in the anatomic pathology reporting of differentiated follicular cell-derived thyroid carcinoma. Cancers 2019, 11, 1100. [Google Scholar] [CrossRef]
- Lloyd, R.V. Endocrine Pathology: Differential Diagnosis and Molecular Advances; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- DeLellis, R.A. Pathology and Genetics of Tumours of Endocrine Organs; IARC: Lyon, France, 2004; Volume 8. [Google Scholar]
- Daniels, G.H. Follicular thyroid carcinoma: A perspective. Thyroid® 2018, 28, 1229–1242. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Sykiotis, G.P.; La Rosa, S.; Bisig, B.; Trimech, M.; Missiaglia, E.; Gremaud, M.; Salvatori Chappuis, V.; De Vito, C.; Sciarra, A. Macrofollicular variant of follicular thyroid carcinoma: A rare underappreciated pitfall in the diagnosis of thyroid carcinoma. Thyroid® 2020, 30, 72–80. [Google Scholar] [CrossRef]
- Feldman, R.P.; Goodrich, J.T. The edwin smith surgical papyrus. Child’s Nerv. Syst. 1999, 15, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, M.; Shaner, A.; Schaefer, S.D. The Edwin Smith Papyrus: The birth of analytical thinking in medicine and otolaryngology. Laryngoscope 2006, 116, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Adams, F. The Genuine Works of Hippocrates; W. Wood: New York, NY, USA, 1886; Volume 1. [Google Scholar]
- Scarani, P. The men who influenced morgagni as anatomist. In Proceedings of the 22nd European Congress of Pathology, Florence, Italy, 4–9 September 2009; Update in Pathology. European Society of Pathology: Brussels, Belgium, 2009. [Google Scholar]
- Worni, M.; Sosa, J.A. Emil Theodor Kocher: 1841–1917. In Surgical Endocrinopathies: Clinical Management and the Founding Figures; Springer: New York, NY, USA, 2015; pp. 13–16. [Google Scholar]
- Söderberg, U. Temporal characteristics of thyroid activity. Physiol. Rev. 1959, 39, 777–810. [Google Scholar] [CrossRef] [PubMed]
- FrancisGary, L.; WaguespackSteven, G.; BauerAndrew, J.; CeruttiJanete, M.; DinauerCatherine, A.; HayIan, D.; ParisiMarguerite, T.; ThompsonGeoffrey, B. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015, 25, 713–868. [Google Scholar]
- Mitchell, A.; Gandhi, A.; Scott-Coombes, D.; Perros, P. Management of thyroid cancer: United Kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 2016, 130, S150–S160. [Google Scholar] [CrossRef]
- Assmus, A. Early history of X rays. Beam Line 1995, 25, 10–24. [Google Scholar]
- Wickbom, I.; Zachrisson, B.; Heimann, P. Thyroid angiography. Acta Radiol. Diagn. 1967, 6, 497–512. [Google Scholar] [CrossRef]
- Atkins, H.L. Technetium-99m pertechnetate uptake and scanning in the evaluation of thyroid function. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Watanabe, K.; Igarashi, T.; Ashida, H.; Ogiwara, S.; Ohta, T.; Uchiyama, M.; Ojiri, H. Diagnostic value of ultrasonography and TI-201/Tc-99m dual scintigraphy in differentiating between benign and malignant thyroid nodules. Endocrine 2019, 63, 301–309. [Google Scholar] [CrossRef]
- De Groot, L.J.; Jameson, J.L. Thyroid imaging. In Endocrinology Adult and Pediatric: The Thyroid Gland; Elsevier: Philadelphia, PA, USA, 2013; pp. e152–e170. [Google Scholar]
- Moreno-Reyes, R.; Kyrilli, A.; Lytrivi, M.; Bourmorck, C.; Chami, R.; Corvilain, B. Is there still a role for thyroid scintigraphy in the workup of a thyroid nodule in the era of fine needle aspiration cytology and molecular testing? F1000Research 2016, 5, 763. [Google Scholar] [CrossRef][Green Version]
- Broome, M.R. Thyroid scintigraphy in hyperthyroidism. Clin. Tech. Small Anim. Pract. 2006, 21, 10–16. [Google Scholar] [CrossRef]
- Schober, B.; Hunt, J. Evaluation of the normal range of values for uptake of radioactive iodine by the thyroid gland. Can. Med. Assoc. J. 1976, 115, 29. [Google Scholar] [PubMed]
- Kim, P.D.; Tran, H.D. I-123 Uptake. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Meier, D.A.; Kaplan, M.M. Radioiodine uptake and thyroid scintiscanning. Endocrinol. Metab. Clin. N. Am. 2001, 30, 291–313. [Google Scholar] [CrossRef]
- Poller, D.N.; Baloch, Z.W.; Fadda, G.; Johnson, S.J.; Bongiovanni, M.; Pontecorvi, A.; Cochand-Priollet, B. Thyroid FNA: New classifications and new interpretations. Cancer Cytopathol. 2016, 124, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Yoel, U.; Angel, S.; Bosin, E.; Elezra, M.; Marks, R.S.; Fraenkel, M. Thyroglobulin Point of Care Assay for Rapid Detection of Metastatic Differentiated Thyroid Carcinoma-A Pilot Study. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2023. [Google Scholar]
- Liu, Q.; Mao, L.; Zhang, Z.; Li, G.; Song, H. Diagnostic efficacy of FNA-Tg in DTC cervical LN metastasis and its impact factors: A large retrospective study. J. Clin. Endocrinol. Metab. 2023, 108, 3311–3319. [Google Scholar] [CrossRef]
- Achille, G.; Garrisi, V.M.; Russo, S.; Guastamacchia, E.; Giagulli, V.A.; Schirosi, L.; Daniele, A.; Tufaro, A.; Cafagna, V.; Centrone, M.; et al. Thyroglobulin determination in fine needle aspiration biopsy washout of suspicious lymph nodes in thyroid carcinoma follow up. Endocr. Metab. Immune Disord.-Drug Targets 2017, 17, 213–218. [Google Scholar] [CrossRef]
- Sorrenti, S.; Ulisse, S.; DI GIOIA, C.R.T.; Ascoli, V.; Bosco, D.; Rullo, E.; Catania, A.; Tartaglia, F.; Carbotta, G.; Nicola, C. Thyroglobulin measurement in the washout of fine needle aspirates for the diagnosis of suspicious cervical lymph nodes. CPQ Cancer 2019, 1, 1–12. [Google Scholar]
- Sinclair, C.F.; Baek, J.H.; Hands, K.E.; Hodak, S.P.; Huber, T.C.; Hussain, I.; Lang, B.H.-H.; Noel, J.E.; Papaleontiou, M.; Patel, K.N. General principles for the safe performance, training, and adoption of ablation techniques for benign thyroid nodules: An American Thyroid Association statement. Thyroid® 2023, 33, 1150–1170. [Google Scholar] [CrossRef]
- Kocjan, G. Fine Needle Aspiration Cytology: Diagnostic Principles and Dilemmas; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Wuertz, F.G.; Kresnik, E.; Malle, P.; Hyden, M.; Lind, P.; Rogatsch, H.; Gallowitsch, H.J. Fine-needle aspiration with immunohistochemistry using a modified scrape cell block technique for the diagnosis of thyroid and parathyroid nodules. Acta Cytol. 2016, 60, 118–130. [Google Scholar] [CrossRef]
- Trimboli, P.; D’Aurizio, F.; Tozzoli, R.; Giovanella, L. Measurement of thyroglobulin, calcitonin, and PTH in FNA washout fluids. cclm 2017, 55, 914–925. [Google Scholar] [CrossRef]
- Liu, R.-B.; Zhou, D.-L.; Xu, B.-H.; Yang, X.-H.; Liu, Q.; Zhang, X.; Tang, T.; Ye, Z.-L.; Li, Y. Comparison of the diagnostic performances of US-guided fine needle aspiration cytology and thyroglobulin measurement for lymph node metastases in patients with differentiated thyroid carcinoma: A meta-analysis. Eur. Radiol. 2021, 31, 2903–2914. [Google Scholar] [CrossRef]
- Bernier, M.; Moisan, C.; Mansour, G.; Aurengo, A.; Ménégaux, F.; Leenhardt, L. Usefulness of fine needle aspiration cytology in the diagnosis of loco-regional recurrence of differentiated thyroid carcinoma. Eur. J. Surg. Oncol. (EJSO) 2005, 31, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, Y.; Li, H.; Yue, K.; Liu, J.; Lai, Q.; Zhou, M.; Ye, B.; Wu, Y.; Zhu, J. Detection of thyroglobulin in fine-needle aspiration for diagnosis of metastatic lateral cervical lymph nodes in papillary thyroid carcinoma: A retrospective study. Front. Oncol. 2022, 12, 909723. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, D.; Wu, W.; Jiang, J.; Xi, C.; Ye, N.; Wang, Y.; Xu, X. Diagnostic value of cytology, thyroglobulin, and combination of them in fine-needle aspiration of metastatic lymph nodes in patients with differentiated thyroid cancer: A systematic review and network meta-analysis. Medicine 2019, 98, e17859. [Google Scholar] [CrossRef] [PubMed]
- Rivera, G.R.; Segura, S.; Suhrland, M. Educational Case: Thyroid Neoplasms: Pathogenesis, Diagnosis, and Treatment. Acad. Pathol. 2018, 5, 2374289518777471. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Martorana, F.; Pennisi, M.S.; Stella, S.; Massimino, M.; Tirrò, E.; Vitale, S.R.; Di Gregorio, S.; Puma, A.; Tomarchio, C. Opportunities and challenges of liquid biopsy in thyroid cancer. Int. J. Mol. Sci. 2021, 22, 7707. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, F. Application of immunohistochemistry in thyroid pathology. Arch. Pathol. Lab. Med. 2015, 139, 67–82. [Google Scholar] [CrossRef]
- Abd Elmageed, Z.Y.; Sholl, A.B.; Tsumagari, K.; Al-Qurayshi, Z.; Basolo, F.; Moroz, K.; Boulares, A.H.; Friedlander, P.; Miccoli, P.; Kandil, E. Immunohistochemistry as an accurate tool for evaluating BRAF-V600E mutation in 130 samples of papillary thyroid cancer. Surgery 2017, 161, 1122–1128. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, C.-S.; Huang, C.-C.; Ku, W.-C.; Shih, H.-Y.; Huang, C.-J. Co-occurrence of differentiated thyroid cancer and second primary malignancy: Correlation with expression profiles of mismatch repair protein and cell cycle regulators. Cancers 2021, 13, 5486. [Google Scholar] [CrossRef]
- Crescenzi, A.; Baloch, Z. Immunohistochemistry in the pathologic diagnosis and management of thyroid neoplasms. Front. Endocrinol. 2023, 14, 1198099. [Google Scholar] [CrossRef]
- Song, S.; Kim, H.; Ahn, S.-H. Role of immunohistochemistry in fine needle aspiration and core needle biopsy of thyroid nodules. Clin. Exp. Otorhinolaryngol. 2019, 12, 224. [Google Scholar] [CrossRef]
- Kresak, A.M. The Technological History of Immunohistochemical Methods and Applications in Clinical Cancer Diagnosis and Research. Master’s Thesis, Case Western Reserve University, Cleveland, OH, USA, 2018. [Google Scholar]
- Policardo, F.; Tralongo, P.; Feraco, A.; Vegni, F.; Carlino, A.; Pontecorvi, A.; Lombardi, C.P.; Raffaelli, M.; Pierconti, F.; Larocca, L.M.; et al. Immunocytochemistry in thyroid cytology and its multiple roles: A systematic review. Diagn. Histopathol. 2023, 29, 386–395. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kim, E.-K. Ultrasound elastography for thyroid nodules: Recent advances. Ultrasonography 2014, 33, 75. [Google Scholar] [CrossRef] [PubMed]
- Fulgham, P.F. Physical principles of ultrasound. In Practical Urological Ultrasound; Springer: Cham, Switzerland, 2021; pp. 13–30. [Google Scholar]
- Frunzac, R.W.; Richards, M. Computed tomography and magnetic resonance imaging of the thyroid and parathyroid glands. Imaging Endocr. Disord. 2016, 45, 16–23. [Google Scholar]
- Abraham, J. Imaging for head and neck cancer. Surg. Oncol. Clin. 2015, 24, 455–471. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Lee, C.; De Gonzalez, A.B. Medical exposure to radiation and thyroid cancer. Clin. Oncol. 2011, 23, 244–250. [Google Scholar] [CrossRef]
- Elangovan, A.; Jeyaseelan, T. Medical imaging modalities: A survey. In Proceedings of the 2016 International Conference on Emerging Trends in Engineering, Technology and Science (ICETETS), Pudukkottai, India, 24–26 February 2016. [Google Scholar]
- Miyakoshi, A.; Dalley, R.W.; Anzai, Y. Magnetic resonance imaging of thyroid cancer. Top. Magn. Reson. Imaging 2007, 18, 293–302. [Google Scholar] [CrossRef]
- Carr, M.W.; Grey, M.L. Magnetic Resonance Imaging: Overview, risks, and safety measures. AJN Am. J. Nurs. 2002, 102, 26–33. [Google Scholar] [CrossRef]
- McDougall, I.; Davidson, J.; Segall, G. Positron emission tomography of the thyroid, with an emphasis on thyroid cancer. Nucl. Med. Commun. 2001, 22, 485–492. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Agress, H., Jr.; Goldsmith, S.J. Clinical role of FDG PET in evaluation of cancer patients. RadioGraphics 2003, 23, 315–340. [Google Scholar] [CrossRef]
- Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef]
- Beckmann, S.; Simanowski, J.H. Update in contrast-enhanced ultrasound. Visc. Med. 2020, 36, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Tamas-Szora, A.; Socaciu, M.A.; Badea, A.F.; Opincariu, I.; Badea, R.I. Noninvasive evaluation of microcirculation under normal and pathological conditions using contrast-enhanced ultrasonography (CEUS). In Microcirculation Revisited-From Molecules to Clinical Practice; IntechOpen: London, UK, 2016. [Google Scholar]
- Papini, E.; Pacella, C.M.; Frasoldati, A.; Hegedüs, L. Ultrasonic imaging of the thyroid gland. In Thyroid Cancer: A Comprehensive Guide to Clinical Management; Springer: New York, NY, USA, 2016; pp. 293–314. [Google Scholar]
- Radzina, M.; Ratniece, M.; Putrins, D.S.; Saule, L.; Cantisani, V. Performance of contrast-enhanced ultrasound in thyroid nodules: Review of current state and future perspectives. Cancers 2021, 13, 5469. [Google Scholar] [CrossRef] [PubMed]
- Nachiappan, A.C.; Metwalli, Z.A.; Hailey, B.S.; Patel, R.A.; Ostrowski, M.L.; Wynne, D.M. The thyroid: Review of imaging features and biopsy techniques with radiologic-pathologic correlation. RadioGraphics 2014, 34, 276–293. [Google Scholar] [CrossRef]
- Ter-Pogossian, M.M. Basic principles of computed axial tomography. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Coca-Pelaz, A.; Rodrigo, J.P.; Shah, J.P.; Nixon, I.J.; Hartl, D.M.; Robbins, K.T.; Kowalski, L.P.; Mäkitie, A.A.; Hamoir, M.; López, F. Recurrent differentiated thyroid cancer: The current treatment options. Cancers 2023, 15, 2692. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, R.; Liu, W.; Chen, Y.; Song, B. Diagnostic efficacy of multiple MRI parameters in differentiating benign vs. malignant thyroid nodules. BMC Med. Imaging 2018, 18, 50. [Google Scholar] [CrossRef]
- Rufini, V.; Baum, R.P.; Castaldi, P.; Treglia, G.; De Gaetano, A.M.; Carreras, C.; Kaemmerer, D.; Hommann, M.; Hörsch, D.; Bonomo, L. Role of PET/CT in the functional imaging of endocrine pancreatic tumors. Abdom. Imaging 2012, 37, 1004–1020. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Nikiforov, Y.E. Molecular diagnostics and predictors in thyroid cancer. Thyroid® 2009, 19, 1351–1361. [Google Scholar] [CrossRef]
- Zatelli, M.C.; Trasforini, G.; Leoni, S.; Frigato, G.; Buratto, M.; Tagliati, F.; Rossi, R.; Cavazzini, L.; Roti, E.; degli Uberti, E.C. BRAF V600E mutation analysis increases diagnostic accuracy for papillary thyroid carcinoma in fine-needle aspiration biopsies. Eur. J. Endocrinol. 2009, 161, 467–473. [Google Scholar] [CrossRef]
- Kim, J.-K.; Seong, C.Y.; Bae, I.E.; Yi, J.W.; Yu, H.W.; Kim, S.-J.; Won, J.-K.; Chai, Y.J.; Choi, J.Y.; Lee, K.E. Comparison of immunohistochemistry and direct sequencing methods for identification of the BRAF V600E mutation in papillary thyroid carcinoma. Ann. Surg. Oncol. 2018, 25, 1775–1781. [Google Scholar] [CrossRef]
- Attia, A.S.; Hussein, M.; Issa, P.P.; Elnahla, A.; Farhoud, A.; Magazine, B.M.; Youssef, M.R.; Aboueisha, M.; Shama, M.; Toraih, E.; et al. Association of BRAFV600E mutation with the aggressive behavior of papillary thyroid microcarcinoma: A meta-analysis of 33 studies. Int. J. Mol. Sci. 2022, 23, 15626. [Google Scholar] [CrossRef]
- Melck, A.L.; Yip, L.; Carty, S.E. The utility of BRAF testing in the management of papillary thyroid cancer. The Oncologist 2010, 15, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.R.; Bentz, B.G.; Bentz, J.S. Detection of BRAF V600E activating mutation in papillary thyroid carcinoma using PCR with allele-specific fluorescent probe melting curve analysis. J. Clin. Pathol. 2007, 60, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Sebo, T.J.; Nakamura, N.; Qian, X.; Oliveira, A.; Majerus, J.A.; Johnson, M.R.; Lloyd, R.V. BRAF mutation analysis in fine needle aspiration (FNA) cytology of the thyroid. Diagn. Mol. Pathol. 2006, 15, 136–143. [Google Scholar] [CrossRef]
- Kansal, R.; Grody, W.W.; Zhou, J.; Dong, L.; Li, X. The Value of T-Cell Receptor γ (TRG) Clonality Evaluation by Next-Generation Sequencing in Clinical Hematolymphoid Tissues: A Descriptive Study of 41 Cases From a Single Institution. Am. J. Clin. Pathol. 2018, 150, 193–223. [Google Scholar] [CrossRef]
- Salk, J.J.; Schmitt, M.W.; Loeb, L.A. Enhancing the accuracy of next-generation sequencing for detecting rare and subclonal mutations. Nat. Rev. Genet. 2018, 19, 269–285. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Next-generation sequencing in thyroid cancer. J. Transl. Med. 2016, 14, 322. [Google Scholar] [CrossRef]
- Capdevila, J.; Matos Garcia, I.; Mancuso, F.M.; Iglesias, C.; Nuciforo, P.; Zafon, C.; Palmer, H.G.; Ogbah, Z.; Muiños, L.; Pena, C.E. RNAseq Analysis of the Sorafenib Phase III Decision Trial in Differentiated Thyroid Cancer (DTC): Correlation with Clinical Outcome; American Society of Clinical Oncology: Alexandria, VA, USA, 2017. [Google Scholar]
- Kukurba, K.R.; Montgomery, S.B. RNA sequencing and analysis. Cold Spring Harb. Protoc. 2015, 2015, pdb.top084970. [Google Scholar] [CrossRef]
- Govindarajan, R.; Duraiyan, J.; Kaliyappan, K.; Palanisamy, M. Microarray and its applications. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. 2), S310–S312. [Google Scholar]
- Huang, Y.; Lin, P.; Liao, J.; Liang, F.; Han, P.; Fu, S.; Jiang, Y.; Yang, Z.; Tan, N.; Huang, J. Next-generation sequencing identified that RET variation associates with lymph node metastasis and the immune microenvironment in thyroid papillary carcinoma. BMC Endocr. Disord. 2024, 24, 68. [Google Scholar] [CrossRef]
- Ren, M.; Yao, Q.; Bao, L.; Wang, Z.; Wei, R.; Bai, Q.; Ping, B.; Chang, C.; Wang, Y.; Zhou, X. Diagnostic performance of next-generation sequencing and genetic profiling in thyroid nodules from a single center in China. Eur. Thyroid. J. 2022, 11, e210124. [Google Scholar] [CrossRef]
- Tarasova, V.D.; Tsai, J.; Masannat, J.; Hernandez Prera, J.C.; Hallanger Johnson, J.; Veloski, C.; Agosto Salgado, S.; McIver, B.; Drusbosky, L.M.; Chung, C.H. Characterization of the Thyroid Cancer Genomic Landscape by Plasma-Based Circulating Tumor DNA Next-Generation Sequencing. Thyroid® 2024, 34, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, E.; Hobo, Y.; Miyauchi, A.; Ito, Y.; Higuchi, M.; Hirokawa, M.; Ito, M.; Fukata, S.; Nishikawa, M.; Akamizu, T. Serum thyroglobulin evaluation on LC-MS/MS and immunoassay in TgAb-positive patients with papillary thyroid carcinoma. Eur. Thyroid. J. 2022, 11, e210041. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Zoerner, A.A. Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: A historical retrospect and a discussion. J. Chromatogr. B 2014, 964, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shuford, C.M.; Johnson, J.S.; Thompson, J.W.; Holland, P.L.; Hoofnagle, A.N.; Grant, R.P. More sensitivity is always better: Measuring sub-clinical levels of serum thyroglobulin on a µLC–MS/MS system. Clin. Mass Spectrom. 2020, 15, 29–35. [Google Scholar] [CrossRef]
- Leung, K.S.-Y.; Fong, B.M.-W. LC–MS/MS in the routine clinical laboratory: Has its time come? Anal. Bioanal. Chem. 2014, 406, 2289–2301. [Google Scholar] [CrossRef]
- Massolt, E.T.; van der Windt, M.; Korevaar, T.I.; Kam, B.L.; Burger, J.; Franssen, G.J.; Lehmphul, I.; Köhrle, J.; Visser, W.E.; Peeters, R.P. Thyroid hormone and its metabolites in relation to quality of life in patients treated for differentiated thyroid cancer. Clin. Endocrinol. 2016, 85, 781–788. [Google Scholar] [CrossRef]
- Fernández-Calle, P.; Díaz-Garzón, J.; Bartlett, W.; Sandberg, S.; Braga, F.; Beatriz, B.; Carobene, A.; Coskun, A.; Gonzalez-Lao, E.; Marques, F.; et al. Biological variation estimates of thyroid related measurands–meta-analysis of BIVAC compliant studies. cclm 2022, 60, 483–493. [Google Scholar] [CrossRef]
- Bartalena, L.; Bogazzi, F.; Brogioni, S.; Burelli, A.; Scarcello, G.; Martino, E. Measurement of serum free thyroid hormone concentrations: An essential tool for the diagnosis of thyroid dysfunction. Horm. Res. 1996, 45, 142–147. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, B.J.; Lee, J.C.; Song, S.H.; Kim, B.H.; Son, S.M.; Kim, I.J.; Kim, Y.K.; Kang, Y.H. Preoperative serum thyroid stimulating hormone levels in well-differentiated thyroid carcinoma is a predictive factor for lateral lymph node metastasis as well as extrathyroidal extension in Korean patients: A single-center experience. Endocrine 2011, 39, 259–265. [Google Scholar] [CrossRef]
- He, L.-Z.; Zeng, T.-S.; Pu, L.; Pan, S.-X.; Xia, W.-F.; Chen, L.-L. Thyroid hormones, autoantibodies, ultrasonography, and clinical parameters for predicting thyroid cancer. Int. J. Endocrinol. 2016, 2016, 8215834. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.M.; Basolo, F. NanoString in the screening of genetic abnormalities associated with thyroid cancer. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Eltzov, E.; Guttel, S.; Low Yuen Kei, A.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays–from paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Axelrod, T.; Eltzov, E.; Marks, R.S. Capture-layer lateral flow immunoassay: A new platform validated in the detection and quantification of dengue NS1. ACS Omega 2020, 5, 10433–10440. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Wang, S. Progress and prospects of elastography techniques in the evaluation of fibrosis in chronic liver disease. Arch. Med. Sci. 2024, 20, 1–9. [Google Scholar] [CrossRef]
- Wijewardene, A.A.; Chehade, M.; Gild, M.L.; Clifton-Bligh, R.J.; Bullock, M. Translational utility of liquid biopsies in thyroid cancer management. Cancers 2021, 13, 3443. [Google Scholar] [CrossRef] [PubMed]
- Assi, T.; Khoury, R.; Ibrahim, R.; Baz, M.; Ibrahim, T.; Cesne, A.L. Overview of the role of liquid biopsy in cancer management. Transl. Oncol. 2023, 34, 101702. [Google Scholar] [CrossRef]
- Abou Daya, S.; Mahfouz, R. Circulating tumor DNA, liquid biopsy, and next generation sequencing: A comprehensive technical and clinical applications review. Meta Gene 2018, 17, 192–201. [Google Scholar] [CrossRef]
- Hashimoto, S.; Bando, H.; Noguchi, E.; Kondo, Y.; Ueda, A.; Terasaki, A.; Okazaki, M.; Tsushima, Y.; Ichioka, E.; Iguchi-Manaka, A. Gene Expression Profiling of Anaplastic Thyroid Carcinoma and Coexisting Differentiated Thyroid Carcinoma Using the NanoString nCounter System; Wolters Kluwer Health: Philadelphia, PA, USA, 2021. [Google Scholar]
- Tsang, H.-F.; Xue, V.W.; Koh, S.-P.; Chiu, Y.-M.; Ng, L.P.-W.; Wong, S.-C.C. NanoString, a novel digital color-coded barcode technology: Current and future applications in molecular diagnostics. Expert Rev. Mol. Diagn. 2017, 17, 95–103. [Google Scholar] [CrossRef]
- Brandenstein, M.; Wiesinger, I.; Künzel, J.; Hornung, M.; Stroszczynski, C.; Jung, E.-M. Multiparametric sonographic imaging of thyroid lesions: Chances of B-mode, elastography and CEUS in relation to preoperative histopathology. Cancers 2022, 14, 4745. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing biosensors with machine learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Paul, A.A.; Aladese, A.D.; Marks, R.S. Additive manufacturing applications in biosensors technologies. Biosensors 2024, 14, 60. [Google Scholar] [CrossRef]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Essays Biochem. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Ribeiro, P.A.; Canário, A.V.; Raposo, M. Biosensors based on stanniocalcin-1 protein antibodies thin films for prostate cancer diagnosis. Biosensors 2023, 13, 981. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xu, H.; Fan, C. Potential diagnostic applications of biosensors: Current and future directions. Int. J. Nanomed. 2006, 1, 433–440. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Dorraji, P.S.; Fotouhi, L.; Hosseini, M.; Khatami, F.; Moazami, H.R.; Omidfar, K.J.S.; Chemical, A.B. Enhanced electrochemiluminescence biosensing of gene-specific methylation in thyroid cancer patients’ plasma based integrated graphitic carbon nitride-encapsulated metal-organic framework nanozyme optimized by central composite design. Sens. Actuators B Chem. 2022, 364, 131895. [Google Scholar] [CrossRef]
- Zhuge, B.; Lin, F. Detection of calcitonin in medullary thyroid carcinoma by an electrochemical sensor. Int. J. Electrochem. Sci. 2017, 12, 10129–10139. [Google Scholar]
- Costante, G.; Durante, C.; Francis, Z.; Schlumberger, M.; Filetti, S. Determination of calcitonin levels in C-cell disease: Clinical interest and potential pitfalls. Nat. Clin. Pr. Endocrinol. Metab. 2009, 5, 35–44. [Google Scholar] [CrossRef]
- Koopman, T.; Niedlich-den Herder, C.; Stegeman, C.A.; Links, T.P.; Bijzet, J.; Hazenberg, B.P.; Diepstra, A. Kidney involvement in systemic calcitonin amyloidosis associated with medullary thyroid carcinoma. Am. J. Kidney Dis. 2017, 69, 546–549. [Google Scholar] [CrossRef]
- Batista, R.L.; Toscanini, A.C.; Brandão, L.G.; Cunha-Neto, M.B.C. False positive results using calcitonin as a screening method for medullary thyroid carcinoma. Indian J. Endocrinol. Metab. 2013, 17, 524–528. [Google Scholar] [CrossRef]
- Salma, U.; Nasreen, F.; Jabin, P.; Roy, S.; Azam, M.N. Association of High TPO Antibody Titer with Differentiated Thyroid Carcinoma: A Case-Control Study. Bangladesh J. Nucl. Med. 2023, 26, 34–38. [Google Scholar] [CrossRef]
- Lubin, I.M.; Astles, J.R.; Shahangian, S.; Madison, B.; Parry, R.; Schmidt, R.L.; Rubinstein, M.L. Bringing the clinical laboratory into the strategy to advance diagnostic excellence. Diagnosis 2021, 8, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Shonka, D.C., Jr.; Ho, A.; Chintakuntlawar, A.V.; Geiger, J.L.; Park, J.C.; Seetharamu, N.; Jasim, S.; Abdelhamid Ahmed, A.H.; Bible, K.C.; Brose, M.S.; et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head Neck 2022, 44, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guan, S.; Ou, Z.; Li, W.; Yan, L.; Situ, B. Advances in AI-based cancer cytopathology. Interdiscip. Med. 2023, 1, e20230013. [Google Scholar] [CrossRef]
- Plebani, M.; Lippi, G. Integrated Diagnostics: The Future of Diagnostic Medicine? In Integrated Diagnostics and Theranostics of Thyroid Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–4. [Google Scholar]
- Baric-Parker, J.; Anderson, E.E. Patient data-sharing for AI: Ethical challenges, catholic solutions. Linacre Q. 2020, 87, 471–481. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Abernethy, A.P. Financial toxicity, part I: A new name for a growing problem. Oncology 2013, 27, 80. [Google Scholar]
- Shah, S.C.; Kayamba, V.; Peek, R.M., Jr.; Heimburger, D. Cancer control in low-and middle-income countries: Is it time to consider screening? J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Baek, H.-S.; Ha, J.; Kim, K.; Bae, J.S.; Kim, J.S.; Kim, S.; Lim, D.-J.; Kim, C.-M. Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population. Endocrinol. Metab. 2024, 39, 310–323. [Google Scholar] [CrossRef]
- Association, A.T. Fine-Needle Aspiration of Thyroid Nodules. 2024. Available online: https://www.thyroid.org/fna-thyroid-nodules/ (accessed on 20 October 2024).
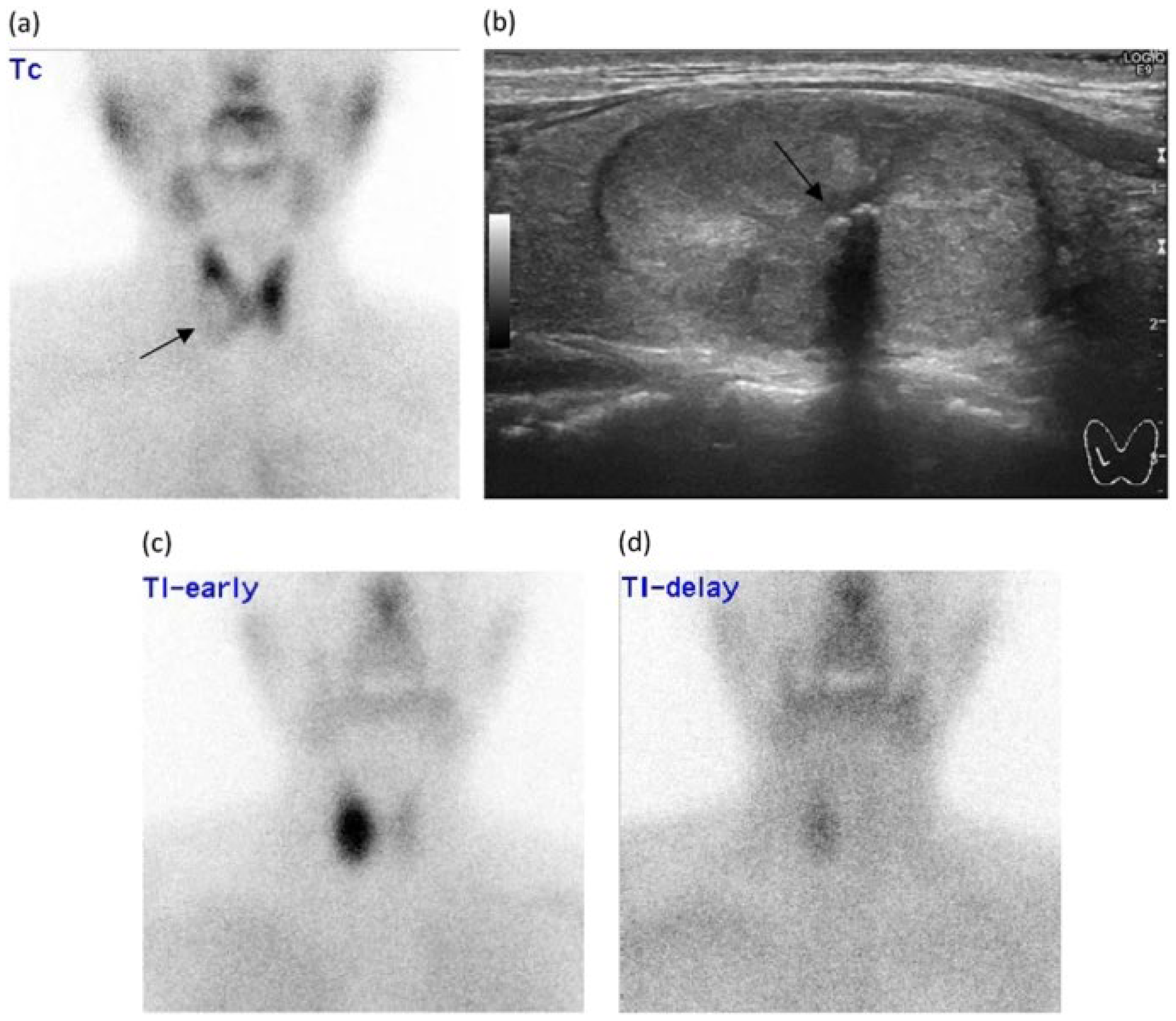
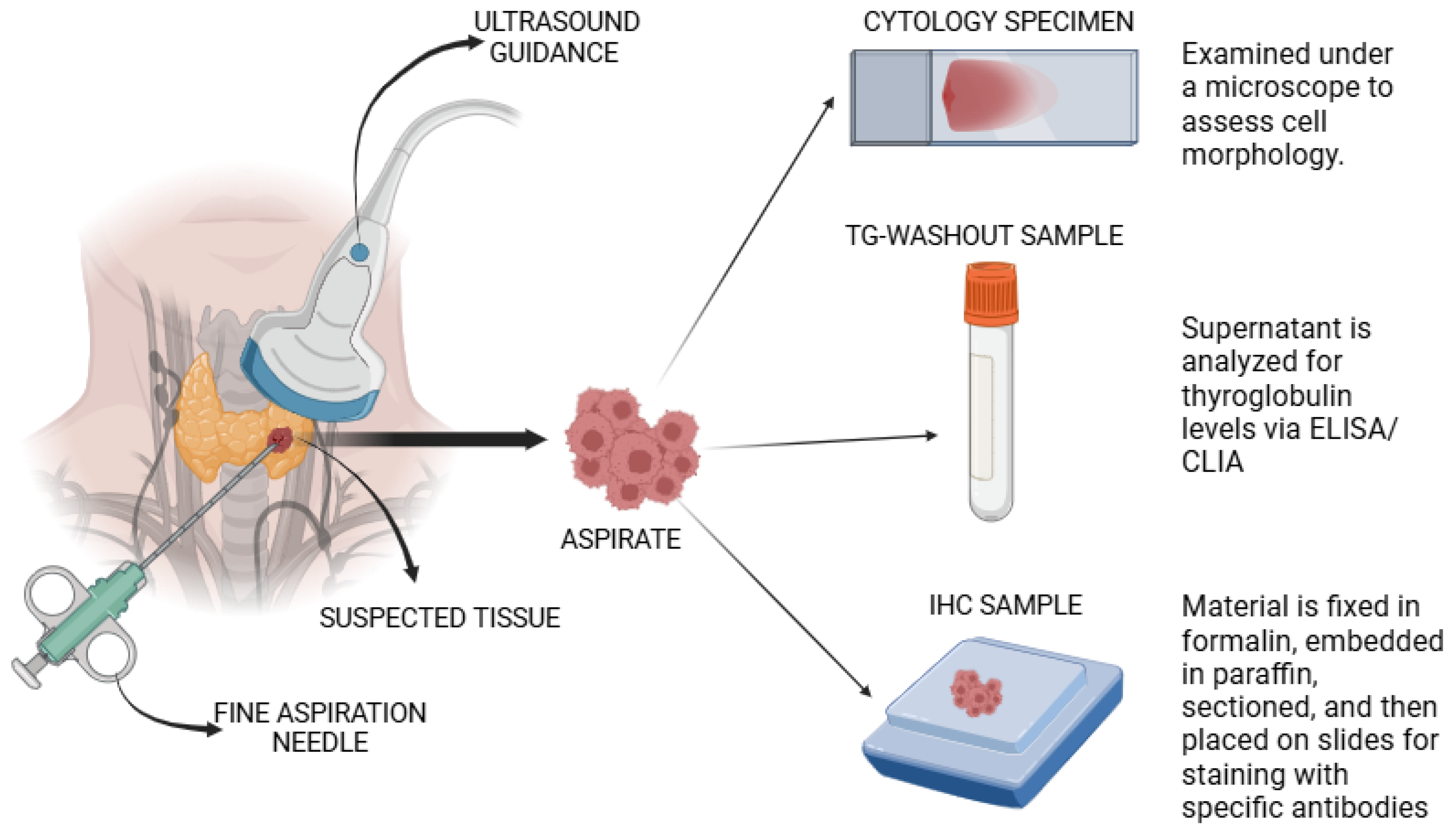
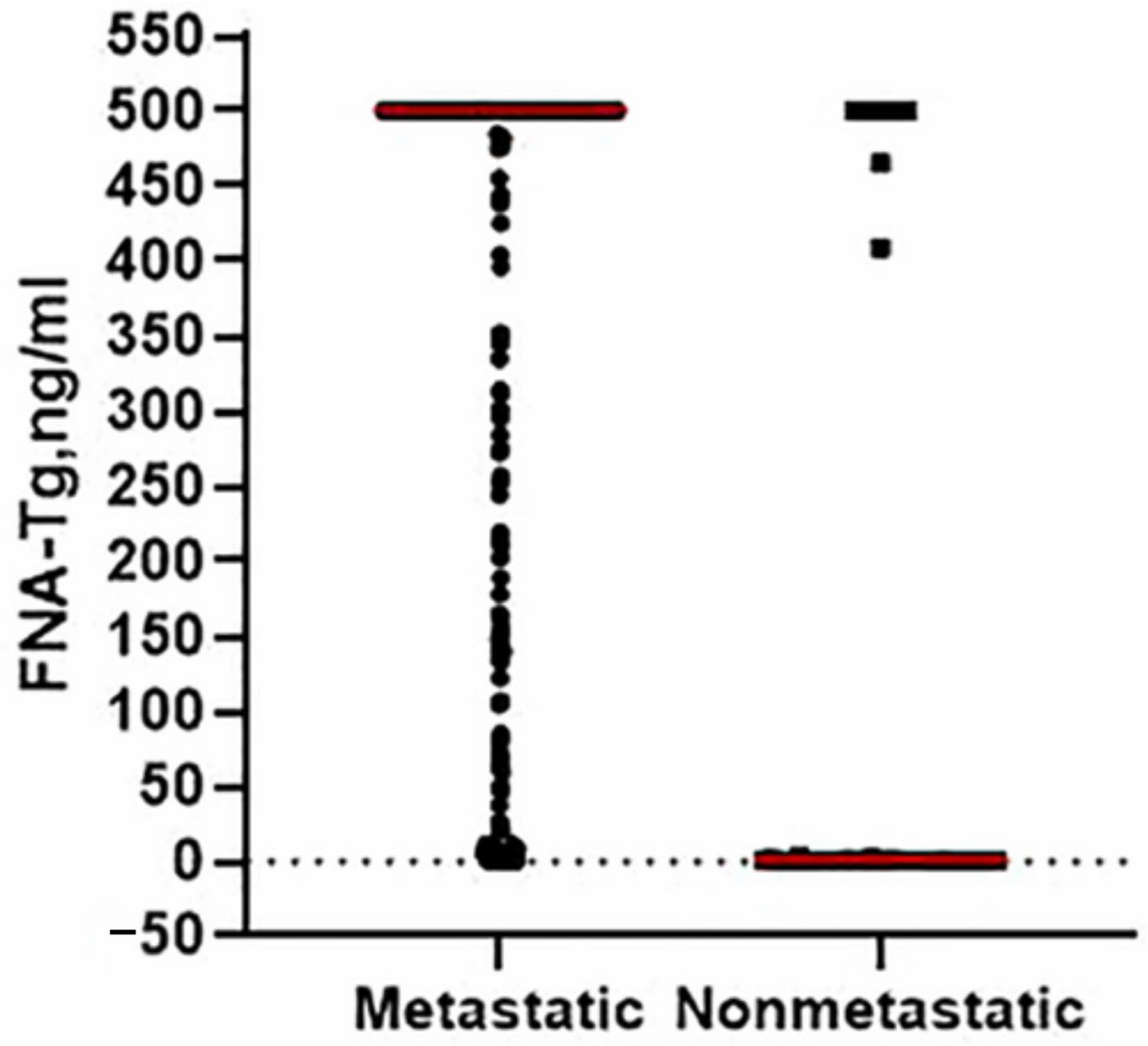
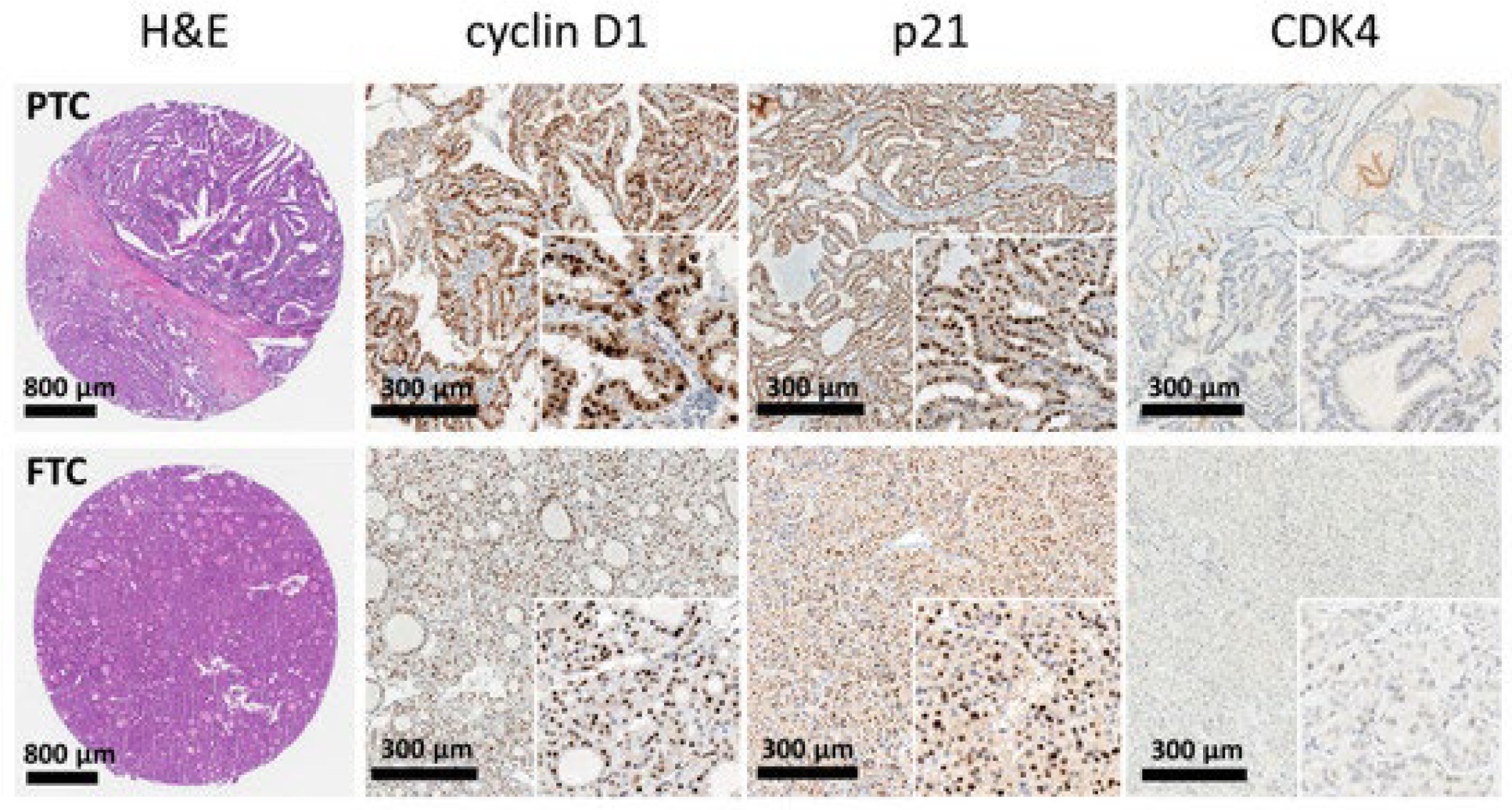


| Imaging Modalities | Technique | Diagnostic Use | Advantages | Limitations | Cost | References |
|---|---|---|---|---|---|---|
| Ultrasound | High-frequency sound waves directed at the thyroid, captured by transducer. | Guiding FNA, visualization of nodules and categorization of risk of malignancy prior FNA. | Painless; non-invasive; no ionizing radiation; real-time visualization. | Limited in assessing deeper nodules, limited sensitivity, and specificity. | Low | [48,76,77] |
| Computed tomography | Based on X-rays, created cross-section image of the thyroid and surrounding tissues. | Complements US results and enhances diagnosis in the case of deep nodules. | Detailed imaging, useful in complex cases. | Ionizing radiation (X-rays) risk of radiation-induced malignancies over time. | Moderate to high | [78,79,80] |
| Magnetic Resonance Imaging | Employs magnetic field and radio waves for detailed imaging. | Evaluates size, location, and characteristics of thyroid nodules. | No ionizing radiations, safe for follow-ups. | Unsafe for patients with metal devices or implants. | High | [81,82,83] |
| Positron Emission Tomography | Detects gamma rays emitted by the annihilation of an electron by the positron emitted by a radiotracer. | Used when metastasis to distant sites is suspected or in cases of recurrent DTC. | High sensitivity for detecting cancerous cells provides functional information for metabolic activity. | Not practical for tumors with low metabolic activity. Ionizing radiation (gamma rays). | High | [84,85,86] |
| Molecular Techniques | Mechanism of DTC Detection | Diagnostic Purpose | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Genetic mutation analysis (BRA, RAS) | LC-PCR; RT-PCR; qPCR; RFLP, etc. | Guides personalized therapy, confirms diagnosis, and predicts prognosis | High specificity: useful for prognosis and guided targeted therapy | Requires high-quality samples, specific only for one said mutation | [97,98,99,100,101,102] |
| Next Generation Sequencing | NGS testing of a panel of genes associated with DTC | Comprehensive gene analysis, detection of rare mutations | Early detection, personalized treatment, patient stratification | Costly; resource-intensive; review by a pathologist required | [103,104,105] |
| Gene expression & profiling | RNA sequencing, microarrays | Identify gene expression patterns | Enhanced diagnostic accuracy; personalized treatment strategies. | Costly; accessibility; interpretation challenges; skilled personnel. | [106,107,108] |
| Recent Technologies | Technique | Diagnostic Purpose | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Liquid Biopsy | PCR-based or NGS-based analysis. | Early detection; monitoring; assessing treatment response. | Non-invasive, using a single sample of blood or saliva. | Requires sensitive methods; need for reliability and standardization. | [68] |
| NanoString | Hybridization-based approach, digital barcoding. | Molecular subtyping of DTC, genetic expression profiling. | High sensitivity and specificity, minimal sample input required. | Requires bioinformatics expertise, initial investment, and operational costs. | [121] |
| POC-Tg | Lateral flow immunoassay | Rapid detection of Tg assessing metastatic LNs. | Rapid; low cost; portable; non-invasive | Higher sensitivity required for patients with recurrent DTC; increased stability required. | [55,122,123] |
| Elastography | Differentiation between benign and malign thyroid nodules. | Measures tissue elasticity and stiffness to detect malignancy. | Non-invasive, real-time assessment. | Specialized equipment required; lower sensitivity/specificity. | [76,124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristollari, K.; Paul, A.A.; Angel, S.; Marks, R.S. Conventional and Emerging Diagnostic Approaches for Differentiated Thyroid Carcinoma. Chemosensors 2024, 12, 229. https://doi.org/10.3390/chemosensors12110229
Kristollari K, Paul AA, Angel S, Marks RS. Conventional and Emerging Diagnostic Approaches for Differentiated Thyroid Carcinoma. Chemosensors. 2024; 12(11):229. https://doi.org/10.3390/chemosensors12110229
Chicago/Turabian StyleKristollari, Kathelina, Abraham Abbey Paul, Sagi Angel, and Robert S. Marks. 2024. "Conventional and Emerging Diagnostic Approaches for Differentiated Thyroid Carcinoma" Chemosensors 12, no. 11: 229. https://doi.org/10.3390/chemosensors12110229
APA StyleKristollari, K., Paul, A. A., Angel, S., & Marks, R. S. (2024). Conventional and Emerging Diagnostic Approaches for Differentiated Thyroid Carcinoma. Chemosensors, 12(11), 229. https://doi.org/10.3390/chemosensors12110229







