Development and Application of an Electrochemical Sensor with 1,10-Phenanthroline-5,6-dione-Modified Electrode for the Detection of Escherichia coli in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Preparation of Electrochemical Sensor
2.4. Culture of E. coli
2.5. Detection of NADH Using As-Prepared Electrochemical Sensor
2.6. Detection of E. coli Using As-Prepared Electrochemical Sensor
2.7. Detection of E. coli Using Colorimetric Method
3. Results and Discussion
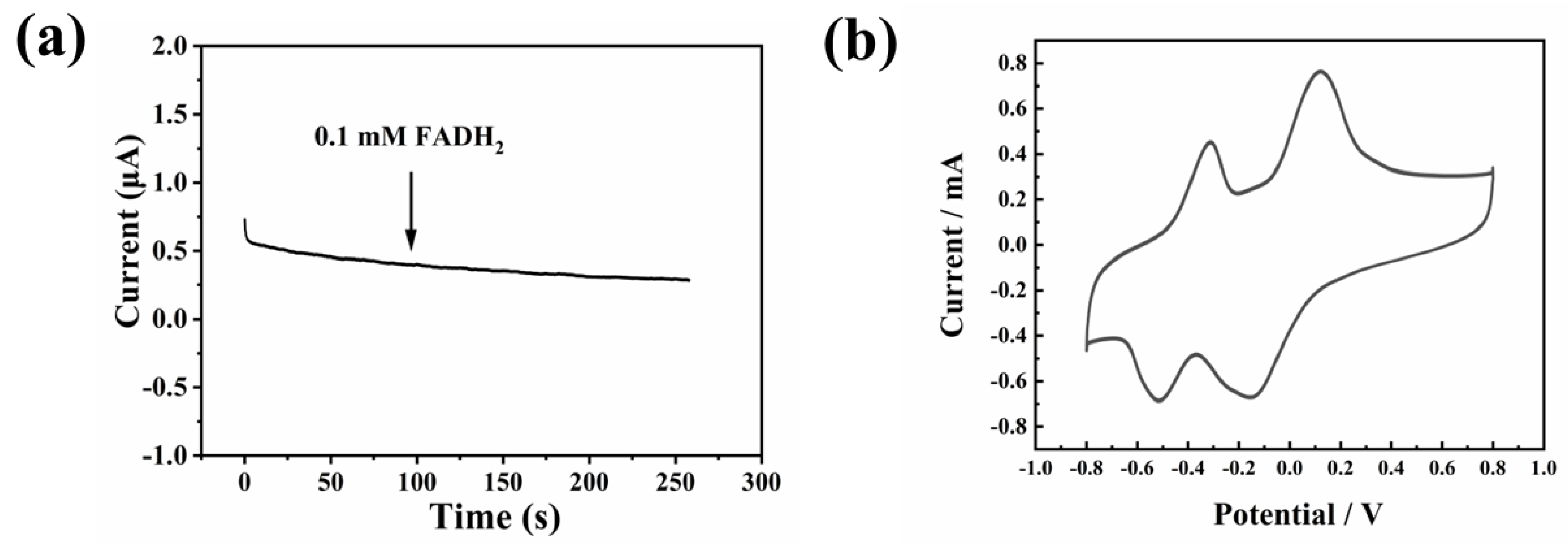
3.1. The Detection Principles of As-Prepared Electrochemical Sensor
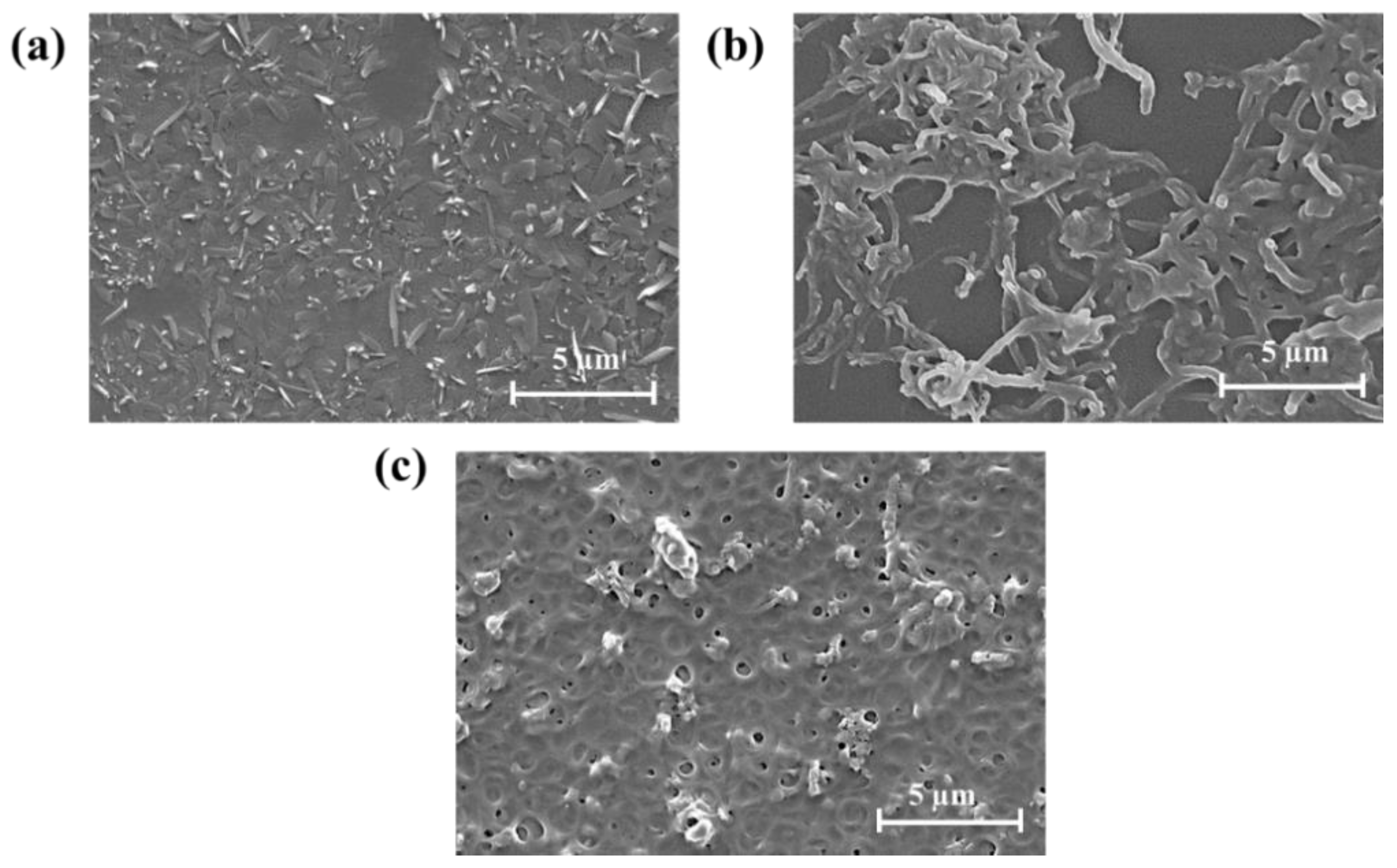
3.2. Surface Characterization of PD-Modified Electrode
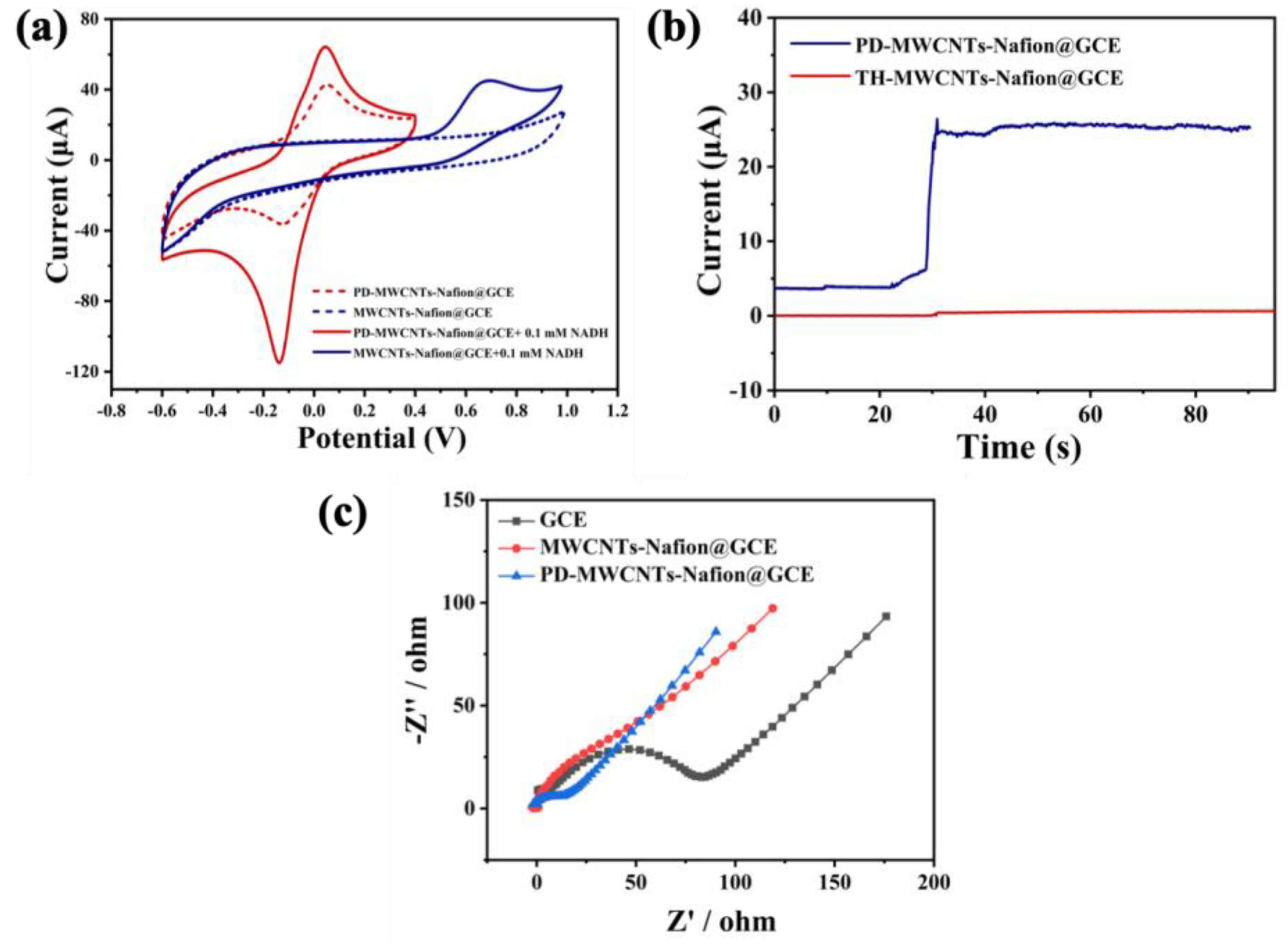
3.3. Electrochemical Characterization of PD-Modified Electrode
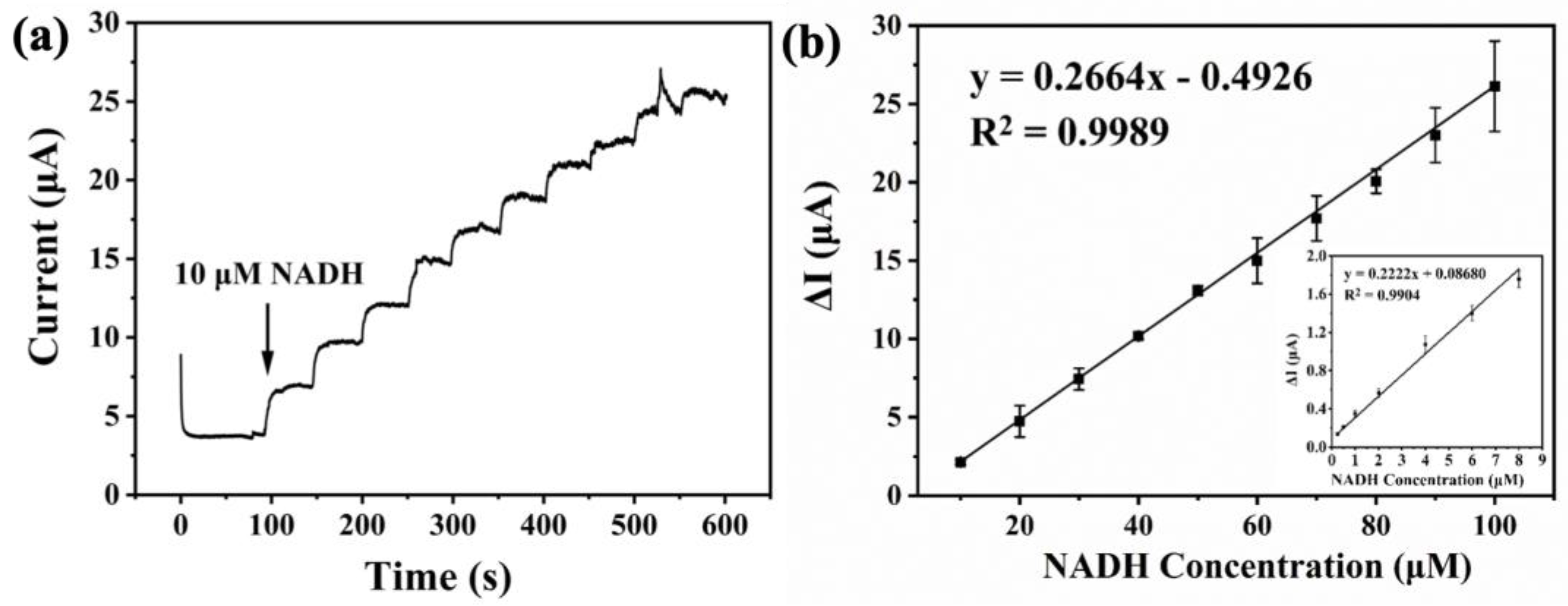
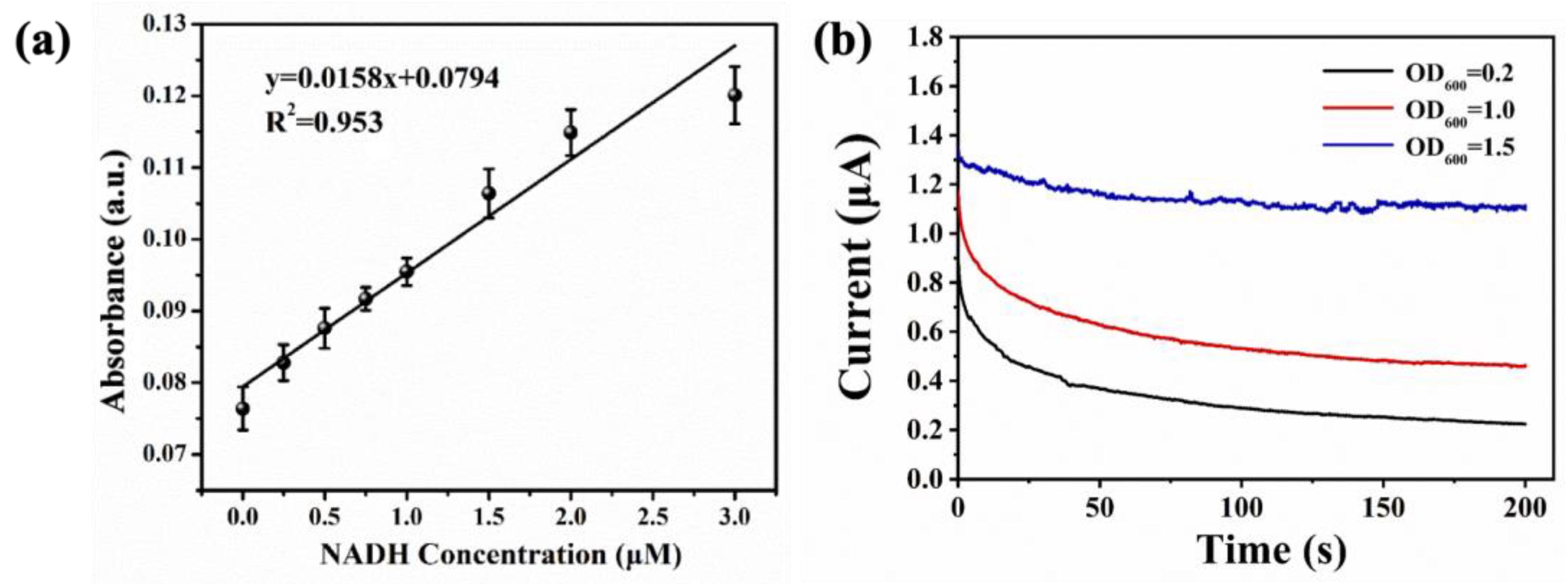
3.4. The Performance of As-Prepared Electrochemical Sensor in NADH Detection
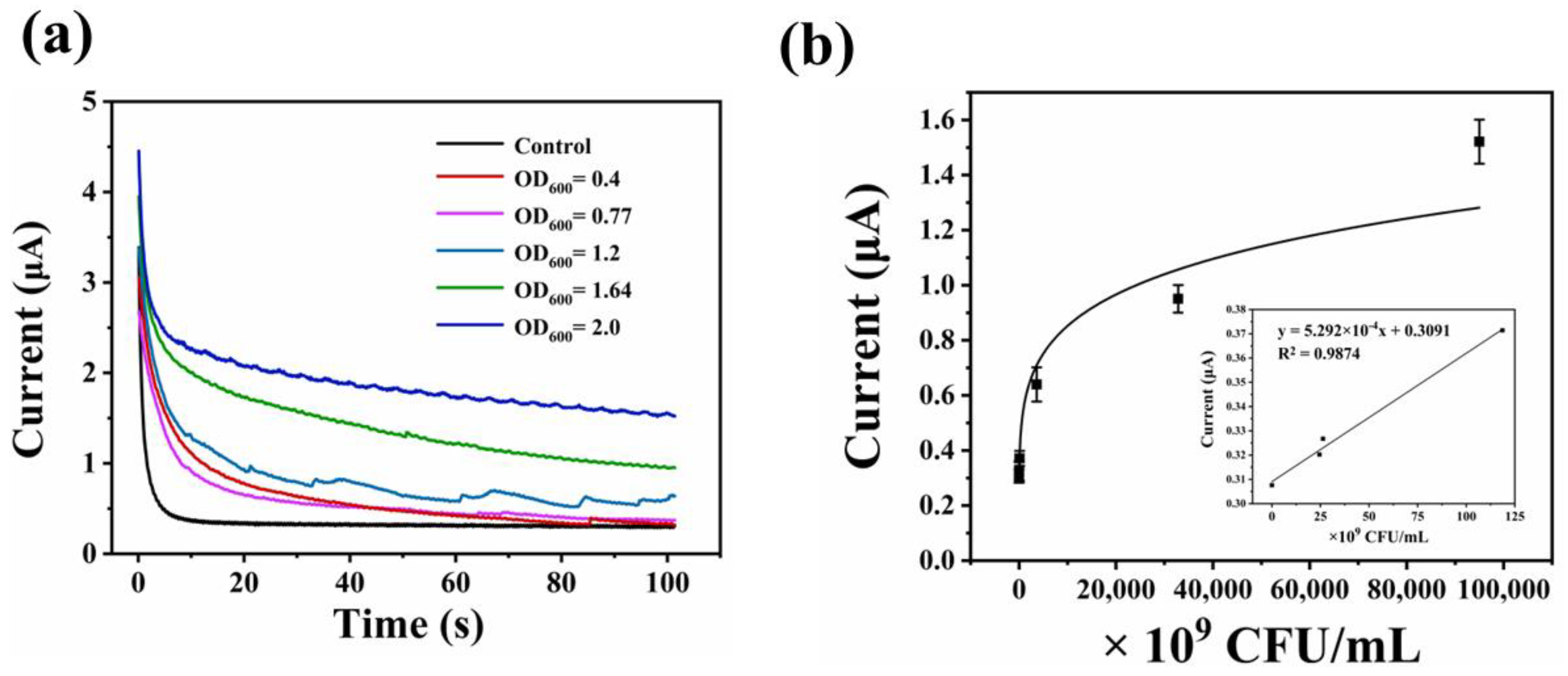
3.5. Detection of E. coli by As-Prepared Electrochemical Sensor
3.6. E. coli Detection in Real Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.D.; Han, J.Z.; Li, J.Y.; Wang, Z.B.; Zhang, Z.H.; Kang, X.; Zhu, W.; Deng, H.B. Water hazard detection: A 20-year review. J. Terramechanics 2023, 105, 53–66. [Google Scholar] [CrossRef]
- Ribeiro, V.H.A.; Moritz, S.; Rehbach, F.; Reynoso-Meza, G. A novel dynamic multi-criteria ensemble selection mechanism applied to drinking water quality anomaly detection. Sci. Total Environ. 2020, 749, 142368. [Google Scholar] [CrossRef]
- Zulkifli, S.N.; Rahim, H.A.; Lau, W.J. Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications. Sens. Actuators B Chem. 2018, 255, 2657–2689. [Google Scholar] [CrossRef] [PubMed]
- Nnachi, R.C.; Sui, N.; Ke, B.W.; Luo, Z.H.; Bhalla, N.; He, D.P.; Yang, Z.G. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Chen, W.J.; Chung, J.; Yin, J.; Yoon, J. Recent progress in fluorescent probes for bacteria. Chem. Soc. Rev. 2021, 50, 7725–7744. [Google Scholar] [CrossRef]
- Syal, K.; Iriya, R.; Yang, Y.Z.; Yu, H.; Wang, S.P.; Haydel, S.E.; Chen, H.Y.; Tao, N.J. Antimicrobial Susceptibility Test with Plasmonic Imaging and Tracking of Single Bacterial Motions on Nanometer Scale. ACS Nano 2016, 10, 845–852. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhu, J.W.; Zhang, H.Y.; Liu, W.M.; Ge, J.C.; Wu, J.S.; Wang, P.F. High sensitivity gram-negative bacteria biosensor based on a small-molecule modified surface plasmon resonance chip studied using a laser scanning confocal imaging-surface plasmon resonance system. Sens. Actuators B Chem. 2018, 259, 492–497. [Google Scholar] [CrossRef]
- Andrei, C.C.; Moraillon, A.; Lau, S.; Felidj, N.; Yamakawa, N.; Bouckaert, J.; Larquet, E.; Boukherroub, R.; Ozanam, F.; Szunerits, S.; et al. Rapid and sensitive identification of uropathogenic Escherichia coli using a surface-enhanced-Raman-scattering-based biochip. Talanta 2020, 219, 121174. [Google Scholar] [CrossRef]
- Tadesse, L.F.; Ho, C.S.; Chen, D.H.; Arami, H.; Banaei, N.; Gambhir, S.S.; Jeffrey, S.S.; Saleh, A.A.E.; Dionne, J. Plasmonic and Electrostatic Interactions Enable Uniformly Enhanced Liquid Bacterial Surface-Enhanced Raman Scattering (SERS). Nano Lett. 2020, 20, 7655–7661. [Google Scholar] [CrossRef]
- Xu, D.K.; Chen, H.Y. Electrochemical Detection for Arrayed Electrode Chips. Prog. Chem. 2009, 21, 2379–2387. [Google Scholar]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Gao, X.L.; Liu, G.S.; Zhu, R.T.; Yang, W.L.; Li, Z.S.; Liu, F.M.; Zhou, K.C.; Yu, Z.M.; Wei, Q.P.; et al. Correlation of the role of boron concentration on the microstructure and electrochemical properties of diamond electrodes. Funct. Diam. 2022, 1, 197–204. [Google Scholar] [CrossRef]
- Dong, X.Y.; Zhao, W.W.; Sun, G.B.; Xu, J.J.; Chen, H.Y. An Electrochemical DNA Biosensor Based on Gold Nanofilm and Stable Y Junction Structure. Acta Chim. Sin. 2012, 70, 1457–1463. [Google Scholar] [CrossRef]
- Tong, P.; Zhang, L.; Xu, J.J.; Chen, H.Y. Simply amplified electrochemical aptasensor of Ochratoxin A based on exonuclease-catalyzed target recycling. Biosens. Bioelectron. 2011, 29, 97–101. [Google Scholar] [CrossRef]
- Du, D.; Ju, H.X.; Zhang, X.J.; Chen, J.; Cai, J.; Chen, H.Y. Electrochemical immunoassay of membrane P-glycoprotein by immobilization of cells on gold nanoparticles modified on a methoxysilyl-terminated butyrylchitosan matrix. Biochemistry 2005, 44, 11539–11545. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Lei, Y.N.; Yan, L.; Yu, T.X.; Li, Q.; Zhang, D.C.; Ding, S.J.; Ju, H.X. A Rapid and Sensitive Aptamer-Based Electrochemical Biosensor for Direct Detection of Escherichia coli O111. Electroanalysis 2012, 24, 1186–1191. [Google Scholar] [CrossRef]
- Guner, A.; Cevik, E.; Senel, M.; Alpsoy, L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef]
- Zou, Y.J.; Liang, J.; She, Z.; Kraatz, H.B. Gold nanoparticles-based multifunctional nanoconjugates for highly sensitive and enzyme-free detection of E. coli K12. Talanta 2019, 193, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, C.J.; Liu, R.; Wang, Y.X.; Li, A.Y.; Tian, S.; Cheng, W.; Ding, S.J.; Li, W.T.; Zhao, M.; et al. A novel CRISPR/Cas14a-based electrochemical biosensor for ultrasensitive detection of Burkholderia pseudomallei with PtPd@PCN-224 nanoenzymes for signal amplification. Biosens. Bioelectron. 2023, 225, 115098. [Google Scholar] [CrossRef]
- Li, L.L.; Yu, S.Q.; Wu, J.; Ju, H.X. Regulation of Target-activated CRISPR/Cas12a on Surface Binding of Polymer Dots for Sensitive Electrochemiluminescence DNA Analysis. Anal. Chem. 2023, 95, 7396–7402. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, W.J.; Li, J.H.; Lu, K.Q.; Wen, H.R.; Ren, J.L. Rapid bacteria electrochemical sensor based on cascade amplification of 3D DNA walking machine and toehold-mediated strand displacement. Talanta 2022, 249, 123646. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kang, H.Y.; Yang, H. Permeabilization-free beta-galactosidase-induction-based electrochemical detection of Escherichia coli. Sens. Actuators B Chem. 2021, 337, 129768. [Google Scholar] [CrossRef]
- Ertl, P.; Mikkelsen, S.R. Electrochemical biosensor array for the identification of microorganisms based on lectin-lipopolysaccharide recognition. Anal. Chem. 2001, 73, 4241–4248. [Google Scholar] [CrossRef]
- Johnson, B.J.; Delehanty, J.B.; Lin, B.; Ligler, F.S. Immobilized proanthocyanidins for the capture of bacterial lipopolysaccharides. Anal. Chem. 2008, 80, 2113–2117. [Google Scholar] [CrossRef]
- Castle, L.M.; Schuh, D.A.; Reynolds, E.E.; Furst, A.L. Electrochemical Sensors to Detect Bacterial Foodborne Pathogens. ACS Sens. 2021, 6, 1717–1730. [Google Scholar] [CrossRef]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Wos, M.L.; Pollard, P.C. Cellular nicotinamide adenine dinucleotide (NADH) as an indicator of bacterial metabolic activity dynamics in activated sludge. Water Sci. Technol. 2009, 60, 783–791. [Google Scholar] [CrossRef]
- Mao, X.Y.; Wu, Y.H.; Xu, L.L.; Cao, X.J.; Cui, X.J.; Zhu, L.D. Electrochemical biosensors based on redox carbon nanotubes prepared by noncovalent functionalization with 1,10-phenanthroline-5,6-dione. Analyst 2011, 136, 293–298. [Google Scholar] [CrossRef]
- Dube, I.; Jimenez, D.; Fedorov, G.; Boyd, A.; Gayduchenko, I.; Paranjape, M.; Barbara, P. Understanding the electrical response and sensing mechanism of carbon-nanotube-based gas sensors. Carbon 2015, 87, 330–337. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhao, D.L.; Guo, X.F.; Correa, J.; Riehl, B.L.; Heineman, W.R. Carbon Nanotube-Loaded Nafion Film Electrochemical Sensor for Metal Ions: Europium. Anal. Chem. 2014, 86, 4354–4361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wang, D.C.; Liu, Y.R.; Gao, G.Y.; Zhi, J.F. Redox activity of single bacteria revealed by electrochemical collision technique. Biosens. Bioelectron. 2021, 176, 112914. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Tan, Y.; Xie, Q.; Ma, M.; Yao, S. Preparation of chitosan–dopamine-multiwalled carbon nanotubes nanocomposite for electrocatalytic oxidation and sensitive electroanalysis of NADH. Sens. Actuators B Chem. 2009, 137, 547–554. [Google Scholar] [CrossRef]
- Liu, A.; Watanabe, T.; Honma, I.; Wang, J.; Zhou, H. Effect of solution pH and ionic strength on the stability of poly(acrylic acid)-encapsulated multiwalled carbon nanotubes aqueous dispersion and its application for NADH sensor. Biosens. Bioelectron. 2006, 22, 694–699. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Chen, L.; Qin, X.; Huang, Z.; Zhou, Y.; Meng, Y.; Li, J.; Huang, S.; Liu, Y.; et al. Amperometric biosensor for NADH and ethanol based on electroreduced graphene oxide–polythionine nanocomposite film. Sens. Actuators B Chem. 2013, 181, 280–287. [Google Scholar] [CrossRef]
- Zhai, X.; Wei, W.; Zeng, J.; Gong, S.; Yin, J. Layer-by-Layer Assembled Film Based on Chitosan/Carbon Nanotubes, and its Application to Electrocatalytic Oxidation of NADH. Microchim. Acta 2006, 154, 315–320. [Google Scholar] [CrossRef]
- Sangamithirai, D.; Narayanan, V.; Muthuraaman, B.; Stephen, A. Investigations on the performance of poly(o-anisidine)/graphene nanocomposites for the electrochemical detection of NADH. Mater. Sci. Eng. C 2015, 55, 579–591. [Google Scholar] [CrossRef]
- Arthisree, D.; Devi, K.S.S.; Devi, S.L.; Meera, K.; Joshi, G.M.; Senthil Kumar, A. A hydrophobic coenzyme Q10 stabilized functionalized-MWCNT modified electrode as an efficient functional biomimetic system for the electron-transfer study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 53–61. [Google Scholar] [CrossRef]
- Eguílaz, M.; Gutiérrez, A.; Rivas, G. Non-covalent functionalization of multi-walled carbon nanotubes with cytochrome c: Enhanced direct electron transfer and analytical applications. Sens. Actuators B Chem. 2016, 225, 74–80. [Google Scholar] [CrossRef]

| Sensor | Detection Potential (V) | LOD (µM) | Linear Range (µM) | Sensitivity (µA/µM) | Ref. |
|---|---|---|---|---|---|
| CS–DA-MWCNTs-COOH/Au | +0.25 vs. SCE | 0.012 | 0.1–600 | 0.009 | [33] |
| PAA-MWNTs/GCE | +0.13 vs. Ag/AgCl | 1 | 4–100 | 0.094 | [34] |
| ERGO–PTH/GC | +0.4 vs. SCE | 0.1 | 10–3900 | 0.143 | [35] |
| CHIT/MWNTs/GC | +0.4 vs. SCE | 0.3 | 0.8–1600 | / | [36] |
| POA/GR@GCE | +0.045 vs. SCE | 1.3 | 0.166–1.772 | 47.1 | [37] |
| PD-MWCNTs-Nafion@GCE | +0.1 vs. Ag/AgCl | 0.0357 | 0.25–8, 10–100 | 0.222 | This work |
| Water Sample | NADH Concentration Detected with Electrochemical Sensor (µM) | Recovery Rate (%) | RSD (%) |
|---|---|---|---|
| Pu River | 0.086 | 89.12 | 8.26 |
| Tangwang River | 0.090 | 93.26 | 2.08 |
| Xueqing River | 0.088 | 91.19 | 4.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Liu, Y.; Gao, G.; Zhang, H.; Zhi, J. Development and Application of an Electrochemical Sensor with 1,10-Phenanthroline-5,6-dione-Modified Electrode for the Detection of Escherichia coli in Water. Chemosensors 2023, 11, 458. https://doi.org/10.3390/chemosensors11080458
Fan Y, Liu Y, Gao G, Zhang H, Zhi J. Development and Application of an Electrochemical Sensor with 1,10-Phenanthroline-5,6-dione-Modified Electrode for the Detection of Escherichia coli in Water. Chemosensors. 2023; 11(8):458. https://doi.org/10.3390/chemosensors11080458
Chicago/Turabian StyleFan, Yining, Yanran Liu, Guanyue Gao, Hanxin Zhang, and Jinfang Zhi. 2023. "Development and Application of an Electrochemical Sensor with 1,10-Phenanthroline-5,6-dione-Modified Electrode for the Detection of Escherichia coli in Water" Chemosensors 11, no. 8: 458. https://doi.org/10.3390/chemosensors11080458
APA StyleFan, Y., Liu, Y., Gao, G., Zhang, H., & Zhi, J. (2023). Development and Application of an Electrochemical Sensor with 1,10-Phenanthroline-5,6-dione-Modified Electrode for the Detection of Escherichia coli in Water. Chemosensors, 11(8), 458. https://doi.org/10.3390/chemosensors11080458






