Abstract
An electrochemical method was developed to investigate the redox properties of zinc oxide (ZnO), zinc peroxide (ZnO2), and sodium-doped zinc peroxide (Na-ZnO2) nanoparticles. The intention was to distinguish the identity of these nanoparticles among themselves, and from other transition metal oxide nanoparticles (TMONPs). Analysis of 3 mM sodium metabisulfite by cyclic voltammetry (CV) produced anodic/cathodic peak currents that are linearly related to the mass of deposited nanoparticles. A graphite working electrode was essential to the oxidation of metabisulfite. ZnO nanoparticles were crucial to the enhancement of metabisulfite oxidation current, and PPy coating could suppress the current enhancement by covering all nanoparticle surfaces. Furthermore, meso-tetrakis(4-carboxyphenyl) porphyrin was demonstrated to be a good chemical reagent that facilitates the differentiation of ZnO from ZnO2 and nanoparticles by CV analysis.
1. Introduction
Non-precious transition metal oxide nanoparticles (TMONPs) offer numerous opportunities for various cost-effective chemical, electrochemical, electronic and fuel cell applications [1,2,3] based on their catalyst, oxidation, pseudo-capacitor, and semiconductor properties [4,5,6]. These nanoparticles can also be used for chemotherapy, implant disinfection, photodynamic therapy, and wound dressing [7,8,9,10,11]. Titanium dioxide (TiO2) nanoparticles were commonly used as an additive in food products until banned by the European Union (EU) in 2021 due to their potential health risks [12]. Zinc oxide (ZnO) nanoparticles have been applied in wastewater treatment based on their ability to adsorb heavy metals and oxidize organic pollutants [13,14]. The optical, structural, and vibrational properties of zinc oxide (ZnO) versus zinc peroxide (ZnO2) nanoparticles are different, as determined by their chemical stoichiometry, size distribution, and surface morphology [15]. ZnO2 is a wide-band-gap semiconductor of the cubic crystal lattice structure that has attracted attention in a variety of scientific fields and industrial applications. Its photocatalytic activity can be enhanced by doping with Sn2+/4+ [16]. Before ZnO2 nanoparticles can be adopted for the next generation of antimicrobial and biomedical applications [17,18], a more insightful understanding of their redox properties must be gained. Cytotoxicity of ZnO2 is related to H2O2 release, alkalinity, and Zn2+ itself [19]. The Zn K-edge X-ray absorption spectroscopy can be used to indicate the decomposition of ZnO2 to ZnO [20]. Scientific investigations into the toxicity of TMONPs these days have largely focused on in vitro, in vivo, in vertebrates, and in invertebrates [21]. Instrumental characteristic techniques include scanning/transmission electron microscopy, photoacoustic imaging, single-particle inductively coupled plasma mass spectrometry [22,23,24,25], and capillary electrophoresis. Unfortunately, these techniques require expensive equipment, high maintenance/operating costs, and long analysis time. There is a pressing need for cost-/time-effective methods to assess the toxicity risk of TMONPs, especially the submicron-granular form of suspended solids in environmental water samples.
In comparison, electrochemical techniques offer the advantages of short analysis time, small sample size, and inexpensive equipment for testing the oxidation toxicity of TMONPs in relation to reactive oxygen species (ROS) [26]. Several electrochemical techniques are suitable for the analysis of ZnO and ZnO2 nanoparticles, including cyclic voltammetry [27], square wave voltammetry, differential pulse voltammetry, electrochemical impedance spectroscopy [28,29,30], and faradaic charge transfer measurement of the pseudocapacitance [31]. Cyclic voltammetry (CV) had previously been reported for the electrochemical sensing of ZnO nanoparticles deposited on a graphite electrode [32], electrochemical deposition of reduced graphene oxide on carbon fibers [33], and electrochemical characterization of Co(III), Ni(II), and Cu(II) mononuclear complexes on a glassy carbon electrode [34]. This electrochemical technique takes advantage of intuitive theory, simple operation, rapid analysis, and easy training. The development of a CV method for the electrochemical analysis of ZnO and ZnO2 nanoparticles in aqueous suspension is beneficial for chemical and environmental toxicology research.
In this work, the research was designed around a novel approach utilizing the unique surface reactivities of individual transition metal oxides toward two chemical reagents. Specifically, the TMONPs deposit was modified with either polypyrrole (PPy) or meso tetrakis-4-carboxyphenyl porphyrin (TCPP) to distinguish between ZnO and ZnO2 nanoparticles. Polypyrrole is a conducting polymer [35] that has been extensively utilized with transition metal oxide/nitride/carbide nanoparticles in advanced applications such as batteries [36], biosensors [37,38], corrosion protection [39], energy storage [40], gamma irradiation shielding [41], gas sensors [42], heavy metals detection [43], optoelectronic devices [44], packaging of perishable food products [45], photoreforming of plastic waste to hydrogen [46], removal of fluoride [47], soft tissue engineering [48], supercapacitors [49], treatment of infections [50], as a uric acid sensor [51], and water treatment [52]. The physicochemical properties and supercapacitance behavior of various PPy/metal oxide composites have recently been reviewed [53]. Both organic or inorganic additives (carbon nanotubes, graphenes, metals, metal oxides, and metal sulfides) in the PPy matrix can improve flammable/toxic gas sensing performance [54]. TCPP is a macromolecular heterocyclic compound that has been increasingly employed in the design of material for cancer therapy. Its strong light absorption in the ultraviolet to visible spectral regions makes it an efficient photodynamic agent for modifying metal oxide nanoparticles to kill tumor cells in photodynamic therapy [55,56]. TCPP has also been employed in chemiresistive gas sensing [57], DNA base sensors [58,59], food allergen detection sensors [60], membrane nanofiltration [61], photocatalytic reduction of CO2 [62], and photoelectrochemical cells [63]. Dentate binding of ZnO nanoparticles with the carboxylic groups of TCPP was applied in the photoelectrochemical biosensing of cysteine [64]. Methodologies to functionalize the ZnO surface with porphyrins were reviewed for the development of sensors [65]. As porphyrins are hydrophobic π-conjugated macrocycles, TCPP can adsorb on nanocrystalline TiO2 by bridging coordinate bonds [66] through an O=C-O-Ti bond [67]. A hybrid of TCPP molecules encapsulated by PPy matrix has been applied as an electrochemical sensor for the detection of cadmium ions [68]. Na-doped ZnO2 nanoparticles were newly synthesized in our lab even though Ag, Ca, Co, Cu, Eu, Fe, Ga, Ho, K, Mg, Mn, Ni, Sm, Sn, and W-doped ZnO nanoparticles had previously been reported in the scientific literature. To the best of our knowledge, the difference in electrochemical properties between Na-doped ZnO2 and ZnO2 nanoparticles has never been reported. In the present work, a new electrochemical method was developed for the quantitative analysis of ZnO and ZnO2 nanoparticles in aqueous suspension by spot-casting them on a graphite SPE. Modification by electropolymerization of pyrrole [69] produced a uniform coating that covered all the nanoparticles, thereby increasing the electrical contact area between ZnO/ZnO2 nanoparticles and graphite. Selectivity was attained using sodium metabisulfite as a molecular probe that helped distinguish ZnO from ZnO2 and Na-doped ZnO2 nanoparticles [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87].
2. Materials and Method
2.1. Materials
Potassium chloride, zinc acetate, ZnO nanoparticles, and TiO2 nanoparticles (<50 nm, >97%) were purchased from Millipore-Sigma (Oakville, ON, Canada). Sodium acetate was obtained from Anachemia (Lachine, QC, Canada). Hydrogen peroxide (35%) was obtained from Caledon (Georgetown, ON, Canada).
2.2. Preparation of Zinc Peroxide Nanoparticles
ZnO2 nanoparticles were synthesized using a sol–gel method adapted from the literature via thermal reaction between zinc acetate and H2O2 in an ultrasonic water bath at 60 ± 1 °C for 30 min [88]. Sodium doping was attained by adding sodium acetate to attain a Na:Zn atomic ratio of 1.0 in the synthesis mixture. Zensor TE100 screen-printed electrodes (SPEs) were obtained from eDAQ (Colorado Springs, CO, USA) with a graphitic working electrode (diameter: 0.3 cm), a graphitic auxiliary electrode, and an Ag/AgCl pseudo-reference electrode.
2.3. Sample Preparation
Standard aqueous suspensions of ZnO or ZnO2 nanoparticles (22–860 μg/mL) were freshly prepared by serial dilution of a stock with distilled deionized water after ultrasonic homogenization at 40 W for 5 min to overcome suspension inhomogeneity [89]. Previously, 30 min was allowed for the larger nanoparticles to precipitate out of the suspension. An aliquot (150 μL) of each suspension was drop-casted on an SPE and allowed to dry out inside a dehydrator (Kwasyo FD-02, 650 watts) at 35 °C. Drop casting is a technique commonly used to modify electrode surfaces for sensor fabrication by depositing a known mass of nanoparticles that can be calculated as the arithmetic product of drop volume and nanoparticle concentration. The adhesion between the nanoparticles and the electrode surface is attributed to a combination of surface, capillary, and van der Waals forces. The drop spread outside the working electrode area to cover the counter and reference electrodes. We tested a smaller volume (than 150 μL) to cover only the working electrode area and observed a very similar shape of the cyclic voltammogram and amplitudes of redox currents, probably due to the penetrable nature of deposited TMONPs.
2.4. Scanning Electron Microscopy
A deposit of nanoparticles on the SPE was confirmed through energy-dispersive X-ray spectroscopy (EDS) in a TESCAN Vega-II XMU VP scanning electron microscope (Warrendale, PA, USA) with an Oxford Advanced AZtechLive EDS detector for elemental composition analysis. Before SEM imaging and EDS analysis, the SPE surface was gently cleaned with compressed air and coated with a thin layer of gold that provided uniform electrical conductivity, inhibited charging, reduced thermal damage, and improved the secondary electron signal required for topographic examination by SEM [90].
2.5. Electrochemical Analysis
A Homiangz μEA160C electrochemical analyzer (Longman, CO, USA) was used for all CV analysis at 25 °C. Unless specified otherwise, typical experimental parameters were initial potential: 0.0 V, high potential: +1.6 V, low potential: −1.6 V, scan rate: 0.100 V/s, initial scan direction: negative for reduction or positive for oxidation, segments: 2, sample interval: 0.001 V, quiescent time: 2 s, and sensitivity: 10 mA. A supporting electrolyte solution (150 μL) containing 1.0 M KCl and 0.003 M sodium metabisulfite (pH of 6–7) was loaded on top of each SPE to obtain both cathodic reduction and anodic oxidation peak currents. Degassing with argon/helium/nitrogen was neither feasible for the small volume of sample loaded nor practical for screening analysis of environmental water in the field.
2.6. Modifications with Polypyrrole and Meso-tetrakis(4-carboxyphenyl) Porphyrin
In situ electrochemical polymerization was carried out by placing 150 μL of pyrrole (0.2 M in 0.1 M KCl supporting electrolyte) solution and applying +0.80 V vs. Ag/AgCl on the graphite working electrode to form a coating of PPy over the nanoparticles deposited on the SPE. After 10, 20, or 30 min, the spent solution was replaced by a fresh solution to continue the electrochemical polymerization for a thicker coating. Alternatively, SPEs were modified by the addition of meso-tetrakis-4-carboxyphenyl porphyrin (TCPP) dissolved in ethanol. An aliquot (10 μL) of TCPP solution was added on the SPE and allowed to dry in air before electrochemical analysis of sodium metabisulfite (0.003 M) by cyclic voltammetry.
3. Results and Discussion
3.1. Structural Characterization of ZnO2 Nanoparticles
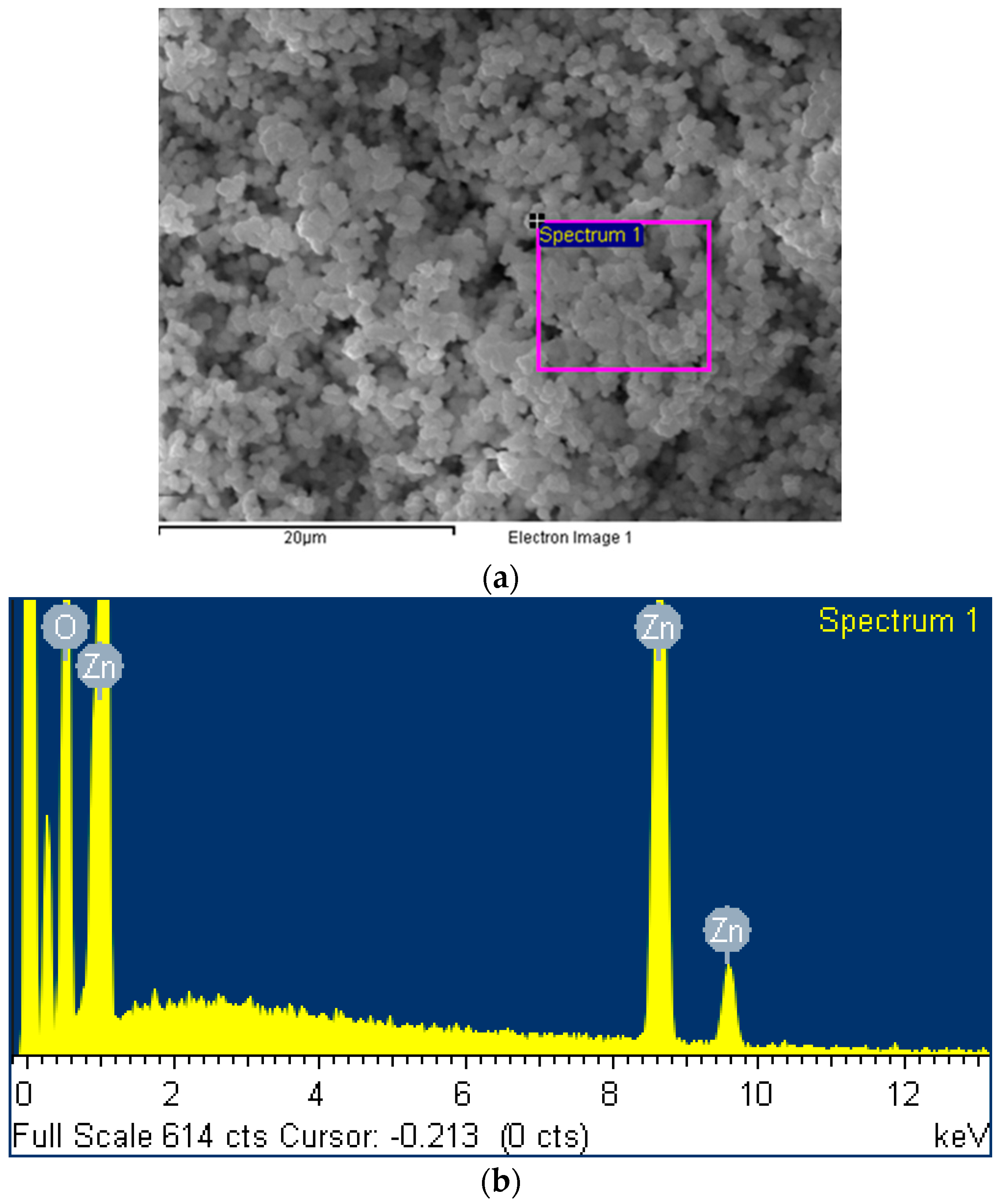
Scanning electron microscopy was used in Figure 1a to characterize the ZnO2 nanoparticles facilely prepared in our lab by following Ramírez et al.’s sol–gel method under ultrasound assistance. The energy dispersive X-ray spectroscopy analysis identified in Figure 1b an elemental composition of 66.75% oxygen and 33.25% zinc, in good consistence with the chemical formula of ZnO2.
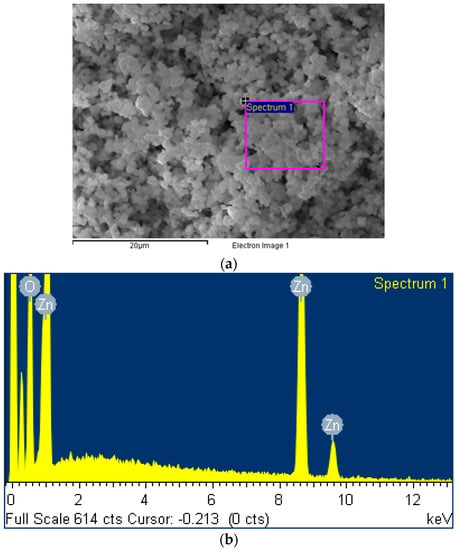
Figure 1.
(a) Scanning electron microscopy and (b) energy dispersive X-ray spectroscopy of ZnO2 nanoparticles prepared in our lab.
3.2. Cyclic Voltammetry
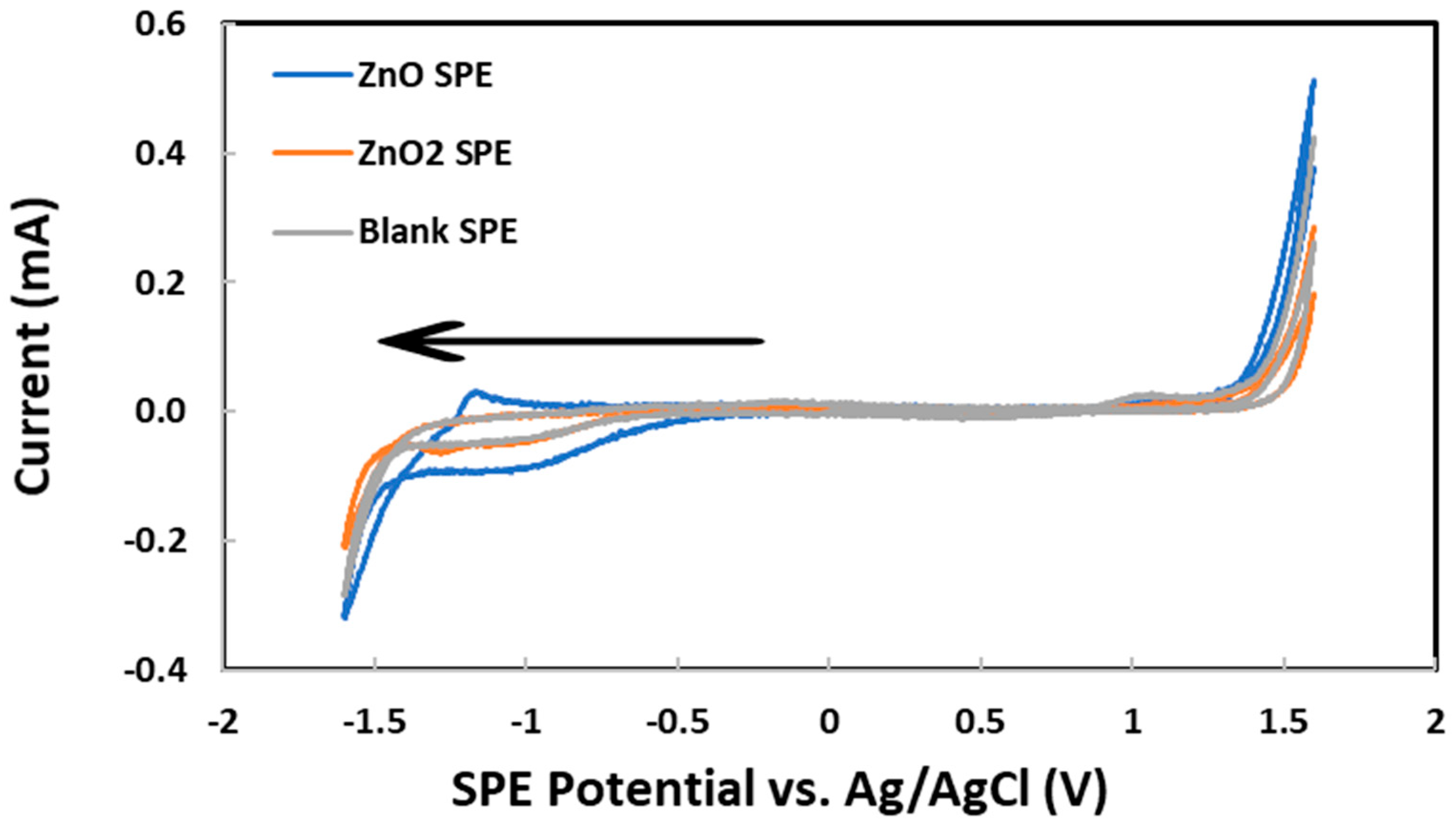
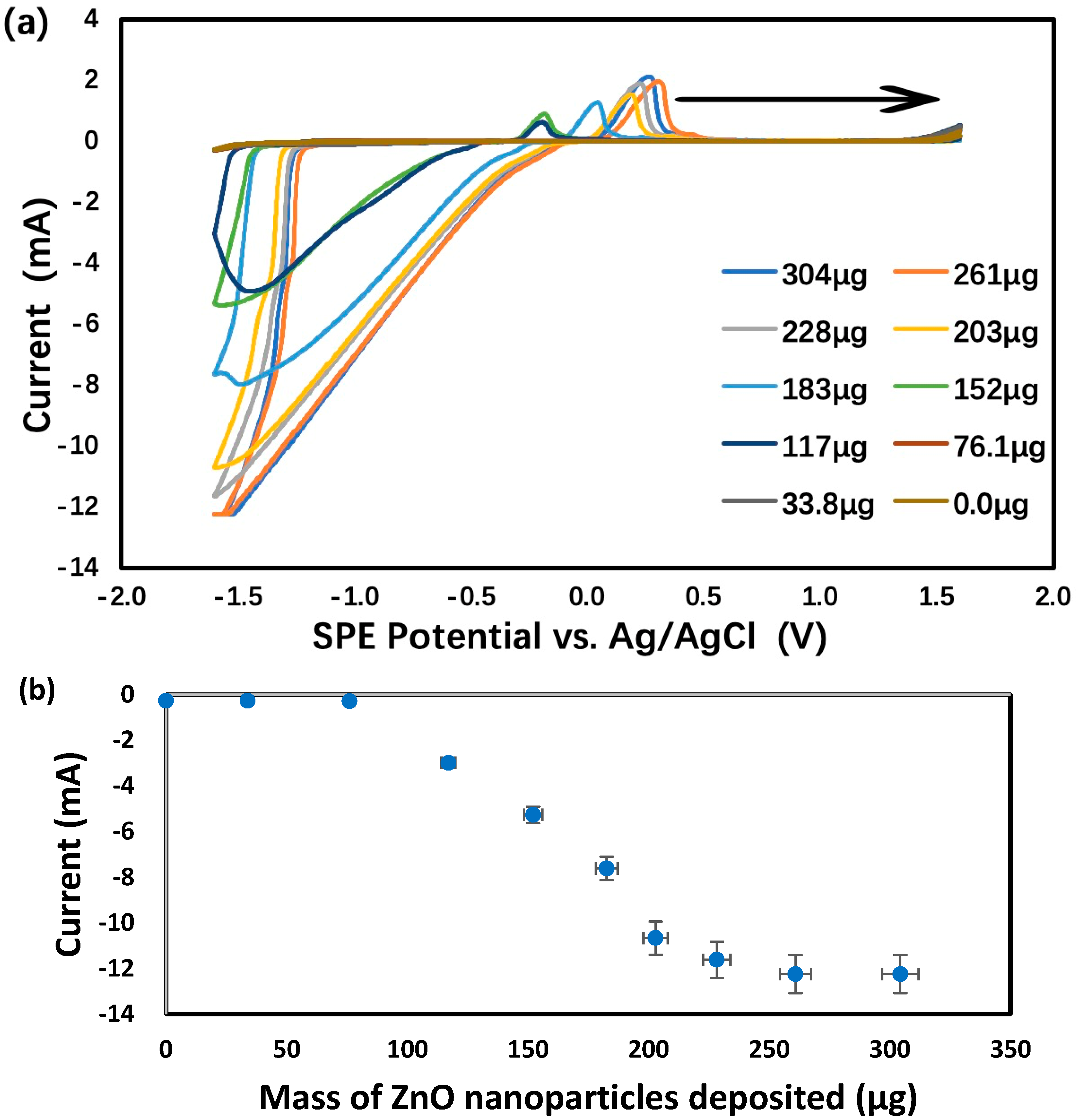
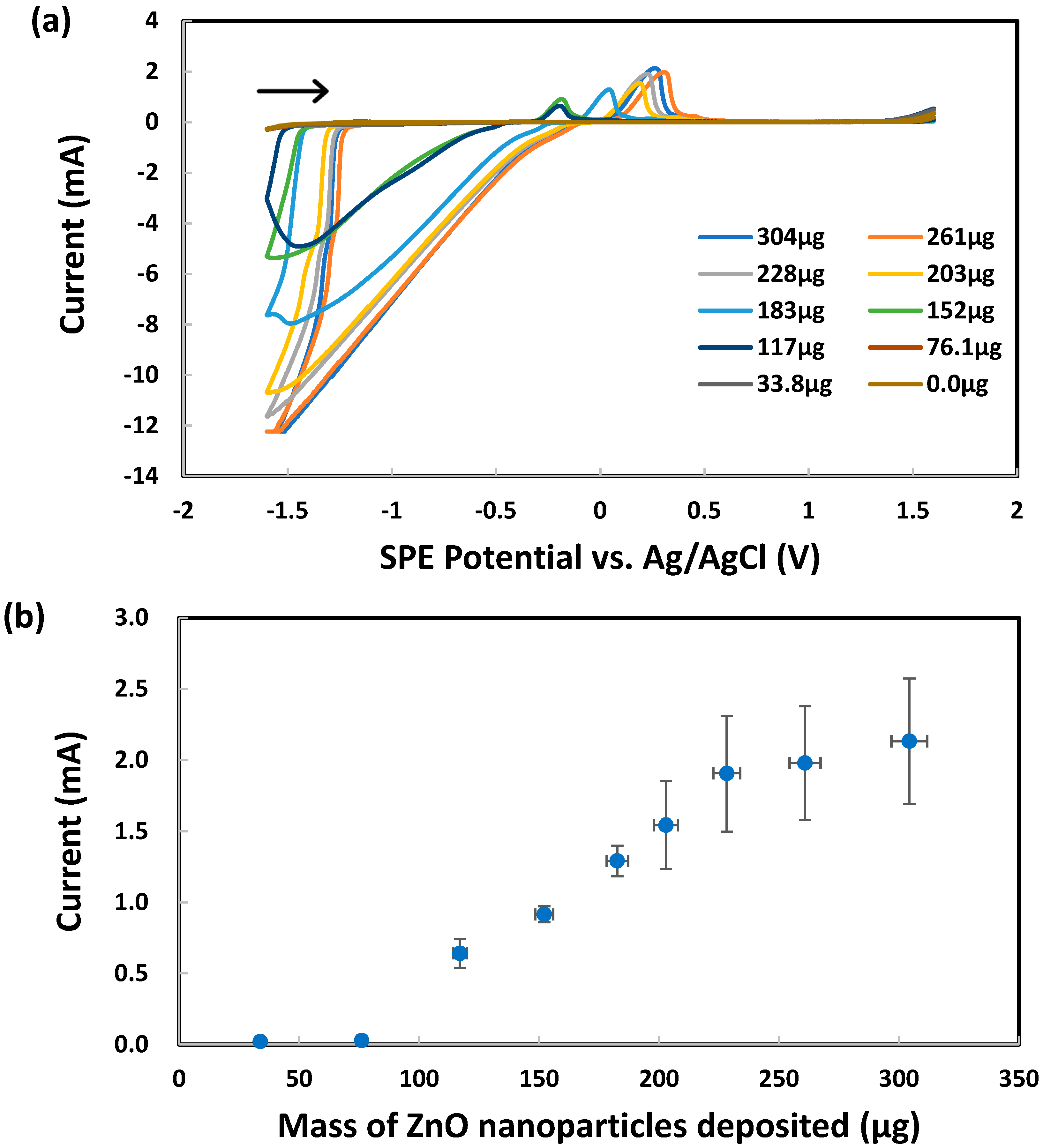
The development of a new electrochemical method for the characterization of ZnO and ZnO2 nanoparticles in aqueous suspension began by drop casting an aliquot (150 μL) on an SPE. After drying out, 1.0 M KCl was loaded on top of the residue before the SPE potential was scanned from 0.0 V to first +1.6 V and then −1.6 V vs. Ag/AgCl. As shown in Figure 2, the reduction currents at −1.0 V were attributed to oxygen dissolved in the KCl supporting electrolyte solution. These results can be explained by both the large total surface area of all nanoparticles and the superior electrical conductivity of ZnO behaving as a semiconductor material [91]. Next, the oxygen reduction current at −1.3 V vs. Ag/AgCl, measured by CV scanning from 0.0 V to first +1.3 V and then −1.3 V, was plotted vs. the mass of ZnO nanoparticles deposited on each SPE, as shown in Figure 3. Below 76 μg of nanoparticles, the current was nearly zero despite the increasing mass of nanoparticles. Apparently, the nanoparticles were falling through the porous graphite electrode surface and stayed underneath. Above 76 μg of nanoparticles, the rough graphite electrode surface was filling up with more nanoparticles with superior electrical conductivity to enhance the reduction current. The linear dynamic range, albeit limited to 76–228 μg, could potentially be used for the quantitative analysis of ZnO nanoparticles. Above 228 μg of nanoparticles, the electrode surface was fully packed with ZnO and the reduction current flattened out in spite of the further mass increase.
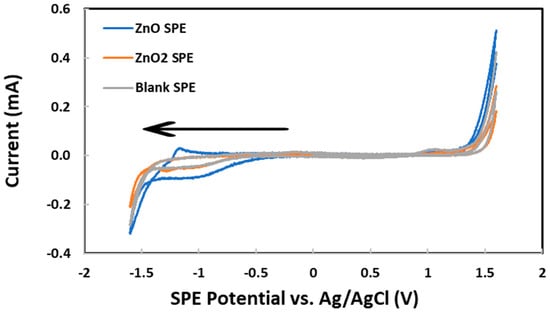
Figure 2.
Cyclic voltammograms for 106 μg of ZnO nanoparticles, 103 μg of ZnO2 nanoparticles, and no nanoparticles deposited on graphite screen-printed electrodes. Initial potential: 0.0 V, low potential: −1.6 V, high potential: +1.6 V, scan rate: 0.100 V/s, initial scan direction: negative (see arrow).
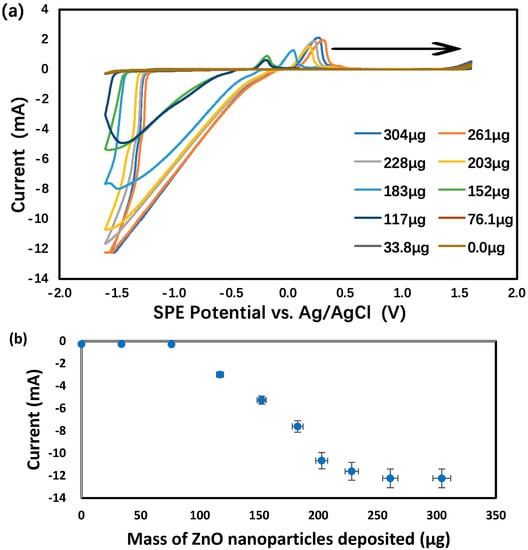
Figure 3.
Analysis of 1.0 M KCl using graphite screen-printed electrodes with different masses of ZnO nanoparticles deposited: (a) cyclic voltammograms, (b) reduction currents for ZnO → Zn under −1.6 V vs. Ag/AgCl. Scan rate: 0.100 V/s. Error bars represent one standard deviation of at least triplicate measurements.
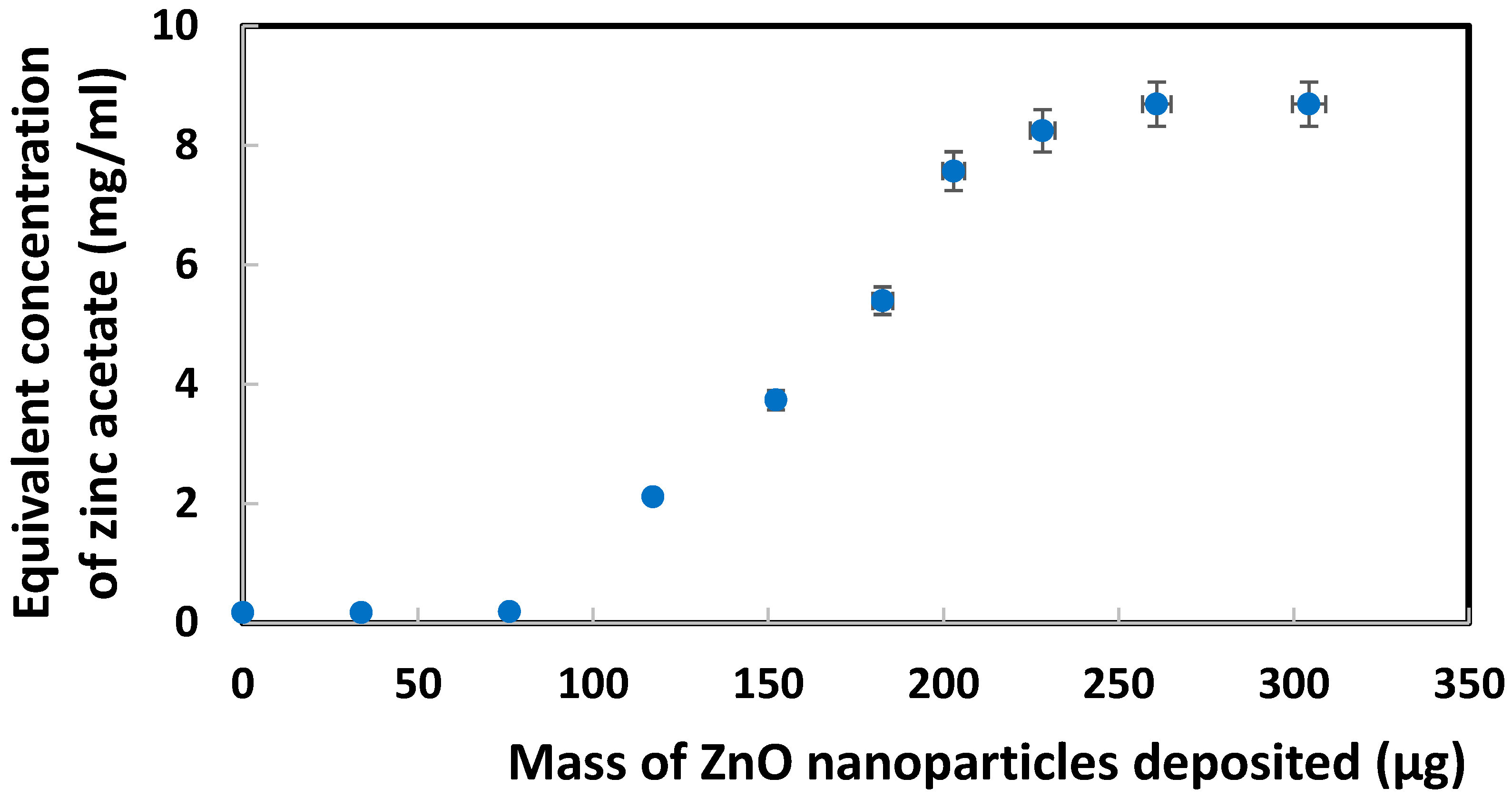
Calibration was constructed by CV analysis of standard zinc acetate solutions in 1.0 M KCl. Based on the reduction peak current for Zn2+ at −1.6 V vs. Ag/AgCl (after subtraction of the blank current due to oxygen/water reduction), a linear regression equation (data not shown) y = −0.001405x − 0.000023 with R² = 0.9927 was obtained. By applying this equation to the reduction currents presented above for ZnO nanoparticles, a correlation can now be demonstrated in Figure 4 between an equivalent Zn acetate concentration and the mass of ZnO nanoparticles deposited on SPE. Although this correlation model seemed possible, it could not adequately explain the sigmoidal trendline to be considered as a major contributor to the current.
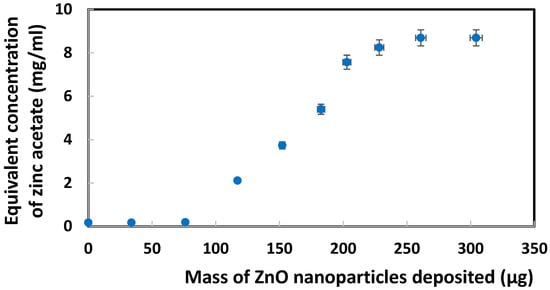
Figure 4.
Correlation between equivalent Zn acetate concentration and mass of ZnO nanoparticles deposited on SPE, based on reduction peak current for Zn2+ at −1.6 V vs. Ag/AgCl.
Upon reversing the scan of working electrode potential in the positive direction, the cathodic current decreased to the baseline level at −1.3 V vs. Ag/AgCl. Then, the anodic current started to increase, producing a re-oxidation peak as the potential reached −0.20 V approximately, as seen in Figure 5a. However, the peak current was significantly lower than that of the oxygen/water reduction peak at −1.6 V before the potential scan reversal. This peak was studied further by varying the mass of ZnO nanoparticles deposited on each SPE. Interestingly, the peak current changed in proportion to the mass of ZnO nanoparticles from 76 mg to 228 mg, as shown in Figure 5b. One plausible explanation is the oxidative stripping of Zn from the SPE to form Zn2+ in the KCl solution. These results afforded direct quantitative analysis of ZnO nanoparticles without the need for any chemical probes. The analytical relationship was linear over a modest range of ZnO nanoparticles. CV analysis of standard zinc acetate solutions in 1.0 M KCl also yielded the same re-oxidation peak for Zn → Zn2+ between −0.4 V and +0.1 V vs. Ag/AgCl. It is herein confirmed that ZnO nanoparticles can dissolve in KCl solution, thereby forming Zn2+ (just like Zn acetate does) that is electrochemically reduced at −1.3 V to form Zn and then re-oxidized eventually at +0.1 V to reform Zn2+. The unusual shift of re-oxidation peak potential might indicate a change in the diffusion zone thickness or the overpotential needed for oxidation as the number of nanoparticles deposited increased [92]. It may be a sign of kinetic effects that require control experiments at different scan speeds to investigate further.
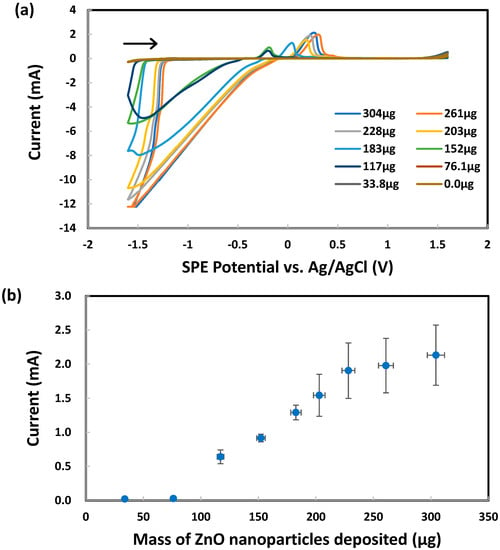
Figure 5.
Cyclic voltammetry analysis of 1.0 M KCl using graphite screen-printed electrodes with different masses of ZnO nanoparticles deposited: (a) cyclic voltammograms, (b) re-oxidation peak current for Zn → Zn2+ between −0.4 V and +0.1 V vs. Ag/AgCl. Scan rate: 0.100 V/s.
As shown above in Figure 2, the cyclic voltammogram for ZnO2 nanoparticles looks different from that for ZnO nanoparticles. One explanation is that ZnO + 2e− → Zn + O2− and ZnO2 + 2e− + H2O → Zn(OH)2 + O2−. Therefore, upon reversal of the potential scan, an anodic stripping peak appeared at −1.28 V vs. Ag/AgCl from the re-oxidation of Zn → Zn2+, but no re-oxidation peak was observed for Zn(OH)2. Further research would be needed to investigate the electrochemical mechanisms behind this difference. Interestingly, ZnO2 nanoparticles afford a working potential window of 3.0 V (from −1.5 V to +1.5 V), nearly as wide as that available from the SPE. In comparison, ZnO nanoparticles can afford a window of only 2.6 V (from −1.2 to + 1.4 V) that is plagued by both the cathodic wave (from −0.5 V to −1.4 V) due to reduction of dissolved oxygen in the KCl solution and the anodic peak (at −1.2 V) arising from the re-oxidation stripping of Zn.
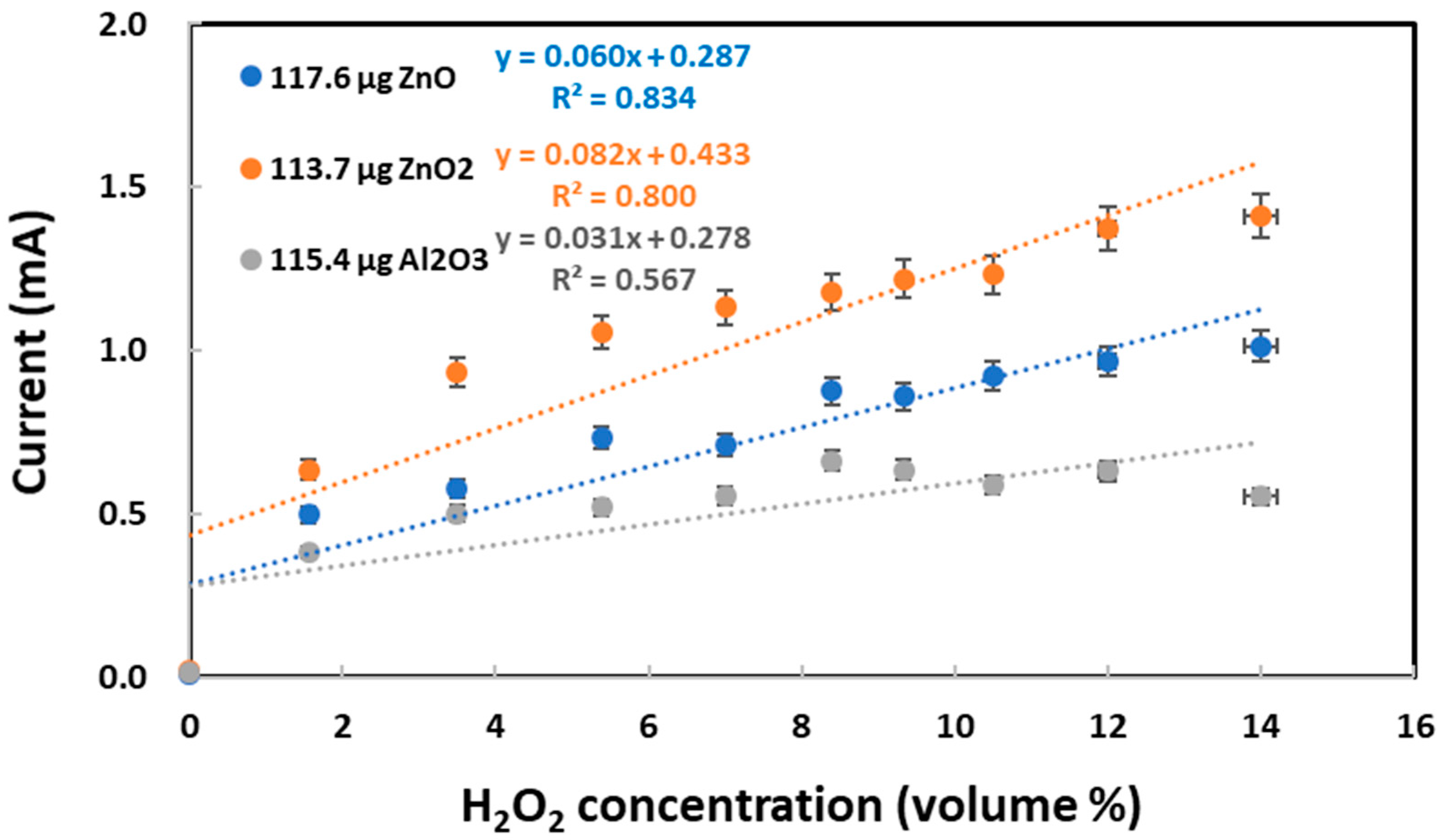
The growing number of commercial applications had brought aluminum oxide nanoparticles under toxicologists’ purview in a previous report [93]. Both ZnO and ZnO2 nanoparticles were compared with Al2O3 nanoparticles for the electrochemical reduction and re-oxidation of varying concentrations of H2O2 as a chemical probe. As shown in Figure 6, ZnO2 nanoparticles produced the highest currents for the H2O2 re-oxidation peak appearing at +0.9 V vs. Ag/AgCl. In contrast, Al2O3 nanoparticles produced the lowest currents that did not seem to increase significantly with increasing H2O2 concentration. Accordingly, the studied oxides/peroxides can be ranked in terms of their oxidation power: ZnO2 > ZnO > Al2O3. This ranking agrees with ZnO2 having a higher toxicity than ZnO nanoparticles [94]. Furthermore, the slope values can assist with the qualitative identification of these three different TMONPs. Regardless of similarity in re-oxidation currents, ZnO and ZnO2 nanoparticles differed in their electrochemical behaviors, as presented in Figure S1. In segment 3 of the CV scan, ZnO nanoparticles reproduced practically the same re-oxidation currents as those observed in segment 1. In stark contrast, ZnO2 nanoparticles attained significantly higher re-oxidation currents in segment 3 than those observed in segment 1.
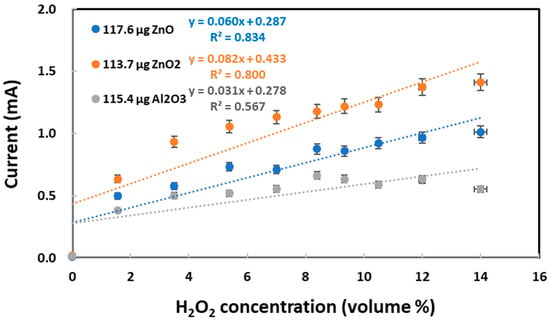
Figure 6.
Re-oxidation peak currents measured at 0.5–1.0 V vs. Ag/AgCl for varying hydrogen peroxide concentrations on graphite screen-printed electrodes with individual deposits of ZnO nanoparticles (118 μg), ZnO2 nanoparticles (114 μg), and Al2O3 nanoparticles (115 μg). The gray regression line (with R2 = 0.56) explicitly shows no linear relationship between current and H2O2 concentration for Al2O3.
3.3. Responsivity of Metabisulfite towards TMONPs
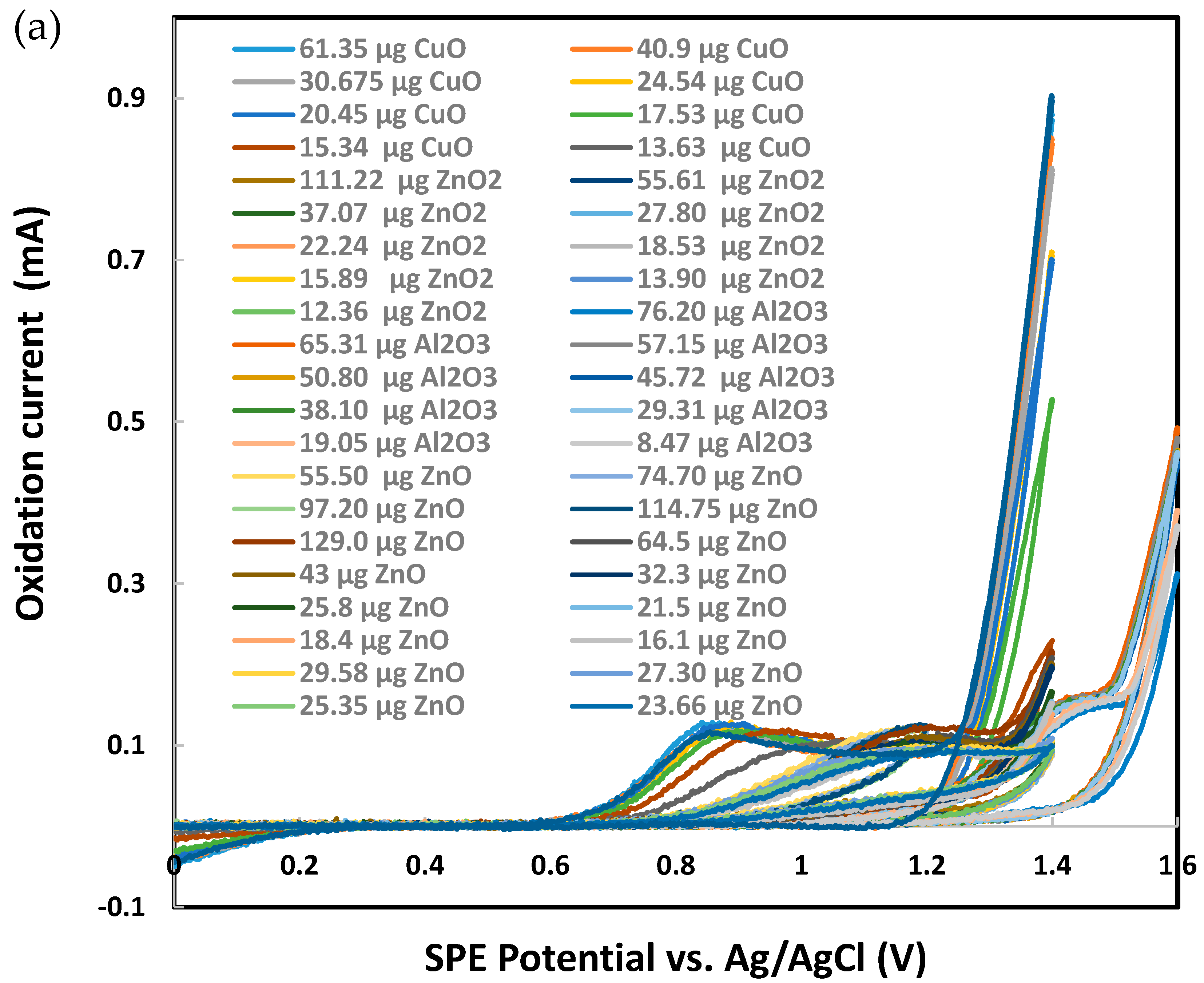
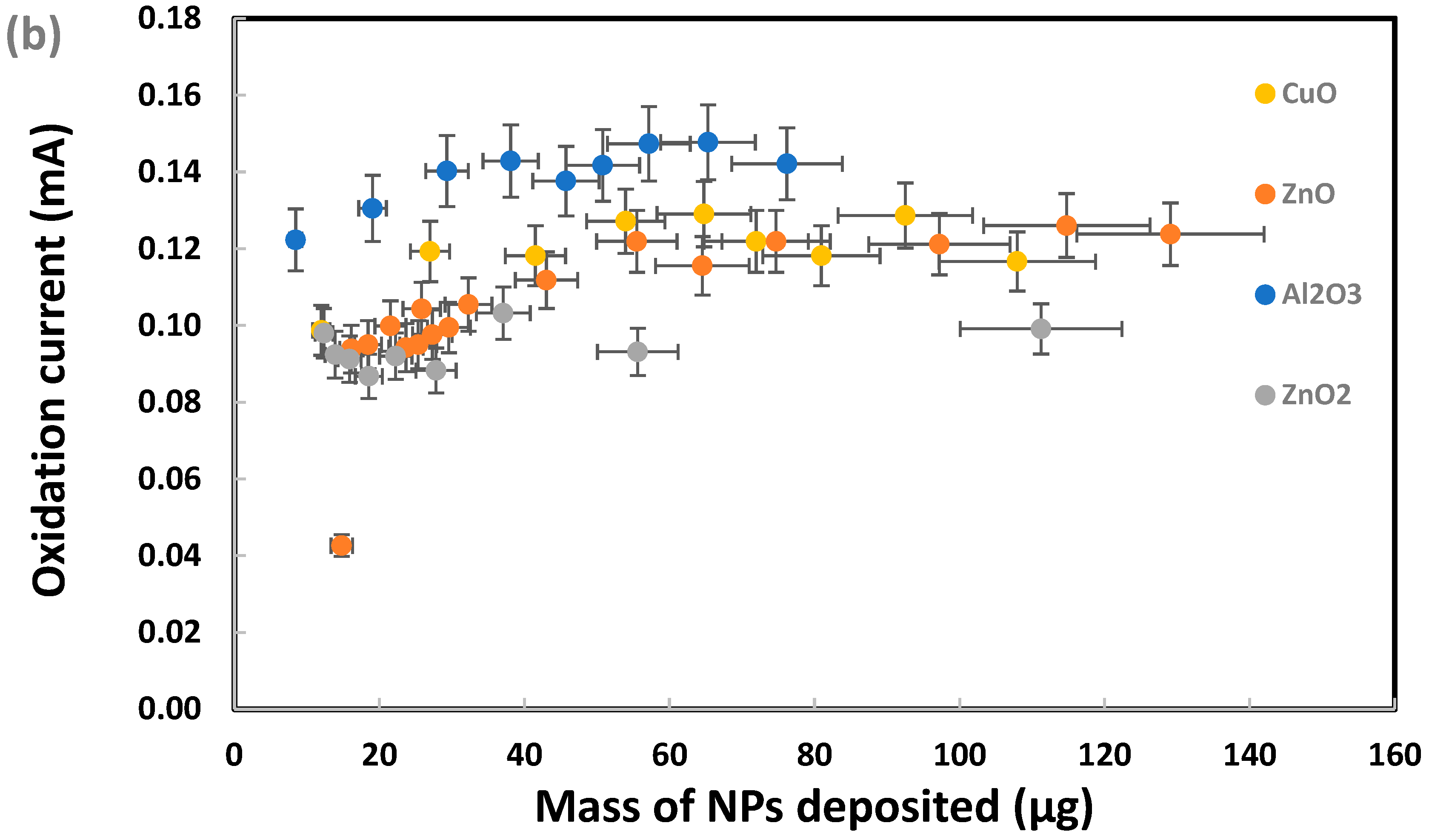
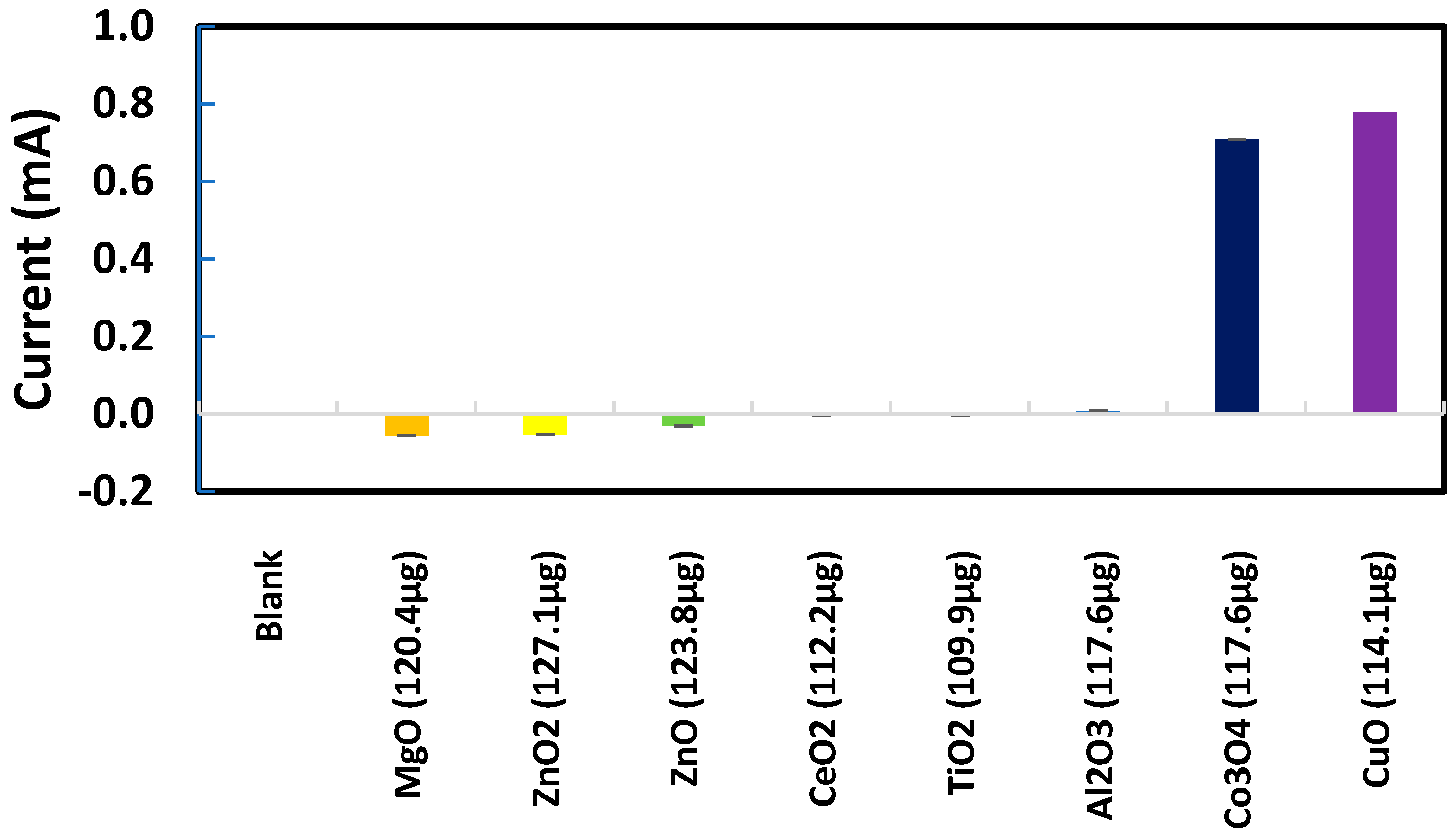
Sodium metabisulfite (0.003 M) was tested as the next chemical probe with Al2O3, CuO, ZnO, and ZnO2 nanoparticles individually deposited on SPEs. As presented in Figure 7, CuO produced significantly higher metasulfite oxidation peak currents than Al2O3, ZnO, and ZnO2. Several other metal oxide nanoparticles were also tested in their response to metabisulfite for their quantitative analysis. Most chosen nanoparticles are widely used in the industrial manufacturing of consumer goods. As summarized in Figure 8, the net metasulfite oxidation peak current measured for each metal oxide nanoparticle (within the controlled mass range of 110–118 μg) varied substantially among the eight different TMONPs studied. Obviously, Co3O4 and CuO stand out among all nanoparticles by generating the two strongest oxidation peak currents from 0.003 M metabisulfite, as measured at +1.4 V vs. Ag/AgCl. This result seems to agree with the significant cytotoxicity (cell death as measured by lactase dehydrogenase levels) previously reported for these two types of nanoparticles [95]. Risk assessment of TMONPs generally comprises in vitro cell-based assays and in vivo animal experimentation. The rapidly increasing number of new composites and varying functionalization makes in vivo toxicity tests undesirable on both ethical and financial grounds. Fortunately, the responsivity of metabisulfite holds promise for development into an in vitro non-cell-based screening test that can predict the toxicity of new TMONP composites with varying functionalization.
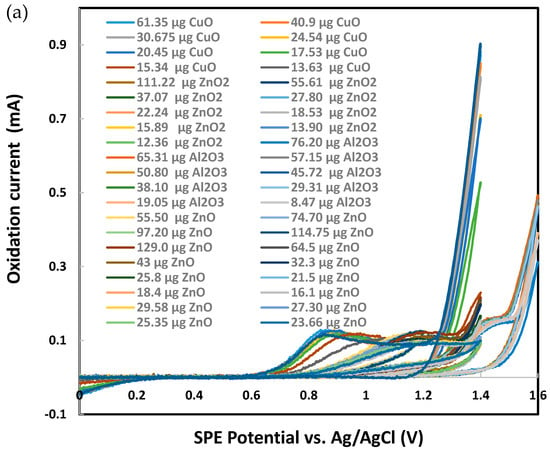
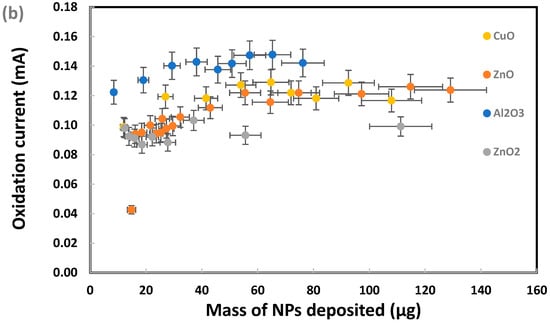
Figure 7.
Cyclic voltammetry analysis of 0.003 M sodium metabisulfite in 1.0 M KCl using graphite screen-printed electrodes with different masses of nanoparticles deposited: (a) cyclic voltammograms, (b) current for metabisulfite oxidation peak. Scan rate: 0.100 V/s. Error bars represent one standard deviation of at least triplicate measurements.
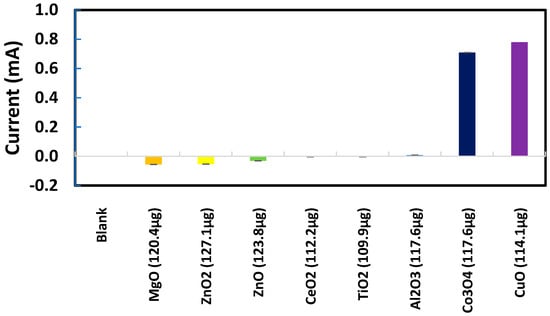
Figure 8.
Net oxidation peak currents from 0.003 M metabisulfite measured at +1.4 V vs. Ag/AgCl for different metal oxide nanoparticles deposited on individual SPEs.
For those SPEs with a deposit of CuO, Co3O4, MgO, or ZnO nanoparticles, one interesting observation was the metabisulfite oxidation peak shifting to a less positive potential range where the blank signal for the bare SPE was nearly zero. No significant shift in the metabisulfite oxidation peak was observed for the other SPEs with a deposit of Al2O3, TiO2, or CeO2 nanoparticles. In the scientific literature, Co3O4 and CuO are known to be redox catalysts that can change their oxidation states (Co(II)/Co(III), Cu(I)/Cu(II)) readily. Further investigation will be needed to gain a full understanding of this discrepancy between electrochemical behaviors of the two groups of metal oxide nanoparticles.
3.4. Modification of SPEs with Polypyrrole Coating
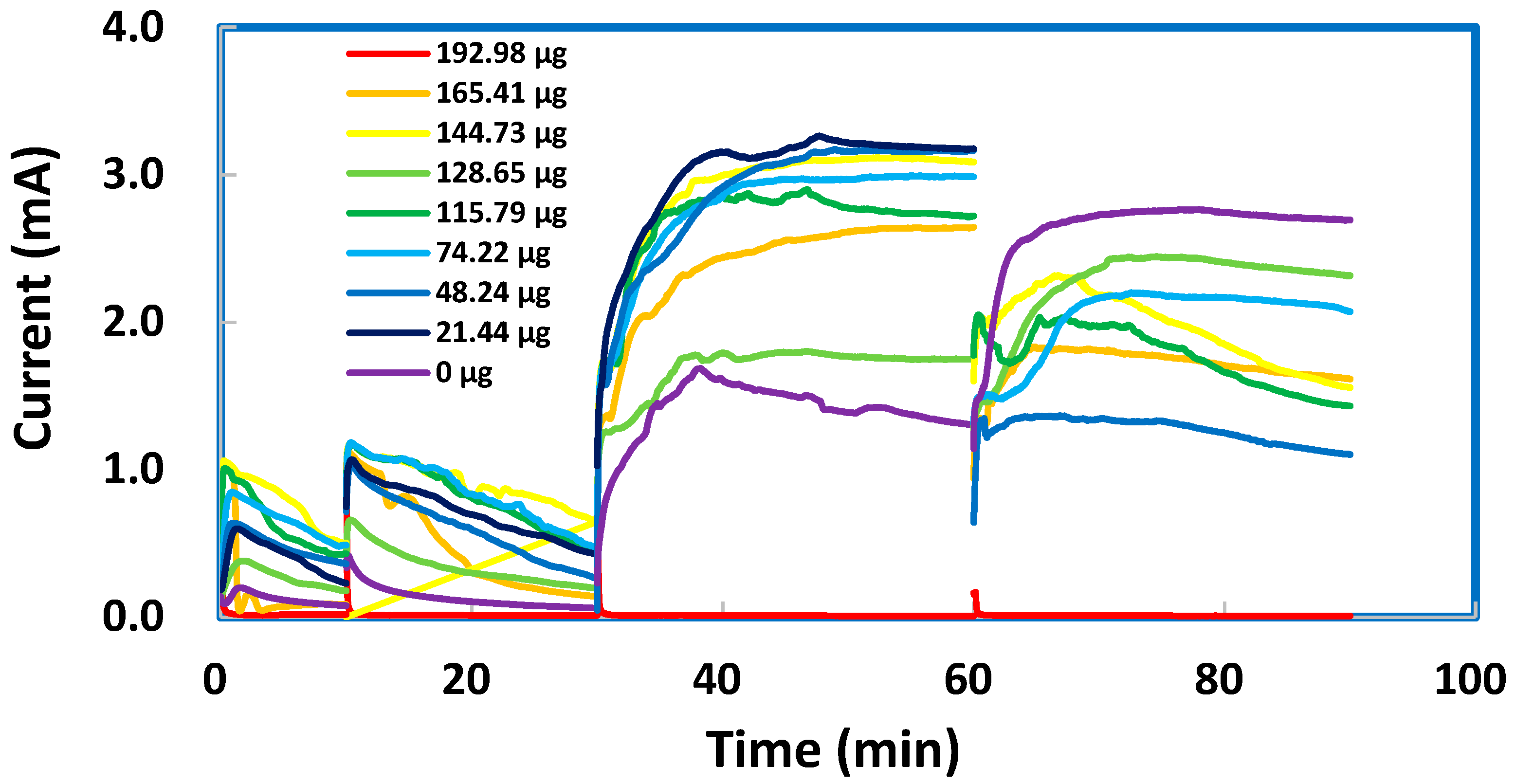
A facile technique was attempted to modify SPEs after the deposition of TMONPs. Several SPEs, each with a deposit of ZnO nanoparticles in varying masses, were coated by PPy via electrochemical oxidation of pyrrole (0.1 M) in 0.1 M KCl at a constant potential of +0.8 V vs. Ag/AgCl during four consecutive time intervals. In the first interval (0–10 min), as shown in Figure 9, the fresh aliquot of pyrrole produced oxidation currents that were all below 0.0010 A. The current represents a flow of electrons from pyrrole molecules into the graphite working electrode (diameter = 3.0 mm) and hence indicates the rate of PPy formation as a coating over the graphite area (7.0 mm2). In the second interval (10–30 min), a refreshed aliquot of pyrrole produced oxidation currents up to 0.0012 A. These slightly higher currents suggested that a more efficient electron transfer was facilitating the oxidative polymerization process. In the third interval (30–60 min), a fresh aliquot of concentrated pyrrole (0.2 M) produced remarkably higher currents, up to 0.0032 A. These strong currents were sustainable without showing any significant decay. In the fourth interval 60–90 min), a fresh aliquot of concentrated pyrrole produced currents up to 0.0027 A only. These less strong currents, although showing a slight decay, indicated the continuous formation of PPy to produce a thicker coating on each SPE. All the PPy-coated SPEs were then tested with 0.003 M sodium metabisulfite (in 1.0 M KCl solution). No well-defined anodic peak can be distinguished for the oxidation of metabisulfite. The anodic currents obtained for various masses of ZnO nanoparticles, albeit strong, did not seem to follow any increasing trend. These results can be explained by a thick PPy coating that covered all the nanoparticles to provide a uniform surface of constant area for electron transfer. Our initial thought was that a thick PPy coating could be useful for the regeneration of spent SPEs. Unfortunately, the PPy coatings on SPEs with different masses of ZnO nanoparticles deposited did not give us similar cyclic voltammograms for 0.003 M sodium metabisulfite in 1.0 M KCl solution, as shown in Figure S2.
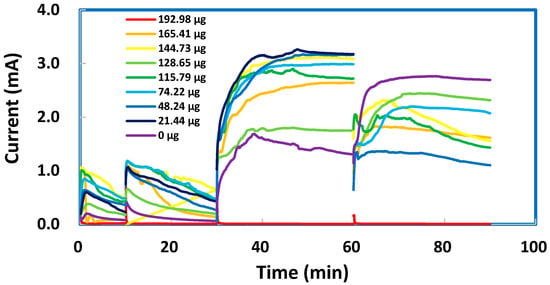
Figure 9.
Potentiostatic I-t plots recorded at +0.8 V vs. Ag/AgCl to show the oxidation currents that represent the formation kinetics as rates of polypyrrole coating on screen-printed electrodes with various masses of ZnO deposited.
3.5. Modification of SPEs with Meso-tetrakis(4-carboxyphenyl) Porphyrin
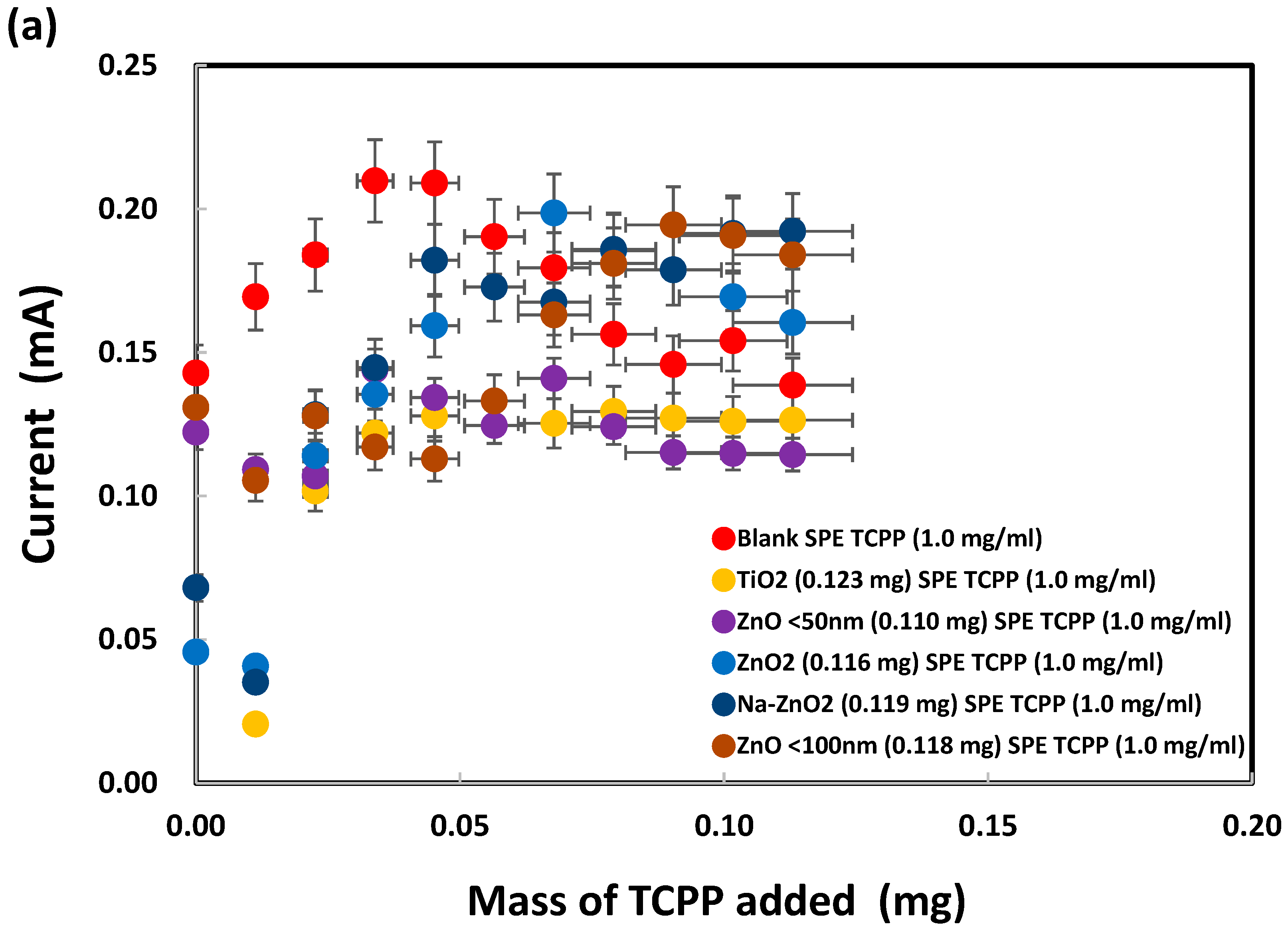
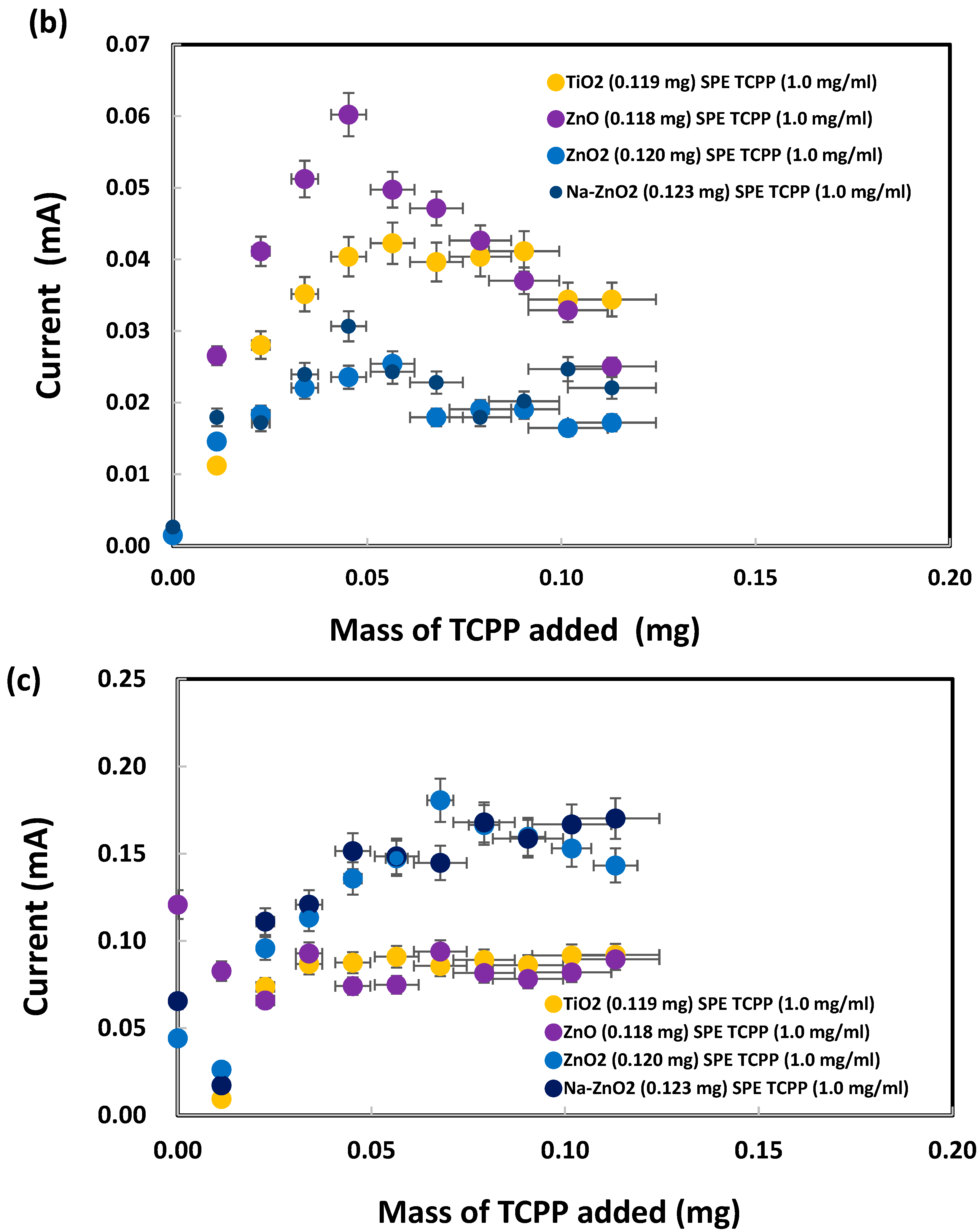
Meso-tetrakis(4-carboxyphenyl) porphyrin was chosen for modification of the ZnO nanoparticles on SPEs because of the dentate binding of ZnO nanoparticles with the carboxylic groups of TCPP, as already explained in the Introduction. SPEs were modified by the addition of TCPP onto bare SPEs before electrochemical analysis of sodium metabisulfite (0.003 M) by cyclic voltammetry, as shown in Figure S3. The oxidation peak currents, measured at +1.05 V vs. Ag/AgCl as presented in Figure 10a, initially increased after addition of TCPP (up to 0.04 mg) but subsequently flattened out or decreased. SPEs with a deposit of either TiO2 or ZnO (<50 nm) nanoparticles generated similar peak currents after TCPP addition (from 0.04 to 0.11 mg). Interestingly, from 0.00 to 0.05 mg of TCPP, large ZnO (<100 nm) nanoparticles produced total currents (~0.00013 A) similar to those (~0.00012 A) for small ZnO (<50 nm) nanoparticles. As shown in Figure 10b, the oxidation peak currents were low when no metabisulfite was present on the TiO2-deposited SPEs, even though TCPP contributed a current proportional to its mass. The TCPP oxidation peak currents for the ZnO-deposited SPEs were slightly higher (up to 0.05 mg of TCPP added), probably due to stronger complex formation between TCPP and ZnO than TCPP and TiO2. Interestingly, the oxidation currents for both ZnO2 and Na-doped ZnO2 were even lower than those for TiO2. Hence TCPP can be used as a chemical reagent to differentiate ZnO from ZnO2 and Na-ZnO2 nanoparticles by cyclic voltammetry.
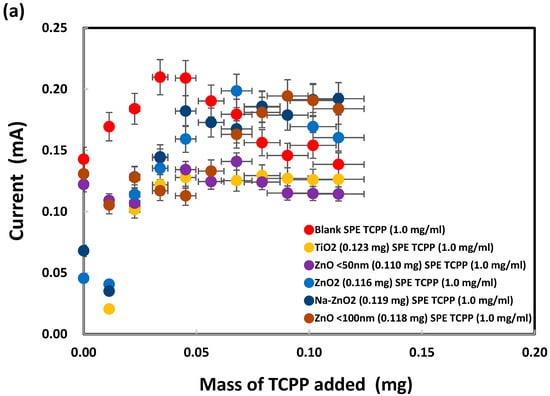
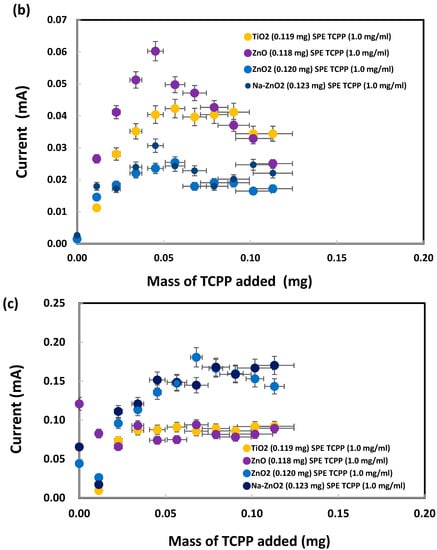
Figure 10.
Oxidation peak currents of (a) 0.003 M and (b) 0.000 M sodium metabisulfite in 1.0 M KCl solution, measured at +1.05 V vs. Ag/AgCl, after addition of TCPP onto SPEs with various nanoparticles deposited. (c) Net metabisulfite oxidation current obtained by subtraction of (b) from (a).
Furthermore, subtraction of the TCPP oxidation currents in Figure 10b from the corresponding total oxidation currents in Figure 10a give the net oxidation currents for metabisulfite, as presented in Figure 10c. ZnO2 and Na-ZnO2 nanoparticles enhanced the metabisulfite oxidation currents, probably due to their lower charge transfer resistance, significantly more than ZnO or TiO2 nanoparticles could do. This new finding is consistent with a previous report that carbon dots/ZnO2 showed higher photocatalytic activity than carbon dots/TiO2, due to its excellent charge separation, larger total pore volume, and faster photoresponsivity [96]. Herein, sodium metabisulfite is proposed as a chemical probe that can work together with TCPP for the sensitive differentiation of ZnO2 and Na-ZnO2 from ZnO nanoparticles. Moreover, despite earlier reports of TCPP’s ability to adsorb onto TiO2 nanoparticles via bidentate coordinate bonds, this study found that such adsorption did not result in a significant current difference between SPEs with TiO2 nanoparticles and those with ZnO nanoparticles. This finding points out ZnO may produce similar TCPP adsorption mechanism as TiO2.
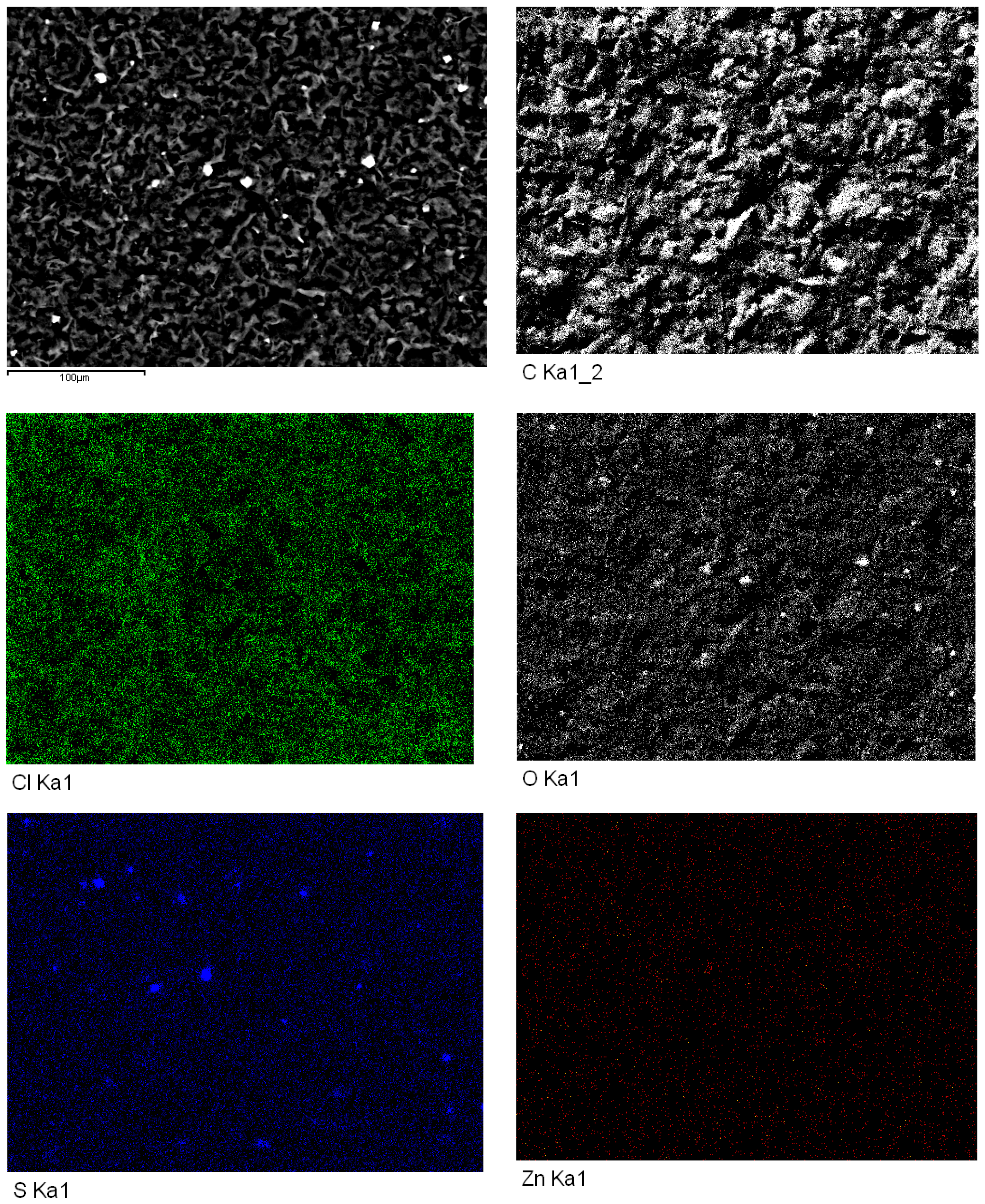
Figure 11 presents the surface distributions for different elements, as examined by high-resolution SEM-EDS, on an SPE with ZnO2 nanoparticles and TCPP, after CV analysis using 1.0 M KCl as the supporting electrolyte. In the SEM-EDS map for Zn, the even distribution of Zn pixels (red dots) confirms the presence of ZnO2 nanoparticles on the graphite surface. The Zn concentration was low because only 116 μg of nanoparticles spread over the SPE. Most importantly, a uniform distribution of O pixels (white dots) was also evidenced in the corresponding SEM-EDS map. By comparison, the Cl map showed a uniform dense distribution of Cl pixels (bright green dots). Other than carbon, zinc, oxide, and chloride that were expected to be present on the graphite working electrode, sulfide also appeared as a contaminant (probably from the commercial production of SPEs) in local aggregations. An atomic ratio of 50:50 was used as our first choice in the synthesis of ZnO2 for the sake of simple design in this feasibility study. When the atomic ratio was analyzed after synthesis using SEM-EDS, an interesting mesoporous crystal lattice was discovered. Knowing Na is monovalent and Zn is divalent, this ratio could produce an imbalance of electronic charges in the doped ZnO2 nanoparticles. A full evaluation of varying atomic ratios is underway in our lab and all new findings will be reported systematically soon.
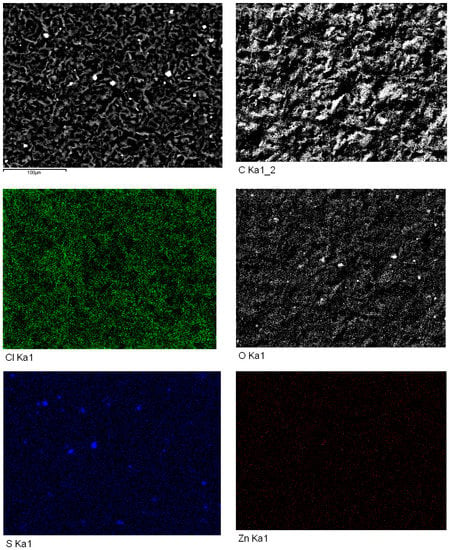
Figure 11.
High-resolution scanning electron microscopy (SEM) images of ZnO2 nanoparticles deposited on SPE after TCPP addition, as detected by energy-dispersive X-ray spectroscopy (EDS) to examine the surface distributions of various elements.
3.6. Potential Shortcomings and Challenges
Several potential shortcomings were noted during the development of this electrochemical method for investigating the redox properties of ZnO, ZnO2, and Na-ZnO2 nanoparticles. First, variations in electrochemical performance existed among SPEs in the same batch and from different batches. Second, the mass of nanoparticles tested was an experimental variable to be optimized. Third, agglutination or agglomeration of nanoparticles could occur when each sample suspension dried up inside the dehydrator. Fourth, the electropolymerization of pyrrole and complexation with TCPP might be sensitive to lab humidity. These shortcomings present certain challenges in the further development of this method. Our ultimate challenge will be to successfully test real environmental suspensions comprising TMONPs and all kinds of mineral nanoparticles. An apparatus of novel design is being constructed in our lab to separate the nanoparticles by size based on sedimentation under gravity and by charge based on electrophoretic migration through parallel microfluidic channels.
4. Conclusions
In this research work, cyclic voltammetry was used to perform electrochemical analysis of ZnO and ZnO2 nanoparticles deposited on SPEs. Both the reductive peak current and the re-oxidation peak current became larger with increasing mass of nanoparticles deposited. Based on the functional relationship between the metasulfite oxidation peak current generated by SPEs having a constant mass of ZnO nanoparticles and the metabisulfite concentration, an electrochemical method was successfully developed for the quantitative analysis in aqueous suspension. These deposits on SPEs were successfully modified with meso-tetrakis-4-carboxyphenyl porphyrin (TCPP) to help distinguish the nanoparticle identities. On the basis of electrochemical behavior, ZnO is similar to TiO2 but different from ZnO2 (whether Na-doped or not). These findings are being applied to the development of a new electrochemical detector that holds the SPE with a constant mass of unknown nanoparticles, followed by modification with TCPP, before flow injection analysis of H2O2 and sodium metabisulfite sequentially. The oxidation currents, measured at an optimal graphite electrode potential, can hopefully reveal the identity of zinc oxide and peroxide nanoparticles from other TMONPs. Among the various TMONPs studied in the present work, some were n-type, some were p-type, and some were insulators. More TMONPs of these types will be studied so as to analyze their results in groups belonging to same category. At this stage of our method development, their redox properties are investigated as received/synthesized and dispersed in distilled deionized water to prepare an aqueous suspension. Organic molecules do not tend to adsorb on the metal oxide surface. Inorganic functional groups will adsorb to varying extents but may not be electrochemically active; they can be removed by solid phase extraction through an ion-exchange cartridge if necessary. The state of metal oxide nanoparticles is a profound issue that requires substantial further research efforts to clarify. It will be investigated as the next part of our study and will be elaborated on in an upcoming report. Our method is feasible for investigating the redox properties of zinc oxide (ZnO), zinc peroxide (ZnO2), and sodium-doped zinc peroxide (Na-ZnO2) nanoparticles. These nanoparticles can be distinguished among themselves and from other TMONPs based on the results being reported in this work. The method is capable of being accomplished in a successful manner within a reasonable period of time, considering all the cost, environmental, instrumental, technological, and training factors. It has much scientific significance in contributing towards the chemical and environmental toxicology research on TMONPs. Future studies will analyze transition metal carbide, nitride, oxide, peroxide, and sulfide nanoparticles in aqueous dispersion towards their environmental toxicology studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors11070369/s1, Figure S1: Re-oxidation peak currents measured at 0.5–1.0 V vs. Ag/AgCl in segment 1 vs. segment 3 for varying hydrogen peroxide concentrations on graphite screen-printed electrodes with a deposit of (a) 118 μg of ZnO nanoparticles, and (b) 114 μg of ZnO2 nanoparticles. Figure S2: Cyclic voltammograms of sodium metabisulfite (0.003 M in 1.0 M KCl solution) using SPEs with different amounts of ZnO nanoparticles deposited after polypyrrole coating. Initial potential: 0.0 V, low potential: −1.6 V, high potential: +1.6 V, segments: 3. Figure S3: Cyclic voltammograms of 0.003 M sodium metabisulfite in 1.0 M KCl solution using SPEs with/without a deposit of TiO2/ZnO nanoparticles after addition of meso-tetrakis(4-carboxyphenyl) porphyrin.
Author Contributions
Conceptualization, E.P.C.L.; methodology, K.W. and E.P.C.L.; validation, E.P.C.L.; formal analysis, K.W.; resources, E.P.C.L.; writing—original draft preparation, K.W.; writing, review and editing, E.P.C.L.; supervision, E.P.C.L.; project administration, E.P.C.L.; funding acquisition, E.P.C.L. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from NSERC Canada (grant number RGPIN-2018-05320) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The raw data generated at Carleton University are available on request from the corresponding author.
Acknowledgments
Special thanks go to Sarah Elabd for her preparation of the Na-doped ZnO2 nanoparticles studied in this work. We thank J.J. Wang, Technical Director of the Nano Imaging Facility at Carleton University, for many helpful discussions of the SEM-EDS images for elemental mapping.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albu, Z.; Alzaid, F.; AlQahtani, S.; AlAbass, N.; Alenazey, F.; Allehyani, I.; AlOtaibi, B. Improving water oxidation performance by implementing heterointerfaces between ceria and metal-oxide nanoparticles. J. Colloid Interface Sci. 2021, 587, 39–46. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Dourandish, Z.; Jahani, P.M.; Sheikhshoaie, I.; Askari, M.B.; Salarizadeh, P.; Nejad, F.G.; Kim, D.; Kim, S.Y.; et al. Applications of non-precious transition metal oxide nanoparticles in electrochemistry. Electroanalysis 2022, 34, 1065–1091. [Google Scholar] [CrossRef]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.M.; Lu, Z.H. Transition metal oxide work functions: The influence of cation oxidation state and oxygen vacancies. Adv. Funct. Mater. 2012, 22, 4557–4568. [Google Scholar] [CrossRef]
- Akbari, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano-Struct. Nano-Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- Sarno, M. Chapter 22—Nanotechnology in energy storage: The supercapacitors. Stud. Surf. Sci. Catal. 2020, 179, 431–458. [Google Scholar]
- Diao, F.; Wang, Y. Transition metal oxide nanostructures: Premeditated fabrication and applications in electronic and photonic devices. Mater. Sci. 2018, 53, 4334–4359. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Vergara-Llanos, D.; Koning, T.; Pavicic, M.F.; Bello-Toledo, H.; Díaz-Gómez, A.; Jaramillo, A.; Melendrez-Castro, M.; Ehrenfeld, P.; Sánchez-Sanhueza, G. Antibacterial and cytotoxic evaluation of copper and zinc oxide nanoparticles as a potential disinfectant material of connections in implant provisional abutments: An in-vitro study. Arch. Oral Biol. 2021, 122, 105031. [Google Scholar] [CrossRef]
- Almaieli, L.M.A.; Khalaf, M.M.; Gouda, M.; Alhayyani, S.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Titanium dioxide/chromium oxide/graphene oxide doped into cellulose acetate for medical applications. Polymers 2023, 15, 485. [Google Scholar] [CrossRef]
- Singh, S.; Pal, K. Exploration of polydopamine capped bimetallic oxide (CuO-NiO) nanoparticles inspired by mussels for enhanced and targeted paclitaxel delivery for synergistic breast cancer therapy. Appl. Surf. Sci. 2023, 626, 157266. [Google Scholar] [CrossRef]
- Bi, X.; Bai, Q.; Liang, M.; Yang, D.; Li, S.; Wang, L.; Liu, J.; Yu, W.W.; Sui, N.; Zhu, Z. Silver peroxide nanoparticles for combined antibacterial sonodynamic and photothermal therapy. Small 2022, 18, 2104160. [Google Scholar] [CrossRef] [PubMed]
- Belder, T.D. Titanium Dioxide Banned as a Food Additive in the EU; report number E42022-0011; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2012.
- Konstantinos, S.; Stefanous, M.; Efthimia, K.; Manassis, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 42–70. [Google Scholar]
- Bedi, P.S.; Kaur, A. An overview on uses of zinc oxide nanoparticles. World J. Pharm. Pharm. Sci. 2015, 4, 1177–1196. [Google Scholar]
- Bocharov, D.; Chesnokov, A.; Chikvaidze, G.; Gabrusenoks, J.; Ignatans, R.; Kalendarev, R.; Krack, M.; Kundzins, K.; Kuzmin, A.; Mironova-Ulmane, N.; et al. A comprehensive study of structure and properties of nanocrystalline zinc peroxide. J. Phys. Chem. Solids 2022, 160, 110318. [Google Scholar] [CrossRef]
- Slušná, M.S.; Smržová, D.; Ecorchard, P.; Tolasz, J.; Motlochová, M.; Jakubec, I.; Maříková, M.; Kormunda, M.; Štengl, V. Photocatalytic activity of Sn-doped ZnO synthesized via peroxide route. J. Phys. Chem. Solids 2022, 160, 110340. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Chen, J.; Duan, X.; Guo, B. Mussel-inspired adhesive antioxidant antibacterial hemostatic composite hydrogel wound dressing via photo-polymerization for infected skin wound healing. Bioact. Mater. 2022, 8, 341–354. [Google Scholar] [CrossRef]
- Azizan, A.; Samsudin, A.A.; Shamshul Baharin, M.B.; Dzulkiflee, M.H.; Rosli, N.R.; Bakar, N.F.A.; Adlim, M. Cellulosic fiber nanocomposite application review with zinc oxide antimicrobial agent nanoparticle: An opt for COVID-19 purpose. Environ. Sci. Pollut. Res. 2022, 30, 16779–16796. [Google Scholar] [CrossRef] [PubMed]
- Rastinfard, A.; Dalisson, B.; Barralet, J. Aqueous decomposition behavior of solid peroxides: Effect of pH and buffer composition on oxygen and hydrogen peroxide formation. Acta Biomater. 2022, 145, 390–402. [Google Scholar] [CrossRef]
- Kuzmin, A.; Pudza, I.; Klementiev, K. In situ study of zinc peroxide decomposition to zinc oxide by X-ray absorption spectroscopy and reverse monte carlo simulations. Solid State Phys. 2022, 259, 2200001. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.; Hsiao, C. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Frechette-Viens, L.; Hadioui, M.; Wilkinson, K.J. Quantification of ZnO nanoparticles and other Zn containing colloids in natural waters using a high sensitivity single particle ICP-MS. Talanta 2019, 200, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Pumera, M. Simultaneous direct voltametric determination of metal-oxide nanoparticles from their mixture (CuO/NiO). ChemElectroChem 2013, 1, 249–253. [Google Scholar] [CrossRef]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In vitro/in vivo toxicity evalution and quantification of iron oxide nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417–24450. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Frey, W.; Emelianov, S. Quantitative photoacoustic imaging of nanoparticles in cells and tissues. ACS Nano 2013, 7, 1272–1280. [Google Scholar] [CrossRef]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M. Experimental study on dielectric relaxation of SiO2 nanoparticle suspensions for developing a particle characterization method based on electrical impedance spectroscopy. Powder Technol. 2015, 281, 200–213. [Google Scholar] [CrossRef]
- Benehkohal, N.P.; Demopoulos, G.P. Electrophoretically self-assembled mixed metal oxide-TiO2 nano-composite film structures for photoelectrochemical energy conversion: Probing of charge recombination and electron transport resistances. J. Power Sources 2013, 240, 667–675. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Lai, E.P.C. Electrochemical impedance spectroscopy of zinc oxide nanoparticles after deposition on screen-printed electrode. J. Nanosci. Nanotechnol. 2021, 21, 5207–5214. [Google Scholar] [CrossRef]
- Ravikumar, C.R.; Kotteeswaran, P.; Murugan, A.; Bheema Raju, V.; Santosh, M.; Nagaswarupa, H.; Prashantha, S.C.; Anil Kumar, M.R.; Gurushantha, K. Electrochemical studies of nano metal oxide reinforced nickel hydroxide materials for energy storage applications. Mater. Today Proc. 2017, 4, 12205–12214. [Google Scholar] [CrossRef]
- Cheng, W.; Compton, R.G. Electrochemical detection of nanoparticles by ‘nano-impact’ methods. Trends Anal. Chem. 2014, 58, 79–89. [Google Scholar] [CrossRef]
- Wang, K.; Lai, E.P.C. Electrochemical oxidation of sodium metabisulfite for sensing zinc oxide nanoparticles deposited on graphite electrode. Chemosensors 2022, 10, 145. [Google Scholar] [CrossRef]
- Sriprasertsuk, S.; Varcoe, J.R.; Crean, C. Reduced Graphene Oxide Fibre Electrodes for Drug Sensing. Available online: https://etextilesconference.files.wordpress.com/2020/10/poster-presentation_ssriprasertsuk_surrey.pdf (accessed on 28 February 2023).
- Pires, B.M.; Silva, D.M.; Visentin, L.C.; Rodrigues, B.L.; Carvalho, N.M.F.; Faria, R.B. Synthesis and characterization of cobalt(III), nickel(II) and copper(II) mononuclear complexes with the ligand 1,3-bis[(2-aminoethyl)amino]-2-propanol and their catalase-like activity. PLoS ONE 2015, 10, 0137926. [Google Scholar] [CrossRef] [PubMed]
- ElNahrawy, A.M.; Haroun, A.A.; Hamadneh, I.; Al-Dujaili, A.H. Conducting cellulose/TiO2 composites by in situ polymerization of pyrrole. Carbohydr. Polym. 2017, 168, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, S.; Liang, Y.; Li, Z.; Wu, S.; Luo, S.; Chang, C.; Cui, Z. 3D N-doped mesoporous carbon/SnO2 with polypyrrole coating layer as high-performance anode material for Li-ion batteries. J. Alloys Compd. 2022, 892, 162083. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC—Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Bahadoran, A.; Baghbadorani, N.B.; Roshan De Lile, J.; Masudy-Panah, S.; Sadeghi, B.; Li, J.; Ramakrishna, S.; Liu, Q.; Janani, B.J.; Fakhri, A. Ag doped Sn3O4 nanostructure and immobilized on hyperbranched polypyrrole for visible light sensitized photocatalytic, antibacterial agent and microbial detection process. J. Photochem. Photobiol. B Biol. 2022, 228, 112393. [Google Scholar] [CrossRef]
- Pasha, A.; Khasim, S.; Darwish, A.A.A.; Hamdalla, T.A.; Al-Ghamdi, S.A. High-performance organic coatings of polypyrrole embedded with manganese iron oxide nanoparticles for corrosion protection of conductive copper surface. J. Inorg. Organomet. Polym. 2022, 32, 499–512. [Google Scholar] [CrossRef]
- Al-hakimi, A.N.; Alminderej, F.; Alhagri, I.A.; Al-Hazmy, S.M.; Farea, M.O.; Abdallah, E.M. Inorganic nanofillers TiO2 nanoparticles reinforced host polymer polypyrrole for microelectronic devices and high-density energy storage systems. J. Mater. Sci. Mater. Electron. 2023, 34, 238. [Google Scholar] [CrossRef]
- Irfan, M.; Aslam, M.; Raza, Z.A. Gamma irradiation protection via flexible polypyrrole coated bismuth oxide nanocomposites. Polym. Bull. 2022, 80, 791–807. [Google Scholar] [CrossRef]
- Gahlot, A.P.S.; Paliwal, A.; Kapoor, A. Theoretical and experimental investigation on SPR gas sensor based on ZnO/polypyrrole interface for ammonia sensing applications. Plasmonics 2022, 17, 1619–1632. [Google Scholar] [CrossRef]
- Lo, M.; Ktari, N.; Gningue-Sall, D.; Madani, A.; Aaron, S.E.; Aaron, J.; Mekhalif, Z.; Delhalle, J.; Chenimi, M.M. Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emerg. Mater. Res. 2020, 3, 815–839. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, R.B. Influence of MnO2 nanoparticles on the optical properties of polypyrrole matrix. Mater. Sci. Semicond. 2022, 139, 106322. [Google Scholar] [CrossRef]
- Fazeli, M.; Alizadeh, M.; Pirsa, S. Nanocomposite film based on gluten modified with heracleum persicum essence/MgO/polypyrrole: Investigation of physicochemical and electrical properties. J. Polym. Environ. 2022, 30, 954–970. [Google Scholar] [CrossRef]
- Gogoi, R.; Singh, A.; Moutam, V.; Sharma, L.; Sharma, K.; Halder, A.; Siril, P.F. Revealing the unexplored effect of residual iron oxide on the photoreforming activities of polypyrrole nanostructures on plastic waste and photocatalytic pollutant degradation. J. Environ. Chem. Eng. 2022, 10, 106649. [Google Scholar] [CrossRef]
- Wang, T.; Yan, L.; He, Y.; Alhassana, S.I.; Gang, H.; Wu, B.; Jin, L.; Wang, H. Application of polypyrrole-based adsorbents in the removal of fluoride: A review. RSC Adv. 2022, 12, 3505–3517. [Google Scholar] [CrossRef]
- Heydari, S.; Asefnejad, A.; Nemati, N.H.; Goodarzi, V.; Vaziri, A. Fabrication of multicomponent cellulose/polypyrrole composed with zinc oxide nanoparticles for improving mechanical and biological properties. React. Funct. Polym. 2022, 170, 105126. [Google Scholar] [CrossRef]
- El Nady, J.; Shokry, A.; Khalil, M.; Ebrahium, S.; Elshaer, A.M.; Anas, M. One-step electrodeposition of a polypyrrole/NiO nanocomposite as a supercapacitor electrode. Sci. Rep. 2022, 12, 3611. [Google Scholar] [CrossRef]
- Mansur, F.A.; Sridewi, N.; Anwar, A.; Anwar, A.; Shahabuddin, S. Polypyrrole-conjugated zinc oxide nanoparticle as antiamoebic drugs against Acanthamoeba castellanii. Mater. Today Proc. 2022, 62, 7077–7081. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Alsareii, S.A.; Harraz, F.A. Highly sensitive and selective non-enzymatic uric acid electrochemical sensor based on novel polypyrrole-carbon black-Co3O4 nanocomposite. Adv. Compos. Mater. 2022, 5, 920–933. [Google Scholar] [CrossRef]
- Shi, X.Y.; Gao, M.H.; Hu, W.W.; Luo, D.; Hu, S.Z.; Huang, T.; Zhang, N.; Wang, Y. Largely enhanced adsorption performance and stability of MXene through in-situ depositing polypyrrole nanoparticles. Sep. Purif. Technol. 2022, 287, 120596. [Google Scholar] [CrossRef]
- Varghese, A.; Devi, K.R.S.; Kausar, F.; Pinheiro, D. Evaluative study on supercapacitance behavior of polyaniline/polypyrrole—Metal oxide based composites electrodes: A review. Mater. Today Chem. 2023, 29, 101424. [Google Scholar] [CrossRef]
- Das, M.; Roy, S. Polypyrrole and associated hybrid nanocomposites as chemiresistive gas sensors: A comprehensive review. Mater. Sci. Semicond. 2021, 121, 105332. [Google Scholar] [CrossRef]
- Chen, D.; Peng, R.; Mo, X.; Jin, Y.; Li, H. Photoelectrochemical sensing of hydrogen peroxide and inhibition of breast cancer cell growth based on a porphyrin derivative modified TiO2 composite. Surf. Interfaces 2023, 36, 102569. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, M.; Sun, Q.; Wang, D.; Li, C. Recent advances in tetrakis (4-carboxyphenyl) porphyrin-based nanocomposites for tomor therapy. Adv. Biomed. Res. 2022, 3, 2200136. [Google Scholar]
- Deng, W.; He, L.; Chen, E.; Wang, G.; Ye, X.; Fu, Z.; Lin, Q.; Xu, G. Crystalline microporous small molecule semiconductors based on porphyrin for high-performance chemiresistive gas sensing. J. Mater. Chem. A 2022, 24, 12977–12983. [Google Scholar] [CrossRef]
- Liu, M.; Yan, X.; Xing, Y.; Xu, Z.; Liu, Y.; Zhao, P.; Zhu, Y.; Lu, N.; Zhai, S.; Zhang, Z.; et al. A novel handy polymerized copper porphyrin sensor detects bases simultaneously. J. Electroanal. Chem. A 2023, 931, 117171. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E. Sensors based on biomimetic porphyrin derivatives and their hybrid combinations. Res. Rev. Electrochem. 2018, 9, 111. [Google Scholar]
- Gao, J.; Sun, X.; Liu, Y.; Niu, B.; Chen, Q.; Fang, X. Ultrathin metal-organic framework nanosheet (Cu-TCPP)-based isothermal nucleic acid amplification for food allergen detection. Food Sci. Hum. Wellness A 2023, 12, 1788–1798. [Google Scholar] [CrossRef]
- Cai, J.; Song, S.; Zhu, L.; Lu, Q.; Lu, Z.; Wei, Y.; Wang, H. Two-dimensional Cu-porphyrin nanosheet membranes for nanofiltration. Nano Res. 2023, 16, 6290–6297. [Google Scholar] [CrossRef]
- Wu, G.; Shen, H.; Li, J.; Guo, J.; Yin, X.; Mu, M. Syntheses of ZnTi-LDH sensitized by tetra (4-carboxyphenyl) porphyrin for accelating photocatalytic reduction of carbon dixoide. J. Solid State Chem. A 2022, 309, 122955. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Sun, X.; Hao, S.; Zhao, P.; Zhu, X.; Dong, S. Bifunctional bio-photoelectrochemical cells: A “trading” platform for simultaneous production of electric power and hydrogen peroxide. J. Mater. Chem. A 2023, 2, 600–608. [Google Scholar] [CrossRef]
- Tu, W.; Lei, J.; Wang, P.; Ju, H. Photoelectrochemistry of free-base-porphyrin-functionalized zinc oxide nanoparticles and their applications in biosensing. Chem. Eur. J. 2011, 17, 9440–9447. [Google Scholar] [CrossRef] [PubMed]
- Ekrami, M.; Magna, G.; Emam-djomeh, Z.; Saeed Yarmand, M.; Paolesse, R.; Di Natale, C. Porphyrin-functionalized zinc oxide nanostructures for sensor applications. Sensors 2018, 18, 2279. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, J.; Feng, L.; Zhang, Y.; Liang, L.; Xing, W.; Liu, C. Photoelectrochemical biofuel cell using porphyrin-sensitized nanocrystalline titanium dioxide mesoporous film as photoanode. Biosens. Bioelectron. 2012, 32, 177–182. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Geng, J.; Zhu, J.; Jiang, Z. Improving visible-light photocatalytic activity of N-doped TiO2 nanoparticles via sensitization by Zn porphyrin. Appl. Surf. Sci. 2008, 255, 2879–2884. [Google Scholar] [CrossRef]
- Peshoria, S.; Narula, A.K. One-pot synthesis of porphyrin@polypyrrole hybrid and its application as an electrochemical sensor. J. Mater. Sci. Eng. B 2018, 229, 53–58. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Lai, E.P.C. Polypyrrole modified stainless steel frits for on-line micro solid phase extraction of ochratoxin A. Anal. Bioanal. Chem. 2005, 381, 948–952. [Google Scholar] [CrossRef]
- Hamidian, K.; Sarani, M.; Sheikhi, E.; Khatami, M. Cytotoxicity evaluation of green synthesized ZnO and Ag-doped ZnO nanoparticles on brain glioblastoma cells. J. Mol. Struct. 2022, 1251, 131962. [Google Scholar] [CrossRef]
- Kareem, M.A.; Bello, I.T.; Shittu, H.A.; Sivaprakash, P.; Adedokun, O.; Arumugam, S. Synthesis, characterization, and photocatalytic application of silver doped zinc oxide nanoparticles. Clean. Mater. 2022, 3, 100041. [Google Scholar] [CrossRef]
- Bembibre, A.; Benamara, M.; Hjiri, M.; Gómez, E.; Alamri, H.R.; Dhahri, R.; Serrà, A. Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. J. Chem. Eng. 2022, 427, 132006. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef] [PubMed]
- Al-Namshah, K.S.; Shkir, M.; Ibrahim, F.A.; Hamdy, M.S. Auto combustion synthesis and characterization of Co doped ZnO nanoparticles with boosted photocatalytic performance. Phys. B Condens. Matter 2022, 625, 413459. [Google Scholar] [CrossRef]
- Vasudevan, J.; Jeyakumar, S.J.; Arunkumar, B.; Jothibas, M.; Muthuvel, A.; Vijayalakshmi, S. Optical and magnetic investigation of Cu doped ZnO nanoparticles synthesized by solid state method. Mater. Today Proc. 2022, 48, 438–442. [Google Scholar] [CrossRef]
- Fathima, A.F.; Mani, R.J.; Roshan, M.M.; Sakthipandi, K. Enhancing structural and optical properties of ZnO nanoparticles induced by the double co-doping of iron and cobalt. Mater. Today Proc. 2022, 49, 2598–2601. [Google Scholar] [CrossRef]
- Kumawat, A.; Chattopadhyay, S.; Verma, R.K.; Misra, K.P. Eu doped ZnO nanoparticles with strong potential of thermal sensing and bioimaging. Mater. Lett. 2022, 308, 131221. [Google Scholar] [CrossRef]
- Islam, M.M.; Yoshida, T.; Fujita, Y. Effects of ambience on thermal-diffusion type Ga-doping process for ZnO nanoparticles. Coatings 2022, 12, 57. [Google Scholar] [CrossRef]
- Ayon, S.A.; Jamal, M.; Billah, M.M.; Neaz, S. Augmentation of magnetic properties and antimicrobial activities of band gap modified Ho3+ and Sm3+ doped ZnO nanoparticles: A comparative experimental study. J. Alloys Compd. 2022, 897, 163179. [Google Scholar] [CrossRef]
- Haghighat, M.; Alijani, H.Q.; Ghasemi, M.; Khosravi, S.; Borhani, F.; Sharifi, F.; Iravani, S.; Najafi, K.; Khatami, M. Cytotoxicity properties of plant-mediated synthesized K-doped ZnO nanostructures. Bioprocess Biosyst. Eng. 2022, 45, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, K.; Sarani, M.; Barani, M.; Khakbaz, F. Cytotoxic performance of green synthesized Ag and Mg dual doped ZnO nanoparticles using Salvadora persica extract against MDA-MB-231 and MCF-10 cells. Arab. J. Chem. 2022, 15, 103792. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; Yadav, S.P.; Behera, P.K.; AlFaify, S. Facile synthesis of Mn-doped ZnO nanoparticles by flash combustion route and their characterizations for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 3849–3869. [Google Scholar] [CrossRef]
- Saleem, S.; Jameel, M.H.; Akhtar, N.; Nazir, N.; Ali, A.; Zaman, A.; Rehman, A.; Butt, S.; Sultana, F.; Mushtaq, M.; et al. Modification in structural, optical, morphological, and electrical properties of zinc oxide nanoparticles by metal dopants for electronic device applications. Arab. J. Chem. 2022, 15, 103518. [Google Scholar] [CrossRef]
- Shkir, M.; Palanivel, B.; Khan, A.; Kumar, M.; Chang, J.H.; Mani, A.; AlFaify, S. Enhanced photocatalytic activities of facile auto-combustion synthesized ZnO nanoparticles for wastewater treatment: An impact of Ni doping. Chemosphere 2022, 291, 132687. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Kosari-Nasab, M.; Divband, B.; Mahjouri, S.; Movafeghi, A. Comparison of the toxicity of pure and samarium-doped zinc oxide nanoparticles to the green microalga Chlorella vulgaris. Environ. Sci. Pollut. Res. 2022, 29, 32002–32015. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sakthivel, P.; Sankaranarayanan, R.K. Influence of Sn4+ ion on band gap tailoring, optical, structural and dielectric behaviors of ZnO nanoparticles. Spectrochim. Acta A Mol. Biomol. 2022, 267, 120487. [Google Scholar] [CrossRef]
- Zandsalimi, Y.; Maleki, A.; Shahmoradi, B.; Dehestani, S.; Rezaee, R.; McKay, G. Photocatalytic removal of 2,4-dichlorophenoxyacetic acid from aqueous solution using tungsten oxide doped zinc oxide nanoparticles immobilised on glass beads. Environ. Technol. 2022, 43, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.I.D.L.; Villegas, V.A.R.; Sicairos, S.P.; Guevara, E.H.; Brito Perea, M.D.C.; Sánchez, B.L. Synthesis and characterization of zinc peroxide nanoparticles for the photodegradation of nitrobenzene assisted by UV light. Catalysts 2020, 10, 1041. [Google Scholar] [CrossRef]
- Pare, A.; Ghosh, S.K. Temperature dependent rheological behavior of zinc oxide based water nanofluid. In Proceedings of the 25th National and 3rd International ISHMT-ASTFE Heat and Mass Transfer Conference 2019, Roorkee, India, 28–31 December 2019; pp. 317–321. [Google Scholar]
- Brief Introduction to Coating Technology for Electron Microscopy. 2013. Available online: https://www.leica-microsystems.com/science-lab/brief-introduction-to-coating-technology-for-electron-microscopy/ (accessed on 28 February 2023).
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-zinc oxide nanocomposite for electrochemical supercapacitor applications. J. Sci. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New direction in screen printed electroanalytical sensors: And overview of recent development. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Prathna, T.C.; Trivedi, S.; Myneni, R.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat. Toxicol. 2013, 132–133, 34–45. [Google Scholar] [CrossRef]
- Roman, L.E.; Maurtua, D.; Paraguay-Delgado, F.; Solis, J.L.; Gómez, M.M. Green synthesis of ZnO2 nanoparticles and their annealing transformation into ZnO nanoparticles: Characterization and antimicrobial activity. J. Nanosci. Nanotechnol. 2016, 16, 9889–9895. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Bradley, M.; Megson, I.L.; MacNee, W.; Lee, J.K.; Jeong, J.; Donaldson, K. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part. Fibre Toxicol. 2013, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- El-Shamy, A.G. New carbon quantum dots nano-particles decorated zinc peroxide nano-composite with superior photocatalytic efficiency for removal of different dyes under UV-A light. Synth. Met. 2020, 267, 116472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).