Abstract
Molecularly imprinted plasmonic nanosensors are robust devices capable of selective target interaction, and in some cases reaction catalysis. Recent advances in control of nanoscale structure have opened the door for development of a wide range of chemosensors for environmental monitoring. The soaring rate of environmental pollution through human activities and its negative impact on the ecosystem demands an urgent interest in developing rapid and efficient techniques that can easily be deployed for in-field assessment and environmental monitoring purposes. Organophosphate pesticides (OPPs) play a significant role for agricultural use; however, they also present environmental threats to human health due to their chemical toxicity. Plasmonic sensors are thus vital analytical detection tools that have been explored for many environmental applications and OPP detection due to their excellent properties such as high sensitivity, selectivity, and rapid recognition capability. Molecularly imprinted polymers (MIPs) have also significantly been recognized as a highly efficient, low-cost, and sensitive synthetic sensing technique that has been adopted for environmental monitoring of a wide array of environmental contaminants, specifically for very small molecule detection. In this review, the general concept of MIPs and their synthesis, a summary of OPPs and environmental pollution, plasmonic sensing with MIPs, surface plasmon resonance (SPR), surface-enhanced Raman spectroscopy (SERS) MIP sensors, and nanomaterial-based sensors for environmental monitoring applications and OPP detection have been elucidated according to the recent literature. In addition, a conclusion and future perspectives section at the end summarizes the scope of molecularly imprinted plasmonic sensors for environmental applications.
1. Introduction
Environmental contamination is a major crisis that has become an albatross for many global economies, such that the deliberate and unintended discharge of pollutants into the environment is a direct repercussion of the economic activities of humans [1]. The alarming rate of release of these toxic, chemical, and biological wastes thus poses a serious threat to human health and the ecosystem such that waterbodies, soil, air, aquatic species, and the food chain have all come under siege in recent times [2]. Due to the devastating impact of environmental pollutants on human health and the ecosystem, it is imperative and dire to develop rapid and sensitive analytical techniques to aid detection and assessment of the impact of these pollutants [3]. These rapid detection mechanisms could thus serve as and provide an early warning signal to support finding lasting solutions to mitigate and address this menace. Environmental monitoring employing rapid detection requires an effective approach to evaluating pollutants at extremely trace levels by employing highly efficient, sensitive, simple, and rapid analytical tools that can be used for real-life applications [1,3]. Although the gold standard approach and the most widely diverse analytical techniques used for environmental analysis include mass spectroscopy and chromatographic methods, their use for infield applications is hindered due to some drawbacks such as their expensive nature and complex instrumentation systems [4]. The advent of nanomaterials for environmental monitoring processes has broken several limitations of the conventional methods of detecting environmental contaminants as reported in numerous studies. Nanomaterial-based concepts that have been generally explored with promising potential for environmental monitoring include electrochemical sensors, surface plasmon resonance (SPR), and surface-enhanced Raman spectroscopy (SERS) sensing techniques [1,2,3,5].
The plasmonic features of noble metals enable them to adequately couple with electromagnetic radiation, thus creating a niche environment of electron plasma that potentially yields electromagnetic hot spots and is very useful for molecular sensing applications. In addition, the formation of these plasmonic hot spots serves as a catalyst for the detection of the vibrational characteristics of molecules at both nano and femtomolar concentrations [3,6].
SERS has been recognized as a promising and efficient analytical technique for the quantitative determination of a myriad of low molecular weight substances in complex matrices. This mechanism of SERS is solely based on the inelastic scattering of light, otherwise classified as Raman scattering. The SERS effect thus depicts the vibrational modes of the detected molecules, and its sensitivity is linked to the formation of an enhanced field of electromagnetic interactions between the analyte in proximity to the SERS substrates or the excited metal nanoparticles under resonance. It is thus noteworthy that the Raman signal enhancement is highly dependent on the chemical and electromagnetic enhancement potential of both the analyte and the substrate [7].
Localized surface plasmon resonance (LSPR) is an important plasmonic attribute of noble metals that results in the oscillations of surface charges that couple with electromagnetic waves. Thus, the wave propagation of these localized surface plasmons between the metal boundary and its adjacent dielectric is what results in this unique feature of the plasmonic effect [6]. However, an efficient LSPR effect significantly depends on the dielectric characteristics of the environment, the metal’s resonant wavelengths, as well as the geometry of the metal particles. Thus, tuning the shape and size of noble metal particles enhances their plasmonic, optical, and electrochemical characteristic properties for an array of sensing applications [6,8]. Hence, a plasmon wavelength shift is observed when there is a variation between the refractive index for a non-absorbent environment and the dielectric function of the surrounding [6]. It is thus evident that during sensing applications, the plasmon wavelength shift helps in signaling molecular binding events while the SERS probes and detects molecular components and their orientation on the surface of SERS nanomaterials [9].
Surface plasmons are generally oscillations of electrons that are propagated at the interface of plasmonic materials such as noble metals and a dielectric substance (for example, in an aqueous medium). The energy generated from surface plasmons is a function of the wavevector of light and the dielectric constants of both the dielectric and the plasmonic material [4,10]. The unique benefit of SPR and LSPR sensors is their label-free recognition mechanism and the variation in refractive indices that occur during the molecule detection on plasmonic surfaces. SPR and LSPR sensors are vital analytical tools, useful for environmental monitoring as they can detect molecular binding events between the target analytes and the plasmonic material and the difference between both SPR and the LSPR aligns with the scale of the length of the plasmonic material [4,10].
Nanoplasmonic sensors are excellent candidates for rapid analytical testing and environmental monitoring applications [5,11]. The SPR sensing effect on the surface of silver and gold, which are classical noble metals, is a result of the excited electrons in the conduction band that undergo coherent oscillations at the metal–dielectric interface by exposure to a wave of light. An important feature of the SPR effect is linked to sensitivity to changes in the refractive index surrounding the metallic structure, as well as the morphological characteristics of the nanostructure [6,12]. In addition, plasmonic sensors with the characteristic SPR effect have superior benefits due to their ease of preparation, cost-effectiveness as well as their unique label-free property which makes them excellent candidates for real-time monitoring applications. The suitability of SPR sensors for several fields of applications has been broadly centered on their non-invasiveness and their enhanced ability in localized sample probing. In addition, the synergistic coupling of plasmonic surfaces or SPR sensors with receptors such as molecularly imprinted polymers (MIPs) can enhance the sensitivity of these developed sensors for a broad range of sensing applications [7,13].
Interestingly, a class of nanochemosensors that has attained immense recognition and has broad-spectrum applications for environmental monitoring are MIPs [8,14]. MIPs are synthetic antibodies with a robust polymeric platform that is highly sensitive and specific in detecting a myriad of environmental targets in various matrices [15]. The development of MIPs as synthetic antibodies has been an idealistic concept; however, thirty (30) years later, it is still a challenge to reproduce MIPs accurately as three-dimensional complexes of either DNA or protein moieties [16]. Although MIPs have limitations such as mimicking the exact functionality of enzymes and antibodies, they have proven to be a better alternative to biological receptors regarding their robust sensing capability and a host of superior characteristics. These distinct excellent characteristics of MIPs that make them attractive for sensing and environmental applications include their inexpensive nature, extreme stability to adverse chemical conditions such as pH, and temperature, as well as their selectivity and sensitivity in detecting small molecules as targets. It is thus not surprising and worth noting that there is a soaring trend of exploring MIP sensors for the detection of various low molecular weight compounds and environmental pollutants as reported in several studies [17].
Over the years, our scientific research group has garnered knowledge in developing plasmonic nanostructured sensors for detecting both small molecules and biomolecules in various matrices. The focal point of our work is to explore nanomaterials and nanostructures to develop efficient analytical tools for rapid testing and monitoring processes and to help address challenges that traditional standard methods encounter in detecting a variety of molecular compounds, such as OPPs [18], protein biomarkers [19,20,21,22] and DNAs [23,24].
This review is structurally organized in sections that elucidate the history of MIPs, a general overview of MIP and their synthesis, plasmonic sensing with MIPs, SPR and SERS MIP sensors, and nanomaterial-based sensors for environmental monitoring applications and OPP detection. In addition, a special emphasis on nanoMIPs for chemosensing of some environmental pollutants was also discussed. Consequently, exploring the potential of molecularly imprinted plasmonic sensors will support and build upon the existing literature in advancing scientific knowledge on alternative rapid detection tools for trace environmental pollutant recognition.
2. History of Molecularly Imprinted Polymers (MIPs)
Ideally, the fundamental concept of molecular imprinting technology is influenced and inspired by the underlying principles of antibody–antigen and enzyme–substrate interactions [25]. As with many chemosensors and biosensors, mechanistic models describe binding using one of the numerous lock-and-key conceptual models described in the literature [25,26]. Section 2.1 provides a detailed discussion of the lock-and-key models for MIPS and nano-MIPS.
Historically, the progenitor of the technology of molecular imprinting is credited to Polyakov, a renowned Soviet chemist who discovered this concept of molecular imprinting in 1930. He observed this technology during the synthesis of polymers based on silica materials and other soluble additives [27]. However, later on, in Europe, German scientists Klaus Mosbach and Wulff Gunter in 1984 and 1985, respectively, resonated the imprinting technology when the scope of their research in MIPs became evident to the global scientific community [28,29]. Generally, these research scientists developed and employed certain techniques for synthesizing the MIPs such as the covalent, semi-covalent, and non-covalent approaches. These techniques were influenced by the type of interactions observed between the functional monomer and the template molecules or target analytes [27]. Interestingly, Mosbach and coworkers focused their research on employing the non-covalent technique for polymer-based imprinting, while Wulff and his research group used the covalent approach in creating the imprints [30]. The main difference between these two techniques is related to the removal or detachment of the template molecules from the polymer framework [27,30]. The template removal plays an integral part of the binding affinity for targets, hence it was confirmed that the covalent technique produced more uniformly distributed binding sites while the non-covalent technique yielded non-homogenous binding sites [27]. In addition, the Mosbach group focused their MIP research on the development of sensing and separation materials while the Wulff research group utilized polymer-based imprints for biochemical applications such as in catalytic reactions [30,31]. The work by the founding scientists Polyakov, Mosbach, and Wulff hence promoted the use of MIPs for analytical sensing applications. Later on, in the 20th century, another researcher by the name of Sergey Piletsky became an acclaimed scientist in the field of developing molecularly imprinted sensors for an array of applications [30,32]. Piletsky is thus a known leading expert in the field of MIPs and has produced multiple approaches in the synthesis of MIP for industrial applications, as well as the progenitor of the commercial MIP company, MIP Diagnostic Limited [15].
2.1. Molecularly Imprinted Polymers: A General Overview
Ideally, MIPs are idealized as antibody mimics that have unique recognition sites that adsorb to specific molecules as their target analytes [33,34,35]. Thus, MIPs function on the principle of the ‘lock and key’ model by selectively interacting through an adsorption and desorption mechanism and binding to specific analytes that serve as templates for the polymer framework [34].
Figure 1 shows a conceptual diagram for describing a few lock-and-key models (with and without reaction) based on particulate schematics by Ullah et al. [36]. In the absence of a reaction (Figure 1A), the rate of MIP target binding is largely governed by Arrhenius behavior, pH, background salt concentration, and binding site occupancy (i.e., cooperativity). Similar to antibody–antigen systems, MIP chemosensors are often diffusion-limited, and systems that are based in multitopic interaction (homobivalent or heterobivalent) may have considerable cooperativity. The signal in such an MIP system (highlighted with a red halo in panel A) is a direct result of target binding and is proportional to the global binding constant (KD). In nanoparticle–MIP ligand systems (Figure 1B), the conceptual idea is that local proximity between the template and doped nanoparticle(s) creates a catalytic site, similar to enzymes. The formation of a target–MIP complex (AB) occurs at the template site, governed by the same principles as non-reactive MIP systems. Using the example of Michaelis–Menten kinetics as a model, a nanoparticle-catalyzed reaction near the template site produces a reaction byproduct (P) which diffuses away and leaves the template site vacant for a subsequent binding/reaction sequence. During the initial interaction, the reverse reaction rate (k-Rxn) is shown for thoroughness, which is thermodynamically possible (particularly in complex media). The overall process is affected by the presence of inhibitors, or insufficient activators (i.e., nanoparticles in proximity of template sites). As discussed by Ullah et al. [36], many other kinetic models exist for describing affinity-based sensors, but the examples below remain the core mathematical models for understanding MIP and nano-MIP systems.
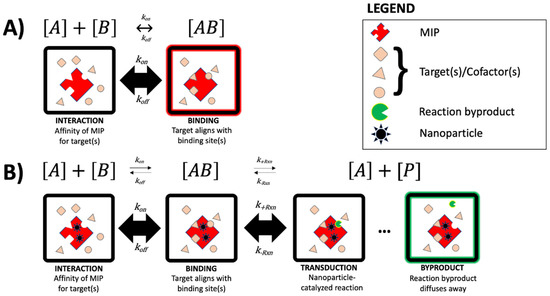
Figure 1.
Particulate schematic of MIPS (top row) and nano-MIPS (bottom row), including an example of one kinetic model for each type of system. In this model, [A] represents a generic templated ligand (MIP), and [B] represents the molecular target. (A) Conceptual interaction between targets/cofactors and MIP. Signal is represented by a red halo, stoichiometrically shown as [AB]. (B) Conceptual diagram depicting nanoparticle-MIP ligand systems, where nanoparticle(s) serve as a reactive site. The signal is represented by a green halo, stoichiometrically shown as [P].
MIPs have unique characteristic features such as high stability compared to other biological receptors. The characteristic enhances their selectivity to diverse targets, and stable in severe environmental conditions such as a broad range of temperatures and pH [34]. The durability, efficiency, and low-cost nature of synthesizing MIPs make them suitable candidates for a myriad of applications such as for simulated enzyme catalysis, environmental contaminant or pesticide residue detection, solid-phase extraction, biotechnological and chromatographic separation processes [35,37]. Among the numerous advantages of MIP, one benefit that has superiority over other receptors employed for sensing or separation techniques includes its ease of reusability of up to several times for any application [37]. Generally, the method of synthesizing MIPs for various technical applications follows a standard principle by (1) creating a polymer that has the target or template molecule incorporated by either a covalent, semi-covalent, or non-covalent interaction to functional monomers, (2) creating and activating the binding sites that are target-specific by the removal of the template molecule from the polymer framework, and (3) selective target molecule detection by the fabricated MIP when exposed to a sample containing the target molecule [34]. MIPs have gained prominence in their near universality as artificial receptors specifically for the detection of extremely minute molecules which enables them to be synthesized for any molecule as a target analyte [34,38]. Biological receptors such as antibodies, enzymes, and aptamers on the other hand, in comparison to MIPs, are limited in their universal functionality due to most of their functional characteristics targeting macromolecules rather than small analytes as targets [38,39,40,41]. Furthermore, MIPs are inexpensive in terms of costs compared to the highly exorbitant prices of obtaining most biological receptors such as antibodies [15,42]. Furthermore, the reported literature [43,44,45,46] has recommended a highly efficient use of MIPs for environmental monitoring applications as they possess a promising potential when coupled with different sensing systems [34,38]. Figure 2 shows a general schematic for the production of MIPs for various applications.
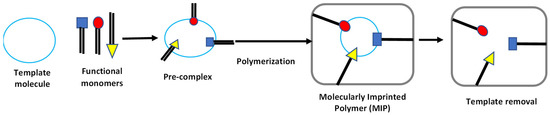
Figure 2.
A generic schematic illustration for MIP synthesis. The first step involves complexation of a functional monomer to a template molecule, followed by polymerization and finally eluting the template molecule to yield the MIP-based sensor platform.
2.2. The Concept of Producing Molecularly Imprinted Polymers
There are numerous established approaches in the available literature by which MIPs are generally synthesized. Some of the notable concepts of producing MIPs include (1) soft lithography techniques, (2) chemical synthesis employing functional monomers and template molecules, and (3) employing a phase inversion technique of precipitating a polymer with an insoluble additive [34]. Among the listed concepts of producing MIPs, the most widely used approach is the chemical synthesis method which is comprised of functional monomers interacting covalently or non-covalently with template molecules to form a complex molecule. Some examples of frequently used MIP functional monomers are acid-based compounds such as methacrylic acid (MAA) and itaconic acid, and some neutral pH compounds such as acrylamide [34,47]. The vital role of the functional monomer is to provide hydrogen bonding or as a reactive species to form a covalent interaction with the template molecule [34]. The formation of the functional monomer–template molecule complex is then supported by the action of a cross-linker compound, and one typically used is ethylene glycol dimethacrylate (EGDMA). For effective polymerization to occur, an initiator and an organic compound are required. For example, azobisisobutyronitrile (AIBN) may be mixed, and polymerization takes effect in the presence of heat or ultraviolet radiation [38]. The polymerization process terminates by producing a powdered substance (MIP) in most cases, which could be employed for an array of applications after the removal of the template molecule by the Soxhlet extraction method which ensures the activation of the binding sites or cavities of the MIP [34,38,47,48]. Some of the commonly used organic compounds employed for MIP synthesis have been outlined (Figure 3).
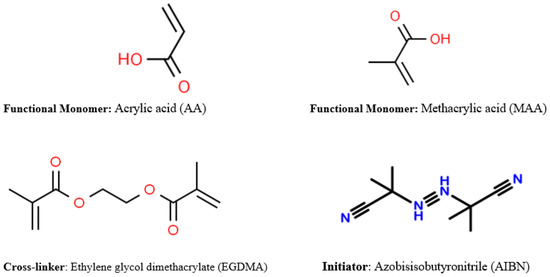
Figure 3.
Some general organic molecules employed for the synthesis of molecularly imprinted polymers.
2.3. Organophosphate Pesticides and Environmental Pollution
Pesticides are generally important control chemicals for pests and diseases, and their use in the agricultural sector cannot be overemphasized. The application of pesticides has a beneficial role in pest management and improving agricultural yield, thus ensuring food security, and as such has become a fundamental agricultural practice in many global economies [49]. OPPs are categorized as the esters of phosphoric acids and are considered the most extensively employed pesticide globally for agricultural purposes [50]. Some examples of OPPs include malathion, chlorpyrifos, dimethoate, and parathion specifically used in the agricultural sector. Inappropriate and excessive use of these OPPs and exposure of these pesticide residues to the environment release toxic compounds into the ecosystem that poses an environmental threat and a gross public health risk [51,52]. Interestingly, these residues are highly toxic to all living strata, especially when OPPs are sprayed on crops; they are able to sparsely diffuse into the environment and can also seep into underground water and contaminate river bodies through agricultural run-off-water [53] (Figure 4). It is also worth noting that OPPs are neurotoxins that potentially inhibit the enzyme acetylcholinesterase (AChE) responsible for supporting the nervous system of all biological species including humans. Hence, OPPs impair neuromuscular transmission and affect the respiratory system [53]. Due to the chronic negative impact of OPPs on the environment and human health, it is critical that rapid analytical tools such as plasmonic sensors are developed [11]. These sensors will help to assess the toxicological impact of these OPPs, which could also support recovery strategies for pesticide removal from the environment and to protect human health [11].
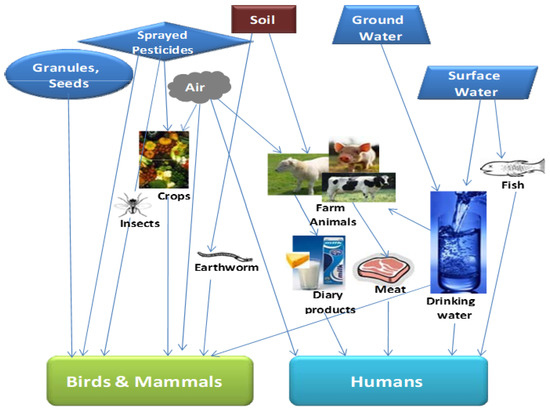
Figure 4.
A schematic of the impact of organophosphate pesticides on humans and the environment. Reprinted/adapted with permission from Ref. [18].
3. Plasmonic Sensing with Molecular Imprinting Technology
Over the recent years, molecular imprinting technology has gained prominence with several applications linked to the analytical sensing of various biological, chemical, and environmental matrices [54]. The adoption of synergistic approaches such as coupling MIPs with plasmonic nanomaterials is an excellent example of enhancing the efficiency of plasmonic sensors [54]. Plasmonic sensors, therefore, consist of sensing materials that have metal or metal-dielectric nanostructures that can generate surface plasmons and are incorporated with recognition materials that tend to distinctively bind to specific targets. Generally, plasmonic sensors are affiliated with the class of optical affinity sensors, such that generated signals become measurable when ligands attach to specific target analytes [55,56]. Figure 5 shows a schematic for the SERS plasmonic detection of a pesticide (cypermethrin) employing gold (Au) MIPs.
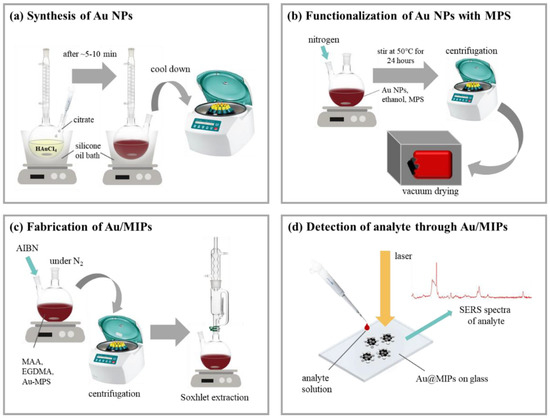
Figure 5.
A gold (Au) nanoparticles MIP illustration for the SERS detection of the analyte cypermethrin [57].
3.1. Surface Plasmon Resonance and Surface-Enhanced Raman Spectroscopy MIP Sensors
SPR and SERS are considered vital plasmonic features and are recognized as superior and ultrasensitive transduction and powerful readout technologies for a myriad of sensing applications [58,59]. These plasmonic features are more appealing to use by coupling with MIP chemosensors largely due to their capability of detecting various analytes such as pesticides, antibiotics, microorganisms, amino acids, and proteins at trace concentration levels [15,38].
Generally, an MIP chemosensor based on SPR has an integration of silver or gold nanocomposites coupled to it, which eventually relays a variation in refractive index and electron density at the MIP’s surface upon binding of the target analyte to the MIP [15,38].
Surface plasmon resonance (SPR): SPR could be defined as a collective oscillation of free electrons in the conduction band of a material that has an interfacial existence between two media with positive and negative dielectric permittivity constants that are triggered by incident light. The mobility of electrons thus generates an electromagnetic wave within and at the peripherals of the structure, therefore depicting a surface plasmon peak in the monitored region of the absorption spectrum [60]. For example, thin films coupled to metallic surfaces create an SPR signal upon analyte binding which is relayed to the SPR transducers, and the signal detected occurs due to a variation in the thickness and refractive index of the thin films. It is noteworthy that most noble metals such as silver and gold are a repository for surface plasmons; however, other suitable alternatives include chromium, copper, and titanium. In MIP sensors, their surfaces are usually functionalized by thiols via in situ thin film polymerization during the MIP’s pre-synthesis phase. A transducer integrated with an MIP sensor laced with silver or gold promotes the SPR signal during a binding event, whereby the applied incident light causes an incident angle shift due to the analyte binding effect altering the MIP layer’s refractive index [44].
Localized surface plasmon resonance (LSPR) also refers to the optical phenomenon whereby there is confinement of an electromagnetic wave or surface plasmon inside conductive nanoparticles such as in silver and gold; however, other suitable nanocomposites of platinum and palladium could also generate LSPR peaks. The LSPR effect occurs predominantly due to the free electrons in the metallic nanoparticles interacting with the incident light or electromagnetic wave, whereas the plasmonic frequency and intensity are dependent on multiple factors such as the dielectric constants of the medium and the morphological characteristic (size and shape) of the nanoparticles [44,60].
MIP–SPR sensors have found important use in many applications such as for the detection of proteins and biomolecules as well as detecting explosives. For example, histamine was successfully detected at a LOD of 25 µg/L by an SPR sensor that was spin-coated with MIP films [44]. Shrivastav et al. [61] developed a synergistic optical fiber MIP-SPR device for the determination of profenofos. This device had enhanced sensitivity and specificity for the target analyte with a LOD of 2.5 × 10−6 μg/L and the linear range for the detection was from 10−4 to 10−1 μg/L. Another study by Saylan et al. [62] also developed an MIP-SPR sensor that was able to detect multiple pesticides such as atrazine, cyanazine, and simazine that were in aqueous form. The MIP sensor was highly selective and was thus able to detect the pesticide with LODs of 0.091, 0.095, and 0.031, respectively. Moreover, it was also observed that the sensor had the capability of being reused four times on average.
Surface-enhanced Raman Spectroscopy (SERS): SERS constitutes the enhancement of Raman scattering and is a very surface-sensitive process that occurs during a binding event and is stimulated by localized surface plasmon coupling with an electromagnetic wave or an incident polarized light on nanostructures or uneven metal surfaces. SERS relies on a signal detection mechanism and when incorporated into MIP-based sensors, this signal is amplified by plasmonic or chemical effects from Raman active molecules that depict a specific vibrational Raman spectrum [38,44]. SERS has distinguished its uniqueness from all other optical transduction mechanisms employed for MIP sensors due to its ability to ultra-sensitively and directly detect targets at solid and liquid interfaces [63,64] as observed in Table 1. In addition, SERS can also potentially detect a wide range of analytes in the femtomolar range and has notably found use in food safety and environmental monitoring applications [15].

Table 1.
Some reported MIP sensors in the literature developed with SPR and SERS for various environmental applications.
Interestingly, SERS measurements can be depicted in coordination with Raman active molecules as well as employed with the LSPR mechanism. Although the SERS mechanism is not completely understood, there are two schools of thought as evidenced in the literature. The first concept is linked to the formation of a chemical bond due to a charge-transfer complex between the metallic structure and the target analyte, thus enhancing its polarization effect. The second concept, on the other hand, revolves around the theory of electromagnetism, in conjunction with the LSPR effect around the surface of the nanoparticles [44]. A summary of some efficient MIP sensors developed with SPR and SERS for the detection of various environmental contaminants is outlined (Table 1).
Furthermore, SERS have been efficiently employed for the non-destructive and rapid identification of a wide array of matrices inclusive of chemical and biological analytes. For example, MIP sensors have successfully explored the efficiency of SERS in detecting histamine (HIS) in canned fish and its reliability and accuracy were unmatched with HIS results ranging from 3 to 90 ppm [85]. The technique of SERS involves an inelastic scattering phenomenon and thus can be used to depict the structural properties of a molecule [86]. Classical noble metals such as silver and gold nanocomposites provide a synergistic SERS effect as these are vital substrates that influence the displayed Raman spectra [86]. The effectiveness of SERS could also be significantly enhanced by coupling it with other techniques such as labeling techniques, colorimetry, and microfluidic systems [86]. In addition, for enhanced accurate analytical sample characterization, SERS could be synergistically used with procedures such as infrared spectroscopy, X-ray photoelectron spectroscopy (XPS), nuclear magnetic resonance (NMR), and mass spectrometry [60]. For example, an MIP–SERS Solid-Phase Extraction (SPE) with silver nanoparticles as a SERS substrate was able to efficiently and rapidly detect 2,4-dichlorophenoxyacetic acid in milk samples [87].
Similarly, another study by Feng et al. [88] employed an MIP chemosensor developed on the principle of SERS for the selective detection of thiabendazole in orange juice samples with LOD 4 ppm. The MIP–SERS sensor setup constituted a SPE cartridge that was laced with the MIPs as sorbents. Interestingly, this sensor system was highly sensitive and very rapid with the complete analytical detection process occurring within twenty-three (23) minutes.
Some optical transduction schemes based on SPR and fluorescence of nanocomposite MIP sensors and their applications in the analytical detection of Sudan dyes and paraquat in environmental samples have been highlighted (Figure 6).
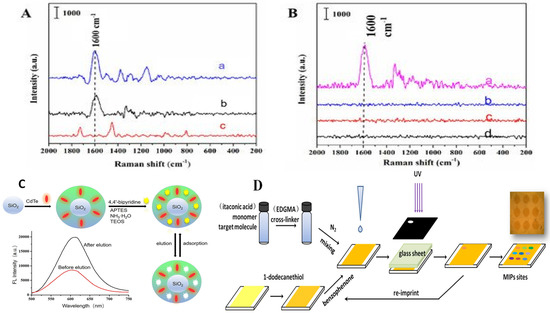
Figure 6.
An illustrative scheme of some optical transduction interactions of nanocomposite MIPs in detecting various environmental analytes. Inserts (A,B) illustrate the surface-enhanced Raman spectroscopy spectra interactions of 1 ppm of Pentachloronitrobenzene (PCNB) with oil-soluble silver-MIP [89]. (A): Surface-enhanced Raman spectroscopy (SERS) spectra of PCNB with oil-soluble silver nanometer as substrate (a), MIPs absorbing 1 µg/mL of PCNB (b), and NIPs absorbing 1 µg/mL of PCNB (c). (B): The SERS spectra acquired using the oil-soluble Ag NPs-embedded MIPs as substrates after the MIPs were respectively incubated with the solutions of PCNB (a), 3,4,5-trichloronitrobenzene (b), 3,5-dichloronitrobenzene (c), and para-chloronitrobenzene (d) for 120 min at the concentration of 1.0 µg/mL. Insert (C) also shows a schematic of a fluorescent silicon dioxide cadmium tellurium quantum dots (SiO2@CdTe QDs) MIP for the detection of paraquat in water samples. Reprinted with permission from Ref. [90] and insert (D) represents a surface plasmon resonance-based molecularly imprinted polymer sensor chip for the detection of antibiotics. Reprinted with permission from Ref. [91].
3.2. Nanomaterial-Based Sensors for Environmental Monitoring
Nanomaterials have become attractive elements for the development of sensors for environmental applications due to their unique surface chemistry and functional properties including their potential to detect environmental pollutants at low concentrations [92]. These nanomaterials are endowed with tunable physico-chemical properties and desirable characteristics such as enhanced catalytic potential, high surface-to-volume ratio, as well as enhanced surface reactivity. These intrinsic properties of nanomaterials promote their suitability as efficient nanosensors for environmental monitoring applications [93]. Nanomaterials could thus play two significant roles in sensors, which include a strong affinity for absorption and sensitive signal transduction. For example, graphene or carbon nanotubes (CNTs) possess a strong adsorptive capacity that could enhance the detection of the targeted analyte; in addition, metal nanoparticles such as gold (Au) or silver (Ag) could efficiently amplify the signal transduction linked with the detection of the targeted analyte.
Another salient application of nanocomposite materials involves Au and Ag nanoparticles for the design of colorimetric and optical sensors, as they function on the principle of color change and excitation of LSPR when a chemical analyte is detected [93]. Nanosensors generally possess superior performance compared to conventional sensors due to their high level of sensitivity, specificity, and rapid response time [94,95,96]. Consequently, nanocomposite materials can be functionalized as nanosensors for ultrasensitive or trace element detection of environmental pollutants in complex matrices [92]. The properties of nanomaterials coupled with analytical signal detection techniques such as SERS, fluorescence, and electrochemistry have caused the field of nanosensors to evolve significantly over the years [92,93].
To date, CNTs, quantum dots, graphene, silicon nanowires, Au, and Ag nanoparticles have been significantly exploited in several studies for the detection of environmental contaminants including pesticides. Graphene and CNTs have been efficiently employed as field-effect transistor (FET) sensors for the detection of heavy metals and pesticides due to their suitable and excellent electrically conductive property [97]. Silver and gold nanoparticles have historically exhibited enhanced extinction coefficients and are suitable candidates as bio- and chemosensors for a wide array of environmental monitoring applications. Generally, the effect of plasmonic coupling in metallic nanoparticles induces electromagnetic enhancement, thus promoting SERS signals of the analytes to be captured and detected in the nanosensors and very much useful for single-molecule sensitivity [98]. Several studies have highlighted the detection of pesticides with nanomaterial-based sensors. For example, Moraes et al. [99] successfully detected carbaryl (1.09 ± 0.02 μg/L) in natural water samples by using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Methyl parathion was also detected in fruits and vegetables with the synergistic coupling of silver and graphene nanoribbon nanocomposite-modified screen-printed electrode [100]. Another study by Liao et al. [101] yielded a sensitive fluorescence immunoassay sensor device that was composed of CdSe/ZnS Quantum dots. The sensor efficiently detected triazophos in apple, pear, cucumber, and rice samples. Au nanoparticles were also used as signal enhancers in optical biosensors and efficiently determined a cocktail of OP compounds (edifenphos, iprobenfos, and diazinon) by a change in coloration of the aggregated Au nanoparticles when they were in contact with the targeted OP compounds. Outstanding results due to the Au nanoparticles were recorded as 27.9 ppb, 53.6 ppb, and 53.3 ppb LODs for edifenphos, iprobenfos, and diazinon, respectively [102].
3.3. Nanoparticle MIPs (NanoMIPs) for Chemosensing of Environmental Pollutants
The quest to enhance analytical sensing techniques has yielded tremendous results with the adoption of MIP systems, especially in the chemosensor and bioassay sectors. The robustness of MIPs with the integration of nanoparticles broadens the functionality of the chemosensor for a wide range of applications. Consequently, the MIP chemosensor should possess the below-recommended features [38]:
- (i)
- High specificity and enhanced affinity that has unparalleled recognition characteristics when coupled with a transducer.
- (ii)
- An intensely sensitive transducer for monitoring and processing the binding potential between the monomer and the imprinted cavities.
MIPs have also distinguished their usefulness in chemical sensing such that targeted analytes in the MIP chemosensors generate physicochemical changes during binding reactions which trigger signals that are transduced and confirmed for the quantitative or qualitative attribute of the toxicant determined. Typical aspects of these transduction signals include a change in optical or fluorescence intensity, and the most employed signal transduction includes SERS, SPR property conductometry, voltammetry, potentiometry, and amperometry [38]. NanoMIPs have a promising potential to detect a myriad of environmental pollutants such as antibiotics in various matrices. For example, Figure 7A,B depicts the sensing potential of a gold SPR chip immobilized with vancomycin-imprinted nanoMIPs, for the targeted detection of vancomycin (a glycopeptide antibiotic) in milk samples.
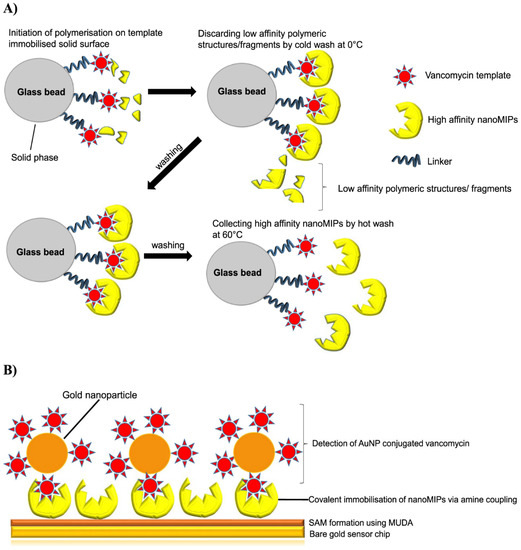
Figure 7.
The efficient use of nanoMIPS for the detection of vancomycin, a glycopeptide antibiotic in milk samples. Insert (A,B) illustrates a schematic of the synthesis of vancomycin nanoMIPs and their chemosensing applications for the detection of vancomycin in complex matrices [103].
Interestingly, MIPs can be synthesized in different modes such as in the form of nano- or microparticles or as films and membranes. In context, nanoparticle MIPs present significant superiority compared to the other forms of bulk material MIPS due to their enhanced binding kinetics, loading capacity and chemical reactivity potential [104,105]. This phenomenon could be attributed to the concept of ‘small is big’, which implies nanoparticles possess a larger surface-to-volume-ratio per unit weight of the polymer matrix [38]. Consequently, the functionality of the MIP system is enhanced by the nanoparticles such that the affinity of the imprinted cavities for the analytes of interest, the kinetics of binding, template removal, as well as molecular recognition characteristics are highly stimulated. Scientific researchers and several authors have determined that nanoparticles embedment in MIPs is very promising, and as such it is considered in sensing assays or for sensor, therapeutic and diagnostic applications. For example, D’Aurelio and coworkers [106] developed a highly sensitive nanoMIP-SPR-based sensor for the detection of β-lactoglobulin. Ashley et al. [107] also synthesized a nanoMIP sensor for the detection of α casein based on the property of SPR. NanoMIPs have also been efficiently developed as biomarkers for the detection of fucose and mannose [108].
For the environmental monitoring of OPPs, novel dual-templated nanoMIPs developed by Abbasi Ghaeni and coworkers were very efficient in detecting diazinon, malathion, glyphosate and dichlorvos in water treatment samples. The nanoMIPs were prepared by the precipitate polymerization technique and the template molecules were the same as the evaluated OPPs. An enhanced binding affinity of the MIP nanoparticles for the detected OPPs were observed in comparison to the non-imprinted polymer nanoparticles [109].
Another fascinating outlook of MIPs is their synergistic interaction with nanoparticles for the development of detection assays. Interestingly, these nanoMIP detection assays have optical and colorimetric properties, thus they are suitable and promising candidates for a wide range of applications [15]. For example, Piletska et al. [110] developed a novel magnetic nanoMIP assay for pepsin detection. In their study, pepsin was the template or target molecule which was synthesized based on a solid-phase technique. The readout of the nanoMIP assay and its sensitivity was also linked to the inclusion of fluorescent polystyrene beads which yielded excellent results and detected pepsin in the quantifiable range between 5 and 50 μg/mL.
In addition, nanoMIPs have become efficient vital tools for the rapid detection of environmental pollutants such as the OPPs, diazinon in food and drinking water. This was evidenced by the development of an MIP nanoparticle-based electrochemical sensor for the analytical determination of diazinon in apple fruits and well water samples [111]. In this study, the precipitate polymerization technique was employed in synthesizing both the MIP and the non-imprinted polymer (NIP). In addition, the MIP nanoparticles were immobilized on a carbon paste electrode and the square wave voltammetry was used for the analytical determination of diazinon in the evaluated samples. It was reported that the sensor was highly efficient for detecting this OPP in the tested samples and the reported LOD of 5.43 × 10−9 M was obtained. Furthermore, the recovery rate of the developed sensor was very promising and was in range of 99.06 to 99.16% [111].
Another study by Wu et al. [112] demonstrated the colorimetric quantitative detection of cartap (pesticide) residue in tea beverages by employing a silver nanoparticle sensor coupled with a magnetic MIP microsphere system. In their design, cartap was the centralized template molecule, mesoporous SiO2 was the intermediate shell, and the recognition element was Fe3O4@mSiO2@MIPs, functional monomer (methacrylic acid), and Fe3O4 was used as the core-shell. The developed nanoMIP thus selectively eluted cartap from the spiked tea samples and the incorporated silver nanoparticles confirmed the quantitative presence of the pesticide (0.1–5 mg/L with LOD 0.01 mg/L) as measured by UV–vis spectroscopy.
In addition, a study by Mankar et al. [113] also developed an efficient nanoMIP system that selectively adsorbed and detected arsenic in groundwater. In their design, polymethacrylate was used as the functional monomer, and imprinting was achieved by the complex of arsenic and fluorescein. The resultant nanoMIP demonstrated a high arsenic adsorption capacity (49 ± 7 mg/L) and had a recovery rate of 98% when the nanoMIP was thoroughly washed with 0.1 M HNO3 solution.
Over the recent years, our research laboratory has developed and demonstrated that organic compounds acting as chemosensors distinctively detected different OPPs through fluorescence emissions. In such work, Guo et al. [114] confirmed that three different OPPs, namely fenthion, malathion, and ethion, uniquely provided fluorescence emissions by binding to two phenanthroline derivatives which include (i) benzodipyrido [3,2-a:2*,3*-c] phenazine (BDPPZ) and (ii) 3,6-dimethylbenzodipyrido-[3,2-a:2*,3*-c] phenazine (DM-BDPPZ), respectively. It was thus confirmed that the BDPPZ and the DM-BDPPZ selectively bounded to the evaluated OPPs and had a significant limit of detection (LOD) of 10−8 M, 10−9 M, and 10−12 M, respectively, for fenthion, malathion, and ethion as shown in Figure 8. The promising outcome of these results confirmed the potential for these chemosensors to selectively detect OPPs and be employed for environmental monitoring purposes.
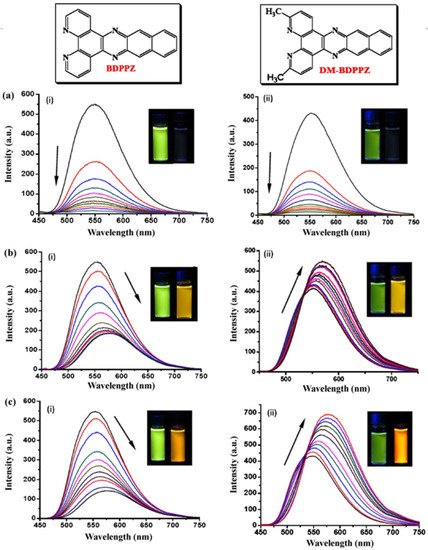
Figure 8.
The fluorescence spectra changes of (i) benzodipyrido [3,2-a:2*,3*-c] phenazine (BDPPZ) (ii) 3,6-dimethylbenzodipyrido-[3,2-a:2*,3*-c] phenazine (DM-BDPPZ) when bound to three different OPP pesticides, (a) fenthion, (b) malathion, and (c) ethion, respectively. The inserts show the corresponding color transformations with the direction of the arrow indicating concentrations of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 µM, respectively [114].
In another study by our group, Obare et al. [18] evaluated the effectiveness of dimethyl-[4-(2-quinolin-2-yl-vinyl)-phenyl]-amine (DQA), an azastilbene derivative, as an efficient chemosensor using fluorescence spectroscopy for the detection of four distinct OPPs, namely ethion, malathion, parathion, and fenthion, respectively. It was observed that fluorescence quenching was evident in all cases at a wavelength of 530 nm for ethion, malathion, and parathion, except no quenching was observed for the fenthion OPP when they were titrated with DQA (Figure 9). With the results of our study, we demonstrated that the sensitivity of fluorescence quenching of the OPPs was in the increasing order of ethion > malathion > parathion. Consequently, organic molecules with enhanced sensitivity to OPPs can potentially serve as chemosensors and be employed for environmental monitoring applications such as for the detection of organophosphate chemical compounds.
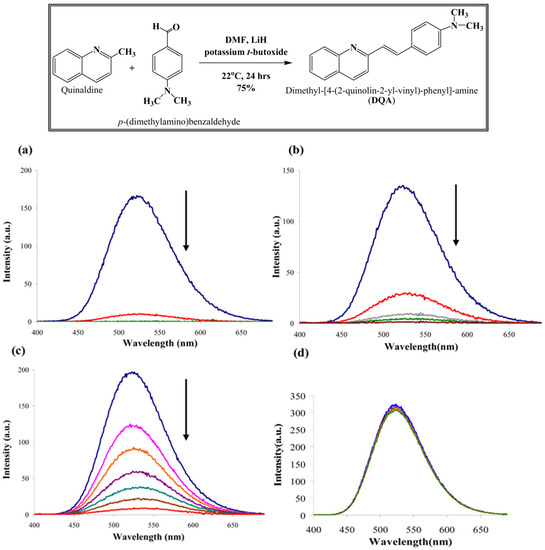
Figure 9.
A schematic illustration of the synthesis of dimethyl-[4-(2-quinolin-2-yl-vinyl)-phenyl]-amine (DQA), an azastilbene chemosensor used for the evaluation of four different organophosphate pesticides in our laboratory. The fluorescence emissions spectra of DQA upon binding to four different OPPs (ethion, malathion, parathion and fenthion). (a) Titration with ethion; from top to bottom, concentration of ethion = 0, 2, 4, 6 µM; (b) titration with malathion; from top to bottom, concentration of malathion = 0, 2, 4, 8 µM; (c) titration with parathion; from top to bottom, concentration of parathion = 0, 2, 4, 6, 8, 10, 12 µM; and (d) titration with fenthion, with up to 24 µM of fenthion added, no change was observed. The direction of the arrow indicates the transformation in intensity of fluorescence [18].
4. Conclusions and Outlook
Molecularly imprinted plasmonic sensors have become a household name as vital environmental monitoring sensing tools. As such, they play an integral and fundamental role in the field of environmental sciences and have an equally huge potential use in the medical and biotechnological sectors as well. Plasmonic sensors in general are recognized as excellent candidates in analytical sensing techniques due to their outstanding ability to effectively detect and monitor several targets in environmental matrices. Plasmonic sensors coupled with MIPs are very cost-effective, ultrasensitive, highly selective, and stable to various extrinsic factors such as extreme temperature and pH compared to conventional sensing approaches. Consequently, some signal transduction features of plasmonic MIP sensors such as SPR, LSPR, and SERS are powerful read-out mechanisms that enhance the sensitivity of these MIP sensors for trace element detection.
Although these sensors have yielded profound results for an array of applications, there are still some hurdles that mitigate against their use, especially for detecting targets in complex environmental matrices. In addition, the challenge of designing plasmonic MIP sensors to efficiently function in different or multi-environments has been daunting. In resolving the challenges of enhanced sensitivity and the limit of detection of these sensors, it is advocated to consider metal nanoparticles that are mono-dispersed morphologically in the design of these MIP plasmonic sensors. In addition, considering a selective domain of monomers that possess multifunctional properties with a host of template molecules can ensure enhanced distinction, specificity, and selectivity of target analytes in different complex environmental matrices.
Irrespective of the highlighted challenges, plasmonic MIP sensors are still considered powerful sensing tools and a force to reckon with in detecting OPPs, as well as for numerous environmental applications. In addition, the advent of high-end technologies in the molecular imprinting field has made it possible for these sensors to be commercialized and employed for real-life applications. Our review thus elucidated the potential of plasmonic MIP sensors for environmental monitoring applications and the detection of OPPs and other environmental pollutants by highlighting their characteristic features of enhanced sensitivity, selectivity, and robustness coupled with their limits of detection of various target analytes in environmental samples.
Author Contributions
Writing: R.D.A., B.O.A., J.W., S.O.O. and E.S.M., Formal analysis & Investigation: R.D.A., B.O.A., J.W., S.O.O. and E.S.M.; Conceptualization: R.D.A., J.W., E.S.M. and S.O.O.; Methodology: R.D.A., J.W., E.S.M. and S.O.O.; Supervision & validation: J.W., E.S.M. and S.O.O.; Funding Acquisition: S.O.O. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support from the US NSF EiR research grant (award#: 1832134) and the National Science Foundation (Grant ECCS-2025462), National Science Foundation of Science and Technologies for Phosphorus Sustainability (STEPS) (CBET-2019435). Financial support for this work was also provided, in part, by Joint School of Nanoscience and Nanoengineering (JSNN), a member of the National Nanotechnology Coordinated Infrastructure (NNCI). Authors also acknowledge the USDA NIFA equipment awarded to acquire Field-Emission SEM (JEOL IT800SEM) with EDS capability.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was conducted at the Joint School of Nanoscience and Nanoengineering (JSNN), a member of the Southeastern Nanotechnology Infrastructure Corridor (SENIC) and National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the US National Science Foundation (ECCS-2025462).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Gruber, K. Cleaning up our future health. Nature 2018, 555, S20–S22. [Google Scholar] [CrossRef]
- Ouyang, L.; Li, D.; Zhu, L.; Tang, H.; Ouyang, L.; Li, D.; Zhu, L.; Tang, H. Precision Target Guide Strategy for Applying SERS into Environmental Monitoring. In Raman Spectroscopy and Applications; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- DLi, W.; Zhai, W.L.; Li, Y.T.; Long, Y.T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2013, 181, 23–43. [Google Scholar] [CrossRef]
- Condorelli, M.; Litti, L.; Pulvirenti, M.; Scardaci, V.; Meneghetti, M.; Compagnini, G. Silver nanoplates paved PMMA cuvettes as a cheap and re-usable plasmonic sensing device. Appl. Surf. Sci. 2021, 566, 150701. [Google Scholar] [CrossRef]
- Jahn, I.J.; Mühlig, A.; Cialla-May, D. Application of molecular SERS nanosensors: Where we stand and where we are headed towards? Anal. Bioanal. Chem. 2020, 412, 5999. [Google Scholar] [CrossRef]
- Zribi, R.; Fazio, E.; Condorelli, M.; D’Urso, L.; Neri, G.; Corsaro, C.; Neri, F.; Compagnini, G.; Neri, G. H2O2 electrochemical sensing properties of size-tunable triangular Ag nanoplates. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2022-Conference Proceedings, Messina, Italy, 22–24 June 2022. [Google Scholar] [CrossRef]
- Potara, M.; Gabudean, A.M.; Astilean, S. Solution-phase, dual LSPR-SERS plasmonic sensors of high sensitivity and stability based on chitosan-coated anisotropic silver nanoparticles. J. Mater. Chem. 2011, 21, 3625–3633. [Google Scholar] [CrossRef]
- Masson, J.F. Portable and field-deployed surface plasmon resonance and plasmonic sensors. Analyst 2020, 145, 3776–3800. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Denizli, A. Highly Sensitive and Selective Plasmonic Sensing Platforms. In Plasmonic Sensors and Their Applications; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 55–69. [Google Scholar] [CrossRef]
- Choi, I. Recent Advances in Nanoplasmonic Sensors for Environmental Detection and Monitoring. J. Nanosci. Nanotechnol. 2016, 16, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Esen, C.; Piletsky, S.A. Surface Plasmon Resonance Sensors Based on Molecularly Imprinted Polymers. In Plasmonic Sensors and Their Applications; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 221–236. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.L.; Manesiotis, P.; Allender, C.J. Twenty years since ‘antibody mimics’ by molecular imprinting were first proposed: A critical perspective. Mol. Impr. 2013, 1, 35–40. [Google Scholar] [CrossRef]
- Rico-Yuste, A.; Carrasco, S. Molecularly Imprinted Polymer-Based Hybrid Materials for the Development of Optical Sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef]
- Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018. [Google Scholar] [CrossRef]
- Bagra, B.; Mabe, T.; Tukur, F.; Wei, J. A plasmonic nanoledge array sensor for detection of anti-insulin antibodies of type 1 diabetes biomarker. Nanotechnology 2020, 31, 325503. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, X.; Mabe, T.; Christie, S.; Gilmore, G.; Smith, A.W.; Wei, J. Protein Trapping in Plasmonic Nanoslit and Nanoledge Cavities: The Behavior and Sensing. Anal. Chem. 2017, 89, 5221–5229. [Google Scholar] [CrossRef]
- Zeng, Z.; Mendis, M.N.; Waldeck, D.H.; Wei, J. A semi-analytical decomposition analysis of surface plasmon generation and the optimal nanoledge plasmonic device. RSC Adv. 2016, 6, 17196–17203. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.; Lin, Y.; Wei, J.; Bono, T.; Lindquist, R.G. An enhanced LSPR fiber-optic nanoprobe for ultrasensitive detection of protein biomarkers. Biosens. Bioelectron. 2014, 61, 95–101. [Google Scholar] [CrossRef]
- Tukur, F.; Bagra, B.; Jayapalan, A.; Liu, M.; Tukur, P.; Wei, J. Plasmon–Exciton Coupling Effect in Nanostructured Arrays for Optical Signal Amplification and SARS-CoV-2 DNA Sensing. ACS Appl. Nano Mater. 2023, 6, 2071–2082. [Google Scholar] [CrossRef]
- Kaye, S.; Zeng, Z.; Sanders, M.; Chittur, K.; Koelle, P.M.; Lindquist, R.; Manne, U.; Lin, Y.; Wei, J. Label-free detection of DNA hybridization with a compact LSPR-based fiber-optic sensor. Analyst 2017, 142, 1974–1981. [Google Scholar] [CrossRef]
- Sajini, T.; Mathew, B. A brief overview of molecularly imprinted polymers: Highlighting computational design, nano and photo-responsive imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. 100 Years “Schlüssel-Schloss-Prinzip”: What Made Emil Fischer Use this Analogy? Angew. Chem. Int. Ed. Engl. 1995, 33, 2364–2374. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G.; Sarhan, A. Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew. Chem. 1972, 84, 364. [Google Scholar] [CrossRef]
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Martínez, I.V.; Ek, J.I.; Ahn, E.C.; Sustaita, A.O. Molecularly imprinted polymers via reversible addition–fragmentation chain-transfer synthesis in sensing and environmental applications. RSC Adv. 2022, 12, 9186–9201. [Google Scholar] [CrossRef]
- Zhang, H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv. Mater. 2020, 32, 1806328. [Google Scholar] [CrossRef]
- Canfarotta, F.; Czulak, J.; Guerreiro, A.; Cruz, A.G.; Piletsky, S.; Bergdahl, G.E.; Hedström, M.; Mattiasson, B. A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2018, 120, 108–114. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Liu, J.; Wang, J.; Liu, J.; Gao, Y.; Ma, N. Chemiluminescence sensors based on molecularly imprinted polymers for the determination of organophosphorus in milk. J. Dairy Sci. 2022, 105, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.F.; Moreira, G.; Datta, S.P.A.; McLamore, E.; Vanegas, D. An Experimental Framework for Developing Point-of-Need Biosensors: Connecting Bio-Layer Interferometry and Electrochemical Impedance Spectroscopy. Biosensors 2022, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, J.; Li, C.; Ma, M.; Yu, W.; Shen, J.; Wang, Z. Molecularly Imprinted Polymer as an Antibody Substitution in Pseudo-immunoassays for Chemical Contaminants in Food and Environmental Samples. J Agric. Food Chem. 2018, 66, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Cecchini, A.; Piletsky, S. CHAPTER 1 Nano-sized Molecularly Imprinted Polymers as Artificial Antibodies. In Molecularly Imprinted Polymers for Analytical Chemistry Applications; Royal Society of Chemistry: London, UK, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Parisi, O.I.; Francomano, F.; Dattilo, M.; Patitucci, F.; Prete, S.; Amone, F.; Puoci, F. The Evolution of Molecular Recognition: From Antibodies to Molecularly Imprinted Polymers (MIPs) as Artificial Counterpart. J. Funct. Biomater. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K. Peer Reviewed: Molecularly Imprinted Polymers: The Next Generation. Anal. Chem. 2003, 75, 376A–383A. [Google Scholar] [CrossRef]
- Smolinska-Kempisty, K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.J.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the ELISA format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef]
- Vasapollo, G.; del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Gao, M.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Lv, J.; Wang, J.; Xu, D.; Liu, G. Recent Advances and Future Trends in the Detection of Contaminants by Molecularly Imprinted Polymers in Food Samples. Front. Chem. 2020, 8, 1142. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Mattiasson, B. MIPs as tools in environmental biotechnology. Adv. Biochem. Eng. Biotechnol. 2015, 150, 183–205. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Molecularly imprinted polymer-based sensors for priority pollutants. Sensors 2021, 21, 2406. [Google Scholar] [CrossRef] [PubMed]
- Marć, M.; Wieczorek, P.P. Introduction to MIP synthesis, characteristics and analytical application. Compr. Anal. Chem. 2019, 86, 1–15. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Q.; Zhou, Q.; Lin, Q.; Wang, C. Template-Monomer Interaction in Molecular Imprinting: Is the Strongest the Best? Open J. Org. Polym. Mater. 2015, 5, 58–68. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Costa, L.G. Organophosphorus Compounds at 80: Some Old and New Issues. Toxicol. Sci. 2018, 162, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Hilbeck, A.; Zurich, E.; Alam, M.J.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Özkara, A.; Akyıl, D.; Konuk, M.; Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, Environmental Pollution, and Health. In Environmental Health Risk-Hazardous Factors to Living Species; Wiley Online Library: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Kaur, K.; Malik, A.K.; Sonne, C.; Lee, S.S.; Kim, K.H. Core-shell structured molecularly imprinted materials for sensing applications. Trends Anal. Chem. 2020, 133, 116043. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M. Chapter 3: Surface Plasmon Resonance Instruments. In Handbook of Surface Plasmon Resonance; RSC Publishing: London, UK, 2017; pp. 60–105. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents. Biosensors 2020, 10, 142. [Google Scholar] [CrossRef]
- Sitjar, J.; Hou, Y.C.; der Liao, J.; Lee, H.; Xu, H.Z.; Fu, W.E.; Chen, G.D. Surface Imprinted Layer of Cypermethrin upon Au Nanoparticle as a Specific and Selective Coating for the Detection of Template Pesticide Molecules. Coatings 2020, 10, 751. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed]
- RPilot; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Coelho, L.C.C.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; de Almeida, J.M.M.M. Biosensors for Biogenic Amines: A Review. Biosensors 2021, 11, 82. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgönüllü, S.; Çimen, D.; Derazshamshir, A.; Bereli, N.; Yılmaz, F.; Denizli, A. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sens. Actuators B Chem. 2017, 241, 446–454. [Google Scholar] [CrossRef]
- Kamra, T.; Zhou, T.; Montelius, L.; Schnadt, J.; Ye, L. Implementation of molecularly imprinted polymer beads for surface enhanced raman detection. Anal. Chem. 2015, 87, 5056–5061. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Oh, B.K.; Lee, W.; Chun, B.S.; Bae, Y.M.; Lee, W.H.; Choi, J.W. The fabrication of protein chip based on surface plasmon resonance for detection of pathogens. Biosens. Bioelectron. 2005, 20, 1847–1850. [Google Scholar] [CrossRef]
- van der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control. 2003, 14, 251–254. [Google Scholar] [CrossRef]
- Decorbie, N.; Tijunelyte, I.; Gam-Derouich, S.; Solard, J.; Lamouri, A.; Decorse, P.; Felidj, N.; Gauchotte-Lindsay, C.; Rinnert, E.; Mangeney, C.; et al. Sensing Polymer/Paracetamol Interaction with an Independent Component Analysis-Based SERS-MIP Nanosensor. Plasmonics 2020, 15, 1533–1539. [Google Scholar] [CrossRef]
- Agrawal, H.; Shrivastav, A.M.; Gupta, B.D. Surface plasmon resonance based optical fiber sensor for atrazine detection using molecular imprinting technique. Sens. Actuators B Chem. 2016, 227, 204–211. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Huang, N.M.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions. Sens. Actuators B Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Ren, X.; Feng, X.; Li, X.; Li, X. Preparation of silver with an ultrathin molecular imprinted layer for detection of carbendazim by SERS. Chem. Pap. 2021, 75, 6477–6485. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Galatus, R.; Bibbò, L.; Pesavento, M.; Zeni, L. Sensors based on surface plasmon resonance in a plastic optical fiber for the detection of trinitrotoluene. Sens. Actuators B Chem. 2013, 188, 221–226. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Optical fiber sensor for the detection of tetracycline using surface plasmon resonance and molecular imprinting. Analyst 2013, 138, 7254–7263. [Google Scholar] [CrossRef]
- Hu, R.; Tang, R.; Xu, J.; Lu, F. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Armutcu, C.; Denizli, A. Molecularly imprinted polymer film based plasmonic sensors for detection of ochratoxin A in dried Figure. Polym. Bull. 2022, 79, 4049–4067. [Google Scholar] [CrossRef]
- Gupta, B.D.; Shrivastav, A.M.; Usha, S.P. Surface Plasmon Resonance-Based Fiber Optic Sensors Utilizing Molecular Imprinting. Sensors 2016, 16, 1381. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Fiber optic SPR sensor for the detection of 3-pyridinecarboxamide (vitamin B3) using molecularly imprinted hydrogel. Sens. Actuators B Chem. 2013, 177, 279–285. [Google Scholar] [CrossRef]
- Castro-Grijalba, A.; Montes-García, V.; Cordero-Ferradás, M.J.; Coronado, E.; Pérez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.S.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S.; Abdullah, J.; Fen, Y.W. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; She, Y.; Cao, X.; Ma, J.; Chen, G.; Hong, S.; Shao, Y.; EI-Aty, A.M.A.; Wang, M.; Wang, J. A molecularly imprinted polymer with integrated gold nanoparticles for surface enhanced Raman scattering based detection of the triazine herbicides, prometryn and simetryn. Mikrochim. Acta 2019, 186, 143. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Localized and propagating surface plasmon resonance based fiber optic sensor for the detection of tetracycline using molecular imprinting. Mater. Res. Express 2015, 2, 035007. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Lai, E.P.C. Interaction of ochratoxin A with molecularly imprinted polypyrrole film on surface plasmon resonance sensor. React. Funct. Polym. 2005, 63, 171–176. [Google Scholar] [CrossRef]
- Xue, Y.; Shao, J.; Sui, G.; Ma, Y.; Li, H. Rapid detection of orange II dyes in water with SERS imprinted sensor based on PDA-modified MOFs@Ag. J. Environ. Chem. Eng. 2021, 9, 106317. [Google Scholar] [CrossRef]
- Yao, G.H.; Liang, R.P.; Huang, C.F.; Wang, Y.; Qiu, J.D. Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of l-nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Gao, F.; Grant, E.; Lu, X. Determination of histamine in canned tuna by molecularly imprinted polymers-surface enhanced Raman spectroscopy. Anal. Chim. Acta 2015, 901, 68–75. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Hua, M.Z.; Feng, S.; Wang, S.; Lu, X. Rapid detection and quantification of 2,4-dichlorophenoxyacetic acid in milk using molecularly imprinted polymers—Surface-enhanced Raman spectroscopy. Food Chem. 2018, 258, 254–259. [Google Scholar] [CrossRef]
- Feng, J.; Hu, Y.; Grant, E.; Lu, X. Determination of thiabendazole in orange juice using an MISPE-SERS chemosensor. Food Chem. 2018, 239, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Liao, C.; Wang, Y.; Wang, Y.; Yang, K. Rapid and Sensitive Detection of Pentachloronitrobenzene by Surface-Enhanced Raman Spectroscopy Combined with Molecularly Imprinted Polymers. Biosensors 2022, 12, 52. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Zhang, Y.; Sun, X. A novel fluorescent molecularly imprinted polymer SiO2 @CdTe QDs@MIP for paraquat detection and adsorption. Luminescence 2021, 36, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yu, N.; Shi, C.; Wang, X.; Wu, J. Surface Plasmon Resonance Sensor for Antibiotics Detection Based on Photo-Initiated Polymerization Molecularly Imprinted Array. Talanta 2016, 161, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 95. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Swierczewska, M.; Liu, G.; Lee, S.; Chen, X. High-sensitivity nanosensors for biomarker detection. Chem. Soc. Rev. 2012, 41, 2641. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H. Nanosensors for Heavy Metal Detection in Environmental Media: Recent Advances and Future Trends. Nanosensors Environ. Food Agric. 2021, 1, 23–51. [Google Scholar] [CrossRef]
- Adam, T.; Dhahi, T.S. Nanosensors: Recent perspectives on attainments and future promise of downstream applications. Process Biochem. 2022, 117, 153–173. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.; Hong, S. Highly selective environmental nanosensors based on anomalous response of carbon nanotube conductance to mercury ions. J. Phys. Chem. C 2009, 113, 19393–19396. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Cheng, J. Molecularly imprinted silica nanospheres embedded Cdse quantum dots for highly selective and sensitive optosensing of pyrethroids. Chem. Mater. 2010, 22, 2451–2457. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Talanta 2009, 79, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, M.; Mani, V.; Chen, S.M.; Chen, T.W.; Sundramoorthy, A.K. Methyl parathion detection in vegetables and fruits using silver@graphene nanoribbons nanocomposite modified screen printed electrode. Sci. Rep. 2017, 7, 46471. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cui, X.; Chen, G.; Wang, Y.; Qin, G.; Li, M.; Zhang, X.; Zhang, Y.; Zhang, C.; Du, P.; et al. Simple and sensitive detection of triazophos pesticide by using quantum dots nanobeads based on immunoassay. Food Agric. Immunol. 2019, 30, 522–532. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, G.W.; Park, T.J. A facile and sensitive detection of organophosphorus chemicals by rapid aggregation of gold nanoparticles using organic compounds. Biosens. Bioelectron. 2015, 67, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z. Surface Plasmon Resonance Based Sensor for the Detection of Glycopeptide Antibiotics in Milk Using Rationally Designed NanoMIPs. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Afzal, A.; Rehman, A.; Dickert, F.L. Nanoparticles for detecting pollutants and degradation processes with mass-sensitive sensors. Sens. Actuators B Chem. 2007, 127, 132–136. [Google Scholar] [CrossRef]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef]
- D’aurelio, R.; Ashley, J.; Rodgers, T.L.; Trinh, L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Development of a NanoMIPs-SPR-Based Sensor for β-Lactoglobulin Detection. Chemosensors 2020, 8, 94. [Google Scholar] [CrossRef]
- Ashley, J.; Shukor, Y.; D’Aurelio, R.; Trinh, L.; Rodgers, T.L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Synthesis of Molecularly Imprinted Polymer Nanoparticles for α-Casein Detection Using Surface Plasmon Resonance as a Milk Allergen Sensor. ACS Sens. 2018, 3, 418–424. [Google Scholar] [CrossRef]
- Garcia, R.; Cabrita, M.J.; Maria, A.; Freitas, C. Application of Molecularly Imprinted Polymers for the Analysis of Pesticide Residues in Food—A Highly Selective and Innovative Approach. Am. J. Analyt. Chem. 2011, 2, 16–25. [Google Scholar] [CrossRef]
- Ghaeni, F.A.; Karimi, G.; Mohsenzadeh, M.S.; Nazarzadeh, M.; Motamedshariaty, V.S.; Mohajeri, S.A. Preparation of dual-template molecularly imprinted nanoparticles for organophosphate pesticides and their application as selective sorbents for water treatment. Sep. Sci. Technol. 2018, 53, 2517–2526. [Google Scholar] [CrossRef]
- Piletska, E.V.; Czulak, J.; Piletsky, S.S.; Guerreiro, A.; Canfarotta, F.; Piletsky, S.A. Novel assay format for proteins based on magnetic molecularly imprinted polymer nanoparticles—Detection of pepsin. J. Chin. Adv. Mater. Soc. 2018, 6, 341–351. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef]
- Wu, M.; Deng, H.; Fan, Y.; Hu, Y.; Guo, Y.; Xie, L. Rapid Colorimetric Detection of Cartap Residues by AgNP Sensor with Magnetic Molecularly Imprinted Microspheres as Recognition Elements. Molecules 2018, 23, 1443. [Google Scholar] [CrossRef]
- Mankar, J.S.; Sharma, M.D.; Krupadam, R.J. Molecularly imprinted nanoparticles (nanoMIPs): An efficient new adsorbent for removal of arsenic from water. J. Mater. Sci. 2020, 55, 6810–6825. [Google Scholar] [CrossRef]
- Guo, W.; Engelman, B.J.; Haywood, T.L.; Blok, N.B.; Beaudoin, D.S.; Obare, S.O. Dual fluorescence and electrochemical detection of the organophosphorus pesticides-ethion, malathion and fenthion. Talanta 2011, 87, 276–283. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).