Molecularly Imprinted Plasmonic Sensors for the Determination of Environmental Water Contaminants: A Review
Abstract
1. Introduction
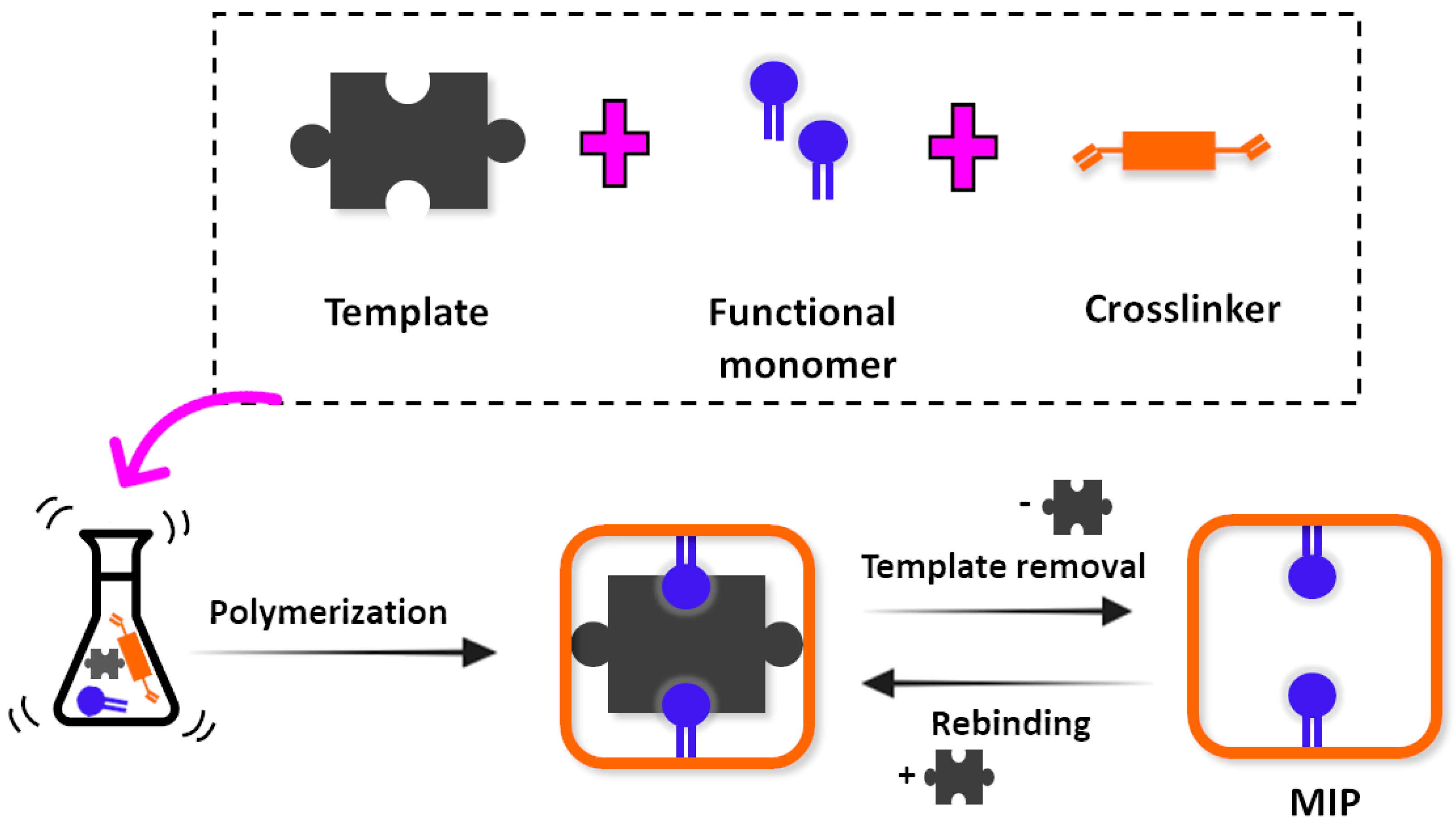
2. Molecular Imprinting Technology
MIPs as Recognition Elements for Sensors
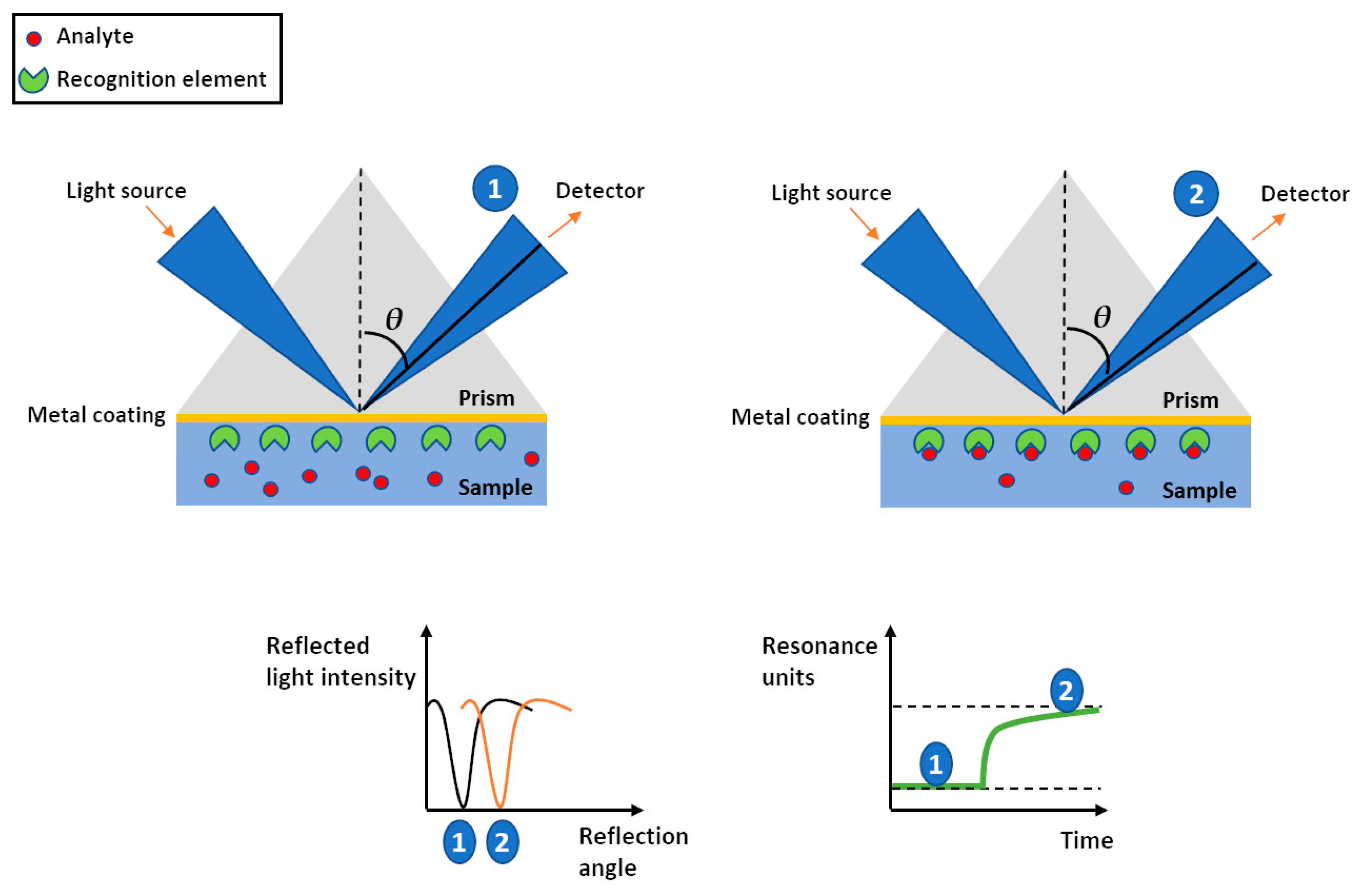
3. SPR Sensors
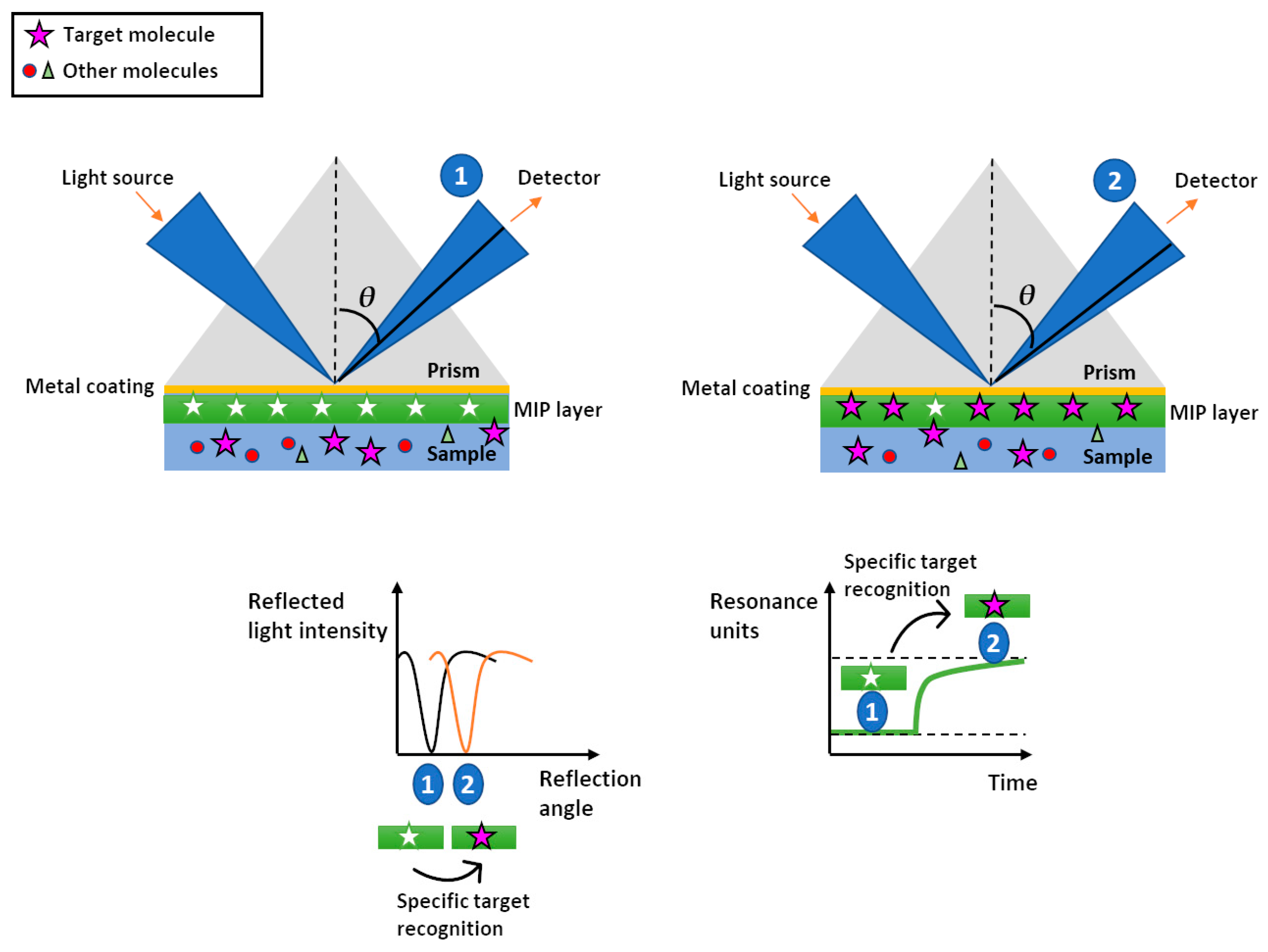
3.1. SPR Based (Bio)sensing-MIPS
Application of MIP-SPR Sensors in Water Contaminant Detection
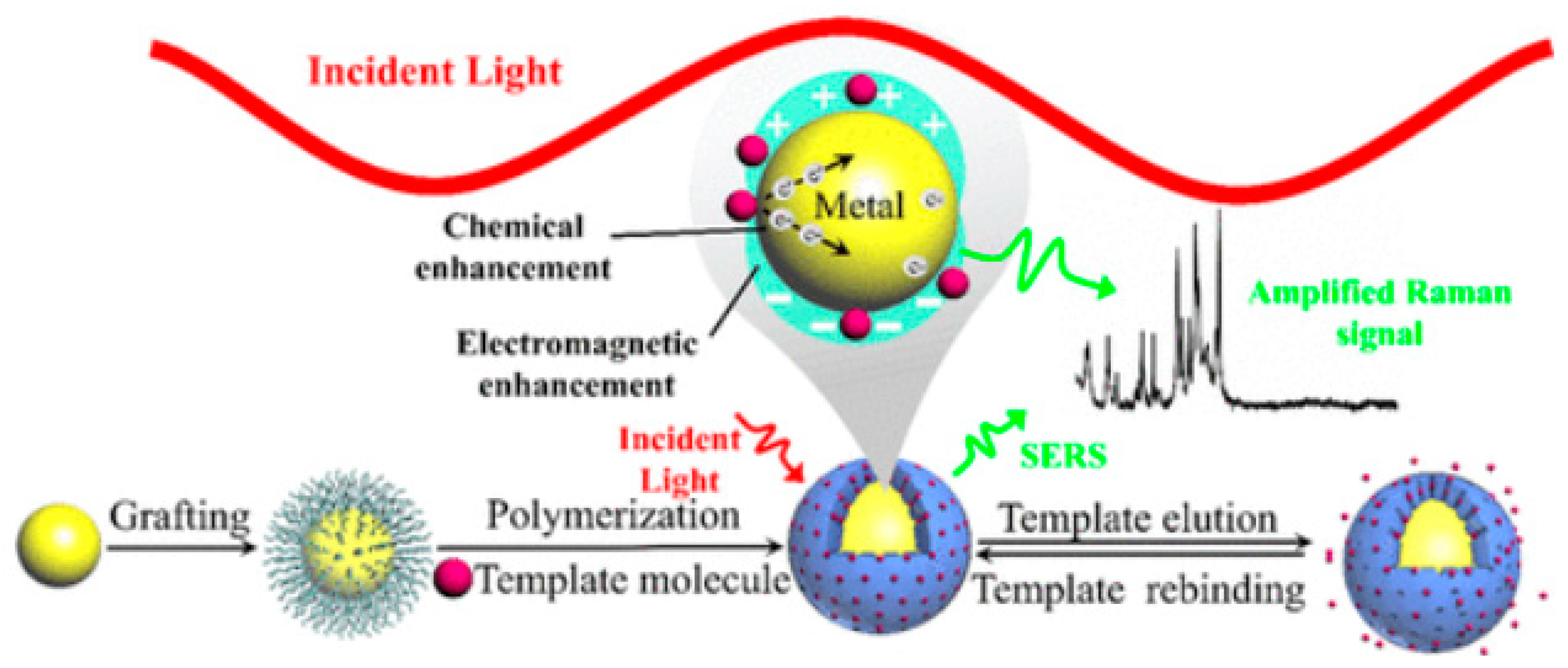
4. SERS Sensors
4.1. MIP-Based SERS
Application of MIP-SERS Sensors in Water Contaminant Detection
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment-A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Fan, L.; Huang, J.; Yang, W.; Wu, H.; Wang, X.; Khanal, R. The gap of water supply-Demand and its driving factors: From water footprint view in Huaihe River Basin. PLoS ONE 2021, 16, e0247604. [Google Scholar] [CrossRef] [PubMed]
- Shoushtarian, F.; Negahban-Azar, M. Worldwide regulations and guidelines for agriculturalwater reuse: A critical review. Water 2020, 12, 971. [Google Scholar] [CrossRef]
- Fito, J.; Van Hulle, S.W.H. Wastewater reclamation and reuse potentials in agriculture: Towards environmental sustainability. Environ. Dev. Sustain. 2021, 23, 2949–2972. [Google Scholar] [CrossRef]
- Lee, K.; Jepson, W. Drivers and barriers to urban water reuse: A systematic review. Water Secur. 2020, 11, 100073. [Google Scholar] [CrossRef]
- Jodar-Abellan, A.; López-Ortiz, M.I.; Melgarejo-Moreno, J. Wastewater Treatment and Water Reuse in Spain. Current Situation and Perspectives. Water 2019, 11, 1551. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Asano, T.; Bahri, A.; Jimenez, B.E.; Tchobanoglous, G. Water Reuse: From Ancient to Modern Times and the Future. Front. Environ. Sci. 2018, 6, 26. [Google Scholar] [CrossRef]
- Voulvoulis, N. Water reuse from a circular economy perspective and potential risks from an unregulated approach. Curr. Opin. Environ. Sci. Health 2018, 2, 32–45. [Google Scholar] [CrossRef]
- Dulio, V.; van Bavel, B.; Brorström-Lundén, E.; Harmsen, J.; Hollender, J.; Schlabach, M.; Slobodnik, J.; Thomas, K.; Koschorreck, J. Emerging pollutants in the EU: 10 years of NORMAN in support of environmental policies and regulations. Environ. Sci. Eur. 2018, 30, 5. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total Environ. 2021, 754, 142122. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Notsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.C.; et al. Removal of Emerging Contaminants from Wastewater Using Advanced Treatments. A Review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Gualda-Alonso, E.; Soriano-Molina, P.; Casas López, J.L.; García Sánchez, J.L.; Plaza-Bolanos, P.; Agüera, A.; Sánchez-Pérez, J.A. Large-scale raceway pond reactor for CEC removal from municipal WWTP effluents by solar photo-Fenton. Appl. Catal. B Environ. 2022, 319, 121908. [Google Scholar] [CrossRef]
- Shah, A.I.; Din Dar, M.U.; Bhat, R.A.; Singh, J.P.; Singh, K.; Bhat, S.A. Prospectives and challenges of wastewater treatment technologies to combat contaminants of emerging concerns. Ecol. Eng. 2020, 152, 105882. [Google Scholar] [CrossRef]
- Zulkifli, S.N.; Rahim, H.A.; Lau, W.J. Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications. Sens. Actuators B Chem. 2018, 255, 2657–2689. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; Duarte, A.C.; Santos, T.A.P.R. Environmental monitoring approaches for the detection of organic contaminants in marine environments: A critical review. Trends Environ. Anal. Chem. 2022, 33, e00154. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Chang, C.; Wang, R.; Liu, Z.; He, L. Recent progress regarding electrochemical sensors for the detection of typical pollutants in water environments. Anal. Sci. 2022, 38, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, B.; Majone, M.; Cavaliere, C.; Montone, C.M.; Fatone, F.; Frison, N.; Laganà, A.; Capriotti, A.L. Determination of multi-class emerging contaminants in sludge and recovery materials from waste water treatment plants: Development of a modified QuEChERS method coupled to LC–MS/MS. Microchem. J. 2020, 155, 104732. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly imprinted polymers-towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Dili, H.; Singla, P.; Peeters, M.; Cleij, T.J.; Van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays–An overview of the current status quo. Sens. Actuactors B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Hamza, M.E.; Othman, M.A.; Swillam, M.A. Plasmonic Biosensors: Review. Biology 2022, 11, 621. [Google Scholar] [CrossRef]
- Dou, X.; Chung, P.Y.; Jiang, P.; Dai, J. Surface plasmon resonance and surface-enhanced Raman scattering sensing enabled by digital versatile discs. Appl. Phys. Lett. 2012, 100, 041116. [Google Scholar] [CrossRef]
- Barchiesi, D.; Lidgi-guigui, N.; de la Chapelle, M.L. Functionalization layer infl uence on the sensitivity of surface plasmon resonance (SPR) biosensor. Opt. Commun. 2012, 285, 1619–1623. [Google Scholar] [CrossRef]
- Divya, J.; Selvendran, S.; Raja, A.S.; Sivasubramanian, A. Surface plasmon based plasmonic sensors: A review on their past, present and future. Biosens. Bioelectron. X 2022, 11, 100175. [Google Scholar] [CrossRef]
- Esen, C.; Piletsky, S.A. Surface Plasmon Resonance Sensors Based on Molecularly Imprinted Polymers. In Plasmonic Sensors and Their Applications; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 221–236. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [PubMed]
- Wisnuwardhani, H.A.; Ibrahim, S.; Mukti, R.R.; Damayanti, S. Molecularly-Imprinted SERS: A Potential Method for Bioanalysis. Sci. Pharm. 2022, 90, 54. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly imprinted polymers for chemical sensing: A tutorial review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Diltemiz, S.E.; Keçili, R.; Ersöz, A.; Say, R. Molecular imprinting technology in Quartz Crystal Microbalance (QCM) sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. The use of polymers with enzymeanalogous structures for the resolution of racemates. Angew. Chem. Int. Ed. Engl. 1972, 11, 341–343. [Google Scholar]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Seguro, I.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Computational Modelling and Sustainable Synthesis of a Highly Selective Electrochemical MIP-Based Sensor for Citalopram Detection. Molecules 2022, 27, 3315. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Arshady, R.; Mosbach, K. Synthesis of Substrate-selective Polymers by Host-Guest Polymerization. Macromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Muller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Ndunda, E.N. Molecularly imprinted polymers—A closer look at the control polymer used in determining the imprinting effect: A mini review. J. Mol. Recognit. 2020, 33, e2855. [Google Scholar] [CrossRef]
- Yan, H.; Kyung, H.R. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R. Advances in fabrication of molecularly imprinted electrochemical sensors for detection of contaminants and toxicants. Environ. Res. 2022, 212, 113359. [Google Scholar] [CrossRef]
- Shaabani, N.; Chan, N.W.C.; Jemere, A.B. A molecularly imprinted sol-gel electrochemical sensor for naloxone determination. Nanomaterials 2021, 11, 631. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Zhang, J.; Sun, J.; Gan, T.; Liu, Y. A disposable molecularly imprinted electrochemical sensor for the ultra-trace detection of the organophosphorus insecticide phosalone employing monodisperse Pt-doped UiO-66 for signal amplification. Analyst 2020, 145, 3245–3256. [Google Scholar] [CrossRef]
- Gao, M.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Lv, J.; Wang, J.; Xu, D.; Liu, G. Recent Advances and Future Trends in the Detection of Contaminants by Molecularly Imprinted Polymers in Food Samples. Front. Chem. 2020, 8, 616326. [Google Scholar] [CrossRef]
- Unger, C.; Lieberzeit, P.A. Molecularly imprinted thin film surfaces in sensing: Chances and challenges. React. Funct. Polym. 2021, 161, 104855. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cetó, X.; del Valle, M. A novel electronic tongue using electropolymerized molecularly imprinted polymers for the simultaneous determination of active pharmaceutical ingredients. Biosens. Bioelectron. 2022, 198, 113807. [Google Scholar] [CrossRef] [PubMed]
- Yarman, A.; Scheller, F.W. How reliable is the electrochemical readout of MIP sensors? Sensors 2020, 20, 2677. [Google Scholar] [CrossRef]
- Caldara, M.; van Wissen, G.; Cleij, T.J.; Diliën, H.; van Grinsven, B.; Eersels, K.; Lowdon, J.W. Deposition Methods for the Integration of Molecularly Imprinted Polymers (MIPs) in Sensor Applications. Adv. Sens. Res. 2023, 2200059, 1–18. [Google Scholar] [CrossRef]
- Caroleo, F.; Magna, G.; Naitana, M.L.; Di Zazzo, L.; Martini, R.; Pizzoli, F.; Muduganti, M.; Lvova, L.; Mandoj, F.; Nardis, S.; et al. Advances in Optical Sensors for Persistent Organic Pollutant Environmental Monitoring. Sensors 2022, 22, 2649. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Teresa Gutierrez-Wing, M.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Oliverio, M.; Perotto, S.; Messina, G.C.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef]
- Soler, M.; Lechuga, L.M. Principles, technologies, and applications of plasmonic biosensors. J. Appl. Phys. 2021, 129, 111102. [Google Scholar] [CrossRef]
- Kashyap, R.; Chakraborty, S.; Zeng, S.; Swarnakar, S.; Kaur, S.; Doley, R.; Mondal, B. Enhanced biosensing activity of bimetallic surface plasmon resonance sensor. Photonics 2019, 6, 108. [Google Scholar] [CrossRef]
- Wood, R.W. XLII. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Proc. Phys. Soc. Lond. 1901, 18, 269–275. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Nat. Sect. J. Phys. Sci. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Yuk, J.S.; Ha, K.S. Proteomic applications of surface plasmon resonance biosensors: Analysis of protein arrays. Exp. Mol. Med. 2005, 37, 1–10. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Advances in surface plasmon resonance biosensor analysis. Curr. Opin. Biotechnol. 2000, 11, 54–61. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar, S.; Yashini, M.; Rajeshwari, S.; Mamathi, C.A.; Nirmal Thirunavookarasu, S.; Sunil, C.K. Recent advances in Surface Plasmon Resonance (SPR) biosensors for food analysis: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 1055–1077. [Google Scholar] [CrossRef]
- Wijaya, E.; Lenaerts, C.; Maricot, S.; Hastanin, J.; Habraken, S.; Vilcot, J.P.; Boukherroub, R.; Szunerits, S. Surface plasmon resonance-based biosensors: From the development of different SPR structures to novel surface functionalization strategies. Curr. Opin. Solid State Mater. Sci. 2011, 15, 208–224. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef]
- Vala, M.; Chadt, K.; Piliarik, M.; Homola, J. High-performance compact SPR sensor for multi-analyte sensing. Sens. Actuators B Chem. 2010, 148, 544–549. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.P.C.; Fafara, A.; Vandernoot, V.A.; Kono, M.; Polsky, B. Surface plasmon resonance sensors using molecularly imprinted polymers for sorbent assay of theophylline, caffeine, and xanthine. Can. J. Chem. 1998, 76, 265–273. [Google Scholar] [CrossRef]
- Fang, L.; Jia, M.; Zhao, H.; Kang, L.; Shi, L.; Zhou, L. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid molecularly imprinted polymer for amoxicillin detection. Biosens. Bioelectron. 2018, 118, 102–107. [Google Scholar] [CrossRef]
- Morin-crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical compounds in aquatic environments— occurrence, fate and bioremediation prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef]
- Papagiannaki, D.; Belay, M.H.; Gonçalves, N.P.F.; Robotti, E.; Bianco-Prevot, A.; Binetti, R.; Calza, P. From monitoring to treatment, how to improve water quality: The pharmaceuticals case. Chem. Eng. J. Adv. 2022, 10, 100245. [Google Scholar] [CrossRef]
- Sari, E.; Üzek, R.; Duman, M.; Denizli, A. Detection of ciprofloxacin through surface plasmon resonance nanosensor with specific recognition sites. J. Biomater. Sci. Polym. Ed. 2018, 29, 1302–1318. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Q.; Wang, Y.; Liu, X.; Wei, T. Surface plasmon resonance sensor for theophylline using a water-compatible molecularly imprinted film. Anal. Methods 2016, 8, 2349–2356. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, T. Detection of 17β-estradiol in water samples by a novel double-layer molecularly imprinted film-based biosensor. Talanta 2015, 141, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.Q.; Chen, X.L.; Yu, J.Y.; Nawaz, T.; Wei, T.X. Surface plasmon resonance sensor based on Bi-monomer System (BMS) molecularly imprinted polymer for detection of 17β-estradiol in aqueous media. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 032017. [Google Scholar] [CrossRef]
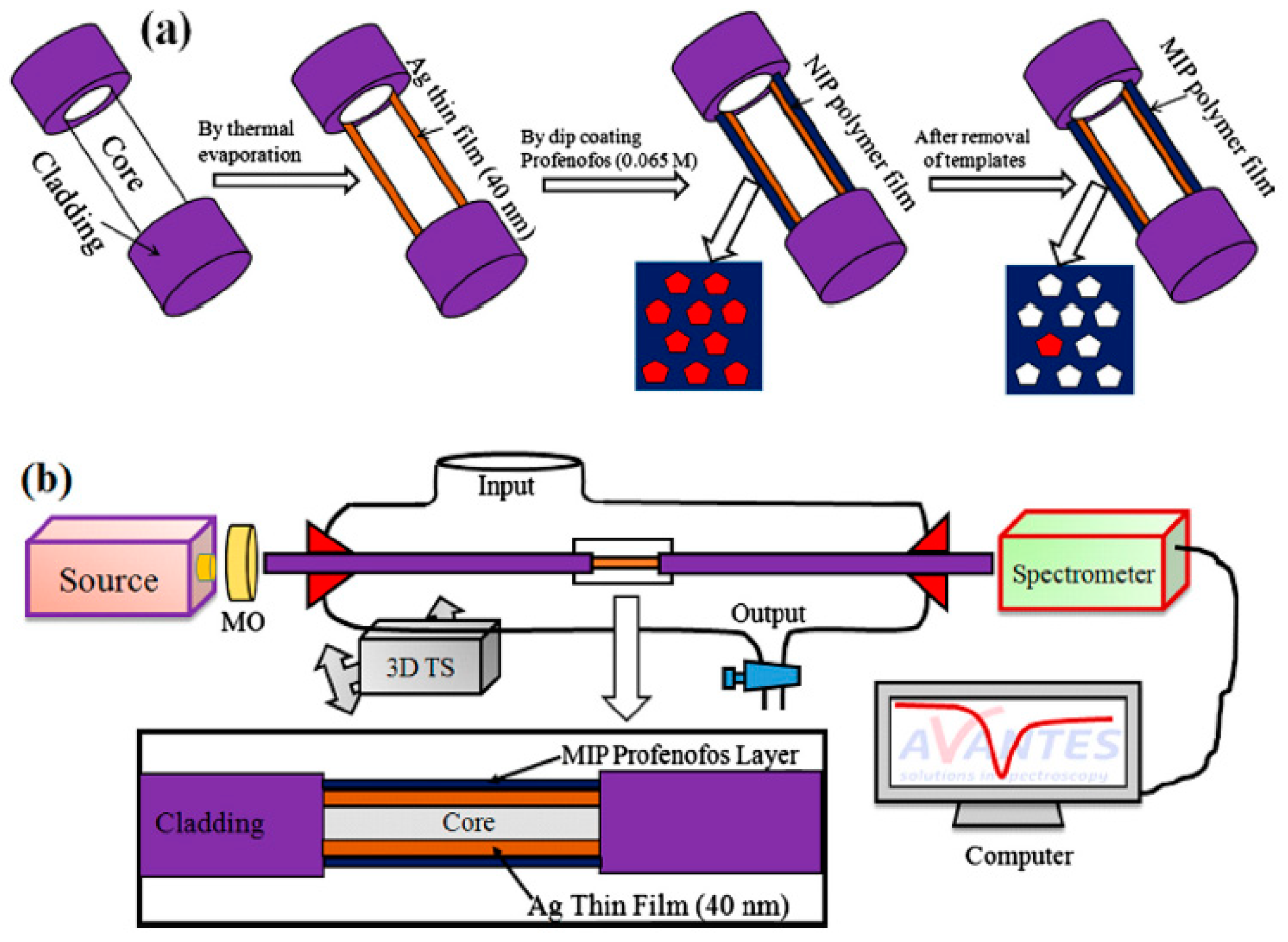
- Dong, J.; Gao, N.; Peng, Y.; Guo, C.; Lv, Z.; Wang, Y.; Zhou, C.; Ning, B.; Liu, M.; Gao, Z. Surface plasmon resonance sensor for profenofos detection using molecularly imprinted thin film as recognition element. Food Control 2012, 25, 543–549. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Denizli, A. Highly sensitive detection of Cd(II) ions using ion-imprinted surface plasmon resonance sensors. Microchem. J. 2020, 159, 105572. [Google Scholar] [CrossRef]
- Shaikh, H.; Sener, G.; Memon, N.; Bhanger, M.I.; Nizamani, S.M.; Üzek, R.; Denizli, A. Molecularly imprinted surface plasmon resonance (SPR) based sensing of bisphenol A for its selective detection in aqueous systems. Anal. Methods 2015, 7, 4661–4670. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Lütfi, M.; Wang, S. A sensitive molecular imprinted surface plasmon resonance nanosensor for selective determination of trace triclosan in wastewater. Sens. Actuators B Chem. 2015, 216, 638–644. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, J.; Zhang, L.; Zhou, J. A 3,3′-dichlorobenzidine-imprinted polymer gel surface plasmon resonance sensor based on template-responsive shrinkage. Anal. Chim. Acta 2014, 812, 129–137. [Google Scholar] [CrossRef]
- Brulé, T.; Granger, G.; Bukar, N.; Deschênes-Rancourt, C.; Havard, T.; Schmitzer, A.R.; Martel, R.; Masson, J.F. A field-deployed surface plasmon resonance (SPR) sensor for RDX quantification in environmental waters. Analyst 2017, 142, 2161–2168. [Google Scholar] [CrossRef]
- Feier, B.; Florea, A.; Cristea, C.; Săndulescu, R. Electrochemical detection and removal of pharmaceuticals in waste waters. Curr. Opin. Electrochem. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Robles-Molina, J.; Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Monitoring of selected priority and emerging contaminants in the Guadalquivir River and other related surface waters in the province ofJaén, South East Spain. Sci. Total Environ. 2014, 479–480, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Paruli, E.; Soppera, O.; Haupt, K.; Gonzato, C. Photopolymerization and Photostructuring of Molecularly Imprinted Polymers. ACS Appl. Polym. Mater. 2021, 3, 4769–4790. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Su, X. Review of optical sensors for pesticides. Trends Anal. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Nesakumar, N.; Suresh, I.; Jegadeesan, G.B.; Rayappan, J.B.B.; Kulandaiswamy, A.J. An efficient electrochemical sensing platform for profenofos detection. Measurement 2022, 202, 111807. [Google Scholar] [CrossRef]
- Vera-Avila, L.E.; García-Ac, A.; Covarrubias-Herrera, R. Trace-level determination of benzidine and 3,3′-dichlorobenzidine in aqueous environmental samples by online solid-phase extraction and liquid chromatography with electrochemical detection. J. Chromatogr. Sci. 2001, 39, 301–307. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Ju, H.; Lu, G. From lab to field: Surface-enhanced Raman scattering-based sensing strategies for on-site analysis. TrAC Trends Anal. Chem. 2022, 146, 116488. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Zhang, Y.; Wang, X.; Ozaki, Y. Recent advances in surface-enhanced Raman scattering-based sensors for the detection of inorganic ions: Sensing mechanism and beyond. J. Raman Spectrosc. 2021, 52, 468–481. [Google Scholar] [CrossRef]
- Tanwar, S.; Kim, J.H.; Bulte, J.W.M.; Barman, I. Surface-enhanced Raman scattering: An emerging tool for sensing cellular function. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1802. [Google Scholar] [CrossRef]
- Bodelón, G.; Pastoriza-Santos, I. Recent Progress in Surface-Enhanced Raman Scattering for the Detection of Chemical Contaminants in Water. Front. Chem. 2020, 8, 478. [Google Scholar] [CrossRef]
- Phuong, N.T.T.; Nguyen, T.-A.; Huong, V.T.; Tho, L.H.; Anh, D.T.; Ta, H.K.T.; Huy, T.H.; Trinh, K.T.L.; Tran, N.H.T. Sensors for Detection of the Synthetic Dye Rhodamine in Environmental Monitoring Based on SERS. Micromachines 2022, 13, 1840. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Zhai, W.L.; Li, Y.T.; Long, Y.T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43. [Google Scholar] [CrossRef]
- Shi, R.; Liu, X.; Ying, Y. Facing Challenges in Real-Life Application of Surface-Enhanced Raman Scattering: Design and Nanofabrication of Surface-Enhanced Raman Scattering Substrates for Rapid Field Test of Food Contaminants. J. Agric. Food Chem. 2018, 66, 6525–6543. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Hu, Y.; Li, G. Recent Progress on Solid Substrates for Surface-Enhanced Raman Spectroscopy Analysis. Biosensors 2022, 12, 941. [Google Scholar] [CrossRef]
- Barbillon, G. Latest Novelties on Plasmonic and Non-Plasmonic Nanomaterials for SERS Sensing. Nanomaterials 2020, 10, 1200. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S. Molecularly imprinted polymers-surface-enhanced Raman spectroscopy: State of the art and prospects. Int. J. Environ. Anal. Chem. 2022, 102, 1385–1415. [Google Scholar] [CrossRef]
- Ma, J.; Yan, M.; Feng, G.; Ying, Y.; Chen, G.; Shao, Y.; She, Y.; Wang, M.; Sun, J.; Zheng, L.; et al. An overview on molecular imprinted polymers combined with surface-enhanced Raman spectroscopy chemical sensors toward analytical applications. Talanta 2021, 225, 122031. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.; Wang, Y.; Dong, W.; Zhao, Y.; Sun, L. Bio-Inspired Imprinting Materials for Biomedical Applications. Adv. Sci. 2022, 9, 2202238. [Google Scholar] [CrossRef]
- Terry, L.R.; Sanders, S.; Potoff, R.H.; Kruel, J.W.; Jain, M.; Guo, H. Applications of surface-enhanced Raman spectroscopy in environmental detection. Anal. Sci. Adv. 2022, 3, 113–145. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, C.; Meng, G.; Wu, N. Review—Surface-Enhanced Raman Scattering Sensors for Food Safety and Environmental Monitoring. J. Electrochem. Soc. 2018, 165, B3098–B3118. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Jiang, W.; Zhou, T.; He, J.; Luan, Y.; Shang, Y.; Liu, C.; Guangbo, C. Magnetically assisted imprinted sensor for selective detection antibiotics in river based on surface-enhanced Raman scattering. Opt. Mater. 2020, 108, 110200. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Cheng, Y.; Huang, Z.; Wei, X.; Feng, J.; Cheng, J. Electrochemical aptasensor based on electrodeposited poly (3,4-ethylenedioxythiophene)-graphene oxide coupled with Au @ Pt nanocrystals for the detection of 17β-estradiol. Microchim. Acta 2022, 189, 178. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, X.; Yu, H.; Ji, L.; Zhou, T.; Liu, C.; Che, G.; Wang, D. A high-performance SERS imprinted membrane based on Ag/CNTs for selective detection of spiramycin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121587. [Google Scholar] [CrossRef]
- Xue, J.Q.; Li, D.W.; Qu, L.L.; Long, Y.T. Surface-imprinted core-shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta 2013, 777, 57–62. [Google Scholar] [CrossRef]
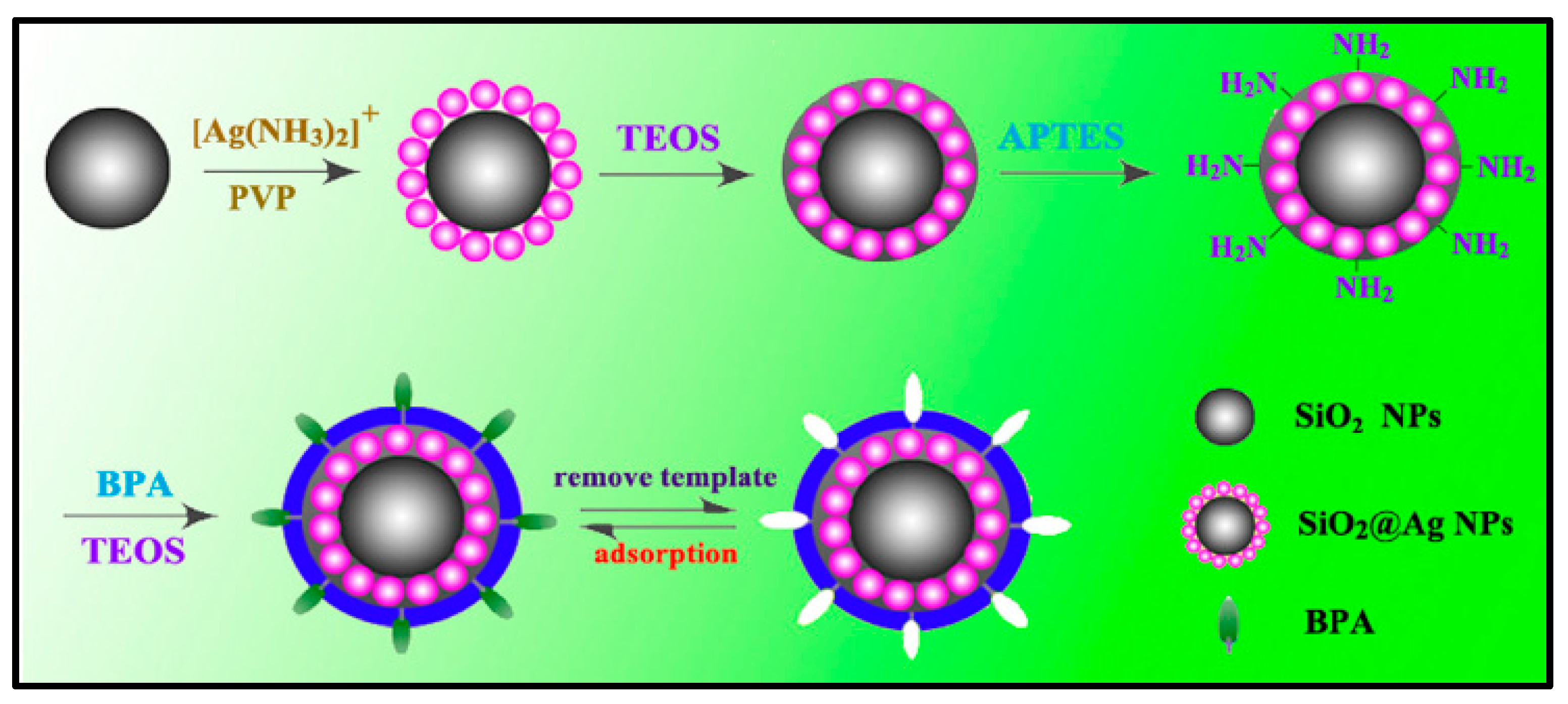
- Yin, W.; Wu, L.; Ding, F.; Li, Q.; Wang, P.; Li, J.; Lu, Z.; Han, H. Surface-imprinted SiO2@Ag nanoparticles for the selective detection of BPA using surface enhanced Raman scattering. Sens. Actuators B Chem. 2018, 258, 566–573. [Google Scholar] [CrossRef]
- Ren, X.; Cheshari, E.C.; Qi, J.; Li, X. Silver microspheres coated with a molecularly imprinted polymer as a SERS substrate for sensitive detection of bisphenol A. Microchim. Acta 2018, 185, 242. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Wang, X.; Jiang, J.; Sun, H.; Wang, L. Determination of 2,6-Dichlorophenol by Surface-Enhanced Raman Scattering with Molecular Imprinting. Anal. Lett. 2018, 51, 2062–2072. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Wang, X.; Jiang, J.; Zheng, J.; Yan, Y.; Li, C. High-performance composite imprinted sensor based on the surface enhanced Raman scattering for selective detection of 2,6-dichlorophenol in water. J. Raman Spectrosc. 2018, 49, 222–229. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Qiao, Y.; Liu, L.; Wang, Q.; Che, G. High-sensitive molecularly imprinted sensor with multilayer nanocomposite for 2,6-dichlorophenol detection based on surface-enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, M.; Zhang, J.; Wang, Q.; Li, H. A molecularly imprinted nanoprobe incorporating Cu2O@Ag nanoparticles with different morphologies for selective SERS based detection of chlorophenols. Microchim. Acta 2020, 187, 59. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Zhang, J.; Li, Y.; Yu, H.; Zhao, Y.; Wang, D.; Li, Y.; Zhu, J. Synthesis of an organic phosphoric acid-based multilayered SERS imprinted sensor for selective detection of dichlorophenol. New J. Chem. 2022, 46, 12069–12076. [Google Scholar] [CrossRef]
- Castro-Grijalba, A.; Montes-García, V.; Cordero-Ferradás, M.J.; Coronado, E.; Pérez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bu, M.; You, X.; Dai, Z.; Shi, J. High-performance detection of p-nitroaniline on defect-graphene SERS substrate utilizing molecular imprinting technique. Microchem. J. 2021, 168, 106536. [Google Scholar] [CrossRef]
- Hu, R.; Tang, R.; Xu, J.; Lu, F. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef]
- Ekmen, E.; Bilici, M.; Turan, E.; Tamer, U.; Zengin, A. Surface molecularly-imprinted magnetic nanoparticles coupled with SERS sensing platform for selective detection of malachite green. Sens. Actuators B Chem. 2020, 325, 128787. [Google Scholar] [CrossRef]
- Cheshari, E.C.; Ren, X.; Li, X. Core-shell magnetic Ag-molecularly imprinted composite for surface enhanced Raman scattering detection of carbaryl. J. Environ. Sci. Health Part B 2021, 56, 222–234. [Google Scholar] [CrossRef]
- Kou, Y.; Wu, T.; Zheng, H.; Kadasala, N.R.; Yang, S.; Guo, C.; Chen, L.; Liu, Y.; Yang, J. Recyclable Magnetic MIP-Based SERS Sensors for Selective, Sensitive, and Reliable Detection of Paclobutrazol Residues in Complex Environments. ACS Sustain. Chem. Eng. 2020, 8, 14549–14556. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Zhang, J.; Qiao, Y.; Wang, Q.; Che, G. Fabrication of pollutant-resistance SERS imprinted sensors based on SiO2@TiO2@Ag composites for selective detection of pyrethroids in water. J. Phys. Chem. Solids 2020, 138, 109254. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, X.; Wang, Z.; Wang, M.; Li, Y.; Wang, Q. Preparation of a high-performance magnetic molecularly imprinted sensor for SERS detection of cyfluthrin in river. J. Raman Spectrosc. 2019, 50, 926–935. [Google Scholar] [CrossRef]
- Li, H.; Ren, C.; Meng, J.; Gao, Y.; Ren, T.; Li, Y.; Qiao, Y. Multifunction Sandwich Composite SERS Imprinted Sensor Based on ZnO/GO/Ag for Selective Detection of Cyfluthrin in River. ChemistrySelect 2020, 5, 6475–6481. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wang, Z.; Jiang, J.; Qiao, Y.; Wei, M.; Yan, Y.; Li, C. A high-performance SERS-imprinted sensor doped with silver particles of different surface morphologies for selective detection of pyrethroids in rivers. New J. Chem. 2017, 41, 14342. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wang, Z.; Wang, Y.; Dai, J.; Gao, L.; Wei, M.; Yan, Y. A polydopamine-based molecularly imprinted polymer on nanoparticles of type SiO2@ rGO@Ag for the detection of λ-cyhalothrin via SERS. Microchim. Acta 2018, 185, 193. [Google Scholar] [CrossRef] [PubMed]
- Alula, M.T.; Mengesha, Z.T.; Mwenesongole, E. Advances in surface-enhanced Raman spectroscopy for analysis of pharmaceuticals: A review. Vib. Spectrosc. 2018, 98, 50–63. [Google Scholar] [CrossRef]
- Orbay, S.; Kocaturk, O.; Sanyal, R.; Sanyal, A. Molecularly Imprinted Polymer-Coated Inorganic Nanoparticles: Fabrication and Biomedical Applications. Micromachines 2022, 13, 1464. [Google Scholar] [CrossRef]
| Analyte | Use | Polymerization Method/ Transducer | Functional Monomer | Water Matrices | Linearity Range (nM) | LOD (nM) | Reference |
|---|---|---|---|---|---|---|---|
| Amoxicillin | Antibiotic | Sol-gel, Au SPR sensor | Methacrylamide | Tap water | 0.1–2.6 | 7.3 × 10−2 | [76] |
| Ciprofloxacin | Antibiotic | Miniemulsion, Au SPR sensor | MAA | Synthetic wastewater | 0.60–3.02 × 102 | 21.4 | [80] |
| Theophilline | Bronchodilator | Visible light, Au SPR sensor | MAA | Wastewater | 0.10–1.0 × 103 | 0.10 | [81] |
| 17β-estradiol | Estrogen steroid hormone | UV light, Au SPR sensor | MAA | Seawater | 2.50 × 10−4–2.50 | 2.50 × 10−4 | [82] |
| UV light, Au SPR sensor | MAA and HEMA | Tap water | 2.50 × 10−7–2.50 | 1.41 × 10−8 | [83] | ||
| Profenofos | Insecticide | Thermal polymerization, Au SPR sensor | MAA | Tap water | 2.7 × 10−1–2.68 | 9.64 × 10−1 | [84] |
| Thermal polymerization, optical fiber | MAA | Tap water and drinking water | 3.02 × 10−8–3.02 × 10−1 | 7.54 × 10−6 | [85] | ||
| Cadmium | Metal | UV light and miniemulsion, Au SPR sensor | N-methacryloyl-L-cysteine | Wastewater | 8.9 × 10−1–4.45 × 102 | 8.9 × 10−2 | [86] |
| Bisphenol A | Manufacturing of plastics and resins | UV light, Au SPR sensor | N-Methacryloyl-L-phenylalanine and 1-vinyl imidazole | Tap water and synthetic wastewater | 8.76 × 10−1–43.8 | 2.63 × 10−1 and 3.50 × 10−1 | [87] |
| Triclosan | Antibacterial and antifungal agent | UV light, Au SPR sensor | Methacryloylamido glutamic acid | Wastewater | 1.73 × 10−1–3.45 | 5.9 × 10−2 | [88] |
| 3,3′-Dichlorobenzidine | Manufacturing of dyes | UV light, Au SPR sensor | MAA | Tap water | 9.0 × 10−3–0.5 | 1.86 × 10−3 | [89] |
| 1,3,5-trinitroperhydro-1,3,5-triazine | Energetic material | Electropolymerization, Au SPR sensor | p-Aminothiophenol | Groundwater | 1.0 × 10−3–50 | 7.20 | [90] |
| Analyte | Use | Polymerization Method/SERS Substrate | Functional Monomer | Water Matrices | Linearity Range (nM) | LOD (nM) | Reference |
|---|---|---|---|---|---|---|---|
| Enrofloxacin hydrochloride | Antibiotic | Self-polymerization/Fe3O4@Ag NPs | Dopamine | Dam water | 1.0–200 | 1.2 × 10−2 | [114] |
| Spiramycin | Antibiotic | Sol-gel/MWCNTs@Ag NPs | APTES | River water | 1.0 × 10−2–1.0 × 103 | 1.0 × 10−2 | [116] |
| Bisphenol A | Manufacturing of plastics and resins | Sol-gel/Au NPs | 3-(triethoxysilyl)propyl isocyanate | River water | 2.19 × 103–9.99 × 104 | 5.26 × 102 | [117] |
| Sol-gel/SiO2@Ag NPs | TEOS | Tap water and lake water | 1.7 × 10−2–1.75 × 102 | 1.5 × 10−2 | [118] | ||
| Precipitation polymerization/Ag NPs | 4-vinylpyridine | Tap water | 1.0–1.0 × 106 | 1.0 | [119] | ||
| 2.6-Dichlorophenol | Precipitation polymerization/SiO2-Au NPs | Acrylamide | Lake water | 1.0–1.0 × 104 | 1.0 | [120] | |
| Precipitation polymerization/Ag-CdTe quantum dots | MAA | Lake water | 1.0–1.0 × 104 | 1.0 | [121] | ||
| Precipitation polymerization/SiO2-rGO-Au NPs | MAA and acrylamide | Dam water | 1.0–100 | 2.0 × 10−2 | [122] | ||
| Precipitation polymerization/Cu2O@Ag NPs | MAA | Lake water and wastewater | 10.0–1.0 × 106 | 5.8 | [123] | ||
| Precipitation polymerization/Ag/IP6@MIL-101(Fe) | Acrylamide | Lake water | 1.0–1.0 × 107 | 1.0 | [124] | ||
| Pyrene | Polycyclic aromatic hydrocarbon | Precipitation polymerization/Au NPS | MAA and divinylbenzene | Creek water and seawater | 0.10–1.0 × 104 | 1.0 | [125] |
| p-Nitroaniline | Manufacturing of dyes | Precipitation polymerization/defect GO-Ag | Methacrylamide | River water | 1.0 × 10−5–1.0 × 105 | 2.5 × 10−6 | [126] |
| Caffeine | Stimulant | Precipitation polymerization/Ag NPs | MAA | River water | 0–5.6 × 102 | 5.6 × 10−1 | [127] |
| Malachite green | Synthetic organic dye | Precipitation polymerization/Fe3O4@Ag NPs | MAA | Tap water | 5.0 × 10−3–100.0 | 1.5 × 10−3 | [128] |
| Carbendazim | Insecticide | Precipitation polymerization/Ag@SiO2 | Methyl acrylamide | Tap water | 1.0–1.0 × 106 | 1.0 | [129] |
| Paclobutrazol | Insecticide | Precipitation polymerization/Fe3O4@SiO2–Au@Ag | Acrylamide | River water | 2.55 × 102–3.49 × 104 | 2.55 × 102 | [130] |
| Fenvalerate | Insecticide | Precipitation polymerization/SiO2@TiO2@Ag | Acrylamide | River water | 1.0–100 | 0.2 | [131] |
| Cyfluthrin | Insecticide | Precipitation polymerization/Fe3O4@GO@Ag | Acrylamide | River water | 10.0–1.0 × 106 | 10.0 | [132] |
| Precipitation polymerization/ZnO@GO@Ag | Acrylamide | River water | 20–500 | 4.0 × 10−2 | [133] | ||
| Cyhalothrin | Insecticide | Precipitation polymerization/Ag NPs | Acrylamide | River water | 100.0–1.0 × 104 | 13 | [134] |
| λ-cyhalothrin | Insecticide | Precipitation polymerization/SiO2@rGO@Ag | MAA and acrylamide | Dam water | 1.0–1.0 × 104 | 3.8 × 10−1 | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebelo, P.; Seguro, I.; Nouws, H.P.A.; Delerue-Matos, C.; Pacheco, J.G. Molecularly Imprinted Plasmonic Sensors for the Determination of Environmental Water Contaminants: A Review. Chemosensors 2023, 11, 318. https://doi.org/10.3390/chemosensors11060318
Rebelo P, Seguro I, Nouws HPA, Delerue-Matos C, Pacheco JG. Molecularly Imprinted Plasmonic Sensors for the Determination of Environmental Water Contaminants: A Review. Chemosensors. 2023; 11(6):318. https://doi.org/10.3390/chemosensors11060318
Chicago/Turabian StyleRebelo, Patrícia, Isabel Seguro, Henri P. A. Nouws, Cristina Delerue-Matos, and João G. Pacheco. 2023. "Molecularly Imprinted Plasmonic Sensors for the Determination of Environmental Water Contaminants: A Review" Chemosensors 11, no. 6: 318. https://doi.org/10.3390/chemosensors11060318
APA StyleRebelo, P., Seguro, I., Nouws, H. P. A., Delerue-Matos, C., & Pacheco, J. G. (2023). Molecularly Imprinted Plasmonic Sensors for the Determination of Environmental Water Contaminants: A Review. Chemosensors, 11(6), 318. https://doi.org/10.3390/chemosensors11060318







