Abstract
Asbestos research, identification, and quantification have been performed over the years, and the relationship between fiber inhalation and lung disease development is well defined. The same cannot be said for the gastroenteric system: the International Agency for Research on Cancer (IARC) believes that colorectal cancer (CRC) could be associated with asbestos exposure, but research has not demonstrated a casual nexus between exposure and CRC, despite highlighting an association tendency. The combination of scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) is the most applied technique in asbestos fiber identification in tissues and intestinal mucosa. In this study, SEM/EDS was applied to evaluate the presence of asbestos fibers and bodies (ABs) inside the tissue of eleven patients affected by CRC who had undergone environmental exposure due to living in an asbestos-polluted area where an Eternit plant had been active in the past. This technique was coupled with optical microscopy (OM) to verify whether the latter could be applied to evaluate the presence of these mineral phases, with the goal of understanding its suitability for identifying fibers and ABs in colon tissues. In addition to verifying the presence of fibers, this study allowed us to identify the deposition site of said fibers within the sample and possibly detect associated tissue reactions using OM, over a shorter time and at lower costs. Despite being a preliminary and descriptive work, the obtained results allowed us to propose a method involving first-sample OM observation to identify regulated (fibers with a length ≥ 5 μm, a thickness ≤ 3 μm, and a length/thickness ratio > 3) asbestos phases and ABs in the extra-respiratory system. In fact, OM and SEM/EDS provided similar information: no asbestiform morphology or ABs were found, but phyllosilicates and other inorganic materials were identified. This research needs to be continued using higher-resolution techniques to definitively rule out the presence of these fibers inside tissues whilst also increasing the number of patients involved.
1. Introduction
The bowel or colorectal tract can be affected by different benign and malign neoplastic pathologies in addition to other non-neoplastic disorders, such as inflammatory bowel diseases. Among the neoplastic pathologies, colorectal cancer (CRC) represents the third most common tumor diagnosis in the world, ranking second in terms of mortality, with etiological associations related to the environment (e.g., eating habits, smoking, pollutant exposure, and drugs) and genetic risk factors [1,2].
According to recent studies, up to 5% of malignant cancers might be attributed to occupational factors, with strong differences between genders and cancer sites [3]. In a recent French study, 3.9% (male) and 0.4% (female) of malignant tumors were estimated to have been caused by working exposure to pollutants. Neoplasia has multifactorial origins, with its professional forms being morphologically and clinically undistinguishable from the non-occupational ones, making their identification very difficult. Due to different contamination sources, evaluating which substances a worker has come into contact with is also complex.
Studies aimed at evaluating asbestos fibers’ presence inside gastroenteric tissue are important to understand these minerals’ role in cancer development. For instance, in a recent review [4], different publications were considered to observe variations in asbestos fibers’ quantity in abdominal organs, demonstrating that colon tissues contained the most fibers. The authors related this trend to the possibility of also ingesting these minerals by means of drinkable water or contaminated food, because high concentrations were detected in both exposed workers and people with unknown exposure. The factors that seem to be most consistently associated with CRC are those fitting asbestos exposure: for example, in the work of Ehrlich et al. in 1991 [5], asbestos fibers and bodies (ABs, i.e., morphologies deriving from pulmonary macrophages’ frustrated phagocytosis of fibers with well-defined morphological parameters, such as a length ≥ 5 μm, a thickness ≤ 3 μm, and a length/thickness ratio > 3 [6,7,8]—so-called breathable fibers) were observed in 14 workers of a cohort composed of 44 people. Moreover, in the literature, there is one case study of an asbestos worker affected by both asbestosis and colon adenocarcinoma [9].
Nevertheless, some studies on cohorts from Casale Monferrato and Balangero (Italy) did not demonstrate this causal link from the epidemiological point of view [10,11,12].
Other sporadic associations have been highlighted in the mechanical industry, a varnish factory, transport, wood workers, and people exposed to pesticides [13].
The association between occupational asbestos exposure and CRC onset was reported for the first time by Selikoff et al. in 1964 [14] in a study of a cohort of 632 male workers in New York and New Jersey, USA, where a surprisingly high CRC mortality was found, higher than expected (17 versus 5.2). The International Agency for Research on Cancer (IARC) Monographs demonstrated a positive correlation between asbestos exposure, gastroenteric neoplasia, and the colorectum [15]; nevertheless, until now, it has not been possible to define whether asbestos is a direct causal agent of this disease [15,16,17,18].
In our preliminary case study [19], after the digestion of tissues derived from a patient affected by colorectal cancer, ABs were detected inside healthy colonic tissue, whereas free asbestos fibers were observed in the neoplastic tissue, corresponding to 3 × 103 and 8 × 103 fibers (or ABs) per gram of wet tissue, respectively.
The above research was a preliminary assessment for the analysis of the 11 cases collected in the present study, considering a cohort of patients living in the National Priority Contaminated Site (NPCS) of Casale Monferrato, an area comprising 48 municipalities which is sadly known for its past heavy asbestos pollution [20].
After the preliminary findings of the abovementioned case study, there was a need to increase the number of analyzed patients. Moreover, the study hereby described aimed to identify asbestos fibers and bodies via optical and scanning electron microscopy and assess the use of optical microscopy (OM) alone as a cheaper and more accessible technique for hospital workers. This study must solely be considered a descriptive and preliminary analysis of the possible application of OM for asbestos identification in extra-respiratory tissues; no epidemiological relationships can be hypothesized due to the low number of participants. OM is useful to identify biological characteristics for the recognition of exposure markers, with the first and second parameters being AB presence and the observation of histiocytes, i.e., the cells involved in AB formation. Histiocytes are morphological markers of asbestos exposure whose presence in tissues is very important. In fact, they are very sporadic in colorectal tissues compared to lung tissues.
Moreover, while OM does not detect nanometric fibers, it might be useful to increase the number of analyzed patients and obtain information about those fibers defined as “breathable” by law [6]. In this work, OM is coupled with scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) techniques to obtain information on breathable fibers and exclude the presence of the longest (>5 μm) and thinnest fibers (OM resolution with a 40× objective is about 400 nm). Indeed, these fibers show the highest carcinogenic effect on tissues, so their identification might be an important source of information for understanding the role of these minerals in colorectal tumor onset. This work aims to show different techniques’ feasibility in defining asbestos-associated diseases and determining the asbestos content inside colorectum tissues, with the further goal of understanding whether OM could be a useful first step in the identification of breathable fibers and ABs, eventually coupled with SEM/EDS analyses.
2. Materials and Methods
Eleven histological samples were collected from patients living in the NPCS of Casale Monferrato—and thus having been exposed to asbestos—who were affected by CRC as diagnosed by the Santo Spirito Hospital in the same town during 2020–2021. This study was approved by the Institutional Review Board of Alessandria Hospital in July 2020. The population enrolled in this research comprised five women, with a median age of 74 years (IQR 71–83, minimum 70 and maximum 84), and six men, whose median age was 79.5 years (IQR 71–82, minimum 56 and maximum 82). Among these eleven people, five were smokers, two lived in houses with asbestos-insulated roofs, and two lived near the Eternit factory. The complete data on this study’s cohort will be published in a future paper (20 patients enrolled in total). The data on the patients considered in the current study are reported in Table 1.

Table 1.
Patient information (N.A. = not applicable).
The histological sections were prepared according to a standardized laboratory protocol utilized for routine manipulations of hospital pathological samples. Formalin-fixed samples were initially macroscopically observed to identify potential neoplastic formations. Samples were collected for paraffin inclusion and subsequent microtome cutting, and they were finally stained using hematoxylin–eosin (H&E).
Histological slides were observed using direct-light OM (Zeiss, Germany; Axioskop model), and the portions of formalin-fixed tissues, from which paraffin blocks would later be prepared, were digested and observed by SEM. The digestion procedure was the same as that applied for lung tissues [21], as it had been previously demonstrated to work well with colon tissues as well [19,22]. OM application is useful for the identification of different characteristics from biological and mineralogical points of view, including the histological confirmation of CRC diagnosis, the detection of phlogosis inside tissues, the observation of histiocytes and ABs, and the analysis of birefringent particles using crossed polarizers.
Regarding SEM/EDS observation, digested samples deposited on polycarbonate filters were observed using a Quanta 200 ESEM (FEI Company, Hillsboro, OR, USA) instrument coupled with an energy-dispersive spectroscope (EDAX, Mahwah, NJ, USA). The analysis protocol and instrument parameters applied for the observation of the digested tissues were the same as those described in Rinaudo et al.’s 2021 work and Croce et al.’s 2023 study [19,22].
Concerning OM observation, the samples were observed at 20× (with a numerical aperture, NA, of 0.60 and a lateral resolution of about 700 nm) magnification to identify the presence of any asbestiform phase. A subsequent observation at 40× (NA 0.75 and lateral resolution of about 400 nm) magnification allowed for the discrimination and/or confirmation of the previously identified ferruginous/carbonaceous bodies.
The Italian Mesothelioma Registry (Registro Nazionale dei Mesoteliomi, ReNaM) questionnaire for the assessment and quantification of asbestos exposure was administered to 10 of the 11 enrolled subjects. The ReNaM questionnaire applies collected asbestos exposure information to the assessment of asbestos exposure and the identification of asbestos-related cancer cases [23]. This work is focused on applying two techniques for asbestos recognition inside colon tissues, so it must only be considered as a descriptive analysis from an epidemiological point of view.
3. Results
The initial part of this study focused on an OM analysis of the case study presented by Rinaudo et al. in 2021 [19] to understand the technique’s applicability in the identification of free asbestos fibers and bodies in both digested tissues and histological samples of colon tissues, among others. The presence of asbestos fibers in this case study was first evaluated by SEM/EDS, and following positive results, the tissues were analyzed for asbestos bodies using OM as well. The obtained data were superimposable, but the observation of only one case was not significant; thus, the same methods were applied to the cases described in this descriptive work about fiber identification in CRC tissues from eleven patients.
In Figure 1, an example of a bundle of fibers, observed inside healthy tissue from case #9, is reported to better display the characteristics of a non-breathable fiber (see Introduction for the morphological parameters). In this case, the length of the particle is about 30 μm, respecting the first parameter. Nevertheless, the diameter is about 9 μm, so it cannot be deemed a “regulated fiber”. Lastly, the aspect ratio is 3.4, meaning that it can be considered a fiber. The EDS-obtained chemical composition allows us to ascribe this elongated particle to tremolite, the Mg end-member of the Ca amphiboles of asbestos, with the theoretical chemical formula Ca2Mg5[Si8O22](OH)2 [24,25]. From the chemical point of view, this particle shows a composition comparable to one of the “asbestos” phases; nevertheless, the morphological parameters do not allow us to classify it as a regulated fiber.
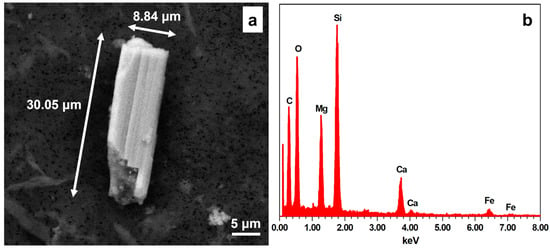
Figure 1.
(a) Fibrous morphology observed in the healthy tissue from case #9; in this particle, only the length and aspect ratio are consistent with the breathable fiber parameters. (b) EDS spectrum obtained from the crystal reported in (a): peak intensities indicate Si > Mg > Ca > Fe, so the mineral can be chemically ascribed to tremolite.
In the eleven cases observed in this work, no well-developed respirable asbestos fibers were detected, although short fibers or small particles with chemical compositions ascribable to mineral phases regulated as “asbestos” by Law 257/1992 [24] were identified. The SEM/EDS principal data of part of the cases reported in this paper were first presented in the work of Croce et al. in 2023 [22]. With respect to the OM data, some of the principal results are reported in Figure 2.
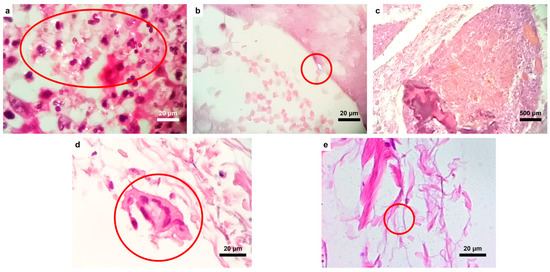
Figure 2.
OM images of the analyzed samples. (a) Numerous birefringent particles (indicated by the red circle), also with an elongated pattern, in the neoplastic tissue from case #4, and (b) a birefringent particle from the same case located inside a vessel. (c) Fecal material with food residue constituting amorphous calcific particles. (d) Multinucleated histocyte (granuloma, in the red circle) phagocytosing amorphous material in case #7. (e) Small, presumably inorganic, fiber (indicated by the red circle) within the serous tunic (case #2).
As shown in the images above, the distribution of inorganic particles (of whatever nature) appeared very scattered; in fact, they were identified within the serosa, submucosa and mucosa, fecal material (Figure 2c), and neoplastic tissues. This finding suggests that particle migration within the tissues is variable, demonstrating that they may be deposited in different anatomical areas, as found in the current study. The topography of their deposition could suggest their origin site.
Furthermore, histological structures typically related to chronic inflammation, such as lymphocytes, histiocytes, plasma cells, and granulomas (Figure 2d), are found close to inorganic deposits. Figure 2d,e show, respectively, a multinucleated histocyte phagocytosing amorphous material and, in the red circle, a small fiber.
The main results obtained by applying optical and scanning electron microscopy to different colon tissues are reported in Table 2, alongside exposure information on the different patients.

Table 2.
Summary of different patients’ exposure information and mineralogical results obtained by OM and SEM/EDS.
As seen in Table 2, some morphological features were ascribed to “possible asbestos” phases under SEM/EDS, but it was not possible to count them as asbestos fibers due to their dimensional parameters. Indeed, length, thickness, and length/thickness ratio (aspect ratio) crtieria must be respected to classify particles as “breathable fibers” (length ≥ 5 μm, thickness ≤ 3 μm, and length/thickness or “aspect” ratio > 3), in addition to their chemical composition [6,24]. In Table 2, phyllosilicates are indicated under the OM data to provide readers with a complete and accurate overview of the obtained information, but these minerals are not attributable to asbestos phases (only chrysotile is a type of phyllosilicate asbestos) [26]. Considering the obtained results, OM is suitable for excluding the presence of breathable asbestos fibers and ABs. The difference in the results between the two techniques is due to the higher resolution of SEM/EDS compared to OM. Moreover, the former also provides chemical information about the analyzed fibers/particles, allowing one to better characterize the mineral phases.
4. Discussion
The study of asbestos-related diseases has made huge leaps in progress regarding respiratory tract pathologies such as asbestosis, malignant pleural mesothelioma (MPM), and lung carcinoma [27,28]. However, thus far, there have not been enough studies and results regarding the correlation between the inhalation or ingestion of asbestiform materials and the onset of neoplastic diseases affecting the gastrointestinal tract. In fact, there is suggestive but insufficient evidence of the association of asbestos exposure with the risk of pharyngeal and stomach cancers and inadequate proof related to esophageal cancer. For instance, the evidence for CRC has been suggestive, but it has left the IARC working group evenly divided on whether it is sufficient to designate causality [15]. A recent scoping review of works on the quantitative assessment of asbestos phases in abdominal organs [4] has highlighted the lack of sufficient representativity of the obtained results due to heterogeneity in aspects such as exposure type, number of cases, samples, and analytical techniques. In the future, it will therefore be important to standardize the samples and methodologies to best assess the analytical results on epidemiological data.
In this paper, the goal was to provide preliminary OM and SEM/EDS results on colic tissue samples from surgical resections performed at the Santo Spirito Hospital in Casale Monferrato to assess which method allowed for faster and less expensive detection of asbestos fibers and bodies inside the tissues. In fact, optical microscopy is currently applied to investigate the presence of asbestos bodies in lung tissues, using first histological sections and then chemical digestion of the biological medium. In this work, this method was applied to colon tissue to test whether the same methodology could be applied to other biological specimens.
In the examined tissues, ABs or fibers were researched, and any histiocytic inflammatory reaction was identified. In fact, the latter might be secondary to fiber penetration, and the inorganic phase cannot be observed by OM due to resolution limitations.
In almost all the cases analyzed by the two techniques, no formations that could be surely ascribed to asbestos bodies were found in either the histological preparations or the tissues digested by sodium hypochlorite. However, particles morphologically related to inorganic fibers, phyllosilicates, or inorganic deposits were evident under the two utilized techniques. In fact, the data reported in Table 1 show that only colon tissues sampled from three patients contained possible asbestos phases from the chemical point of view; however, their morphological characteristics did not allow them to be classified as “breathable fibers” (as already highlighted in [22]). Moreover, due to these characteristics, the OM-observed phyllosilicate phases were not ascribable to asbestos phases, and no serpentine elongated phases were detected by SEM/EDS in the tissues. Asbestos exposure was estimated in two people, one of them also living near the Eternit factory; statistical considerations were impossible due to the small number of patients considered. The three patients with possible chemically identified asbestos phases were two men and one woman who, alongside being smokers, had reported sure, possible, and familiar (for the female subject) exposure based on the ReNaM questionnaire.
The observed asbestos particles were detected only by SEM/EDS, thanks to its higher resolution compared to OM. However, both OM and SEM/EDS were able to exclude the presence of (breathable) asbestos fibers and ABs, suggesting the possibility of a quicker and less costly first-stage OM analysis to determine whether breathable fibers or ABs are contained in certain tissues. From a biological point of view, OM allowed the histological characterization of colorectum carcinoma, as well as the observation of other inorganic phases (e.g., phyllosilicates), confirming its feasibility for the localization of particles/fibers. Moreover, a definitive characterization of these phases could be carried out via the coupled use of other techniques (e.g., SEM/EDS and micro-Raman spectroscopy) on the same morphologies to study their chemical characteristics and detect “non-breathable” asbestos fibers (identifying, for example, very short fibers). This was not one of the objectives of the current project, which instead focused on the research, identification, and quantification of asbestos fibers inside tissues taken from a cohort of patients affected by CRC.
Comparing the SEM/EDS and OM data, they can be deemed to be most likely complementary techniques with regard to “breathable” asbestos fibers, confirming that optical microscopy is a technique providing useful results, considering the cost/benefit ratio. Based on these results, OM could be proposed as a technique to be applied during a first step of tissue observation to exclude the presence of breathable asbestos fibers or ABs in extra-respiratory organs, as routinely performed for lung samples. In this scenario, an electron microscope will certainly remain necessary for the detection of nanometer-scale fibers and the collection of EDS spectra for the chemical identification of mineral phases. Moreover, OM could be coupled with Raman spectroscopy to carry out spectroscopical analyses using the same histological sections or prepared filters of digested tissues [8].
5. Conclusions
In this work, OM was applied to understand whether the obtained results would be comparable to those from SEM/EDS, with the final goal of applying the former in the identification of regulated asbestos morphologies and also ABs in extra-respiratory organs.
Considering our findings regarding asbestos phases, it is possible to conclude that OM and SEM/EDS showed congruent results, except for three cases (in which SEM/EDS detected the presence of possible asbestos phases). This disagreement was due to OM’s resolution limitations and the fact that SEM/EDS analysis allowed us to also determine the chemical composition of the analyzed inorganic particles. In fact, the asbestos phases were recognized by SEM/EDS from the chemical point of view, but their dimensional characteristics did not allow their classification as “breathable” asbestos fibers [22]. Moreover, asbestos bodies were not detected inside the tissues, both directly in the histological sections and after tissue digestion, as opposed to the case study presented by Rinaudo et al. in 2021 [19]. Due to the state of the art, the comprehension of this difference in fiber detection between the 12 (the first case study reported by Rinaudo et al. in 2021 plus the 11 cases in this work) patients analyzed had been, until now, impossible [19].
The OM technique appears to be more cost-effective than SEM/EDS when used as a preliminary means of investigation to identify possible asbestiform fibers inside organic tissues. However, it presents resolution and magnification limitations as, theoretically, its highest optical resolution is around 200 nm, considering the numerical aperture of conventional lenses [29] in optimal conditions, meaning that the thinnest fibers might be undetectable by OM. In our case, the operative conditions allowed for a highest resolution of about 400 nm. Moreover, the highest magnification that can be applied to analyze histological sections by OM is 63×, so its resolution is even lower than the theoretical maximum (about 300 nm, achievable using a 100× magnification objective lens). There are also limitations depending on the sample type: in fact, the observation of histological slides is difficult due to eye fatigue in the operators performing the analyses, leading to less detailed and careful observations over time.
However, the absence of ABs in the samples analyzed in this work does not exclude asbestos presence in the tissues sampled from patients affected by CRC (e.g., fibers with nanometric dimensional parameters). The number of analyzed samples in this study is still too limited, making further research necessary to obtain results with higher statistical significance. Moreover, it must be remembered that the patients considered in this work had undergone much lower asbestos exposure than the patients considered in past works (see, for example, the work of Ehrlich et al., 1991 [5]) at the time of cancer diagnosis, so the number of inhaled and/or ingested fibers might also be lower. In fact, in Italy, asbestos was banned in 1992 [24], so it can be assumed that the exposure dose withstood during the last few decades by the patients in this work was lower than that suffered by an asbestos worker.
The study results are similar to the findings reported by Rosen et al., 1974 [30], where the tissues sampled from 16 patients affected by colonic carcinoma were analyzed. In that study, no classical ferruginous bodies and fibers were found, suggesting that another technique (e.g., SEM/EDS) might be helpful for the detection of shorter or thinner fibers due to minerals breaking into fragments or not being able to form ABs inside colon tissues. Another key point hypothesized by the authors was linked to the exposure dose undergone by their patients.
In our case, the results obtained on the 11 CRC cases are comparable: the OM observations allowed us to identify neither asbestos fibers nor asbestos bodies, whereas SEM/EDS enabled us to identify some small fibers or fragments. In our experience, the only patient showing any differences was the case study reported in [19], where well-developed asbestos bodies and fibers were observed. Nevertheless, their dimensional and morphological parameters made them easily detectable by OM as well, so we could postulate that the only difference between our twelve cases might have been the exposure dose, allowing AB formation and partial chrysotile fiber dissolution.
To definitively exclude this point, future samples could be analyzed using various, more sensitive techniques, possibly providing positive results, especially considering the shortest and thinnest mineral fibers. Applying different microscopy techniques may provide further supporting epidemiological evidence of the role of asbestos exposure in colon cancer development. Indeed, very recent works have not yet defined whether this exposure increases CRC risk; for example, in 2023, a work concerning 35 asbestos workers concluded that there was no sufficient evidence to support this relationship [31], proposing a specific screening program in its conclusion. Additionally, mineralogical microscopy analyses might be useful to understand whether asbestos is linked to CRC development, coupled with histopathological and immunohistochemical analyses of tissues from autopsies of asbestos workers affected by CRC. By following the above recommendations and applying SEM/EDS, it will be possible to understand whether asbestos fibers and bodies can reach colon tissues and define which mineral phase is associated with these elongated particles, understanding the exposure source for the different subjects affected by this disease.
Author Contributions
Conceptualization, A.C.; methodology, A.C.; software, A.C.; validation, A.C.; formal analysis, A.G., D.B. and A.C.; investigation, A.G., D.B. and A.C.; resources, A.M.; data curation, A.G. and A.C.; writing—original draft preparation, A.C., A.G., C.B., M.F., F.G. and D.B.; writing—review and editing, A.C., A.G., C.B., M.F., F.G. and D.B.; visualization, A.C. and A.G.; supervision, A.R., M.B. and A.M.; project administration, A.R. and M.B.; funding acquisition, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was funded by the CRT foundation.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo of Alessandria (protocol no. 0016369 on 29 July 2020).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in this study.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors wish to thank Caterina Rinaudo, Marco Francesco Amisano, Elisabetta Nada, Stefania Erra, and Federica Grosso for their support during sample acquisition, protocol writing, and analysis checking.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Slattery, M.L.; Edwards, S.L.; Boucher, K.M.; Anderson, K.; Caan, B.J. Lifestyle and Colon Cancer: An Assessment of Factors Associated with Risk. Am. J. Epidemiol. 1999, 150, 869–877. [Google Scholar] [CrossRef][Green Version]
- Lotfollahzadeh, S.; Recio-Boiles, A.; Cagir, B. Colon cancer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Marant Micallef, C.; Shield, K.D.; Vignat, J.; Baldi, I.; Charbotel, B.; Fervers, B.; Gilg Soit Ilg, A.; Guénel, P.; Olsson, A.; Rushton, L.; et al. Cancers in France in 2015 attributable to occupational exposures. Int. J. Hyg. Environ. Health 2019, 222, 22–29. [Google Scholar] [CrossRef]
- Caraballo-Arias, Y.; Roccuzzo, F.; Graziosi, F.; Danilevskaia, N.; Rota, S.; Zunarelli, C.; Caffaro, P.; Boffetta, P.; Bonetti, M.; Violante, F.S. Quantitative assessment of asbestos fibers in abdominal organs: A scoping review. Med. Lav. 2023, 114, e2023048. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.; Gordon, R.E.; Dikman, S.H. Carcinoma of the colon in asbestos-exposed workers: Analysis of asbestos content in colon tissue. Am. J. Ind. Med. 1991, 19, 626–636. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Environmental Health Criteria 53—Asbestos and Other Natural Mineral Fibres; International Programme for Chemical Safety: Geneva, Switzerland, 1986; ISBN 92 4 154193 8. [Google Scholar]
- Murai, Y.; Kitagawa, M.; Hiraoka, T. Asbestos body formation in the human lung: Distinctions, by type and size. Arch. Environ. Health 1995, 50, 19–25. [Google Scholar] [CrossRef]
- Croce, A.; Gatti, G.; Calisi, A.; Cagna, L.; Bellis, D.; Bertolotti, M.; Rinaudo, C.; Maconi, A. Asbestos bodies in human lung: Localization of iron and carbon in the coating. Geosciences 2024, 14, 58. [Google Scholar] [CrossRef]
- Ehrlich, A.; Rohl, A.N.; Holstein, E.C. Asbestos bodies in carcinoma of colon in an insulation worker with asbestosis. JAMA 1985, 254, 2932–2933. [Google Scholar] [CrossRef]
- Magnani, C.; Terracini, B.; Bertolone, G.P.; Castagneto, B.; Cocito, V.; De Giovanni, D.; Paglieri, P.; Botta, M. Mortality from tumors and other diseases of the respiratory system in cement-asbestos workers in Casale Monferrato. A historical cohort study. Med. Lav. 1987, 78, 441–453. [Google Scholar] [PubMed]
- Magnani, C.; Terracini, B.; Ivaldi, C.; Botta, M.; Budel, P.; Mancini, A.; Zanetti, R. A cohort study on mortality among wives of workers in the asbestos cement industry in Casale Monferrato, Italy. Br. J. Ind. Med. 1993, 50, 779–784. [Google Scholar] [CrossRef]
- Ferrante, D.; Mirabelli, D.; Silvestri, S.; Azzolina, D.; Giovannini, A.; Tribaudino, P.; Magnani, C. Mortality and mesothelioma incidence among chrysotile asbestos miners in Balangero, Italy: A cohort study. Am. J. Ind. Med. 2020, 63, 135–145. [Google Scholar] [CrossRef]
- Potter, J.D.; Slattery, M.L.; Bostick, R.M.; Gapstur, S.M. Colon cancer: A review of the epidemiology. Epidemiol. Rev. 1993, 15, 499–545. [Google Scholar] [CrossRef] [PubMed]
- Selikoff, I.J.; Churg, J.; Hammond, E.C. Asbestos exposure and neoplasia. JAMA 1964, 188, 22–26. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100C, pp. 219–309. ISBN 978-92-832-1320-8. [Google Scholar]
- Huang, Q.; Lan, Y. Colorectal cancer and asbestos exposure—An overview. Ind. Health 2020, 58, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.J.; More, S.L.; Maddaloni, M.A.; Fung, E.S. Evaluation of potential gastrointestinal carcinogenicity associated with the ingestion of asbestos. Rev. Environ. Health 2021, 36, 15–26. [Google Scholar] [CrossRef]
- Ramada Rodilla, J.M.; Calvo Cerrada, B.; Serra Pujadas, C.; Delclos, G.L.; Benavides, F.G. Fiber burden and asbestos-related diseases: An umbrella review. Gac. Sanit. 2022, 36, 173–183. [Google Scholar] [CrossRef]
- Rinaudo, C.; Croce, A.; Erra, S.; Nada, E.; Bertolotti, M.; Grosso, F.; Maconi, A.; Amisano, M. Asbestos Fibers and Ferruginous Bodies Detected by VP-SEM/EDS in Colon Tissues of a Patient Affected by Colon-Rectum Cancer: A Case Study. Minerals 2021, 11, 658. [Google Scholar] [CrossRef]
- Marsili, D.; Canepa, A.; Mossone, N.; Comba, P. Environmental Health Education for Asbestos-Contaminated Communities in Italy: The Casale Monferrato Case Study. Ann. Glob. Health 2019, 85, 84. [Google Scholar] [CrossRef]
- Belluso, E.; Bellis, D.; Fornero, E.; Capella, S.; Ferraris, G.; Coverlizza, S. Assessment of inorganic fibre burden in biological samples by scanning electron microscopy—Energy dispersive spectroscopy. Microchim. Acta 2006, 155, 95–100. [Google Scholar] [CrossRef]
- Croce, A.; Bertolotti, M.; Crivellari, S.; Amisano, M.; Nada, E.; Grosso, F.; Cagna, L.; Rinaudo, C.; Gatti, G.; Maconi, A. Scanning Electron Microscopy coupled with Energy Dispersive Spectroscopy applied to the analysis of fibers and particles in tissues from colon adenocarcinomas. Work. Pap. Public Health 2023, 11, 9586. [Google Scholar] [CrossRef]
- Airoldi, C.; Ferrante, D.; Miligi, L.; Piro, S.; Stoppa, G.; Migliore, E.; Chellini, E.; Romanelli, A.; Sciacchitano, C.; Mensi, C.; et al. Estimation of Occupational Exposure to Asbestos in Italy by the Linkage of Mesothelioma Registry (ReNaM) and National Insurance Archives. Methodology and Results. Int. J. Environ. Res. Public Health 2020, 17, 1020. [Google Scholar] [CrossRef]
- Italian Government. Regulations Concerning the Cessation of the Use of Asbestos. S. Ord. to G.U.N. 087 General Series Part One of 13/04/1992 Supplement 064 of 13/04/1992—Law no. 257. 27 March 1992. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Rinaudo, C.; Gastaldi, D.; Belluso, E. Characterization of chrysotile, antigorite and lizardite by FT-Raman spectroscopy. Can. Mineral. 2003, 41, 883–890. [Google Scholar] [CrossRef]
- Kamp, D.W. Asbestos-induced lung diseases: An update. Transl. Res. 2009, 153, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Prazakova, S.; Thomas, P.S.; Sandrini, A.; Yates, D.H. Asbestos and the lung in the 21st century: An update. Clin. Resp. J. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Van Putten, E.G.; Akbulut, D.; Bertolotti, J.; Vos, W.L.; Lagendijk, A.; Mosk, A.P. Scattering lens resolves sub-100 nm structures with visible light. Phys. Rev. Lett. 2011, 106, 193905. [Google Scholar] [CrossRef]
- Rosen, P.; Savino, A.; Melamed, M. Ferruginous (asbestos) bodies and primary carcinoma of the colon. Am. J. Clin. Pathol. 1974, 61, 135–138. [Google Scholar] [CrossRef]
- Porzio, A.; Feola, A.; Parisi, G.; Lauro, A.; Campobasso, C.P. Colorectal cancer: 35 cases in asbestos-exposed workers. Healthcare 2023, 11, 3077. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).