Evaluation of the Essential Oil Composition of Five Thymus Species Native to Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Isolation of the Essential Oil
2.4. GC-MS Analysis Conditions
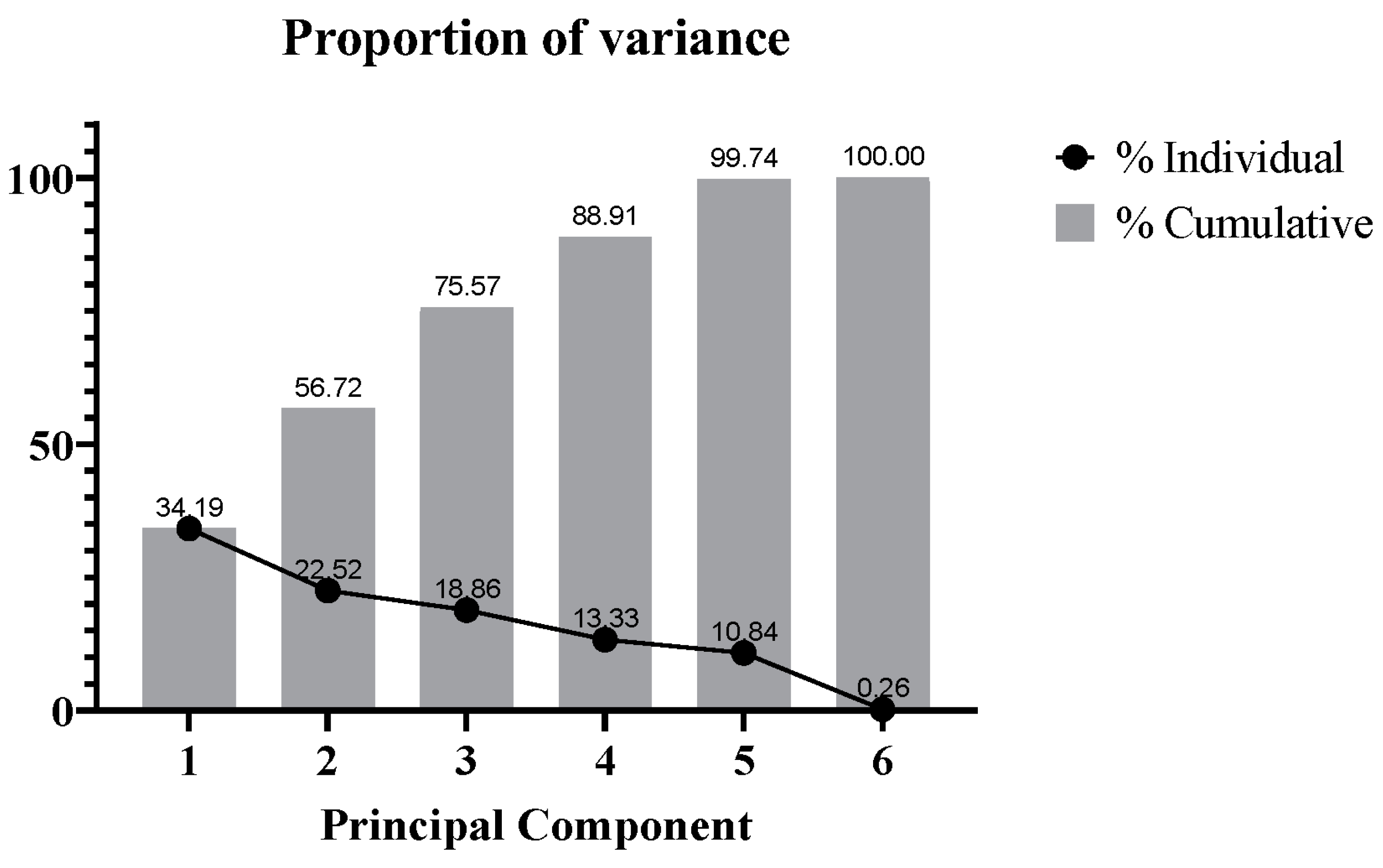
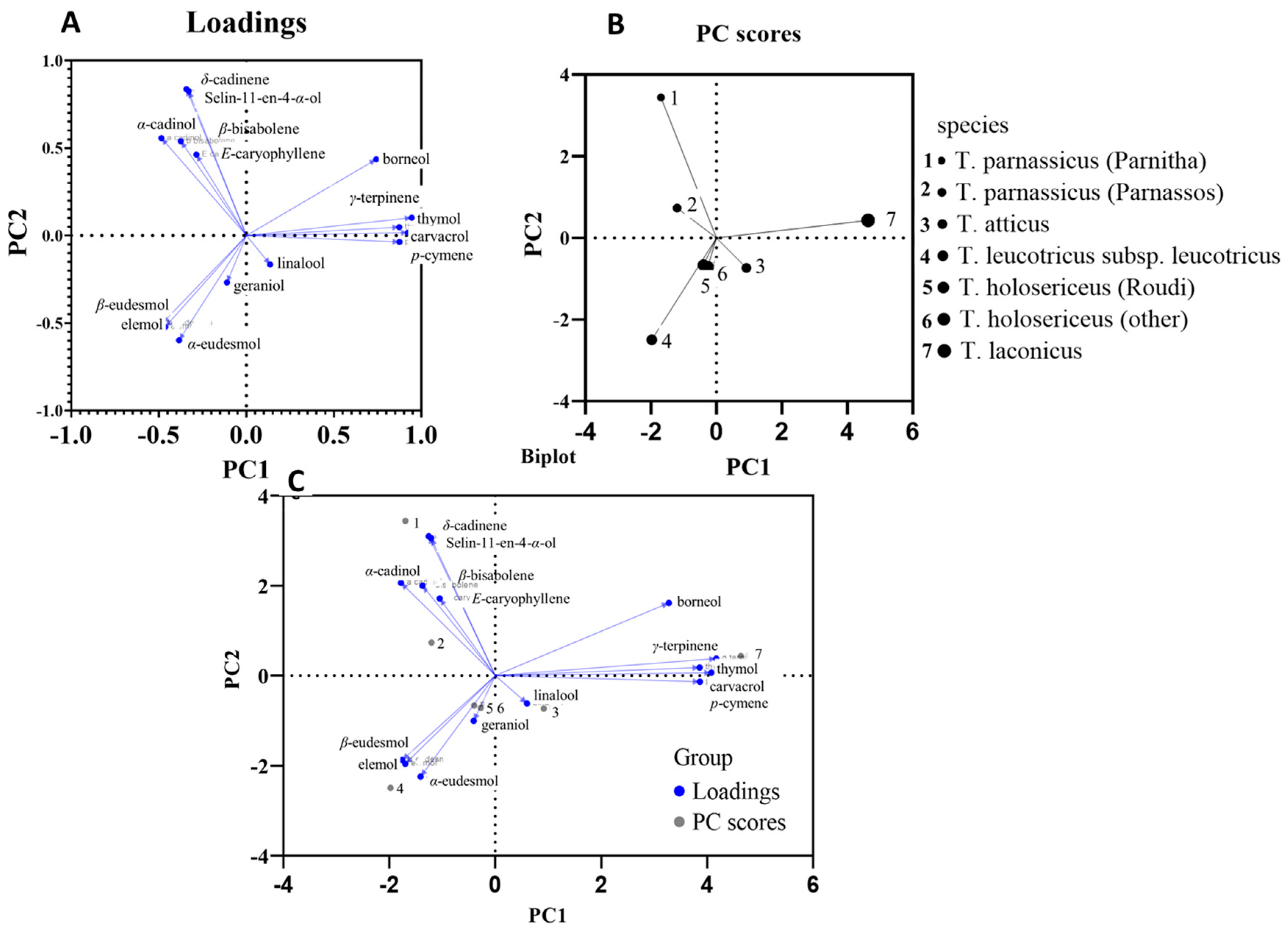
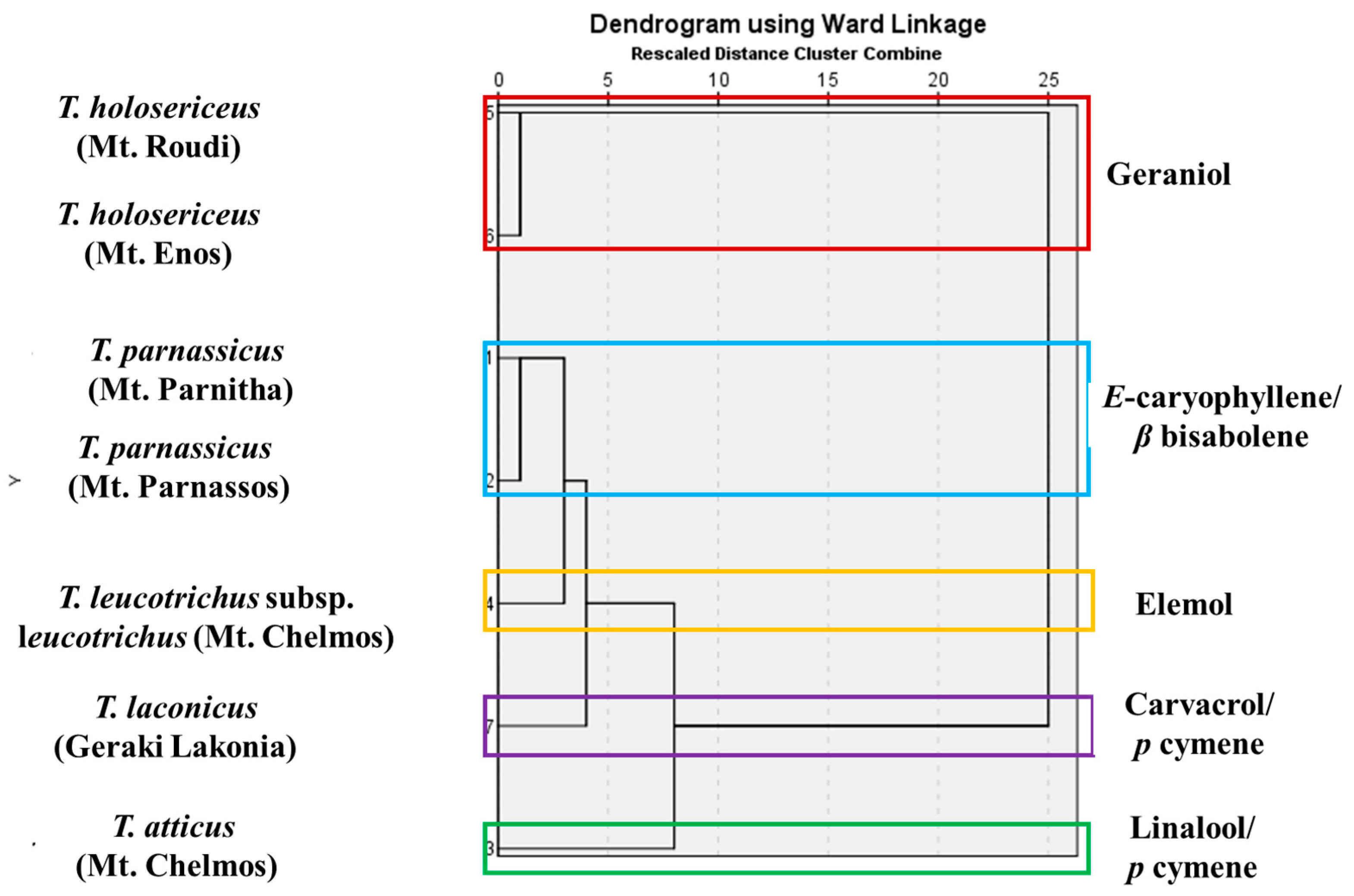
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morales, R. The History, Botany and Taxonomy of the Genus Thymus. In Thyme: The Genus Thymus, 1st ed.; Stahl-Biskup, E., Sáez, F., Eds.; Taylor and Francis, Inc.: London, UK, 2002; pp. 1–43. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece, an Annotated Checklist; Botanic Garden and Botanical Museum: Berlin, Germany; Hellenic Botanical Society: Athens, Greece, 2013; pp. 1–372. [Google Scholar]
- Bouyahya, A.; Chamkhi, I.; Guaouguaou, F.E.; Benali, T.; Balahbib, A.; El-Omari, N.; Taha, D.; El-Shazly, M.; El-Menyiy, N. Ethnomedicinal use, phytochemistry, pharmacology, and food benefits of Thymus capitatus. J. Ethnopharmacol. 2020, 259, 112925. [Google Scholar] [CrossRef] [PubMed]
- Dauqan, E.M.; Abdullah, A. Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. Appl. Biol. Biotechnol. 2017, 5, 17–22. [Google Scholar] [CrossRef]
- Hossain, M.A.; Alrashdi, Y.B.A.; Al-Touby, S. A review on essential oil analyses and biological activities of the traditionally used medicinal plant Thymus vulgaris L. Int. J. Second. Metab. 2022, 9, 103–111. [Google Scholar] [CrossRef]
- Damianova, S.; Tasheva, S.; Stoyanova, A.; Damianov, D. Investigation of extracts from thyme (Thymus vulgaris L.) for application in cosmetics. J. Essent. Oil-Bear. Plants. 2008, 11, 443–450. [Google Scholar] [CrossRef]
- Nieto, G. A review on applications and uses of thymus in the food industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Unlü, G.; Candan, F.; Sökmen, A.; Daferera, D.; Polissiou, M.; Sökmen, M.; Dönmez, E.; Tepe, B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J. Agric. Food Chem. 2003, 51, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Granger, R.; Passet, J. Thymus vulgaris spontane de France: Races chimiques et chemotaxonomie. Phytochemistry 1973, 12, 1683–1691. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Alternat. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.M.; Polissiou, G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R.; Michitsch, H.; Franz, C. Antioxidative properties of Thymus vulgaris leaves: Comparison of different extracts and essential oil chemotypes. J. Agric. Food Chem. 2008, 56, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Čmiková, N.; Kačániová, M. Thymus vulgaris essential oil and its biological activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Babotă, M.; Frumuzachi, O.; Nicolescu, A.; Dias, M.I.; Pinela, J.; Barros, L.; Añibarro-Ortega, M.; Stojković, D.; Carević, T.; Mocan, A.; et al. Thymus Species from Romanian Spontaneous Flora as Promising Source of Phenolic Secondary Metabolites with Health-Related Benefits. Antioxidants 2023, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Hanlidou, E.; Lazari, D. Essential oils of Thymus leucospermus Hartvig, a Greek endemic rich in phenolic monoterpenes. Nat. Prod. Res. 2013, 27, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Daferera, D.; Polissiou, M.; Sokmen, A. Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J. Food Eng. 2005, 66, 447–454. [Google Scholar] [CrossRef]
- Miloš, N.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Azaz, A.D.; Irtem, H.A.; Kurkcuoǧlu, M.; Can Baser, K.H. Composition and the in vitro antimicrobial activities of the essential oils of some Thymus species. Z. Naturforsch. C J. Biosci. 2004, 59, 75–80. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Volatiles from Thymbra and Thymus species of the western Mediterranean basin, Portugal and Macaronesia. Nat. Prod. Commun. 2010, 5, 1934578X1000500924. [Google Scholar] [CrossRef]
- European Medicine Agency (EMA). Assessment Report on Thymus vulgaris L., vulgaris zygis L., aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis-l-aetheroleum-revision-1_en.pdf (accessed on 12 December 2023).
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of essential oil in aromatic plants under changing environment. J. Appl. Res. Med. Aromat. Plants. 2022, 32, 100441. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Quantifying how climatic factors influence essential oil yield in wild-growing plants. Arab. J. Geosci. 2021, 14, 1257. [Google Scholar] [CrossRef]
- Abdelmajeed, N.A.; Danial, E.N.; Ayad, H.S. The effect of environmental stress on qualitative and quantitative essential oil of aromatic and medicinal plants. Arch. Sci. 2013, 66, 100–120. [Google Scholar]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Singh-Sangwan, N.; Abad Farooqi, A.H.; Singh Sangwan, R. Effect of drought stress on growth and essential oil metabolism in Lemongrasses. New Phytol. 1994, 128, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Morales, R. Synopsis of the genus Thymus L. in the Mediterranean area. Lagascalia 1997, 19, 249–262. [Google Scholar]
- Tsiftsoglou, O.S.; Stagiopoulou, R.; Krigas, N.; Lazari, D. Exploring the Ecological Preferences and Essential Oil Variability in Wild-Growing Populations of the Endangered Local Greek Endemic Thymus holosericeus (Lamiaceae). Plants 2023, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O.; Constantinidis, T. Chemotaxonomic significance of volatile compounds in Thymus samius and its related species Thymus atticus and Thymus parnassicus. Biochem. Syst. Ecol. 2005, 33, 1131–1140. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomi, F.; Tomas, X.; Canigueral, S.; Casanova, J.; da Chuna, A.P.; Adzet, T. Composition and infraspecific variability of the essential oil from Thymus camphoratus. Phytochemistry 1997, 45, 1177–1183. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomas, X.; Canigueral, S.; Paiva, J.; da Chuna, A.P.; Adzet, T. Chemotaxonomic study on Thymus villosus from Portugal. Biochem. Syst. Ecol. 2000, 28, 471–482. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomas, X.; Canigueral, S.; Paiva, J.; da Chuna, A.P.; Adzet, T. Essential oil composition and variability of Thymus lotocephalus and Thymus mourae. Biochem. Syst. Ecol. 2000, 28, 457–470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous influences on plant secondary metabolite levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential Oil Composition of Five Thymus Species from Bulgaria. Chem. Biodivers. 2021, 18, e2100498. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.G.; Evergetis, E.T.; Aligiannis, N.; Mitakou, S.; Nychas, G.J.E.; Haroutounian, S.A. Correlation between Chemical Composition of Greek Essential Oils and their Antibacterial Activity against Food-borne Pathogens. Nat. Prod. Commun. 2007, 2, 1934578X0700200413. [Google Scholar] [CrossRef]
- Maresca, V.; Badalamenti, N.; Ilardi, V.; Bruno, M.; Bontempo, P.; Basile, A. Chemical Composition of Thymus leucotrichus var. creticus Essential Oil and Its Protective Effects on Both Damage and Oxidative Stress in Leptodictyum riparium Hedw. Induced by Cadmium. Plants 2022, 11, 3529. [Google Scholar] [CrossRef] [PubMed]
- Cüce, M.; Basançelebi, O. Comparison of Volatile Constituents, Antioxidant and Antimicrobial Activities of Thymus leucotrichus (Lamiaceae) Stem and Leaves Essential Oils from Both Natural Resources and In vitro Derived Shoots. J. Essent. Oil-Bear. Plants 2021, 24, 1097–1112. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Reichard, S.H. Current practice in the use of subspecies, variety, and forma in the classification of wild plants. Taxon 1992, 41, 485–498. [Google Scholar] [CrossRef]
- Tümen, G.; Kirimer, N.; Kürkçüoglu, M.; Baser, K.H.C. Composition of the Essential Oils of Thymus atticus and Thymus roegneri from Turkey. Essent. Oil Res. 1997, 9, 473–474. [Google Scholar] [CrossRef]
- Boira, H.; Blanquer, A. Environmental factors affecting chemical variability of essential oils in Thymus piperella L. Biochem. Syst. Ecol. 1998, 26, 811–822. [Google Scholar] [CrossRef]
- Ložienė, K.; Vaičiūnienė, J.; Venskutonis, P.R. Chemical composition of the essential oil of different varieties of thyme (Thymus pulegioides L.) growing wild in Lithuania. Biochem Syst Ecol. 2003, 31, 249–259. [Google Scholar] [CrossRef]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Förster, C.; Leidecker, F.; Wiese, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The biosynthesis of thymol, carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of gamma-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Stahl Biskup, E.; Sáez, F. Thyme: The Genus Thymus; Medicinal and Aromatic Plants—Industrial Profiles Series; Taylor and Francis: London, UK; New York, NY, USA, 2002. [Google Scholar]
- Polatoglu, K. “Chemotypes”—A Fact that should not be Ignored in Natural Product Studies. J. Nat. Prod. 2013, 3, 10–14. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. Linalool–A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1934578X0800300727. [Google Scholar] [CrossRef]
- Yang, H.; Jung, E.M.; Ahn, C.; Lee, G.S.; Lee, S.Y.; Kim, S.H.; Choi, I.G.; Park, M.J.; Lee, S.S.; Choi, D.H.; et al. Elemol from Chamaecyparis obtusa ameliorates 2, 4-dinitrochlorobenzene-induced atopic dermatitis. Int. J. Mol. Med. 2015, 36, 463–472. [Google Scholar] [CrossRef]
- Santos, E.L.; Freitas, P.R.; Araújo, A.C.J.; Almeida, R.S.; Tintino, S.R.; Paulo, C.L.R.; Silva, A.C.A.; Silva, L.E.; do Amaral, W.; Deschamps, C.; et al. Enhanced antibacterial effect of antibiotics by the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. and its major constituent beta-caryophyllene. Phytomed. Plus. 2021, 1, 100100. [Google Scholar] [CrossRef]
| Taxon | Collection Site | Latitude | Longitude | Elevation (m) |
|---|---|---|---|---|
| T.parnassicus | Mt. Parnitha | 38°10′23″ | 23°43′41″ Ε | 1300 |
| T.parnassicus | Mt. Parnassos | 38°33′39″ | 22°34′26″ Ε | 1700 |
| T. atticus | Mt. Chelmos | 38°05′14″ | 22°10′30″ | 950 |
| T. leucotrichus subsp. leucotrichus | Mt. Chelmos | 37°59′20″ | 22°11′25″ | 2150 |
| T. holosericeus | Κephalonia (Mt. Roudi) | 38°10′31″ | 20°36′41″ | 870 |
| T. holosericeus | Κephalonia (Mt. Enos) | 38°07′35″ | 20°42′04″ | 1110 |
| T. laconicus | Peloponnese (Geraki Lakonias) | 36°58′53″ | 22°44′26″ | 340 |
| Subsect. Subbracteati | ||||||||
|---|---|---|---|---|---|---|---|---|
| % Composition | ||||||||
| No | Classification [a] | Compound Identification | A.I Experimental | A.I Literature | T. parnassicus (Mt. Parnitha) | T. parnassicus (Mt. Parnassos) | T. atticus (Mt. Chelmos) | T. leucotrichus subsp. leucotrichus (Mt. Chelmos) |
| 1 | MH | α-pinene [b] | 931 | 932 | 0.63 | 1.89 | - [c] | - |
| 2 | MH | camphene | 947 | 946 | - | 0.08 | - | - |
| 3 | others | benzaldehyde | 955 | 952 | - | - | - | 0.22 |
| 4 | AL | 1-octen-3-ol | 974 | 974 | 0.13 | 0.42 | 0.68 | 0.72 |
| 5 | AL | 6-methyl-5-hepten-2-one | 979 | 981 | tr. * | - | - | - |
| 6 | MH | myrcene | 986 | 988 | 0.11 | tr. | - | - |
| 7 | AL | 3-octanol [b] | 991 | 988 | tr. | 0.26 | tr. | - |
| 8 | AL | (2E, 4E)-Heptadienal | 1006 | 1005 | - | - | - | 0.11 |
| 9 | MH | α-terpinene [b] | 1015 | 1014 | - | tr. | 0.25 | - |
| 10 | MH | p-cymene [b] [d] | 1022 | 1020 | 0.15 | tr. | 25.63 | - |
| 11 | MH | limonene | 1027 | 1024 | 0.22 | 1.10 | 0.08 | - |
| 12 | OM | eucalyptol | 1029 | 1026 | 0.84 | 3.91 | - | - |
| 13 | others | benzylalcohol | 1034 | 1026 | - | - | - | 0.44 |
| 14 | others | benzene acetaldehyde | 1038 | 1036 | tr. | 0.08 | - | 2.22 |
| 15 | MH | (Ε)-β-ocimene | 1042 | 1044 | tr. | -- | - | - |
| 16 | MH | γ-terpinene [b] | 1055 | 1054 | 0.21 | 0.21 | 1.36 | - |
| 17 | OM | cis-sabinene hydrate | 1065 | 1064 | - | - | 0.30 | - |
| 18 | OM | cis-linalool oxide | 1067 | 1067 | tr. | tr. | 0.39 | - |
| 19 | OM | trans-linalool oxide | 1083 | 1084 | tr. | - | 0.37 | - |
| 20 | OM | linalool [b] | 1097 | 1095 | 3.11 | 5.30 | 63.04 | 4.04 |
| 21 | AL | 1-octen-1-ol, acetate | 1105 | 1112 | 0.10 | tr. | - | - |
| 22 | others | 2,6-dimethyl-cyclohexanol | 1108 | - | - | - | - | 0.15 |
| 23 | PHP | phenyl ethyl alcohol | 1114 | 1106 | - | - | - | 0.15 |
| 24 | OM | camphor | 1143 | 1141 | 2.02 | 3.53 | - | 0.08 |
| 25 | OM | lavandulol | 1163 | 1165 | - | - | 0.13 | - |
| 26 | OM | borneol | 1168 | 1165 | 2.73 | 2.80 | - | - |
| 27 | OM | terpinen-4-ol [b] | 1177 | 1174 | 0.69 | 2.52 | 0.30 | 0.28 |
| 28 | OM | α-terpineol [b] | 1191 | 1186 | 2.06 | 1.74 | 0.19 | 0.22 |
| 29 | OM | cis-dihydrocarvone | 1194 | 1191 | - | 0.20 | - | - |
| 30 | OM | trans-dihydrocarvone | 1200 | 1200 | - | 0.31 | - | - |
| 31 | AL | decanal | 1203 | 1201 | tr. | tr. | - | - |
| 32 | OM | trans-carveol | 1215 | 1215 | - | tr. | - | - |
| 33 | others | 2,3-dihydro-benzofuran | 1218 | - | - | - | - | 0.60 |
| 34 | OM | citronellol | 1222 | 1223 | tr. | - | - | - |
| 35 | OM | nerol | 1225 | 1227 | tr. | - | - | tr. |
| 36 | OM | thymol methyl ether | 1226 | 1232 | tr. | tr. | - | - |
| 37 | OM | pulegone | 1234 | 1233 | - | tr. | - | - |
| 38 | OM | carvone | 1239 | 1239 | tr. | 1.09 | - | - |
| 39 | OM | thymoquinone | 1242 | 1248 | - | - | 0.26 | - |
| 40 | OM | geraniol | 1247 | 1249 | 0.38 | - | - | 2.45 |
| 41 | OM | geranial | 1264 | 1264 | tr. | - | - | 0.09 |
| 42 | OM | bornyl acetate | 1280 | 1284 | 0.64 | 1.99 | - | - |
| 43 | OM | thymol | 1287 | 1289 | 0.42 | - | 0.11 | 0.82 |
| 44 | OM | carvacrol | 1295 | 1298 | 0.21 | 0.11 | 5.82 | 3.27 |
| 45 | OM | 2-methoxy-4-vinylphenol | 1305 | 1309 | tr. | - | - | 0.77 |
| 46 | SEH | δ-elemene | 1329 | 1335 | tr. | - | - | - |
| 47 | SEH | α-cubebene | 1344 | 1348 | 0.12 | tr. | - | - |
| 48 | OM | eugenol | 1356 | 1356 | - | - | - | 0.05 |
| 49 | OM | neryl acetate | 1355 | 1359 | 0.32 | tr. | - | - |
| 50 | OM | carvacrol acetate | 1366 | 1370 | - | - | 0.62 | - |
| 51 | SEH | α-copaene | 1372 | 1374 | 0.98 | 0.10 | - | - |
| 52 | OM | geranyl acetate | 1374 | 1379 | 0.92 | 0.17 | - | - |
| 53 | SEH | β-bourbonene | 1379 | 1387 | 0.22 | 0.16 | - | - |
| 54 | SEH | β-elemene | 1385 | 1389 | 0.86 | 0.17 | - | - |
| 55 | SEH | (Z)-caryophyllene | 1400 | 1408 | tr. | 0.10 | - | - |
| 56 | SEH | α-gurjunene | 1403 | 1409 | tr. | - | - | - |
| 57 | SEH | E-caryophyllene [b] | 1415 | 1417 | 11.83 | 35.20 | 0.16 | - |
| 58 | SEH | β-copaene | 1425 | 1430 | 0.07 | tr. | - | - |
| 59 | SEH | trans-α-bergamotene | 1429 | 1432 | tr. | tr. | - | - |
| 60 | SEH | aromadendrene | 1433 | 1439 | tr. | - | - | - |
| 61 | SEH | cis-β-farnesene | 1437 | 1440 | tr. | - | - | - |
| 62 | SEH | α-humulene | 1450 | 1452 | 1.09 | 1.85 | - | - |
| 63 | OM | E-geranylacetone | 1443 | 1453 | - | 0.09 | - | - |
| 64 | SEH | allo-aromadendrene | 1455 | 1458 | 0.61 | - | - | - |
| 65 | SEH | Dauca-5,8-diene | 1467 | 1471 | 0.16 | - | - | - |
| 66 | SEH | γ-muurolene | 1470 | 1478 | 0.24 | tr. | - | - |
| 67 | SEH | δ-germacrene | 1475 | 1480 | 2.08 | 1.20 | - | - |
| 68 | SEH | β-selinene | 1483 | 1489 | tr. | - | - | - |
| 69 | SEH | γ-amorphene | 1485 | 1495 | 0.45 | - | - | - |
| 70 | OM | 5,6-epoxy-β-ionone | 1490 | - | - | - | - | 0.12 |
| 71 | SEH | bicyclogermacrene | 1490 | 1500 | 1.09 | - | - | - |
| 72 | SEH | α-muurolene | 1494 | 1500 | 0.66 | - | - | - |
| 73 | SEH | β-bisabolene | 1504 | 1505 | 4.77 | 10.41 | - | - |
| 74 | SEH | γ-cadinene | 1507 | 1513 | 0.64 | - | - | - |
| 75 | SEH | δ-cadinene | 1513 | 1522 | 6.54 | 0.24 | - | - |
| 76 | SEH | zonarene | 1518 | 1528 | 0.21 | - | - | - |
| 77 | SEH | β-sesquiphellandrene | 1519 | 1521 | - | 0.14 | - | - |
| 78 | SEH | α-cadinene | 1531 | 1537 | 0.13 | - | - | - |
| 79 | SEH | α-calacorene | 1535 | 1544 | 0.09 | - | - | - |
| 80 | others | 5,6,7,7α-tetrahydro-4,4,7α-trimethyl-2(4H)-benzo-furanone | 1538 | - | - | - | - | 0.18 |
| 81 | SEO | elemol | 1543 | 1548 | 2.36 | 6.92 | - | 35.56 |
| 82 | SEO | (E)-nerolidol | 1557 | 1561 | 0.12 | 1.90 | - | - |
| 83 | AL | dodecanoic acid | 1574 | 1565 | - | - | - | 0.55 |
| 84 | SEO | palustrol | 1563 | 1567 | 0.12 | - | - | - |
| 85 | SEO | spathulenol | 1570 | 1577 | 0.94 | - | - | 0.77 |
| 86 | SEO | caryophyllene oxide [b] | 1575 | 1582 | 3.21 | 1.72 | 0.17 | 0.59 |
| 87 | SEO | viridiflorol | 1587 | 1592 | 0.23 | - | - | - |
| 88 | SEO | 1,10-di-epi-Cubenol | 1621 | 1618 | - | - | - | 0.25 |
| 89 | SEO | 1-epi-cubenol | 1621 | 1627 | 0.71 | - | - | |
| 90 | SEO | γ-eudesmol | 1625 | 1630 | 0.77 | 1.32 | - | 2.42 |
| 91 | SEO | caryophylla-4(12),8(13)-dien-5-ol | 1632 | 1639 | 0.49 | - | - | - |
| 92 | SEO | epi-α-muurolol | 1637 | 1640 | 6.49 | - | - | - |
| 93 | SEO | α-muurolol | 1640 | 1644 | 0.94 | - | - | - |
| 94 | SEO | β-eudesmol | 1648 | 1649 | - | 2.87 | - | 6.11 |
| 95 | SEO | α-cadinol | 1649 | 1652 | 13.53 | - | - | 5.82 |
| 96 | SEO | selin-11-en-4α-ol | 1656 | 1658 | 7.29 | - | - | - |
| 97 | SEO | α-eudesmol | 1666 | 1652 | - | - | - | 11.15 |
| 98 | SEO | α-bisabolol | 1679 | 1685 | - | tr. | - | - |
| 99 | SEO | shyobunol | 1703 | 1688 | - | - | - | 3.16 |
| 100 | SEO | (2E, 6Z)-farnesal | 1703 | 1713 | 1.02 | 0.10 | - | - |
| 101 | SEO | (2E, 6Z)-farnesol | 1714 | 1714 | 3.34 | 2.16 | - | - |
| 102 | SEO | (2Z, 6E)-farnesol | 1725 | 1722 | - | - | - | 0.26 |
| 103 | SEO | (2E, 6E)-farnesal | 1730 | 1740 | 1.8 | 0.47 | - | - |
| 104 | others | benzyl benzoate | 1756 | 1759 | - | tr. | - | - |
| 105 | AL | tetradecanoic acid | 1769 | - | - | - | - | 0.92 |
| 106 | SEO | cryptomeridiol | 1820 | 1813 | - | - | - | 0.27 |
| 107 | SEO | (2Z, 6E)-farnesyl acetate | 1828 | 1821 | 2.13 | - | - | - |
| 108 | AL | hexadecanoic acid | 1975 | 1959 | - | - | - | 4.13 |
| %Yield (mL/100 g of dry plant material) | 0.35 | 0.32 | 0.3 | 0.5 | ||||
| AL | Aliphatic compounds | 0.23 | 0.68 | 0.68 | 6.43 | |||
| MH | Monoterpene hydrocarbons | 1.32 | 3.28 | 27.32 | - | |||
| OM | Oxygenated monoterpenes | 14.34 | 23.76 | 71.53 | 12.19 | |||
| SEH | Sesquiterpenes hydrocarbons | 32.84 | 49.57 | 0.16 | - | |||
| SEO | Sesquiterpenes oxygenated | 46.43 | 17.46 | 0.17 | 66.36 | |||
| others | - | 0.08 | - | 3.96 | ||||
| TOTAL | 95.51 | 94.83 | 99.24 | 88.94 | ||||
| Subsect. Thymbropsis | |||||||
|---|---|---|---|---|---|---|---|
| % Composition | |||||||
| No | Classification [a] | Compound Identification | A.Ι Experimental | A.Ι Literature | T. holosericeus (Mt. Roudi) | T.holosericeus (Mt. Enos) | T. laconicus Peloponnesse (Geraki Laconias) |
| 1 | MH | α-thujene | 925 | 924 | - [c] | - | 0.3 |
| 2 | MH | α-pinene [b] | 932 | 932 | - | - | 1.1 |
| 3 | MH | camphene | 948 | 946 | - | - | 2.0 |
| 4 | AL | 1-octen-3-ol | 976 | 974 | - | - | 1.1 |
| 5 | MH | myrcene | 989 | 988 | - | 0.1 | 0.4 |
| 6 | MH | α-phellandrene | 1005 | 1002 | - | - | tr.* |
| 7 | MH | α-terpinene | 1017 | 1014 | - | - | 0.7 |
| 8 | MH | p-cymene [b][d] | 1029 | 1020 | - | - | 29.7 |
| 9 | others | benzene acetaldehyde | 1037 | 1036 | tr. | - | - |
| 10 | MH | (E)-β-ocimene | 1049 | 1044 | - | tr. | - |
| 11 | MH | γ-terpinene [b] | 1060 | 1054 | - | - | 6.8 |
| 12 | OM | cis-linalool oxide | 1070 | 1067 | 0.1 | - | - |
| 13 | OM | trans-linalool oxide | 1083 | 1084 | tr. | - | - |
| 14 | MH | terpinolene | 1089 | 1086 | - | - | tr. |
| 15 | MH | p-cymenene | 1089 | 1089 | - | - | 0.3 |
| 16 | OM | Linalool [b] | 1100 | 1095 | 0.4 | 0.5 | 1.0 |
| 17 | OM | camphor | 1145 | 1141 | - | - | 0.1 |
| 18 | OM | borneol | 1171 | 1165 | 1.2 | 0.2 | 8.2 |
| 19 | OM | terpinen-4-ol [b] | 1181 | 1174 | - | tr. | 2.1 |
| 20 | OM | p-cymen-8-ol | 1185 | 1179 | - | - | 0.2 |
| 21 | OM | α-terpineol [b] | 1192 | 1186 | - | - | 0.1 |
| 22 | OM | verbenone | 1207 | 1204 | 0.1 | - | - |
| 23 | OM | nerol | 1227 | 1227 | 0.5 | 0.4 | - |
| 24 | OM | neral | 1238 | 1235 | tr. | 0.6 | - |
| 25 | OM | geraniol | 1247 | 1249 | 89.9 | 87.7 | - |
| 26 | OM | geranial | 1275 | 1264 | 1.1 | - | - |
| 27 | OM | bornyl acetate | 1287 | 1284 | - | - | 0.3 |
| 28 | OM | lavandulyl acetate | 1299 | 1288 | - | 0.1 | - |
| 29 | OM | thymol | 1303 | 1289 | tr. | 1.7 | 7.9 |
| 30 | OM | carvacrol | 1311 | 1298 | - | 0.4 | 32.7 |
| 31 | OM | ethyl nerolate | 1361 | 1351 | - | tr. | - |
| 32 | OM | carvacrol acetate | 1366 | 1370 | - | - | tr. |
| 33 | OM | geranyl acetate | 1385 | 1379 | 0.7 | 0.2 | - |
| 34 | SEH | β-bourbonene | 1389 | 1387 | 0.2 | - | - |
| 35 | SEH | E-caryophyllene [b] | 1424 | 1417 | 0.7 | 2.0 | 2.8 |
| 36 | SEH | α-humulene | 1460 | 1452 | - | 0.1 | tr. |
| 37 | OM | geranyl propanoate | 1474 | 1476 | - | 0.1 | - |
| 38 | SEH | δ-germacrene | 1487 | 1480 | 0.2 | tr. | - |
| 39 | SEH | bicyclogermacrene | 1502 | 1500 | 0.4 | 0.1 | - |
| 40 | SEH | δ-Cadinene | 1527 | 1522 | 0.1 | -- | - |
| 41 | OM | geranyl butanoate | 1562 | 1562 | - | 1.2 | - |
| 42 | SEO | spathulenol | 1586 | 1577 | 0.8 | 0.1 | - |
| 43 | SEO | caryophyllene oxide [b] | 1592 | 1582 | 1.0 | 1.1 | 1.4 |
| 44 | OM | geranyl isovalerate | 1603 | 1606 | - | 0.5 | - |
| 45 | SEO | caryophylla-4(12),8(13)-dien-5-ol | 1645 | 1639 | - | 0.1 | tr. |
| 46 | OM | geranyl valerate | 1656 | 1655 | - | tr. | - |
| 47 | SEO | epi-β-bisabolol | 1672 | 1670 | 0.4 | - | - |
| % Yield (mL/100 g of dry plant material) | 2 | 3.3 | 2 | ||||
| AL | Aliphatic compounds | - | - | 1.1 | |||
| MH | Monoterpene hydrocarbons | - | 0.1 | 41.3 | |||
| OM | Oxygenated monoterpene | 94 | 93.6 | 52.6 | |||
| SEH | Sesquiterpenes hydrocarbons | 1.6 | 2.2 | 2.8 | |||
| SEO | Sesquiterpenes oxygenated | 2.2 | 1.3 | 1.4 | |||
| TOTAL | 97.8 | 97.2 | 99.2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakouri, E.; Daferera, D.; Andriopoulou, A.; Trigas, P.; Tarantilis, P.A. Evaluation of the Essential Oil Composition of Five Thymus Species Native to Greece. Chemosensors 2024, 12, 7. https://doi.org/10.3390/chemosensors12010007
Kakouri E, Daferera D, Andriopoulou A, Trigas P, Tarantilis PA. Evaluation of the Essential Oil Composition of Five Thymus Species Native to Greece. Chemosensors. 2024; 12(1):7. https://doi.org/10.3390/chemosensors12010007
Chicago/Turabian StyleKakouri, Eleni, Dimitra Daferera, Anastasia Andriopoulou, Panayiotis Trigas, and Petros A. Tarantilis. 2024. "Evaluation of the Essential Oil Composition of Five Thymus Species Native to Greece" Chemosensors 12, no. 1: 7. https://doi.org/10.3390/chemosensors12010007
APA StyleKakouri, E., Daferera, D., Andriopoulou, A., Trigas, P., & Tarantilis, P. A. (2024). Evaluation of the Essential Oil Composition of Five Thymus Species Native to Greece. Chemosensors, 12(1), 7. https://doi.org/10.3390/chemosensors12010007









