An Innovative Electrochemical Immuno-Platform for Monitoring Chronic Conditions Using the Biosensing of Hyaluronic Acid in Human Plasma Samples
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentations
2.3. Preparation of the Pt@Au Nanoparticle Deposition Solution
2.4. Gold Electrode Pre-Treatment before the Electrodeposition of Pt@Au Alloys
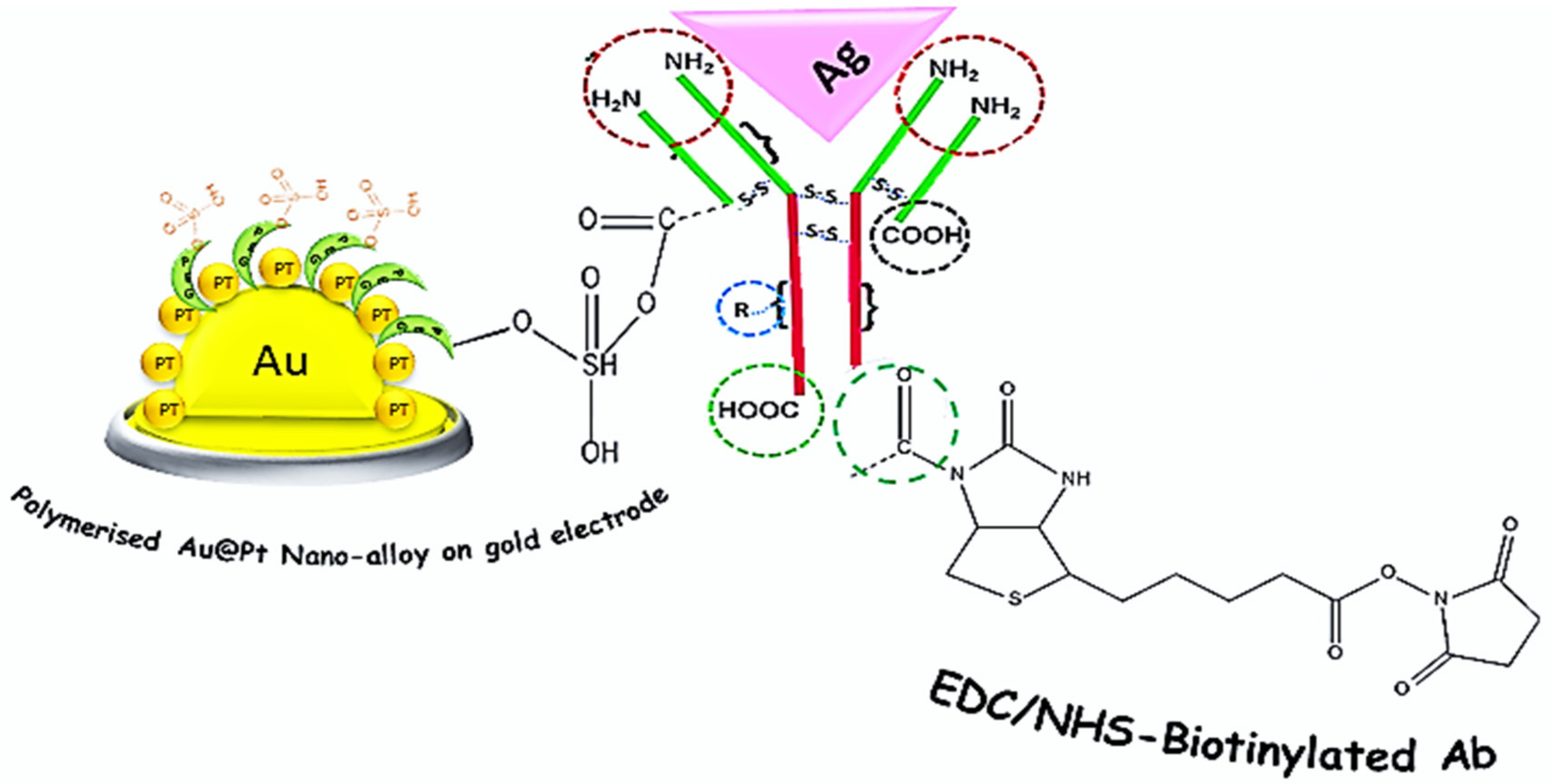
2.5. (Pt@Au)-Based Gold Electrodes Preparation via the CHA Technique
3. Characterization
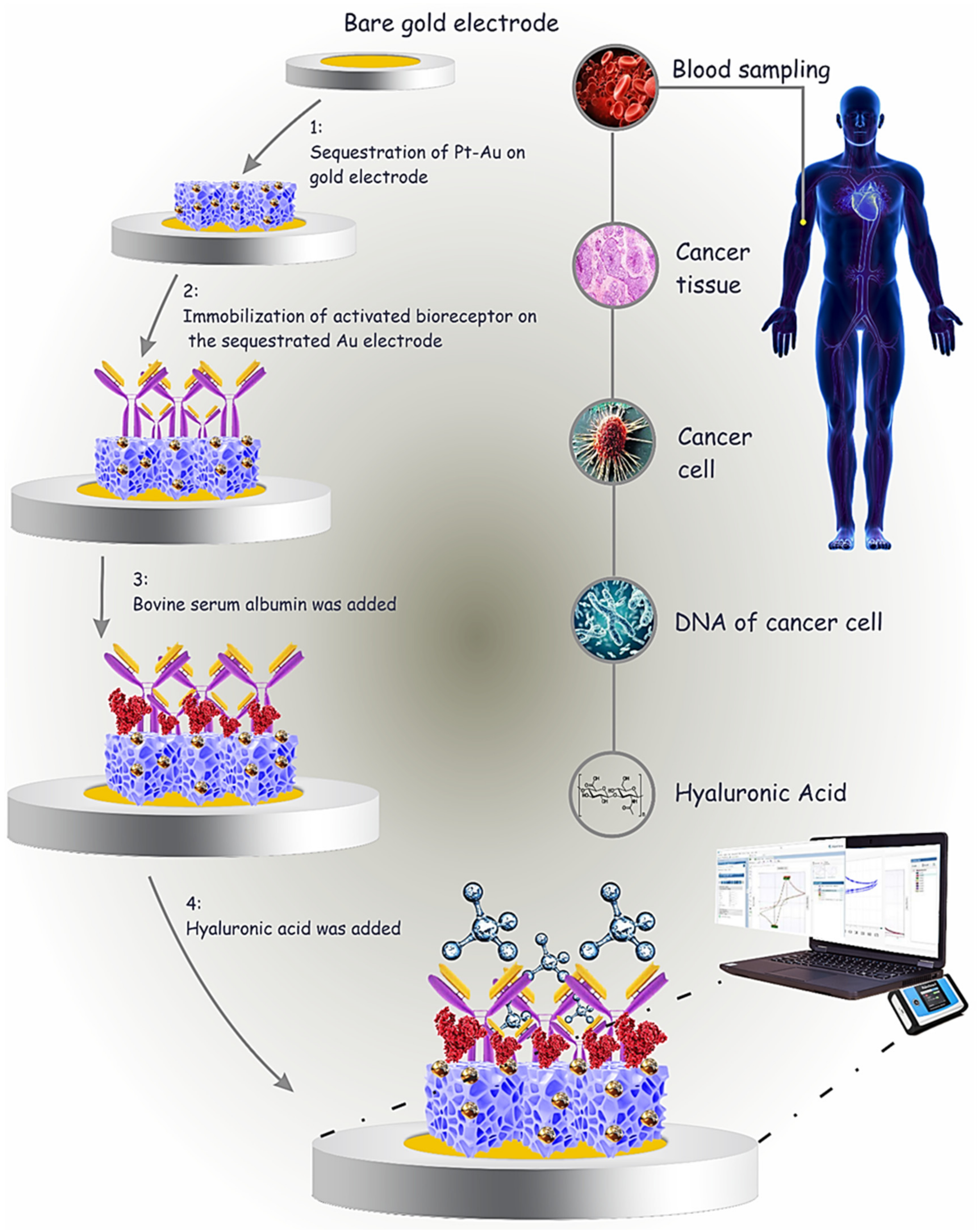
3.1. Fabrication of Immunosensor
3.2. Investigation of the Electrochemical Behaviors of the Immunosensor during Different Stages of the Preparation
4. Results and Discussion
4.1. Analytical Study
4.2. Investigation of Selectivity
4.3. Stability of Immunosensor’s Substrate
4.4. Investigation of Reproducibility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2019, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Velesiotis, C.; Vasileiou, S.; Vynios, D.H. A guide to hyaluronan and related enzymes in breast cancer: Biological significance and diagnostic value. FEBS J. 2019, 286, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Spadea, A.; de la Rosa, J.M.R.; Tirella, A.; Ashford, M.B.; Williams, K.J.; Stratford, I.J.; Tirelli, N.; Mehibel, M. Evaluating the Efficiency of Hyaluronic Acid for Tumor Targeting via CD44. Mol. Pharm. 2019, 16, 2481–2493. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Veter- Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Neuman, M.G.; Cohen, L.B.; Nanau, R.M. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin. Biochem. 2016, 49, 302–315. [Google Scholar] [CrossRef]
- Schwertfeger, K.; Cowman, M.; Telmer, P.; Turley, E.; McCarthy, J. Hyaluronan, inflammation, and breast cancer progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Mobed, A.; Dolati, S.; Shakouri, S.; Eftekharsadat, B.; Izadseresht, B. Recent advances in biosensors for detection of Osteoar-thritis and Rheumatoid Arthritis biomarkers. Sens. Actuators A Phys. 2021, 331, 112975. [Google Scholar] [CrossRef]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis—A five-year prospective cohort study. Thromb. Haemost. 2015, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Saruga, T.; Sasaki, E.; Inoue, R.; Chiba, D.; Ota, S.; Iwasaki, H.; Uesato, R.; Nakaji, S.; Ishibashi, Y. Usefulness of serum hyaluronic acid levels as a predictor of incidence of hand osteoarthritis analyzed by longitudinal analysis from the Iwaki cohort. Sci. Rep. 2021, 11, 4074. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, D.; Sharma, N. Role of hyaluronic acid in early diagnosis of knee osteoarthritis. J. Clin. Diagn. Res. 2014, 8, LC04. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, C.J.; Farid, W.R.; de Jonge, J.; Metselaar, H.J.; Kazemier, G.; van der Laan, L.J. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J. Hepatol. 2014, 61, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Gudowska, M.; Cylwik, B.; Chrostek, L. The role of serum hyaluronic acid determination in the diagnosis of liver diseases. Acta Biochim. Pol. 2017, 64, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Orasan, O.H.; Ciulei, G.; Cozma, A.; Sava, M.; Dumitrascu, D.L. Hyaluronic acid as a biomarker of fibrosis in chronic liver diseases of different etiologies. Med. Pharm. Rep. 2016, 89, 24–31. [Google Scholar] [CrossRef]
- Rostved, A.A.; Ostrowski, S.R.; Peters, L.; Lundgren, J.D.; Hillingsø, J.; Johansson, P.I.; Rasmussen, A. Hyaluronic Acid Is a Biomarker for Allograft Dysfunction and Predicts 1-Year Graft Loss After Liver Transplantation. Transplant. Proc. 2018, 50, 3635–3643. [Google Scholar] [CrossRef]
- Haserodt, S.; Aytekin, M.; Dweik, R.A. A comparison of the sensitivity, specificity, and molecular weight accuracy of three different commercially available Hyaluronan ELISA-like assays. Glycobiology 2010, 21, 175–183. [Google Scholar] [CrossRef]
- Suzuki, A.; Angulo, P.; Lymp, J.; Li, D.; Satomura, S.; Lindor, K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005, 25, 779–786. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Guo, S.-W. Plasma High Mobility Group Box 1 (HMGB1), Osteopontin (OPN), and Hyaluronic Acid (HA) as Admissible Biomarkers for Endometriosis. Sci. Rep. 2019, 9, 9272. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, D.; Liang, J.; Meltzer, E.B.; Gray, A.; Miura, R.; Wogensen, L.; Yamaguchi, Y.; Noble, P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med. 2011, 208, 1459–1471. [Google Scholar] [CrossRef]
- Peng, C.; Wallwiener, M.; Rudolph, A.; Ćuk, K.; Eilber, U.; Celik, M.; Modugno, C.; Trumpp, A.; Heil, J.; Marmé, F. Plasma hyaluronic acid level as a prognostic and monitoring marker of metastatic breast cancer. Int. J. Cancer 2016, 138, 2499–2509. [Google Scholar] [CrossRef]
- Skarmoutsos, I.; Skarmoutsos, A.; Katafigiotis, I.; Tataki, E.; Giagini, A.; Adamakis, I.; Alamanis, C.; Duvdevani, M.; Sitaras, N.; Constantinides, C. Hyaluronic acid and hyaluronidase as possible novel urine biomarkers for the diagnosis of prostate cancer. Med. Oncol. 2018, 35, 97. [Google Scholar] [CrossRef]
- Morera, D.; Hennig, M.S.; Talukder, A.; Lokeshwar, S.D.; Wang, J.; Garcia-Roig, M.; Ortiz, N.; Yates, T.J.; Lopez, L.E.; Kallifatidis, G.; et al. Hyaluronic acid family in bladder cancer: Potential prognostic biomarkers and therapeutic targets. Br. J. Cancer 2017, 117, 1507–1517. [Google Scholar] [CrossRef]
- Yahya, R.; El-Bindary, A.; El-Mezayen, H.; Abdelmasseh, H.; Eissa, M. Biochemical Evaluation of Hyaluronic Acid in Breast Cancer. Clin. Lab. 2014, 60, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S. Accumulation of small hyaluronan oligosaccharides in tumour interstitial fluid correlates with lymphatic invasion and lymph node metastasis. Br. J. Cancer 2014, 111, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Aghcheli, K.; Parsian, H.; Qujeq, D.; Talebi, M.; Mosapour, A.; Khalilipour, E.; Islami, F.; Semnani, S.; Malekzadeh, R. Serum hyaluronic acid and laminin as potential tumor markers for upper gastrointestinal cancers. Eur. J. Intern. Med. 2012, 23, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; Stern, R. An ELISA-like assay for hyaluronidase and hyaluronidase inhibitors. Matrix 1992, 12, 397–403. [Google Scholar] [CrossRef]
- Ge, M.; Bai, P.; Chen, M.; Tian, J.; Hu, J.; Zhi, X.; Yin, H.; Yin, J. Utilizing hyaluronic acid as a versatile platform for fluorescence resonance energy transfer-based glucose sensing. Anal. Bioanal. Chem. 2018, 410, 2413–2421. [Google Scholar] [CrossRef]
- Liu, X.; Huang, R.; Su, R.; Qi, W.; Wang, L.; He, Z. Grafting Hyaluronic Acid onto Gold Surface to Achieve Low Protein Fouling in Surface Plasmon Resonance Biosensors. ACS Appl. Mater. Interfaces 2014, 6, 13034–13042. [Google Scholar] [CrossRef]
- Eliezer, M.; Sculean, A.; Miron, R.J.; Nemcovsky, C.; Weinberg, E.; Weinreb, M.; Zoabi, H.; Bosshardt, D.D.; Fujioka-Kobayashi, M.; Moses, O. Hyaluronic acid slows down collagen membrane degradation in uncontrolled diabetic rats. J. Periodontal Res. 2019, 54, 644–652. [Google Scholar] [CrossRef]
- Pepeliaev, S.; Hrudíková, R.; Jílková, J.; Pavlík, J.; Smirnou, D.; Černý, Z.; Franke, L. Colorimetric enzyme-coupled assay for hyaluronic acid determination in complex samples. Eur. Polym. J. 2017, 94, 460–470. [Google Scholar] [CrossRef]
- Buledi, J.A.; Mahar, N.; Mallah, A.; Solangi, A.R.; Palabiyik, I.M.; Qambrani, N.; Karimi, F.; Vasseghian, Y.; Karimi-Maleh, H. Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: A potential method for environmental remediation. Food Chem. Toxicol. 2022, 161, 112843. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Raman, P.K.Y.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2021, 287, 132187. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qin, J.; Lv, J.; Yang, J.; Yan, G. “Turn on” room-temperature phosphorescent biosensors for detection of hyaluronic acid based on manganese-doped ZnS quantum dots. RSC Adv. 2018, 8, 2873–2879. [Google Scholar] [CrossRef]
- Hayase, S.; Oda, Y.; Honda, S.; Kakehi, K. High-performance capillary electrophoresis of hyaluronic acid: Determination of its amount and molecular mass. J. Chromatogr. A 1997, 768, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Hokputsa, S.; Jumel, K.; Alexander, C.; Harding, S.E. A comparison of molecular mass determination of hyaluronic acid using SEC/MALLS and sedimentation equilibrium. Eur. Biophys. J. 2003, 32, 450–456. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.; Shabbir, M.A.B.; Cheng, G.; Yuan, Z. Current advances in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Gonzales, W.V.; Mobashsher, A.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef]
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit. Rev. Anal. Chem. 2017, 47, 138–153. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2015, 78, 497–504. [Google Scholar] [CrossRef]
- Vashist, S.K. Comparison of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Based Strategies to Crosslink Antibodies on Amine-Functionalized Platforms for Immunodiagnostic Applications. Diagnostics 2012, 2, 23–33. [Google Scholar] [CrossRef]



| Technique | Advantages | Disadvantage and Limitation | Ref. |
|---|---|---|---|
| Radioimmunoassay | Simple, convenient, noninvasive credible, low-cost method, small sample volume | Low specificity, low automation, absence of separation step, short half-life label, high health hazards due to the radioactivity | [36] |
| Fluorescent-Based Immunoassays | High specificity due to the exceptional optical properties of the molecules, measurement of analyte concentration using fluorescence and decay time, excellent reproducibility | Susceptible to interference due to pH changes and oxygen levels, costs are substantially high, skilled personnel, fluorescent labeling | [37] |
| ELISA | Sensitive, rapid, low-cost method, time saving, strong affinity | Need a large sample size, antibody variability, cross reactivity, time consuming, not sensitive enough to detect sample volumes that are too small, false positive | [36] |
| Colorimetric | Fast, low-cost method, small sample size, ability to customize array for specific analyte, flexible array size, potential to analyze liquid samples | Low reproducibility of imaging and printing, sample application may vary, low selectivity, low stability | [38] |
| Platform/Technique | Sample/Model | Electrode | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| EC/CV | Animal | GCE | 1–800 μM | 0.42 μM | [32] |
| HPLC | Biological | NA | 0.01 mg/mL to 3.3 mg/mL | 1.0 μg/mL | [34] |
| SEC/MALLS | Synthetic | NA | (16.5–21.1) × 104 | Unavailable | [35] |
| Colorimetric | Complex samples | NA_ | 3–2000 mg/L | 0.3 mg/L | [30] |
| RTP/QDs | Real samples | NP | 0.08–2.8 μg mL−1 | 0.03 μg mL−1 | [33] |
| EC/Pt@Au Nano | Real samples | Gold | 0.156–160 ng.mL−1 | 0.039 ng.mL−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mobed, A.; Kohansal, F.; Dolati, S.; Hasanzadeh, M.; Shakouri, S.K. An Innovative Electrochemical Immuno-Platform for Monitoring Chronic Conditions Using the Biosensing of Hyaluronic Acid in Human Plasma Samples. Chemosensors 2023, 11, 367. https://doi.org/10.3390/chemosensors11070367
Mobed A, Kohansal F, Dolati S, Hasanzadeh M, Shakouri SK. An Innovative Electrochemical Immuno-Platform for Monitoring Chronic Conditions Using the Biosensing of Hyaluronic Acid in Human Plasma Samples. Chemosensors. 2023; 11(7):367. https://doi.org/10.3390/chemosensors11070367
Chicago/Turabian StyleMobed, Ahmad, Fereshteh Kohansal, Sanam Dolati, Mohammad Hasanzadeh, and Seyed Kazem Shakouri. 2023. "An Innovative Electrochemical Immuno-Platform for Monitoring Chronic Conditions Using the Biosensing of Hyaluronic Acid in Human Plasma Samples" Chemosensors 11, no. 7: 367. https://doi.org/10.3390/chemosensors11070367
APA StyleMobed, A., Kohansal, F., Dolati, S., Hasanzadeh, M., & Shakouri, S. K. (2023). An Innovative Electrochemical Immuno-Platform for Monitoring Chronic Conditions Using the Biosensing of Hyaluronic Acid in Human Plasma Samples. Chemosensors, 11(7), 367. https://doi.org/10.3390/chemosensors11070367







