Abstract
The species Zanthoxylum caribaeum belongs to the Rutaceae family, from which several chemical nuclei are known, including alkaloids and coumarins. In addition, its essential oil has been characterized, showing differences in composition and various antimicrobial activities. In the present study, the essential oil of Z. caribaeum collected in the department of Tolima, central Colombia, was characterized by gas chromatography with mass selective detector (GC-MS). The essential oil showed a composition of about 43 compounds (including major and minor), whose main components, according to their abundance, are the following: germacrene D (228.0 ± 1.6 mg/g EO), (E)-β-farnesene (128.0 ± 1.5 mg/g EO), β-elemene (116.0 ± 1.6 mg/g EO) and (E)-nerolidol (74.0 ± 2.2 mg/g EO). This oil was tested against microorganisms that affect cocoa production in Colombia and in tropical countries where the production of this commodity is very important for the economy. The antifungal tests were performed on the fungal species Moniliophthora roreri and showed promising and significant activity, inhibiting growth by more than 95% at concentrations of 50 µL/mL and 100 µL/mL. This remarkable antifungal activity could be due to the presence of major and minor compounds that synergistically enhance the activity.
1. Introduction
The genus Zanthoxylum belongs to the Rutaceae family. Worldwide, 250 species have been described, distributed mainly in the tropical and subtropical zones of the planet [1]. In Colombia, it is mainly found in the Andean region, the Caribbean and part of the Amazon [2]. Some species belonging to these genera have shown a variety of biological activities such due to their high content of secondary metabolites including alkaloids, terpenes, lignans, steroids, coumarins and flavonoids, such as anti-inflammatory, anticancer, antimalarial, antioxidant, anti-HIV and antimicrobial activities [3,4,5,6,7,8]. These metabolites are distributed throughout the plant but have been found in higher concentrations in bark, roots and leaves.
One of the plants that is cultivated annually in Colombia is Theobroma cacao (cocoa), an ancient plant of the American continent [9,10] that has acquired great cultural, environmental and economic importance. It belongs to the family Malvaceae, which includes more than 22 species, and is divided into criollo, forastero and trinitario cocoa with different physical, chemical and functional properties. The dried beans are obtained from the fruits, and their aromatic and compositional quality is determined by factors such as origin, processing and the influence of soil and climatic conditions [11,12]. According to some studies, cocoa originated in the headwaters of the Amazon basin, and a natural cocoa population spread westward and northward in the central part of the Amazon-Guayana region, forming the Forastero-Amazon group and the second group, called Criollo, which is well accepted in the market due to its high organoleptic qualities [13]. Currently, this tree is commercially grown in Asia and Oceania, Central and South America and Africa, with a global share of production of 12.5%, 12.7% and 74.8%, respectively. Most cocoa for international trade is grown in Africa, with Côte d’Ivoire being the largest producer and cocoa from Ghana being the highest quality [14].
Phytosanitary problems are the main factors that have contributed to the decline in cocoa production and the deterioration of quality of the final product. These include diseases caused by phytopathogenic fungi [15]. This problem has increased recently, favored by the lack of proper cultivation management and by man-made environmental changes [16]. The main diseases affecting the cocoa plant in Colombia include: Moniliasis, a disease caused by the basidiomycete fungus Moniliophthora roreri (Figure 1), which affects about 40% of the annual cocoa production in Colombia, with a total production of 62,000 MT averaged over the last 5 years [17,18]. It has high survivability in different environments, with rapid growth and spread, in general, commercial genotypes are highly susceptible to this pathogen [19] and current disease control methods are inefficient and increase production costs [20]. This situation threatens the sustainability of national production of this crop. Some phytosanitary conditions related to the agro-ecological zone, the severity of the inoculum and inadequate crop management favor damage up to 100% in a plantation, which is why the disease is considered the most prevalent and severe.
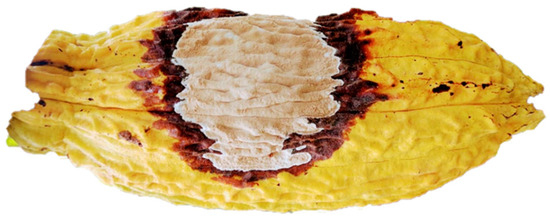
Figure 1.
Fruit of Theobroma cacao affected by the fungus Moniliophthora roreri.
Essential oils (EOs) have a natural potential for plant defense mechanisms, are volatile compounds produced by many species, and can act against various plant pathogenic microorganisms [5]. Their use is emerging as a sustainable alternative for disease control in today’s agriculture, where agricultural practices allow the use of certain substances to combat pathogens [6]. Natural products such as essential oils have emerged as an “environmentally friendly” alternative for use as effective as antimicrobial agents because they are easy to obtain and have low toxicity to non-target organisms. In this study we isolated the fungus M. roreri, causal agent of Moniliasis disease in cocoa plantations in Colombia and tropical countries. The microorganism was cultured, and biological assays were developed using essential oils extracted from the leaves of Z. caribaeum as active principle, obtaining promising results for the development of bioproducts.
2. Materials and Methods
2.1. Plant Material
The species Z. caribaeum was collected in January 2022 in the municipality of Piedra, Department of Tolima-Colombia (4°28′57″ N 74°59′17″ W, altitude 593 masl), the species were collected by Andrea Jiménez-González, Marcial Fuentes-Estrada and Olimpo García-Beltrán, and identified and classified by botanist Héctor Esquivel and a specimen in the TOLI Herbarium of the Universidad del Tolima (Colombia) with voucher number N° 28543.
2.2. Extraction of Essential Oil
Z. caribaeum leaf samples were pre-cleaned. The essential oils of Z. caribaeum were extracted from the leaves using a microwave-assisted hydrodistillation (MWHD) system as the extraction method [21]. The heating source for the system was a conventional microwave radiation source (SAMSUNG, model MS23J5133AG, Malaysia), set at 2450 MHz, 1.2 kW. 750 g of plant material was mixed with 500 mL of distilled water, plant material, especially the leaves, were placed in a 2 L reaction balloon. Then, a heating program was carried out; the first stage was 10 min at 100% power for preheating; the second stage of 45 min distillation at 80% power. A maximum condensation temperature of 13 °C was maintained.
2.3. Chromatographic Analysis
The extracted essential oil of Z. caribaeum (20 mg) was dissolved in CH2Cl2 (1 mL), and an aliquot of this dilution (2 µL) was injected into a gas chromatograph coupled to a mass-selective detector and a flame detection system.
The analysis was performed with a gas chromatograph, GC 6890 Plus (Agilent Technologies, AT, Palo Alto, CA, USA), equipped with a mass-selective detector MS 5973 Network (AT, Palo Alto, CA, USA) using electron ionization (EI, 70 eV). Helium (99.995%, AP gas, Messer, Bogotá, Colombia) was used as the carrier gas, with an initial inlet pressure at the column head of 113.5 kPa; the volumetric flow rate of the carrier gas was kept constant (1 mL/min) during the chromatographic run. The injection mode was split (30:1) and the temperature of the injector was maintained at 250 °C.
Compounds were separated on two capillary columns, one containing the polar stationary phase of poly (ethylene glycol), PEG (DB-WAX, J & W Scientific, Folsom, CA, USA) of 60 m × 0.25 mm (i.d.) × 0.25 μm (df) of 60 m × 0.25 mm (i.d.) × 0.25 μm (df) and the other with the stationary phase (s.f.) apolar 5%-phenyl-poly(methylsiloxane), 5%-Ph-PDMS (DB-5MS, J & W Scientific, Folsom, CA, USA) with the same dimensions. On the polar column (DB-WAX), the oven temperature was programmed from 50 °C (5 min) to 150 °C (7 min), at 4 °C/min, and then to 230 °C (50 min), at 4 °C/min. Same conditions was used for the analysis via flame detection system (GC/FID). On the apolar column (DB-5MS), the chromatographic oven temperature was programmed from 45 °C (5 min) to 150 °C (2 min) at 4 °C/min, and then to 300 °C (10 min) at 5 °C/min. The GC/MS transfer line temperature was set to 230 °C when using the polar column and 300 °C for the nonpolar column. The ionization chamber and quadrupole temperatures were 250 °C and 150 °C, respectively. The mass range for ion current acquisition was m/z 45–450 u, with an acquisition rate of 3.58 scan/s. Data were processed using MSDChemStation G1701DA software (AT, Palo Alto, CA, USA). Integration parameters were as follows: threshold = 18 and “rejection area” of the peak above baseline less than 1%. Compounds were identified based on linear retention indices (LRI) and comparison of experimentally obtained mass spectra with those reported in Adams 2007, NIST 2017 and Wiley 2008 databases.
where: n is the number of carbon atoms in the n-paraffin eluting before the compound of interest (its retention time is tRx); tRn and tRN are the retention times of the n-paraffins with the number of carbon atoms n and N, respectively, eluting immediately before and after the analyte of interest.
Compound quantification was performed with the external standardization by GC/FID. Standard substances were analyzed under the same chromatographic conditions as the Z. caribeum EO. In the case for which a reference substance was not available, quantification was performed using the calibration curve obtained for a structurally similar molecule.
2.4. Antimicrobial Activity
In vitro antimicrobial activity was performed against the fungus M. roreri, which affects cocoa kernel production in cocoa-producing countries.
2.4.1. Isolation of Moniliophthora roreri
In order to obtain the inoculum of M. roreri, infested fruits with early symptoms and signs of the formation of a dark brown spot with mycelium on the shell of the cocoa kernel (fruits) must be collected in the field.
The harvested fruits were washed and disinfected with hypochlorite 1% for two minutes. Then, they were rinsed with distilled water and dried with sterile absorbent paper. The disinfected fruits were then segmented into 5 mm diameter portions (endodermal tissue) in a laminar flow chamber, and the tissue segments infested with M. roreri were placed in Petri dishes containing potato dextrose agar (PDA) culture medium to incubate at 25 °C until fungal growth appeared in the samples to identify them by their macroscopic and microscopic characteristics, which was repeated until pure cultures were obtained.
2.4.2. Antifungal Activity against Moniliophthora roreri
In vitro assay of antifungal activity of Z. caribaaeum essential oils on M. roreri was performed under controlled conditions using the poisoning technique [22,23], the concentrations evaluated in the test were 5 µL, 10 µL, 50 µL, 100 µL and 495 µL and tests were performed in triplicate. After the preparation of the poisoned medium, mycelial discs with a diameter of 5 mm from pure cultures grown for 7 to 10 days were sown in the center of the boxes containing the treatments. Slices of the pathogen on PDA agar medium without essential oils were used as a negative control. Copper oxychloride (Cu2(OH)3Cl), a commercial fungicide with known activity on M. roreri was used as the positive control. Petri dishes were incubated at 25 °C. The plates were daily evaluated by measuring their radial growth in cm (Roque et al., 2001). The measurement was completed when the mycelium of the pathogen completely covered the negative control plate.
The inhibition mycelial growth of the pathogen is calculated as the percentage of radial growth relative to the control (Equation (2)).
where: NCD = Negative control diameter, TD = Treatment diameter.
The measurement shall be concluded when the pathogen’s mycelium completely covers the control treatment plate. The evaluation of the efficacy of the product shall be expressed as a percentage inhibition of mycelial growth.
2.5. Statistical Analysis
Statistical analysis was performed using a single factor experimental design with the software IBM SPSS version 25.0 [24,25,26]. This analysis was applied to the in vitro biometric measurement data obtained from the application of Z. caribaeum essential oil, with 5 treatments (in triplicate) corresponding to the established dosages of 5 µL (T1), 10 µL (T2), 50 µL (T3), 100 µL (T4) and 495 µL (T5). In addition, 2 control treatments were performed; M. roreri on PDA, treatment 6 is PDA only (T6) and a commercial fungicide, phosphorus oxychloride (Cu2(OH)3Cl) as treatment 7 (T7).
To demonstrate the normality, the Shapiro–Wilk (S-W) test [27] was applied (p-value (S-W test) > 0.05). In addition, Levene’s test for homogeneity of variances was applied to detect equality or difference between at least one pair of means of the treatments, and thus ANOVA of a single factor was used to test the specific differences between treatments by means of Post hoc tests (DSM or T2-Tamhane) accepting significant differences with a p-value < 0.05 [28,29].
3. Results and Discussion
The essential oil of Z. caribaeum consisted of 43 compounds, mainly sesquiterpenes (67%), monoterpenes (18%), in addition, three unidentified compounds with m/z 152 (C15H16O), 204 (C15H24) and 220 (C15H24O). However, in this work, we highlight six main compounds that have a proportion higher than 5%, including three sesquiterpenes, germacrene D (228.0 ± 1.6 mg/g EO), (E)-β-farnesene (128.0 ± 1.5 mg/g EO), β-elemene (116.0 ± 1.6 mg/g EO), one monoterpene, limonene (73.0 ± 1.9 mg/g EO) and one oxygenated monoterpene, (E)-nerolidol (74.0 ± 2.2 mg/g EO). (Table 1, Figure 2). Comparing the composition and abundance of essential oil metabolites in this study with those reported in the literature [30,31,32], it was clear that the concentration of germacrene D in essential oil of Z. caribaeum was higher in the individuals used in this study.

Table 1.
Chemical characterization via GC/FID and GC/MS of the essential oil distilled from Z. caribeum.
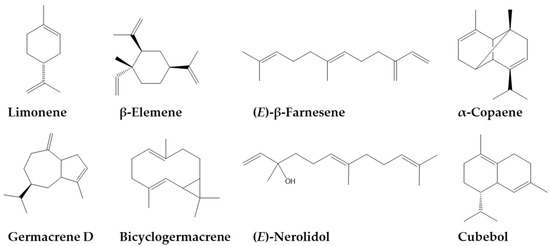
Figure 2.
Main components of Zanthoxylum caribaeum essential oils.
In order to obtain reliable and verified data, two chromatographic studies were performed using two capillary columns, the first with a DB-5MS is apolar phenyl arylene polymer column with 5% phenyl-poly(methylsiloxane) stationary phase, this type of column has excellent performance with its signal/noise ratio, very important for the development of analytical applications, in addition, it shows high sensitivity and mass spectral integrity. The second with a DB-WAX high-polar column with poly (ethylene glycol) stationary phase, the use of this column allows it to be used in food, fragrance and flavor applications. Its use at low temperatures shows excellent resolution of low boiling point active ingredients. The chromatograms obtained from both columns showed the same components with different rates, and the experimental values differed from those reported in the literature (Table 1; Figure 3).
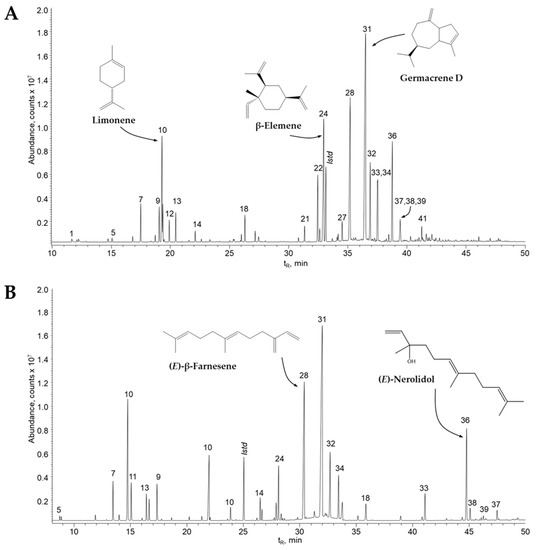
Figure 3.
Chromatographic profiles of the essential oil distilled from Z. caribaeum obtained by GC/MS (full scan). (A) DB-5MS column (60 m), split injection 1:30, MSD (EI, 70 eV); (B) DB-WAX column (60 m), split injection 1:30, MSD (EI, 70 eV).
Inhibition of mycelial growth was determined macroscopically by placing a fragment of the fungal colony in a Petri dish containing PDA supplemented with different dilutions of essential oil (5, 10, 50, 50, 100 to 496 µL/mL), negative control (PDA without treatment) and positive control (copper oxychloride); each test was performed in triplicate. Mycelial growth was determined by measuring colony diameter (cm) for 7 days, and its inhibition percentage was determined (Table 2). When calculating the percentage of inhibition, it is evident that the essential oil of Z. caribaeum is active; it presents a high inhibition of the growth of M. roreri at low concentrations of 10 µL/mL, which inhibits 88.3%. The concentration/percentage of inhibition relationship is notorious, observing a proportionality with the increase in the concentration to 50 µL/mL, determining that an inhibition of 96% of the fungus growth was reached. This determines that concentrations ranging between 10–50 µL/mL are promising for future development of bioproducts involving essential oils with comparable chemical profile.

Table 2.
Shapiro–Wilk normality test and percentage inhibition values per treatment.
The statistical test performed shows that the data are normal (Supplementary Material Figure S1), determined by the p-value being greater than 0.05, therefore the null hypothesis is rejected [38]. With the exception of treatment T5 and T7 (0.000), but it is accepted that the behavior is obtained normal for the other data. To choose the best treatment, a multiple comparison between treatments was performed, for which the Tamhane test was used (Supplementary Material Table S2), because the analyzed data did not show homogeneity between variances. This showed that T5 has significant differences compared to the other treatments, this would be the one with the highest degree of inhibition, since it produces an average diameter growth of 0.04667 cm less than T4, the latter being the second-best treatment in terms of inhibition of the phytopathogen. As for the other treatments that showed significant differences, T6 and T7 were considered as control treatments (Table 2). The antifungal assays showed inhibition of M. roreri growth by the T2 treatment (10 µL/mL); however, the activity of the essential oils was more stable in the T4 treatment (100 µL/mL) over the study period (7 days) (Figure 4).
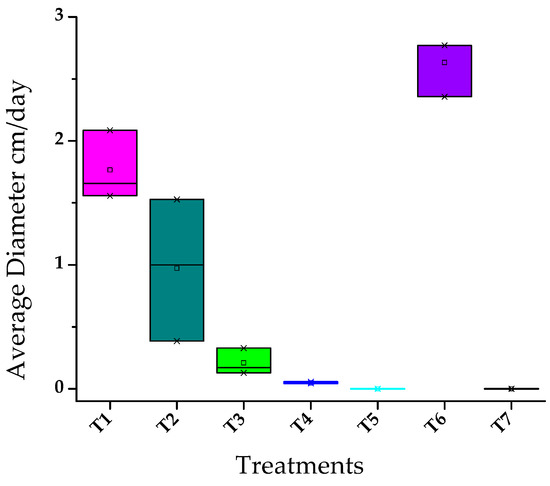
Figure 4.
Comparative boxplot of biometric growth at day 7 between treatments evaluated against M. roreri.
The main compounds of Z. caribaeum are germacrene D, (E)-β-farnesene, (E)-nerolidol and bicyclogermacrene. However, it should be noted that the compounds (E)-β-farnesene, (E)-nerolidol and bicyclogermacrene are components of essential oils that exhibit antimicrobial activity, but no such activity can be attributed to them [39]. Studies have evaluated the inhibitory effect of the essential oil (EO) of Z. armatum on the filamentous fungus Aspergillus flavus. The essential oil showed a chemical composition where its major compounds were linalool (41.73%), D-limonene (13.24%), β-phellandrene (7.53%), trans-nerolidol (6.30%) and terpinen-4-ol (5.33%). When the oil was evaluated, it showed a decrease in the radial growth of A. flavus and also when a microscopic study was carried out, the mycelium was observed to be considerably reduced, as well as the number of colonies at room temperature [40]. In contrast, the high concentration of germacrene D is remarkable, and it should be noted that there is a direct relationship between the presence of this substance and antifungal activity. Several studies have shown antifungal activity on different groups of fungi. An example of this is the germacrene-rich essential oils extracted from species such as Artemisia campestris, which have shown in vitro antifungal activity against Fusarium graminearum, which attacks crops such as rice, oats and corn [40]. It has also been shown to inhibit the growth of plant pathogenic fungi, including a number of Fusarium species, Botrytis cinerea and Alternaria solani [41,42,43]. In Glechon species, the antifungal capacity against fungi of human interest such as Candida has been noted, and the extracted oil has in common that germacrene D is one of its main components [44]. In Buddleja perfoliata and Pelargonium graveolens species, the extracted essential oil showed antifungal activity against various fungi affecting the postharvest of the plants. In particular, this oil showed broad activity against strains of Aspergillus amylovorus, A. flavus, A nomius, A ostianus, Eurotium halophilicum, Eupenicillum hirayamae, Penicillium cinnamopurpureum and P. viridicatum var. ii [45].
4. Conclusions
In the essential oil of Z. caribaeum, the main compounds were identified, mainly molecules of germacrene D (228.0 ± 1.6 mg/g EO), (E)-β-farnesene (128.0 ± 1.5 mg/g EO), β-elemene (116.0 ± 1.6 mg/g EO) and (E)-nerolidol (74.0 ± 2.2 mg/g EO) were found in its chemical composition. The essential oil was tested against the basidiomycete M. roreri, a fungus that affects cocoa production worldwide and therefore has a particular impact on family economy. The results show a promising potential of this oil at concentrations between 50 µL/mL and 100 µL/mL, where it showed an inhibition percentage of 96% and 99%, respectively, sufficient concentrations to keep the growth of the fungus under control. In addition, it should be noted that, although four main compounds were found, germacrene D is the substance attributed with 26.4% antifungal activity based on the background shown. The other three compounds were found in oils showing antimicrobial activity; however, their concentrations are not appreciable, but it can be speculated that their combined effect enhances the antimicrobial activity. This is a step towards the development of bioproducts for sustainable phytosanitary control of microorganisms infesting cocoa crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11080447/s1, Table S1: Calibration curves for compound quantification in Z. caribaeum essential oil; Table S2: Descriptive analysis of the growth of M. roreri in the different concentrations of essential oil of Z. caribaeum; Figure S1: Normal Q-Q diagram of the mean growth diameter of M. roreri treated with essential oils of Z. caribaeum.
Author Contributions
Conceptualization, M.F.-E., A.J.-G., C.A., E.S., N.P.B. and O.G.-B.; methodology, D.D., M.F.-E., R.S.-B. and O.G.-B.; validation, C.A., E.S., N.P.B., J.C., D.B.-P. and O.G.-B., data curation, R.S.-B. and M.F.-E.; writing—M.F.-E., A.J.-G., O.G.-B.; writing—review and editing, O.G.-B., C.A., J.C. and D.B.-P.; project administration, E.S., N.P.B. and O.G.-B.; funding acquisition, E.S., N.P.B. and O.G.-B. All authors have read and agreed to the published version of the manuscript.
Funding
E.S., N.P.B. and O.G.-B., thanks the Ministry of Science, Technology and Innovation (Minciencias), the Ministry of Education, the Ministry of Industry, Commerce and Tourism and ICETEX, Program Ecosistema Científico–Colombia Científica, from the Francisco José de Caldas Fund (Grant RC-FP44842-212-2018). National Authority of Environmental Licensing of Colombia supported the Universidad de Ibagué. Framework Permit for the Collection of Specimens of Species of Wild Species of Biological Specimens of Wild Species of Biological Diversity for Non-Commercial Scientific Research Purposes, and other determinations are made (Resolution No. 01003-2019). The funders were not involved in technical aspects of the research development and C.A., Grand FONDECYT REGULAR 1230414 from Chile.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors may share the datasets obtained in this study upon request and on reasonable and good terms.
Acknowledgments
The authors would like to thank Hector Esquivel for the identification of the species Z. caribaeum and FEDECACAO for allowing the biological activity work to be carried out at its facilities in the municipality of San Vicente de Chucurí (Santander-Colombia) and CENIVAM of the Universidad Industrial de Santander.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, interpretation of data; in the writing of the manuscript and in the decision to publish the results.
References
- Appelhans, M.S.; Reichelt, N.; Groppo, M.; Paetzold, C.; Wen, J. Molecular Phylogenetics and Evolution Phylogeny and biogeography of the pantropical genus Zanthoxylum and its closest relatives in the proto-Rutaceae group (Rutaceae). Mol. Phylogenet. Evol. 2018, 126, 31–44. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Rutaceae, Z.L.; Rivas-arancibia, S.P. Distribution patterns of the genus Zanthoxylum L. (Rutaceae) in Mexico. Rev. Mex. Biodivers. 2013, 84, 1179–1188. [Google Scholar] [CrossRef]
- Syowai, E.; Kimutai, F.; Mbandi, E.; Nyongesa, E.; Ochieng, W.; Nanjala, C.; Njambi, C.; Kirega, M.; Muguci, M.; Wahiti, R.; et al. Ethnobotanical uses, phytochemistry and pharmacology of pantropical genus Zanthoxylum L. (Rutaceae): An update nuclear Magnetic Resonance Spectroscopy. J. Ethnopharmacol. 2023, 303, 115895. [Google Scholar] [CrossRef]
- Tan, M.A.; Sharma, N. Phyto-Carbazole Alkaloids from the Rutaceae Family as Potential Protective Agents against Neurodegenerative Diseases. Antioxidants 2022, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Zhou, Q.; Zhou, Q.; Xie, Y.; Khan, A.; Zhou, Z.; Lv, X.; Liu, L. Fitoterapia (±)-Zanthonitidumines A and B: Two new benzophenanthridine alkaloids enantiomers from Zanthoxylum nitidum and their anti-inflammatory activity. Fitoterapia 2023, 164, 105362. [Google Scholar] [CrossRef]
- Qin, F.; Wang, F.; Wang, C.; Chen, Y.; Li, M.; Zhu, Y.; Huang, X.; Fan, C.; Wang, H. Fitoterapia The neurotrophic and antineuroinflammatory effects of phenylpropanoids from Zanthoxylum nitidum var. tomentosum (Rutaceae). Fitoterapia 2021, 153, 104990. [Google Scholar] [CrossRef]
- Kerubo, L.; Nchiozem-ngnitedem, V.; Guefack, M.F. South African Journal of Botany Antibacterial activities of thirteen naturally occuring compounds from two Kenyan medicinal plants: Zanthoxylum paracanthum (Mildbr). Kokwaro (Rutaceae) and Dracaena usambarensis Engl. (Asparagaceae) against MDR phenotypes. S. Afr. J. Bot. 2022, 151, 756–762. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Shi, X.; Xia, X.; He, Y.; Zhu, Y.; Xie, T.; Liu, T.; Xu, X.; Luo, X. Food Bioscience Comparison of chemical constituents in diverse zanthoxylum herbs, and evaluation of their relative antibacterial and nematicidal activity. Food Biosci. 2021, 42, 101206. [Google Scholar] [CrossRef]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef]
- Dillinger, T.L.; Barriga, P.; Esca, S.; Jimenez, M.; Lowe, D.S.; Grivetti, L.E. Chocolate: Modern Science Investigates an Ancient Medicine Food of the Gods: Cure for Humanity? A Cultural History of the Medicinal and Ritual Use of Chocolate 1. J. Nutr. 2000, 130, 2057–2072. [Google Scholar] [CrossRef]
- Pérez-Vicente, L. Moniliophthora roreri H.C. Evans et al. y Moniliophthora perniciosa (Stahel) Aime: Impacto, síntomas, diagnóstico, epidemiología y manejo. Rev. Protección Veg. 2018, 33, 1–13. [Google Scholar]
- de Brito, E.S.; García, N.H.P.; Gallão, M.I.; Cortelazzo, A.L.; Fevereiro, P.S.; Braga, M.R. Structural and chemical changes in cocoa (Theobroma cacao L.) during fermentation, drying and roasting. J. Sci. Food Agric. 2001, 288, 281–288. [Google Scholar] [CrossRef]
- Bari, V.; Cihat, N.; Akyil, S.; Said, O. Trends in Food Science & Technology Cocoa based beverages–Composition, nutritional value, processing, quality problems and new perspectives. Trends Food Sci. Technol. 2023, 132, 65–75. [Google Scholar] [CrossRef]
- Swaray, R. Commodity buffer stock redux: The role of International Cocoa Organization in prices and incomes. J. Policy Model. 2011, 33, 361–369. [Google Scholar] [CrossRef]
- Hebbar, P.K. e-X tra * Cacao Diseases: Important Threats to Chocolate Production Worldwide Cacao Diseases: A Global Perspective from an Industry Point of View. 1997. [Google Scholar]
- Cubillos, G. Frosty Pod Rot, disease that affects the cocoa (Theobroma cacao) crops in Colombia. Crop. Prot. 2017, 96, 77–82. [Google Scholar] [CrossRef]
- Hütz-Adams, F.; Campos, P.; Fountain, A.C. Barómetro del cacao Base de referencia para Latinoamérica; Consorcio del Barómetro del Cacao. 2022. [Google Scholar]
- Wuellins, D. Cadena del Valor del Cacao; FONTAGRO: Washington, DC, USA, 2019; ISBN 9789942364654. [Google Scholar]
- Correa Alvarez, J.; Castro Martínez, S.; Coy, J. Estado de la Moniliasis del cacao causada por Moniliophthora roreri en Colombia. Acta Agronómica 2014, 63, 388–399. [Google Scholar] [CrossRef]
- Guillermo, J.; Gil, R. Pérdidas económicas asociadas a la pudrición de la mazorca del cacao causada por Phytophthora spp., y Moniliophthora roreri (Cif y Par) Evans et al., en la hacienda Theobroma, Colombia. Rev. De Protección Veg. 2016, 31, 42–49. [Google Scholar]
- Manrique-moreno, M.; Klaiss-luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar]
- Palumbo, J.D.; Keeffe, T.L.O. Method for high-throughput antifungal activity screening of bacterial strain libraries. J. Microbiol. Methods 2021, 189, 106311. [Google Scholar] [CrossRef]
- Garnier, L.; Salas, M.L.; Pinon, N.; Wiernasz, N.; Pawtowski, A.; Coton, E.; Mounier, J.; Valence, F. Technical note: High-throughput method for antifungal activity screening in a cheese-mimicking model. J. Dairy Sci. 2018, 101, 4971–4976. [Google Scholar] [CrossRef]
- Hornby, B.D.; Bateman, G.L.; Payne, R.W.; Brown, M.E. Field tests of bacteria and soil-applied fungicides as control agents for take-all in winter wheat. Ann. Appl. Biol. 1993, 122, 253–270. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Dinh, M.H.; Chi, H.T.; Wang, S.L.; Nguyen, Q.V.; Tran, T.D.; Nguyen, A.D. Antioxidant and cytotoxic activity of lichens collected from Bidoup Nui Ba National Park, Vietnam. Res. Chem. Intermed. 2019, 45, 33–49. [Google Scholar] [CrossRef]
- Maric, M.; de Haan, E.; Huizenga, H.M. ScienceDirect Evaluating Statistical and Clinical Significance of Intervention Effects in Single-Case Experimental Designs: An SPSS Method to Analyze Univariate Data. Behav. Ther. 2015, 46, 230–241. [Google Scholar] [CrossRef]
- Liang, J.; Tang, M.; Chan, P.S. A generalized Shapiro–Wilk W statistic for testing high-dimensional. Comput. Stat. Data Anal. 2009, 53, 3883–3891. [Google Scholar] [CrossRef]
- Sesaazi, C.D.; Peter, E.L.; Mtewa, A.G. The anti-nociceptive effects of ethanol extract of aerial parts of Schkuhria pinnata in mice. J. Ethnopharmacol. 2021, 271, 113913. [Google Scholar] [CrossRef]
- Shirani, M.; Savabi, O.; Mosharraf, R. Comparison of translucency and opalescence among different dental monolithic ceramics. J. Prosthet. Dent. 2021, 126, 446.e1–446.e6. [Google Scholar] [CrossRef]
- Nogueira, J.; Mourão, S.C.; Dolabela, I.B. Zanthoxylum caribaeum (Rutaceae) essential oil: Chemical investigation and biological effects on Rhodnius prolixus nymph. Parasitol. Res. 2014, 113, 4271–4279. [Google Scholar] [CrossRef]
- Farouil, L.; Dias, R.P.; Popotte-julisson, G.; Bibian, G.; Adou, A.I.; de Mata, A.P.; Sylvestre, M.; Harynuk, J.J.; Cebri, G. The Metabolomic Profile of the Essential Oil from Zanthoxylum caribaeum (syn. chiloperone) Growing in Guadeloupe FWI using GC × GC-TOFMS. Metabolites 2022, 12, 1293. [Google Scholar] [CrossRef]
- de Lara de Souza, J.G.; Toledo, A.G.; Walerius, A.H.; Jann Favreto, W.A.; da Costa, W.F.; da Silva Pinto, F.G. Chemical Composition, Antimicrobial, Repellent and Antioxidant Activity of Essential Oil of Zanthoxylum caribaeum Lam. J. Essent. Oil Bear. Plants 2019, 22, 380–390. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Spectrometry, M.; Adams, R.P. Identification of Essential Oil Components by Gas Chromatography; Texensis Publishing: Gruver, TX, USA, 2017; ISBN 9781932633214. [Google Scholar]
- Le, N.V.; Sam, L.N.; Huong, L.T.; Ogunwande, I.A. Chemical Compositions of Essential Oils and Antimicrobial Activity of Piper albispicum C. DC. from Vietnam. J. Essent. Oil Bear. Plants 2022, 25, 82–92. [Google Scholar] [CrossRef]
- NIST Standard Reference Database. NIST/EPA/NIH Spectral Library with Search Program, Version 2.3; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- McLafferty, F.W.; Douglas, B.S. The Wiley/NBS Registry of Mass Spectral Data, 2nd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Echeverri, L.I.; Arroyave, E.A.; Barajas, F.H. Comparación de pruebas de normalidad. XXI Simp. Int. Estad 2015, 8–11. [Google Scholar]
- Li, T.; Chen, M.; Ren, G.; Hua, G.; Mi, J.; Jiang, D. Antifungal Activity of Essential Oil From Zanthoxylum armatum DC. on Aspergillus flavus and Aflatoxins in Stored Platycladi Semen. Front. Microbiol. 2021, 12, 633714. [Google Scholar] [CrossRef] [PubMed]
- Houicher, A.; Hechachna, H.; Özogul, F. In Vitro Determination of the Antifungal Activity of Artemisia campestris Essential Oil from Algeria In Vitro Determination of the Antifungal Activity of Artemisia campestris Essential Oil from Algeria. Int. J. Food Prop. 2016, 19, 1749–1756. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D.; Biomolecolari, S.; Biologia, S.; Carlo, U. Essential oil composition and antifungal activity of aerial parts of Ballota nigra ssp foetida collected at flowering and fruiting times. Nat. Prod. Commun. 2014, 9, 1934578X1400900733. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Halima, N.B.; Abdelkafi, S.; Hamdi, N. Essential Oil from Artemisia phaeolepis: Chemical Composition and Antimicrobial Activities. J. Oleo Sci. 2013, 980, 973–980. [Google Scholar] [CrossRef]
- Alvarenga, E.S.; Moreira, C.; Barreto, R.W. Chemical Characterization of Volatile Compounds of Lantana camara L. and L. radula Sw. and Their Antifungal Activity. Molecules 2012, 17, 11447–11455. [Google Scholar] [CrossRef]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.L.; Roehe, P.M.; Alexandre, M.; Henriques, A.T.; Venturi, C.R.; et al. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef]
- Juárez, Z.N.; Bach, H.; Sánchez-Arreola, E.; Hernández, L.R. Protective antifungal activity of essential oils extracted from Buddleja perfoliata and Pelargonium graveolens against fungi isolated from stored grains. J. Appl. Microbiol. 2016, 120, 1264–1270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).