The Bray–Liebhafsky Oscillatory Reaction as a Chemosensor for Benzenediols
Abstract
1. Introduction
2. Materials and Methods
2.1. Bray–Liebhafsky Reaction Experimental Setup
2.2. EPR Spectroscopy Measurements of Hydroxyl Radical Scavenging by Benzenediols
2.3. Computational Methods
3. Results
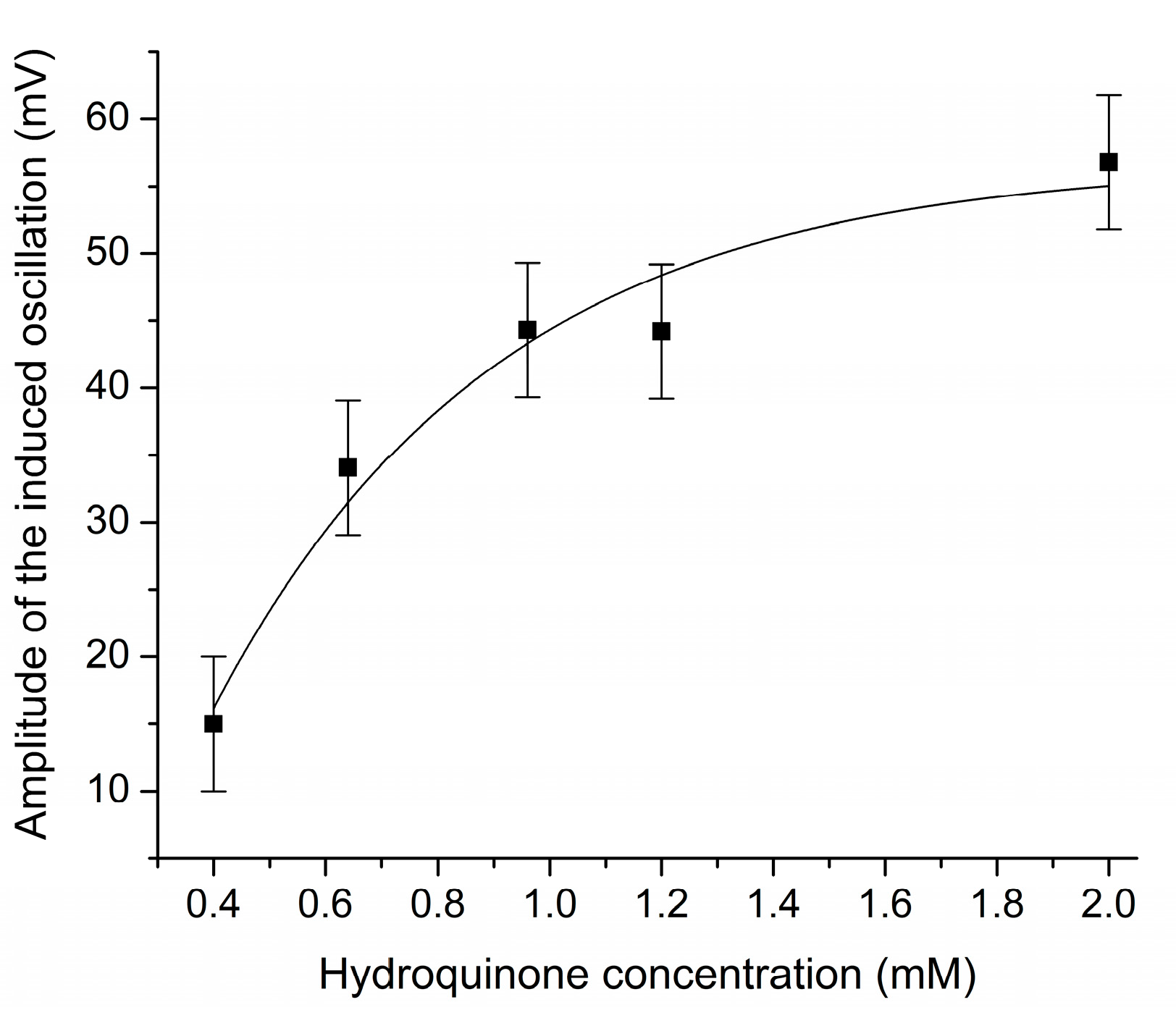
3.1. The Effect of Benzenediol Isomers on Bray–Liebhafsky Reaction
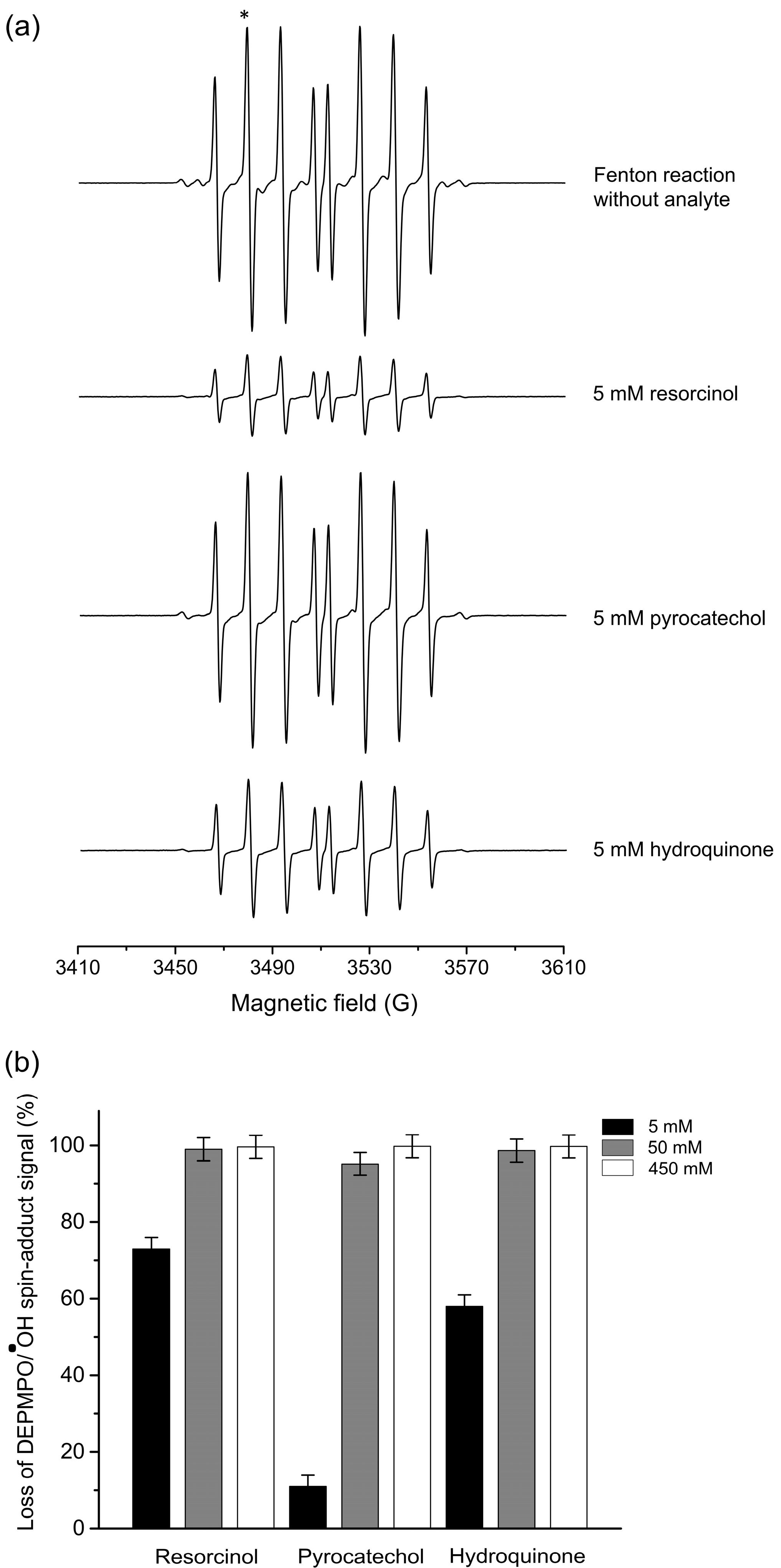
3.2. Hydroxyl Radical Scavenging by Benzenediols—EPR Measurements
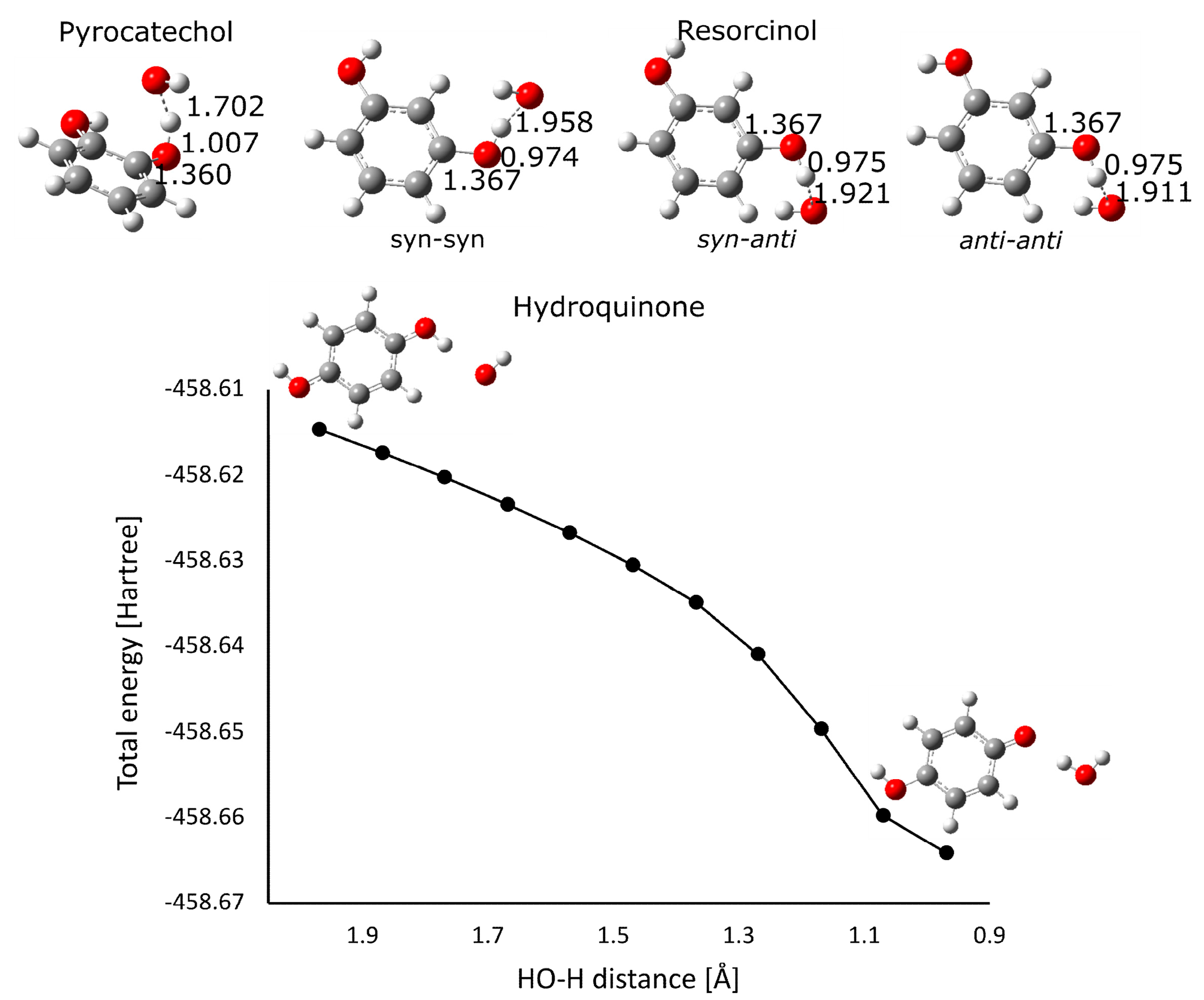
3.3. Computational Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, W.C. A periodic reaction in homogeneous solution and its relation to catalysis. J. Am. Chem. Soc. 1921, 43, 1262–1267. [Google Scholar] [CrossRef]
- Bray, W.C.; Liebhafsky, H.A. Reactions involving hydrogen peroxide, iodine and iodate ion. i. introduction. J. Am. Chem. Soc. 1931, 53, 38–44. [Google Scholar] [CrossRef]
- Sharma, K.R.; Noyes, R.M. Oscillations in Chemical Systems. 13. A Detailed Molecular Mechanism for the Bray-Liebhafsky Reaction of Iodate and Hydrogen Peroxide. J. Am. Chem. Soc. 1976, 98, 4345–4361. [Google Scholar] [CrossRef]
- Kolar-Anić, L.; Schmitz, G. Mechanism of the Bray–Liebhafsky Reaction: Effect of the Oxidation of Iodous Acid by Hydrogen Peroxide. J. Chem. Soc. Faraday Trans. 1992, 88, 2343–2349. [Google Scholar] [CrossRef]
- Treindl, L.; Noyes, R.M. A New Explanation of the Oscillations in the Bray-Liebhafsky Reaction. J. Phys. Chem. 1993, 97, 11354–11362. [Google Scholar] [CrossRef]
- Kolar-Anić, L.; Misljenović, D.; Anić, S.; Nicolis, G. Influence of the Reduction of Iodate Ion by Hydrogen Peroxide on the Model of the Bray-Liebhafsky Reaction. React. Kinet. Catal. Lett. 1995, 54, 35–41. [Google Scholar] [CrossRef]
- Schmitz, G.; Noszticzius, Z.; Hollo, G.; Wittmann, M.; Furrow, S.D. Reactions of Iodate with Iodine in Concentrated Sulfuric Acid. Formation of I(+3) and I(+1) Compounds. Chem. Phys. Lett. 2018, 691, 44–50. [Google Scholar] [CrossRef]
- Noyes, R.M. Some Models of Chemical Oscillators. J. Chem. Educ. 1989, 66, 190. [Google Scholar] [CrossRef][Green Version]
- Kéki, S.; Székely, G.; Beck, M.T. The Effect of Light on the Bray−Liebhafsky Reaction. J. Phys. Chem. A 2003, 107, 73–75. [Google Scholar] [CrossRef]
- Stanisavljev, D.R.; Milenković, M.C.; Popović-Bijelić, A.D.; Mojović, M.D. Radicals in the Bray–Liebhafsky Oscillatory Reaction. J. Phys. Chem. A 2013, 117, 3292–3295. [Google Scholar] [CrossRef]
- Pagnacco, M.C.; Mojović, M.D.; Popović-Bijelić, A.D.; Horváth, A.K. Investigation of the Halogenate–Hydrogen Peroxide Reactions Using the Electron Paramagnetic Resonance Spin Trapping Technique. J. Phys. Chem. A 2017, 121, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Kolar-Anic, L.Z.; Misljenovic, D.M.; Stanisavljev, D.R.; Anic, S.R. Applicability of Schmitz’s Model to Dilution-Reinitiated Oscillations in the Bray-Liebhafsky Reaction. J. Phys. Chem. 1990, 94, 8144–8146. [Google Scholar] [CrossRef]
- Valent, I.; Ševčík, P. Simulations of the Iodine Interphase Transport Effect on the Oscillating Bray−Liebhafsky Reaction. J. Phys. Chem. A 1998, 102, 7576–7579. [Google Scholar] [CrossRef]
- Láňová, B.; Vřešt’ál, J. Study of the Bray−Liebhafsky Reaction by On-Line Mass Spectrometry. J. Phys. Chem. A 2002, 106, 1228–1232. [Google Scholar] [CrossRef]
- Schmitz, G.; Kolar-Anić, L.; Anić, S.; Grozdić, T.; Vukojević, V. Complex and Chaotic Oscillations in a Model for the Catalytic Hydrogen Peroxide Decomposition under Open Reactor Conditions. J. Phys. Chem. A 2006, 110, 10361–10368. [Google Scholar] [CrossRef]
- Ševčík, P.; Adamčíková, L. Effect of a Pressure Decrease and Stirring on the Oscillating Bray-Liebhafsky Reaction. Chem. Phys. Lett. 1997, 267, 307–312. [Google Scholar] [CrossRef]
- Ševčík, P. Effect of a Gas Bubbling and Stirring on the Oscillating Bray−Liebhafsky Reaction. J. Phys. Chem. A 1998, 102, 1288–1291. [Google Scholar] [CrossRef]
- Stanisavljev, D.R.; Djordjević, A.R.; Likar-Smiljanić, V.D. Investigation of Microwave Effects on the Oscillatory Bray–Liebhafsky Reaction. Chem. Phys. Lett. 2005, 412, 420–424. [Google Scholar] [CrossRef]
- Stanisavljev, D.R.; Djordjević, A.R.; Likar-Smiljanić, V.D. Microwaves and Coherence in the Bray–Liebhafsky Oscillatory Reaction. Chem. Phys. Lett. 2006, 423, 59–62. [Google Scholar] [CrossRef]
- Ševčík, P.; Kissimonová, K.; Adamčíková, L. Oxygen Production in the Oscillatory Bray−Liebhafsky Reaction. J. Phys. Chem. A 2000, 104, 3958–3963. [Google Scholar] [CrossRef]
- Kissimonová, K.; Valent, I.; Adamčíková, L.; Ševčík, P. Numerical Simulations of the Oxygen Production in the Oscillating Bray–Liebhafsky Reaction. Chem. Phys. Lett. 2001, 341, 345–350. [Google Scholar] [CrossRef]
- Pejić, N.; Kolar-Anić, L.; Maksimović, J.; Janković, M.; Vukojević, V.; Anić, S. Dynamic Transitions in the Bray–Liebhafsky Oscillating Reaction. Effect of Hydrogen Peroxide and Temperature on Bifurcation. React. Kinet. Mech. Cat. 2016, 118, 15–26. [Google Scholar] [CrossRef]
- Vukojević, V.; Anić, S.; Kolar-Anić, L. Investigation of Dynamic Behavior of the Bray−Liebhafsky Reaction in the CSTR. Determination of Bifurcation Points. J. Phys. Chem. A 2000, 104, 10731–10739. [Google Scholar] [CrossRef]
- Ren, J.; Gao, J.; Qu, J.; Wei, X.; Chen, X.; Yang, W. Nonlinear behavior in Bray-Libhafsky Chemical Reaction. J. Chil. Chem. Soc. 2008, 53, 1620–1623. [Google Scholar] [CrossRef]
- Negrojević, L.; Lončar, A.; Maksimović, J.; Anić, S.; Čupić, Ž.; Kolar-Anić, L.; Pejić, N. Bray–Liebhafsky Oscillatory Reaction in a Continuous-Flow Stirred Tank Reactor as the Matrix System for Determination of Tyrosine. Reac. Kinet. Mech. Cat. 2022, 135, 1147–1162. [Google Scholar] [CrossRef]
- Maksimović, J.; Čupić, Ž.; Manojlović, N.; Đerić, A.; Anić, S.; Kolar-Anić, L. Bray–Liebhafsky Oscillatory Reaction as the Matrix System for the Kinetic Determination of Microquantities of Alizarin and Purpurin. Reac. Kinet. Mech. Cat. 2020, 130, 655–668. [Google Scholar] [CrossRef]
- Pejić, N.; Kolar-Anić, L.; Anić, S.; Stanisavljev, D. Determination of Paracetamol in Pure and Pharmaceutical Dosage Forms by Pulse Perturbation Technique. J. Pharm. Biomed. Anal. 2006, 41, 610–615. [Google Scholar] [CrossRef]
- Pejić, N.D.; Blagojević, S.M.; Anić, S.R.; Vukojević, V.B.; Mijatović, M.D.; Ćirić, J.S.; Marković, Z.S.; Marković, S.D.; Kolar-Anić, L.Z. Kinetic Determination of Morphine by Means of Bray–Liebhafsky Oscillatory Reaction System Using Analyte Pulse Perturbation Technique. Anal. Chim. Acta 2007, 582, 367–374. [Google Scholar] [CrossRef]
- Pejic, N.; Blagojevic, S.; Vukelic, J.; Kolar-Anic, L.; Anic, S. Analyte Pulse Perturbation Technique for the Determination of 6-O-Acetylmorphine in Seized Street Drug Samples. BCSJ 2007, 80, 1942–1948. [Google Scholar] [CrossRef]
- Pejić, N.; Blagojević, S.; Anić, S.; Kolar-Anić, L. Determination of Ascorbic Acid in Pharmaceutical Dosage Forms and Urine by Means of an Oscillatory Reaction System Using the Pulse Perturbation Technique. Anal. Bioanal. Chem. 2007, 389, 2009–2017. [Google Scholar] [CrossRef]
- Maksimović, J.P.; Kolar-Anić, L.Z.; Anić, S.R.; Ribić, D.D.; Pejić, N.D. Quantitative Determination of Some Water-Soluble B Vitamins by Kinetic Analytical Method Based on the Perturbation of an Oscillatory Reaction. J. Braz. Chem. Soc. 2011, 22, 38–48. [Google Scholar] [CrossRef]
- Sr, R.J.L. Sax’s Dangerous Properties of Industrial Materials, 11th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004; ISBN 978-0-471-47662-7. [Google Scholar]
- Krumenacker, L.; Costantini, M.; Pontal, P.; Sentenac, J. Hydroquinone, Resorcinol, and Catechol. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 978-0-471-23896-6. [Google Scholar]
- Körbahti, B.K.; Tanyolaç, A. Continuous Electrochemical Treatment of Phenolic Wastewater in a Tubular Reactor. Water Res. 2003, 37, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Kumar, S. Adsorption of Resorcinol and Catechol on Granular Activated Carbon: Equilibrium and Kinetics. Carbon 2003, 41, 3015–3025. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical Properties of Catechols and Their Molecular Modes of Toxic Action in Cells, from Microorganisms to Mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Dellinger, B.; Pryor, W.A.; Cueto, R.; Squadrito, G.L.; Hegde, V.; Deutsch, W.A. Role of Free Radicals in the Toxicity of Airborne Fine Particulate Matter. Chem. Res. Toxicol. 2001, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Centennial ed.; Merck: Rahway, NJ, USA, 1989; ISBN 978-0-911910-28-5. [Google Scholar]
- Milligan, P.W.; Häggblom, M.M. Biodegradation of Resorcinol and Catechol by Denitrifying Enrichment Cultures. Environ. Toxicol. Chem. 1998, 17, 1456–1461. [Google Scholar] [CrossRef]
- Hays, M.D.; Fine, P.M.; Geron, C.D.; Kleeman, M.J.; Gullett, B.K. Open Burning of Agricultural Biomass: Physical and Chemical Properties of Particle-Phase Emissions. Atmos. Environ. 2005, 39, 6747–6764. [Google Scholar] [CrossRef]
- Maksimović, J.P.; Tošović, J.; Pagnacco, M.C. Insight into the Origin of Pyrocatechol Inhibition on Oscillating Bray-Liebhafsky Reaction: Combined Experimental and Theoretical Study. BCSJ 2020, 93, 676–684. [Google Scholar] [CrossRef]
- Jackson, S.K.; Liu, K.J.; Liu, M.; Timmins, G.S. Detection and Removal of Contaminating Hydroxylamines from the Spin Trap DEPMPO, and Re-Evaluation of Its Use to Indicate Nitrone Radical Cation Formation and SN1 Reactions. Free Radic. Biol. Med. 2002, 32, 228–232. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post-d Group 16–18 Elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Eckart, C. The Penetration of a Potential Barrier by Electrons. Phys. Rev. 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Duncan, W.T.; Bell, R.L.; Truong, T.N. TheRate: Program for Ab Initio Direct Dynamics Calculations of Thermal and Vibrational-State-Selected Rate Constants. J. Comput. Chem. 1998, 19, 1039–1052. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative Mechanisms in Chlorogenic Acid. Food Chem. 2017, 237, 390–398. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S. Antioxidative Activity of Chlorogenic Acid Relative to Trolox in Aqueous Solution—DFT Study. Food Chem. 2019, 278, 469–475. [Google Scholar] [CrossRef]
- Tošović, J.; Bren, U. Antioxidative Action of Ellagic Acid—A Kinetic DFT Study. Antioxidants 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Furrow, S.D.; Schmitz, G.E. I2O in Solution and Volatility. Chem. Phys. Lett. 2019, 730, 186–190. [Google Scholar] [CrossRef]
- Schwartz, N.A.; Boaz, N.C.; Kalman, S.E.; Zhuang, T.; Goldberg, J.M.; Fu, R.; Nielsen, R.J.; Goddard, W.A.I.; Groves, J.T.; Gunnoe, T.B. Mechanism of Hydrocarbon Functionalization by an Iodate/Chloride System: The Role of Ester Protection. ACS Catal. 2018, 8, 3138–3149. [Google Scholar] [CrossRef]
- Zagrean-Tuza, C.; Dorneanu, S.; Mot, A.C. The Strange Case of Polyphenols Inhibiting the Briggs-Rauscher Reaction: pH-Modulated Reactivity of the Superoxide Radical. Free Radic. Biol. Med. 2020, 146, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, R.; Furrow, S.D. Effects of Additives on the Oscillations of the Briggs-Rauscher Reaction. Russ. J. Phys. Chem. 2013, 87, 2121–2126. [Google Scholar] [CrossRef]
- Uddin, W.; Hu, G.; Hu, L.; Hu, Y.; Fang, Z.; Ullah, R.; Sun, X.; Zhang, Y.; Song, J. Identification of Two Aromatic Isomers between 2- and 3-Hydroxy Benzoic Acid by Using a Briggs-Rauscher Oscillator. J. Electroanal. Chem. 2017, 803, 135–140. [Google Scholar] [CrossRef]
- Chen, J.; Hu, L.; Hu, G.; Zhang, Y.; Hu, Y.; Song, J. An Application of Chemical Oscillation: Distinguishing Two Isomers between Cyclohexane-1,3-Dione and 1,4-Cyclohexanedione. Electrochim. Acta 2016, 195, 223–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Hu, L.; Song, J. Identification of Two Aliphatic Position Isomers between α- and β-Ketoglutaric Acid by Using a Briggs–Rauscher Oscillating System. Anal. Chem. 2015, 87, 10040–10046. [Google Scholar] [CrossRef]
- Zhang, W.; Uddin, W.; Hu, G.; Hu, L.; Fang, Z. Identification of Four Isomers of Dihydroxynaphthalene by Using a Briggs-Rauscher Oscillating System. J. Electroanal. Chem. 2018, 823, 378–387. [Google Scholar] [CrossRef]
- Uddin, W.; Hu, G.; Hu, L.; Hu, Y.; Fang, Z.; Ullah, S.; Sun, X.; Shen, X.; Song, J. Identification of Two Positional Isomers between Ortho-Vanillin and Para-Vanillin by Their Inhibitory Effects on a Briggs-Rauscher Oscillator. Int. J. Electrochem. Sci. 2017, 12, 4193–4203. [Google Scholar] [CrossRef]
- Nawabi, M.Y.; Uddin, W.; Hu, G. Identification of the Three Isomers of Monochlorophenol: Application of Briggs-Rauscher Oscillation. Int. J. Electrochem. Sci. 2022, 17, 220425. [Google Scholar] [CrossRef]
- Cervellati, R.; Höner, K.; Furrow, S.D.; Neddens, C.; Costa, S. The Briggs-Rauscher Reaction as a Test to Measure the Activity of Antioxidants. Helv. Chim. Acta 2001, 84, 3533–3547. [Google Scholar] [CrossRef]
- Cervellati, R.; Greco, E.; Blagojević, S.M.; Blagojević, S.N.; Anić, S.; Čupić, Ž.D. Experimental and Mechanistic Study of the Inhibitory Effects by Phenolics on the Oscillations of the Orbàn–Epstein Reaction. Reac. Kinet. Mech. Cat. 2018, 123, 125–139. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, S.; Wang, D.; An, Q. Fenton-Like Reaction: Recent Advances and New Trends. Chem. A Eur. J. 2024, 30, e202304337. [Google Scholar] [CrossRef] [PubMed]

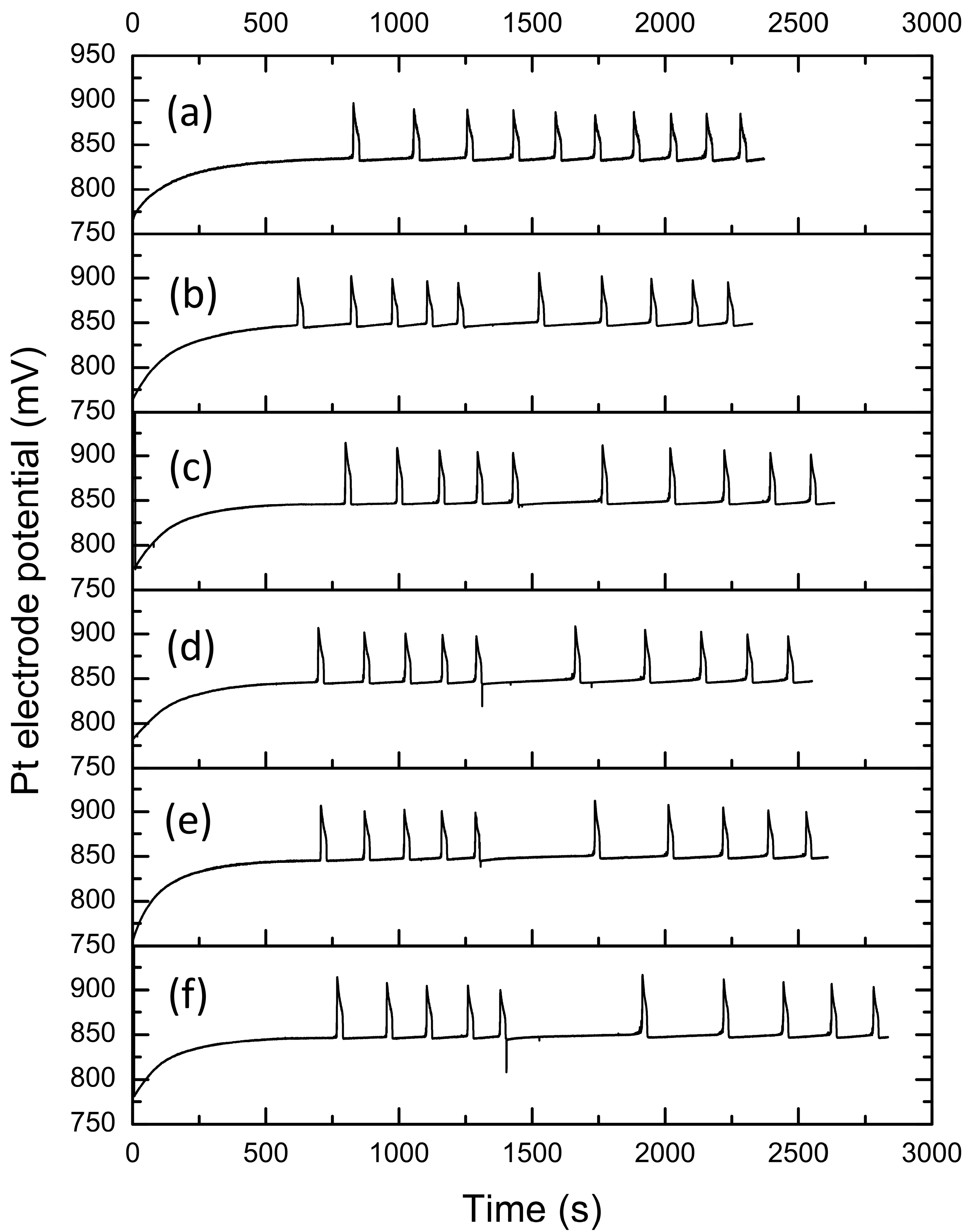


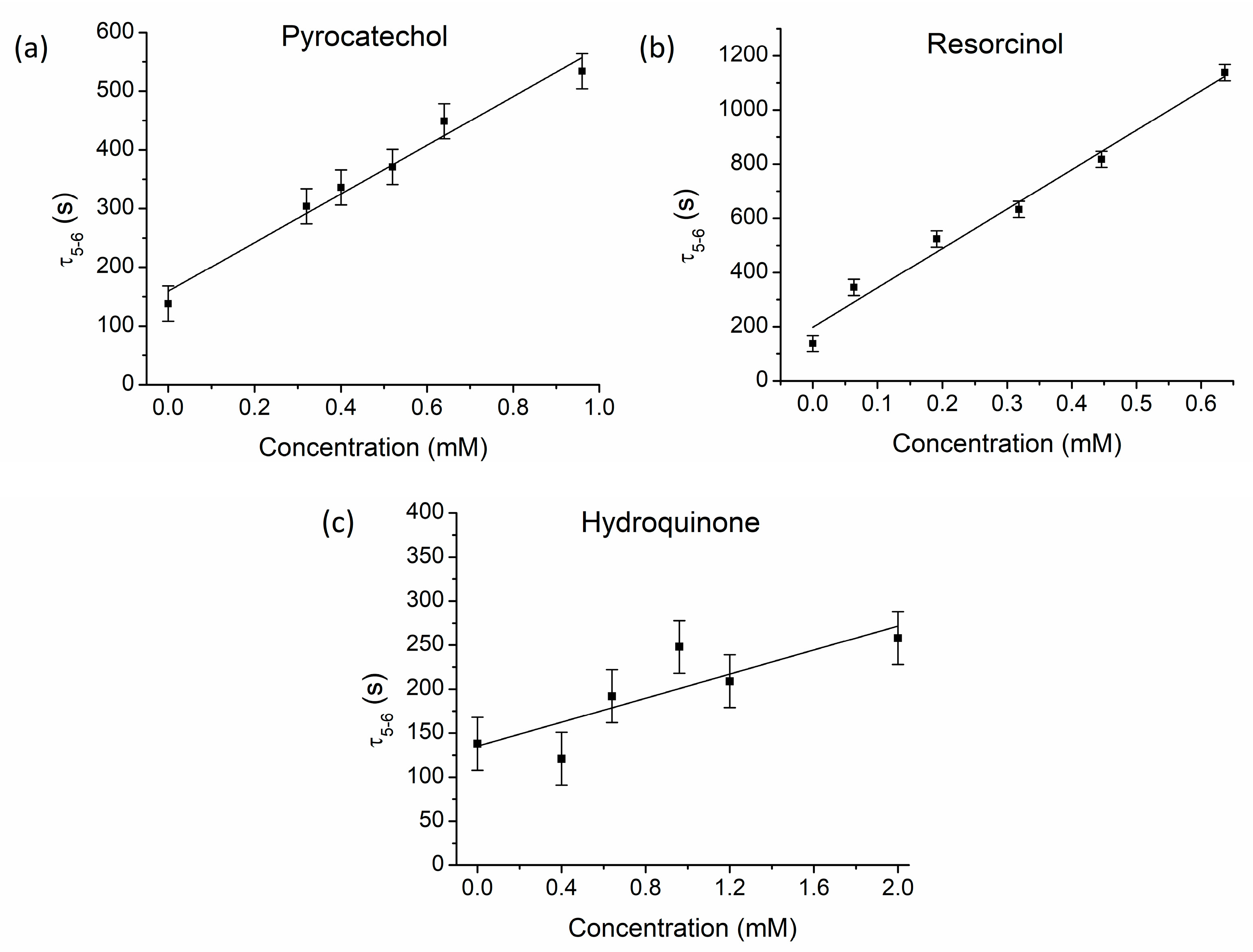
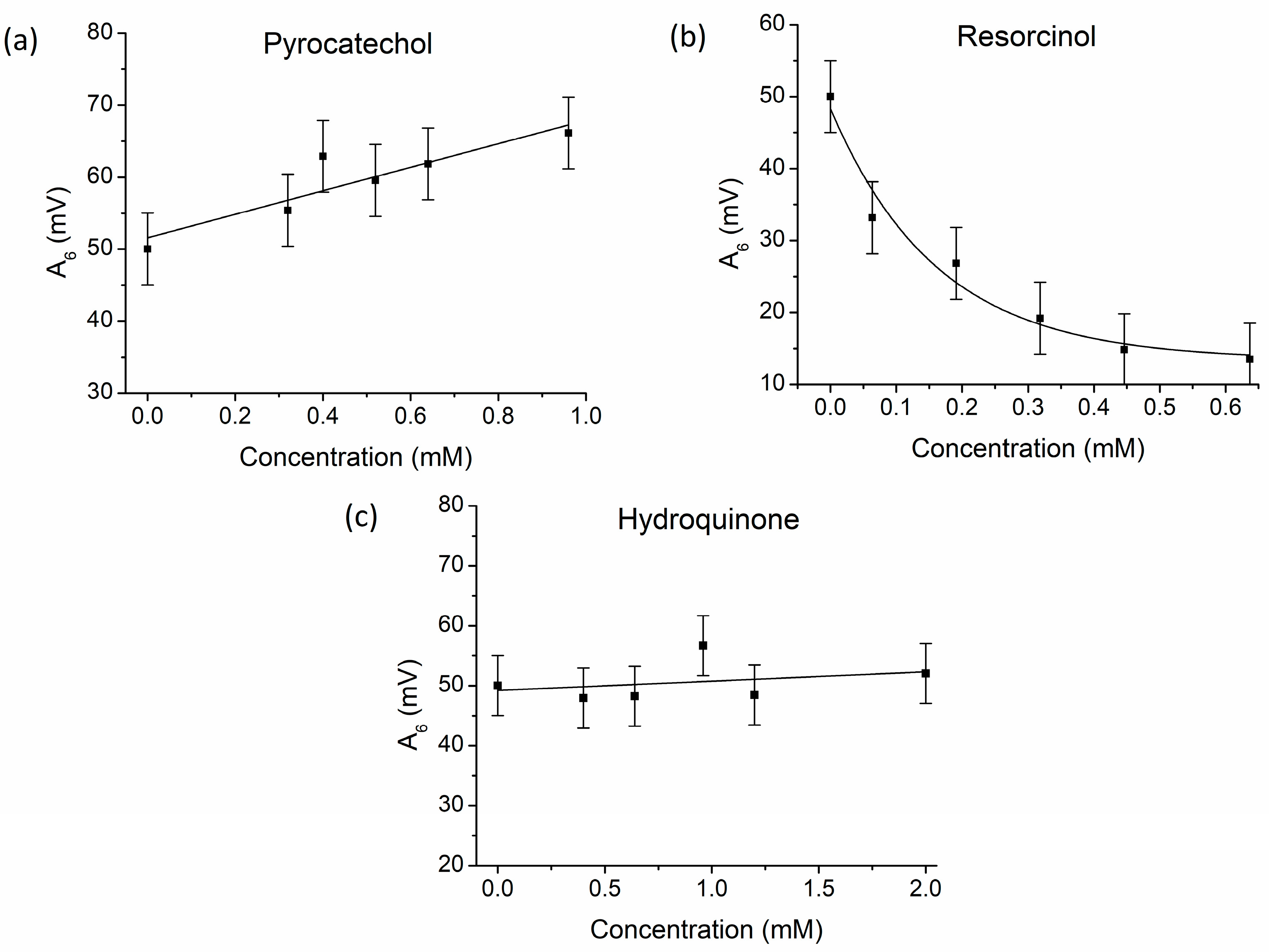
| τind (s) | τend (s) | τ5–6 (s) | A6 (mV) | |
|---|---|---|---|---|
| Mean value of BL reaction parameter | 820 ± 40 | 2200 ± 400 | 140 ± 30 | 51 ± 9 |
| Benzenediol Isomer | LOD (mM) | LOQ (mM) |
|---|---|---|
| Pyrocatechol | 0.16 | 0.49 |
| Resorcinol | 0.14 | 0.41 |
| Hydroquinone | 1.6 | 4.8 |
| Compounds | HO• (1) | HOO• (1) | I• (1) | IO• (1) | IO2• (1) | I2O (2) | I2O (3) | HIO (4) | HIO2 (5) | HIO2 (6) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cat | −174.7 | −35.6 | −4.5 | −75.5 | −17.7 | −35.9 | −181.3 | −36.8 | −144.2 | 60.9 |
| syn-syn Res | −151.2 | −12.1 | 19.0 | −52.0 | 5.8 | 113.2 | −32.2 | 112.3 | 4.9 | 210.0 |
| syn-anti Res | −151.5 | −12.4 | 18.7 | −52.3 | 5.5 | 112.8 | −32.5 | 111.9 | 4.5 | 209.7 |
| anti-anti Res | −152.1 | −13.0 | 18.1 | −52.9 | 5.0 | 113.3 | −32.0 | 112.4 | 5.1 | 210.2 |
| cis-Hq | −172.7 | −33.6 | −2.5 | −73.5 | −15.6 | −56.0 | −201.3 | −56.9 | −164.3 | 40.8 |
| trans-Hq | −173.4 | −34.3 | −3.1 | −74.2 | −16.3 | −56.7 | −202.0 | −57.6 | −165.0 | 40.2 |
| Free Radicals | HO• | HOO• | I• | IO• | IO2• |
|---|---|---|---|---|---|
| Pyrocatechol | |||||
| 16.3 | 49.6 | 53.63 | 19.0 | 44.2 | |
| kZCT_0 | 5.52 × 107 | 3.93 × 106 | 1.87 × 105 | 3.89 × 107 | 1.70 × 106 |
| syn-syn Resorcinol | |||||
| 25.4 | 68.0 | / | 25.2 | 55.7 | |
| kZCT_0 | 3.33 × 108 | 1.55 × 105 | / | 1.65 × 107 | 1.38 × 105 |
| syn-anti Resorcinol | |||||
| 25.8 | 63.1 | / | 20.8 | 53.7 | |
| kZCT_0 | 3.73 × 108 | 1.06 × 105 | / | 2.32 × 107 | 4.99 × 105 |
| anti-anti Resorcinol | |||||
| 23.2 | 63.2 | / | 22.7 | 57.5 | |
| kZCT_0 | 8.54 × 108 | 0.80 × 105 | / | 1.69 × 107 | 0.75 × 105 |
| cis-Hydroquinone | |||||
| ≈0.0 | 49.4 | 97.91 | 22.3 | 39.2 | |
| kZCT_0 | ≈1.9 × 109 | 4.16 × 106 | 1.04 × 10−2 | 2.49 × 107 | 3.25 × 106 |
| trans-Hydroquinone | |||||
| ≈0.0 | 49.3 | 100.12 | 21.1 | 38.7 | |
| kZCT_0 | ≈1.9 × 109 | 5.46 × 106 | 5.92 × 10−3 | 3.13 × 107 | 3.57 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavićević, A.; Veles, M.; Maksimović, J.; Tošović, J.; Bren, U.; Čakar, U.; Pagnacco, M. The Bray–Liebhafsky Oscillatory Reaction as a Chemosensor for Benzenediols. Chemosensors 2024, 12, 211. https://doi.org/10.3390/chemosensors12100211
Pavićević A, Veles M, Maksimović J, Tošović J, Bren U, Čakar U, Pagnacco M. The Bray–Liebhafsky Oscillatory Reaction as a Chemosensor for Benzenediols. Chemosensors. 2024; 12(10):211. https://doi.org/10.3390/chemosensors12100211
Chicago/Turabian StylePavićević, Aleksandra, Marija Veles, Jelena Maksimović, Jelena Tošović, Urban Bren, Uroš Čakar, and Maja Pagnacco. 2024. "The Bray–Liebhafsky Oscillatory Reaction as a Chemosensor for Benzenediols" Chemosensors 12, no. 10: 211. https://doi.org/10.3390/chemosensors12100211
APA StylePavićević, A., Veles, M., Maksimović, J., Tošović, J., Bren, U., Čakar, U., & Pagnacco, M. (2024). The Bray–Liebhafsky Oscillatory Reaction as a Chemosensor for Benzenediols. Chemosensors, 12(10), 211. https://doi.org/10.3390/chemosensors12100211







