Biosensing for Autoimmune Chronic Disease—A Review
Abstract
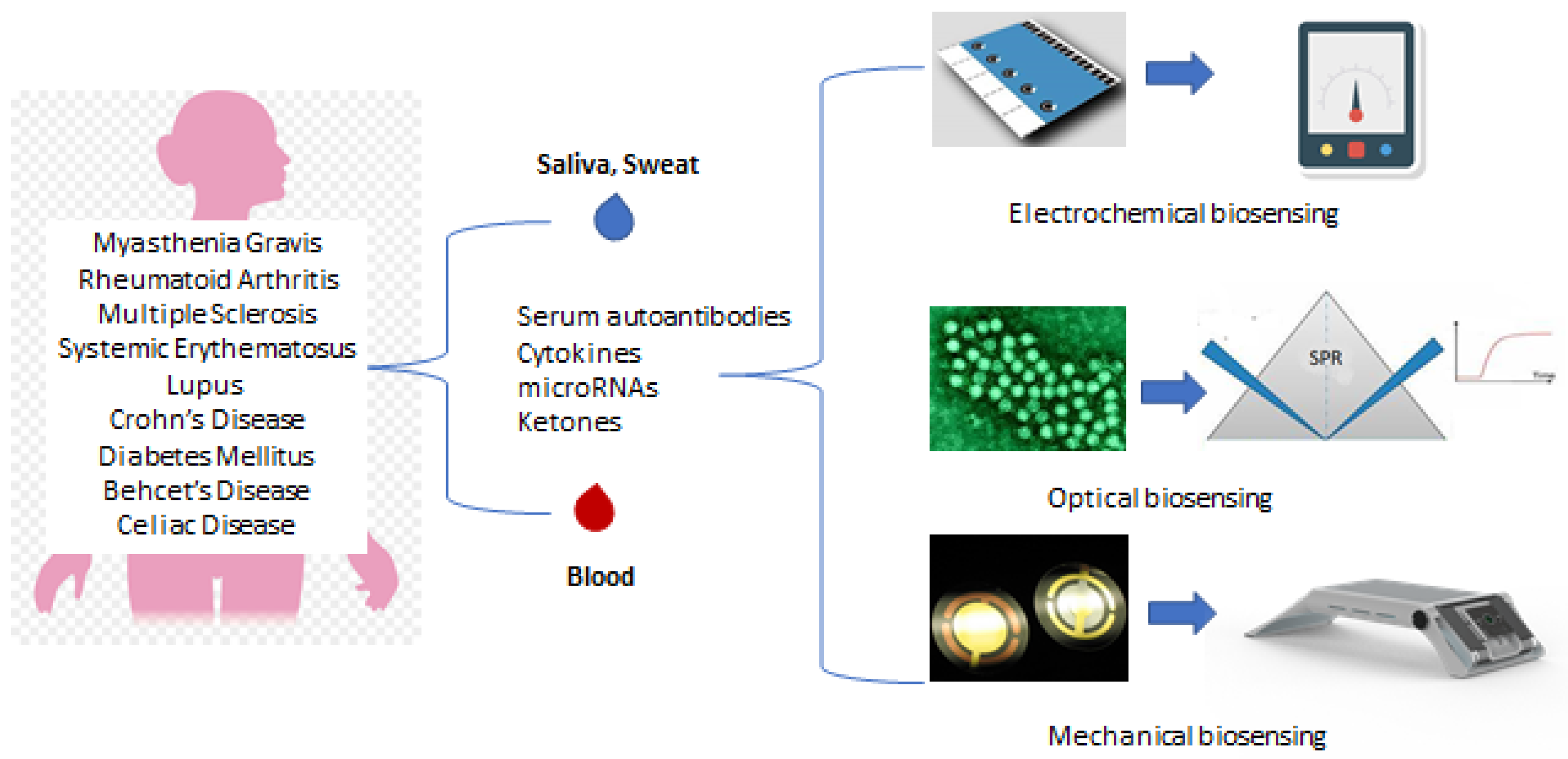
1. Introduction
2. Biosensors
2.1. Categories
2.1.1. Electrochemical
2.1.2. Optical Biosensors
2.1.3. Mechanical Biosensors
3. Nanomaterials in Biosensors
4. Bioelement Immobilization
5. Chronic Diseases and Biosensors
6. Biosensors for Autoimmune Diseases Based on Antibodies, Antigens and Peptides
7. Discussion and General Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tozzoli, R. The diagnostic role of autoantibodies in the prediction of organ-specific autoimmune diseases. Clin. Chem. Lab. Med. 2008, 46, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Magro, R.; Borg, A.A. Characterisation of Patients with Systemic Lupus Erythematosus in Malta: A Population Based Cohort Cross-Sectional Study. Biomed. Res. Int. 2018, 2018, 2385386. [Google Scholar] [CrossRef]
- Anstey, N.M.; Bastian, I.; Dunckley, H.; Currie, B.J. Systemic lupus erythematosus in Australian Aborigines: High prevalence, morbidity and mortality. Aust. N. Z. J. Med. 1993, 23, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.W.; Falasinnu, T.; Ramsey-Goldman, R.; Clarke, A.E. The global epidemiology of SLE: Narrowing the knowledge gaps. Rheumatology 2023, 62, i4–i9. [Google Scholar] [CrossRef]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef]
- Zhang, X.; Zambrano, A.; Lin, Z.-T.; Xing, Y.; Rippy, J.; Wu, T. Immunosensors for Biomarker Detection in Autoimmune Diseases. Arch. Immunol. Ther. Exp. 2017, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; Roggenbuck, D.; Reinhold, D.; Sack, U. Autoantibody diagnostics in clinical practice. Autoimmun. Rev. 2012, 11, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Pedrero, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensors for autoantibodies in autoimmune and cancer diseases. Anal. Methods 2019, 11, 871–887. [Google Scholar] [CrossRef]
- Thaler, M.; Buhl, A.; Welter, H.; Schreiegg, A.; Kehrel, M.; Alber, B.; Metzger, J.; Luppa, P.B. Biosensor analyses of serum autoantibodies: Application to antiphospholipid syndrome and systemic lupus erythematosus. Anal. Bioanal. Chem. 2009, 393, 1417–1429. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.R.; Cumba, R.L. Optimizing Glucose Sensing for Diabetes Monitoring. In Electronic and Optical Materials, Bioelectronics and Medical Devices; Pal, K., Kraatz, H.-B., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 765–778. [Google Scholar] [CrossRef]
- Jones, A.; Dhanapala, L.; Kankanamage, R.N.T.; Kumar, C.V.; Rusling, J.F. Multiplexed Immunosensors and Immunoarrays. Anal. Chem. 2020, 92, 345–362. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Jiang, X.; Li, D.; Xu, X.; Ying, Y.; Li, Y.; Ye, Z.; Wang, J. Immunosensors for detection of pesticide residues. Biosens. Bioelectron. 2008, 23, 1577–1587. [Google Scholar] [CrossRef]
- Gauglitz, G. Direct optical sensors: Principles and selected applications. Anal. Bioanal. Chem. 2005, 381, 141–155. [Google Scholar] [CrossRef]
- Leca-Bouvier, B.; Blum, L.J. Biosensors for Protein Detection: A Review. Anal. Lett. 2005, 38, 1491–1517. [Google Scholar] [CrossRef]
- Mascini, M.; Tombelli, S. Biosensors for biomarkers in medical diagnostics. Biomarkers 2008, 13, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Spain, E.; Keyes, T.E.; Forster, R.J. DNA sensor based on vapour polymerised pedot films functionalised with gold nanoparticles. Biosens. Bioelectron. 2013, 41, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, J.; Liu, J.; Wang, X.; Chen, B. Disease-Related Detection with Electrochemical Biosensors: A Review. Sensors 2017, 17, 2375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Li, P.; Yi, W.; Zhang, Z.; Chen, S.; Su, S.; Zhao, L.; Hu, C. Development of a novel method to measure macrophage migration inhibitory factor (MIF) in sera of patients with rheumatoid arthritis by combined electrochemical immunosensor. Int. Immunopharmacol. 2008, 8, 859–865. [Google Scholar] [CrossRef]
- de Gracia Villa, M.; Jiménez-Jorquera, C.; Haro, I.; Gomara, M.J.; Sanmartí, R.; Fernández-Sánchez, C.; Mendoza, E. Carbon nanotube composite peptide-based biosensors as putative diagnostic tools for rheumatoid arthritis. Biosens. Bioelectron. 2011, 27, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Fagúndez, P.; Brañas, G.; Cairoli, E.; Laíz, J.; Tosar, J.P. An electrochemical biosensor for rapid detection of anti-dsDNA antibodies in absolute scale. Analyst 2018, 143, 3874–3882. [Google Scholar] [CrossRef]
- Bhavsar, K.; Fairchild, A.; Alonas, E.; Bishop, D.K.; La Belle, J.T.; Sweeney, J.; Alford, T.; Joshi, L. A cytokine immunosensor for Multiple Sclerosis detection based upon label-free electrochemical impedance spectroscopy using electroplated printed circuit board electrodes. Biosens. Bioelectron. 2009, 25, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Derkus, B.; Emregul, E.; Yucesan, C.; Emregul, K.C. Myelin basic protein immunosensor for multiple sclerosis detection based upon label-free electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013, 46, 53–60. [Google Scholar] [CrossRef]
- Derkus, B.; Bozkurt, P.A.; Tulu, M.; Emregul, K.C.; Yucesan, C.; Emregul, E. Simultaneous quantification of Myelin Basic Protein and Tau proteins in cerebrospinal fluid and serum of Multiple Sclerosis patients using nanoimmunosensor. Biosens. Bioelectron. 2017, 89, 781–788. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Chapter One—Advances in celiac disease testing. Adv. Clin. Chem. 2019, 91, 1–29. [Google Scholar] [CrossRef]
- Gupta, S.; Kaushal, A.; Kumar, A.; Kumar, D. Ultrasensitive transglutaminase based nanosensor for early detection of celiac disease in human. Int. J. Biol. Macromol. 2017, 105, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.M.; González-García, M.B.; Nouws, H.P.; Costa-García, A. Celiac disease detection using a transglutaminase electrochemical immunosensor fabricated on nanohybrid screen-printed carbon electrodes. Biosens. Bioelectron. 2012, 31, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Mazzanti, B.; Pazzagli, M.; Peroni, E.; Alcaro, M.C.; Sabatino, G.; Lanzillo, R.; Morra, V.B.; Santoro, L.; Gasperini, C.; et al. The glycopeptide CSF114(Glc) detects serum antibodies in multiple sclerosis. J. Neuroimmunol. 2005, 167, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bellagha-Chenchah, W.; Sella, C.; Fernandez, F.R.; Peroni, E.; Lolli, F.; Amatore, C.; Thouin, L.; Papini, A. Interactions between Human Antibodies and Synthetic Conformational Peptide Epitopes: Innovative Approach for Electrochemical Detection of Biomarkers of Multiple Sclerosis at Platinum Electrodes. Electrochim. Acta 2015, 176, 1239–1247. [Google Scholar] [CrossRef]
- Abolhasan, R.; Mehdizadeh, A.; Rashidi, M.R.; Aghebati-Maleki, L.; Yousefi, M. Application of hairpin DNA-based biosensors with various signal amplification strategies in clinical diagnosis. Biosens. Bioelectron. 2019, 129, 164–174. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, Y.; Pan, J.; Zhao, Y.; Chen, Y.; Ren, X.; Ma, H.; Wei, Q.; Du, B. A photoelectrochemical biosensor for fibroblast-like synoviocyte cell using visible light-activated NCQDs sensitized-ZnO/CH3NH3PbI3 heterojunction. Biosens. Bioelectron. 2016, 77, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Application of various optical and electrochemical aptasensors for detection of human prostate specific antigen: A review. Biosens. Bioelectron. 2019, 142, 111484. [Google Scholar] [CrossRef] [PubMed]
- Damborsky, P.; Svitel, J.; Katrlik, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [PubMed]
- Liu, S.; Tong, Z.; Mu, X.; Liu, B.; Du, B.; Liu, Z.; Gao, C. Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors 2018, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Li, X.; Wang, H.; Zhang, Y.; Ma, H.; Wei, Q. Label-free ECL immunosensor for the early diagnosis of rheumatoid arthritis based on asymmetric heterogeneous polyaniline-gold nanomaterial. Sens. Actuators B Chem. 2018, 257, 354–361. [Google Scholar] [CrossRef]
- Mansourian, N.; Rahaie, M.; Hosseini, M. A Nanobiosensor Based on Fluorescent DNA-Hosted Silver Nanocluster and HCR Amplification for Detection of MicroRNA Involved in Progression of Multiple Sclerosis. J. Fluoresc. 2017, 27, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hao, K.; Tian, Y.; Jin, S.; Lu, H.; Zhou, S.-F.; Zhang, X. Label-free and ultrasensitive microRNA detection based on novel molecular beacon binding readout and target recycling amplification. Biosens. Bioelectron. 2014, 53, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Campu, A.; Susu, L.; Orzan, F.; Maniu, D.; Craciun, A.M.; Vulpoi, A.; Roiban, L.; Focsan, M.; Astilean, S. Multimodal Biosensing on Paper-Based Platform Fabricated by Plasmonic Calligraphy Using Gold Nanobypiramids Ink. Front. Chem. 2019, 7, 55. [Google Scholar] [CrossRef]
- Susu, L.; Campu, A.; Astilean, S.; Focsan, M. Calligraphed Selective Plasmonic Arrays on Paper Platforms for Complementary Dual Optical “ON/OFF Switch” Sensing. Nanomaterials 2020, 10, 1025. [Google Scholar] [CrossRef]
- Itoh, T.; Procházka, M.; Dong, Z.-C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a New Era of SERS and TERS at the Nanometer Scale: From Fundamentals to Innovative Applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef]
- Debreczeni, M.L.; Szekacs, I.; Kovacs, B.; Saftics, A.; Kurunczi, S.; Gál, P.; Dobó, J.; Cervenak, L.; Horvath, R. Human primary endothelial label-free biochip assay reveals unpredicted functions of plasma serine proteases. Sci. Rep. 2020, 10, 3303. [Google Scholar] [CrossRef]
- Avram, L.; Iancu, S.D.; Stefancu, A.; Moisoiu, V.; Colnita, A.; Marconi, D.; Donca, V.; Buzdugan, E.; Craciun, R.; Leopold, N.; et al. SERS-Based Liquid Biopsy of Gastrointestinal Tumors Using a Portable Raman Device Operating in a Clinical Environment. J. Clin. Med. 2020, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef]
- Vega, B.; Calle, A.; Sánchez, M.A.; Lechuga, L.M.; Ortiz, A.M.; Armelles, G.; Rodríguez-Frade, J.M.; Mellado, M. Real-time detection of the chemokine CXCL12 in urine samples by surface plasmon resonance. Talanta 2013, 109, 209–215. [Google Scholar] [CrossRef]
- Sguassero, A.; Artiga, Á.; Morasso, C.; Jimenez, R.R.; Rapún, R.M.; Mancuso, R.; Agostini, S.; Hernis, A.; Abols, A.; Linē, A.; et al. A simple and universal enzyme-free approach for the detection of multiple microRNAs using a single nanostructured enhancer of surface plasmon resonance imaging. Anal. Bioanal. Chem. 2019, 411, 1873–1885. [Google Scholar] [CrossRef]
- Yerrapragada, R.M.; Mampallil, D. Interferon-gamma detection in point of care diagnostics: Short review. Talanta 2022, 245, 123428. [Google Scholar] [CrossRef]
- Breese, E.; Braegger, C.P.; Corrigan, C.J.; Walker-Smith, J.A.; MacDonald, T.T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology 1993, 78, 127–131. [Google Scholar]
- Manivasagam, S.; Williams, J.L.; Vollmer, L.L.; Bollman, B.; Bartleson, J.M.; Ai, S.; Wu, G.F.; Klein, R.S. Targeting IFN-lambda Signaling Promotes Recovery from Central Nervous System Autoimmunity. J. Immunol. 2022, 208, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, S.; Nahak, S.K.; Padhi, S.; Pradhan, B.; Nayak, N.; Pati, A.; Panda, A.K. Interferon-gamma (IFN-γ) intronic variant (rs2430561) is a risk factor for systemic lupus erythematosus: Observation from a meta-analysis. Lupus 2023, 32, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Šípová, H.; Ševců, V.; Kuchař, M.; Ahmad, J.N.; Mikulecký, P.; Osička, R.; Malý, P.; Homola, J. Surface plasmon resonance biosensor based on engineered proteins for direct detection of interferon-gamma in diluted blood plasma. Sens. Actuators B Chem. 2012, 174, 306–311. [Google Scholar] [CrossRef]
- Šípová, H.; Ševců, V.; Kuchař, M.; Ahmad, J.N.; Mikulecký, P.; Šebo, P.; Malý, P.; Homola, J. Sensitive Detection of Interferon-Gamma with Engineered Proteins and Surface Plasmon Resonance Biosensor. Procedia Eng. 2011, 25, 940–943. [Google Scholar] [CrossRef]
- Stigter, E.C.; de Jong, G.J.; van Bennekom, W.P. An improved coating for the isolation and quantitation of interferon-gamma in spiked plasma using surface plasmon resonance (SPR). Biosens. Bioelectron. 2005, 21, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-L.; Chang, C.-C.; Chu-Su, Y.; Wei, S.-C.; Zhao, X.-H.; Hsueh, P.-R.; Lin, C.-W. Disposable surface plasmon resonance aptasensor with membrane-based sample handling design for quantitative interferon-gamma detection. Lab Chip 2014, 14, 2968–2977. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Golfinopoulou, R.; Papageorgiou, L.; Efthimiadou, A.; Bacopoulou, F.; Chrousos, G.P.; Eliopoulos, E.; Vlachakis, D. Clinical Genomic, phenotype and epigenetic insights into the pathology, autoimmunity and weight management of patients with Myasthenia Gravis (Review). Mol. Med. Rep. 2021, 24, 512. [Google Scholar] [CrossRef]
- Tamayo, J.; Kosaka, P.M.; Ruz, J.J.; Paulo, Á.S.; Calleja, M. Biosensors based on nanomechanical systems. Chem. Soc. Rev. 2013, 42, 1287–1311. [Google Scholar] [CrossRef]
- Arlett, J.; Myers, E.; Roukes, M. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef]
- Drouvalakis, K.A.; Bangsaruntip, S.; Hueber, W.; Kozar, L.G.; Utz, P.J.; Dai, H. Peptide-coated nanotube-based biosensor for the detection of disease-specific autoantibodies in human serum. Biosens. Bioelectron. 2008, 23, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, K.L. Electromechanical Transducers at the Nanoscale: Actuation and Sensing of Motion in Nanoelectromechanical Systems (NEMS). Small 2005, 1, 786–797. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Melinte, G.; Simon, I.; Cristea, C. Electrochemical Biosensors as Potential Diagnostic Devices for Autoimmune Diseases. Biosensors 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Tang, X.; Bagarozzi, D.A., Jr.; Pan, D.; Locascio, L.; Walker, A.; Barron, F.; et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88. [Google Scholar] [CrossRef]
- Cernat, A.; Tertiș, M.; Păpară, C.N.; Bodoki, E.; Săndulescu, R. Nanostructured Platform Based on Graphene-polypyrrole Composite for Immunosensor Fabrication. Procedia Technol. 2017, 27, 108–109. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Wang, L.; Xie, H.; Lin, Y.; Wang, M.; Sha, L.; Yu, X.; Yang, J.; Zhao, J.; Li, G. Covalent organic frameworks (COFs)-based biosensors for the assay of disease biomarkers with clinical applications. Biosens. Bioelectron. 2022, 217, 114668. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Rizwan, K. Metal-organic frameworks based hybrid nanocomposites as state-of–the-art analytical tools for electrochemical sensing applications. Biosens. Bioelectron. 2022, 199, 113867. [Google Scholar] [CrossRef] [PubMed]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. A fully integrated electrochemical biosensor platform fabrication process for cytokines detection. Biosens. Bioelectron. 2017, 93, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Balkenhohl, T.; Lisdat, F. Screen-printed electrodes as impedimetric immunosensors for the detection of anti-transglutaminase antibodies in human sera. Anal. Chim. Acta 2007, 597, 50–57. [Google Scholar] [CrossRef]
- Taleat, Z.; Ravalli, A.; Mazloum-Ardakani, M.; Marrazza, G. CA 125 Immunosensor Based on Poly-Anthranilic Acid Modified Screen-Printed Electrodes. Electroanalysis 2012, 25, 269–277. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Bioaffinity Immobilization. In Immobilization of Enzymes and Cells; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 107–116. [Google Scholar]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef]
- Asal, M.; Özen, Ö.; Şahinler, M.; Baysal, H.T.; Polatoğlu, I. An overview of biomolecules, immobilization methods and support materials of biosensors. Sens. Rev. 2019, 39, 377–386. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Kutcherlapati, S.R.; Arunbabu, D.; Jana, T. Chapter Seven—Armored Urease: Enzyme-Bioconjugated Poly(acrylamide) Hydrogel as a Storage and Sensing Platform. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 143–167. [Google Scholar]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of Enzymes: A Literature Survey. Methods Mol. Biol. 2013, 1051, 15–31. [Google Scholar] [CrossRef]
- Fraas, R.; Franzreb, M. Reversible covalent enzyme immobilization methods for reuse of carriers. Biocatal. Biotransform. 2017, 35, 337–348. [Google Scholar] [CrossRef]
- Liébana, S.; Drago, G.A. Bioconjugation and stabilisation of biomolecules in biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2016, 1, 166–177. [Google Scholar] [CrossRef]
- Prieto-Simon, B.; Campas, M.; Marty, J.-L. Biomolecule Immobilization in Biosensor Development: Tailored Strategies Based on Affinity Interactions. Protein Pept. Lett. 2008, 15, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Fabrication of Electrochemical Immunosensor for Interferon-γ Determination and Its Application of Tuberculosis Diagnosis. Int. J. Electrochem. Sci. 2017, 12, 7262–7271. [Google Scholar] [CrossRef]
- Yan, G.; Wang, Y.; He, X.; Wang, K.; Liu, J.; Du, Y. A highly sensitive label-free electrochemical aptasensor for interferon-gamma detection based on graphene controlled assembly and nuclease cleavage-assisted target recycling amplification. Biosens. Bioelectron. 2013, 44, 57–63. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, J.; Dai, H.; Li, J.; Shen, J.; Jiao, X.; Hu, X.; Ju, H. Graphene oxide based ultrasensitive flow-through chemiluminescent immunoassay for sub-picogram level detection of chicken interferon-gamma. Biosens. Bioelectron. 2014, 51, 356–361. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Yazdian-Robati, R.; Abnous, K. An amplified fluorescent aptasensor based on single-stranded DNA binding protein, copper and silica nanoparticles for sensitive detection of interferon-gamma. Anal. Chim. Acta 2017, 984, 162–167. [Google Scholar] [CrossRef]
- Wen, D.; Liu, Q.; Cui, Y.; Kong, J.; Yang, H.; Liu, Q. DNA based click polymerization for ultrasensitive IFN-γ fluorescent detection. Sens. Actuators B Chem. 2018, 276, 279–287. [Google Scholar] [CrossRef]
- Jeong, H.-H.; Erdene, N.; Park, J.-H.; Jeong, D.-H.; Lee, H.-Y.; Lee, S.-K. Real-time label-free immunoassay of interferon-gamma and prostate-specific antigen using a Fiber-Optic Localized Surface Plasmon Resonance sensor. Biosens. Bioelectron. 2013, 39, 346–351. [Google Scholar] [CrossRef]
- Jiang, J.; He, Y.; Yu, X.; Zhao, J.; Cui, H. A homogeneous hemin/G-quadruplex DNAzyme based turn-on chemiluminescence aptasensor for interferon-gamma detection via in-situ assembly of luminol functionalized gold nanoparticles, deoxyribonucleic acid, interferon-gamma and hemin. Anal. Chim. Acta 2013, 791, 60–64. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, Y.; Wen, Q.; Li, J.; Yang, P. Dynamic evaluation of cell-secreted interferon gamma in response to drug stimulation via a sensitive electro-chemiluminescence immunosensor based on a glassy carbon electrode modified with graphene oxide, polyaniline nanofibers, magnetic beads, and gold nanoparticles. Microchim. Acta 2016, 183, 1739–1748. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Guang, Z.; Miao, T.; Ali, Z.; Qiao, D.; Yao, Y.; Wu, K.; Zhou, L.; Meng, C.; et al. Ultra-High Sensitivity Terahertz Microstructured Fiber Biosensor for Diabetes Mellitus and Coronary Heart Disease Marker Detection. Sensors 2023, 23, 2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lindley-Hatcher, H.; Chen, X.; Pickwell-MacPherson, E. THz Sensing of Human Skin: A Review of Skin Modeling Approaches. Sensors 2021, 21, 3624. [Google Scholar] [CrossRef]
- Habib, M.A.; Anower, M.S.; Abdulrazak, L.F.; Reza, M.S. Hollow core photonic crystal fiber for chemical identification in terahertz regime. Opt. Fiber Technol. 2019, 52, 101933. [Google Scholar] [CrossRef]
- Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Wearable non-invasive monitors of diabetes and hypoxia through continuous analysis of sweat. Talanta 2020, 215, 120922. [Google Scholar] [CrossRef]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef]
- Karpova, E.V.; Shcherbacheva, E.V.; Galushin, A.A.; Vokhmyanina, D.V.; Karyakina, E.E.; Karyakin, A.A. Noninvasive Diabetes Monitoring through Continuous Analysis of Sweat Using Flow-Through Glucose Biosensor. Anal. Chem. 2019, 91, 3778–3783. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Liu, Y.; Mugo, S.M.; Zhang, Q. Integrated Wearable Sensors for Sensing Physiological Pressure Signals and β-Hydroxybutyrate in Physiological Fluids. Anal. Chem. 2022, 94, 993–1002. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, L.; Su, G.; Kijlstra, A.; Yang, P. Specific sweat metabolite profile in ocular Behcet’s disease. Int. Immunopharmacol. 2021, 97, 107812. [Google Scholar] [CrossRef] [PubMed]
- Hladek, M.; Gill, J.M.; Lai, C.; Bandeen-Roche, K.; Xue, Q.-L.; Allen, J.; Leyden, C.; Kanefsky, R.; Szanton, S.L. High Social Coping Self-Efficacy Associated With Lower Sweat Interleukin-6 in Older Adults With Chronic Illness. J. Appl. Gerontol. 2021, 41, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, R.; Yao, W.; Matteini, A.; Beamer, B.A.; Xue, Q.-L.; Yang, H.; Manwani, B.; Reiner, A.; Jenny, N.; Parekh, N.; et al. Simple Biologically Informed Inflammatory Index of Two Serum Cytokines Predicts 10 Year All-Cause Mortality in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15.e1, Erratum in 2017, 181, S1–S3. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Kim, J.; de Araujo, W.R.; Samek, I.A.; Bandodkar, A.J.; Jia, W.; Brunetti, B.; Paixão, T.R.; Wang, J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 2015, 51, 41–45. [Google Scholar] [CrossRef]
- Sato, K.; Leidal, R.; Sato, F. Morphology and development of an apoeccrine sweat gland in human axillae. Am. J. Physiol. Content 1987, 252 Pt 2, R166–R180. [Google Scholar] [CrossRef]
- Huynh, V.L.; Trung, T.Q.; Meeseepong, M.; Lee, H.; Nguyen, T.D.; Lee, N. Hollow Microfibers of Elastomeric Nanocomposites for Fully Stretchable and Highly Sensitive Microfluidic Immunobiosensor Patch. Adv. Funct. Mater. 2020, 30, 2004684. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- An, J.E.; Kim, K.H.; Park, S.J.; Seo, S.E.; Kim, J.; Ha, S.; Bae, J.; Kwon, O.S. Wearable Cortisol Aptasensor for Simple and Rapid Real-Time Monitoring. ACS Sens. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Bae, C.W.; Toi, P.T.; Kim, B.Y.; Lee, W.I.; Lee, H.B.; Hanif, A.; Lee, E.H.; Lee, N.-E. Fully Stretchable Capillary Microfluidics-Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [Google Scholar] [CrossRef]
- Santiago-Malagón, S.; Río-Colín, D.; Azizkhani, H.; Aller-Pellitero, M.; Guirado, G.; del Campo, F.J. A self-powered skin-patch electrochromic biosensor. Biosens. Bioelectron. 2021, 175, 112879. [Google Scholar] [CrossRef]
- Neves, M.M.; González-García, M.B.; Delerue-Matos, C.; Costa-García, A. Multiplexed electrochemical immunosensor for detection of celiac disease serological markers. Sens. Actuators B Chem. 2013, 187, 33–39. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A double signal electrochemical human immunoglobulin G immunosensor based on gold nanoparticles-polydopamine functionalized reduced graphene oxide as a sensor platform and AgNPs/carbon nanocomposite as signal probe and catalytic substrate. Biosens. Bioelectron. 2016, 77, 1078–1085. [Google Scholar] [CrossRef]
- Real-Fernández, F.; Passalacqua, I.; Peroni, E.; Chelli, M.; Lolli, F.; Papini, A.M.; Rovero, P. Glycopeptide-Based Antibody Detection in Multiple Sclerosis by Surface Plasmon Resonance. Sensors 2012, 12, 5596–5607. [Google Scholar] [CrossRef]
- Takeuchi, T. Biomarkers as a treatment guide in rheumatoid arthritis. Clin. Immunol. 2018, 186, 59–62. [Google Scholar] [CrossRef]
- Hu, H.; Pan, D.; Xue, H.; Zhang, M.; Zhang, Y.; Shen, Y. A photoelectrochemical immunoassay for tumor necrosis factor-α using a GO-PTCNH2 nanohybrid as a probe. J. Electroanal. Chem. 2018, 824, 195–200. [Google Scholar] [CrossRef]
- Real-Fernández, F.; Rossi, G.; Lolli, F.; Papini, A.M.; Rovero, P. Label-free method for anti-glucopeptide antibody detection in Multiple Sclerosis. MethodsX 2015, 2, 141–144. [Google Scholar] [CrossRef]
- Yuan, X.; Li, C.; Yin, X.; Yang, Y.; Ji, B.; Niu, Y.; Ren, L. Epidermal Wearable Biosensors for Monitoring Biomarkers of Chronic Disease in Sweat. Biosensors 2023, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Dahlbom, I.; Olsson, M.; Forooz, N.K.; Sjöholm, A.G.; Truedsson, L.; Hansson, T. Immunoglobulin G (IgG) Anti-Tissue Transglutaminase Antibodies used as Markers for IgA-Deficient Celiac Disease Patients. Clin. Diagn. Lab Immunol. 2005, 12, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.M.; González-García, M.B.; Santos-Silva, A.; Costa-García, A. Voltammetric immunosensor for the diagnosis of celiac disease based on the quantification of anti-gliadin antibodies. Sens. Actuators B Chem. 2012, 163, 253–259. [Google Scholar] [CrossRef]
- Dulay, S.; Lozano-Sánchez, P.; Iwuoha, E.; Katakis, I.; O’Sullivan, C.K. Electrochemical detection of celiac disease-related anti-tissue transglutaminase antibodies using thiol based surface chemistry. Biosens. Bioelectron. 2011, 26, 3852–3856. [Google Scholar] [CrossRef]
- Cennamo, N.; Varriale, A.; Pennacchio, A.; Staiano, M.; Massarotti, D.; Zeni, L.; D’auria, S. An innovative plastic optical fiber-based biosensor for new bio/applications. The case of celiac disease. Sens. Actuators B Chem. 2012, 176, 1008–1014. [Google Scholar] [CrossRef]

| Disease | Target Biomarker | Biosensor Technology | Sample | LOD | Reference |
|---|---|---|---|---|---|
| Rheumatoid Arthritis | macrophage migration inhibitory factor (MIF) | EC | Human Serum | N/A | [21] |
| anti-citrullinated peptide antibodies (ACPAs) | EC | Rabbit Serum/Human Serum | N/A | [22] | |
| fibroblast-like synoviocyte (FLS) cells | optical | - | 2 cell/mL | [33] | |
| anti-CCP (anti-cyclic citrullinated peptide) | optical | - | 0.2 pg mL−1 | [37] | |
| interferon-γ | optical | CNS Tissue | N/A | [52,53] | |
| Multiple Sclerosis | interleukin-12 | EC | - | <100 fm | [24] |
| anti-myelin basic protein | EC | Human Serum | 0.15 ng mL−1 | [25] | |
| miR-145 | Fluorescence spectrophotometer | Human Serum | 0.1 nM | [38] | |
| multiple microRNAs (miR-422, miR-223, miR-126 and miR-23a) | Phase shift in surface plasmon resonance imaging | Human Serum | 0.55 pM–1.79 pM for the different miRNAs | [49] | |
| MS specific autoantibodies | SPR | Human Serum | N/A | [120,123] | |
| Diabetes Mellitus | Glucose | EC | Sweat | 3.84 μΜ | [124] |
| Glucose | Colorimetric | Sweat | 7 μΜ | [124] | |
| Depression, anxiety | Cortisol | EC | Sweat | 10 pM | [124] |
| Cortisol | Colorimetric | Sweat | 6.76 ng/mL | [124] | |
| Celiac Disease | IgA anti-tTG IgB anti-tTG | Differential Pulse Voltammetry (DPV) | Human Serum | 3.2 AU mL−1 1.4 AU mL−1 | [125] |
| IgA anti-tTG IgB anti-tTG | cyclic voltammetry (CV) | Human Serum | 1.7 AU mL−1 2.7 AU mL−1 | [126] | |
| Anti-tTG | Electrochemical Impedance Spectroscopy (EIS) | Serum | N/A | [75] | |
| anti-IgG-HRP | amperometry | Serum | 390 ng/mL | [127] | |
| tTG | SPR | Serum | N/A | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golfinopoulou, R.; Kintzios, S. Biosensing for Autoimmune Chronic Disease—A Review. Chemosensors 2023, 11, 366. https://doi.org/10.3390/chemosensors11070366
Golfinopoulou R, Kintzios S. Biosensing for Autoimmune Chronic Disease—A Review. Chemosensors. 2023; 11(7):366. https://doi.org/10.3390/chemosensors11070366
Chicago/Turabian StyleGolfinopoulou, Rebecca, and Spyridon Kintzios. 2023. "Biosensing for Autoimmune Chronic Disease—A Review" Chemosensors 11, no. 7: 366. https://doi.org/10.3390/chemosensors11070366
APA StyleGolfinopoulou, R., & Kintzios, S. (2023). Biosensing for Autoimmune Chronic Disease—A Review. Chemosensors, 11(7), 366. https://doi.org/10.3390/chemosensors11070366







