Screening the Specific Surface Area for Metal-Organic Frameworks by Cataluminescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of the Narrow-Pore (np) Form of Al-Based MOF
2.3. Synthesis of the Large-Pore (lp) Form of Al-Based MOF
2.4. Synthesis of the Medium-Pore (mp) Phase of Al-Based MOF
2.5. Apparatus and Characterization
2.6. Fourier Transform Infrared (FT-IR) Measurements
2.7. Gas Chromatography–Mass Spectrometry (GC–MS) Measurements
2.8. Brunauer–Emmett–Teller (BET) Measurements
2.9. Thermogravimetric Analysis (TGA) Measurements
2.10. Cataluminescence (CTL) Measurements
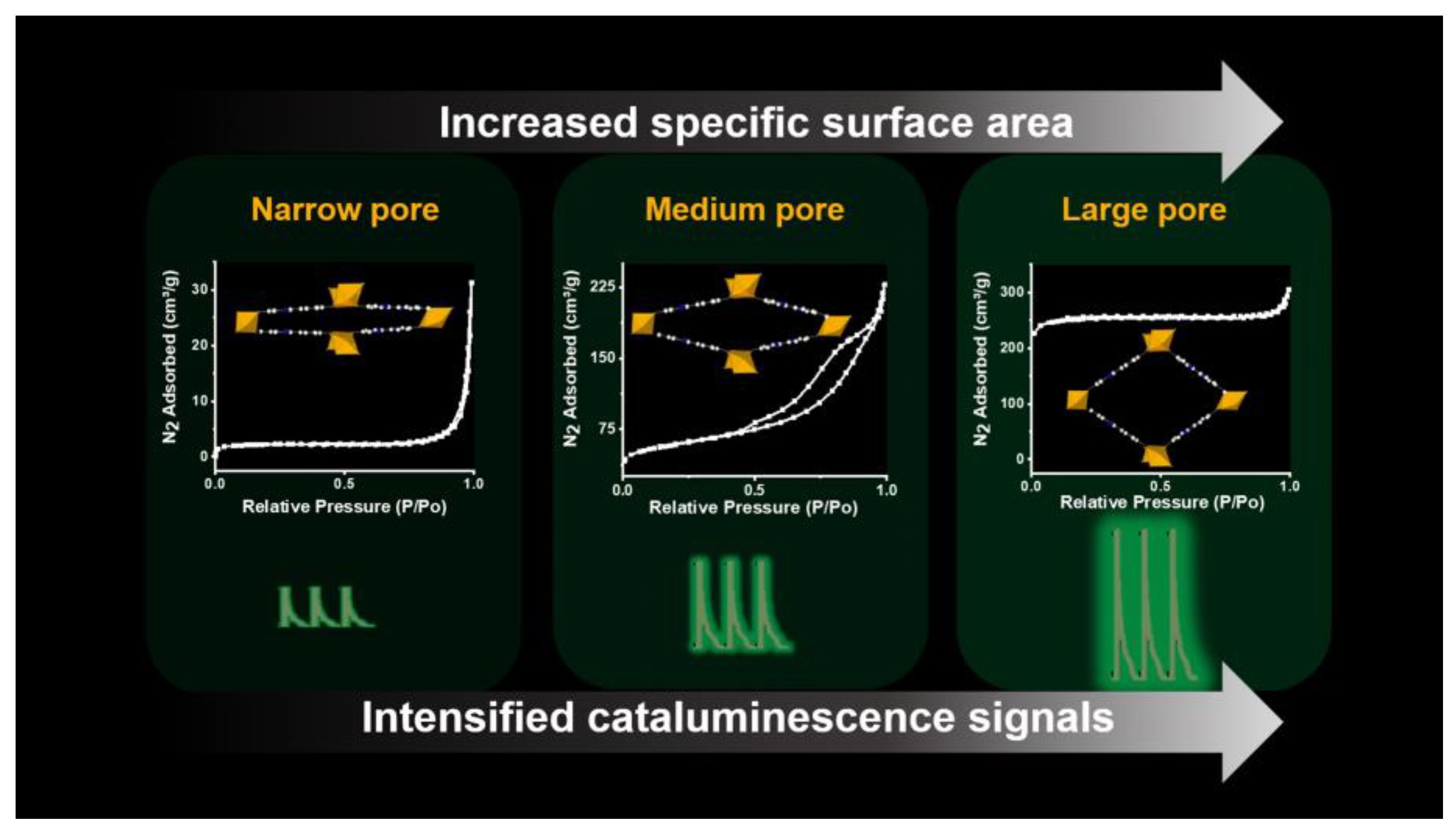
3. Results and Discussion
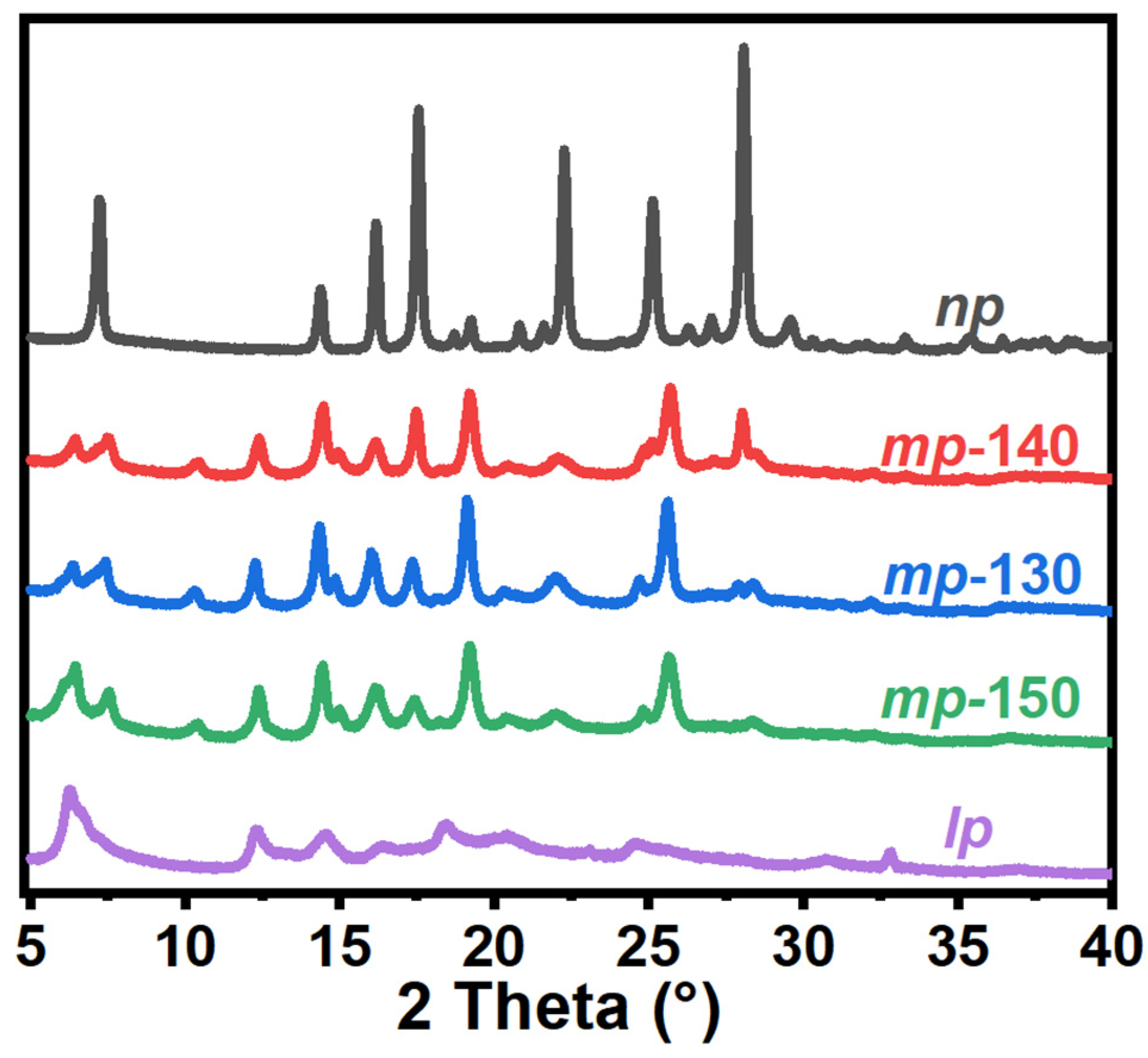
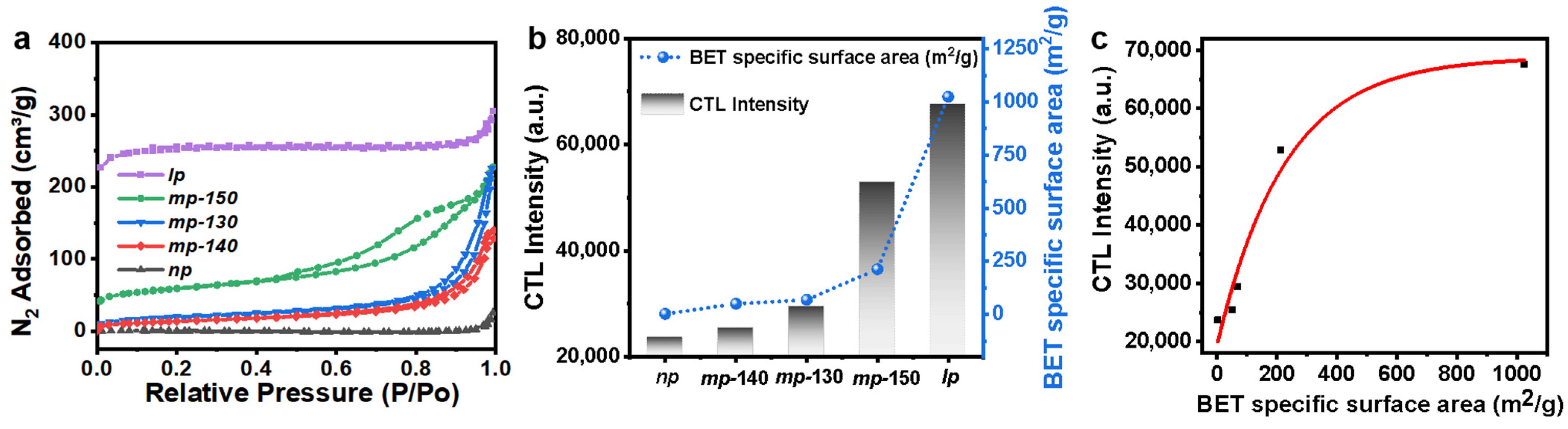
3.1. Structural and Morphological Characterization of Porous MOFs
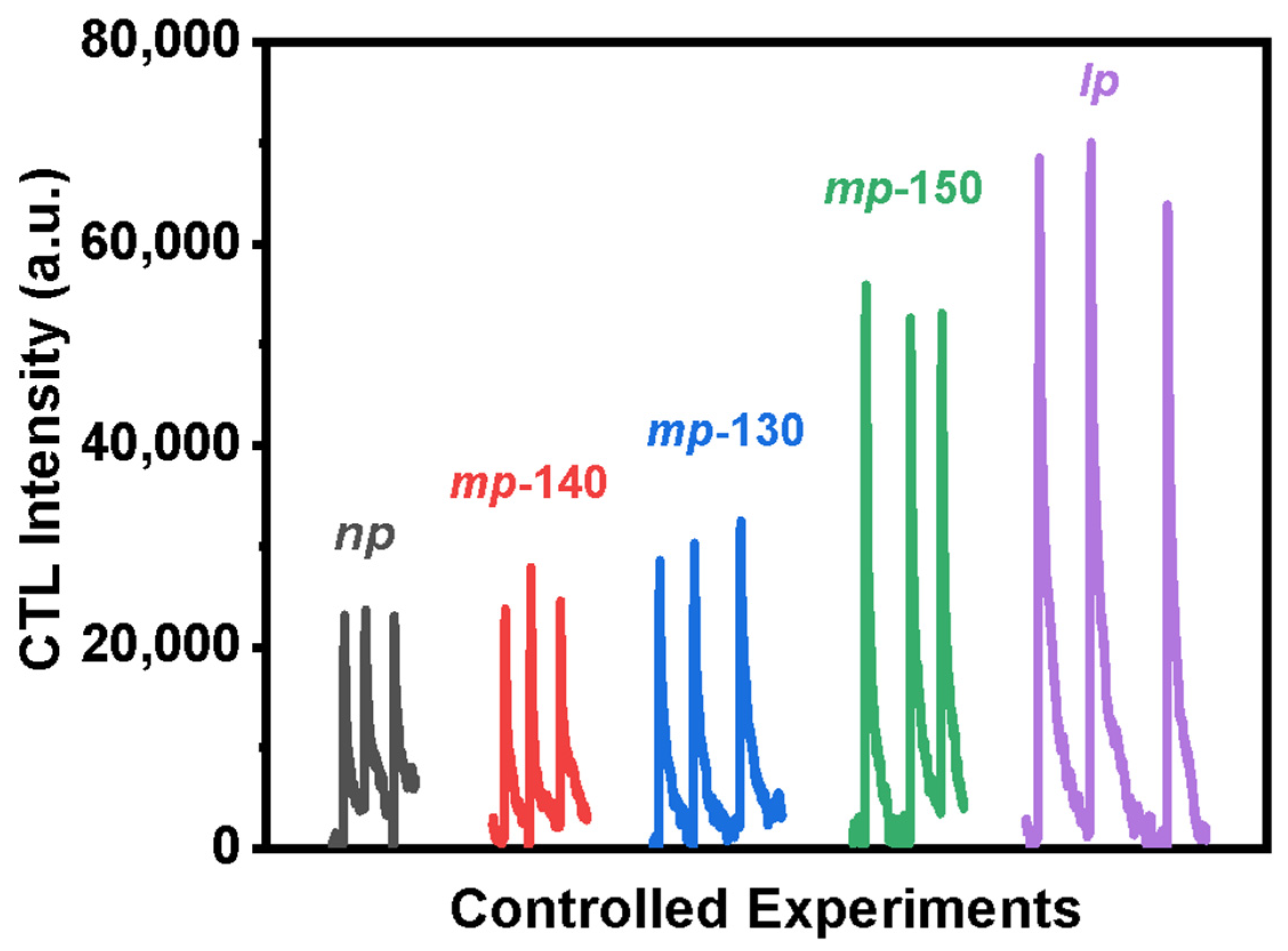
3.2. Cataluminescence of Ethanol Catalyzed by MOFs
3.3. Cataluminesence of Ethanol Catalyzed by MOFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurisingal, J.F.; Li, Y.; Sagynbayeva, Y.; Chitumalla, R.K.; Park, D.-W. Porous aluminum-based DUT metal-organic frameworks for the transformation of CO2 into cyclic carbonates: A computationally supported study. Catal. Today 2019, 352, 227–236. [Google Scholar] [CrossRef]
- Kustov, L.M.; Isaeva, V.I.; Přech, J.; Bisht, K.K. Metal-organic frameworks as materials for applications in sensors. Mendeleev Commun. 2019, 29, 361–368. [Google Scholar] [CrossRef]
- An, B.; Li, Z.; Song, Y.; Zhang, J.; Lin, W. Cooperative copper centres in a metal-organic framework for selective conversion of CO2 to ethanol. Nat. Catal. 2019, 2, 709–717. [Google Scholar] [CrossRef]
- Nath, K.; Ahmed, A.; Siegel, D.J.; Matzger, A.J. Microscale determination of binary gas adsorption isotherms in MOFs. J. Am. Chem. Soc. 2022, 144, 20939–20946. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Strategies for hydrogen storage in metal-organic frameworks. Angew. Chem. Int. Ed. 2004, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, X.; Xi, R.; Shan, G.; Li, P.-Z.; Liu, J.; Zhao, Y.; Zou, R. A Robust aluminum metal-organic framework with temperature-induced breathing effect. ACS Mater. Lett. 2020, 2, 220–226. [Google Scholar] [CrossRef]
- Senkovska, I.; Hoffmann, F.; FröBa, M.; Getzschmann, J.; BöHlmann, W.; Kaskel, S. New highly porous aluminium based metal-organic frameworks: Al(OH)(ndc) (ndc=2,6-naphthalene dicarboxylate) and Al(OH)(bpdc) (bpdc=4,4′-biphenyl dicarboxylate). Microporous Mesoporous Mater. 2009, 122, 93–98. [Google Scholar] [CrossRef]
- Wang, H.; Warren, M.; Jagiello, J.; Jensen, S.; Ghose, S.K.; Tan, K.; Yu, L.; Emge, T.J.; Thonhauser, T.; Li, J. Crystallizing atomic xenon in a flexible MOF to probe and understand its temperature-dependent breathing behavior and unusual gas adsorption phenomenon. J. Am. Chem. Soc. 2020, 142, 20088–20097. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.; Zhang, L.; Liu, R.; Lv, Y. Highly efficient cataluminescence gas sensor for acetone vapor based on UIO-66 metal-organic frameworks as preconcentrator. Sens. Actuators B Chem. 2020, 312, 127952. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Duyne, R.P.V.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, B.; Li, J.; Qian, L.; Liu, L.; Liu, Z.; Fang, P.; He, C. Dependence of dye molecules adsorption behaviors on pore characteristics of mesostructured MOFs fabricated by surfactant template. ACS Appl. Mater. Interfaces 2019, 11, 31441–31451. [Google Scholar] [CrossRef]
- Srivastava, S.; Shet, S.P.; Priya, S.S.; Sudhakar, K.; Tahir, M. Molecular simulation of copper based metal-organic framework (Cu-MOF) for hydrogen adsorption. Int. J. Hydrogen Energy 2022, 47, 15820–15831. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Yavuz, E.; Demir, S.; Patat, Ş. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, R.; Yang, Z.; Chai, L.; Jin, L.; Alhassan, S.I.; Ren, L.; Wang, H.; Huang, L. Preparation of MOFs and MOFs derived materials and their catalytic application in air pollution: A review. Catal. Today 2021, 375, 10–29. [Google Scholar] [CrossRef]
- Deng, X.; Qin, Y.; Hao, M.; Li, Z. MOF-253-supported Ru complex for photocatalytic CO2 reduction by coupling with semidehydrogenation of 1,2,3,4-Tetrahydroisoquinoline (THIQ). Inorg. Chem. 2019, 58, 16574–16580. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gualdrón, D.A.; Moghadam, P.Z.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Application of consistency criteria to calculate BET areas of micro- and mesoporous metal-organic frameworks. J. Am. Chem. Soc. 2016, 138, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Datar, A.; Chung, Y.G.; Lin, L.-C. Beyond the BET analysis: The surface area prediction of nanoporous materials using a machine learning method. J. Phys. Chem. Lett. 2020, 11, 5412–5417. [Google Scholar] [CrossRef]
- Thommes, M. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Islamoglu, T.; Idrees, K.B.; Son, F.A.; Chen, Z.; Lee, S.-J.; Li, P.; Farha, O.K. Are you using the right probe molecules for assessing the textural properties of metal-organic frameworks? J. Mater. Chem. A 2022, 10, 157–173. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The use of van der waals adsorption isotherms in determining the surface arer of iron synthetic ammonia catalysts. J. Am. Chem. Soc. 1935, 57, 1754–1755. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET method for determining surface areas of microporous metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET theory for the characterization of meso and microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Bau, S.; Witschger, O.; Gensdarmes, F.; Rastoix, O.; Thomas, D. A TEM-based method as an alternative to the BET method for measuring off-line the specific surface area of nanoaerosols. Powder Technol. 2010, 200, 190–201. [Google Scholar] [CrossRef]
- Kockler, K.B.; Frisch, H.; Barner-Kowollik, C. Making and breaking chemical bonds by chemiluminescence. Macromol. Rapid Commun. 2018, 39, 1800516. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Jang, G.; Bilby, D.; Lee, T.S.; Kim, J. Design principles of chemiluminescence (CL) chemodosimeter for self-signaling detection: Luminol protective approach. RSC Adv. 2014, 4, 46488–46493. [Google Scholar] [CrossRef]
- Meng, F.; Lu, Z.; Zhang, R.; Li, G. Cataluminescence sensor for highly sensitive and selective detection of iso-butanol. Talanta 2019, 194, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, Z.; Zhang, L.; Lin, Y.; Lu, C. Recent advances in cataluminescence-based optical sensing systems. Analyst 2017, 142, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Guan, W.; Lin, Y.; Lu, C. Rapid discrimination of adsorbed oxygen and lattice oxygen in catalysts by the cataluminescence method. Anal. Chem. 2022, 94, 1382–1389. [Google Scholar] [CrossRef]
- Zhang, L.; Song, H.; Su, Y.; Lv, Y. Advances in nanomaterial-assisted cataluminescence and its sensing applications. Trends Anal. Chem. 2015, 67, 107–127. [Google Scholar] [CrossRef]
- Zeng, N.; Long, Y.; Wang, J.; Sun, J.; Ouyang, N.; Na, N. An Acetone Sensor Based on Plasma-Assisted Cataluminescence and Mechanism Studies by Online Ionizations. Anal. Chem. 2019, 91, 15763–15768. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Britt, D.; Lee, C.; Doonan, C.J.; Uribe-Romo, F.J.; Furukawa, H.; Long, J.R.; Yaghi, O.M. Metal insertion in a microporous metal-organic framework lined with 2,2′-bipyridine. J. Am. Chem. Soc. 2010, 132, 14382–14384. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Huang, L.; Huang, Z.; Sun, D.; Qin, J.-S.; Feng, L.; Li, J.; Zou, X.; Cagin, T.; Zhou, H.-C. Continuous variation of lattice dimensions and pore sizes in metal-organic frameworks. J. Am. Chem. Soc. 2020, 142, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Gao, Q.; Zhang, X.-R.; Xu, B.-Q. Cataluminescence and catalytic reactions of ethanol oxidation over nanosized Ce1−xZrxO2 (0≤x≤1) catalysts. Catal. Commun. 2006, 7, 589–592. [Google Scholar] [CrossRef]
- de Souza, M.J.B.; Silva, T.H.A.; Ribeiro, T.R.S.; da Silva, A.O.S.; Pedrosa, A.M.G. Thermal and catalytic pyrolysis of polyvinyl chloride using micro/mesoporous ZSM-35/MCM-41 catalysts. J. Therm. Anal. Calorim. 2020, 140, 167–175. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Energy-efficient sonochemical approach for the preparation of nanohybrid composites from graphene oxide and metal-organic framework. Inorg. Chem. Commun. 2019, 102, 185–191. [Google Scholar] [CrossRef]
- Yan, B.; Qin, S.-J. A facile indicator box based on Eu3+ functionalized MOF hybrid for the determination of 1-naphthol, a biomarker for carbaryl in urine. Sens. Actuators B Chem. 2018, 259, 125–132. [Google Scholar]
- Visa, A.; Mracec, M.; Maranescu, B.; Maranescu, V.; Ilia, G.; Popa, A.; Mracec, M. Structure simulation into a lamellar supramolecular network and calculation of the metal ions/ligands ratio. Chem. Cent. J. 2012, 6, 91. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Pei, D.; Tian, R.; Lu, C. Screening the Specific Surface Area for Metal-Organic Frameworks by Cataluminescence. Chemosensors 2023, 11, 292. https://doi.org/10.3390/chemosensors11050292
Li Z, Pei D, Tian R, Lu C. Screening the Specific Surface Area for Metal-Organic Frameworks by Cataluminescence. Chemosensors. 2023; 11(5):292. https://doi.org/10.3390/chemosensors11050292
Chicago/Turabian StyleLi, Zenghe, Danning Pei, Rui Tian, and Chao Lu. 2023. "Screening the Specific Surface Area for Metal-Organic Frameworks by Cataluminescence" Chemosensors 11, no. 5: 292. https://doi.org/10.3390/chemosensors11050292
APA StyleLi, Z., Pei, D., Tian, R., & Lu, C. (2023). Screening the Specific Surface Area for Metal-Organic Frameworks by Cataluminescence. Chemosensors, 11(5), 292. https://doi.org/10.3390/chemosensors11050292






