Functionalized Carbon-Nanotubes-Based Thin-Film Transistor Sensor for Highly Selective Detection of Methane at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
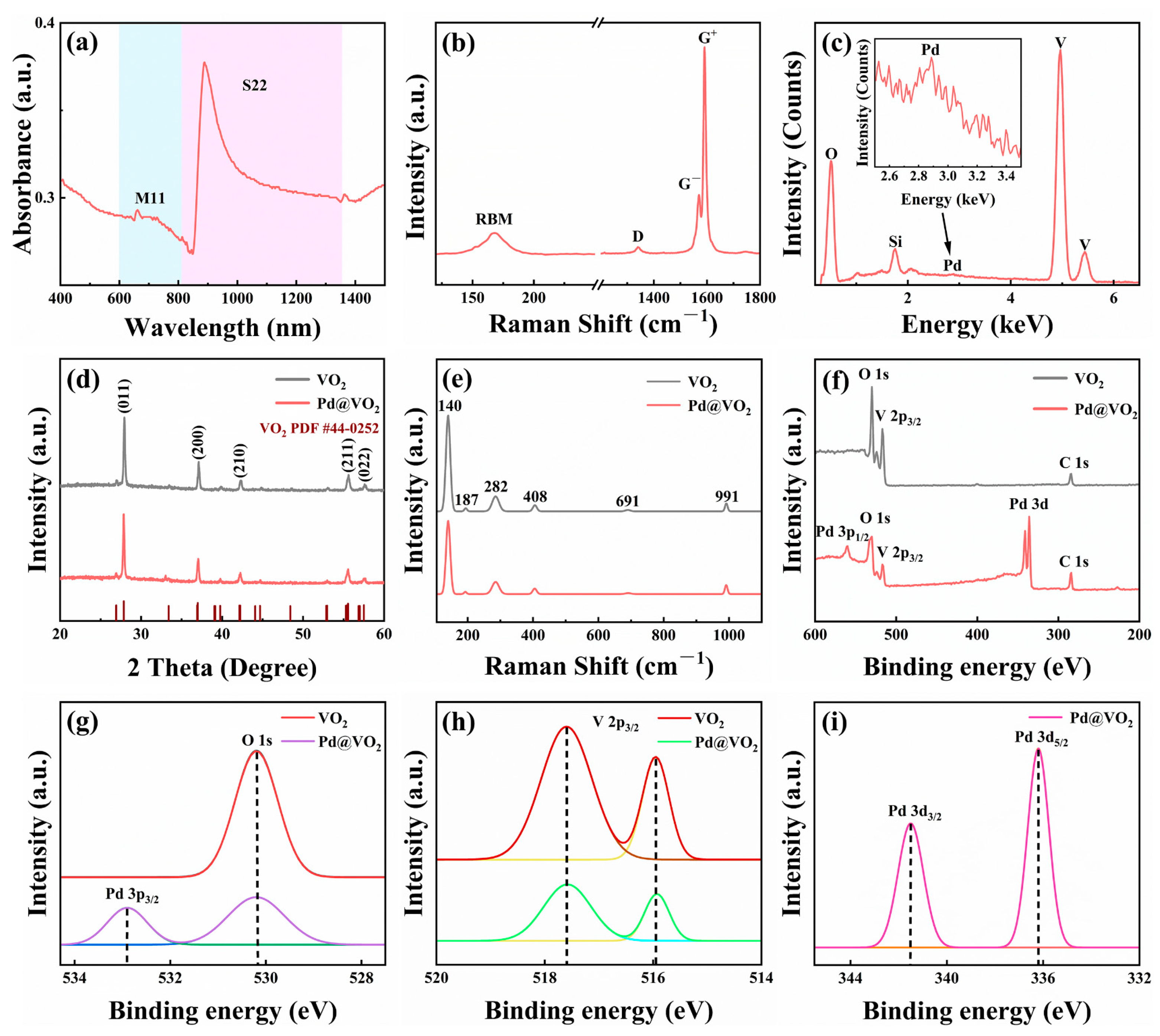
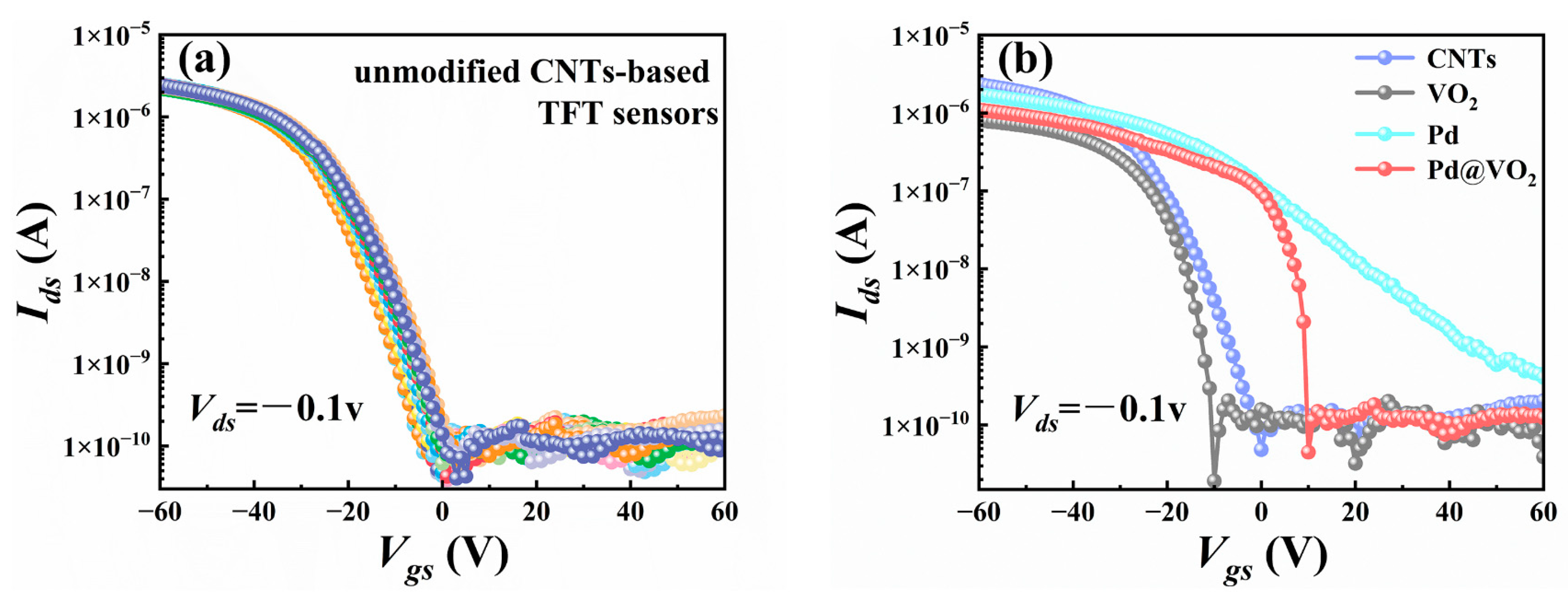
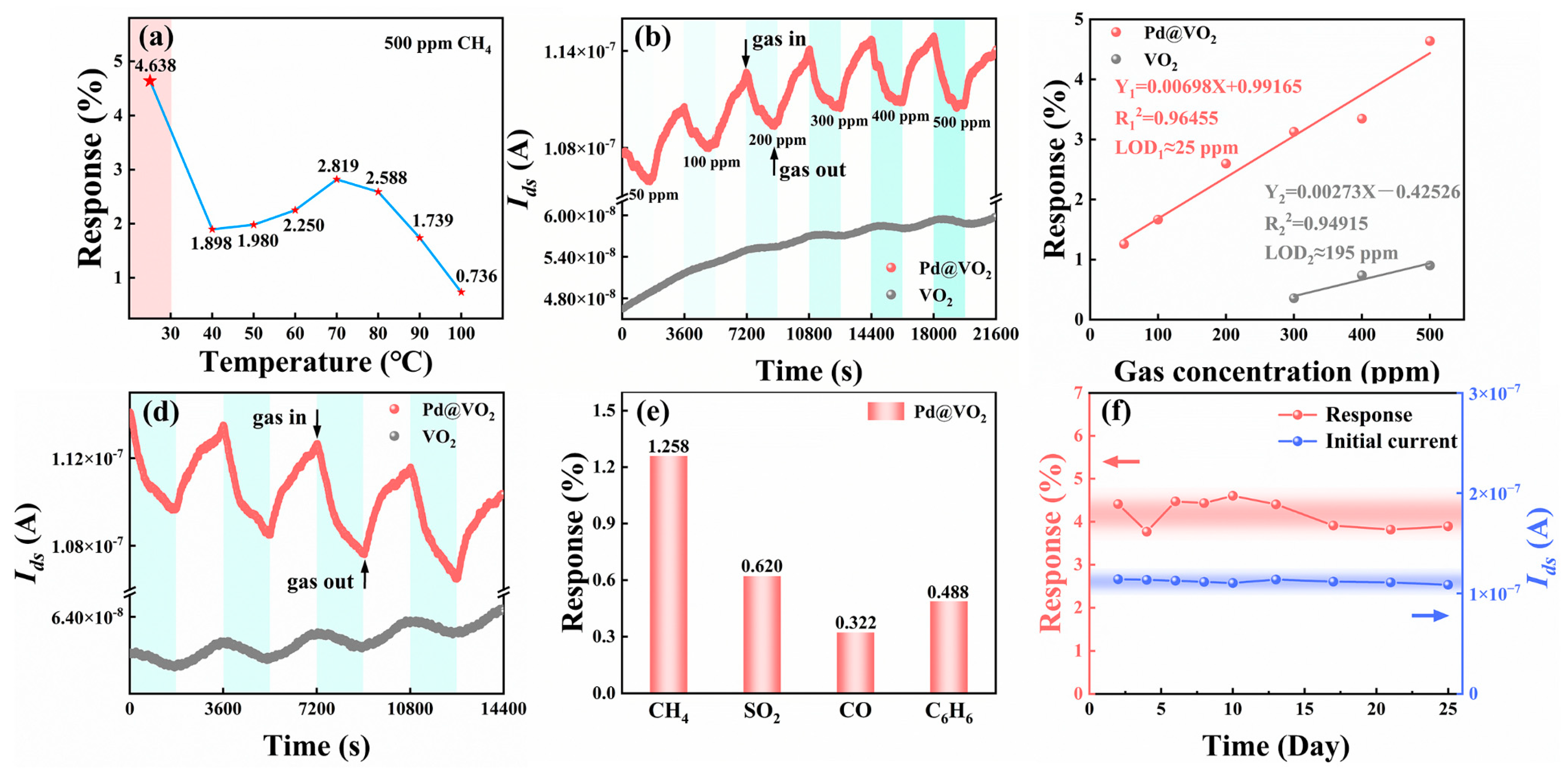
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chimowa, G.; Tshabalala, Z.P.; Akande, A.A.; Bepete, G.; Mwakikunga, B.; Ray, S.S.; Benecha, E.M. Improving CH4 gas sens-ing properties of multi-walled carbon nanotubes by vanadium oxide filling. Sens. Actuators B Chem. 2017, 247, 11–18. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Yao, M.; Sun, G.; Zhang, Z. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Li, N.; Li, W. Magnetron sputtered Au-decorated vanadium oxides composite thin films for CH4-sensing properties at room temperature. J. Alloys Compd. 2016, 671, 283–290. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Liu, J.; Hu, M. Room temperature CH4 sensing properties of Au decorated VO2 nanosheets. Mater. Lett. 2016, 184, 92–95. [Google Scholar] [CrossRef]
- Kim, W.-T.; Kim, I.-H.; Choi, W.-Y. Fabrication of TiO2 Nanotube Arrays and Their Application to a Gas Sensor. J. Nanosci. Nanotechnol. 2015, 15, 8161–8165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Liu, W.; Li, F.; Zhan, Z. Design of SnO2-based CH4 sensors with reactive anti-poisoning layers: Excellent stability and high resistance to hexamethyldisiloxane. J. Mater. Sci. 2023, 58, 2140–2155. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, J.; Zhang, Y. Visible light assisted trace gaseous NO2 sensor with anti-humidity ability via LSPR enhancement effect. Sens. Actuators B Chem. 2022, 367. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Room-Temperature Gas Sensing Based on Electron Transfer between Discrete Tin Oxide Nano-crystals and Multiwalled Carbon Nanotubes. Adv. Mater. 2009, 21, 2487–2491. [Google Scholar] [CrossRef]
- Hu, J.; Liu, X.; Zhang, J.; Gu, X.; Zhang, Y. Plasmon-activated NO2 sensor based on Au@MoS2 core-shell nanoparticles with heightened sensitivity and full recoverability. Sens. Actuators B Chem. 2023, 382, 133505. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Zhang, B.; Sun, G.; Zhang, Z. Synthesis of g-C3N4-Decorated ZnO Porous Hollow Microspheres for Room-Temperature Detection of CH4 under UV-Light Illumination. Nanomaterials 2019, 9, 1507. [Google Scholar] [CrossRef]
- Xiao, M.; Liang, S.; Han, J.; Zhong, D.; Liu, J.; Zhang, Z.; Peng, L. Batch Fabrication of Ultrasensitive Carbon Nanotube Hydro-gen Sensors with Sub-ppm Detection Limit. ACS Sens. 2018, 3, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiao, M.; Ning, Y.; Zhou, S.; He, J.; Lin, Y.; Zhang, Z. Toward practical gas sensing with rapid recovery semiconducting carbon nanotube film sensors. Sci. China Inf. Sci. 2022, 65, 162402. [Google Scholar] [CrossRef]
- Abd, I.K.; Shano, A.M.; Khodair, Z.T. MWCNT Thin Films by CVD Method and Some Applications. J. Nano-Electron. Phys. 2022, 14, 06002. [Google Scholar] [CrossRef]
- Shukla, P.; Saxena, P.; Madhwal, D.; Bhardwaj, N.; Jain, V.K. Electrostatically functionalized CVD grown multiwalled carbon nanotube/palladium polymer nanocomposite (MWCNT/Pd) for CH4 detection at room temperature. Chem. Eng. Sci. 2022, 264, 118191. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Han, J.; Ng, H.T.; Binder, C.; Partridge, C. Meyyappan, room temperature CH4 detection using palladium loaded single-walled carbon nanotube sensors. Chem. Phys. Lett. 2004, 391, 344–348. [Google Scholar] [CrossRef]
- Aroutiounian, V.M. Metal oxide gas sensors decorated with carbon nanotubes. Lith. J. Phys. 2015, 55, 4. [Google Scholar] [CrossRef]
- Afrin, R.; Shah, N. Room temperature gas sensors based on carboxyl and thiol functionalized carbon nanotubes buckypapers. Diam. Relat. Mater. 2015, 60, 42–49. [Google Scholar] [CrossRef]
- Humayun, T.; Divan, R.; Liu, Y.; Gundel, L.; Solomon, P.A.; Paprotny, I. Novel chemoresistive CH4 sensor with 10 ppm sensitivity based on multiwalled carbon nanotubes functionalized with SnO2 nanocrystals. J. Vac. Sci. Technol. A 2016, 34, 01A131. [Google Scholar] [CrossRef]
- Humayun, M.T.; Divan, R.; Stan, L.; Gupta, A.; Rosenmann, D.; Gundel, L.; Solomon, P.A.; Paprotny, I. ZnO functionalization of multiwalled carbon nanotubes for CH4 sensing at single parts per million concentration levels. J. Vac. Sci-Ence Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 06FF01. [Google Scholar]
- Chen, C.; Jiang, M.; Luo, X.; Tai, H.; Jiang, Y.; Yang, M.; Xie, G.; Su, Y. Ni-Co-P hollow nanobricks enabled humidity sensor for respiratory analysis and human-machine interfacing. Sens. Actuators B Chem. 2022, 370, 132441. [Google Scholar] [CrossRef]
- Prasad, A.K.; Amirthapandian, S.; Dhara, S.; Dash, S.; Murali, N.; Tyagi, A.K. Novel single phase vanadium dioxide nanostructured films for CH4 sensing near room temperature. Sens. Actuators B Chem. 2014, 191, 252–256. [Google Scholar] [CrossRef]
- Li, W.; Liang, J.; Liu, J.; Zhou, L.; Yang, R.; Hu, M. Synthesis and room temperature CH4 gas sensing properties of vanadium dioxide nanorods. Mater. Lett. 2016, 173, 199–202. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Luo, N.; Wang, W.; Xu, J. Defective ZnO Nanoflowers Decorated by Ultra-Fine Pd Clusters for Low-Concentration CH4 Sensing: Controllable Preparation and Sensing Mechanism Analysis. Coatings 2022, 12, 677. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Zhao, D. GaN Nanowires Decorated with Pd for CH4 Gas Sensor. In IOP Conference Series: Earth and Envi-ronmental Science; IOP Publishing: Bristol, UK, 2020; p. 042037. [Google Scholar]
- Luo, S.; Chen, R.; Wang, J.; Xiang, L. ZnO/Pd@ ZIF-7-Based Gas Sensors for Selective CH4 Sensing. ACS Appl. Nano Mate-Rials 2023, 67, 5808–5816. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Wu, G.; Cao, J.; Zhang, Z.; Zhang, Y. Carbon Nanotube-Based Field-Effect Transistor-Type Sensor with a Sens-ing Gate for Ppb-Level Formaldehyde Detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319. [Google Scholar] [CrossRef]
- Kim, C.-H.; Cho, I.-T.; Shin, J.-M.; Choi, K.-B.; Lee, J.-K.; Lee, J.-H. A New Gas Sensor Based on MOSFET Having a Horizontal Floating-Gate. IEEE Electron Device Lett. 2013, 35, 265–267. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, C.-H.; Shin, J.; Kim, K.Y.; Kim, J.S.; Hwang, C.S.; Lee, J.-H. Highly selective ZnO gas sensor based on MOSFET having a horizontal floating-gate. Sens. Actuators B Chem. 2016, 232, 653–659. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, M.; Wu, D.; Lin, Y.; Liu, L.; He, J.; Zhang, G.-J.; Peng, L.-M.; Zhang, Z. Wafer-Scale Uniform Carbon Nanotube Transistors for Ultrasensitive and Label-Free Detection of Disease Biomarkers. ACS Nano 2020, 14, 8866–8874. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, M.; Liu, F.; He, J.; Lin, Y.; Zhang, Z. Sub-10 parts per billion detection of hydrogen with floating gate transis-tors built on semiconducting carbon nanotube film. Carbon 2021, 180, 41–47. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, J.; Kang, Y.; Ai, Y.; Li, C.M. Two dimensional atomically thin MoS2 nanosheets and their sensing applica-tions. Nanoscale 2015, 7, 19358–19376. [Google Scholar] [CrossRef]
- Biswas, C.; Lee, S.Y.; Ly, T.H.; Ghosh, A.; Dang, Q.N.; Lee, Y.H. Chemically Doped Random Network Carbon Nanotube p–n Junction Diode for Rectifier. ACS Nano 2011, 5, 9817–9823. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Tan, Y.; Mo, R.; Zhang, Y. A CuO thin film type sensor via inkjet printing technology with high reproducibil-ity for ppb-level formaldehyde detection. Sens. Actuators B Chem. 2022, 362, 131775. [Google Scholar] [CrossRef]
- Braun, F.; Tarditi, A.M.; Miller, J.B.; Cornaglia, L.M. Pd-based binary and ternary alloy membranes: Morphological and perm-selective characterization in the presence of H2S. J. Membr. Sci. 2014, 450, 299–307. [Google Scholar] [CrossRef]
- Alie, D.; Gedvilas, L.; Wang, Z.; Tenent, R.; Engtrakul, C.; Yan, Y.; Shaheen, S.E.; Dillon, A.C.; Ban, C. Direct synthesis of ther-mochromic VO2 through hydrothermal reaction. J. Solid State Chem. 2014, 212, 237–241. [Google Scholar] [CrossRef]
- Kong, F.; Li, M.; Pan, S.; Zhang, Y.; Li, G. Synthesis and thermal stability of W-doped VO2 nanocrystals. Mater. Res. Bull. 2011, 46, 2100–2104. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, Y.; Zhang, G.; Wei, Q.; Jiang, Y.; Xiong, T.; He, P.; Yang, W.; Yan, M.; An, Q.; et al. Ultrastable and High-Performance Zn/VO2 Battery Based on a Reversible Single-Phase Reaction. Chem. Mater. 2019, 31, 699–706. [Google Scholar] [CrossRef]
- Li, R.; Liu, C.-Y. VO2(B) nanospheres: Hydrothermal synthesis and electrochemical properties. Mater. Res. Bull. 2010, 45, 688–692. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cheong, H.M.; Seong, M.J.; Liu, P.; Tracy, C.; Mascarenhas, A.; Pitts, J.; Deb, S.K. Raman spectroscopic studies of amorphous vanadium oxide thin films. Solid State Ion. 2003, 165, 111–116. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J. XPS, AES and Auger parameter of Pd and PdO. J. Electron Spectrosc. Relat-Ed Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Powell, M.J.; Godfrey, I.J.; Quesada-Cabrera, R.; Malarde, D.; Teixeira, D.; Emerich, H.; Palgrave, R.G.; Carmalt, C.J.; Parkin, I.P.; Sankar, G. Qualitative XANES and XPS Analysis of Substrate Effects in VO2 Thin Films: A Route to Improving Chemical Vapor Deposition Synthetic Methods? J. Phys. Chem. C 2017, 121, 20345–20352. [Google Scholar] [CrossRef]
- Luo, M.; Zhu, M.; Wei, M.; Shao, S.; Robin, M.; Wei, C.; Cui, Z.; Zhao, J.; Zhang, Z. Radiation-hard and repairable complemen-tary metal–oxide–semiconductor circuits integrating n-type indium oxide and p-type carbon nanotube field-effect transis-tors. ACS Appl. Mater. Interfaces 2020, 12, 49963–49970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, S.; Ding, L.; Liang, X.; Pei, T.; Shen, J.; Xu, H.; Chen, Q.; Cui, R.; Li, Y.; et al. Self-Aligned Ballistic n-Type Single-Walled Carbon Nanotube Field-Effect Transistors with Adjustable Threshold Voltage. Nano Lett. 2008, 8, 3696–3701. [Google Scholar] [CrossRef]
- Xu, X.; He, X.; Wang, G.; Yuan, X.; Liu, X.; Huang, H.; Chu, J. The study of optimal oxidation time and different tempera-tures for high quality VO2 thin film based on the sputtering oxidation coupling method. Appl. Surf. Sci. 2011, 257, 8824–8827. [Google Scholar] [CrossRef]
- Bertrand, J. Spectroscopic and Electrical Studies of the Influence of Electrodes on SnO2 Based Sensors. Ph.D. Thesis, Tübingen, Univ., Tübingen, Germany, 2008. [Google Scholar]
- Liu, J.; Hu, Z.; Zhang, Y.; Li, H.-Y.; Gao, N.; Tian, Z.; Zhou, L.; Zhang, B.; Tang, J.; Zhang, J.; et al. MoS2 Nanosheets Sensitized with Quantum Dots for Room-Temperature Gas Sensors. Nano-Micro Lett. 2020, 12, 219–242. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hwang, I.-S.; Kang, Y.C.; Lee, J.-H. Design of Selective Gas Sensors Using Additive-Loaded In2O3 Hollow Spheres Prepared by Combinatorial Hydrothermal Reactions. Sensors 2011, 11, 10603–10614. [Google Scholar] [CrossRef] [PubMed]
- Horastani, Z.K.; Sayedi, S.M.; Sheikhi, M.H.; Rahimi, E. Effect of silver additive on electrical conductivity and CH4 sensitivity of SnO2. Mater. Sci. Semicond. Process. 2015, 35, 38–44. [Google Scholar] [CrossRef]
- Mounasamy, V.; Mani, G.K.; Madanagurusamy, S. Vanadium oxide nanostructures for chemiresistive gas and vapour sens-ing: A review on state of the art. Microchim. Acta 2020, 187, 253. [Google Scholar] [CrossRef]
- Nasresfahani, S.; Sheikhi, M.; Tohidi, M.; Zarifkar, A. CH4 gas sensing properties of Pd-doped SnO2/reduced graphene oxide synthesized by a facile hydrothermal route. Mater. Res. Bull. 2017, 89, 161–169. [Google Scholar] [CrossRef]
- Liu, C.; Suematsu, K.; Uchiyama, A.; Watanabe, K.; Guo, Y.; Wang, D.; Shimanoe, K. Impact of Pd nanoparticle loading meth-od on SnO2 surface for natural gas detection in humid atmosphere. J. Mater. Sci. 2021, 56, 13975–13988. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, F.; Hu, J.; Zhang, Y. Functionalized Carbon-Nanotubes-Based Thin-Film Transistor Sensor for Highly Selective Detection of Methane at Room Temperature. Chemosensors 2023, 11, 365. https://doi.org/10.3390/chemosensors11070365
Ji F, Hu J, Zhang Y. Functionalized Carbon-Nanotubes-Based Thin-Film Transistor Sensor for Highly Selective Detection of Methane at Room Temperature. Chemosensors. 2023; 11(7):365. https://doi.org/10.3390/chemosensors11070365
Chicago/Turabian StyleJi, Feifan, Jinyong Hu, and Yong Zhang. 2023. "Functionalized Carbon-Nanotubes-Based Thin-Film Transistor Sensor for Highly Selective Detection of Methane at Room Temperature" Chemosensors 11, no. 7: 365. https://doi.org/10.3390/chemosensors11070365
APA StyleJi, F., Hu, J., & Zhang, Y. (2023). Functionalized Carbon-Nanotubes-Based Thin-Film Transistor Sensor for Highly Selective Detection of Methane at Room Temperature. Chemosensors, 11(7), 365. https://doi.org/10.3390/chemosensors11070365





