Abstract
The development of flexible electronic technology has led to significant advancements in wearable sensors. In the past decades, wearable chemosensors have received much attention from researchers worldwide due to their high portability, flexibility, lightweight, and adaptability. It allows real-time access to the user’s physiological status at the molecular level to analyze their health status. Therefore, it can be widely used in the field of precision medicine. This review introduces the sensing mechanisms of wearable chemosensors and recent progress in wearable sweat and interstitial fluid-based chemosensors. The complexities of wearable chemosensors are not to be underestimated, as there are considerable challenges in this field. This review aims to shed light on the difficulties associated with designing wearable sweat and interstitial fluid-based chemosensors and their potential development directions.
1. Introduction
Owing to the aging population and society’s development, people have higher demands for healthcare. Traditional medical care requires the patient/examinee to undergo continuous physical examinations in the hospital. The doctor assesses the physical condition of the patient based on the knowledge learned and the results of the examinations, as well as the experience of previous treatments. However, this approach often lacks individualized diagnosis and management. There is a need for a patient-specific approach that takes current status and examination results into account [1]. This is where the burgeoning field of Precision Medicine and Point of Care comes into play. From clinical practice to evidence-based care, precision medicine refers to a medical approach based on an individual’s genetics, biomarkers, current psychological status, and social status. The significant difference from traditional methods is that it considers the applicability of treatments for different conditions to varying individuals while reducing the misjudgments and omissions associated with the traditional approach [2,3]. Point of Care means that biochemical tests can be performed anytime and anywhere when patients need medical care [4]. This remote and instantaneous test offers prompt access to a patient’s physical condition, compared with a hospital-based test. When combined with the latest information technology, it enables diagnosis at home. It offers a promising way to analyze physical status and care. As information technology advances, the Internet of Medical Things (IOMT)—a complex system of medical sensors and actuators connected via the Internet—has received attention from researchers in the past as a type of Internet of Things [5]. IOMT has great potential to change the current state of healthcare by obtaining the physical status of users through IOMT devices and forming a system of prevention and treatment that can help improve the health of society as a whole [5,6,7,8]. With the innovation of materials, sensing methods, and energy supply, personalized wearable sensors can be used as a new medical tool in Precision Medicine and Point of Care [9,10,11,12].
Wearable sensors are capable of acquiring the user’s physiological status at the molecular level in real time without affecting activities in daily life. These sensors can collect physiological data in a non-invasive manner and assist in clinical diagnosis or even replace it to a certain extent. Previous research has demonstrated that wearable sensors have many applications in disease monitoring and control, physiological signal acquisition, and health state monitoring [13,14,15,16,17,18,19,20]. The first generation of wearable sensors primarily focuses on the physical signals generated by user activities such as bioelectrical signals (electrocardiogram (ECG), electromyogram (EMG), electroencephalogram (EEG)), physical activity, heartbeat, body temperature, etc. [21,22,23,24,25,26,27,28,29,30]. The first generation of wearable sensors can measure physical signals with great sensitivity but lacks a comprehensive molecular perspective on monitoring. The study of physiological mechanisms recognizes that biomarkers in the body fluid secreted by humans contain much information and that the biomarkers can reflect the current physiological conditions [31,32]. The emergence of a new generation of wearable sensors that use biofluid to convert the concentration values of specific analytes into electrical signals or colors has addressed this issue. Prototype sensors in the laboratory have demonstrated the ability to monitor metabolites, electrolytes, drugs, etc., in the user’s body fluids [11,33,34,35,36,37,38,39,40,41,42]. Successfully commercialized products such as FREESTYLE LIBRE, Gx Sweat Patch, Dexcom G7, etc., also validate the usefulness of the next generation of sensors. Both types of wearable sensors mentioned above track only a single physical or chemical aspect, limiting our ability to analyze the body’s current state. Given that the human body undergoes complex changes when moving or sitting still [43,44], a multimodal sensing approach has been developed that can provide multi-perspective monitoring from both physical and chemical aspects [45]. This approach offers a more comprehensive view of the body’s state, allowing for more precise monitoring and better-informed decisions.
In this review, we will focus on the latest advancements in wearable chemosensors that allow for non-invasive health monitoring through real-time analysis of body fluids. Our primary focus will be on two key areas of research: continuous and non-invasive biomarker monitoring of human sweat and interstitial fluids. As we explore this topic, we will examine various electrode and sensing materials, system-level configurations, and working principles employed for continuous biomarker monitoring. Furthermore, we will discuss the challenges and opportunities presented by wearable chemosensors, as well as potential avenues for their future development (Figure 1).
Figure 1.
Wearable chemosensors in physiological monitoring.
2. Sensing Methods
A chemical sensor is defined as a device that converts information about the chemical composition of an analyte into a signal that can be processed [11,46]. A general chemical sensor can be divided into two main parts: (1) a specific identification element: it has high selectivity and sensitivity for the analyzed components under complex chemical environmental conditions. (2) a chemical-physical transducer device that converts chemical information, such as the concentration or chemical composition of the analyte, into measurable and analyzable physical information under test conditions. We can classify chemical sensors by signal conversion into chemosensors and optical/visual sensors [47]. Currently, urine and blood tests are standard methods for clinical use to obtain complete health information about being tested for medical treatment. However, we cannot access real-time health information and comprehensive knowledge of the patient’s treatment status in this way. Additionally, professionals are required to perform the tests in the laboratory using specialized, bulky, and expensive testing instruments to test the collected samples, in addition to the possible need to pre-treat the samples. Further, assays that utilize blood as a sample for analysis require invasive sampling of the subject, which can pose hygiene and psychological problems. Moreover, colorimetric methods to acquire the user’s real-time physiological status have also been widely used in clinical applications. Colorimetric sensing has been applied to monitor biomarkers such as glucose [48,49], proteins [48,50], nucleic acids [51], and urea [52]. This technology offers several advantages, including its low cost and ease of use, and has the potential to provide rapid diagnostic tests without requiring specialized training. However, the complexity of the samples and the deficient concentrations of biomarkers make the colorimetric method challenging. At the same time, colorimetric detection cannot achieve high-precision quantitative detection of the target compared to labor-intensive laboratory quantitative methods.
In summary, the currently prevalent methods impose strict conditions for real-time and complete health monitoring of the general population, so developing a technology that can continuously monitor health status at the molecular level is urgently needed. Chemosensors are attracting the attention of many researchers worldwide as an important avenue for future health monitoring and personalized medicine because of their label-free, miniaturization, high portability, real-time tracking, no need for detection reagents, and non-invasive features. In this section, we introduce the amperometry, potentiometry, voltammetry, and conductometry methods, which convert the chemical information of the test object into electrical signals that can be measured and displayed accurately in real-time.
2.1. Amperometric Sensors
Current electrochemical sensors are typically configured as a three-electrode system, consisting of a working electrode where the redox reaction of the analyzed sample takes place, a reference electrode used to maintain a fixed voltage at the working electrode, and a counter electrode, which completes the current circuit and is used to detect the magnitude of the current generated by the reaction [53,54]. Amperometric sensors are effective in detecting samples with low redox voltages, such as glucose, lactate, uric acid, etc. Also, due to the high selectivity and sensitivity of enzymes for detection, various enzymes are used as modification materials for the working electrode in amperometric sensors, such as glucose oxidase, lactate oxidase, etc.
By applying a constant working voltage between the working electrode and the reference electrode, the molecules selected by the enzyme at the working electrode undergo a redox reaction. The electrons exchanged between the detected substance and the electrode during the redox reaction generate a current, the magnitude of which is proportional to the concentration of the detected molecule. There have been three generations of amperometric sensors. Here, we introduce the detection principles of the three generations of sensors, using the glucose sensor as an example. In the first generation of sensors, the concentration of glucose is calculated by detecting the hydrogen peroxide produced by the glucose oxidation reaction. In the second generation of sensors, a small-molecule substance, such as Prussian Blue, is used as a medium for electron transfer for glucose oxidation, acting as a bridge of electrons between the reaction center of the enzyme and the electrode. It can minimize the voltage applied to the reaction to reduce the error caused by the response of impurities. Third-generation sensors directly measure the electron exchange between glucose and the electrode. Amperometric sensors have been successfully used in commercial sensors (Figure 2a,b).
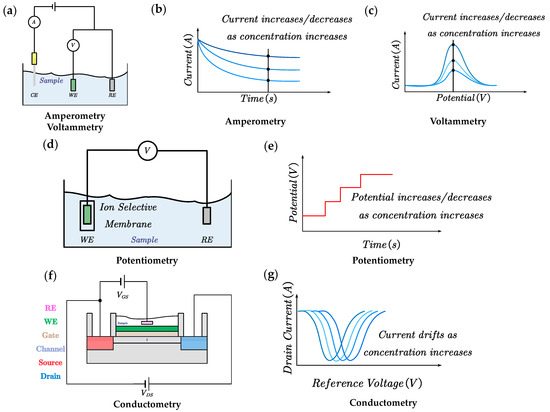
Figure 2.
Sensing methods for wearable chemosensors. (a) System diagram of amperometric sensors; (b,c) The generated current signal increases/decreases with the increase in concentration. (d) System diagram of potentiometic sensors. (e) The generated voltage signal increases/decreases with the increase in concentration. Here we demonstrate how the sensing method relies on the ‘signal-on’ mechanism. (f) System diagram of conductometric sensors. (g) The generated current signal drifts with the increase in concentration. Abbreviations: CE, counter electrode; WE, working electrode; RE, reference electrode. VGS, voltage across gate and source; VDS, voltage across drain and source.
2.2. Voltammetric Sensors
Voltammetric and amperometric sensors are three-electrode systems widely used to detect substances such as metabolites, nutrients, and drugs. These electroactive substances undergo a direct chemical reaction on the surface of the electrode, gaining or losing electrons upon applying the corresponding voltage. The concentration of the detected substance is reflected by measuring the current level. According to the waveform of the voltage applied on the electrode, we can divide voltammetry into many subcategories, such as cyclic voltammetry, differential pulse, square wave voltammetry, and other methods [54]. The current variation in voltammetry arises from two components: (1) Faraday current, which comes from the chemical reaction and directly reflects the information of the analyte at the electrode. (2) Charging current, also known as background current, comes from the ion rearrangement in the electrode solution. Compared with amperometric sensors, the magnitude of the background current generated during the detection process can be reduced by applying a variable voltage, improving the detection sensitivity of voltammetry. In particular, wearable detection can use anodic dissolution voltammetry to detect minimal amounts of heavy metal ions in body fluids [55]. However, the absence of specific functional material, such as enzymes, may affect the detection of the current by interfering with substances with redox voltages similar to those of the detected material (Figure 2a,c).
2.3. Potentiometric Sensors
Potentiometric sensors are commonly employed in electrochemical sensors to detect ions in biological fluids such as pH, Na+, K+, etc. Like amperometric sensors, potentiometric sensors typically consist of a working electrode and a reference electrode, but they do not apply a voltage between the electrodes or measure the reaction current [53,54]. Instead, the concentration of the estimated analyte is usually detected by measuring the voltage between the working electrode and the reference electrode. According to the Nernst equation, the voltage generated between the two-electrode system is linearly related to the logarithmic value of the ion concentration. In general, potentiometric sensors usually consist of an ion-selective electrode and an Ag/AgCl reference electrode. The ion-selective electrode (ISE) is usually functionalized by an ion-selective membrane (only ions of a specific size and charge can migrate through the membrane, like a sodium ionophore X for Na+ or valinomycin for K+), combined with an ion-to-electron transducer that converts the resulting interfacial potential difference between the ISE and the solution into a voltage signal [56]. The ISE provides high selectivity and sensitivity to the measured species (Figure 2d,e).
2.4. Conductometric Sensors
Conductometric sensors typically employ field-effect transistors (FETs) that operate with an affinity-based detection principle. The gate of the FET is modified with aptamers, antibodies, receptor proteins, etc. A measurable surface charge perturbation is generated when the targeted analyte binds to the sensing element. The change in the surface charge of the semiconductor is expressed as a change in the effective VGS and, subsequently, as a change in the IDS. The detected material’s concentration can be reflected by measuring the IDS of the FET [57]. This method is compatible with current CMOS (complementary metal–oxide–semiconductor) manufacturing processes [56]. However, it has yet to be widely used in wearable devices due to the requirement of instrumentation for FET detection (Figure 2f,g).
3. Sweat-Based Sensors
Sweat is a vital and readily available biological fluid. The sweat produced by the human body is rich in chemical components that reflect the health status [58]. The sweat glands are widely distributed throughout the body, and we can collect sweat in a simple way. In wearable sensors, we mainly consider the eccrine sweat glands (used for body temperature, electrolyte, and pH balance regulation) rather than the apocrine sweat glands. Because the location of the apocrine sweat glands is not conducive to sensor design, the apocrine sweat glands are susceptible to being interfered with by lipids and bacteria, which prevents accurate results [59]. The sweat produced by eccrine sweat glands has electrolytes (Na+, Cl−, K+, Ca2+, etc.) [60,61,62], metabolic substances (glucose, lactic acid, alcohol, uric acid, etc.), drugs (caffeine, Levodopa, etc.) [63,64], trace metals (zinc, copper, etc.) [65], proteins and hormones [66,67,68], etc. In contrast to traditional sampling methods, such as blood sampling and catheters, we can use sweat glands all over the skin to non-invasively obtain the sweat secreted by the body. Also, with the development of technology, we can stimulate the production of sweat at the sensor site by iontophoresis to meet the needs of the sensor. In addition, most sweat sensors utilize appropriate sensing enzymes, allowing us to improve the sensor’s response time for real-time monitoring. We can use the concentration of abnormal sweat chemistry to monitor, prevent, and treat human diseases and conditions [69,70,71]. For example, the chloride and sodium ions in the sweat of patients with cystic fibrosis are significantly higher than in normal subjects [72]. A strong correlation exists between ethanol concentrations in human sweat and blood [73]. Furthermore, significant changes in sweat composition can be observed in athletes’ sweat when they are in different body states [74]. Although sweat detection has shown great potential in healthcare, it has many problems. (1) Without iontophoresis to stimulate sweating, the sweat rate and location of sweat secretion in the human body are very heterogeneous, which makes it difficult to set up sensors. (2) The sweat secreted in normal individuals is generally weakly acidic, affecting the activity of the enzymes on the sensor, which affects the sensor’s accuracy. (3) Since the sweat glands of the skin are more susceptible to evaporation and pollution interference than other biological fluid secretions, it affects the real-time accuracy of the sensor. Sweat sensing may still have some unresolved issues, but it has garnered enough attention from researchers. The research on sweat sensors is shown in Table 1, which lists the current research papers on sweat sensors in terms of sensed objects, sensing methods, identification materials, and system structures.

Table 1.
Wearable chemosensors for continuous sweat analysis.
Along with the research and development investment related to electrochemical sensing mechanisms, sensing recognition materials, electrode design, and system integration, sweat electrochemical sensor platforms have advanced from single-sensing objects to complex multimodal sensing platforms, including electrochemical and physical-chemical sensing platforms for a variety of measured things. The current sweat sensor platforms mainly use working electrodes functionalized by specific identification materials to convert the information of the analyte into electrical signals, which are processed by back-end circuits and transmitted to mobile devices before visualization at the user end. For sweat composition, the functional material for the monitoring of electrolytes is the ion-selective electrode (ISE); the monitoring of metabolic substances generally utilizes its enzymes, and the tracking of drugs, trace metals, proteins, and hormones in sweat is directly achieved using voltammetry, corresponding aptamers, and polymer-selective membranes. As early as 2014, Joseph Wang’s group used screen printing technology to develop a wearable tattoo system based on sodium ion-selective membranes that can monitor sodium ions in sweat in real time [62]. In order to improve the sensitivity and stability of the sensor while increasing its ability to be reused multiple times, Gao’s group improved the long-term stability by designing a three-dimensional gradient porous graphene electrode to limit the water layer generated on the ion-selective electrode after multiple uses of the sensor, while reducing the diffusion path of ions in the ion-selective membrane and enhancing the electroactive surface to improve the sensitivity, as shown in Figure 3a [75]. For stand-alone drug monitoring, a wearable system for caffeine monitoring was designed using carbon nanotubes and Nafion-modified carbon electrodes fabricated by Javey’s group through a roll-to-roll process [63]. The working electrode material is critical for the electrode surface to monitor the electrochemical reactions. The carbon electrode was selected due to its high scanning voltage stability under drug monitoring and good biocompatibility. In order to apply the sensors to a wide range of wearable health applications, a method capable of mass fabrication of sensors was needed. Gao’s group proposed a method using laser engraving capable of large-scale fabrication of graphene sensor arrays with both fast production and low cost while using immune-sensing methods. A correlation between cortisol in serum and sweat was found, as shown in Figure 3b [67]. In addition, Cheng et al. proposed a fiber-based wearable glucose sensor in which the electrochemical fabric sensor achieved high electrical conductivity in the fiber system, was able to bind well with enzymes, and was also able to perform the monitoring function under large deformation of the fiber, as shown in Figure 3c [82]. In addition to the sensors mentioned above, there are also electrochemical sensors based on transistor structures with a single analyte. Salleo et al. used molecularly imprinted polymers to create an MS-OECT (molecularly selective organic electrochemical transistor) to detect cortisol in human sweat by combining molecularly selective membranes with the channel of OECT. The specific binding of cortisol in sweat to the molecularly selective membrane alters the electrical properties of the OECT, which influences the electrical properties of the transistor to determine cortisol concentration by changes in the IDS [68]. Wang et al. used a novel cortisol aptamer combined with a transistor with In2O3 as the channel to achieve highly selective cortisol detection at low concentrations, as shown in Figure 3d [86]. Due to the semiconductor gating effect, the response generated by the FET is nonlinear, so the sensing concentration range is used as the standard of the sensor. Jai Eun An et al. designed a flexible, wearable cortisol sensing system using a liquid-ion-gated FET system. The nanofibers produced by electrospinning were conjugated with cortisol aptamers. The sensor exhibited a low detection limit of 10 pM with high selectivity. The sensor was transferred to an actual application scenario and also detected cortisol at a level of 1 nM from actual sweat [107]. However, using FET-type sensors also leads to new problems. The detection of the FET current under different static gate voltage biases will produce different sensor results, so the data need to be calibrated. In order to solve the problem of sensor power supply, NFC, BFC, TENG, photovoltaic cells, flexible cells, supercapacitors, etc., have been introduced into wearable systems [76,78,79]. By integrating photovoltaic cells and flexible cells into a wearable sensor system, Ali Javey’s group achieved the first sensing platform with self-powered capability on a single platform and also realized the functions of signal processing and display and in situ electrochemical detection of sweat glucose, as shown in Figure 3e [79]. Lu Yin et al. used a sweat-based biofuel cell and triboelectric generator to collect both chemical and physical energy and a designed supercapacitor to regulate the accumulated energy for high power output to meet the system requirements and also improve the energy collection efficiency, as shown in Figure 3f [76]. Min et al. designed a wearable chemosensor with integrated perovskite solar cells. The system utilizes a flexible quasi-two-dimensional perovskite solar cell module, which is capable of simultaneously monitoring the user’s glucose, pH, sodium, sweat rate, and temperature under different lighting conditions. The combination of a solar cell module greatly reduces the size of the system and provides a novel solution to address the power needs of wearable systems [108]. Yu et al. designed an ultra-thin and fabric-integrated sweat-active battery for wearable electrochemical devices that has good power output and can continuously supply power to the system for about 3 h, solving the power problem of wearable chemosensors. At the same time, the miniaturized system is designed to be well integrated with current sports gear and utilizes NFC for data transfer [109].
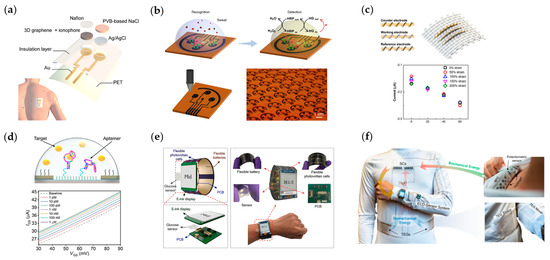
Figure 3.
Wearable and flexible sweat chemosensors. (a) Gradient porous graphene−based wearable chemosensor for health-care applications. Reproduced with permission from [75]. (b) Laser-engraving-graphene-based wireless system for monitoring cortisol in human sweat. Reproduced with permission from [67]. (c) Highly stretchable and strain−insensitive fiber-based wearable chemosensors for monitoring glucose in sweat. Reproduced with permission from [82]. (d) Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Reproduced with permission from [86]. (e) A fully integrated and self-powered smartwatch for continuous sweat glucose monitoring. Reproduced with permission from [79]. (f) A self-sustainable wearable multi-modular E-textile bioenergy microgrid system. Reproduced with permission from [76].
As research progresses, owing to the complex relationship between multiple biomarkers and current physiological status in the human body, as well as the need to calibrate biomarkers’ concentrations by measuring physical information such as sweat rate and temperature, because the dilution of analytes during sweat excretion is influenced by the sweat rate and temperature also affects enzyme activity, it is necessary to design a multimodal sensing platform [71]. Gao et al. constructed the first multifunctional sensor array-based platform to demonstrate the detection of sweat metabolites (glucose, lactate) and electrolytes (Na+, K+) and skin temperature, as shown in Figure 4a [14]. This study demonstrates the promising application of multimodal sensors in the field of physiological monitoring. Promoting the development of multi-analyte wearable sensors for sensing, signal transduction and processing, and system morphology and integration, enabling prolonged monitoring in everyday applications. Similarly, FET-based sensors based on ion-selective membranes have been extensively investigated in multi-analyte sensor fabrication. Adrian M. Ionescu’s group has designed and validated electrochemical biosensors compatible with CMOS processes using current semiconductor fabrication processes. By heterogeneously integrating an ultrathin silicon-on-insulator ion-selective FET with a biocompatible microfluidic structure for sweat sensing on a single silicon wafer, the amount of sweat used for sensing is significantly reduced, while the operating current of the sensor is lowered [56]. Xue Mantian et al. developed an ion-selective membrane-modified graphene channel-based FET-type sensor to detect potassium, sodium, and calcium ions; see Figure 4b. In this work, the researchers applied a random forest algorithm to quantify ion concentrations in the presence of multiple ionic analytes, which improved the sensor’s performance by combining the sensor array with the algorithm [60]. The rational design of the working electrode material is the key to the sensing performance of wearable electrochemical sensors. Zhang’s group synthesized a novel silk fabric-derived carbon textile that has high electrical conductivity due to its inherent nanostructure and porous structure. It can be used directly as a working electrode after being modified with functional materials. The electrochemical sensor array based on this material has high sensitivity, selectivity, and long-term stability; see Figure 4c [92]. The group of John A. Rogers combined electrochemical and optical methods to monitor glucose, lactate, chloride, and pH in sweat, using NFC to power the system and transmit the results to mobile devices; see Figure 4d [91]. The article also proposed a new current detection method for enzyme-based sensors—biofuel cell-based electrochemical sensors. This method generates a current between the anode and cathode that is proportional to the concentration of the analyte and converts the current into a corresponding voltage by using the resistance. The combination of this monitoring method and NFC can significantly reduce the size of the system for wearable applications. Due to the low power consumption of this detection method, Lu Yin et al. designed a stretchable printed battery and used this detection method to detect glucose and lactate in sweat, which greatly reduced the size of the system and improved the flexibility of the wearable system. In addition, for the detection of pH and Na ions, since the voltage generated by the detected ions on the sensor is proportional to their concentration, the research group introduced a fully flexible electrochromic material to convert the concentration of the detected substance into a logic output to solve the problem of information transfer with the user in the sweat sensing system [88]. For the detection of biomarkers in sweat, researchers are not only focused on the concentration of the analyte. Dae-Hyeong Kim’s group has designed a graphene-based glucose sensor that can also detect the pH of the functional surface, and the temperature is used to calibrate the readings of the glucose sensor. In this system, the researchers designed a bioresorbable, thermally responsive microneedle that enables controlled transcutaneous drug delivery of glucose, forming a Sense-act model with great application potential, as shown in Figure 4e [18]. For wearable sweat sensors, most of them are based on enzymes; how to improve the stability of enzyme-based sensors is the focus of research. Lei Yongjiu et al. designed a MXene/Prussian Blue-based sensor electrode, using superhydrophobic carbon fiber membranes as the substrate of the electrode. The electrode can form a solid–liquid–air three-phase interface, and because the diffusion coefficient of oxygen in the air is much higher than that of liquid, the oxygen level in the reaction system can be maintained at a high level of constancy, enhancing the current signal generated by the sensor, as shown in Figure 4f [96]. Similarly, to improve the stability and robustness of the sensor readout, Sam Emaminejad’s group designed a freestanding electrochemical sensing system device to directly connect body fluids on the skin to a flexible circuit that can detect glucose, lactate, and choline in sweat. The group analyzed an out-of-plane interconnection method to connect the sensor to the circuit in a region close to 0 strain, as shown in Figure 4g [90]. Using Au/Bi as the working electrode, Wei Gao et al. used square wave anodic dissolution voltammetry to partially reduce the metal ions measured by dissolving them into the microelectrode or precipitating them on the surface of the electrode, and then applied a reverse voltage to the electrode to oxidize the metal on the microelectrode and generate an oxidation current. The current-voltage curve of the oxidation process is used to detect the metal ions with very low concentrations in the sweat, as shown in Figure 4h [65]. Daniel Mukasa et al. have proposed QuantumDock, an automated computational framework for universal MIP development toward wearable applications. QuantumDock can optimize the design of molecularly imprinted polymers and improve the selectivity of target molecules. In this article, QuantumDock-based molecularly imprinted polymers in combination with LEG enabled the first non-invasive monitoring of phenylalanine in a human experiment [110].
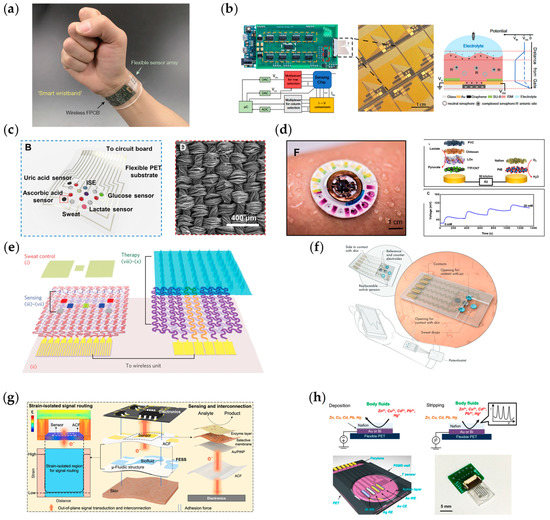
Figure 4.
Multifunctional flexible sweat chemosensors. (a) Fully integrated smart wristband with a flexible sensor array and a flexible PCB for multiplexed in situ sweat analysis. Reproduced with permission from [14]. (b) Integrated chemosensor platform based on graphene transistor arrays for real-time high-accuracy ion sensing. Reproduced with permission from [60]. (c) Integrated textile sensor patch for real-time and multiplex sweat analysis. B: Multiplex electrochemical sensor array integrated in the patch. D: SEM images of the carbonized silk fabric. Reproduced with permission from [92]. (d) Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous monitoring of sweat. F: Image illustrating the device during sweating. Reproduced with permission from [91]. (e) A graphene-based chemosensor with thermoresponsive microneedles for diabetes monitoring and therapy. Reproduced with permission from [18]. (f) Wearable chemosensor system for high-performance in vitro perspiration analysis. Reproduced with permission from [96]. (g) A wearable, freestanding electrochemical sensing system. Reproduced with permission from [90]. (h) Flexible sensor array for multiplexed heavy metal monitoring in sweat. Reproduced with permission from [65].
Sweat samples can be generated by passive or active approaches. The above sensors are based on passive methods, e.g., physical movement such as running, cycling, etc. In order to generate the amount of sweat required for sensing, the user needs to achieve a certain level of movement, which somewhat hinders the application of sensors in the disabled population. One method of active stimulation of sweating that is currently widely used by researchers in wearable sensors is iontophoresis, which stimulates sweat production at a fixed rate in a specific area of the stimulation electrode. As shown in Figure 5a, by placing a hydrogel that contains a stimulating agonist (such as methacholine, acetylcholine, or pilocarpine) underneath the stimulation electrode and generating a small current between the electrodes, the stimulant is conveyed to sweat glands near the anode, resulting in sweat production [99]. Based on iontophoresis, Sam Emaminejad et al. designed a multi-analyte active sweat detection platform using methacholine as a stimulant capable of modulating sweat rate and secretion interval by modulating different current input patterns. The electrochemical platform is capable of detecting the concentration of sodium, chloride, and glucose in sweat through sweat stimulation, as shown in Figure 5b [99]. Joseph Wang’s group achieved iontophoresis using pilocarpine nitrate as the stimulating agent, which was the first work to achieve a platform that can simultaneously sample and analyze sweat and ISF independently, detecting both alcohols in sweat and glucose in ISF. This parallel sweat and ISF monitoring combine both advantages, expanding the range of detectable biomarkers and improving the accuracy of the results. From the experiments of this platform on humans, we can obtain a good correlation between alcohol in sweat and blood, as shown in Figure 5c [41]. Gao’s group designed a wearable patch capable of detecting C-reactive protein in sweat in real-time using integrated sensor arrays with iontophoresis, stimulating sweat secretion, microfluidic channels for mixing and transportation of sweat and reagents, and laser-engraved graphene. The specific binding of the capture antibody and detection antibody to the C-reactive protein was utilized. At the same time, the working electrode was modified with a redox label, thionine, to achieve one-step direct electrochemical detection. The linear trend of C-reactive protein by square wave voltammetry at different concentrations of the test samples proves that this study is expected to be used in practical clinical testing [111].
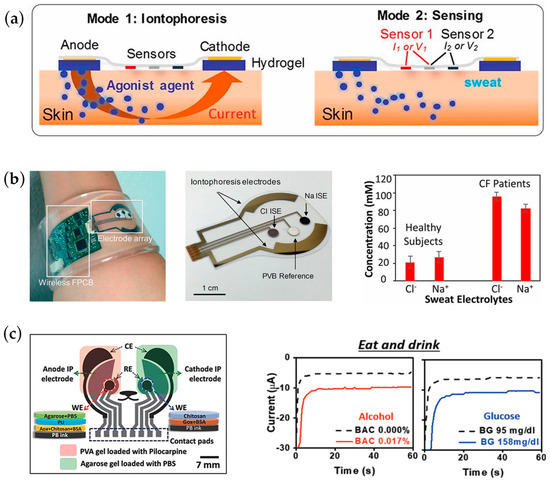
Figure 5.
Iontophoresis for wearable applications. (a) Mechanism of iontophoresis in sweat sensing applications. (b) A fully integrated platform for controlled iontophoresis sweat induction and real-time sweat sensing; real-time monitoring of cystic fibrosis patients and healthy participants. Reproduced with permission from [99]. (c) Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. A significantly increased current response of the chemosensor was observed after alcohol and glucose intake. Reproduced with permission from [41].
The sweat secretion rate from the sweat glands on the human skin surface is affected by many factors, such as age, temperature, and location, with significant individual and regional differences. With the development of sweat glucose sensors, the focus is increasingly on the active sampling and control of sweat, and sensors based on sweat microfluidics have been proposed. The key to a microfluidic device is a region defined by empty space, such as reservoirs, chambers, and microchannels [112]. The small volume of sweat secreted on the body’s surface and the low level of biomarkers create new difficulties in real-time sweat monitoring. By incorporating a microfluidic device in the sensor, we can avoid contamination of sweat by microscopic debris and lipids present on the skin surface, as well as prevent contamination of newly secreted sweat by old sweat and reduce evaporation of newly secreted sweat, increasing the ability of the sensor to monitor sweat in real-time. In addition, designing the microfluidic device’s inlet/outlet size and microchannel geometry according to a specific strategy can accurately direct the small amount of secreted sweat to different and isolated response areas to improve the signal-to-noise ratio and reduce the crosstalk from different regions. Joseph Wang’s research team has expanded the application of microfluidic devices in sweat sensing by combining a three-electrode system with the reservoirs of microfluidic devices. The designed microfluidic device increases the rate of sweat infusion on the skin surface, improves the sampling rate of sweat, and can continuously provide a sufficient amount of fresh sweat to the sensing electrode system to eliminate the influence of raw sweat, enabling real-time monitoring of sweat glucose and lactate, as shown in Figure 6a [100]. Inspired by biology, Jonghyun Son et al. designed a sweat collection patch inspired by the cactus spine with a pattern that combines with superhydrophobic/superhydrophilic materials to reduce evaporation and sweat loss during transport. At the same time, the shape of the optimally designed microchannel enables the collected sweat to be automatically transported to the sensing area, enabling real-time monitoring of glucose and lactate in sweat, as shown in Figure 6b [106]. Using the characteristics of microfluidic devices, Ali Javey’s group has developed a patch system that allows real-time monitoring of thermoregulatory sweat in everyday life. The combination of the designed microfluidic system and the superhydrophilic material can achieve monitoring under low sweat secretion rates with small amounts of thermoregulatory sweat. The system comprises three parts: an electrochemical sensing layer, a microfluidic layer, and a superhydrophilic layer, as shown in Figure 6c [64]. The system can play the role of real-time monitoring when the human body is at rest, further promoting the application of wearable sweat in daily life. By introducing a thermally responsive hydrogel as the valve of the channel in the microfluidic system, Haisong Lin et al. realized the active sampling and classification of sweat. They proposed the concept of active biofluid management (AFM). The researchers integrated this system with a flexible printed circuit board and communicated with mobile devices to monitor glucose and lactate in user sweat in real-time, and the structure of the system is shown in Figure 6d [97]. One of the challenges to overcome for microfluidic sweat sensing devices is the manufacturing of the sensor and the consistency between the sensor parts. To solve this problem, Ali Javey’s group has been able to manufacture wearable sweat sensors on a large scale by introducing the Roll-to-Roll process in the sensor manufacturing and maintaining good consistency, allowing real-time monitoring of Na+, K+, glucose, and sweat rate in real-time, as shown in Figure 6e [103]. With the sensors designed and manufactured by the group, the researchers analyzed the relationship between sweat composition and sweat properties and applications, such as the positive correlation between the concentration of sodium ions and the rate of sweat secretion in various parts of the body; the relationship between the loss of body fluids in some areas and the general area, which can be applied to monitor the state of dehydration; and the relationship between the concentration of sodium and potassium ions. A more comprehensive longitudinal study of the relationship between sweat and physiological state was conducted to improve the personalized relationship between sweat parameters and physiological state. Lin et al. designed a wearable electrochemical glucose sensing system capable of sampling natural sweat in daily life by combining a hydrogel patch with an electrochemical monitoring component. Through the hydrogel patch, a hydrophilic channel is formed between the sweat gland and the working electrode, which helps in the passive transportation of sweat and metabolite extraction. The designed Prussian Blue-PEDOT modification also improves the monitoring capability of the working electrode [83]. Joseph Wang’s group has designed a touch-based sensor that simultaneously detects glucose and β-hydroxybutyrate (HB) levels in fingertip sweat. The electrochemical detection range was extended by introducing the HB enzymes and glucose oxidase. Porous PVA hydrogel collected sweat produced by the finger in real-time, eliminating the need for an invasive measurement of HB. The article also demonstrates the correlation between HB in sweat and capillaries in healthy humans by taking commercial ketone supplements [113].
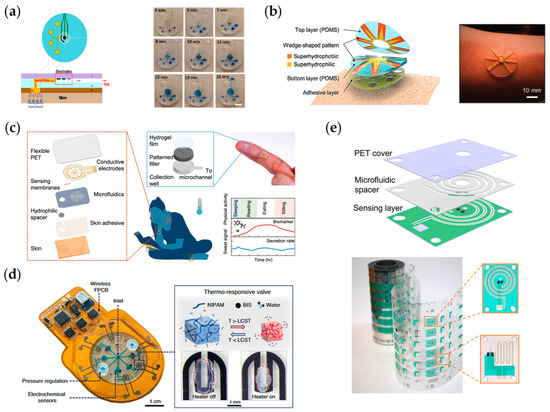
Figure 6.
Microfluidic sweat chemosensors. (a) Epidermal microfluidic electrochemical detection system for metabolite detection. Reproduced with permission from [100]. (b) Cactus-spine-inspired sweat-collecting patch for fast and continuous monitoring of sweat. Reproduced with permission from [106]. (c) A wearable patch for continuous analysis of thermoregulatory sweat at rest. Reproduced with permission from [64]. (d) A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Reproduced with permission from [97]. (e) High-throughput microfluidic sensing patches for monitoring sweat. Reproduced with permission from [103].
The above describes the recent research progress on sweat sensors. The wearable electrochemical sweat monitoring platform has made progress in the types of sensing substances, the sensitivity, the integration strategy and multimodality of sweat monitoring systems, and the energy supply. In the future, sweat is expected to be used as a new generation of suitable health-monitoring biofluid. Although sweat and sweat monitoring systems have made significant progress, there are still many problems in their practical application. (1) For some specific analytes in sweat, such as Na+, Cl−, etc., their concentrations are often related to the physical properties of sweat (secretion rate, temperature, volume, etc.) and the mode of secretion (movement, iontophoresis stimulation, thermal stimulation, active thermal regulation, etc.), requiring compensation and calibration of the analyte concentrations in the sweat with different properties. Also, the concentration of specific biomarkers in sweat, such as heavy metal ions and glucose in thermally regulated sweat, is much lower than that of blood, which poses new requirements for sensor design and monitoring system design. At the same time, since wearable sweat monitoring platforms primarily work on the skin surface, this also brings the problem of detecting environmental pollution to the sensor. In addition, the prolonged secretion of sweat under continuous exercise or stimulated sweating also brings the problem of old sweat adhering to the skin surface and contaminating newly generated sweat, which is currently a major problem affecting the real-time performance of sweat sensors. The small volume of sweat may be directly exposed to the air and thus lead to evaporation, affecting the detection concentration. (2) The current wearable electrochemical system has the problem of robust heterogeneous integration. The wearable sweat system is mainly a rigid integrated circuit chip mounted on a flexible circuit board (FPCB) and incorporated with the flexible sensor through the interface. Due to the difference in Young’s modulus between the wearable flexible sensor and the rigid circuit and interface, the sensing system will generate interference signals such as motion artifacts and affect the sensor system’s service life. In order to solve this problem, Bao Zhenan’s research group has proposed a highly stretchable BIND interface that enables a reliable mechanical connection and electrical path for wearable electrochemical sensing systems [114]. In addition, multimodal sensing systems often have multiple active regions with potential crosstalk between sensors. Designers must isolate possible interference and filter it well in the back-end circuit design. As for the sensor design, researchers should pay more attention to the compatibility between different manufacturing materials and manufacturing processes. Furthermore, the active region of the chemical sensor needs to be in direct contact with sweat. Contrarily, the circuitry cannot have any wet parts, which also puts a new challenge on the system packaging. (3) There are compatibility difficulties between the current monitoring needs of sweat sensors and wearable platforms. For example, monitoring ion concentration produces different potentials that need to be experimented with and calibrated to be able to produce a linear response to the concentration, which affects the consistency of the response of sensors. In addition, some sensors need to be stored in a specific environment. Otherwise, the activity of functional enzymes can be influenced, thus affecting the sensing results. Currently, data processing in wearable platforms is often implemented by using a microcontroller (MCU) in the circuit, which limits the performance of the system in multiple sensing due to the limitations of the MCU. (4) More critically, the current secretion pathways of biomarkers in sweat may differ significantly from each other. More research is needed to understand the secretion pathways and mechanisms of biomarkers in sweat from blood vessels to form research from mechanism to potential physiological relationships to final detection and application, which can expand the ability related to accurate diagnosis, treatment, and prevention capabilities of disease.
4. Interstitial Fluid-Based Sensors
Although sweat-based electrochemical sensors have achieved promising results in the laboratory and in some commercial products, the lack of understanding of the principles and pathways of biomarker secretion in sweat has prevented sweat from becoming the ‘gold standard’ for widespread use [11,115]. Similarly, blood is the ‘gold standard’ for biochemical testing because it contains many biomarkers, but most of the biomarkers present in the blood are obtained through invasive assays. Interstitial fluids provide a compromise solution to this issue. Researchers have used non-invasive or minimally invasive methods to monitor biomarkers in the interstitial fluids by studying the extraction method and the relationship between biomarkers in the interstitial fluid and blood [116]. Interstitial fluid is wrapped around the outside of human cells. It serves as an intermediary for exchanging nutrients between cells and the ends of blood vessels, secreting metabolic wastes, and transmitting chemical substances for intermolecular information exchange. As a medium for the exchange between cells and blood vessels, interstitial fluid and blood often contain the same biomarkers and have a certain equilibrium in their concentrations due to diffusion, compared to other biological fluids such as sweat, saliva, tears, etc. [117,118]. At the same time, interstitial fluids are more suitable for prolonged monitoring than blood extracts, which can cause pain and coagulation problems. In addition, interstitial fluid can provide unique information owing to its distinct protein composition [118,119]. Interstitial fluid is now widely studied and used in medical applications such as drug monitoring, metabolic disease research, and organ failure research [120,121,122,123]. The volume of interstitial fluid in the human body is roughly three times larger than blood [124]. As the largest organ in the human body, the skin is rich in interstitial fluid resources, so the wearable interstitial fluid sensor is mainly applied to the skin, and its structure is shown in Figure 7 below [125]. The interstitial fluid in the skin mainly exists in the dermis [126]. Biomarkers of interstitial fluid are primarily derived from continuous capillaries, and there are three methods to enter interstitial fluid from blood vessels: (1) Transcellular: directly through the outer cell of capillaries by diffusion. (2) Paracellular: transport through the gaps between cells. (3) Transcytosis: transport through vesicles [59,127]. However, the filtering properties present in the extracellular matrix (ECM) can lead to hindrances in the extraction of specific macromolecular biomarkers, which in turn prevents the quantification of their total concentrations. Wearable interstitial fluid sensors are widely used for continuous glucose monitoring in commercial applications. Because glucose can freely diffuse between interstitial fluid and capillaries, the glucose concentration in the interstitial fluid can better reflect that in the blood. Compared to monitoring sweat, although interstitial fluid is less readily available on the skin surface, it avoids interference from the body’s excretory processes. Established commercial products exist, such as Abbott’s FreeStyle Libre and Cygnus’ GlucoWatch. Reverse iontophoresis (RI) is a non-invasive method of interstitial fluid extraction based on applying a small current to the skin surface and the movement of ions and macromolecules in the dermis toward the anode and cathode under the force of an electric field. Reverse iontophoresis can extract urea, glucose, sodium ions, and other substances from interstitial fluid [120,128,129,130]. However, the liquid produced by this method is the product of the actual interstitial fluid after filtration due to the filtering effect of the extracellular matrix [59]. Therefore, a correction algorithm is needed to obtain the actual concentration of the biomarkers with a long lag time [131]. This poses difficulties for subsequent data processing. At the same time, products based on reverse iontophoresis can cause skin irritation, inflammation, and discomfort, so this type of product was withdrawn from the market [132]. Similar to the FreeStyle Libre, microneedles (MN) are used to penetrate the stratum corneum for sensing directly in the interstitial fluid space, or hollow/porous/hydrophilic microneedles are used to extract interstitial fluid for monitoring. Using this method provides a painless, minimally invasive, and effective way for interstitial fluid extraction. The design of the microneedle array also allows us to monitor multiple biomarkers simultaneously in one sensing device or to obtain concentration information for multiple batches of one biomarker to improve the accuracy of monitoring [125,131,133,134]. Microneedle-based sensors can be affected by the internal skin environment, reducing the accuracy and lifetime of the sensor. Advances in interstitial fluid sensors from both RI and MN will also be presented next. The research on ISF sensors is shown in Table 2, which contains a representative study of two major interstitial fluid extraction methods.
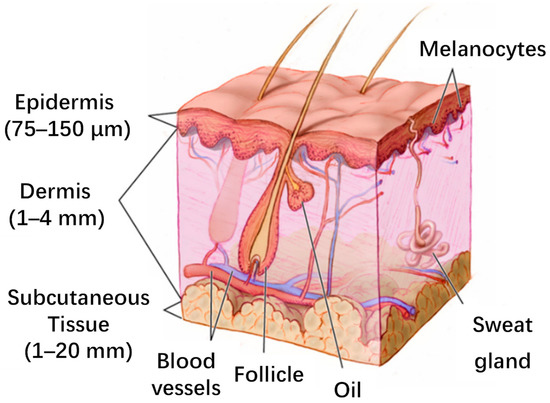
Figure 7.
Skin structure. Reproduced with permission from [125].

Table 2.
Wearable chemosensors for continuous ISF analysis.
Bandodkar et al. introduced a fully printed tattoo patch-based flexible electrochemical monitoring patch that, for the first time, combined the sensor electrode with the electrode of the RI. It used Prussian Blue as an intermediary layer for the enzymatic reaction, reducing the voltage for detection and also the current density required for the RI, showing higher glucose results in individuals after a meal; Figure 8a [135]. Further, Sempionatto et al. designed a wearable device capable of extracting interstitial fluid and detecting biomarkers through RI while being able to monitor blood pressure and heart rate using ultrasonic sensors. The developed system is able to reduce crosstalk and motion artifacts between different sensors, enrich the understanding of the body’s response to daily activities by integrating wearable devices for continuous and simultaneous acoustic and electrochemical sensing, and contribute to the early prediction of abnormal physiological changes [35]. Using gelatin methacryloyl, Zhu et al. designed a porous wearable patch with good breathability, flexibility, and biocompatibility for prolonged monitoring applications. It shows good monitoring capability in human experiments, providing the research direction of the wearable platform from the perspective of substrate material; Figure 8b [136]. Pu et al. introduced a thermal stimulation method in RI to achieve interstitial fluid extraction at a low current density to reduce the possibility of inflammation. Meanwhile, a temperature control module was designed to keep the system at a constant 37 degrees to improve the sensor’s lifetime and accuracy by increasing the stability of the enzymatic reaction. The glucose sensor was combined with the Na+ sensor to calibrate the glucose sensing results in the interstitial fluid by Na+ concentration data, solving the problems caused by individual differences; Figure 8c [137]. Chen et al. developed a non-invasive interstitial fluid glucose detection platform based on an electrochemical dual channel (ETC) with RI. Hyaluronic acid (HA) was applied to the RI electrode. It increased the osmotic pressure of interstitial fluid during the RI to obtain a concentration similar to the blood glucose concentration; Figure 8d [139]. Due to the potential interference of RI-derived interstitial fluid, Lipani et al. developed a non-invasive, transdermal, path-selective glucose wearable system based on graphene patches. Glucose in the interstitial fluid was collected and measured directly by graphene pixels without fingertip blood collection correction, improving the accuracy of the measurement; Figure 8e [140]. Yao Yao et al. designed a two-electrode system with a working electrode and a counter electrode based on graphene/carbon nanotubes/glucose oxidase composite textile and graphene/carbon nanotube/silver/silver chloride composite textile, respectively. The textile electrodes proposed in this paper have highly durable characteristics, flexibility, and biocompatibility [36]. Yanxiang Chen et al. proposed “Skin penetration-reverse iontophoresis (RI) extraction-electrochemical detection” and designed interstitial fluid-based chemosensors integrated with RI and MN array. Micro-needles array that painlessly penetrates the skin provides access for glucose extraction from interstitial fluids, increasing the amount of glucose produced in reverse iontophoresis and with a high correlation to commercial assay instruments [141]. Emily Kemp et al. utilized magnetohydrodynamic extraction for interstitial fluids in this paper. The extracted interstitial fluid is efficiently delivered to the working surface for precise detection of glucose concentration by optimizing enzyme immobilization and the interfacial layer between the sensor and the skin. Magnetohydrodynamic extraction has the advantages of low current and short stimulation time. These factors improve the representativeness of interstitial fluid samples and speed up analysis [142].
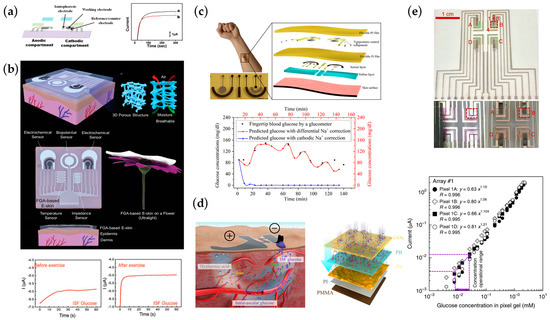
Figure 8.
Wearable and flexible chemosensors for monitoring interstitial fluid by RI. (a) Tattoo-based noninvasive glucose monitoring, Reproduced with permission from [135]. (b) A breathable, passive-cooling, electronic skin for wearable physical-electrophysiological-chemical analysis. Reproduced with permission from [136]. (c) A thermally activated and self-calibrated flexible epidermal chemosensor for ISF glucose monitoring. Reproduced with permission from [137]. (d) Skin-like electrochemical twin channels (ETC) system for noninvasive ISF glucose monitoring; Reproduced with permission from [139]. (e) Non-invasive, transdermal, path-selective, and specific glucose monitoring via a graphene-based platform. A–D: ISF extraction region. Reproduced with permission from [140].
Joseph Wang’s group developed a highly integrated wearable electrochemical detection system based on MN, which includes a reusable electronic system with a disposable microneedle array. The detection results of this system are closely related to the corresponding gold standard, solving the problem of system integration and crosstalk. The design of the microneedles enabled noninvasive personalization of metabolic monitoring; Figure 9a [37]. Wu et al. developed an electrochemical Aptamer-Based sensor. This platform achieves molecular recognition based on affinity interactions and extends the range of analytes. A microfluidic system simulating in vivo excretion kinetics was also developed to monitor the functional ability of the sensor; Figure 9b [143]. Li et al. designed a small hollow microneedle sensor for real-time detection in interstitial fluid space that can monitor multiple electrolytes in artificial ISF with fast response time and high selectivity [144]. In order to obtain comprehensive information about the biomarkers, Goud et al. designed an orthogonal microneedle assay system for real-time monitoring of Levodopa, using microneedle arrays for simultaneous enzymatic current monitoring and voltammetric monitoring of Levodopa. This parallel monitoring method provides redundant information about the biomarker, improves monitoring ability in complex environments, and reduces the detection limit of the biomarker, as shown in practical tests [121]. A novel interstitial fluid sensing mode was designed by Zheng et al. with an extended-gate FET-based biosensor. The designed flexible MN is able to penetrate the skin to reach the interstitial fluid space to measure Na+ with high selectivity and good biocompatibility. The FET structure directly converts the obtained concentration signal into an electrical signal for subsequent processing, which will ultimately help to provide efficient medical care and accurate clinical decision-making for patients in this study; Figure 9c [147]. Zhu et al. designed a microneedle-coupled epidermal electrochemical electrode that can be used to monitor electrolytes present in the human body. The developed microneedle array is able to penetrate the stratum corneum while being highly swellable, enabling rapid extraction of interstitial fluid for monitoring at the patch electrode. It also shows high selectivity in human experiments and can be applied to personalized medicine; Figure 9d [145]. Muamer Dervisevic et al. presented a three-electrode patch based on a high-density microneedle array for electrochemical glucose monitoring. Due to the high density of microneedles, the system provides a large surface area for contacting the transdermal ISF without affecting the normal skin structure and, at the same time, provides good electrochemical properties [148]. Xiaofeng Jin et al. chemically synthesized water-soluble poly (N-phenylalanine) (PPG) for the immobilization of glucose oxidase, designing a miniaturized electrochemical system with an excellent linear range to glucose and long-term stability under normal conditions of use [149]. Marc Parrilla et al. fabricated a hollow microneedle patch by 3D printing, modified with conductive ink. They designed a miniaturized pH sensor, while pH monitoring of ISF was demonstrated in human trials [150]. Peng Chen’s group proposed a swellable microneedle patch that could painlessly extract ISF from the epidermis and, through specific enzyme functionalization, was able to detect the concentration of multiple analytes using a colorimetric method while validating the feasibility of its use using in vitro and in vivo experiments [151].
Figure 9.
Wearable and flexible chemosensors for monitoring interstitial fluid by MN. (a) An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Reproduced with permission from [37]. (b) Microneedle aptamer-based sensors for continuous, real−time therapeutic drug monitoring. Reproduced with permission from [143]. (c) A wearable microneedle-based extended gate transistor for real−time detection of sodium in interstitial fluids. Reproduced with permission from [147]. (d) Microneedle−coupled epidermal sensors for in situ-multiplexed ion detection in interstitial fluids. Reproduced with permission from [145].
As mentioned above, RI- and MN-based interstitial fluid sensors have a wide range of physiological state detection applications and great potential. Interstitial fluid sensors need to be used in daily applications to get alternative monitoring of critical biomarkers in the blood. However, current wearable interstitial fluid sensors still have problems in practical applications. First, because the primary source of biomarkers in skin interstitial fluid is the subcutaneous capillaries and capillaries in the dermis have a large specific surface area but a slow metabolic rate due to their small size, this results in an inherent delay between interstitial fluid and blood, which may lead to certain risks in diabetes monitoring. Secondly, the small volume of interstitial fluid present in the skin at the region of the sensor size, as well as the possibility of changing the pressure in the subcutaneous capillaries when collecting interstitial fluid using RI or MN, make the collected interstitial fluid not raw, which poses a challenge to the monitoring sensitivity and subsequent algorithmic processing. Similarly, there is an urgent need for a mature and fully integrated platform for interstitial fluid monitoring that combines sensing with data processing and signal transmission in a single platform for practical personalized medicine applications.
5. Summary and Outlook
This review provides an overview of the current state-of-the-art wearable chemosensors for physiological applications. The development of sweat and interstitial fluid-based electrochemical sensors is mainly described. A large number of sensors based on new materials, new system structures, or new detection methods emerge. The application of wearable electrochemical sensors for monitoring metabolites (glucose, lactate, etc.), electrolytes (Na+, K+, Ca2+ in Biofluid), or drugs (Levodopa, phenytoin, etc.) has been demonstrated in various human trials. Table 3 details the information and concentrations in blood, sweat, and interstitial fluids for several representative analytes.

Table 3.
Comparison of analytes found in blood, sweat, and ISF.
To extend the use of wearable devices in personalized medicine, the device needs to obtain adequate physiological information about the user at a specific temporal resolution. Therefore, in recent years, wearable electrochemical sensors have been developed for multi-analyte, real-time monitoring with hybrid sensing, dramatically improving sensor detection results’ reliability and enhancing monitoring capabilities. Despite significant progress in the development of wearable chemosensors, most of the systems have only been used in human experiments in the laboratory environment, testing in simulated human environments, or verification of the proof-of-concept stage for certain specific markers. Therefore, there is still a considerable gap between actual clinical application and commercialization. Several challenges need to be addressed and performance needs to be verified for these sensors to be clinically viable.
For one, the current sensors are oriented toward a limited variety of objects, and research on multiple analytes for robust, continuous sensing is still limited. The developed sensors are still mainly focused on primary metabolites and electrolytes such as glucose, lactate, and Na+, while monitoring other biomarkers such as proteins, peptides, hormones, and DNA/RNA still needs to be well studied. As the human body is a complex system, accurate determination of current physiological status for clinical monitoring or disease screening requires the precise concentration of multiple biomarkers in the biofluid, allowing monitoring at the molecular level to provide comprehensive information for future medical applications. At the same time, blood is the ‘gold standard’ for clinical applications. It is vital to correctly obtain the concentration of biomarkers in noninvasive biofluids such as sweat and interstitial fluid with blood. Studying new samples, such as urine, semen, and pus, can expand the range of detectable substances, providing new physiological states with biomarkers and thus broadening the scope of application. The concentration of biomarkers can significantly differ between different individuals and their physiological states. Some biomarkers, such as heavy metals and proteins, have low concentrations in the noninvasive biofluid, which usually requires proper pretreatment before detection, making the application of the sensor difficult and reducing the temporal resolution. The new generation of wearable sensors can extend the detection range by utilizing novel functional materials (e.g., aptamers, MIPs, nanozymes, MOFs, etc.) for sensing.
Secondly, the body surface is the main operating scenario for wearable sensors, which presents unique challenges compared to traditional laboratory-based detection. Motion artifacts and exposed working environments can lead to significant variations in sensing results, which is unacceptable in clinical medical applications. Therefore, designing sensing systems to ensure accurate and reliable results under different application conditions is critical for commercialization. Suitable packaging of the functional part system is needed. Most of the current wearable sensors utilize electrochemical detection based on enzymatic reactions, which are highly susceptible to bias due to the temperature and pH of the reaction system. In order to solve this problem, the primary trend of the latest development is to incorporate physical sensors (e.g., temperature and pH) in the system to do an on-site active correction to determine the relationship between the results of enzymatic reactions and environmental conditions. Additionally, non-environmentally sensitive materials, such as MIPs, can also be utilized for detection. Alternatively, redundant detection of the analytes under sensing arrays can be used to calibrate the result. For wearable devices, prolonged monitoring can lead to the accumulation of collected samples at the sensor, which affects the results. Currently, these issues are mainly addressed by using surface microfluidic technology so that the functional area of the sensor is always in contact with FRESH samples. Also, the signal drift of the sensor over time can be relieved by modifying the functional region of the electrode with protective materials (e.g., semi-permeable membranes, etc.) or self-cleaning materials. Alternatively, a system consisting of a reusable circuit and disposable sensors can be developed to improve the accuracy of sensing while reducing the system’s cost.
Currently, the integration of wearable sensors at the system level is also a significant concern. The wearable chemosensor system typically comprises a front-end sensor and back-end signal processing and transmission circuits. The signal obtained from the electrochemical sensor needs to undergo signal processing before being transmitted to the user for display. Along with the increased complexity of wearable signal processing systems, the sensing arrays require a suitable power supply module. Currently, rigid lithium-ion batteries are the most common power supply method. These batteries can provide a stable power supply to the system and extend its working time. However, they present a potential risk of discomfort and electrical irritation. Researchers have adopted flexible, stretchable batteries, piezoelectric devices, and biofuel cells to develop the system further. In particular, wearable supercapacitors (WSCs) act as ‘energy depots,’ using their high energy density and flexibility to power the system continuously in self-powered systems. At the same time, the system’s power policy needs to be explicitly arranged for practical use. Nevertheless, there is still a need to study the problem of the long-term stability of this new power supply.
Moreover, signal processing and transmission are also essential parts of the wearable system. Raw electrical signals generated by electrochemical sensors often contain interference, and signal processing can yield more accurate information. For wearable platforms, microprocessors can handle simple processing tasks, while complex processing and display work needs to be conducted on computing platforms (e.g., cell phones, PCs). Bluetooth Low Energy (BLE) and Near Field Communication (NFC) have been widely used in wearable platforms. Both technologies have inherent drawbacks. While NFC can supply energy to the system during the transmission of signals, it requires a close communication distance, which is inconvenient. BLE technology often takes up a significant portion of the energy required in wearable systems. At the same time, both technologies need to balance communication rates and energy consumption to meet practical needs. Future wearable systems need to maintain effective information processing and transmission capabilities under prolonged senseless applications, and a new type of interaction is urgently needed.
Wearable chemosensors are an interdisciplinary field whose development has been accompanied by advances in materials science, electronics, medicine, and other branches. In the foreseeable future, wearable chemosensors will become more compact and participate in daily life as part of personalized medicine. Sensor systems will evolve into a “Sense-Act” model, providing clinical treatment to the system through a closed-loop system. For instance, by sensing the glucose concentration, insulin release can be regulated autonomously based on the information obtained from the sensors. Along with the development of AI technology, intelligent terminals will also play a crucial role in wearable chemosensors. The data generated by a large number of users can be used as a database for AI learning. The future sensors can act as “Doctors by Your Side” to give current medical advice based on the collected physiological information and predict the user’s future health condition so that patients can participate in treatment at the early stage of the disease. Future ASIC designs will also improve system integration, integrate signal processing processes, and reduce power consumption. The research and market for wearable chemosensors still have much room for development and are gradually becoming involved in medical applications.
Author Contributions
Conceptualization, T.R. and Y.Y.; methodology, Z.T.; validation, Z.T., T.C. and H.L.; formal analysis, J.J.; investigation, Z.T. and J.J.; resources, T.R. and Y.Y.; data curation, Z.T.; writing—original draft preparation, Z.T. and T.C.; writing—review and editing, D.L. and H.L.; visualization, Z.T. and J.J.; supervision, T.R.; project administration, Y.Y.; funding acquisition, T.R. and Y.Y., Z.T. and T.C. are co-first authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program (2021YFC3002200, 2022YFB3204100) and the National Natural Science Foundation (U20A20168, 51861145202, 61874065, 62022047) of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gray, J.A.M. The Shift to Personalised and Population Medicine. Lancet 2013, 382, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L. Precision Medicine Personalized, Problematic, and Promising. N. Engl. J. Med. 2015, 372, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Bierman, A.S.; Tinetti, M.E. Precision Medicine to Precision Care: Managing Multimorbidity. Lancet 2016, 388, 2721–2723. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Haghi Kashani, M.; Madanipour, M.; Nikravan, M.; Asghari, P.; Mahdipour, E. A Systematic Review of IoT in Healthcare: Applications, Techniques, and Trends. J. Netw. Comput. Appl. 2021, 192, 103–164. [Google Scholar] [CrossRef]
- Chamola, V.; Hassija, V.; Gupta, V.; Guizani, M. A Comprehensive Review of the COVID-19 Pandemic and the Role of IoT, Drones, AI, Blockchain, and 5G in Managing Its Impact. IEEE Access 2020, 8, 90225–90265. [Google Scholar] [CrossRef]
- Dang, L.M.; Piran, M.J.; Han, D.; Min, K.; Moon, H. A Survey on Internet of Things and Cloud Computing for Healthcare. Electronics 2019, 8, 768. [Google Scholar] [CrossRef]
- Hassanalieragh, M.; Page, A.; Soyata, T.; Sharma, G.; Aktas, M.; Mateos, G.; Kantarci, B.; Andreescu, S. Health Monitoring and Management Using Internet-of-Things (IoT) Sensing with Cloud-Based Processing: Opportunities and Challenges. In Proceedings of the 2015 IEEE International Conference on Services Computing, Washington, DC, USA, 27 June–2 July 2015; pp. 285–292. [Google Scholar]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narvez, E.; Gder, F.; Collins, J.J.; Dincer, C. End-to-End Design of Wearable Sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Lyu, Q.; Gong, S.; Yin, J.; Dyson, J.M.; Cheng, W. Soft Wearable Healthcare Materials and Devices. Adv. Healthc. Mater. 2021, 10, e2100577. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Vila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in Healthcare Wearable Devices. NPJ Flex. Electron. 2021, 5, 9. [Google Scholar] [CrossRef]
- Fan, F.-R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent Triboelectric Nanogenerators and Self-Powered Pressure Sensors Based on Micropatterned Plastic Films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwdiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An Ultra-Lightweight Design for Imperceptible Plastic Electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ghaffari, R.; Lu, N.; Rogers, J.A. Flexible and Stretchable Electronics for Biointegrated Devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A Graphene-Based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible Polymer Transistors with High Pressure Sensitivity for Application in Electronic Skin and Health Monitoring. Nat. Commun. 2013, 4, 1859–1867. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Suo, Z. Highly Stretchable and Tough Hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Tao, L.-Q.; Tian, H.; Liu, Y.; Ju, Z.-Y.; Pang, Y.; Chen, Y.-Q.; Wang, D.-Y.; Tian, X.-G.; Yan, J.-C.; Deng, N.-Q.; et al. An Intelligent Artificial Throat with Sound-Sensing Ability Based on Laser Induced Graphene. Nat. Commun. 2017, 8, 14579. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, K.; Yang, Z.; Jiang, S.; Ju, Z.; Li, Y.; Wang, X.; Wang, D.; Jian, M.; Zhang, Y.; et al. Epidermis Microstructure Inspired Graphene Pressure Sensor with Random Distributed Spinosum for High Sensitivity and Large Linearity. ACS Nano 2018, 12, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.-E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoringand Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pang, Y.; Han, X.; Yang, Y.; Ling, J.; Jian, M.; Zhang, Y.; Yang, Y.; Ren, T.-L. Graphene Textile Strain Sensor with Negative Resistance Variation for Human Motion Detection. ACS Nano 2018, 12, 9134–9141. [Google Scholar] [CrossRef]
- Tao, L.-Q.; Zhang, K.-N.; Tian, H.; Liu, Y.; Wang, D.-Y.; Chen, Y.-Q.; Yang, Y.; Ren, T.-L. Graphene-Paper Pressure Sensor for Detecting Human Motions. ACS Nano 2017, 11, 8790–8795. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, R.; Ji, S.; Zhao, B.; Cui, T.; Tan, X.; Gou, G.; Jian, J.; Xu, H.; Qiao, Y.; et al. Multifunctional Graphene Microstructures Inspired by Honeycomb for Ultrahigh Performance Electromagnetic Interference Shielding and Wearable Applications. ACS Nano 2021, 15, 8907–8918. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, Y.; Jian, J.; Li, M.; Jiang, G.; Li, X.; Deng, G.; Ji, S.; Wei, Y.; Pang, Y.; et al. Multifunctional and high-performance electronic skin based on silver nanowires bridging graphene. Carbon 2020, 156, 253–260. [Google Scholar] [CrossRef]
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-Inspired Highly Stretchable and Conformable Matrix Networks for Multifunctional Sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.-U.; Park, I.; Sitti, M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater. 2022, 34, e2109357. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Zhou, X.; Yuan, M.; Qu, D.; Zheng, Y.; Vishinkin, R.; Khatib, M.; Wu, W.; Haick, H. Disease Detection with Molecular Biomarkers: From Chemistry of Body Fluids to Nature-Inspired Chemical Sensors. Chem. Rev. 2019, 119, 11761–11817. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramrez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable Chemical Sensors for Biomarker Discovery in the Omics Era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Kammarchedu, V.; Butler, D.; Ebrahimi, A. A Machine Learning-Based Multimodal Electrochemical Analytical Device Based on EMoSx-LIG for Multiplexed Detection of Tyrosine and Uric Acid in Sweat and Saliva. Anal. Chim. Acta 2022, 1232, 340–447. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A Soft, Wearable Microfluidic Device for the Capture, Storage, and Colorimetric Sensing of Sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De La Paz, E.; Pei, K.; Sonsa-ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An Epidermal Patch for the Simultaneous Monitoring of Haemodynamic and Metabolic Biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, J.; Guo, Y.; Lv, T.; Chen, Z.; Li, N.; Cao, S.; Chen, B.; Chen, T. Integration of Interstitial Fluid Extraction and Glucose Detection in One Device for Wearable Non-Invasive Blood Glucose Sensors. Biosens. Bioelectron. 2021, 179, 113078. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Aghavali, R.; Hosseini-Toudeshki, H.; Brown, C.; Zhang, F.; et al. An Integrated Wearable Microneedle Array for the Continuous Monitoring of Multiple Biomarkers in Interstitial Fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Kim, J.; Valds-Ramrez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramrez, J.; Mercier, P.; Wang, J. Non-Invasive Mouthguard Biosensor for Continuous Salivary Monitoring of Metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Zong, S.; Cui, Y. Rapid and Reproducible Analysis of Thiocyanate in Real Human Serum and Saliva Using a Droplet SERS-Microfluidic Chip. Biosens. Bioelectron. 2014, 62, 13–18. [Google Scholar] [CrossRef]
- Kim, J.; Sempionatto, J.R.; Imani, S.; Hartel, M.C.; Barfidokht, A.; Tang, G.; Campbell, A.S.; Mercier, P.P.; Wang, J. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 2018, 5, 1800880. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valds-Ramrez, G.; Paixo, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- Rittweger, J.; Beller, G.; Felsenberg, D. Acute Physiological Effects of Exhaustive Whole-Body Vibration Exercise in Man. Clin. Physiol. Funct. Imaging 2000, 20, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, A.L.; Burton, G.G.; O’Connor, S.M.; Wong, J.D.; Donelan, J.M.; Wilcox, S.L.; Broxterman, R.M.; Barstow, T.J.; Arad, A.D.; DiMenna, F.J.; et al. Interaction of physiological mechanisms during exercise. J. Appl. Physiol. 1967, 22, 71–85. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeang, W.J.; Ghaffari, R.; Rogers, J.A. Wearable Sensors for Biochemical Sweat Analysis. Annu. Rev. Anal. Chem. 2019, 12, 1–22. [Google Scholar] [CrossRef]
- Nilghaz, A.; Bagherbaigi, S.; Lam, C.L.; Mousavi, S.M.; Crcoles, E.P.; Wicaksono, D.H.B. Multiple Semi-Quantitative Colorimetric Assays in Compact Embeddable Microfluidic Cloth-Based Analytical Device (CAD) for Effective Point-of-Care Diagnostic. Microfluid. Nanofluidics 2015, 19, 317–333. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.U.; Deng, Y.; Krishnan, S.R.; Choi, J.; Jang, H.; Lee, K.; Su, C.-J.; Yoo, I.; Wu, Y.; et al. An On-Skin Platform for Wireless Monitoring of Flow Rate, Cumulative Loss and Temperature of Sweat in Real Time. Nat. Electron. 2021, 4, 302–312. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, J.H.; Chung, S.J.; Chung, B.H. An Operationally Simple Colorimetric Assay of Hyaluronidase Activity Using Cationic Gold Nanoparticles. Analyst 2009, 134, 1291–1293. [Google Scholar] [CrossRef]
- Huang, P.; Wang, H.; Cao, Z.; Jin, H.; Chi, H.; Zhao, J.; Yu, B.; Yan, F.; Hu, X.; Wu, F.; et al. A Rapid and Specific Assay for the Detection of MERS-CoV. Front. Microbiol. 2018, 9, 1101. [Google Scholar] [CrossRef]
- Yue, X.; Xu, F.; Zhang, L.; Ren, G.; Sheng, H.; Wang, J.; Wang, K.; Yu, L.; Wang, J.; Li, G.; et al. Simple, Skin-Attachable, and Multifunctional Colorimetric Sweat Sensor. ACS Sens. 2022, 7, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry | Wiley Online Books. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/0471790303 (accessed on 20 February 2023).
- Brad, A.; Faulkner, L. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2000; Volume 12. [Google Scholar]
- Electrochemical Stripping Analysis | Nature Reviews Methods Primers. Available online: https://www.nature.com/articles/s43586-022-00155-1 (accessed on 20 February 2023).
- Garcia-Cordero, E.; Bellando, F.; Zhang, J.; Wildhaber, F.; Longo, J.; Gurin, H.; Ionescu, A.M. Three-Dimensional Integrated Ultra-Low-Volume Passive Microfluidics with Ion-Sensitive Field-Effect Transistors for Multiparameter Wearable Sweat Analyzers. ACS Nano 2018, 12, 12646–12656. [Google Scholar] [CrossRef] [PubMed]
- Takaloo, S.; Moghimi Zand, M. Wearable Electrochemical Flexible Biosensors: With the Focus on Affinity Biosensors. Sens. Bio-Sens. Res. 2021, 32, 100403. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Suzuki, M.; Tamiya, E.; Karube, I. Analysis of Metabolites in Sweat as a Measure of Physical Condition. Anal. Chim. Acta 1994, 289, 2734. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing Analytes in Biofluids for Peripheral Biochemical Monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Xue, M.; Mackin, C.; Weng, W.-H.; Zhu, J.; Luo, Y.; Luo, S.-X.L.; Lu, A.-Y.; Hempel, M.; McVay, E.; Kong, J.; et al. Integrated Biosensor Platform Based on Graphene Transistor Arrays for Real-Time High-Accuracy Ion Sensing. Nat. Commun. 2022, 13, 50–64. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Tai, L.-C.; Ngo, Q.P.; Chao, M.; Zhang, G.B.; Gao, W.; Bariya, M.; Bullock, J.; Kim, H.; Fahad, H.M.; et al. A Wearable Microfluidic Sensing Patch for Dynamic Sweat Secretion Analysis. ACS Sens. 2018, 3, 944–952. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valds-Ramrez, G.; Andrade, F.J.; Schning, M.J.; Wang, J. Epidermal Tattoo Potentiometric Sodium Sensors with Wireless Signal Transduction for Continuous Non-Invasive Sweat Monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef]
- Tai, L.-C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, e1707442. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.; Davis, N.; Javey, A. A Wearable Patch for Continuous Analysis of Thermoregulatory Sweat at Rest. Nat. Commun. 2021, 12, 18–23. [Google Scholar] [CrossRef]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.-C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef]
- Torrente-Rodrguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless MHealth System. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly Selective Nanoporous Membrane-Based Wearable Organic Electrochemical Device for Noninvasive Cortisol Sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B. Physiology of Sweat Gland Function: The Roles of Sweating and Sweat Composition in Human Health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-Invasive Analyte Access and Sensing through Eccrine Sweat: Challenges and Outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The Microfluidics of the Eccrine Sweat Gland, Including Biomarker Partitioning, Transport, and Biosensing Implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [PubMed]
- Sant’agnese, P.D.; Darling, R.C.; Perara, G.A.; Shea, E.C. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas. Pediatrics 1953, 12, 549563. [Google Scholar]
- Buono, M.J. Sweat Ethanol Concentrations Are Highly Correlated with Co-Existing Blood Values in Humans. Exp. Physiol. 1999, 84, 401–404. [Google Scholar] [CrossRef]
- Alvear-Ordenes, I.; Garca-Lpez, D.; Paz, J.A.D.; Gonzlez-Gallego, J. Sweat Lactate, Ammonia, and Urea in Rugby Players. Int. J. Sports Med. 2005, 26, 632–637. [Google Scholar] [CrossRef]
- Yeung, K.K.; Li, J.; Huang, T.; Hosseini, I.I.; Al Mahdi, R.; Alam, M.M.; Sun, H.; Mahshid, S.; Yang, J.; Ye, T.T.; et al. Utilizing Gradient Porous Graphene Substrate as the Solid-Contact Layer To Enhance Wearable Electrochemical Sweat Sensor Sensitivity. Nano Lett. 2022, 22, 6647–6654. [Google Scholar] [CrossRef]
- Yin, L.; Kim, K.N.; Lv, J.; Tehrani, F.; Lin, M.; Lin, Z.; Moon, J.-M.; Ma, J.; Yu, J.; Xu, S.; et al. A Self-Sustainable Wearable Multi-Modular E-Textile Bioenergy Microgrid System. Nat. Commun. 2021, 12, 154. [Google Scholar] [CrossRef]
- Liang, B.; Cao, Q.; Mao, X.; Pan, W.; Tu, T.; Fang, L.; Ye, X. An Integrated Paper-Based Microfluidic Device for Real-Time Sweat Potassium Monitoring. IEEE Sens. J. 2021, 21, 9642–9648. [Google Scholar] [CrossRef]
- Dang, W.; Manjakkal, L.; Navaraj, W.T.; Lorenzelli, L.; Vinciguerra, V.; Dahiya, R. Stretchable Wireless System for Sweat PH Monitoring. Biosens. Bioelectron. 2018, 107, 192–202. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, Y.; Wu, J.; Nyein, H.Y.Y.; Bariya, M.; Tai, L.-C.; Chao, M.; Ji, W.; Zhang, G.; Fan, Z.; et al. A Fully Integrated and Self-Powered Smartwatch for Continuous Sweat Glucose Monitoring. ACS Sens. 2019, 4, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Abelln-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Moralln, E. A Stretchable and Screen-Printed Electrochemical Sensor for Glucose Determination in Human Perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A Wearable Electrochemical Glucose Sensor Based on Simple and Low-Cost Fabrication Supported Micro-Patterned Reduced Graphene Oxide Nanocomposite Electrode on Flexible Substrate. Biosens. Bioelectron. 2018, 109, 7582. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable Hydrogel Patch with Noninvasive, Electrochemical Glucose Sensor for Natural Sweat Detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valds-Ramrez, G.; Windmiller, J.R.; Yang, Z.; Ramrez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Xu, K.; Zhong, Y.; He, C.; Jiang, L.; Sun, J.; Rao, Z.; Zhu, J.; Huang, J.; et al. Natural Oxidase-Mimicking Copper-Organic Frameworks for Targeted Identification of Ascorbate in Sensitive Sweat Sensing. Nat. Commun. 2023, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, C.; Wang, Z.; Yang, K.-A.; Cheng, X.; Liu, W.; Yu, W.; Lin, S.; Zhao, Y.; Cheung, K.M.; et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022, 8, eabk0967. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixo, T.R.L.C.; Wang, J. Wearable Temporary Tattoo Sensor for Real-Time Trace Metal Monitoring in Human Sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Yin, L.; Cao, M.; Kim, K.N.; Lin, M.; Moon, J.-M.; Sempionatto, J.R.; Yu, J.; Liu, R.; Wicker, C.; Trifonov, A.; et al. A Stretchable Epidermal Sweat Sensing Platform with an Integrated Printed Battery and Electrochromic Display. Nat. Electron. 2022, 5, 694705. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.; Zhang, N.; Zheng, J.; Chen, X.; Wen, Q.; Luo, X.; Lee, C.-Y.; Liu, X.; Zhang, X.; et al. A Non-Printed Integrated-Circuit Textile for Wireless Theranostics. Nat. Commun. 2021, 12, 4876. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, B.; Hojaiji, H.; Wang, Z.; Lin, S.; Yeung, C.; Lin, H.; Nguyen, P.; Chiu, K.; Salahi, K.; et al. A Wearable Freestanding Electrochemical Sensing System. Sci. Adv. 2020, 6, eaaz0007. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Gutruf, P.; Choi, J.; Lee, K.; Sekine, Y.; Reeder, J.T.; Jeang, W.J.; Aranyosi, A.J.; Lee, S.P.; Model, J.B.; et al. Battery-Free, Skin-Interfaced Microfluidic/Electronic Systems for Simultaneous Electrochemical, Colorimetric, and Volumetric Analysis of Sweat. Sci. Adv. 2019, 5, eaav3294. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated Textile Sensor Patch for Real-Time and Multiplex Sweat Analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Fan, Z.; Sun, Y.; Sun, Y.; Yang, Y.; Zhang, Y.; Ma, J.; Wang, Z.; Zhu, Z. A Dual-Function Wearable Electrochemical Sensor for Uric Acid and Glucose Sensing in Sweat. Biosensors 2023, 13, 105. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef]
- Yuan, Z.; Hou, L.; Bariya, M.; Nyein, H.Y.Y.; Tai, L.-C.; Ji, W.; Li, L.; Javey, A. A Multi-Modal Sweat Sensing Patch for Cross-Verification of Sweat Rate, Total Ionic Charge, and Na+ Concentration. Lab. Chip 2019, 19, 3179–3189. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, e1901190. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tan, J.; Zhu, J.; Lin, S.; Zhao, Y.; Yu, W.; Hojaiji, H.; Wang, B.; Yang, S.; Cheng, X.; et al. A Programmable Epidermal Microfluidic Valving System for Wearable Biofluid Management and Contextual Biomarker Analysis. Nat. Commun. 2020, 11, 4405. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and PH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Martn, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Bariya, M.; Li, L.; Ghattamaneni, R.; Ahn, C.H.; Nyein, H.Y.Y.; Tai, L.-C.; Javey, A. Glove-Based Sensors for Multimodal Monitoring of Natural Sweat. Sci. Adv. 2020, 6, eabb8308. [Google Scholar] [CrossRef]
- Yu, M.; Li, Y.-T.; Hu, Y.; Tang, L.; Yang, F.; Lv, W.-L.; Zhang, Z.-Y.; Zhang, G.-J. Gold Nanostructure-Programmed Flexible Electrochemical Biosensor for Detection of Glucose and Lactate in Sweat. J. Electroanal. Chem. 2021, 882, 115029. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Bariya, M.; Kivimki, L.; Uusitalo, S.; Liaw, T.S.; Jansson, E.; Ahn, C.H.; Hangasky, J.A.; Zhao, J.; Lin, Y.; et al. Regional and Correlative Sweat Analysis Using High-Throughput Microfluidic Sensing Patches toward Decoding Sweat. Sci. Adv. 2019, 5, eaaw9906. [Google Scholar] [CrossRef]
- Oh, S.Y.; Hong, S.Y.; Jeong, Y.R.; Yun, J.; Park, H.; Jin, S.W.; Lee, G.; Oh, J.H.; Lee, H.; Lee, S.-S.; et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and PH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/Disposable Sweat-Based Glucose Monitoring Device with Multistage Transdermal Drug Delivery Module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Bae, G.Y.; Lee, S.; Lee, G.; Kim, S.W.; Kim, D.; Chung, S.; Cho, K. Cactus Spine Inspired Sweat Collecting Patch for Fast and Continuous Monitoring of Sweat. Adv. Mater. 2021, 33, 2102740. [Google Scholar] [CrossRef] [PubMed]
- An, J.E.; Kim, K.H.; Park, S.J.; Seo, S.E.; Kim, J.; Ha, S.; Bae, J.; Kwon, O.S. Wearable Cortisol Aptasensor for Simple and Rapid Real-Time Monitoring. ACS Sens. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Min, J.; Demchyshyn, S.; Sempionatto, J.R.; Song, Y.; Hailegnaw, B.; Xu, C.; Yang, Y.; Solomon, S.; Putz, C.; Lehner, L.E.; et al. An Autonomous Wearable Biosensor Powered by a Perovskite Solar Cell. Nat. Electron. 2023, 112. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zhou, J.; Nejad, S.K.; Wong, T.H.; Huang, Y.; Li, H.; Yiu, C.K.; Park, W.; Li, J.; et al. Garment Embedded Sweat-Activated Batteries in Wearable Electronics for Continuous Sweat Monitoring. Npj Flex. Electron. 2022, 6, 10. [Google Scholar] [CrossRef]
- Mukasa, D.; Wang, M.; Min, J.; Yang, Y.; Solomon, S.A.; Han, H.; Ye, C.; Gao, W. A Computationally Assisted Approach for Designing Wearable Biosensors toward Non-Invasive Personalized Molecular Analysis. Adv. Mater. 2023, 9, e2212161. [Google Scholar] [CrossRef]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.-Y.; et al. A Wireless Patch for the Monitoring of C-Reactive Protein in Sweat. Nat. Biomed. Eng. 2023; 1–14, Online ahead of print. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef]
- Moon, J.-M.; Del Cao, R.; Moonla, C.; Sakdaphetsiri, K.; Saha, T.; Francine Mendes, L.; Yin, L.; Chang, A.-Y.; Seker, S.; Wang, J. Self-Testing of Ketone Bodies, along with Glucose, Using Touch-Based Sweat Analysis. ACS Sens. 2022, 7, 3973–3981. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, S.; Sun, J.; Huang, J.; Li, Y.; Zou, G.; Salim, T.; Wang, C.; Li, W.; Jin, H.; et al. A Universal Interface for Plug-and-Play Assembly of Stretchable Devices. Nature 2023, 614, 456–462. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev. 2019, 48, 14651491. [Google Scholar] [CrossRef]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and Challenges in the Diagnostic Utility of Dermal Interstitial Fluid. Nat. Biomed. Eng. 2023; 1–15, Online ahead of print. [Google Scholar] [CrossRef]
- Fogh-Andersen, N.; Altura, B.M.; Altura, B.T.; Siggaard-Andersen, O. Composition of Interstitial Fluid. Clin. Chem. 1995, 41, 1522–1525. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.Q.; Miller, P.R.; Taylor, R.M.; Boyd, G.; Mach, P.M.; Rosenzweig, C.N.; Baca, J.T.; Polsky, R.; Glaros, T. Proteomic Characterization of Dermal Interstitial Fluid Extracted Using a Novel Microneedle-Assisted Technique. J. Proteome Res. 2018, 17, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Krishnakumar, R.; Sinha, A.; Poorey, K.; Williams, K.P.; et al. Extraction and Biomolecular Analysis of Dermal Interstitial Fluid Collected with Hollow Microneedles. Commun. Biol. 2018, 1, 173. [Google Scholar] [CrossRef]
- Degim, I.T.; Ilbasmis, S.; Dundaroz, R.; Oguz, Y. Reverse Iontophoresis: A Non-Invasive Technique for Measuring Blood Urea Level. Pediatr. Nephrol. 2003, 18, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Leboulanger, B.; Guy, R.H.; Delgado-Charro, M.B. Non-Invasive Monitoring of Phenytoin by Reverse Iontophoresis. Eur. J. Pharm. Sci. 2004, 22, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Sieg, A.; Jeanneret, F.; Fathi, M.; Hochstrasser, D.; Rudaz, S.; Veuthey, J.-L.; Guy, R.H.; Begoa Delgado-Charro, M. Extraction of Amino Acids by Reverse Iontophoresis in Vivo. Eur. J. Pharm. Biopharm. 2009, 72, 226–231. [Google Scholar] [CrossRef]
- Tobias, A.; Ballard, B.D.; Mohiuddin, S.S. Physiology, Water Balance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Madden, J.; OMahony, C.; Thompson, M.; ORiordan, A.; Galvin, P. Biosensing in Dermal Interstitial Fluid Using Microneedle Based Electrochemical Devices. Sens. Bio-Sens. Res. 2020, 29, 100348. [Google Scholar] [CrossRef]
- Groenendaal, W.; von Basum, G.; Schmidt, K.A.; Hilbers, P.A.J.; van Riel, N.A.W. Quantifying the Composition of Human Skin for Glucose Sensor Development. J. Diabetes Sci. Technol. 2010, 4, 1032–1040. [Google Scholar] [CrossRef]
- Anderson, J.M.; Itallie, C.M.V. Physiology and Function of the Tight Junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Rao, G.; Glikfeld, P.; Guy, R.H. Reverse Iontophoresis: Development of a Noninvasive Approach for Glucose Monitoring. Pharm. Res. 1993, 10, 1751–1755. [Google Scholar] [CrossRef]
- Prausnitz, M.R. The Effects of Electric Current Applied to Skin: A Review for Transdermal Drug Delivery. Adv. Drug Deliv. Rev. 1996, 18, 395–425. [Google Scholar] [CrossRef]
- Glikfeld, P.; Hinz, R.S.; Guy, R.H. Noninvasive Sampling of Biological Fluids by Iontophoresis. Pharm. Res. 1989, 6, 988–990. [Google Scholar] [CrossRef]
- Teymourian, H.; Tehrani, F.; Mahato, K.; Wang, J. Lab under the Skin: Microneedle Based Wearable Devices. Adv. Healthc. Mater. 2021, 10, 2002255. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Non-Invasive Glucose Monitoring Technology in Diabetes Management: A Review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Garca-Guzmn, J.J.; Prez-Rfols, C.; Cuartero, M.; Crespo, G.A. Microneedle Based Electrochemical (Bio)Sensing: Towards Decentralized and Continuous Health Status Monitoring. TrAC Trends Anal. Chem. 2021, 135, 116–148. [Google Scholar] [CrossRef]
- Himawan, A.; Vora, L.K.; Permana, A.D.; Sudir, S.; Nurdin, A.R.; Nislawati, R.; Hasyim, R.; Scott, C.J.; Donnelly, R.F. Where Microneedle Meets Biomarkers: Futuristic Application for Diagnosing and Monitoring Localized External Organ Diseases. Adv. Healthc. Mater. 2023, 12, e2202066. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardmc, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Haghniaz, R.; Hartel, M.C.; Guan, S.; Bahari, J.; Li, Z.; Baidya, A.; Cao, K.; Gao, X.; Li, J.; et al. A Breathable, Passive Cooling, NonInflammatory, and Biodegradable Aerogel Electronic Skin for Wearable Physical Electrophysiological Chemical Analysis. Adv. Mater. 2023, 35, e2209300. [Google Scholar] [CrossRef]
- Pu, Z.; Zhang, X.; Yu, H.; Tu, J.; Chen, H.; Liu, Y.; Su, X.; Wang, R.; Zhang, L.; Li, D. A Thermal Activated and Differential Self-Calibrated Flexible Epidermal Biomicrofluidic Device for Wearable Accurate Blood Glucose Monitoring. Sci. Adv. 2021, 7, eabd0199. [Google Scholar] [CrossRef]
- De la Paz, E.; Barfidokht, A.; Rios, S.; Brown, C.; Chao, E.; Wang, J. Extended Noninvasive Glucose Monitoring in the Interstitial Fluid Using an Epidermal Biosensing Patch. Anal. Chem. 2021, 93, 12767–12775. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Zhang, S.; Li, Y.; Qu, Z.; Chen, Y.; Lu, B.; Wang, X.; Feng, X. Skin-like Biosensor System via Electrochemical Channels for Noninvasive Blood Glucose Monitoring. Sci. Adv. 2017, 3, e1701629. [Google Scholar] [CrossRef] [PubMed]
- Lipani, L.; Dupont, B.G.R.; Doungmene, F.; Marken, F.; Tyrrell, R.M.; Guy, R.H.; Ilie, A. Non-Invasive, Transdermal, Path-Selective and Specific Glucose Monitoring via a Graphene-Based Platform. Nat. Nanotechnol. 2018, 13, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A Touch-Actuated Glucose Sensor Fully Integrated with Microneedle Array and Reverse Iontophoresis for Diabetes Monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef] [PubMed]
- Kemp, E.; Palomki, T.; Ruuth, I.A.; Boeva, Z.A.; Nurminen, T.A.; Vnsk, R.T.; Zschaechner, L.K.; Prez, A.G.; Hakala, T.A.; Wardale, M.; et al. Influence of Enzyme Immobilization and Skin-Sensor Interface on Non-Invasive Glucose Determination from Interstitial Fluid Obtained by Magnetohydrodynamic Extraction. Biosens. Bioelectron. 2022, 206, 114–123. [Google Scholar] [CrossRef]
- Wu, Y.; Tehrani, F.; Teymourian, H.; Mack, J.; Shaver, A.; Reynoso, M.; Kavner, J.; Huang, N.; Furmidge, A.; Duvvuri, A.; et al. Microneedle Aptamer-Based Sensors for Continuous, Real-Time Therapeutic Drug Monitoring. Anal. Chem. 2022, 94, 8335–8345. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Weng, Z.; Sun, H.; Nistala, R.; Zhang, Y. Microneedle-Based Potentiometric Sensing System for Continuous Monitoring of Multiple Electrolytes in Skin Interstitial Fluids. ACS Sens. 2021, 6, 2181–2190. [Google Scholar] [CrossRef]
- Zhu, D.D.; Tan, Y.R.; Zheng, L.W.; Lao, J.Z.; Liu, J.Y.; Yu, J.; Chen, P. Microneedle-Coupled Epidermal Sensors for In-Situ-Multiplexed Ion Detection in Interstitial Fluids. ACS Appl. Mater. Interfaces 2023, 15, 14146–14154. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, X.; Zhu, J.; Wang, B.; Jelinek, D.; Zhao, Y.; Wu, T.-Y.; Horrillo, A.; Tan, J.; Yeung, J.; et al. Wearable Microneedle-Based Electrochemical Aptamer Biosensing for Precision Dosing of Drugs with Narrow Therapeutic Windows. Sci. Adv. 2022, 8, eabq4539. [Google Scholar] [CrossRef]
- Zheng, Y.; Omar, R.; Zhang, R.; Tang, N.; Khatib, M.; Xu, Q.; Milyutin, Y.; Saliba, W.; Broza, Y.Y.; Wu, W.; et al. A Wearable Microneedle-Based Extended Gate Transistor for Real-Time Detection of Sodium in Interstitial Fluids. Adv. Mater. 2022, 34, 2108607. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Yan, L.; Senel, M.; Gengenbach, T.R.; Prieto-Simon, B.; Voelcker, N.H. Transdermal Electrochemical Monitoring of Glucose via High-Density Silicon Microneedle Array Patch. Adv. Funct. Mater. 2022, 32, 2009850. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Fully Integrated Flexible Biosensor for Wearable Continuous Glucose Monitoring. Biosens. Bioelectron. 2022, 196, 113760. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Vanhooydonck, A.; Johns, M.; Watts, R.; De Wael, K. 3D-Printed Microneedle-Based Potentiometric Sensor for PH Monitoring in Skin Interstitial Fluid. Sens. Actuators B Chem. 2023, 378, 133–159. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zheng, L.W.; Duong, P.K.; Cheah, R.H.; Liu, X.Y.; Wong, J.R.; Wang, W.J.; Tien Guan, S.T.; Zheng, X.T.; Chen, P. Colorimetric Microneedle Patches for Multiplexed Transdermal Detection of Metabolites. Biosens. Bioelectron. 2022, 212, 114412. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, H.; Li, J.; Bandodkar, A.J.; Rogers, J.A. Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids. Trends Chem. 2019, 1, 559–571. [Google Scholar] [CrossRef]
- Sim, D.; Brothers, M.C.; Slocik, J.M.; Islam, A.E.; Maruyama, B.; Grigsby, C.C.; Naik, R.R.; Kim, S.S. Biomarkers and Detection Platforms for Human Health and Performance Monitoring: A Review. Adv. Sci. 2022, 9, e2104426. [Google Scholar] [CrossRef]
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable Electrochemical Biosensors in North America. Biosens. Bioelectron. 2021, 172, 112750. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Crespo, G.A. Wearable Potentiometric Ion Sensors. TrAC Trends Anal. Chem. 2019, 110, 303–320. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry. Anal. Chem. 2020, 92, 378–396. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Pearlmutter, P.; DeRose, G.; Samson, C.; Linehan, N.; Cen, Y.; Begdache, L.; Won, D.; Koh, A. Sweat and Saliva Cortisol Response to Stress and Nutrition Factors. Sci. Rep. 2020, 10, 19050. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, W.; Wang, B.; Zhao, Y.; En, K.; Zhu, J.; Cheng, X.; Zhou, C.; Lin, H.; Wang, Z.; et al. Noninvasive Wearable Electroactive Pharmaceutical Monitoring for Personalized Therapeutics. Proc. Natl. Acad. Sci. 2020, 117, 1901719025. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Schlenvoigt, G.; Ladwig, K.; Herrmann, D.; Moths, C.; Linse, R.; Neumann, C. The Sweat of Patients with Atopic Dermatitis Contains Specific IgE Antibodies to Inhalant Allergens. Clin. Exp. Dermatol. 1996, 21, 347–350. [Google Scholar] [CrossRef]
- Katchman, B.A.; Zhu, M.; Blain Christen, J.; Anderson, K.S. Eccrine Sweat as a Biofluid for Profiling Immune Biomarkers. PROTEOMICS Clin. Appl. 2018, 12, e1800010. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).