Advances in the Application of Nano-Enzymes in the Electrochemical Detection of Reactive Oxygen Species: A Review
Abstract
1. Introduction
2. Selection and Construction of Nanozymes with Highly Active Sites for Electrochemical Detection of ROS
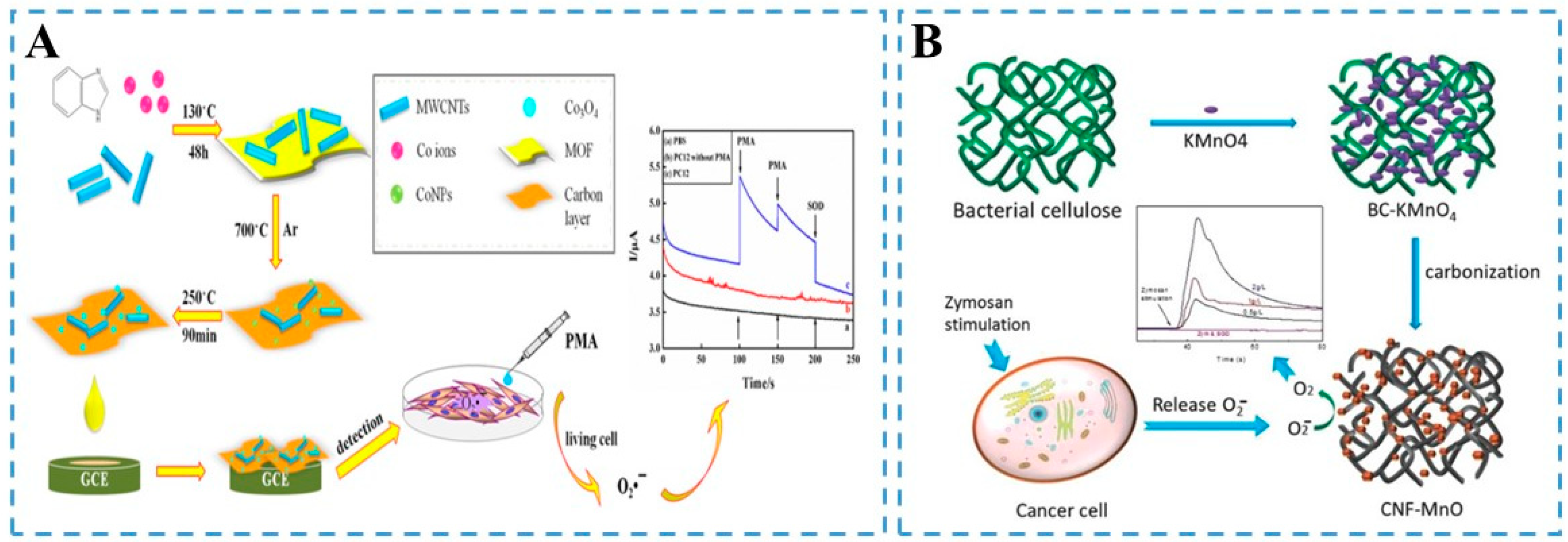
2.1. Carbon-Based Nanozymes
2.2. Noble-Metal-Based Nanozymes
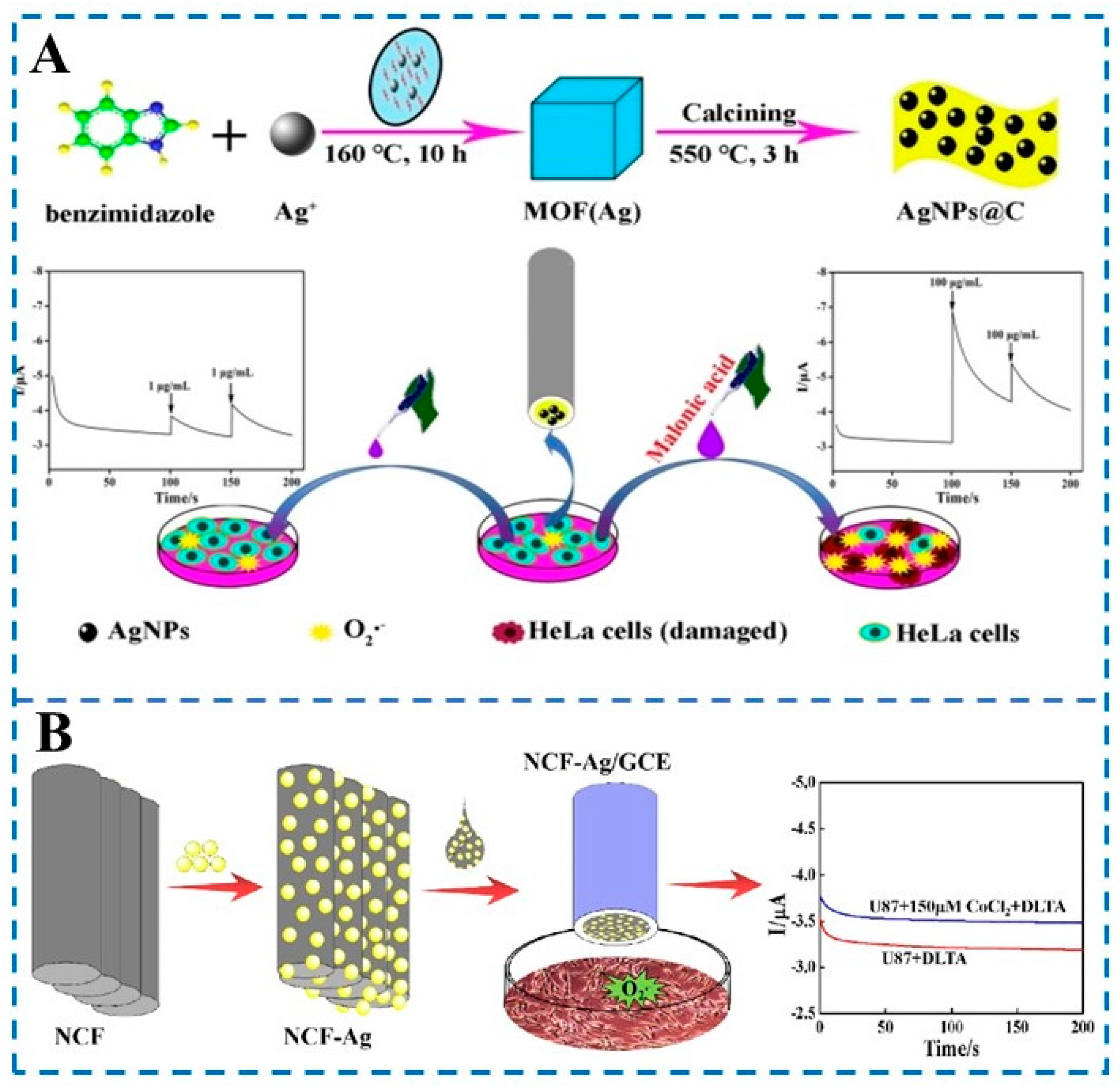
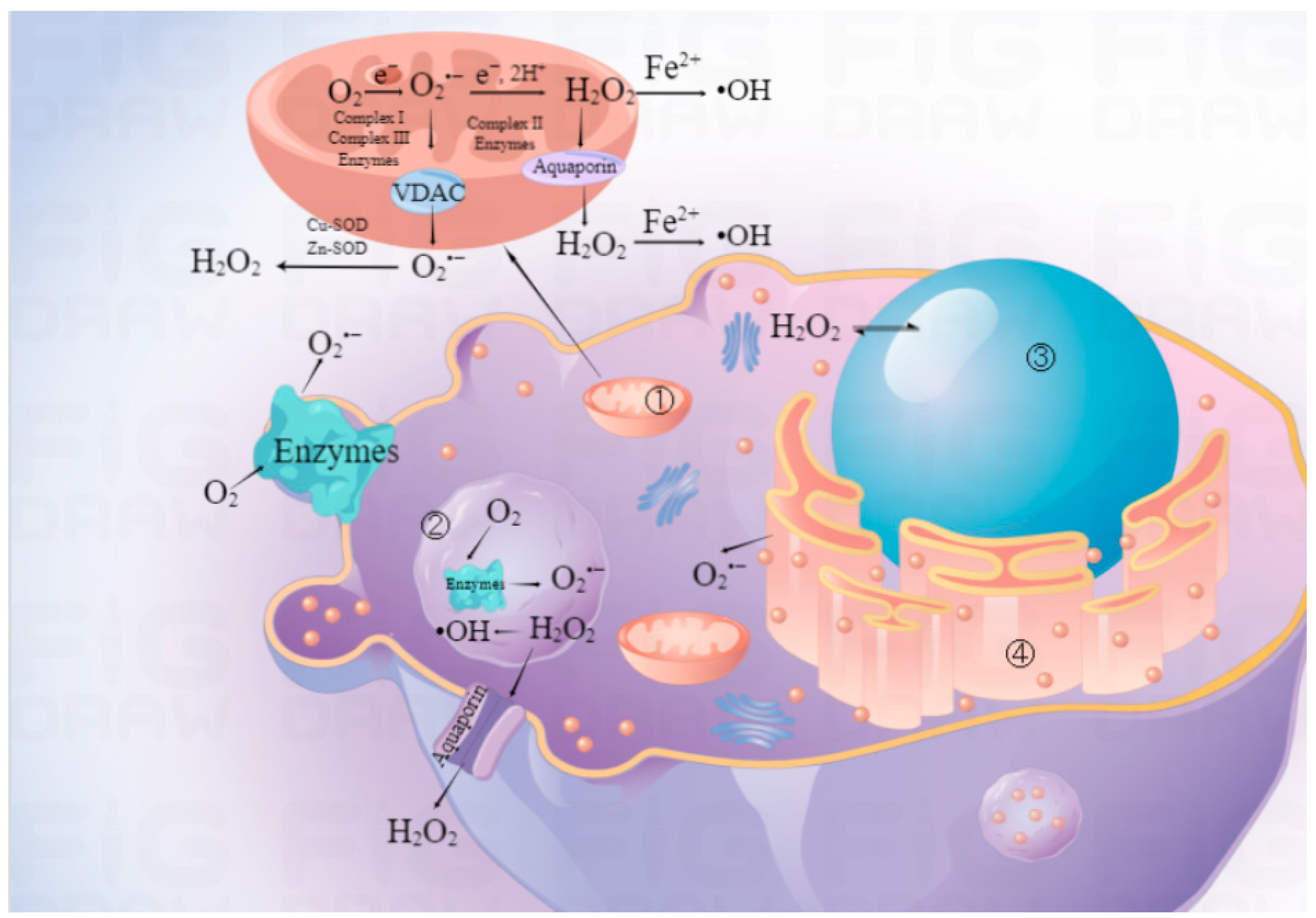
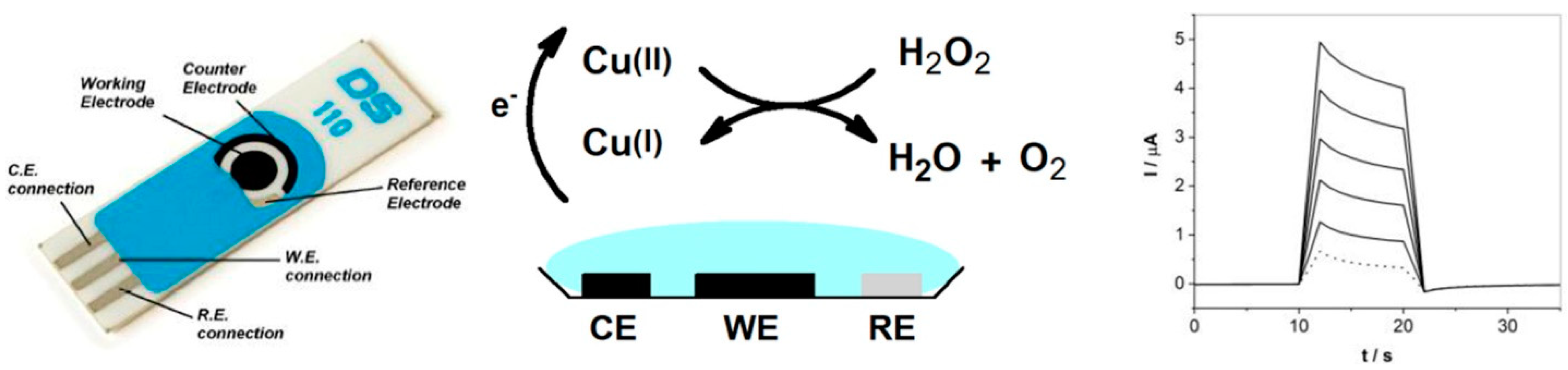
2.3. Transition-Metal-Based Nanozymes
2.3.1. Metallic Oxides
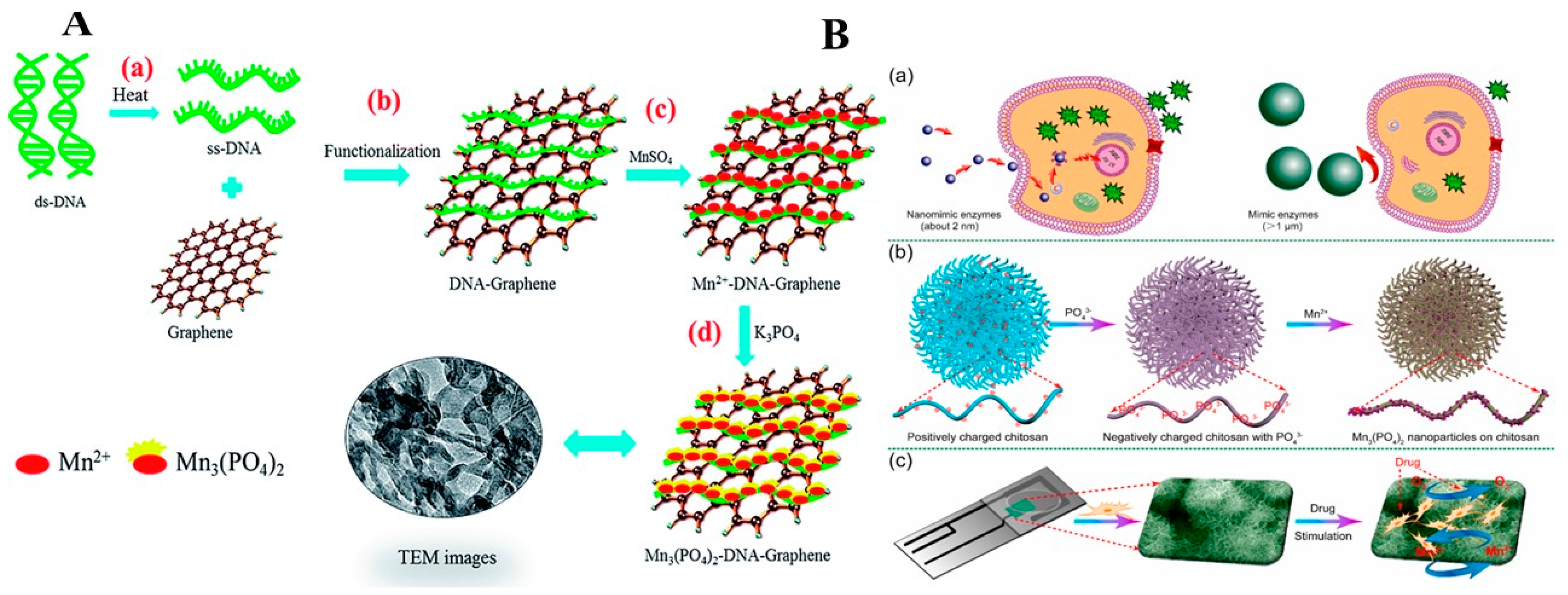
2.3.2. Transition Metal Phosphates
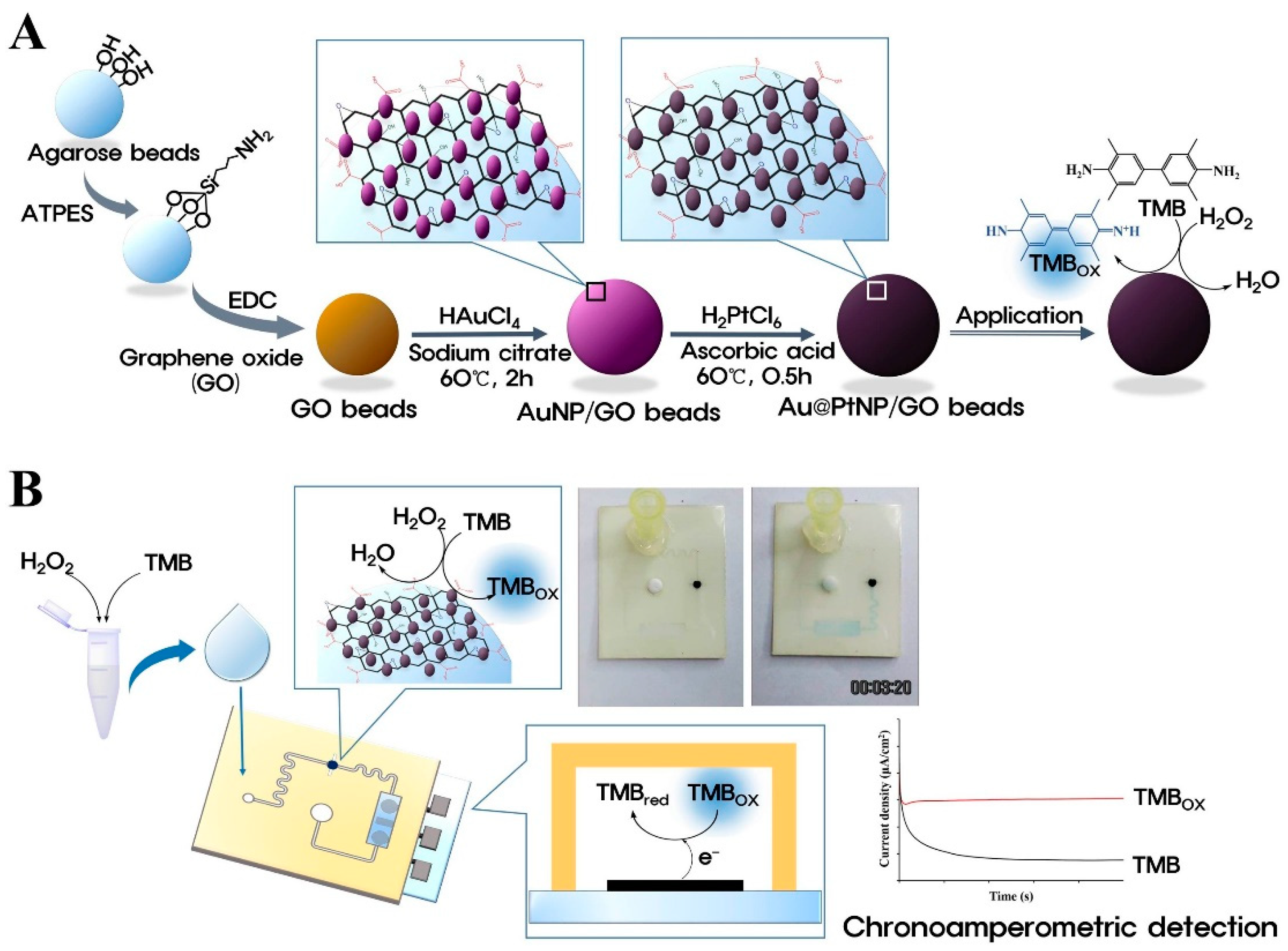
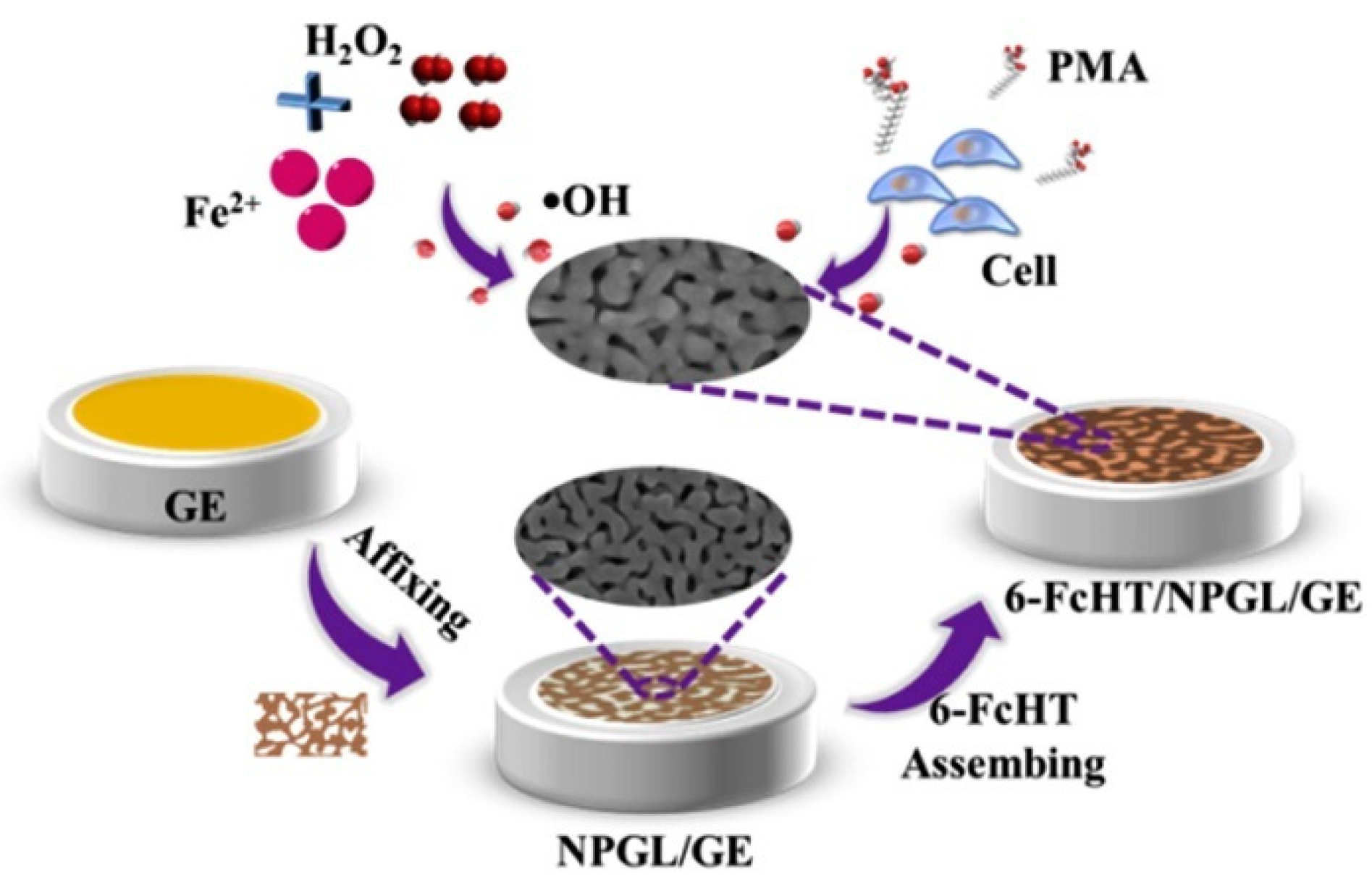
2.4. Metal–Organic-Framework-Based Nanozymes
3. Selective Challenges of Nanozymes for Electrochemical Detection of ROS
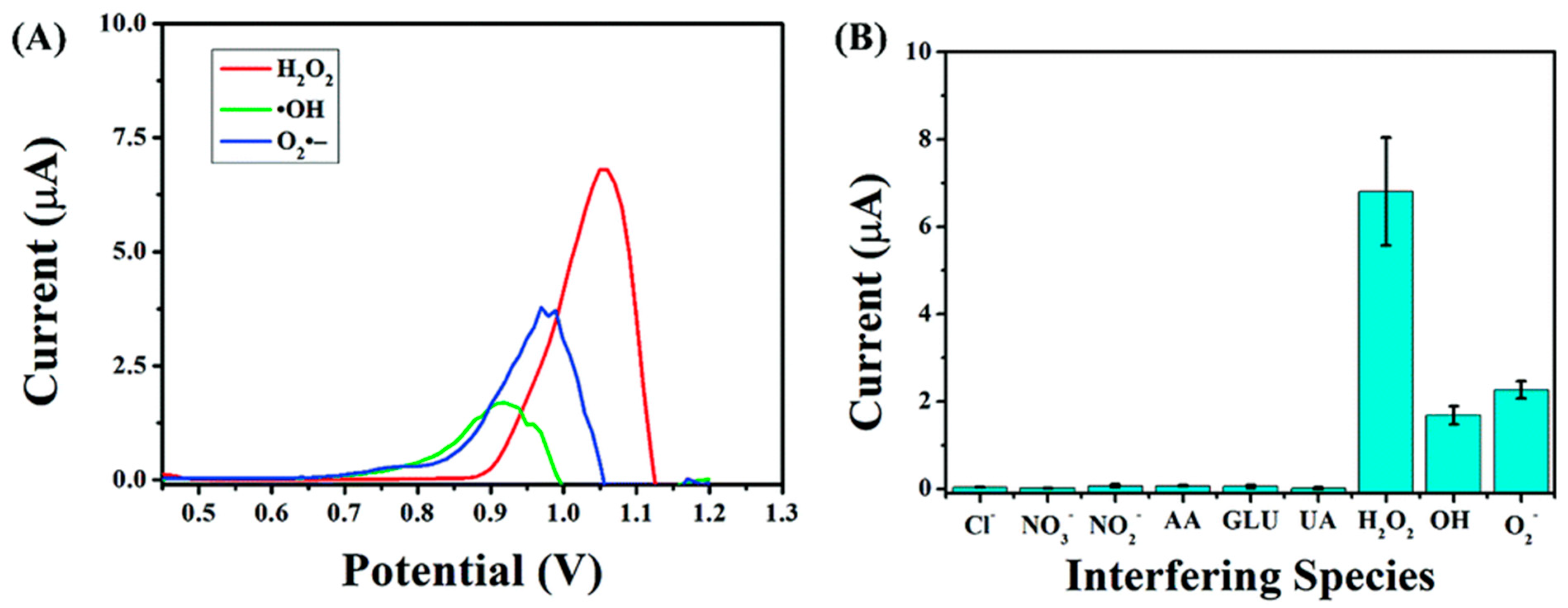
3.1. Selective Challenge of the Same Nanozymes
3.2. Selective Challenge of Electrochemical Technology
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef]
- Geraskevich, A.V.; Solomonenko, A.N.; Dorozhko, E.V.; Korotkova, E.I.; Barek, J. Electrochemical Sensors for the Detection of Reactive Oxygen Species in Biological Systems: A Critical Review. Crit. Rev. Anal. Chem. 2022, 1–33. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef]
- Gao, R.; Yang, X.; Yang, Q.; Wu, Y.; Wang, F.; Xia, Q.; Bao, S.-J. Design of an amperometric glucose oxidase biosensor with added protective and adhesion layers. Microchim. Acta 2021, 188, 312. [Google Scholar] [CrossRef]
- Kaur, J.; Choudhary, S.; Chaudhari, R.; Jayant, R.; Joshi, A. Enzyme-Based Biosensors; ResearchGate: Berlin, Germany, 2019; pp. 211–240. [Google Scholar]
- Wang, X.; Dong, S.; Wei, H. Recent Advances on Nanozyme-based Electrochemical Biosensors. Electroanalysis 2022, 35, 38–39. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, e1805368. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in electrochemical affinity biosensing. Microchim. Acta 2020, 187, 423. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Pacheco, J.C.; Seraphim, P.M.; Teixeira, M.F.S. Synergistic effect of reduced graphene oxide/azo-polymer layers on electrochemical performance and application as nonenzymatic chemiresistor sensors for detecting superoxide anion radicals. J. Electroanal. Chem. 2019, 852, 113520. [Google Scholar] [CrossRef]
- Cai, X.; Chen, H.L.; Wang, Z.X.; Sun, W.Q.; Shi, L.B.; Zhao, H.L.; Lan, M.B. 3D graphene-based foam induced by phytic acid: An effective enzyme-mimic catalyst for electrochemical detection of cell-released superoxide anion. Biosens. Bioelectron. 2019, 123, 101–107. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.L.; Shi, L.B.; Lan, M.B.; Zhang, H.W.; Yu, C.Z. Enzyme- and metal-free electrochemical sensor for highly sensitive superoxide anion detection based on nitrogen doped hollow mesoporous carbon spheres. Electrochim. Acta 2017, 227, 69–76. [Google Scholar] [CrossRef]
- Tian, J.Q.; Liu, Q.; Ge, C.J.; Xing, Z.C.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Ultrathin graphitic carbon nitride nanosheets: A low-cost, green, and highly efficient electrocatalyst toward the reduction of hydrogen peroxide and its glucose biosensing application. Nanoscale 2013, 5, 8921–8924. [Google Scholar] [CrossRef]
- Bai, J.; Sun, C.H.; Jiang, X.E. Carbon dots-decorated multiwalled carbon nanotubes nanocomposites as a high-performance electrochemical sensor for detection of H2O2 in living cells. Anal. Bioanal. Chem. 2016, 408, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.Z.; Liu, X.H.; Wu, J.S.; Liu, Q.; Ding, L.; Liu, X.H. Development of a Novel Silver-based Sensing Platform for Detecting Superoxide Anion Released from HeLa Cells Directly. Electroanalysis 2022, 34, 987–994. [Google Scholar] [CrossRef]
- Welch, C.M.; Banks, C.E.; Simm, A.O.; Compton, R.G. Silver nanoparticle assemblies supported on glassy-carbon electrodes for the electro-analytical detection of hydrogen peroxide. Anal. Bioanal. Chem. 2005, 382, 12–21. [Google Scholar] [CrossRef]
- Wu, T.D.; Li, L.; Jiang, X.C.; Liu, F.X.; Liu, Q.; Liu, X.H. Construction of silver-cotton carbon fiber sensing interface and study on the protective effect of antioxidants on hypoxia-induced cell damage. Microchem. J. 2020, 159, 105345. [Google Scholar] [CrossRef]
- Zhu, D.Z.; He, P.; Kong, H.; Yang, G.Z.; Luan, X.; Wei, G. Biomimetic graphene-supported ultrafine platinum nanowires for colorimetric and electrochemical detection of hydrogen peroxide. J. Mater. Chem. B 2022, 10, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Van-Khue, T.; Son, S.E.; Hur, W.; Choi, H.; Seong, G.H. Characterization of Au@PtNP/GO nanozyme and its application to electrochemical microfluidic devices for quantification of hydrogen peroxide. Sens. Actuators B-Chem. 2019, 294, 166–176. [Google Scholar] [CrossRef]
- Xu, Y.W.; Wang, D.Y.; Zhang, Y.; Zhang, J.J.; Jiang, S.Y.; Liang, W.C.; Zhu, T.T.; Ye, B.C. A novel electrochemical sensor for determination of hydroxyl radicals in living cells by coupling nanoporous gold layer with self-assembled 6-(Ferrocenyl) hexanethiol. Anal. Chim. Acta 2020, 1096, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Wei, H.W.; Jiang, X.C.; Guo, H.X.; Liu, X.H. Synthesis of metal-organic frameworks derived nanocomposites for superoxide anion radical sensing and cell monitoring upon oxidative stress. J. Electroanal. Chem. 2018, 820, 51–59. [Google Scholar] [CrossRef]
- Ding, A.L.; Liu, F.; Zheng, J.S.; Chen, J.C.; Li, C.M.; Wang, B. Synthesis of Manganese Oxide Embedded Carbon Nanofibers as Effective Biomimetic Enzymes for Sensitive Detection of Superoxide Anions Released from Living Cells. Macromol. Mater. Eng. 2018, 303, 1800079. [Google Scholar] [CrossRef]
- Klun, U.; Zorko, D.; Stojanov, L.; Mirceski, V.; Jovanovski, V. Amperometric sensor for gaseous H2O2 based on copper redox mediator incorporated electrolyte. Sens. Actuators Rep. 2023, 5, 100144. [Google Scholar] [CrossRef]
- Ghaedamini, H.; Alba-Rubio, A.C.; Kim, D.S. A Novel Electrochemical Sensor Based on a Cerium Oxide/Gold/Carbon Nanocomposite for the Detection of Hydroxyl Free Radicals. J. Electrochem. Soc. 2023, 170, 047510. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Alateeq, F.A.O.; Kim, S.S.; Kim, D.-S.; Alba-Rubio, A.C. The effects of size and content of cerium oxide nanoparticles on a composite sensor for hydroxyl radicals detection. Sens. Actuators B Chem. 2020, 321, 128467. [Google Scholar] [CrossRef]
- Barnese, K.; Gralla, E.B.; Cabelli, D.E.; Selverstone Valentine, J. Manganous Phosphate Acts as a Superoxide Dismutase. J. Am. Chem. Soc. 2008, 130, 4604–4606. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ye, C.; Bao, S.J.; Xu, M.W. Controlled synthesis of Mn3(PO4)2 hollow spheres as biomimetic enzymes for selective detection of superoxide anions released by living cells. Microchim. Acta 2017, 184, 1177–1184. [Google Scholar] [CrossRef]
- Ding, A.L.; Wang, B.; Ma, X.Q.; Diao, J.L.; Zheng, J.S.; Chen, J.C.; Li, C.M. DNA-induced synthesis of biomimetic enzyme for sensitive detection of superoxide anions released from live cell. Rsc Adv. 2018, 8, 12354–12359. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Sun, L.H.; Xue, P.; Wang, M.Q.; Lu, Z.S.; Wang, F.; Xia, Q.Y.; Xu, M.W.; Bao, S.J. Constructing high effective nano-Mn3(PO4)2-chitosan in situ electrochemical detection interface for superoxide anions released from living cell. Biosens. Bioelectron. 2019, 133, 133–140. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Z.X.; Zhang, H.H.; Li, Y.F.; Chen, K.C.; Zhao, H.L.; Lan, M.B. Carbon-mediated synthesis of shape-controllable manganese phosphate as nanozymes for modulation of superoxide anions in HeLa cells. J. Mater. Chem. B 2019, 7, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Ye, C.; Bao, S.J.; Xu, M.W.; Zhang, Y.; Wang, L.; Ma, X.Q.; Guo, J.; Li, C.M. Nanostructured cobalt phosphates as excellent biomimetic enzymes to sensitively detect superoxide anions released from living cells. Biosens. Bioelectron. 2017, 87, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.Q.; Lei, L.L.; Chen, Z.Y.; Liu, Y.S.; Bao, S.J. FePO4 embedded in nanofibers consisting of amorphous carbon and reduced graphene oxide as an enzyme mimetic for monitoring superoxide anions released by living cells. Microchim. Acta 2018, 185, 140. [Google Scholar] [CrossRef]
- Cui, M.; Zhao, H.Y.; Wen, X.F.; Li, N.; Ren, J.J.; Zhang, C. Facile synthesis of nickel phosphate nanorods as biomimetic enzyme with excellent electrocatalytic activity for highly sensitive detection of superoxide anion released from living cells. J. Pharm. Biomed. Anal. 2022, 212, 114653. [Google Scholar] [CrossRef]
- Rao, D.J.; Zhang, J.; Zheng, J.B. Synthesis of silver nanoparticles-decorated FePO4 nanosphere at a gas-liquid interface for the electrochemical detection of Hydrogen peroxide. J. Chem. Sci. 2016, 128, 839–847. [Google Scholar] [CrossRef]
- Peng, L.J.; Zhou, H.Y.; Zhang, C.Y.; Yang, F.Q. Study on the peroxidase-like activity of cobalt phosphate and its application in colorimetric detection of hydrogen peroxide. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 647, 129031. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.X.; Du, H.; Wang, X.; Liu, L.; Li, C.M. Solvent-engineered morphologies of Mn-MOF toward ultrasensitive sensing cell superoxide. Electrochim. Acta 2022, 431, 141147. [Google Scholar] [CrossRef]
- Ling, P.; Cheng, S.; Chen, N.; Qian, C.; Gao, F. Nanozyme-Modified Metal-Organic Frameworks with Multienzymes Activity as Biomimetic Catalysts and Electrocatalytic Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 17185–17192. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Li, W.; Yang, H.; Li, T.; He, S.N.; Wang, Y.; Hu, Y.L. Synthesis of 3D Co-based Zeolitic Imidazolate Framework and Application as Electrochemical Sensor for H2O2 Detection. Int. J. Electrochem. Sci. 2022, 17, 221132. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, M.H.; Zhang, X.Y.; Li, Q.; Liu, X.Y.; Zhang, W.J.; Qin, X.; Qu, F.L. An Enzyme-free Electrochemical H2O2 Sensor Based on a Nickel Metal-organic Framework Nanosheet Array. Electroanalysis 2022, 34, 369–374. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, W.; Gao, R.; Wang, Y.; Bai, Y.; Xu, Z.; Bao, S.-J. MIL-47(V) catalytic conversion of H2O2 for sensitive H2O2 detection and tumor cell inhibition. Sens. Actuators B Chem. 2022, 354, 131201. [Google Scholar] [CrossRef]
- Yu, K.; Li, M.J.; Chai, H.N.; Liu, Q.; Hai, X.; Tian, M.W.; Qu, L.J.; Xu, T.L.; Zhang, G.Y.; Zhang, X.J. MOF-818 nanozyme-based colorimetric and electrochemical dual-mode smartphone sensing platform for in situ detection of H2O2 and H2S released from living cells. Chem. Eng. J. 2023, 451, 138321. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Chen, K.; Zhou, F.; Magdassi, S.; Lan, M. Two-dimensional mesoporous nitrogen-rich carbon nanosheets loaded with CeO2 nanoclusters as nanozymes for the electrochemical detection of superoxide anions in HepG2 cells. Biosens Bioelectron 2022, 209, 114229. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Yu, R.; Wei, H.W.; Wu, J.S.; He, N.; Liu, X.H. Construction of a novel electrochemical sensing platform to investigate the effect of temperature on superoxide anions from cells and superoxide dismutase enzyme activity. Anal. Chim. Acta 2022, 1198, 339561. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.X.; Jiang, X.C.; He, N.; Yu, R.; Xue, Z.H.; Liu, X.H. Electrochemical investigation for enhancing cellular antioxidant defense system based on a superoxide anion sensor. Sens. Actuators B-Chem. 2022, 368, 132190. [Google Scholar] [CrossRef]
- Zou, Z.; Shi, Z.Z.; Yuan, C.S.; Tang, C.Y.; Wu, C.; Liang, T.T.; Tang, K.L.; Chen, H.; Yang, H.B.; Li, C.M. Steric shelter-free cobalt nanoparticle-based high-sensitive biomimetic superoxide anion sensor. Mater. Chem. Front. 2021, 5, 6860–6864. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, J.; Shi, Z.Z.; Yuan, C.S.; Zhou, G.D.; Liu, Q.; Chen, H.; Zeng, Q.X.; Liang, T.T.; Tang, K.L.; et al. Cobalt Phosphates Loaded into Iodine-Spaced Reduced Graphene Oxide Nanolayers for Electrochemical Measurement of Superoxide Generated by Cells. ACS Appl. Nano Mater. 2021, 4, 3631–3638. [Google Scholar] [CrossRef]
- Cai, X.; Shi, L.; Sun, W.; Zhao, H.; Li, H.; He, H.; Lan, M. A facile way to fabricate manganese phosphate self-assembled carbon networks as efficient electrochemical catalysts for real-time monitoring of superoxide anions released from HepG2 cells. Biosens. Bioelectron. 2018, 102, 171–178. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, P.; Zheng, J.L.; Chen, Y.Y.; Liu, Y.Y.; Zheng, J.; Luo, X.G.; Huo, D.Q.; Hou, C.J. Fe Single-Atom Electrochemical Sensors for H2O2 Produced by Living Cells. ACS Appl. Nano Mater. 2022, 5, 11852–11863. [Google Scholar] [CrossRef]
- Li, H.X.; Jiang, L.L.; Shao, D.; Wu, C.S.; Gao, Y.J.; Yang, Z.Q.; Yang, Z.J. Facile synthesis of Cu@Cu2O aerogel for an effective electrochemical hydrogen peroxide sensor. Chin. J. Anal. Chem. 2022, 50, 100060. [Google Scholar] [CrossRef]
- Patella, B.; Buscetta, M.; Di Vincenzo, S.; Ferraro, M.; Aiello, G.; Sunseri, C.; Pace, E.; Inguanta, R.; Cipollina, C. Electrochemical sensor based on rGO/Au nanoparticles for monitoring H2O2 released by human macrophages. Sens. Actuators B-Chem. 2021, 327, 128901. [Google Scholar] [CrossRef]
- Othmani, A.; Derbali, M.; Kalfat, R.; Touati, F.; Dhaouadi, H. A novel 1D/2D Bi2S3/g-C3N4 core-shell nanocomposite as a highly performing H2O2 non-enzymatic electrochemical sensor. J. Mater. Res. Technol. -JmrT 2021, 15, 5762–5775. [Google Scholar] [CrossRef]
- Lu, Z.W.; Wu, L.; Dai, X.X.; Wang, Y.Y.; Sun, M.M.; Zhou, C.L.; Du, H.J.; Rao, H.B. Novel flexible bifunctional amperometric biosensor based on laser engraved porous graphene array electrodes: Highly sensitive electrochemical determination of hydrogen peroxide and glucose. J. Hazard. Mater. 2021, 402, 123774. [Google Scholar] [CrossRef]
- Banavath, R.; Srivastava, R.; Bhargava, P. Nanoporous Cobalt Hexacyanoferrate Nanospheres for Screen-Printed H2O2 Sensors. ACS Appl. Nano Mater. 2021, 4, 5564–5576. [Google Scholar] [CrossRef]
- Saidu, F.K.; Joseph, A.; Varghese, E.V.; Thomas, G.V. Silver nanoparticles-embedded poly(1-naphthylamine) nanospheres for low-cost non-enzymatic electrochemical H2O2 sensor. Polym. Bull. 2020, 77, 5825–5846. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Bai, X.Y.; Wang, X.M.; Shiu, K.K.; Zhu, Y.L.; Jiang, H. Highly Sensitive Graphene-Pt Nanocomposites Amperometric Biosensor and Its Application in Living Cell H2O2 Detection. Anal. Chem. 2014, 86, 9459–9465. [Google Scholar] [CrossRef]
- Han, L.; Cui, S.F.; Deng, D.M.; Li, Y.Y.; Yan, X.X.; He, H.B.; Luo, L.Q. Synthesis of Ag-Au/Reduced Graphene Oxide/TiO2 Nanocomposites: Application as a Non-enzymatic Amperometric H2O2 Sensor. Curr. Anal. Chem. 2020, 16, 485–492. [Google Scholar] [CrossRef]
- Wang, H.F.; Chen, W.X.; Chen, Q.Y.; Liu, N.; Cheng, H.J.; Li, T. Metal-organic framework (MOF)-Au@Pt nanoflowers composite material for electrochemical sensing of H2O2 in living cells. J. Electroanal. Chem. 2021, 897, 115603. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, H.Y.; Wang, M.X.; Li, Z. Non-enzymatic H2O2 electrochemical sensor based on NiAl-LDH/PPy-Ag composite. Ionics 2022, 28, 5561–5570. [Google Scholar] [CrossRef]
- Zhou, K.W.; Li, Y.; Zhuang, S.J.; Ren, J.; Tang, F.; Mu, J.L.; Wang, P. A novel electrochemical sensor based on CuO-CeO2/MXene nanocomposite for quantitative and continuous detection of H2O2. J. Electroanal. Chem. 2022, 921, 116655. [Google Scholar] [CrossRef]
- Shafa, M.; Ahmad, I.; Hussain, S.; Asif, M.; Pan, Y.; Zairov, R.; Alothman, A.A.; Ouladsmane, M.; Ullah, Z.; Ullah, N.; et al. Ag-Cu nanoalloys: An electrochemical sensor for H2O2 detection. Surf. Interfaces 2023, 36, 102616. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xie, Y.X.; Guo, X.J.; Sun, D.P. Self-supporting electrochemical sensors for monitoring of cell-released H2O2 based on metal nanoparticle/MOF nanozymes. Microchem. J. 2022, 181, 107715. [Google Scholar] [CrossRef]
- Derbali, M.; Othmani, A.; Kouass, S.; Touati, F.; Dhaouadi, H. BiVO4/TiO2 nanocomposite: Electrochemical sensor for hydrogen peroxide. Mater. Res. Bull. 2020, 125, 110771. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.W.; Guan, X.L.; Peng, W.C.; Fan, X.B.; Zhang, G.L.; Zhang, F.B.; Li, Y. A novel H2O2 electrochemical sensor based on NiCo2S4 functionalized reduced graphene oxide. J. Alloys Compd. 2019, 784, 827–833. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Ipekci, H.H. The use of CeO2-modified Pt/C catalyst inks for the construction of high-performance enzyme-free H2O2 sensors. J. Electroanal. Chem. 2019, 848, 113302. [Google Scholar] [CrossRef]
- Xie, F.Y.; Cao, X.Q.; Qu, F.L.; Asiri, A.M.; Sun, X.P. Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection. Sens. Actuators B-Chem. 2018, 255, 1254–1261. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Renitta, A.; Alagusundaram, K.; Monamary, A. Novel two-step process for the fabrication of MnO2 nanostructures on tantalum for enhanced electrochemical H2O2 detection. Mater. Chem. Phys. 2018, 214, 431–439. [Google Scholar] [CrossRef]
- Fan, K.L.; Wang, H.; Xi, J.Q.; Liu, Q.; Meng, X.Q.; Duan, D.M.; Gao, L.Z.; Yan, X.Y. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Commun. 2017, 53, 424–427. [Google Scholar] [CrossRef]
- Xu, D.Q.; Hou, B.B.; Qian, L.S.; Zhang, X.J.; Liu, G.D. Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes. Molecules 2019, 24, 3411. [Google Scholar] [CrossRef]
- Liu, X.; Ran, M.; Liu, G.; Liu, X.; Xue, Z.; Lu, X. A sensitively non-enzymatic amperometric sensor and its application in living cell superoxide anion radical detection. Talanta 2018, 186, 248–255. [Google Scholar] [CrossRef]
- Rajendran, S.; Manoj, D.; Suresh, R.; Vasseghian, Y.; Ghfar, A.A.; Sharma, G.; Soto-Moscoso, M. Electrochemical detection of hydrogen peroxide using micro and nanoporous CeO2 catalysts. Environ. Res. 2022, 214, 113961. [Google Scholar] [CrossRef]
- Shu, Y.; Xu, J.; Chen, J.; Xu, Q.; Xiao, X.; Jin, D.; Pang, H.; Hu, X. Ultrasensitive electrochemical detection of H2O2 in living cells based on ultrathin MnO2 nanosheets. Sens. Actuators B Chem. 2017, 252, 72–78. [Google Scholar] [CrossRef]
- Gan, Y.; Hu, N.; He, C.; Zhou, S.; Tu, J.; Liang, T.; Pan, Y.; Kirsanov, D.; Legin, A.; Wan, H.; et al. MnO2 nanosheets as the biomimetic oxidase for rapid and sensitive oxalate detection combining with bionic E-eye. Biosens. Bioelectron. 2019, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.G.; Aloisio, D.; Donato, N.; Russo, P.A.; Ferro, M.C.; Pinna, N.; Neri, G. Amperometric Sensing of H2O2 using Pt-TiO2/Reduced Graphene Oxide Nanocomposites. Chemelectrochem 2014, 1, 617–624. [Google Scholar] [CrossRef]
- Patra, D.C.; Deka, N.; Dash, A.; Khan, S.A.; Misra, T.K.; Mondal, S.P. Noninvasive Electrochemical Nitric Oxide Detection in Human Saliva Using Pt and TiO2 Nanoparticle Composite Electrodes. ACS Appl. Electron. Mater. 2023, 5, 832–845. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, J.; Liu, T.; Ying, S.; Kong, Y.; Chai, N.; Yi, F.-Y. UiO-66-Derived PBA Composite as Multifunctional Electrochemical Non-Enzymatic Sensor Realizing High-Performance Detection of Hydrogen Peroxide and Glucose. Inorg. Chem. 2023, 62, 7014–7023. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sachdev, A.; Matai, I. Self-assembled reduced graphene oxide-cerium oxide nanocomposite@cytochrome c hydrogel as a solid electrochemical reactive oxygen species detection platform. New J. Chem. 2020, 44, 11248–11255. [Google Scholar] [CrossRef]




| Electrode | Linear Range (μM) | LOD (nM) | Sensitivity (μA cm−2 mM−1) | Stability (Days) | Application Potential (V) | Ref. |
|---|---|---|---|---|---|---|
| AgNPs@C/GCE | 7.422 × 10−4–0.5719 | 1.011 × 10−4 | - | 7 | −0.7 | [17] |
| Co3O4@CMWCNTs/GCE | 5 × 10−9–10 | 1.6767 × 10−9 | - | - | - | [23] |
| Mn-MPSA-HCS/SPCE | 0–1257.4 | 1.25 | 224 | - | 0.75 | [32] |
| Ni(PO4)NRs/C-MWCNTs/GCE | 1–80 | 97 | 5.67 × 104 | 25 | −0.3 | [35] |
| 2D-mNC@CeO2/SPCEs | 8–536 | 179 | 401.4 | 20 | −0.5 | [44] |
| PAMAM-Au/GCE | 3.69 × 10−5–37.2 | 0.0123 | - | - | −0.7 | [45] |
| AgNPs-MC/GCE | 1.68 × 10−3–30.6 | 0.012 | - | 15 | −0.5 | [46] |
| Co-NPs–NG/GCE | 1.67 × 10−3–0.575 | 1.67 | 628.86 | - | 0.9 | [47] |
| Co3(PO4)2/I-rGO/GCE | 2.4 × 10−3–2.195 | 2.4 | 177.14 | 30 | 0.6 | [48] |
| Mn-MPSA-MWCNTs/SPCE | 0–1817 | 127 | 77.47 | 30 | 0.7 | [49] |
| Electrode | Linear Range (μM) | LOD (nM) | Sensitivity (μA cm−2 mM−1) | Stability (Days) | Application Potential (V) | Ref. |
|---|---|---|---|---|---|---|
| Fe SAs-N/C/GCE | 764–9664 | 340 | 22.1 | - | −0.05 | [50] |
| Cu@Cu2O/GCE | 2–860 | 460 | 1855.53 | - | −0.5 | [51] |
| rGO/Au-NPs/GCE | 25–3000 | 6.55 | 0.0641 | - | −0.8 | [52] |
| Bi2S3/g-C3N4/GCE | 0.5–950 | 78 | 1011 | 7 | 0.26 | [53] |
| Pt-LEPG/GCE | 0.01 × 10−3–0.029 | 0.65 | 575.75 | 14 | 0.5 | [54] |
| CoHCF-NSp’s /GCE | 2–1130 | 2.1 | 329 | - | 0.8 | [55] |
| Ag/PNA/GCE | 1–3000 | 0.972 | 1844.76 | 10 | −0.42 | [56] |
| RGO–Pt NPs/GCE | 0.5–3475 | 0.2 | 459 | 14 | −0.08 | [57] |
| Ag-Au/RGO/TiO2/GCE | 10–30,000 | 3 | - | - | - | [58] |
| MOF-Au@Pt/GCE | 0.8–3000 | 86 | 24.14 | - | −0.12 | [59] |
| LDH/PPy-Ag/GCE | 30–800 | 280 | 257.64 | 30 | −0.3 | [60] |
| CuO-CeO2/MXene/GCE | 5–100 | 1.67 | 84.44 | - | −0.3 | [61] |
| Ag-Cu nanoalloys/GCE | 2000–961,000 | 152 | - | - | −0.07 | [62] |
| AgNPs/2D Zn-MOFs/GCE | 5–70,000 | 1.67 × 103 | 358.7 | 6 | −0.55 | [63] |
| BiVO4/TiO2/GCE | 5–400 | 5 × 103 | 3014 | 90 | 0.5 | [64] |
| NiCo2S4/rGO/GCE | 25–11,250 | 190 | 118.5 | 14 | −0.45 | [65] |
| Pt/C-CeO2/GCE | 10–30,000 | 2 × 103 | 185.4 | 15 | −0.4 | [66] |
| Co3N NW/TM/GCE | 2–28 | 1 × 103 | 139.9 | 30 | −0.7 | [67] |
| MnO2/Ta/GCE | 1–2 | 60 | 1111.09 | - | −1.21 | [68] |
| Nanozyme | Analyte | Linear Range (μM) | LOD (nM) | Sensitivity (μA cm−2 mM−1) | Ref. |
|---|---|---|---|---|---|
| AgNPs-MWCNT | H2O2 | 1–1000 | 380 | 2556 | [70] |
| AgNPs-MWCNT | O2•− | 3.65 × 10−7–5.59 × 10−4 | 0.1192 | 80.22 | [71] |
| CeO2 | H2O2 | 0.001–0.125 | 0.4 | 141.96 | [72] |
| CeO2 | O2•− | 8–536 | 179 | 401.4 | [44] |
| MnO2 | H2O2 | 25 × 10−3–2 | 5 | 3261 | [73] |
| MnO2 | oxalate | 7.8–250 | 910 | - | [74] |
| Pt/TiO2 nanotube | H2O2 | 0–20 | 400 | 40 | [75] |
| Pt-TiO2 NPs | NO | 0.01–17,790 | 2.47 | 7.81 | [76] |
| PBA/UiO-66/NF | H2O2 | 50–3500 | 0.02 | 1903 | [77] |
| PBA/UiO-66/NF | Glucose | 200–450 | 0.28 | 22,800 | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Bao, S. Advances in the Application of Nano-Enzymes in the Electrochemical Detection of Reactive Oxygen Species: A Review. Chemosensors 2023, 11, 440. https://doi.org/10.3390/chemosensors11080440
Gao R, Bao S. Advances in the Application of Nano-Enzymes in the Electrochemical Detection of Reactive Oxygen Species: A Review. Chemosensors. 2023; 11(8):440. https://doi.org/10.3390/chemosensors11080440
Chicago/Turabian StyleGao, Rongwei, and Shujuan Bao. 2023. "Advances in the Application of Nano-Enzymes in the Electrochemical Detection of Reactive Oxygen Species: A Review" Chemosensors 11, no. 8: 440. https://doi.org/10.3390/chemosensors11080440
APA StyleGao, R., & Bao, S. (2023). Advances in the Application of Nano-Enzymes in the Electrochemical Detection of Reactive Oxygen Species: A Review. Chemosensors, 11(8), 440. https://doi.org/10.3390/chemosensors11080440





