Rapid Fabrication of Homogeneous Submicron Silver Particles via a Microfluidic Chip and Use as a SERS Detection Substrate
Abstract
1. Introduction
2. Methodology and Characterizations
2.1. Materials
2.2. Instruments
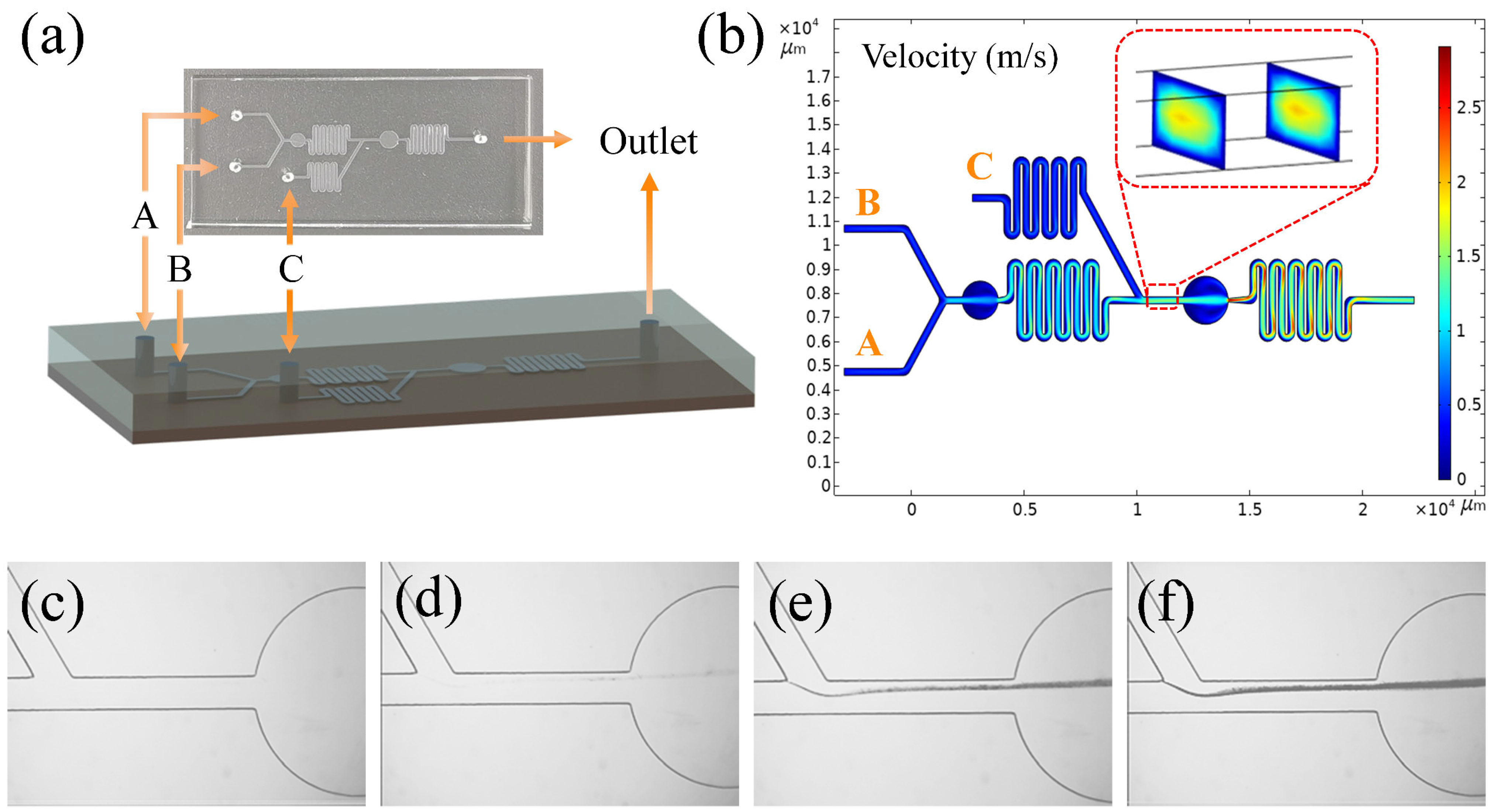
2.3. Microfluidic Devices
2.4. Manufacturing Submicron Silver Particles by Microfluidics
2.5. SERS Measurement
3. Results and Discussion
3.1. Reduction Reaction in Microchips
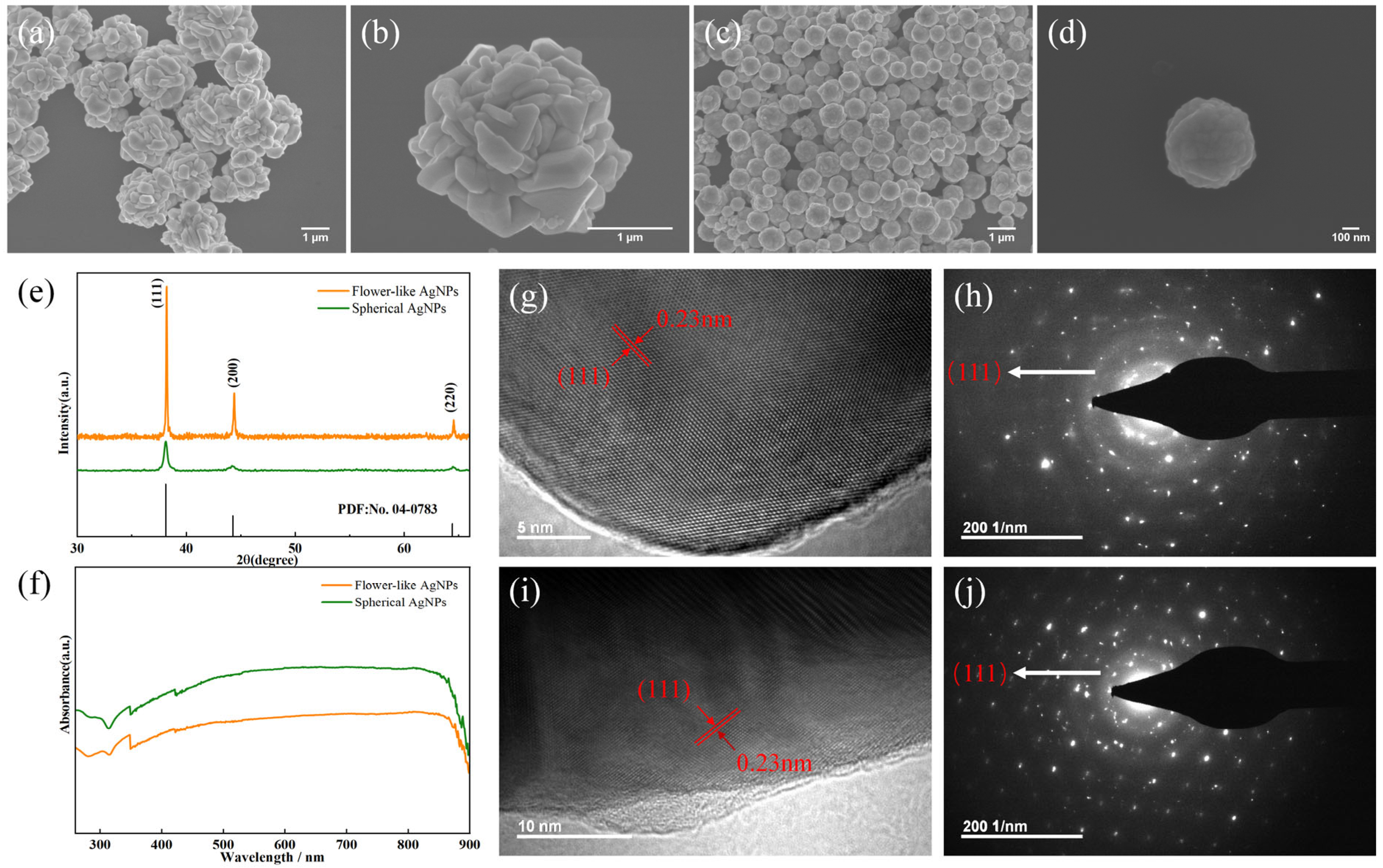
3.2. Characterization of Submicron Silver Particles
3.3. Factors Affecting the Morphology of the Silver Particles
3.4. Comparison of Silver Particles Prepared by the Microfluidic Chip and the Conventional Method
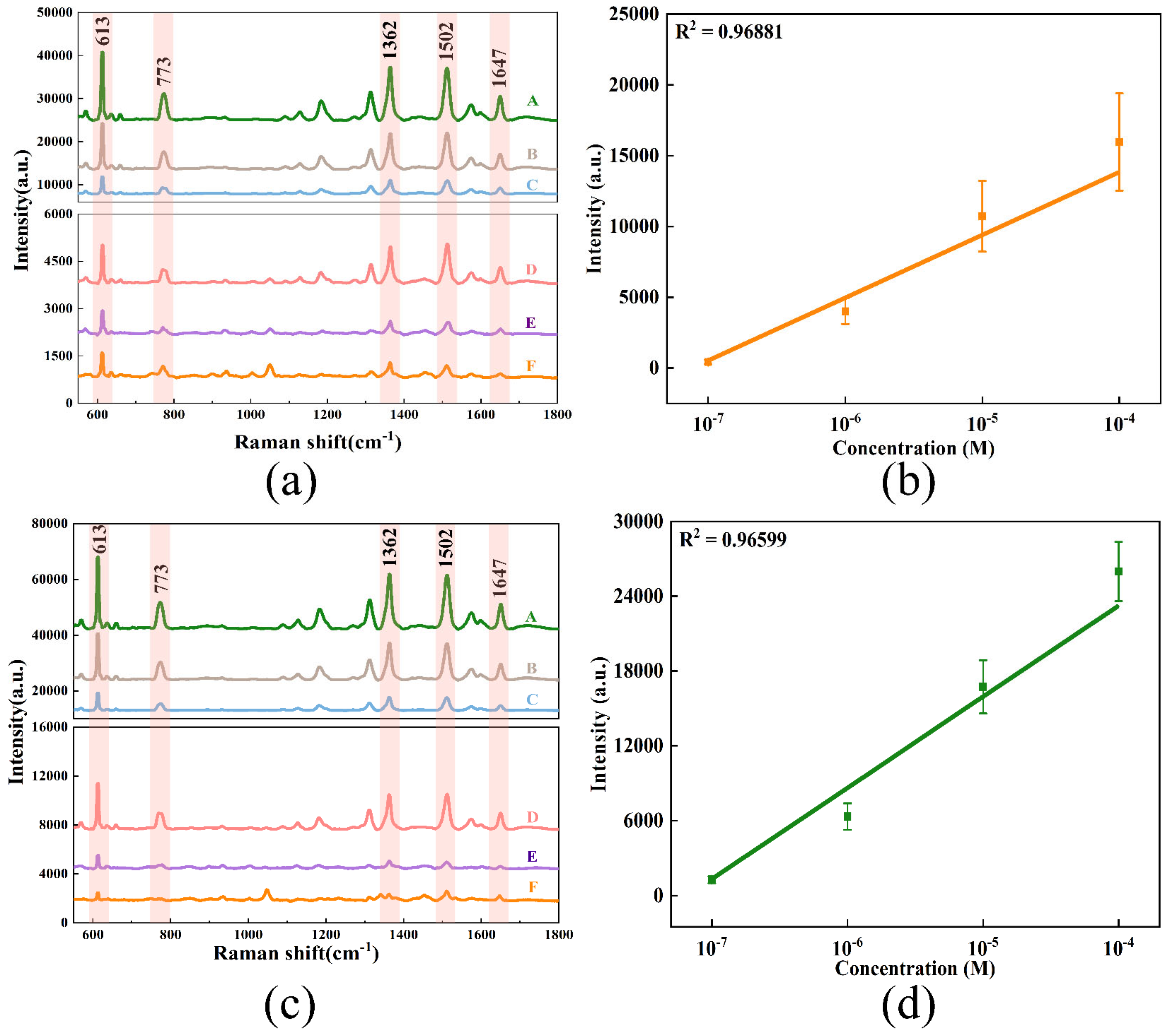
3.5. SERS Enhancement Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, N.; Paramasivam, K.; Rajesh, T.; Purusothamanan, V.S.; Iyahraja, S. Silver nanoparticles for enhanced thermal energy storage of phase change materials. Mater. Today-Proc. 2021, 45, 607–611. [Google Scholar] [CrossRef]
- Shankar, A.; Salcedo, E.; Berndt, A.; Choi, D.; Ryu, J.E. Pulsed light sintering of silver nanoparticles for large deformation of printed stretchable electronics. Adv. Compos. Hybrid Mater. 2017, 1, 193–198. [Google Scholar] [CrossRef]
- Zhang, J.P.; Li, H.T.; Xu, T.; Wu, J.J.; Zhou, S.L.; Hang, Z.H.; Zhang, X.H.; Yang, Z.H. Homogeneous silver nanoparticles decorating 3D carbon nanotube sponges as flexible high-performance electromagnetic shielding composite materials. Carbon 2020, 165, 404–411. [Google Scholar] [CrossRef]
- Qi, L.B.; Zhang, K.M.; Qin, W.; Hu, Y.X. Highly efficient flow-through catalytic reduction of methylene blue using silver nanoparticles functionalized cotton. Chem. Eng. J. 2020, 388, 124252. [Google Scholar] [CrossRef]
- Vega-Baudrit, J.; Gamboa, S.M.; Rojas, E.R.; Martinez, V.V. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosens. Bioelectron. 2019, 5, 166–173. [Google Scholar] [CrossRef]
- Panacek, A.; Kolar, M.; Vecerova, R.; Prucek, R.; Soukupova, J.; Krystof, V.; Hamal, P.; Zboril, R.; Kvitek, L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Quinten, M.; Leitner, A.; Krenn, J.R.; Aussenegg, F.R. Electromagnetic energy transport via linear chains of silver nanoparticles. Opt. Lett. 1998, 23, 1331–1333. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Nguyen, T.V.; Chu, A.D.; Tran, H.V.; Tran, L.T.; Huynh, C.D. A label-free colorimetric sensor based on silver nanoparticles directed to hydrogen peroxide and glucose. Arab. J. Chem. 2018, 11, 1134–1143. [Google Scholar] [CrossRef]
- Eising, R.; Elias, W.C.; Albuquerque, B.L.; Fort, S.; Domingos, J.B. Synthesis of silver glyconanoparticles from new sugar-based amphiphiles and their catalytic application. Langmuir 2014, 30, 6011–6020. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver nanoparticle catalysed redox reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
- Ren, X.; Meng, X.; Chen, D.; Tang, F.; Jiao, J. Using silver nanoparticle to enhance current response of biosensor. Biosens Bioelectron 2005, 21, 433–437. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Haes, A.J.; Duyne, R.P. Preliminary studies and potential applications of localized surface plasmon resonance spectroscopy in medical diagnostics. Expert Rev. Mol. Diagn. 2004, 4, 527–537. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jayaramudu, T.; Varaprasad, K.; Sadiku, R.; Ray, S.S.; Mohana Raju, K. Cellulose-polymer-Ag nanocomposite fibers for antibacterial fabrics/skin scaffolds. Carbohydr. Polym. 2013, 93, 553–560. [Google Scholar] [CrossRef]
- Vu, X.H.; Duong, T.T.T.; Pham, T.T.H.; Trinh, D.K.; Nguyen, X.H.; Dang, V.-S. Synthesis and study of silver nanoparticles for antibacterial activity against Escherichia coli and Staphylococcus aureus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025019. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Noguez, C. Plasmonic Optical Properties and Applications of Metal Nanostructures. Plasmonics 2008, 3, 127–150. [Google Scholar] [CrossRef]
- Zhang, M.L.; Fan, X.; Zhou, H.W.; Shao, M.W.; Zapien, J.A.; Wong, N.B.; Lee, S.T. A High-Efficiency Surface-Enhanced Raman Scattering Substrate Based on Silicon Nanowires Array Decorated with Silver Nanoparticles. J. Phys. Chem. C 2010, 114, 1969–1975. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.a.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Kibis, L.S.; Stadnichenko, A.I.; Pajetnov, E.M.; Koscheev, S.V.; Zaykovskii, V.I.; Boronin, A.I. The investigation of oxidized silver nanoparticles prepared by thermal evaporation and radio-frequency sputtering of metallic silver under oxygen. Appl. Surf. Sci. 2010, 257, 404–413. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Mangini, V.; Valenza, G.; De Pascale, O.; De Stradis, A.; Natile, G.; Arnesano, F.; De Giacomo, A. Silver and gold nanoparticles produced by pulsed laser ablation in liquid to investigate their interaction with Ubiquitin. Appl. Surf. Sci. 2016, 374, 297–304. [Google Scholar] [CrossRef]
- Jung, J.H.; Cheol Oh, H.; Soo Noh, H.; Ji, J.H.; Soo Kim, S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. 2006, 37, 1662–1670. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Liu, X. Ultrafine silver nanoparticles obtained from ethylene glycol at room temperature: Catalyzed by tungstate ions. Dalton Trans. 2014, 43, 132–137. [Google Scholar] [CrossRef]
- Nasiriboroumand, M.; Montazer, M.; Barani, H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J. Photochem. Photobiol. B Biol. 2018, 179, 98–104. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Biomol. Eng. 2009, 2, 104–111. [Google Scholar]
- Toisawa, K.; Hayashi, Y.; Takizawa, H. Synthesis of Highly Concentrated Ag Nanoparticles in a Heterogeneous Solid-Liquid System under Ultrasonic Irradiation. Mater. Trans. 2010, 51, 1764–1768. [Google Scholar] [CrossRef]
- Wagner, J.; Kohler, J.M. Continuous synthesis of gold nanoparticles in a microreactor. Nano Lett. 2005, 5, 685–691. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Xu, P.F.; Liu, Z.H.; Duan, Y.H.; Sun, Q.; Wang, D.; Zeng, X.F.; Wang, J.X. Microfluidic controllable synthesis of monodispersed sulfur nanoparticles with enhanced antibacterial activities. Chem. Eng. J. 2020, 398, 125293. [Google Scholar] [CrossRef]
- Luo, G.S.; Du, L.; Wang, Y.J.; Lu, Y.C.; Xu, J.H. Controllable preparation of particles with microfluidics. Particuology 2011, 9, 545–558. [Google Scholar] [CrossRef]
- Jahn, A.; Reiner, J.E.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Preparation of nanoparticles by continuous-flow microfluidics. J. Nanoparticle Res. 2008, 10, 925–934. [Google Scholar] [CrossRef]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Jiang, L.G.; Wang, W.P.; Chau, Y.; Yao, S.H. Controllable formation of aromatic nanoparticles in a three-dimensional hydrodynamic flow focusing microfluidic device. Rsc Adv. 2013, 3, 17762–17769. [Google Scholar] [CrossRef]
- Lazarus, L.L.; Riche, C.T.; Marin, B.C.; Gupta, M.; Malmstadt, N.; Brutchey, R.L. Two-phase microfluidic droplet flows of ionic liquids for the synthesis of gold and silver nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 3077–3083. [Google Scholar] [CrossRef]
- Lazarus, L.L.; Yang, A.S.; Chu, S.; Brutchey, R.L.; Malmstadt, N. Flow-focused synthesis of monodisperse gold nanoparticles using ionic liquids on a microfluidic platform. Lab A Chip 2010, 10, 3377–3379. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding whether to go with the flow: Evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Li, X.D.; Wang, H. Microfluidic Synthesis of Nanomaterials for Biomedical Applications. Small Methods 2017, 1, 1700140. [Google Scholar] [CrossRef]
- Brivio, M.; Verboom, W.; Reinhoudt, D.N. Miniaturized continuous flow reaction vessels: Influence on chemical reactions. Lab A Chip 2006, 6, 329–344. [Google Scholar] [CrossRef]
- deMello, J.; deMello, A. Microscale reactors: Nanoscale products. Lab Chip 2004, 4, 11N–15N. [Google Scholar] [CrossRef]
- Li, G.X.; Li, Q.; Cheng, R.; Chen, S. Synthesis of quantum dots based on microfluidic technology. Curr. Opin. Chem. Eng. 2020, 29, 34–41. [Google Scholar] [CrossRef]
- Amreen, K.; Goel, S. Review-Miniaturized and Microfluidic Devices for Automated Nanoparticle Synthesis. Ecs J. Solid State Sci. Technol. 2021, 10, 017002. [Google Scholar] [CrossRef]
- Zhang, M.J.; Wang, W.; Xie, R.; Ju, X.J.; Liu, Z.; Jiang, L.; Chen, Q.M.; Chu, L.Y. Controllable microfluidic strategies for fabricating microparticles using emulsions as templates. Particuology 2016, 24, 18–31. [Google Scholar] [CrossRef]
- Chen, C.H.; Shah, R.K.; Abate, A.R.; Weitz, D.A. Janus particles templated from double emulsion droplets generated using microfluidics. Langmuir 2009, 25, 4320–4323. [Google Scholar] [CrossRef]
- Shum, H.C.; Abate, A.R.; Lee, D.; Studart, A.R.; Wang, B.; Chen, C.H.; Thiele, J.; Shah, R.K.; Krummel, A.; Weitz, D.A. Droplet microfluidics for fabrication of non-spherical particles. Macromol. Rapid Commun. 2010, 31, 108–118. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, X.; Li, P.; Lin, X.; Wang, J.; Hu, Z.; Zhang, P.; Chen, D.; Cai, H.; Niessner, R.; et al. Ultrasensitive and Simultaneous SERS Detection of Multiplex MicroRNA Using Fractal Gold Nanotags for Early Diagnosis and Prognosis of Hepatocellular Carcinoma. Anal. Chem. 2021, 93, 8799–8809. [Google Scholar] [CrossRef]
- Xu, L.; Peng, J.H.; Yan, M.; Zhang, D.; Shen, A.Q. Droplet synthesis of silver nanoparticles by a microfluidic device. Chem. Eng. Process.-Process Intensif. 2016, 102, 186–193. [Google Scholar] [CrossRef]
- Magdalene, D.J.; Muthuselvam, D.; Pravinraj, T. Microfluidics-based green synthesis of silver nanoparticle from the aqueous leaf extract of Ipomea quamoclit L. Appl. Nanosci. 2021, 11, 2073–2084. [Google Scholar] [CrossRef]
- Liu, G.J.; Ma, X.; Sun, X.D.; Jia, Y.H.; Wang, T.F. Controllable Synthesis of Silver Nanoparticles Using Three-Phase Flow Pulsating Mixing Microfluidic Chip. Adv. Mater. Sci. Eng. 2018, 2018, 3758161. [Google Scholar] [CrossRef]
- Ryan, N.W.; Johnson, M.M. Transistion from laminar to turbulent flow in pipes. AIChE J. 1959, 5, 433–435. [Google Scholar] [CrossRef]
- Murphy, C.J. Materials science. Nanocubes and nanoboxes. Science 2002, 298, 2139–2141. [Google Scholar] [CrossRef]
- Wang, W.; Efrima, S.; Regev, O. Directing Silver Nanoparticles into Colloid−Surfactant Lyotropic Lamellar Systems. J. Phys. Chem. B 1999, 103, 5613–5621. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles, and silver ions induce reactive oxygen species, apoptosis, and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Li, Y.; Mo, F.; Li, Z.; Chai, R.; Wang, H. Dispersibility and Size Control of Silver Nanoparticles with Anti-Algal Potential Based on Coupling Effects of Polyvinylpyrrolidone and Sodium Tripolyphosphate. Nanomaterials 2020, 10, 1042. [Google Scholar] [CrossRef]
- Munoz-Davila, M.J. Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections. Antibiotics 2014, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, S.; Yao, F.; Xu, W.; Li, Y.; Chen, Q.; Liang, P. Rapid Fabrication of Homogeneous Submicron Silver Particles via a Microfluidic Chip and Use as a SERS Detection Substrate. Chemosensors 2023, 11, 232. https://doi.org/10.3390/chemosensors11040232
Chen J, Li S, Yao F, Xu W, Li Y, Chen Q, Liang P. Rapid Fabrication of Homogeneous Submicron Silver Particles via a Microfluidic Chip and Use as a SERS Detection Substrate. Chemosensors. 2023; 11(4):232. https://doi.org/10.3390/chemosensors11040232
Chicago/Turabian StyleChen, Junjie, Suyang Li, Fuqi Yao, Wanbing Xu, Yunfeng Li, Qiang Chen, and Pei Liang. 2023. "Rapid Fabrication of Homogeneous Submicron Silver Particles via a Microfluidic Chip and Use as a SERS Detection Substrate" Chemosensors 11, no. 4: 232. https://doi.org/10.3390/chemosensors11040232
APA StyleChen, J., Li, S., Yao, F., Xu, W., Li, Y., Chen, Q., & Liang, P. (2023). Rapid Fabrication of Homogeneous Submicron Silver Particles via a Microfluidic Chip and Use as a SERS Detection Substrate. Chemosensors, 11(4), 232. https://doi.org/10.3390/chemosensors11040232






