Facile Electrochemical Approach Based on Hydrogen-Bonded MOFs-Derived Tungsten Ethoxide/Polypyrrole-Reduced GO Nanocrystal for ppb Level Ammonium Ions Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polypyrrole-Reduced Graphene Oxide
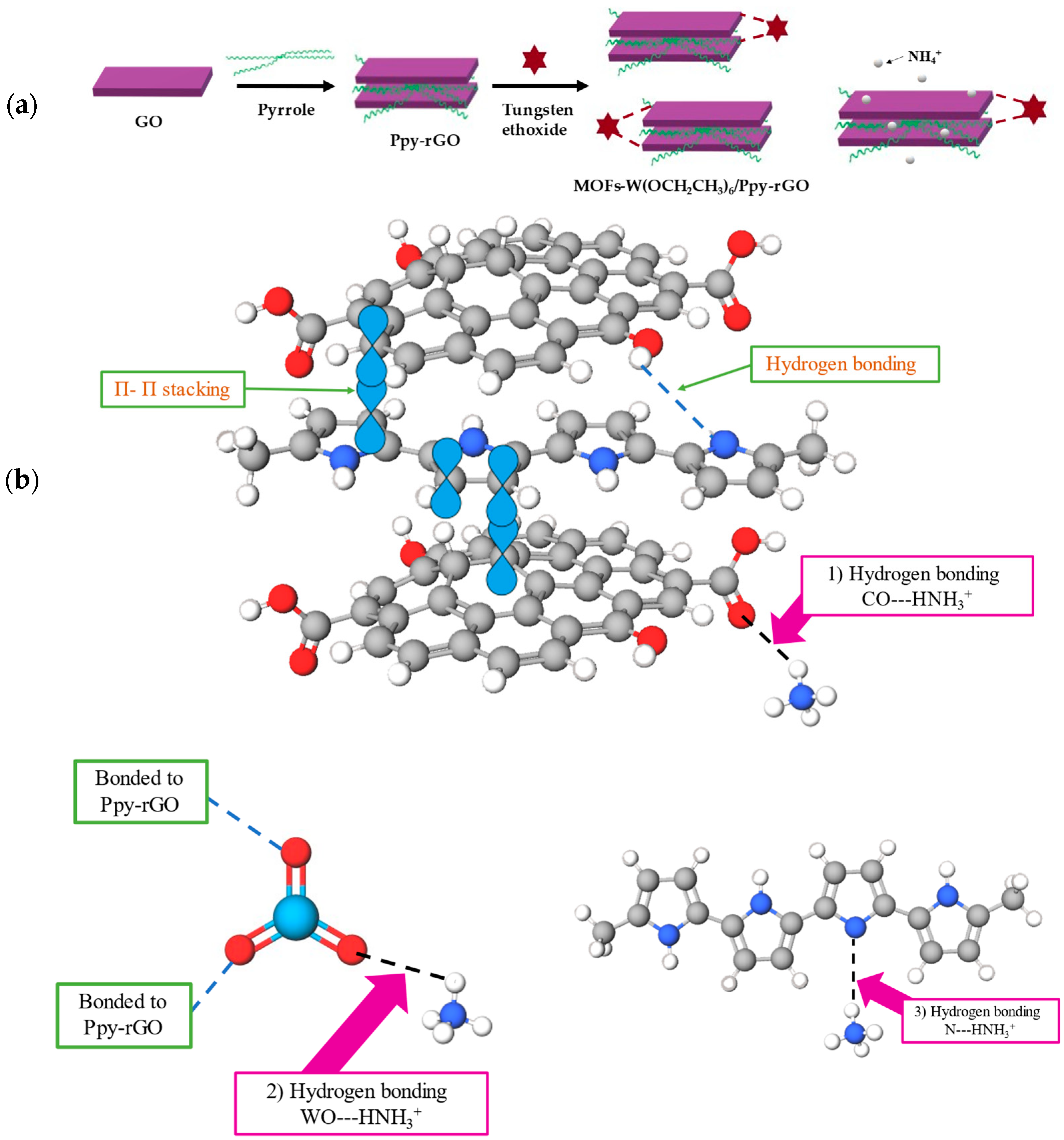
2.3. Preparation of Metal-Organic Frameworks Derived Tungsten Ethoxide/Polypyrrole-Reduced Graphene Oxide
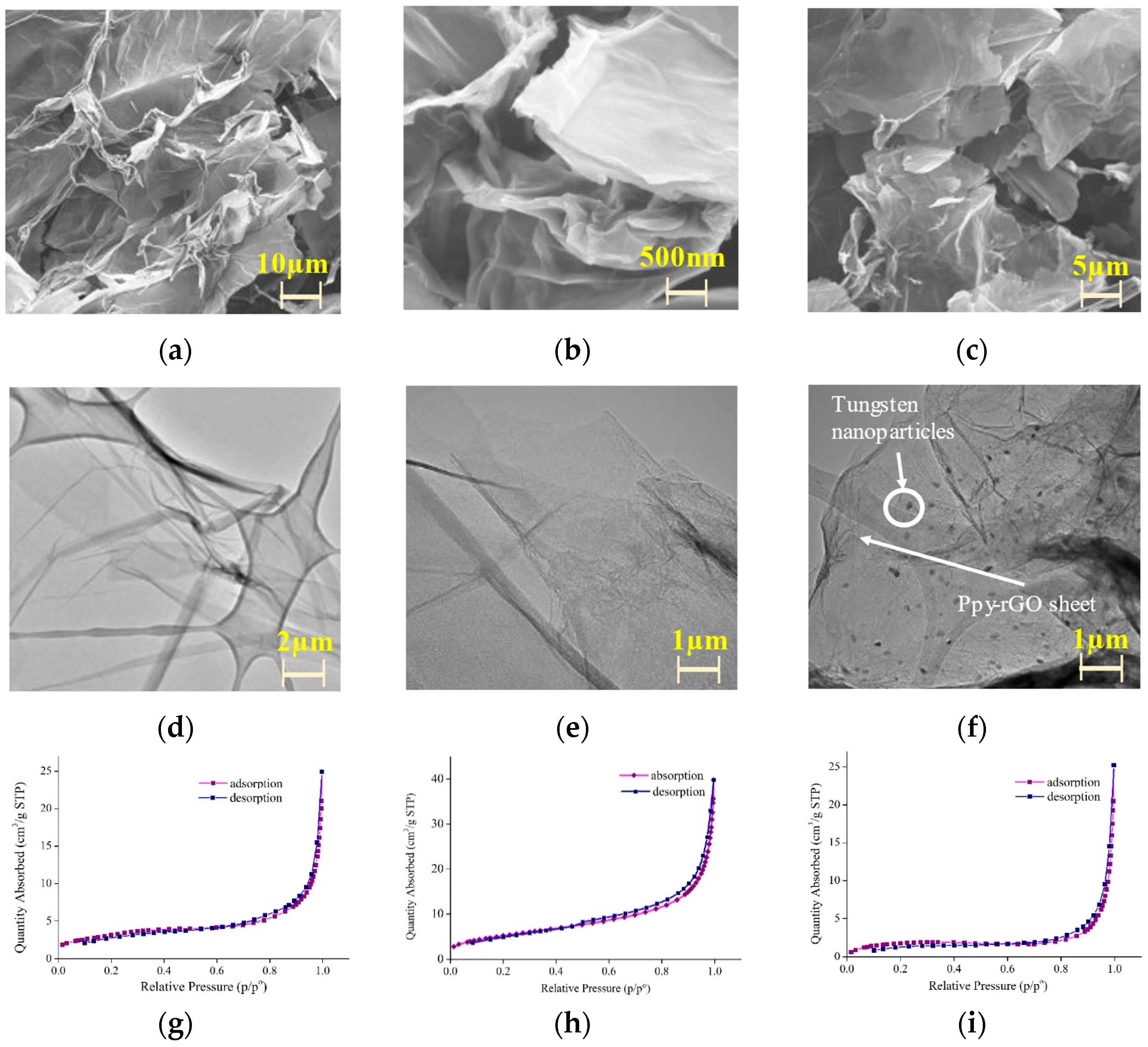
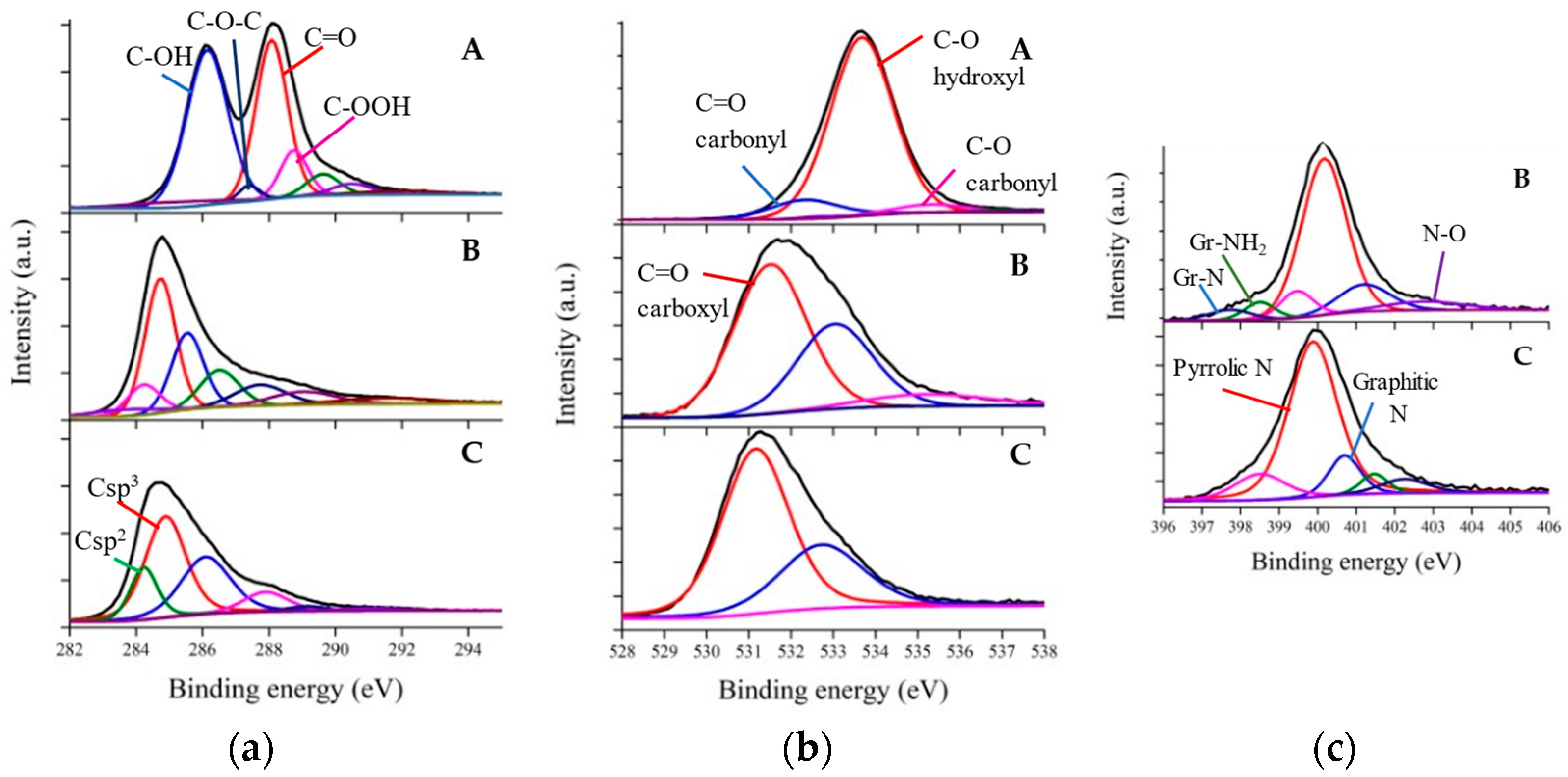
2.4. Characterizations
2.5. Ammonium Ion Sensing Measurement
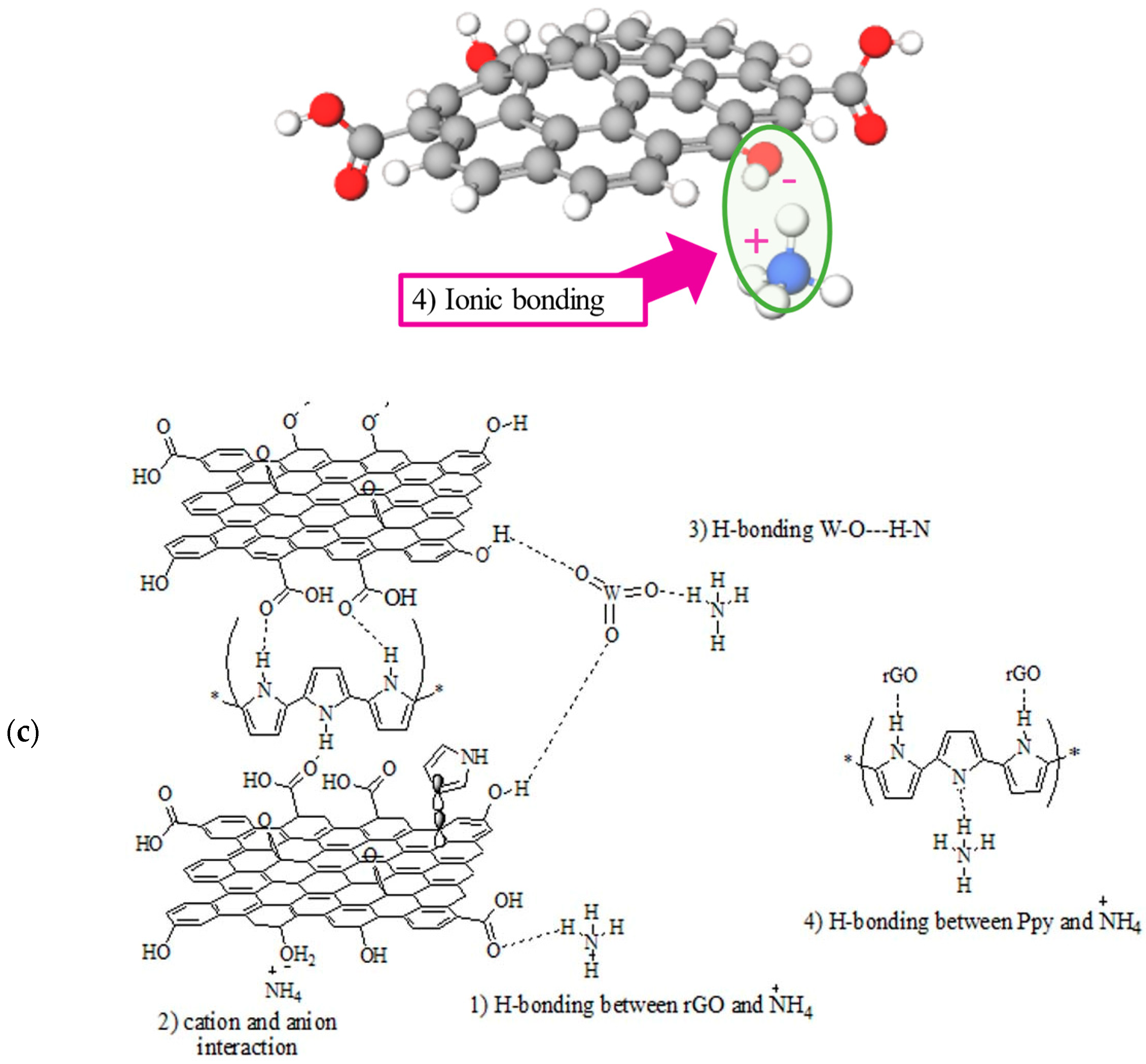
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef]
- Vega-Mas, I.; Marino, D.; Sanchez-Zabala, J.; Gonzalez-Murua, C.; Estavillo, J.M.; González-Moro, M.B. CO2 enrichment modulates ammonium nutrition in tomato adjusting carbon and nitrogen metabolism to stomatal conductance. Plant Sci. 2015, 241, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Framework as an Efficient Adsorbent for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Amirjani, A.; Fatmehsari, D.H. Colorimetric detection of ammonia using smartphones based on localized surface plasmon resonance of silver nanoparticles. Talanta 2018, 176, 242–246. [Google Scholar] [CrossRef]
- Ling, T.L.; Ahmad, M.; Heng, L.Y. UV-vis spectrophotometric and artificial neural network for estimation of ammonia in aqueous environment using cobalt (II) ions. Anal. Methods 2013, 5, 6709–6714. [Google Scholar] [CrossRef]
- Azmi, N.E.; Ahmad, M.; Abdullah, J.; Sidek, H.; Heng, L.Y.; Karuppiah, N. Biosensor based on glutamate dehydrogenase immobilized in chitosan for the determination of ammonium in water samples. Anal. Biochem. 2009, 388, 28–32. [Google Scholar] [CrossRef]
- Jackson, P.E. Ion chromatography in environmental analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 2779–2801. [Google Scholar]
- Kazanskaya, N.; Kukhtin, A.; Manenkova, M.; Reshetilov, N.; Yarysheva, L.; Arzhakova, O.; Volynskii, A.; Bakeyev, N. FET-based sensors with robust photosensitive polymer membranes for detection of ammonium ions and urea. Biosens. Bioelectron. 1996, 11, 253–261. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Mohanta, G.C.; Sharma, A.L.; Kim, K.-H.; Deep, A. A three-phase copper MOF-graphene-polyaniline composite for effective sensing of ammonia. Anal. Chim. Acta 2018, 1043, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Shustova, N.B.; Cozzolino, A.F.; Reineke, S.; Baldo, M.; Dincă, M. Selective turn-on ammonia sensing enabled by high-temperature fluorescence in metal–organic frameworks with open metal sites. J. Am. Chem. Soc. 2013, 135, 13326–13329. [Google Scholar] [CrossRef]
- Li, Y.-P.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C.; Zhai, Q.-G. A semiconductor and fluorescence dual-mode room-temperature ammonia sensor achieved by decorating hydroquinone into a metal–organic framework. Chem. Commun. 2018, 54, 9789–9792. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, L.; Yu, S.; Liang, X.; Li, Z.; Li, G. Enhancing Proton Conductivity of a 3D Metal–Organic Framework by Attaching Guest NH3 Molecules. Inorg. Chem. 2018, 57, 11560–11568. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Howarth, A.J.; Moghadam, P.Z.; DeCoste, J.B.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. High volumetric uptake of ammonia using Cu-MOF-74/Cu-CPO-27. Dalton Trans. 2016, 45, 4150–4153. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Demon, S.Z.N.; Kamisan, A.I.; Abdullah, N.; Noor, S.A.M.; Khim, O.K.; Kasim, N.A.M.; Yahya, M.Z.A.; Manaf, N.A.A.; Azmi, A.F.M.; Halim, N.A. Graphene-based Materials in Gas Sensor Applications: A Review. Sens. Mater. 2020, 32, 759–777. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef]
- Gong, X.; Liu, G.; Li, Y.; Yu, D.Y.W.; Teoh, W.Y. Functionalized-graphene composites: Fabrication and applications in sustainable energy and environment. Chem. Mater. 2016, 28, 8082–8118. [Google Scholar] [CrossRef]
- Zheng, H.; Tachibana, Y.; Kalantar-Zadeh, K. Dye-sensitized solar cells based on WO3. Langmuir 2010, 26, 19148–19152. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Yue, Y.; Wang, X.; Srikhaow, A.; Sriprachuabwong, C.; Tuantranont, A.; Zhang, X.; Wu, Z.-S.; Qin, J. Strongly coupled tungsten oxide/carbide heterogeneous hybrid for ultrastable aqueous rocking-chair zinc-ion batteries. Chem. Eng. J. 2021, 426, 131893. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, X.; Cheng, G.; Chen, R.; Xiong, J.; Li, W.; Wei, Y. Engineered tungsten oxide-based photocatalysts for CO2 reduction: Categories and roles. J. Mater. Chem. A 2021, 9, 22781–22809. [Google Scholar] [CrossRef]
- Han, W.; Shi, Q.; Hu, R. Advances in electrochemical energy devices constructed with tungsten oxide-based nanomaterials. Nanomaterials 2021, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yun, S.; Wang, C.; Wang, Z.; Han, F.; Si, Y. Bio-based carbon-enhanced tungsten-based bimetal oxides as counter electrodes for dye-sensitized solar cells. J. Power Sources 2019, 423, 339–348. [Google Scholar] [CrossRef]
- Jeevitha, G.; Mangalaraj, D. Ammonia sensing at ambient temperature using tungsten oxide (WO3) nanoparticles. Mater. Today Proc. 2019, 18, 1602–1609. [Google Scholar] [CrossRef]
- Gupta, S.P.; More, M.A.; Late, D.J.; Walke, P.S. High-rate quasi-solid-state hybrid supercapacitor of hierarchical flowers of hydrated tungsten oxide nanosheets. Electrochim. Acta 2021, 366, 137389. [Google Scholar] [CrossRef]
- Jaroenapibal, P.; Boonma, P.; Saksilaporn, N.; Horprathum, M.; Amornkitbamrung, V.; Triroj, N. Improved NO2 sensing performance of electrospun WO3 nanofibers with silver doping. Sens. Actuators B Chem. 2018, 255, 1831–1840. [Google Scholar] [CrossRef]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: Gas sensing performance and mechanism study. Adv. Funct. Mater. 2018, 28, 1705268. [Google Scholar] [CrossRef]
- Ban, F.; Majid, S.R.; Huang, N.M.; Lim, H. Graphene oxide and its electrochemical performance. Int. J. Electrochem. Sci. 2012, 7, 4345–4351. [Google Scholar]
- Hizam, S.M.M.; Soaid, N.I.; Saheed, M.S.M.; Mohamed, N.M.; Kait, C.F. Study of Electrical Conductivity of Pyrrole-Reduced Graphene Oxide Pellet. In Proceedings of the 2021 IEEE International Conference on Sensors and Nanotechnology (SENNANO), Port Dickson, Malaysia, 22–24 September 2021; pp. 150–154. [Google Scholar]
- Wang, Y.; Zhang, L.; Hu, N.; Wang, Y.; Zhang, Y.; Zhou, Z.; Liu, Y.; Shen, S.; Peng, C. Ammonia gas sensors based on chemically reduced graphene oxide sheets self-assembled on Au electrodes. Nanoscale Res. Lett. 2014, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Esfandiar, A.; Irajizad, A.; Akhavan, O.; Ghasemi, S.; Gholami, M.R. Pd–WO3/reduced graphene oxide hierarchical nanostructures as efficient hydrogen gas sensors. Int. J. Hydrogen Energy 2014, 39, 8169–8179. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman study of D* band in graphene oxide and its correlation with reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Xu, H.; Wu, X.; Li, X.; Luo, C.; Liang, F.; Orignac, E.; Zhang, J.; Chu, J. Properties of graphene-metal contacts probed by Raman spectroscopy. Carbon 2018, 127, 491–497. [Google Scholar] [CrossRef]
- Vidano, R.; Fischbach, D.; Willis, L.; Loehr, T. Observation of Raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Saheed, M.S.M.; Mohamed, N.M.; Singh, B.S.M.; Saheed, M.S.M. Precursor and pressure dependent 3D graphene: A study on layer formation and type of carbon material. Diamond Relat. Mater. 2017, 79, 93–101. [Google Scholar] [CrossRef]
- Peng, F.; Wang, S.; Yu, W.; Huang, T.; Sun, Y.; Cheng, C.; Chen, X.; Hao, J.; Dai, N. Ultrasensitive ppb-level H2S gas sensor at room temperature based on WO3/rGO hybrids. J. Mater. Sci. Mater. Electron. 2020, 31, 5008–5016. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Chen, X.; Kalenczuk, R.J.; Wajda, A.; Łapczuk, J.; Kurzewski, M.; Drozdzik, M.; Chu, P.K.; Borowiak-Palen, E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B 2012, 89, 79–85. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. In situ shear-induced mercapto group-activated graphite nanoplatelets for fabricating mechanically strong and thermally conductive elastomer composites for thermal management applications. Compos. Part A Appl. Sci. Manuf. 2018, 112, 40–48. [Google Scholar] [CrossRef]
- Ansari, S.M.; Khan, M.Z.; Anwar, H.; Ikram, M.; Sarfraz, Z.; Alam, N.; Khan, Y. Tungsten Oxide–reduced Graphene Oxide Composites for Photoelectrochemical Water Splitting. Arab. J. Sci. Eng. 2021, 46, 813–825. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. In New Developments in Photon and Materials Research; Nova Science: Hauppauge, NY, USA, 2013; Volume 1, pp. 1–20. [Google Scholar]
- Hu, N.; Yang, Z.; Wang, Y.; Zhang, L.; Wang, Y.; Huang, X.; Wei, H.; Wei, L.; Zhang, Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 2013, 25, 025502. [Google Scholar] [CrossRef] [PubMed]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Tiwari, D.C.; Atri, P.; Sharma, R. Sensitive detection of ammonia by reduced graphene oxide/polypyrrole nanocomposites. Synth. Met. 2015, 203, 228–234. [Google Scholar] [CrossRef]
- Kang, M.-A.; Ji, S.; Kim, S.; Park, C.-Y.; Myung, S.; Song, W.; Lee, S.S.; Lim, J.; An, K.-S. Highly sensitive and wearable gas sensors consisting of chemically functionalized graphene oxide assembled on cotton yarn. RSC Adv. 2018, 8, 11991–11996. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Soultati, A.; Georgiadou, D.; Stergiopoulos, T.; Palilis, L.; Kennou, S.; Stathopoulos, N.; Davazoglou, D.; Argitis, P. Correction: Hydrogenated under-stoichiometric tungsten oxide anode interlayers for efficient and stable organic photovoltaics. J. Mater. Chem. A 2016, 4, 17875. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Li, T.; Gao, X.; Li, W.; Zhang, Z.; Wang, Y.; Zhang, X. Preparation and electrochemical performances of graphene/polypyrrole nanocomposite with anthraquinone-graphene oxide as active oxidant. Carbon 2017, 119, 111–118. [Google Scholar] [CrossRef]
- Dai, S.; Liu, Z.; Zhao, B.; Zeng, J.; Hu, H.; Zhang, Q.; Chen, D.; Qu, C.; Dang, D.; Liu, M. A high-performance supercapacitor electrode based on N-doped porous graphene. J. Power Sources 2018, 387, 43–48. [Google Scholar] [CrossRef]
- Ganbavle, V.; Agawane, G.; Moholkar, A.; Kim, J.; Rajpure, K. Structural, optical, electrical, and dielectric properties of the spray-deposited WO 3 thin films. J. Mater. Eng. Perform. 2014, 23, 1204–1213. [Google Scholar] [CrossRef]
- Kalanur, S.S. Structural, optical, band edge and enhanced photoelectrochemical water splitting properties of tin-doped WO3. Catalysts 2019, 9, 456. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Meng, F.; Li, Y.; Li, R.; Chang, Y.; Zhao, J.; Han, E.; Wang, S. Highly Sensitive Ammonia Sensors Based on Ag-Decorated WO 3 Nanorods. IEEE Trans. Nanotechnol. 2018, 17, 1252–1258. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chen, H.-I.; Hsu, C.-S.; Huang, C.-C.; Wu, J.-S.; Chou, P.-C.; Liu, W.-C. Characteristics of ZnO nanorods-based ammonia gas sensors with a cross-linked configuration. Sens. Actuators B Chem. 2015, 221, 491–498. [Google Scholar] [CrossRef]
- Zhybak, M.T.; Vagin, M.Y.; Beni, V.; Liu, X.; Dempsey, E.; Turner, A.P.; Korpan, Y.I. Direct detection of ammonium ion by means of oxygen electrocatalysis at a copper-polyaniline composite on a screen-printed electrode. Microchim. Acta 2016, 183, 1981–1987. [Google Scholar] [CrossRef]
- Biswas, S.; Drzal, L.T. Multilayered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Khataee, A.; Dražević, E.; Catalano, J.; Bentien, A. Performance optimization of differential pH quinone-bromide redox flow battery. J. Electrochem. Soc. 2018, 165, A3918. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Naderi, H.R.; Bahrani, S.; Arjmand, M.; Hagfeldt, A.; Chiang, W.-H.; Ramakrishna, S. Reinforced polypyrrole with 2D graphene flakes decorated with interconnected nickel-tungsten metal oxide complex toward superiorly stable supercapacitor. Chem. Eng. J. 2021, 418, 129396. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Khan, M.Y.; Bhat, K.S.; Ahn, M.-s.; Hahn, Y.-B. Ammonium ion detection in solution using vertically grown ZnO nanorod based field-effect transistor. RSC Adv. 2016, 6, 54836–54840. [Google Scholar] [CrossRef]
- Ribeiro, A.; Silva, F.; Pereira, C.M. Electrochemical sensing of ammonium ion at the water/1, 6-dichlorohexane interface. Talanta 2012, 88, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zazoua, A.; Kazane, I.; Khedimallah, N.; Dernane, C.; Errachid, A.; Jaffrezic-Renault, N. Evidence of ammonium ion-exchange properties of natural bentonite and application to ammonium detection. Mater. Sci. Eng. C 2013, 33, 5084–5089. [Google Scholar] [CrossRef] [PubMed]
- Heng, L.Y.; Alva, S.; Ahmad, M. Ammonium ion sensor based on photocured and self-plasticising acrylic films for the analysis of sewage. Sens. Actuators B 2004, 98, 160–165. [Google Scholar] [CrossRef]
- Ling, T.L.; Ahmad, M.; Heng, L.Y.; Seng, T.C. The effect of multilayer gold nanoparticles on the electrochemical response of ammonium ion biosensor based on alanine dehydrogenase enzyme. J. Sens. 2011, 2011, 754171. [Google Scholar] [CrossRef]
- Tan, L.L.; Musa, A.; Lee, Y.H. Reflectance based optical fibre sensor for ammonium ion using solid-state Riegler’s reagent. Sens. Actuators B 2012, 173, 614–619. [Google Scholar] [CrossRef]
- Tan, L.L.; Ahmad, M.; Lee, Y.H. A novel optical ammonia sensor based on reflectance measurements for highly polluted and coloured water. Sens. Actuators B 2012, 171, 994–1000. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Giakisikli, G.; Anthemidis, A.N. Automatic pressure-assisted dual-headspace gas-liquid microextraction. Lab-in-syringe platform for membraneless gas separation of ammonia coupled with fluorimetric sequential injection analysis. Anal. Chim. Acta 2018, 1033, 73–80. [Google Scholar] [CrossRef]
- Bushra, K.A.; Prasad, K.S. Based field-effect transistor sensors. Talanta 2022, 239, 123085. [Google Scholar] [CrossRef]
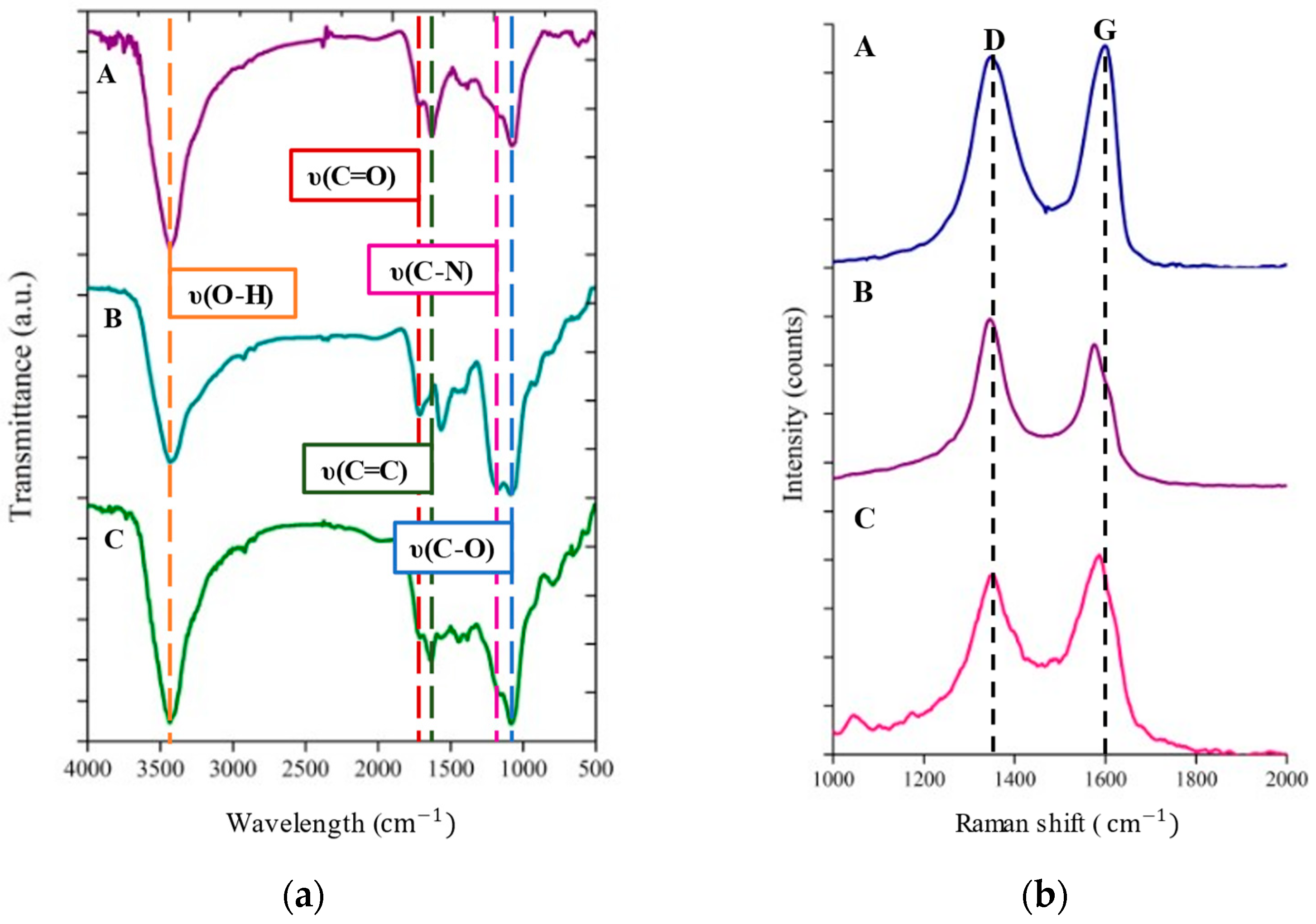


| Nanocomposites | (cm−1) | ID/IG Ratio | |
|---|---|---|---|
| D Band | G Band | ||
| GO | 1352.7 | 1603.0 | 0.949 |
| Ppy-rGO | 1340.5 | 1572.8 | 1.146 |
| MOFs-W(OCH2CH3)6/Ppy-rGO | 1357.7 | 1587.9 | 0.887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hizam, S.M.; Mohamed Saheed, M.S. Facile Electrochemical Approach Based on Hydrogen-Bonded MOFs-Derived Tungsten Ethoxide/Polypyrrole-Reduced GO Nanocrystal for ppb Level Ammonium Ions Detection. Chemosensors 2023, 11, 201. https://doi.org/10.3390/chemosensors11030201
Mohd Hizam SM, Mohamed Saheed MS. Facile Electrochemical Approach Based on Hydrogen-Bonded MOFs-Derived Tungsten Ethoxide/Polypyrrole-Reduced GO Nanocrystal for ppb Level Ammonium Ions Detection. Chemosensors. 2023; 11(3):201. https://doi.org/10.3390/chemosensors11030201
Chicago/Turabian StyleMohd Hizam, Sara Maira, and Mohamed Shuaib Mohamed Saheed. 2023. "Facile Electrochemical Approach Based on Hydrogen-Bonded MOFs-Derived Tungsten Ethoxide/Polypyrrole-Reduced GO Nanocrystal for ppb Level Ammonium Ions Detection" Chemosensors 11, no. 3: 201. https://doi.org/10.3390/chemosensors11030201
APA StyleMohd Hizam, S. M., & Mohamed Saheed, M. S. (2023). Facile Electrochemical Approach Based on Hydrogen-Bonded MOFs-Derived Tungsten Ethoxide/Polypyrrole-Reduced GO Nanocrystal for ppb Level Ammonium Ions Detection. Chemosensors, 11(3), 201. https://doi.org/10.3390/chemosensors11030201








