Flexible Humidity Sensor Based on Au Nanoparticles/Organosilica-Containing Polyelectrolyte Composite
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Fabrication of Au NPs
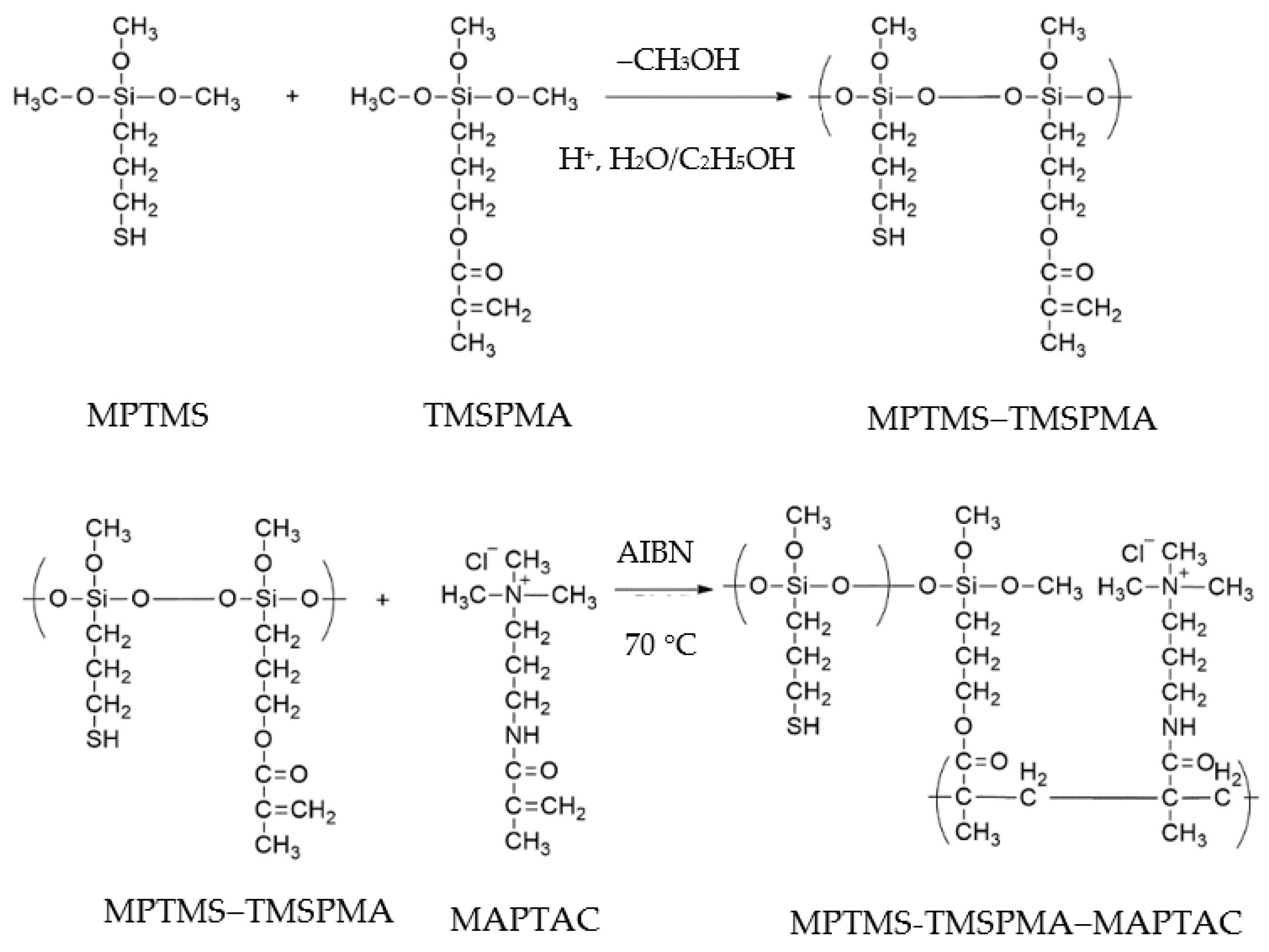
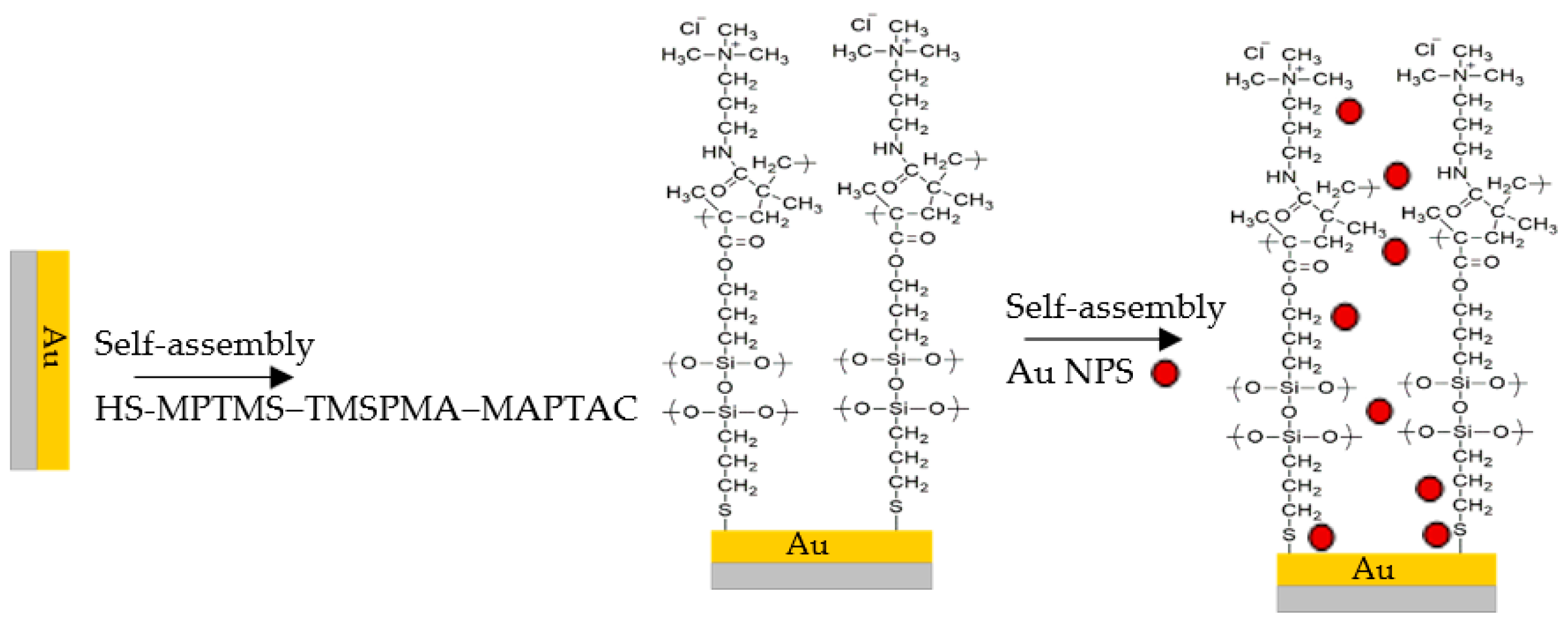
2.3. Fabrication of Flexible Humidity Sensor Based on Au NPs/MPTMS-TMSPMA-MAPTAC Composite
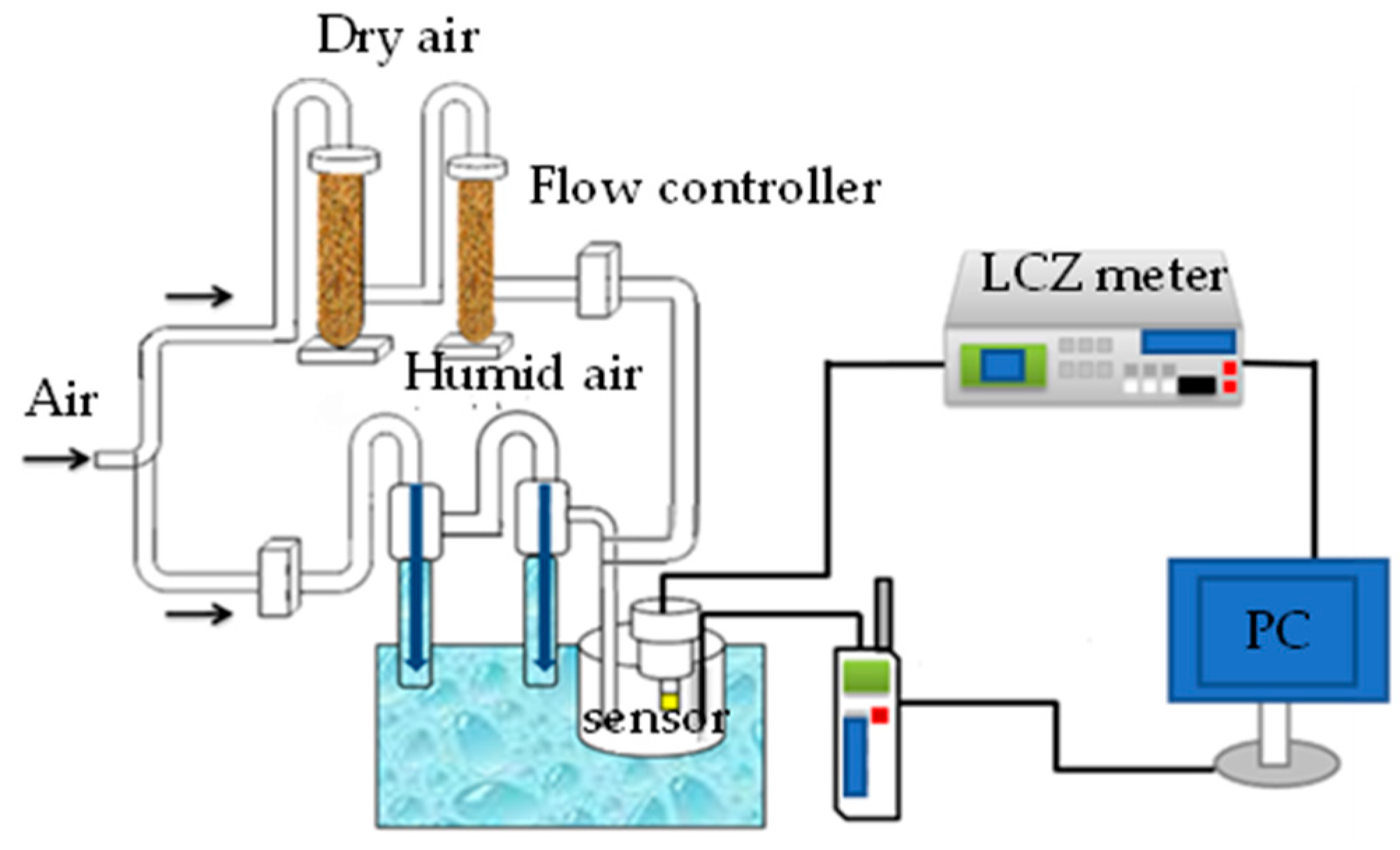
2.4. Instruments and Analysis
3. Results
3.1. Preparation and Characterization of Au NPs/MPTMS-TMSPMA-MAPTAC Composite
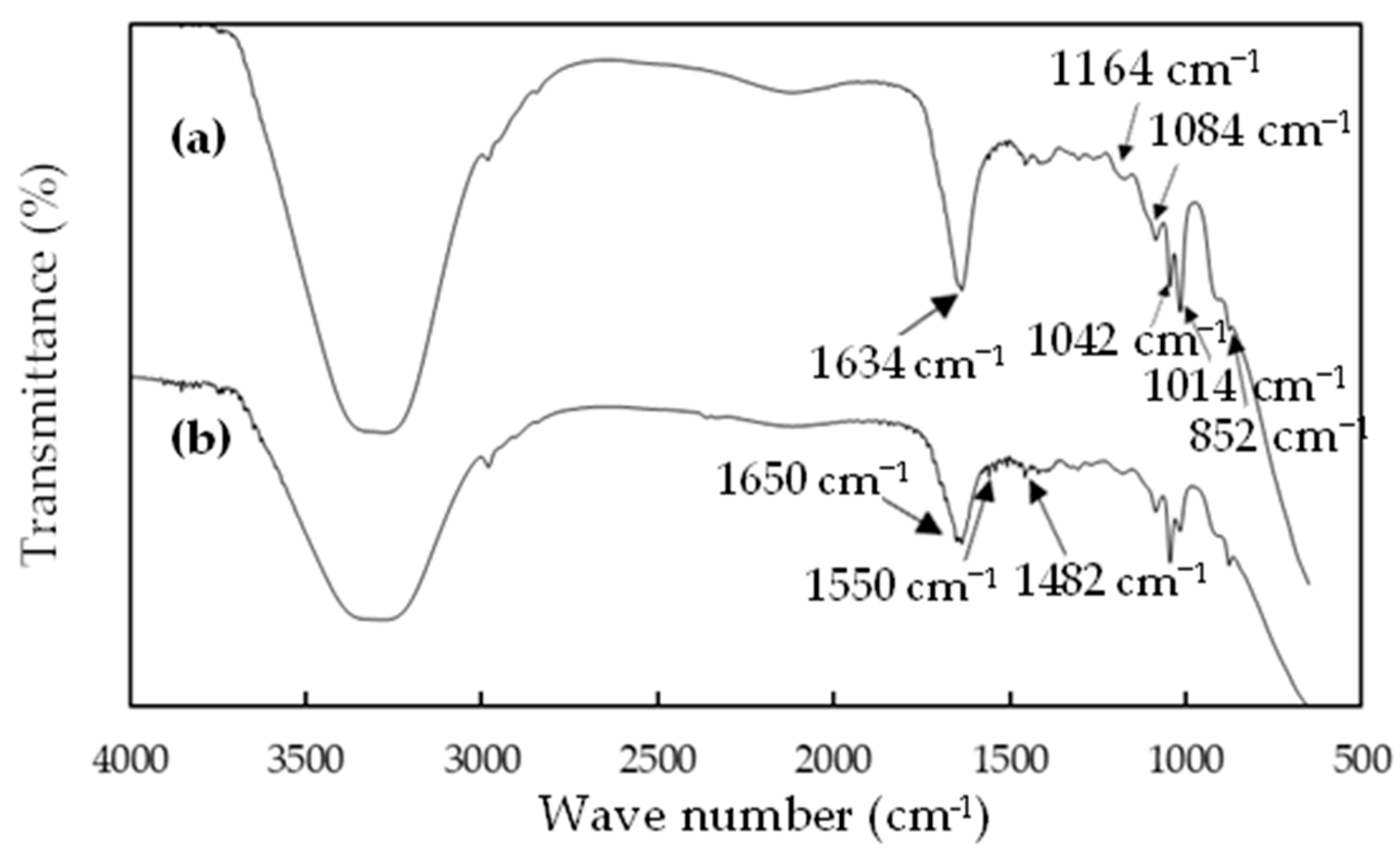
3.1.1. IR Analysis
3.1.2. SEM Analyses
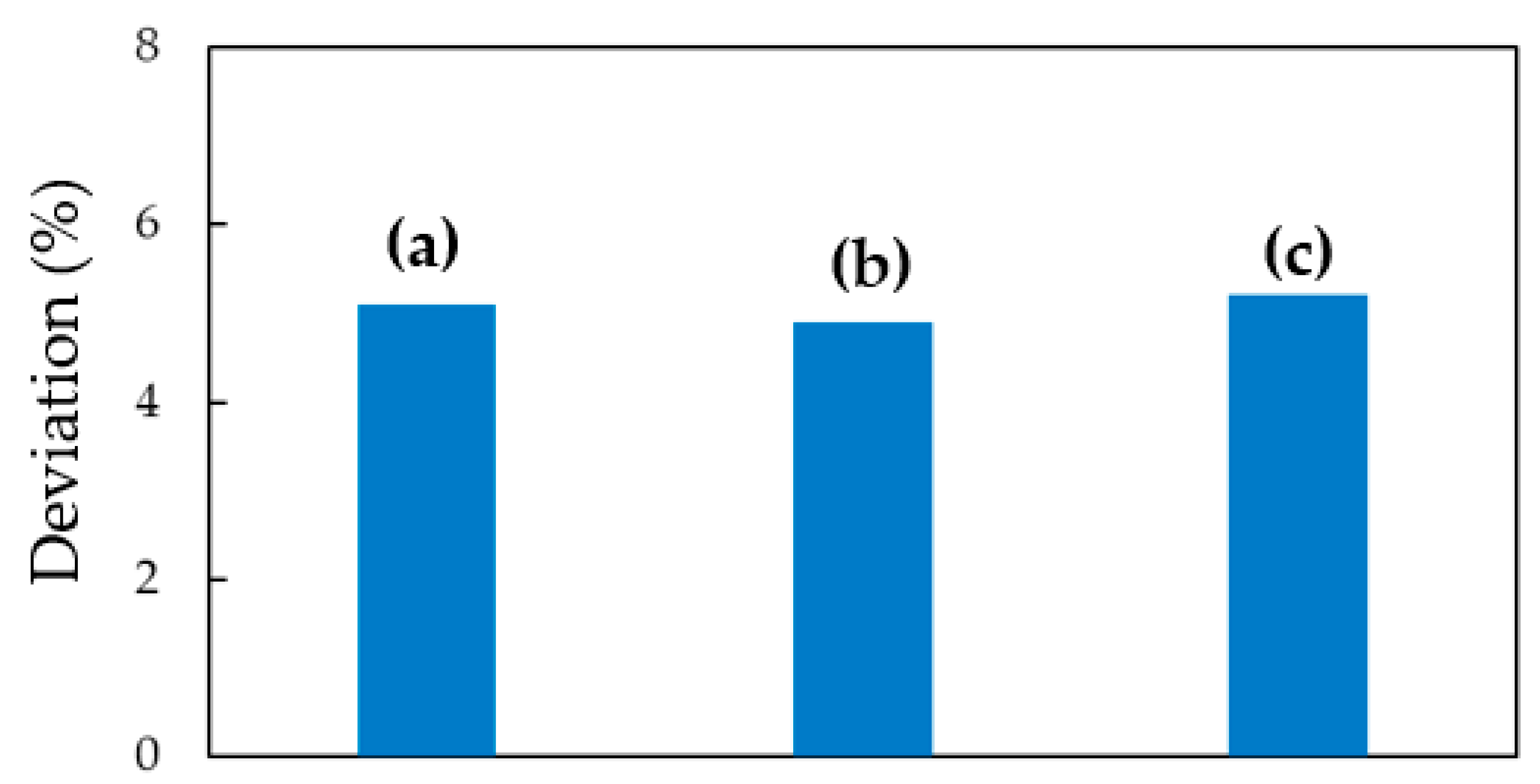
3.2. Water-Resistance and Flexibility Properties of Au NPs/MPTMS-TMSPMA-MAPTAC Composite Film
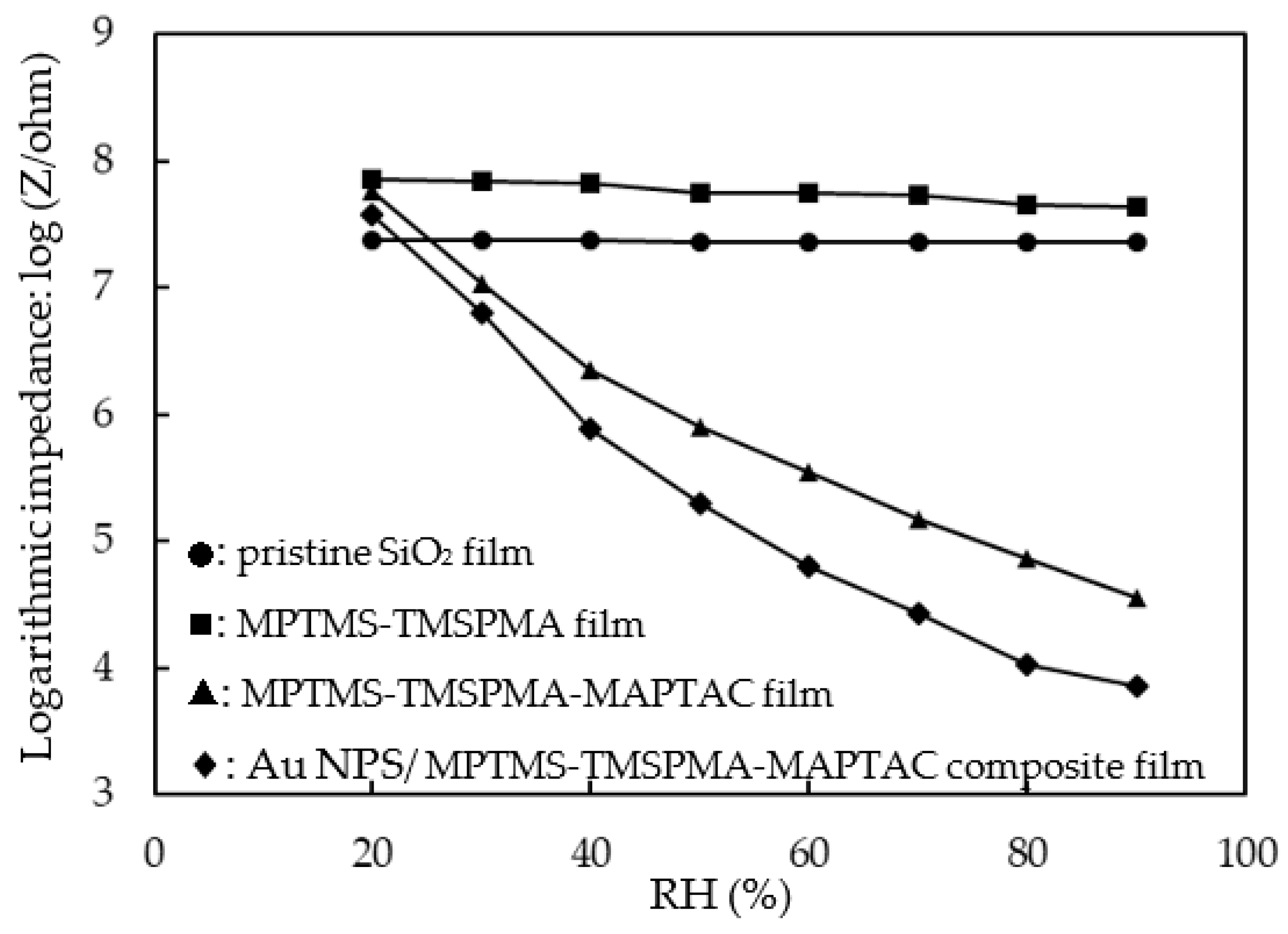
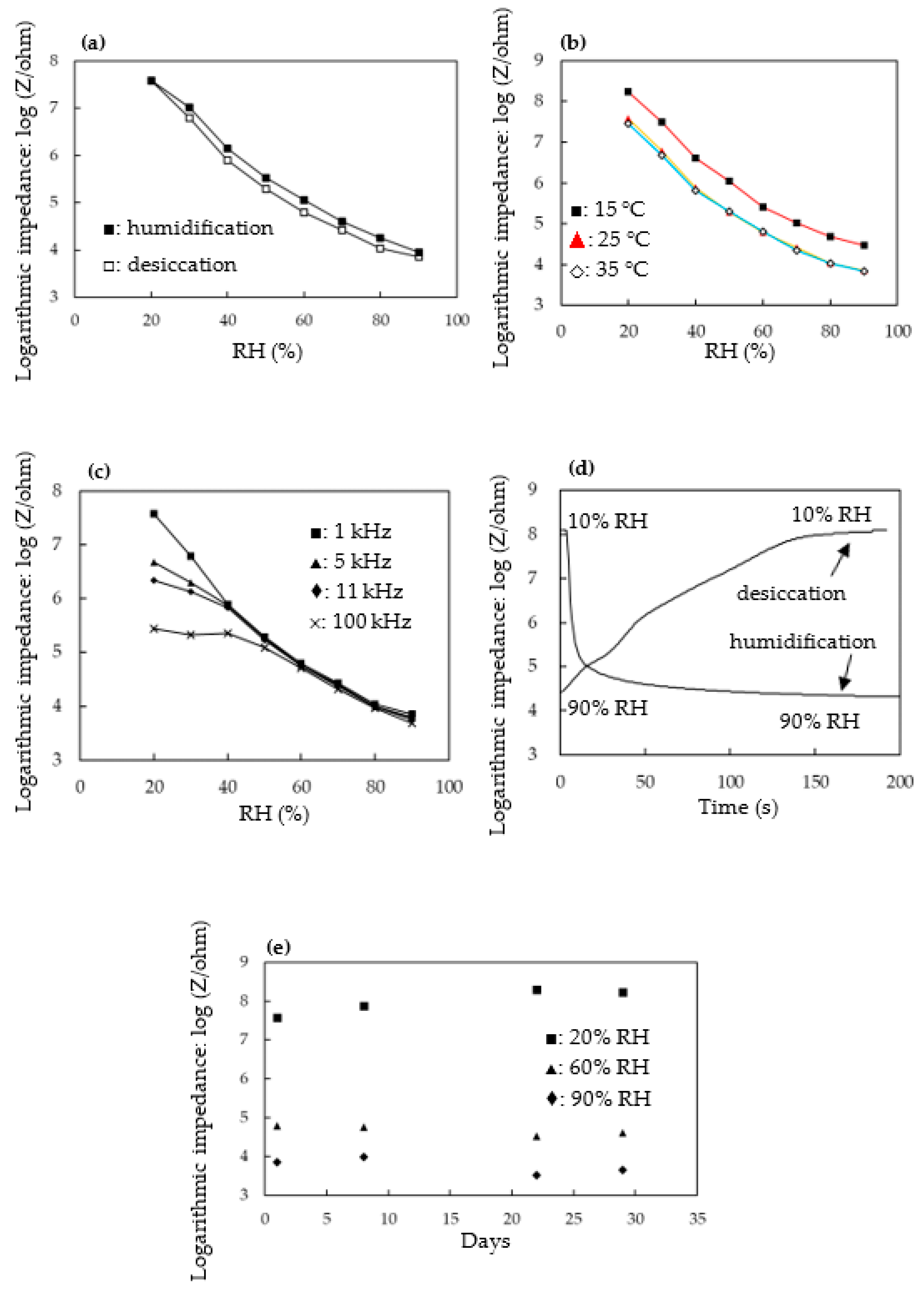
3.3. Electrical and Humidity-Sensing Properties of Au NPs/MPTMS-TMSPMA-MAPTAC Composite Film
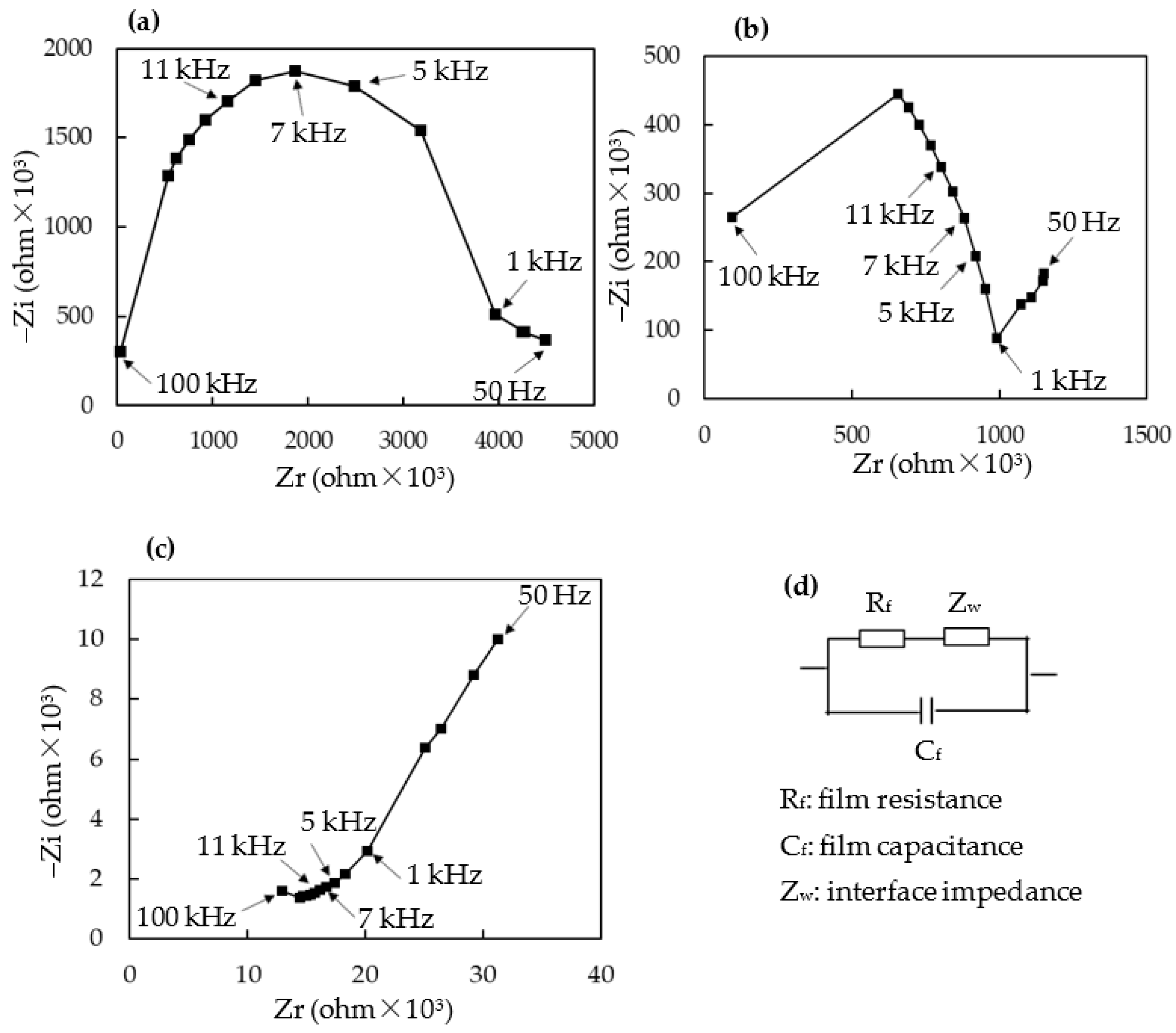
3.4. Humidity-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cochrane, C.; Hertleer, C.; Schwarz-Pfeiffer, A. Smart Textiles in Health: An Overview, in Smart Textiles and Their Applications; Koncar, V., Ed.; Woodhead Publishing: Sawston, UK, 2016; Volume 178, pp. 9–32. [Google Scholar]
- Wasson, T.; Choudhury, T.; Sharma, S.; Kumar, P. Integration of RFID and sensor in agriculture using IOT. In Proceedings of the International Conference on Smart Technologies for Smart Nation (SmartTechCon), Bengaluru, India, 17–19 August 2017; pp. 217–222. [Google Scholar]
- Mezzanotte, P.; Palazzi, V.; Alimenti, F.; Roselli, L. Innovative RFID sensors for Internet of Things applications. IEEE J. Microw. 2021, 1, 55–65. [Google Scholar] [CrossRef]
- Zuo, J.; Feng, J.; Gameiro, M.G.; Tian, Y.; Liang, J.; Wang, Y.; Ding, J.; He, Q. RFID-based sensing in smart packaging for food applications: A review. Future Foods 2022, 6, 100198. [Google Scholar] [CrossRef]
- Amin, E.M.; Bhuiyan, M.S.; Karmakar, N.C.; Winther-Jensen, B. Development of a low cost printable chipless RFID humidity sensor. IEEE Sens. J. 2013, 14, 140–149. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, L.; Chen, Q.; Zheng, L.R. Low-cost printed chipless RFID humidity sensor tag for intelligent packaging. IEEE Sens. J. 2014, 15, 3201–3208. [Google Scholar] [CrossRef]
- Delipinar, T.; Shafique, A.; Gohar, M.S.; Yapici, M.K. Fabrication and materials integration of flexible humidity sensors for emerging applications. ACS Omega 2021, 6, 8744–8753. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Costa, J.C.; Spina, F.; Lugoda, P.; Garcia-Garcia, L.; Roggen, D.; Münzenrieder, N. Flexible sensors—From materials to applications. Technologies 2019, 7, 35. [Google Scholar] [CrossRef]
- Kuzubasoglu, B.A. Recent studies on the humidity sensor: A mini review. ACS Appl. Electron. Mater. 2022, 4, 4797–4807. [Google Scholar] [CrossRef]
- Su, P.G.; Shiu, W.L.; Tsai, M.S. Flexible humidity sensor based on Au nanoparticles/graphene oxide/thiolated silica sol-gel film. Sens. Actuators B 2015, 216, 467–475. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Lee, D. Highly stable and sensitive resistive flexible humidity sensors by means of roll-to-roll printed electrodes and flower-like TiO2 nanostructures. Ceram. Int. 2019, 45, 985–992. [Google Scholar] [CrossRef]
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A. Organic thin-film capacitive and resistive humidity sensors: A focus review. Adv. Mater. Interfaces 2018, 5, 1800969. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, Y.; Mei, C.; Zhai, S.; Xuan, Y.; Liu, Z.; Pan, M.; Rojas, O.J. Chiral nematic coatings based on cellulose nanocrystals as a multiplexing platform for humidity sensing and dual anticounterfeiting. Small 2021, 17, 2103936. [Google Scholar] [CrossRef]
- Assunçaõ da Silva, E.; Duc, C.; Redon, N.; Wojkiewicz, J. Humidity sensor based on PEO/PEDOT: PSS blends for breath monitoring. Macromol. Mater. Eng. 2021, 306, 2100489. [Google Scholar] [CrossRef]
- Sobhanimatin, M.B.; Pourmahdian, S.; Tehranchi, M.M. Fast inverse opal humidity sensor based on acrylamide/AMPS hydrogel. Mater. Today Commun. 2021, 26, 101997. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, M.; Alem, S.; Tao, Y.; Chu, T.-Y.; Xiao, G.; Ramful, C.; Griffin, R. Printed flexible capacitive humidity sensors for field application. Sens. Actuators B 2022, 359, 131620. [Google Scholar] [CrossRef]
- Tekcin, M.; Sayar, E.; Yalcin, M.K.; Bahadir, S.K. Wearable and flexible humidity sensor integrated to disposable diapers for wetness monitoring and urinary incontinence. Electronics 2022, 11, 1025. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiong, Y.; Pan, M.; Li, L.; Dong, L. A flexible, sensitive and stable humidity sensor based on an all-polymer nanofiber film. Mater. Lett. 2023, 330, 133268. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Chen, X.; Ding, X.; Zhao, X. High-sensitive humidity sensor based on graphene oxide with evenly dispersed multiwalled carbon nanotubes. Mater. Chem. Phys. 2018, 207, 135–140. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Q.; Nie, J.; Liang, J.; Joshi, N.; Hayasaka, T.; Zhao, S.; Zhang, M.; Wang, X.; Lin, L. All-carbon based flexible humidity sensor. J. Nanosci. Nanotechnol. 2019, 19, 5310–5316. [Google Scholar] [CrossRef] [PubMed]
- Tulliani, J.M.; Inserra, B.; Ziegler, D. Carbon-based materials for humidity sensing: A short review. Micromachines 2019, 10, 232. [Google Scholar] [CrossRef]
- Liang, R.; Luo, A.; Zhang, Z.; Li, Z.; Han, C.; Wu, W. Research progress of graphene-based flexible humidity sensor. Sensors 2020, 20, 5601. [Google Scholar] [CrossRef]
- Anichini, C.; Aliprandi, A.; Gali, S.M.; Liscio, F.; Morandi, V.; Minoia, A.; Beljonne, D.; Ciesielski, A.; Samorì, P. Ultrafast and highly sensitive chemically functionalized graphene oxide-based humidity sensors: Harnessing device performances via the supramolecular approach. ACS Appl. Mater. Interfaces 2020, 12, 44017–44025. [Google Scholar] [CrossRef] [PubMed]
- Songkeaw, P.; Onlaor, K.; Thiwawong, T.; Tunhoo, B. Transparent and flexible humidity sensor based on graphene oxide thin films prepared by electrostatic spray deposition technique. J. Mater. Sci. Mater. Electron. 2020, 31, 12206–12215. [Google Scholar] [CrossRef]
- Hajian, S.; Zhang, X.; Khakbaz, P.; Tabatabaei, S.M.; Maddipatla, D.; Narakathu, B.B.; Blar, R.G.; Atashbar, M.Z. Development of a fluorinated graphene-based resistive humidity sensor. IEEE Sens. J. 2020, 20, 7517–7524. [Google Scholar] [CrossRef]
- Gu, Y.; Jiang, H.; Ye, Z.; Sun, N.; Kuang, X.; Liu, W.; Li, G.; Song, X.; Zhang, L.; Bai, W. Impact of size on humidity sensing property of copper oxide nanoparticles. Electron. Mater. Lett. 2020, 16, 61–71. [Google Scholar] [CrossRef]
- Li, P.; Yu, S.; Zhang, H. Preparation and performance analysis of Ag/ZnO humidity sensor. Sensors 2021, 21, 857. [Google Scholar] [CrossRef] [PubMed]
- Nitta, R.; Lin, H.E.; Kubota, Y.; Kishi, T.; Yano, T.; Matsushita, N. CuO nanostructure-based flexible humidity sensors fabricated on PET substrates by spin-spray method. Appl. Surf. Sci. 2022, 572, 151352. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z. Fabrication of tin disulfide/graphene oxide nanoflower on flexible substrate for ultrasensitive humidity sensing with ultralow hysteresis and good reversibility. Sens. Actuators B 2019, 287, 398–407. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Z.; Yang, Z.; Song, X. High-performance flexible self-powered tin disulfide nanoflowers/reduced graphene oxide nanohybrid-based humidity sensor driven by triboelectric nanogenerator. Nano Energy 2020, 67, 104251. [Google Scholar] [CrossRef]
- Chaloeipote, G.; Samarnwong, J.; Traiwatcharanon, P.; Kerdcharoen, T.; Wongchoosuk, C. High-performance resistive humidity sensor based on Ag nanoparticles decorated with graphene quantum dots. R. Soc. Open Sci. 2021, 8, 210407. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; Morsy, M.; Ateia, M.A.; Abdel-Haleem, F.M. Ultrafast response humidity sensors based on polyvinyl chloride/ graphene oxide nanocomposites for intelligent food packaging. Sens. Actuators A 2021, 331, 112918. [Google Scholar] [CrossRef]
- Cai, C.; Zhao, W.; Yang, J.; Zhang, L. Sensitive and flexible humidity sensor based on sodium hyaluronate/MWCNTs composite film. Cellulose 2021, 28, 6361–6371. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.K.; Mathuriya, A.S.; Sachdeva, A. Synthesis, characterisation and humidity sensing properties of PVA-NaI composite. Mater. Today Proc. 2022, 49, 3365–3369. [Google Scholar] [CrossRef]
- Afnas, V.M.; Unnikrishnan, G.; Budhe, S.; Manaf, O.; Ameen, J. PVA/gelatin/chitin ternary blend as a humidity sensing material. J. Mater. Sci. Mater. Electron. 2022, 33, 2031–2043. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Z.; Wang, Q.; Liu, R.; Xu, Y.; Wang, K.; Shi, X.; Ye, S. Surface engineering on polyimide−silver films in low-cost, flexible humidity sensors. ACS Appl. Mater. Interfaces 2022, 14, 16621–16630. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.Y.; Lee, S.H.; Yi, S.H. Humidity sensors using porous silicon layer with mesa structure. J. Phys. D Appl. Phys. 2000, 33, 1781–1784. [Google Scholar] [CrossRef]
- Fürjes, P.; Kovács, A.; Dücso, C.; Ádáma, M.; Müller, B.; Mescheder, U. Porous silicon-based humidity sensor with interdigital electrodes and internal heaters. Sens. Actuators B 2003, 95, 140–144. [Google Scholar] [CrossRef]
- Harraz, F.A. Porous silicon chemical sensors and biosensors: A review. Sens. Actuators B 2014, 202, 897–912. [Google Scholar] [CrossRef]
- He, X.W.; Geng, W.C.; Zhang, B.L.; Jia, L.M.; Duan, L.B.; Zhang, Q.Y. Ultrahigh humidity sensitivity of NaCl-added 3D mesoporous silica KIT-6 and its sensing mechanism. RSC Adv. 2016, 6, 38391–38398. [Google Scholar] [CrossRef]
- Su, P.G.; Chen, I.C.; Wu, R.J. Use of poly(2-acrylamido-2-methylpropane sulfonate) modified with tetraethyl orthosilicate as sensing material for measurement of humidity. Anal. Chim. Acta 2001, 449, 103–109. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Hong, L.; Luo, D.; Yang, M. A highly water-resistive humidity sensor based on silicon-containing polyelectrolytes prepared by one-pot method. Sens. Actuators B 2007, 124, 347–351. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Dai, J.; Jiang, K.; Fei, T. Preparation of hydrophilic organic groups modified mesoporous silica materials and their humidity sensitive properties. Sens. Actuators B 2017, 240, 681–688. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Qi, R.; Dai, J.; Liu, S.; Fei, T.; Lu, G. Organic-inorganic hybrid materials based on mesoporous silica derivatives for humidity sensing. Sens. Actuators B 2017, 248, 803–811. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, T.; Guan, X.; Dai, J.; Liu, S.; Zhao, H.; Fei, T. Capacitive humidity sensors based on mesoporous silica and poly (3, 4-ethylenedioxythiophene) composites. J. Colloid Interface Sci. 2020, 565, 592–600. [Google Scholar] [CrossRef]
- Su, P.G.; Huang, S.C. Electrical and humidity sensing properties of carbon nanotubes-SiO2-poly(2-acrylamido-2-methylpropane sulfonate) composite material. Sens. Actuators B 2006, 113, 142–149. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Xiong, Y.; Dong, F. Rational design, synthesis, and application of silica/graphene-based nanocomposite: A review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, N.; Yin, Y.; Xu, B.; Zhang, W.; Wang, C. High-sensitivity and low-hysteresis GO-NH2/mesoporous SiO2 nanosphere-fabric-based humidity sensor for respiratory monitoring and noncontact sensing. Adv. Mater. Interfaces 2022, 9, 2101498. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.L.; Qing, Q.; Yang, Y.L.; Li, Q.W.; Liu, Z.F.; Quo, X.Y.; Du, Z.L. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Casalbore-Miceli, G.; Yang, M.J.; Camaioni, N.; Mari, C.M.; Li, Y.; Sun, H.; Ling, M. Investigations on the ion transport mechanism in conduction polymer films. Solid State Ion. 2000, 131, 311–321. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.K.; Ruan, S.P.; Wang, S.P. Preparation and electrical properties of humidity sensing films of BaTiO3/polystrene sulfonic sodium. Mater. Chem. Phys. 2003, 78, 746–750. [Google Scholar] [CrossRef]



| Sensing Curve | ||
|---|---|---|
| Materials | Sensitivity (log Z/%RH) a | Linearity (R2) b |
| Pristine SiO2 | –0.0003 | 0.9646 |
| MPTMS-TMSPMA | –0.0034 | 0.9609 |
| MPTMS-TMSPMA-MAPTAC | –0.0442 | 0.9694 |
| Au NPs/MPTMS-TMSPMA-MAPTAC | –0.0533 | 0.9562 |
| Sensing Material | Working Range (%RH) | Sensitivity −(log Z/% RH) | Hysteresis (% RH) | Flexibility (%) (log Z Deviation) | Response Time (s) | References |
|---|---|---|---|---|---|---|
| SiO2-poly-AMPS | 30–90 | 0.0571 | 2% | - | - | [43] |
| APTOS a/n-butyl bromide | 11–97 | - | 1% | - | 13 | [44] |
| SBA-15/MATMAC b | 11–95 | - | 2% | - | 11 | [45] |
| SBA-15/sodium p-styrenesulfonate | 11–95 | - | - | - | 5 | [46] |
| MCM-41/PEDOT | 11–95 | - | 6 | - | 165 | [47] |
| Au NPs/MPTMS-TMSPMA-MAPTAC | 20–90 | 0.0533 | 3.5% | <10% | 15 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.-G.; Hsu, C.-C. Flexible Humidity Sensor Based on Au Nanoparticles/Organosilica-Containing Polyelectrolyte Composite. Chemosensors 2023, 11, 291. https://doi.org/10.3390/chemosensors11050291
Su P-G, Hsu C-C. Flexible Humidity Sensor Based on Au Nanoparticles/Organosilica-Containing Polyelectrolyte Composite. Chemosensors. 2023; 11(5):291. https://doi.org/10.3390/chemosensors11050291
Chicago/Turabian StyleSu, Pi-Guey, and Chih-Chang Hsu. 2023. "Flexible Humidity Sensor Based on Au Nanoparticles/Organosilica-Containing Polyelectrolyte Composite" Chemosensors 11, no. 5: 291. https://doi.org/10.3390/chemosensors11050291
APA StyleSu, P.-G., & Hsu, C.-C. (2023). Flexible Humidity Sensor Based on Au Nanoparticles/Organosilica-Containing Polyelectrolyte Composite. Chemosensors, 11(5), 291. https://doi.org/10.3390/chemosensors11050291







