Hydrogel-Based Electrodeposition of Copper Nanoparticles for Selective Detection for Hydrogen Peroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
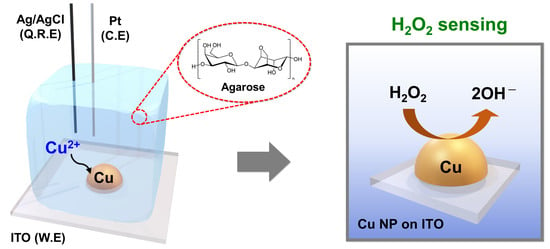
2.2. Preparation of Agarose Hydrogel for Electrochemistry
2.3. Electrochemical and FE-SEM Measurement
2.4. Electrodeposition of Cu on ITO and Electrochemical H2O2 Sensing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleijn, S.E.; Lai, S.C.; Koper, M.T.; Unwin, P.R. Electrochemistry of nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 3558–3586. [Google Scholar] [CrossRef]
- Zhao, M.; Balachandran, R.; Patterson, Z.; Gouk, R.; Verhaverbeke, S.; Shadman, F.; Keswani, M. Contactless bottom-up electrodeposition of nickel for 3D integrated circuits. RSC Adv. 2015, 5, 45291–45299. [Google Scholar] [CrossRef]
- Shoja, Y.; Isoaho, N.; Jokinen, V.; Franssila, S. Microfabrication atomic layer deposited Pt NPs/TiN thin film on silicon as a nanostructure signal Transducer: Electrochemical characterization toward neurotransmitter sensing. Appl. Surf. Sci. 2022, 573, 151444. [Google Scholar] [CrossRef]
- Fu, G.; Lee, J.M. Ternary metal sulfides for electrocatalytic energy conversion. J. Mater. Chem. A 2019, 7, 9386–9405. [Google Scholar] [CrossRef]
- Jin, H.; Song, T.; Paik, U.; Qiao, S.Z. Metastable Two-Dimensional Materials for Electrocatalytic Energy Conversions. Acc. Mater. Res. 2021, 2, 559–573. [Google Scholar] [CrossRef]
- Li, H.; Qin, X.; Jiang, T.; Ma, X.Y.; Jiang, K.; Cai, W.B. Changing the Product Selectivity for Electrocatalysis of CO2 Reduction Reaction on Plated Cu Electrodes. ChemCatChem 2019, 11, 6139–6146. [Google Scholar] [CrossRef]
- Li, S.; Yu, Q.; Chen, Z.; Zhu, W.; Han, L.; Li, S.; Wu, Y.; Lu, X.; Yuan, J.; Lv, Z.; et al. Co-doping of SiO2 and ZrO2 for the synthesis of energy-saving PbO2 anode material for trivalent chromium electroplating. Int. J. Hydrogen Energy 2022, 47, 37694–37707. [Google Scholar] [CrossRef]
- Wu, F.; Pan, C.; He, C.T.; Han, Y.; Ma, W.; Wei, H.; Ji, W.; Chen, W.; Mao, J.; Yu, P.; et al. Single-Atom Co-N4 Electrocatalyst Enabling Four-Electron Oxygen Reduction with Enhanced Hydrogen Peroxide Tolerance for Selective Sensing. J. Am. Chem. Soc. 2020, 142, 16861–16867. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Karimi, F.; Orooji, Y.; Mansouri, G.; Razmjou, A.; Aygun, A.; Sen, F. A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 2020, 10, 11699. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Lee, M.W.; Hong, S.; Ahn, H.S.; Kim, B.K. Electrosynthesis of palladium nanocatalysts using single droplet reactors and catalytic activity for formic acid oxidation. Electrochim. Acta 2022, 401, 139446. [Google Scholar] [CrossRef]
- Kim, S.D.; Park, J.H.; Ahn, H.; Lee, J.; Shin, C.H.; Jang, W.D.; Kim, B.K.; Ahn, H.S. The discrete single-entity electrochemistry of Pickering emulsions. Nanoscale 2022, 14, 6981–6989. [Google Scholar] [CrossRef]
- Cioffi, N.; Colaianni, L.; Ieva, E.; Pilolli, R.; Ditaranto, N.; Angione, M.D.; Cotrone, S.; Buchholt, K.; Spetz, A.L.; Sabbatini, L.; et al. Electrosynthesis and characterization of gold nanoparticles for electronic capacitance sensing of pollutants. Electrochim. Acta 2011, 56, 3713–3720. [Google Scholar] [CrossRef]
- Caschera, D.; Federici, F.; Zane, D.; Focanti, F.; Curulli, A.; Padeletti, G. Gold nanoparticles modified GC electrodes: Electrochemical behaviour dependence of different neurotransmitters and molecules of biological interest on the particles size and shape. J. Nanopart. Res. 2009, 11, 1925–1936. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Abbott, A.P.; Griffith, J.; Nandhra, S.; O’Connor, C.; Postlethwaite, S.; Ryder, K.S.; Smith, E.L. Sustained electroless deposition of metallic silver from a choline chloride-based ionic liquid. Surf. Coat. Technol. 2008, 202, 2033–2039. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random Alloyed versus Intermetallic Nanoparticles: A Comparison of Electrocatalytic Performance. Adv. Mater. 2018, 30, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Aldeghi, M.; Wu, T.C.; Aspuru-Guzik, A.; Kumacheva, E. Nanoparticle synthesis assisted by machine learning. Nat. Rev. Mater. 2021, 6, 701–716. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- Park, K.; Kim, E.; Park, J.H.; Hwang, S. Influence of an active vibration isolator and electrochemical cell design on electrochemical measurements to minimize natural convection. Electrochem. Commun. 2017, 82, 93–97. [Google Scholar] [CrossRef]
- Fedorov, R.G.; Mandler, D. Effect of Self-Assembled Monolayers on the Locally Electrodeposited Silver Thin Layers. J. Phys. Chem. C 2016, 120, 15608–15617. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chem. Sci. 2020, 11, 10614–10625. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Liu, G.; Huang, C.; Tang, R.; Liu, Y.; Shi, C. The influence of pH and current density for nano-silver electrodeposited in cyanide-free succinimide bath. Surf. Interfaces 2021, 27, 101487. [Google Scholar] [CrossRef]
- Wasiewska, L.A.; Seymour, I.; Patella, B.; Inguanta, R.; Burgess, C.M.; Duffy, G.; O’Riordan, A. Reagent free electrochemical-based detection of silver ions at interdigitated microelectrodes using in-situ pH control. Sens. Actuators B Chem. 2021, 333, 129531. [Google Scholar] [CrossRef]
- Shin, S.J.; An, S.; Lee, S.; Lee, J.G.; Chung, T.D. Direct electrodeposition of various metal nanocrystals on silicon oxide dielectric layer and insights into electrochemical behavior. Bull. Korean Chem. Soc. 2022, 43, 227–231. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.Y.; Park, J.H.; Kwak, J. Electrochemical detection of dopamine using a bare indium-tin oxide electrode and scan rate control. J. Electroanal. Chem. 2013, 708, 7–12. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, C.; Xi, F. Disposable Amperometric Label-Free Immunosensor on Chitosan-Graphene-Modified Patterned ITO Electrodes for Prostate Specific Antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.K.; Park, K. Electrodeposition of Silver Nanoparticles on Indium-Doped Tin Oxide Using Hydrogel Electrolyte for Hydrogen Peroxide Sensing. Nanomaterials 2022, 13, 48. [Google Scholar] [CrossRef]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef]
- Hacker, M.C.; Nawaz, H.A. Multi-Functional Macromers for Hydrogel Design in Biomedical Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2015, 16, 27677–27706. [Google Scholar] [CrossRef] [PubMed]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef]
- Khajouei, S.; Ravan, H.; Ebrahimi, A. DNA hydrogel-empowered biosensing. Adv. Colloid Interface Sci. 2020, 275, 102060. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Islam, M.M.; Alarcon, E.I.; Samanta, A.; Wang, S.; Lundstrom, P.; Hilborn, J.; Griffith, M.; Phopase, J. Functionalised type-I collagen as a hydrogel building block for bio-orthogonal tissue engineering applications. J. Mater. Chem. B 2016, 4, 318–326. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, G. Engineering Hydrogels for Efficient Solar Desalination and Water Purification. Acc. Mater. Res. 2021, 2, 374–384. [Google Scholar] [CrossRef]
- Garcia-Astrain, C.; Averous, L. Synthesis and behavior of click cross-linked alginate hydrogels: Effect of cross-linker length and functionality. Int. J. Biol. Macromol. 2019, 137, 612–619. [Google Scholar] [CrossRef]
- Kang, H.; Hwang, S.; Kwak, J. Hydrogel Pen for Electrochemical Reaction and Its Applications for 3D Printing. Nanoscale 2015, 7, 994–1001. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y.D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Hurdis, E.C.; Romeyn, J. Accuracy of Determination of Hydrogen Peroxide by Cerate Oxidimetry. Anal. Chem. 1954, 26, 320. [Google Scholar] [CrossRef]
- Matsubara, C.; Kawamoto, N.; Takamura, K. Oxo [5, 10, 15, 20-tetra(4-pyridyl)porphyrinato]titanium(IV): An ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst 1992, 117, 1781–1784. [Google Scholar] [CrossRef]
- Abo, M.; Urano, Y.; Hanaoka, K.; Terai, T.; Komatsu, T.; Nagano, T. Development of a highly sensitive fluorescence probe for hydrogen peroxide. J. Am. Chem. Soc. 2011, 133, 10629–10637. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Tian, Y.; Wang, H. A novel hydrogen peroxide sensor based on LaB6 electrode. Electrochim. Acta 2012, 63, 64–68. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Chai, X.; Wang, T.; Cao, T.; Li, Y.; Zhang, L.; Fan, F.; Fu, Y.; Qi, W. An Electrochemical Sensor for H2O2 Based on Au Nanoparticles Embedded in UiO-66 Metal–Organic Framework Films. ACS Appl. Nano Mater. 2021, 4, 6103–6110. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, J.; Liu, T.; Ying, S.; Kong, Y.; Chai, N.; Yi, F.Y. UiO-66-Derived PBA Composite as Multifunctional Electrochemical Non-Enzymatic Sensor Realizing High-Performance Detection of Hydrogen Peroxide and Glucose. Inorg. Chem. 2023, 62, 7014–7023. [Google Scholar] [CrossRef]
- Waqas, M.; Hui, Y.; Wang, L.; Fan, F.; Mahmood, K.; Chen, W.; Chen, D.H.; Fan, Y.; Yasmeen, G. ZnO@C Microballs Wrapped with Ni(OH)2 Nanofilms for Electrochemical Sensing of Glucose and Hydrogen Peroxide. ChemPlusChem 2023, 88, e202300065. [Google Scholar] [CrossRef]
- Niu, B.; Wnag, H.; Zhang, Y.; Nie, B.; Wang, H.; Lian, X.; Li, W. Green synthesis and characterization of Ag nanoparticles in a phytic acid/ascorbic acid/sodium hydroxide system and their application in the electrochemical detection of H2O2. New J. Chem. 2023, 47, 8797–8808. [Google Scholar] [CrossRef]
- Gu, T.; Hasebe, Y. DNA-Cu(II) poly(amine) complex membrane as novel catalytic layer for highly sensitive amperometric determination of hydrogen peroxide. Biosens. Bioelectron. 2006, 21, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Park, K. Mass Transport Properties and Influence of Natural Convection for Voltammetry at the Agarose Hydrogel Interface. J. Electrochem. Sci. Technol. 2022, 13, 347–353. [Google Scholar] [CrossRef]
- Su, C.; An, M.; Yang, P.; Gu, H.; Guo, X. Electrochemical behavior of cobalt from 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. Appl. Surf. Sci. 2010, 256, 4888–4893. [Google Scholar] [CrossRef]
- Cui, K.; Song, Y.; Yao, Y.; Huang, Z.; Wang, L. A novel hydrogen peroxide sensor based on Ag nanoparticles electrodeposited on DNA-networks modified glassy carbon electrode. Electrochem. Commun. 2008, 10, 663–667. [Google Scholar] [CrossRef]
- Meng, F.; Yan, X.; Liu, J.; Gu, J.; Zou, Z. Nanoporous gold as non-enzymatic sensor for hydrogen peroxide. Electrochim. Acta 2011, 56, 4657–4662. [Google Scholar] [CrossRef]
- Reyter, D.; Chamoulaud, G.; Bélanger, D.; Roué, L. Electrocatalytic reduction of nitrate on copper electrodes prepared by high-energy ball milling. J. Electroanal. Chem. 2006, 596, 13–24. [Google Scholar] [CrossRef]
- Waszczuk, P.; Zelenay, P.; Sobkowski, J. Surface Interaction of Benzoic Acid with A Copper Electrode. Electrochim. Acta 1995, 40, 1717–1721. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, L.; Hou, X.; Chen, J.; Chou, K.C. Bare and boron-doped cubic silicon carbide nanowires for electrochemical detection of nitrite sensitively. Sci. Rep. 2016, 6, 24872. [Google Scholar] [CrossRef] [PubMed]
- Anson, F.C. Chronocoulometry: A Convenient, Rapid and Reliable Technique for Detection and Determination of Adsorbed Reactants. J. Chem. Educ. 1983, 60, 293–296. [Google Scholar] [CrossRef]
- Stanković, V.; Đurđić, S.; Ognjanović, M.; Mutić, J.; Kalcher, K.; Stanković, D.M. A novel nonenzymatic hydrogen peroxide amperometric sensor based on AgNp@GNR nanocomposites modified screen-printed carbon electrode. J. Electroanal. Chem. 2020, 876, 114487. [Google Scholar] [CrossRef]
- Sophia, J.; Muralidharan, G. Amperometric sensing of hydrogen peroxide using glassy carbon electrode modified with copper nanoparticles. Mater. Res. Bull. 2015, 70, 315–320. [Google Scholar]
- Aparicio-Martínez, E.; Ibarra, A.; Estrada-Moreno, I.A.; Osuna, V.; Dominguez, R.B. Flexible electrochemical sensor based on laser scribed Graphene/Ag nanoparticles for non-enzymatic hydrogen peroxide detection. Sens. Actuators B Chem. 2019, 301, 127101. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Wang, W.; Zhang, J.; Li, G.; Liu, J.; Lian, K.; Hu, J.; Zhuiykov, S. Ag Functionalized Molybdenum Disulfide Hybrid Nanostructures for Selective and Sensitive Amperometric Hydrogen Peroxide Detection. Int. J. Electrochem. Sci. 2017, 12, 8761–8776. [Google Scholar] [CrossRef]
- Riaz, M.A.; Yuan, Z.; Mahmood, A.; Liu, F.; Sui, X.; Chen, J.; Huang, Q.; Liao, X.; Wei, L.; Chen, Y. Hierarchically porous carbon nanofibers embedded with cobalt nanoparticles for efficient H2O2 detection on multiple sensor platforms. Sens. Actuators B Chem. 2020, 319, 128243. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.J.; Kim, J.H.; Lee, G.J. Electrochemical Detection of H2O2 Released from Prostate Cancer Cells Using Pt Nanoparticle-Decorated rGO–CNT Nanocomposite-Modified Screen-Printed Carbon Electrodes. Chemosensors 2020, 8, 63. [Google Scholar] [CrossRef]
- Njima, M.A.B.; Legrand, L. Ag nanoparticles-oxidized green rust nanohybrids for novel and efficient non-enzymatic H2O2 electrochemical sensor. J. Electroanal. Chem. 2022, 906, 116015. [Google Scholar] [CrossRef]
- Sun, D.; Yang, D.; Wei, P.; Liu, B.; Chen, Z.; Zhang, L.; Lu, J. One-Step Electrodeposition of Silver Nanostructures on 2D/3D Metal-Organic Framework ZIF-67: Comparison and Application in Electrochemical Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2020, 12, 41960–41968. [Google Scholar] [CrossRef]








| Sensor Type | Dynamic Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|
| AgNPs@GNR/SPCE | 50–5000 | 20 | [61] |
| CuNPs@GCE | 8–70 | 3.45 | [62] |
| AgNPs@LSG | 10–10,000 | 7.9 | [63] |
| AgNPs/MoS2@GCE | 25–135,200 | 3.5 | [64] |
| CoNPs/CNF@GCE | 10–5000 | 10 | [65] |
| PtNP/rGO-CNT/PtNP@SPCE | 25–1000 | 4.3 | [66] |
| Ag-exGRc-CI@SS | 100–8000 | 5 | [67] |
| Ag/H-ZIF-67@GCE | 5–7000 | 1.1 | [68] |
| CuNp@ITO | 1–500 | 1.73 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Kim, J.; Kim, B.-K.; Park, K. Hydrogel-Based Electrodeposition of Copper Nanoparticles for Selective Detection for Hydrogen Peroxide. Chemosensors 2023, 11, 384. https://doi.org/10.3390/chemosensors11070384
Han J, Kim J, Kim B-K, Park K. Hydrogel-Based Electrodeposition of Copper Nanoparticles for Selective Detection for Hydrogen Peroxide. Chemosensors. 2023; 11(7):384. https://doi.org/10.3390/chemosensors11070384
Chicago/Turabian StyleHan, Jihun, Jihyeon Kim, Byung-Kwon Kim, and Kyungsoon Park. 2023. "Hydrogel-Based Electrodeposition of Copper Nanoparticles for Selective Detection for Hydrogen Peroxide" Chemosensors 11, no. 7: 384. https://doi.org/10.3390/chemosensors11070384
APA StyleHan, J., Kim, J., Kim, B.-K., & Park, K. (2023). Hydrogel-Based Electrodeposition of Copper Nanoparticles for Selective Detection for Hydrogen Peroxide. Chemosensors, 11(7), 384. https://doi.org/10.3390/chemosensors11070384