Self-Powered Wearable Breath-Monitoring Sensor Enabled by Electromagnetic Harvesting Based on Nano-Structured Electrochemically Active Aluminum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Activation
2.3. Longevity
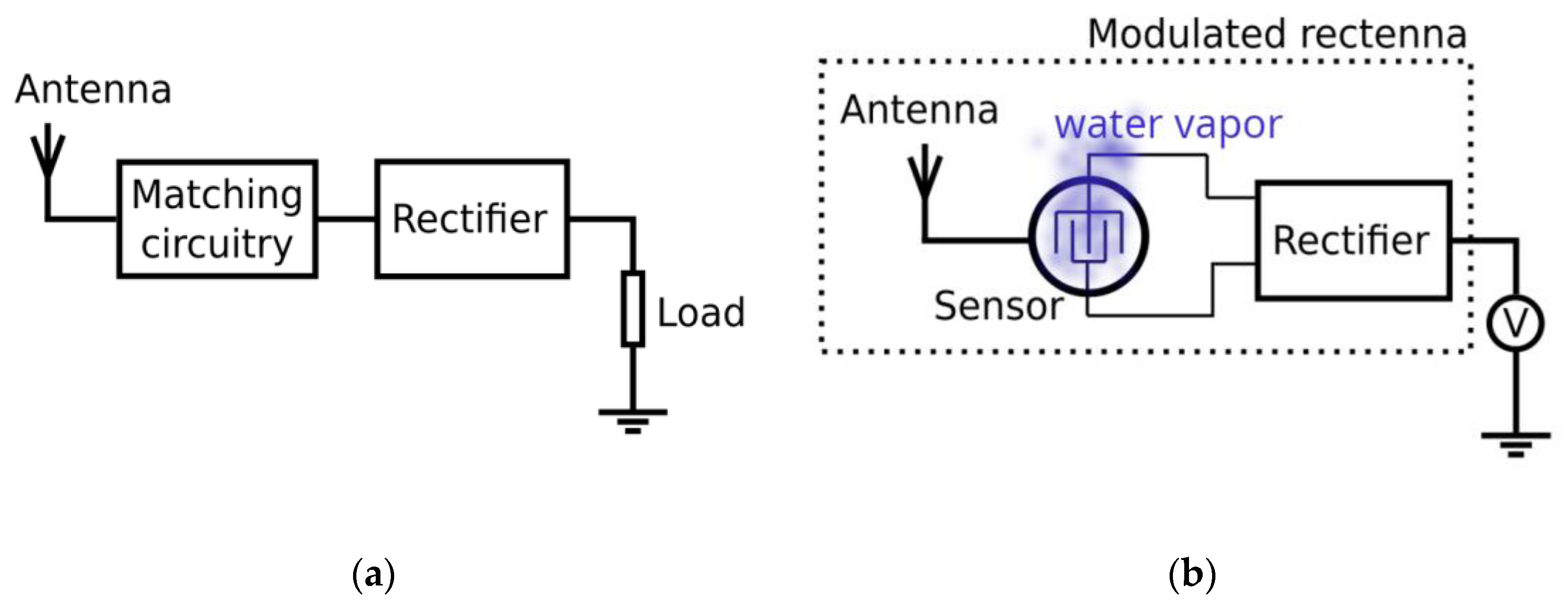
2.4. Concept of the Sensor Operation
2.5. EM Energy Harvesting Quantification
2.6. Direct Breath Blow Test
2.7. Software
3. Results
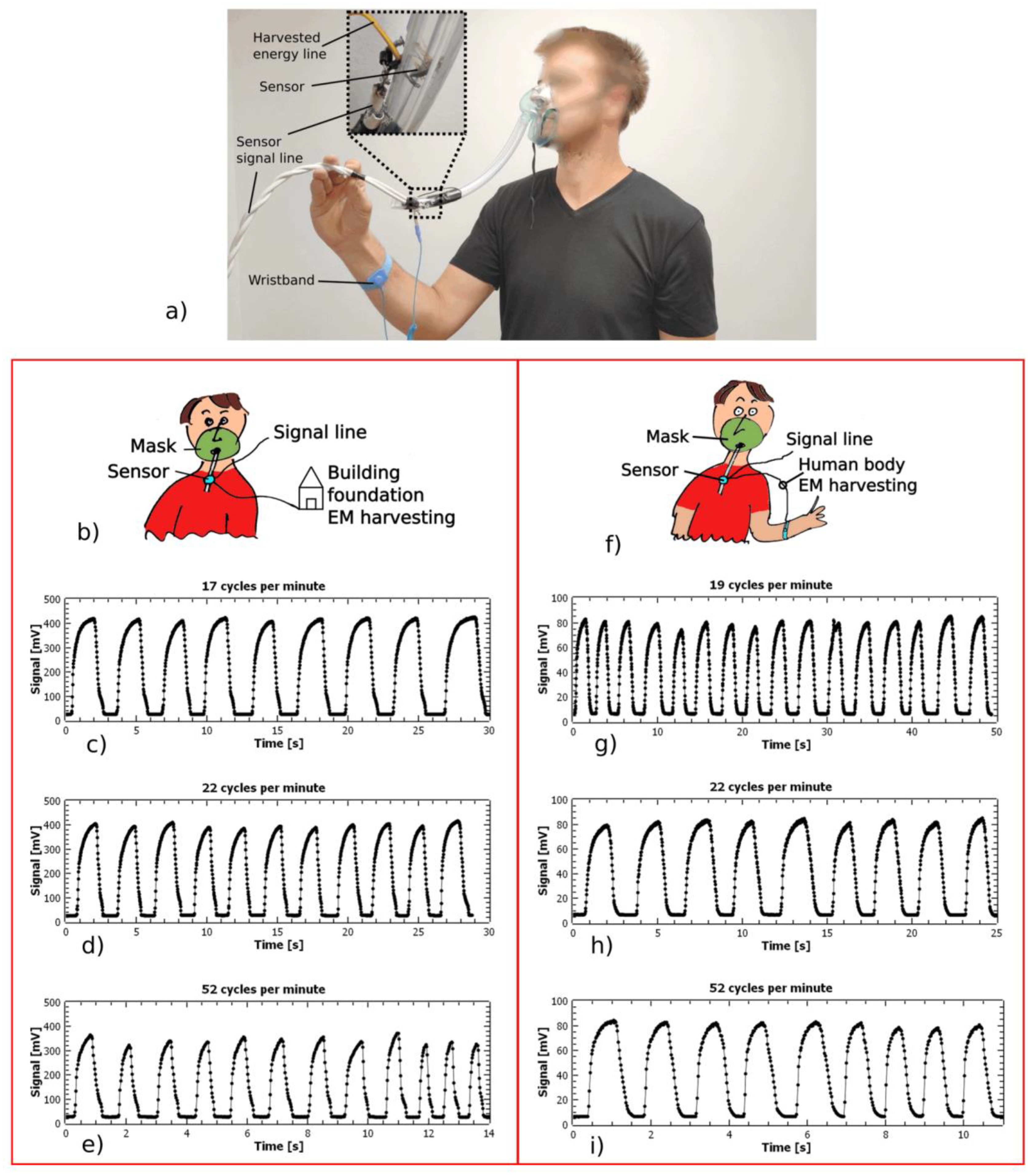
3.1. Mask Breathing and Respiration Monitoring
3.2. Wearable Applications
3.3. SEM and EDS Characterization of Activated and Non-Activated Samples
4. Discussion
4.1. Electrochemical Activity of the Thin Film
4.2. Comparison with Other Respiration Monitoring Solutions Based on Humidity Detection
4.3. Opportunity for Fully Independent Operation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, J.; Li, L.; Shi, B.; Li, Z. Recent progress of self-powered respiration monitoring systems. Biosens. Bioelectron. 2021, 194, 113609. [Google Scholar] [CrossRef]
- Beduk, T.; Durmus, C.; Hanoglu, S.B.; Beduk, D.; Salama, K.N.; Goksel, T.; Turhan, K.; Timur, S. Breath as the mirror of our body is the answer really blowing in the wind? Recent technologies in exhaled breath analysis systems as non-invasive sensing platforms. TrAC Trends Anal. Chem. 2021, 143, 116329. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sensors Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.J.; Whitesides, G.M. Paper-Based Electrical Respiration Sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef]
- Sun, A.C.; Yao, C.; Venkatesh, A.G.; Hall, D.A. An efficient power harvesting mobile phone-based electrochemical biosensor for point-of-care health monitoring. Sensors Actuators B Chem. 2016, 235, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent Advances in Graphene-Based Humidity Sensors. Nanomaterials 2019, 9, 422. [Google Scholar] [CrossRef]
- Andrić, S.; Tomašević-Ilić, T.; Bošković, M.V.; Sarajlić, M.; Vasiljević-Radović, D.; Smiljanic, M.M.; Spasenović, M. Ultrafast humidity sensor based on liquid phase exfoliated graphene. Nanotechnology 2020, 32, 025505. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Y.; Chen, M.-Q.; Xia, F. Research Advances in Microfiber Humidity Sensors. Small 2018, 14, e1800524. [Google Scholar] [CrossRef]
- Li, Y. Temperature and humidity sensors based on luminescent metal-organic frameworks. Polyhedron 2020, 179, 114413. [Google Scholar] [CrossRef]
- Malook, K.; Ali, M.; Ul-Haque, I. Elucidation of room temperature humidity sensing properties of Mn2O3 particles. Appl. Phys. A 2021, 127, 758. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, J. Synthesis of high response gold/titanium dioxide humidity sensor and its application in human respiration. Ceram. Int. 2021, 47, 30880–30887. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review. Carbohydr. Polym. 2021, 270, 118385. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef]
- Delipinar, T.; Shafique, A.; Gohar, M.S.; Yapici, M.K. Fabrication and Materials Integration of Flexible Humidity Sensors for Emerging Applications. ACS Omega 2021, 6, 8744–8753. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xiao, M.; Xiao, Y.; Zou, G.; Hu, L.; Zhao, B.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-Powered, Rapid-Response, and Highly Flexible Humidity Sensors Based on Moisture-Dependent Voltage Generation. ACS Appl. Mater. Interfaces 2019, 11, 14249–14255. [Google Scholar] [CrossRef]
- Kassal, P.; Steinberg, M.D.; Steinberg, I.M. Wireless chemical sensors and biosensors: A review. Sensors Actuators B Chem. 2018, 266, 228–245. [Google Scholar] [CrossRef]
- Xu, L.; Xuan, W.; Chen, J.; Zhang, C.; Tang, Y.; Huang, X.; Li, W.; Jin, H.; Dong, S.; Yin, W.; et al. Fully self-powered instantaneous wireless humidity sensing system based on triboelectric nanogenerator. Nano Energy 2021, 83, 105814. [Google Scholar] [CrossRef]
- Grattieri, M.; Minteer, S.D. Self-Powered Biosensors. ACS Sensors 2017, 3, 44–53. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, Q.; Sun, X. Nanogenerators for Self-Powered Gas Sensing. Nano-Micro Lett. 2017, 9, 45. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, C.; Gong, Q.; Xie, G.; Yao, M.; Tai, H.; Jiang, Y.; Chen, J. Self-Powered Respiration Monitoring Enabled By a Triboelectric Nanogenerator. Adv. Mater. 2021, 33, e2101262. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, M.; Wu, H.; Xu, L.; Zhang, T.; Chen, W.; Wang, Z.L.; Yang, Y. Moisture induced electricity for self-powered microrobots. Nano Energy 2021, 90, 106499. [Google Scholar] [CrossRef]
- Fan, F.R.; Wu, W. Emerging Devices Based on Two-Dimensional Monolayer Materials for Energy Harvesting. Research 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, H.; Ward, J.E.; Liu, X.; Yin, B.; Fu, T.; Chen, J.; Lovley, D.R.; Yao, J. Power generation from ambient humidity using protein nanowires. Nature 2020, 578, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cheng, H.; Zhang, Z.; Jiang, L.; Qu, L. Direct Power Generation from a Graphene Oxide Film under Moisture. Adv. Mater. 2015, 27, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kuang, Y.; Shi, D.; Hou, M.; Chen, X.; Jiang, L.; Gao, J.; Zhang, L.; He, Y.; Wong, C.-P. A triboelectric nanogenerator design for harvesting environmental mechanical energy from water mist. Nano Energy 2020, 73, 104765. [Google Scholar] [CrossRef]
- Shao, C.; Gao, J.; Xu, T.; Ji, B.; Xiao, Y.; Gao, C.; Zhao, Y.; Qu, L. Wearable fiberform hygroelectric generator. Nano Energy 2018, 53, 698–705. [Google Scholar] [CrossRef]
- Chang, T.-H.; Peng, Y.-W.; Chen, C.-H.; Wu, J.-M.; Hwang, J.-C.; Gan, J.-Y.; Lin, Z.-H. Protein-based contact electrification and its uses for mechanical energy harvesting and humidity detecting. Nano Energy 2016, 21, 238–246. [Google Scholar] [CrossRef]
- Li, L.; Hao, M.; Yang, X.; Sun, F.; Bai, Y.; Ding, H.; Wang, S.; Zhang, T. Sustainable and flexible hydrovoltaic power generator for wearable sensing electronics. Nano Energy 2020, 72, 104663. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Yin, J.; Xu, Y.; Fei, W.; Xue, M.; Wang, Q.; Zhou, J.; Guo, W. Emerging hydrovoltaic technology. Nat. Nanotechnol. 2018, 13, 1109–1119. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, D.; Zou, G.; Wu, A.; Liu, L.; Duley, W.W.; Zhou, Y.N. Self-powered, flexible and remote-controlled breath monitor based on TiO2nanowire networks. Nanotechnology 2019, 30, 325503. [Google Scholar] [CrossRef]
- Bošković, M.V.; Sarajlić, M.; Frantlović, M.; Smiljanić, M.M.; Randjelović, D.V.; Zobenica, K.C.; Radović, D.V. Aluminum-based self-powered hyper-fast miniaturized sensor for breath humidity detection. Sensors Actuators B Chem. 2020, 321, 128635. [Google Scholar] [CrossRef]
- Bošković, M.; Šljukić, B.; Radović, D.V.; Radulović, K.; Rafajilović, M.R.; Frantlović, M.; Sarajlić, M. Full-Self-Powered Humidity Sensor Based on Electrochemical Aluminum–Water Reaction. Sensors 2021, 21, 3486. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J. Metal–Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Zhang, X.; Grajal, J.; López-Vallejo, M.; McVay, E.; Palacios, T. Opportunities and Challenges of Ambient Radio-Frequency Energy Harvesting. Joule 2020, 4, 1148–1152. [Google Scholar] [CrossRef]
- Cambero, E.V.V.; da Paz, H.P.; da Silva, V.S.; Consonni, D.; Capovilla, C.E.; Casella, I.R.S. A revised methodology to analyze the rectenna power conversion efficiency based on antenna/rectifier interface losses. AEU-Int. J. Electron. Commun. 2021, 134, 153686. [Google Scholar] [CrossRef]
- Song, C.; Huang, Y.; Zhou, J.; Zhang, J.; Yuan, S.; Carter, P. A High-Efficiency Broadband Rectenna for Ambient Wireless Energy Harvesting. IEEE Trans. Antennas Propag. 2015, 63, 3486–3495. [Google Scholar] [CrossRef]
- Lopez, O.L.A.; Clerckx, B.; Latva-Aho, M. Dynamic RF Combining for Multi-Antenna Ambient Energy Harvesting. IEEE Wirel. Commun. Lett. 2021, 11, 493–497. [Google Scholar] [CrossRef]
- Assimonis, S.D.; Fusco, V.; Georgiadis, A.; Samaras, T. Efficient and Sensitive Electrically Small Rectenna for Ultra-Low Power RF Energy Harvesting. Sci. Rep. 2018, 8, 15038. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sriramdas, R.; Kumar, P.; Sanghadasa, M.; Kang, M.G.; Priya, S. Maximizing power generation from ambient stray magnetic fields around smart infrastructures enabling self-powered wireless devices. Energy Environ. Sci. 2020, 13, 1462–1472. [Google Scholar] [CrossRef]
- Gao, M.; Wang, P.; Jiang, L.; Wang, B.; Yao, Y.; Liu, S.; Chu, D.; Cheng, W.; Lu, Y. Power generation for wearable systems. Energy Environ. Sci. 2021, 14, 2114–2157. [Google Scholar] [CrossRef]
- Tian, X.; Lee, P.M.; Tan, Y.J.; Wu, T.L.Y.; Yao, H.; Zhang, M.; Li, Z.; Ng, K.A.; Tee, B.C.K.; Ho, J.S. Wireless body sensor networks based on metamaterial textiles. Nat. Electron. 2019, 2, 243–251. [Google Scholar] [CrossRef]
- Kravchenko, O.; Semenenko, K.; Bulychev, B.; Kalmykov, K. Activation of aluminum metal and its reaction with water. J. Alloy. Compd. 2005, 397, 58–62. [Google Scholar] [CrossRef]
- Trenikhin, M.V.; Kozlov, A.G.; Nizovskii, A.I.; Drozdov, V.A.; Lavrenov, A.V.; Bubnov, A.V.; Finevich, V.P.; Duplyakin, V.K. Activated aluminum: Features of production and application in the synthesis of catalysts for pertochemistry and oil processing. Russ. J. Gen. Chem. 2007, 77, 2320–2327. [Google Scholar] [CrossRef]
- STPS1150. 150 V, 1 A Power Schottky Rectifier. Available online: https://www.st.com/en/diodes-and-rectifiers/stps1150.html#documentation (accessed on 20 September 2022).
- Canepa, P.; Hanson, R.M.; Ugliengo, P.; Alfredsson, M. J-ICE: A new Jmol interface for handling and visualizing crystallographic and electronic properties. J. Appl. Crystallogr. 2010, 44, 225–229. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 10353966, Aluminum carbonate” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum-carbonate (accessed on 12 September 2022).
- Tamez, M.; Yu, J.H. Aluminum—Air Battery. J. Chem. Educ. 2007, 84, 1936A. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Moon, S.-M. Corrosion mechanism of pure aluminium in aqueous alkaline solution. J. Solid State Electrochem. 2000, 4, 267–272. [Google Scholar] [CrossRef]
- Godart, P.; Fischman, J.; Seto, K.; Hart, D. Hydrogen production from aluminum-water reactions subject to varied pressures and temperatures. Int. J. Hydrogen Energy 2019, 44, 11448–11458. [Google Scholar] [CrossRef]
- Bošković, M.; Milinković, E.; Radović, D.V.; Stevanović, J.; Sarajlić, M. Self-powered wearable breath-monitoring sensor based on electrochemically modulated electromagnetic energy harvesting, Supplementary data 1. Mendeley Data, 2022; V1. [Google Scholar] [CrossRef]
- Sarajlic, M.; Bošković, M. Self-powered wearable breath-monitoring sensor based on electrochemically modulated electromagnetic energy harvesting, Supplementary data 2. Mendeley Data, 2022; V1. [Google Scholar] [CrossRef]





| Non-Activated | Activated | |||
|---|---|---|---|---|
| Element | Counts | Percentage Counts [%] | Counts | Percentage Counts [%] |
| C | 3230 | 1.549 | 5089 | 2.401 |
| O | 32,869 | 15.76 | 38,892 | 18.35 |
| Al | 157,694 | 75.64 | 152,960 | 72.17 |
| Si | 14,666 | 7.035 | 14,985 | 7.071 |
| Total | 208,459 | 100 | 211,926 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bošković, M.V.; Frantlović, M.; Milinković, E.; Poljak, P.D.; Radović, D.V.; Stevanović, J.N.; Sarajlić, M. Self-Powered Wearable Breath-Monitoring Sensor Enabled by Electromagnetic Harvesting Based on Nano-Structured Electrochemically Active Aluminum. Chemosensors 2023, 11, 51. https://doi.org/10.3390/chemosensors11010051
Bošković MV, Frantlović M, Milinković E, Poljak PD, Radović DV, Stevanović JN, Sarajlić M. Self-Powered Wearable Breath-Monitoring Sensor Enabled by Electromagnetic Harvesting Based on Nano-Structured Electrochemically Active Aluminum. Chemosensors. 2023; 11(1):51. https://doi.org/10.3390/chemosensors11010051
Chicago/Turabian StyleBošković, Marko V., Miloš Frantlović, Evgenija Milinković, Predrag D. Poljak, Dana Vasiljević Radović, Jelena N. Stevanović, and Milija Sarajlić. 2023. "Self-Powered Wearable Breath-Monitoring Sensor Enabled by Electromagnetic Harvesting Based on Nano-Structured Electrochemically Active Aluminum" Chemosensors 11, no. 1: 51. https://doi.org/10.3390/chemosensors11010051
APA StyleBošković, M. V., Frantlović, M., Milinković, E., Poljak, P. D., Radović, D. V., Stevanović, J. N., & Sarajlić, M. (2023). Self-Powered Wearable Breath-Monitoring Sensor Enabled by Electromagnetic Harvesting Based on Nano-Structured Electrochemically Active Aluminum. Chemosensors, 11(1), 51. https://doi.org/10.3390/chemosensors11010051







