Highly Sensitive p-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanomaterials
2.2. Materials Characterization
2.3. Fabrication and Measurement of Gas Sensors
3. Results
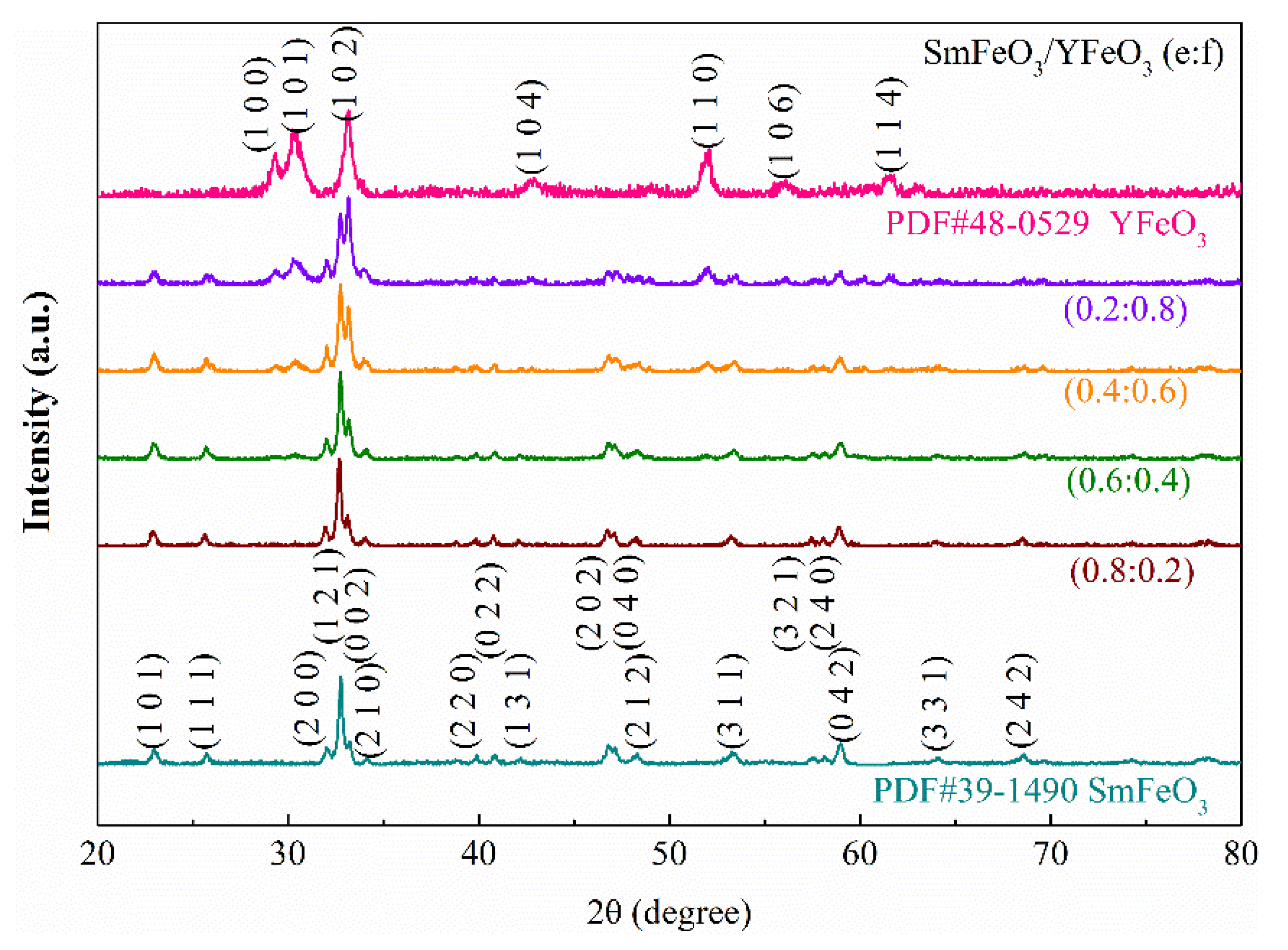
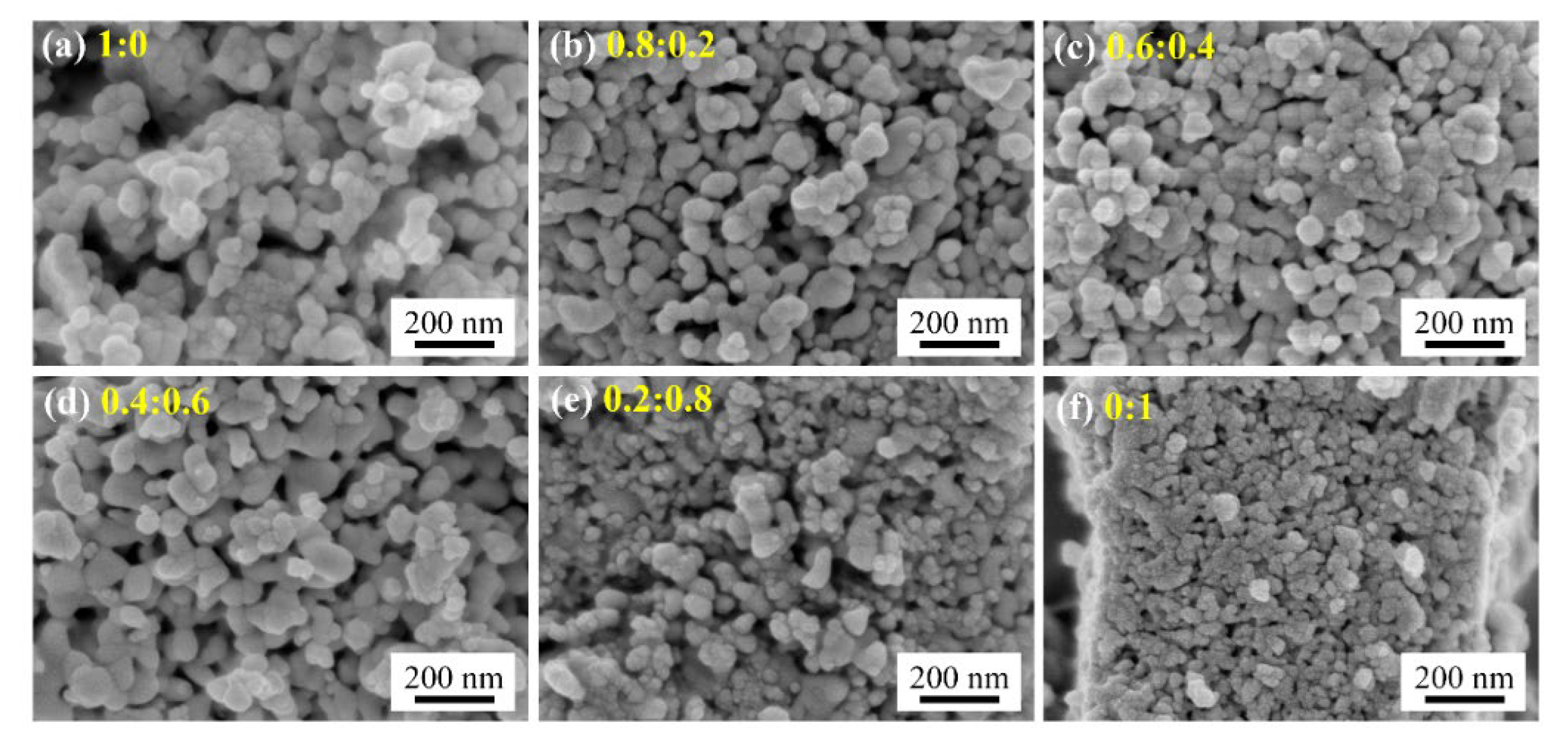
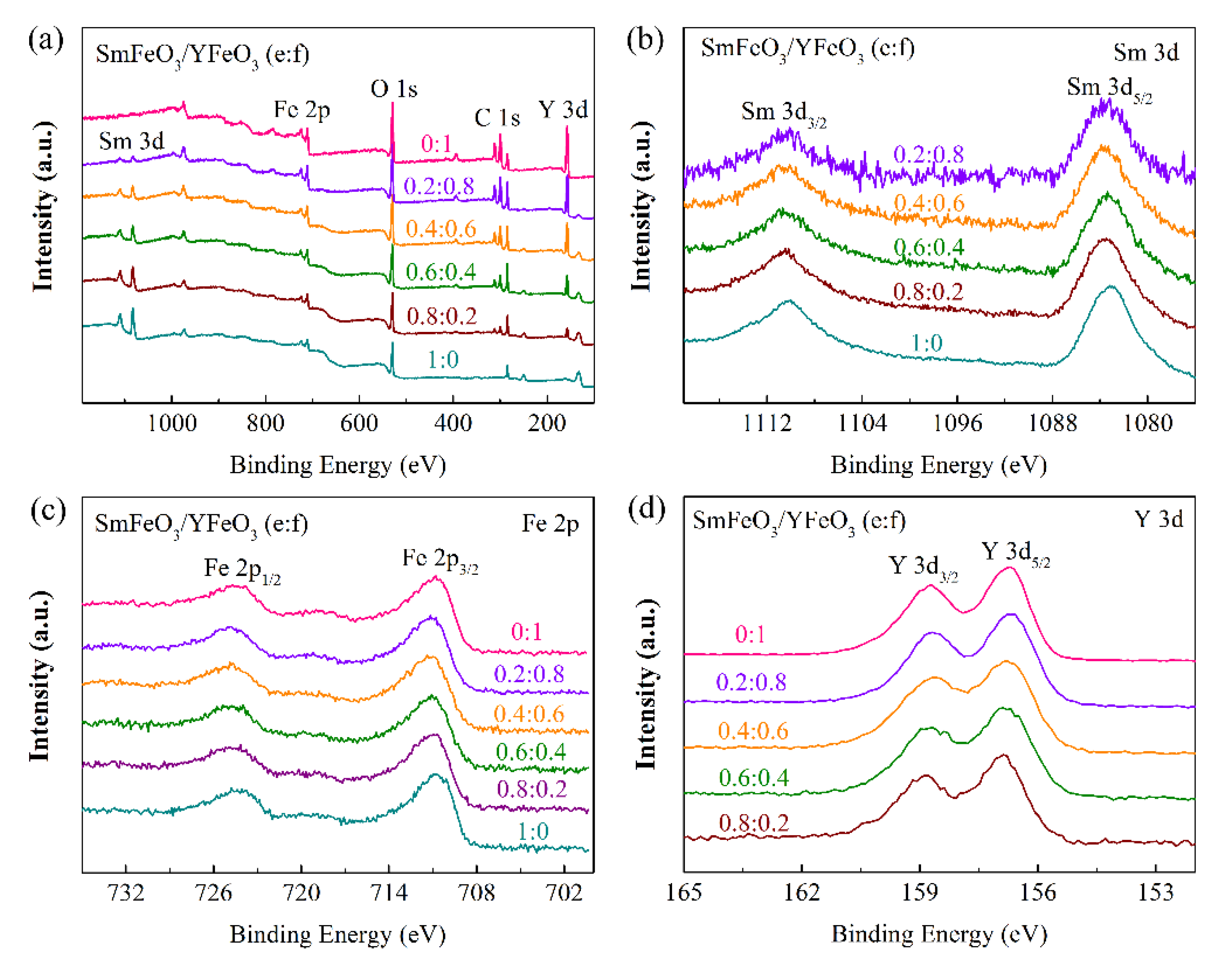
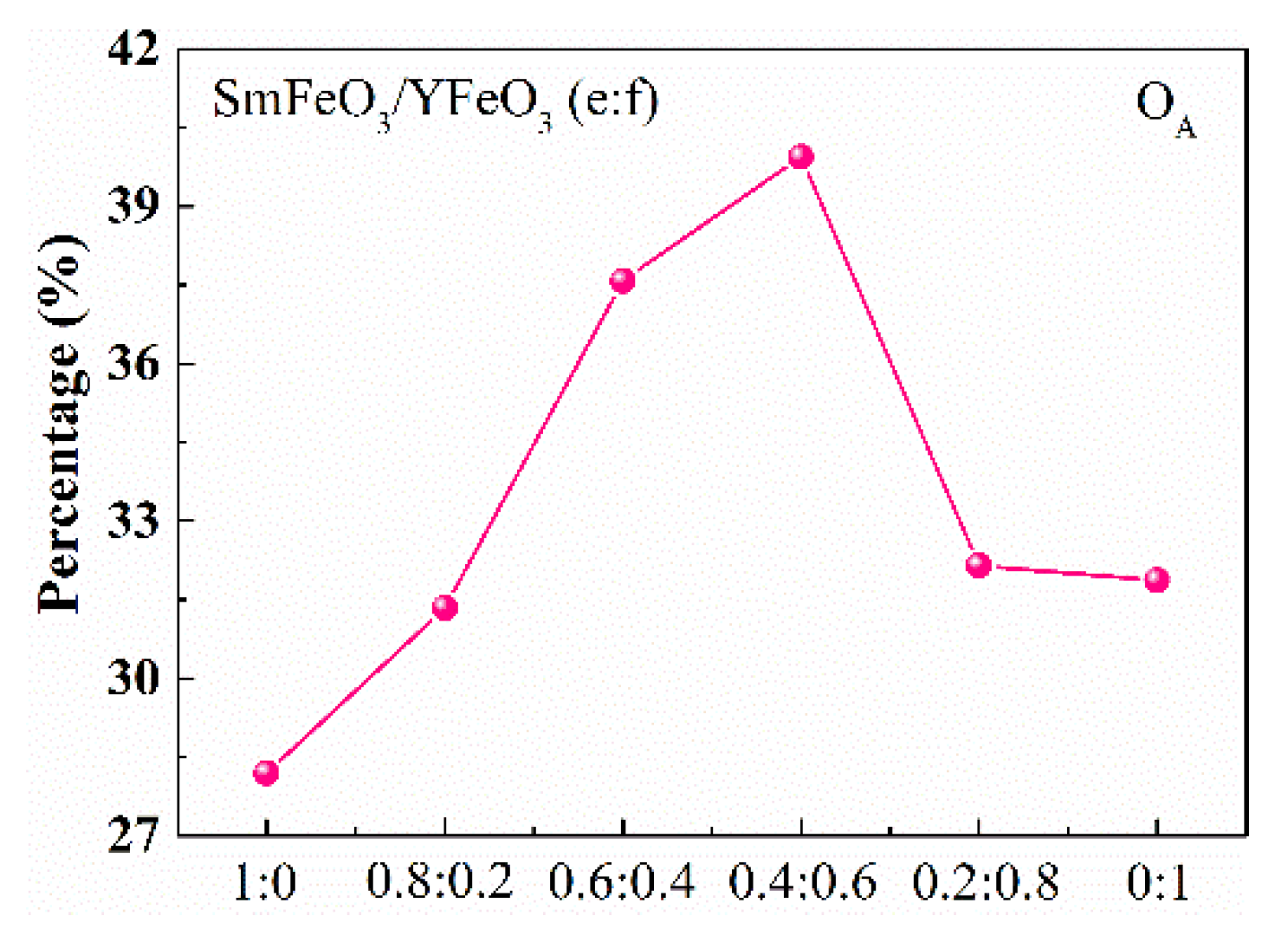
3.1. Nanomaterial Characterization
3.2. Gas-Sensing Characteristics
3.3. Mechanism Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boots, A.W.; van Berkel, J.J.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath. Res. 2012, 6, 027108. [Google Scholar] [CrossRef]
- Park, A.S.; Ritz, B.; Ling, C.; Cockburn, M.; Heck, J.E. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int. J. Hyg. Environ. Health 2017, 220, 1133–1140. [Google Scholar] [CrossRef]
- Int Panis, L.; de Geus, B.; Vandenbulcke, G.; Willems, H.; Degraeuwe, B.; Bleux, N.; Mishra, V.; Thomas, I.; Meeusen, R. Exposure to particulate matter in traffic: A comparison of cyclists and car passengers. Atmos. Environ. 2010, 44, 2263–2270. [Google Scholar] [CrossRef]
- Jiang, Q.; Guo, X.; Wang, C.; Jia, L.; Zhao, Z.; Yang, R.; Wang, P.; Deng, Q. Polyvinylpyrrolidone-mediated Co3O4 microspheres assembled in size-tunable submicron spheres with porous core-shell structure for high-performance gases sensing. J. Alloys Compd. 2023, 935, 167976. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, C.; Wu, L.; Liu, H.; Shi, F.; Zhao, J.; Liu, F.; Fu, K.; Wang, F.; Wang, Z.; et al. Construction of Pt/Ce-In2O3 hierarchical microspheres for superior triethylamine detection at low temperature. Colloids Surf. A 2023, 659, 130738. [Google Scholar] [CrossRef]
- Sun, B.; Shi, G.; Tang, Z.; Zhang, P.; Guo, Y.; Zhu, S.; Liu, J. Rapid gas-sensing detection of carbon disulfide by a CdS/SnS nanocomposite-based cataluminescence sensor. Chemosensors 2023, 11, 10. [Google Scholar] [CrossRef]
- Liu, M.; Xue, L.; Feng, Q.; Wang, Y.; Liu, J.; Zhang, S.; Hu, W. Facile preparation of Co3O4 hollow dodecahedron with superior peroxidase-like activity for selective detection of cholesterol. Chemosensors 2023, 11, 27. [Google Scholar] [CrossRef]
- Cui, X.; Lu, Z.; Wang, Z.; Zeng, W.; Zhou, Q. Highly sensitive SF6 decomposition byproducts sensing platform based on CuO/ZnO heterojunction nanofibers. Chemosensors 2023, 11, 58. [Google Scholar] [CrossRef]
- Torai, S.; Ueda, T.; Kamada, K.; Hyodo, T.; Shimizu, Y. Effects of addition of CuxO to porous SnO2 microspheres prepared by ultrasonic spray pyrolysis on sensing properties to volatile organic compounds. Chemosensors 2023, 11, 59. [Google Scholar] [CrossRef]
- Li, J.; Jin, Z.; Chao, Y.; Wang, A.; Wang, D.; Chen, S.; Qian, Q. Synthesis of graphene-oxide-decorated porous ZnO nanosheet composites and their gas sensing properties. Chemosensors 2023, 11, 65. [Google Scholar] [CrossRef]
- Ur Rahman, Z.; Shah, U.; Alam, A.; Shah, Z.; Shaheen, K.; Bahadar Khan, S.; Ali Khan, S. Photocatalytic degradation of cefixime using CuO-NiO nanocomposite photocatalyst. Inorg. Chem. Commun. 2023, 148, 110312. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Cao, Y.; Zhang, H.; Hu, J. Acetone sensing properties and mechanism of SnO2 thick-films. Sensors 2018, 18, 3425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Yu, Y. DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I-V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar] [CrossRef]
- Castello Lux, K.; Fajerwerg, K.; Hot, J.; Ringot, E.; Bertron, A.; Collière, V.; Kahn, M.L.; Loridant, S.; Coppel, Y.; Fau, P. Nano-structuration of WO3 nanoleaves by localized hydrolysis of an organometallic Zn precursor: Application to photocatalytic NO2 abatement. Nanomaterials 2022, 12, 4360. [Google Scholar] [CrossRef] [PubMed]
- Chumakova, V.; Marikutsa, A.; Platonov, V.; Khmelevsky, N.; Rumyantseva, M. Distinct roles of additives in the improved sensitivity to CO of Ag- and Pd-modified nanosized LaFeO3. Chemosensors 2023, 11, 60. [Google Scholar] [CrossRef]
- Sheng, H.; Ma, S.; Han, T.; Yun, P.; Yang, T.; Ren, J. A highly sensitivity and anti-humidity gas sensor for ethanol detection with NdFeO3 nano-coral granules. Vacuum 2022, 195, 110642. [Google Scholar] [CrossRef]
- Rajaitha, P.M.; Hajra, S.; Padhan, A.M.; Panda, S.; Sahu, M.; Kim, H.J. An electrochemical sensor based on multiferroic NdFeO3 particles modified electrode for the detection of H2O2. J. Alloys Compd. 2022, 915, 165402. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Chen, J.; Zhang, L.; Zhang, Y.; Jin, P. Au modified PrFeO3 with hollow tubular structure can be efficient sensing material for H2S detection. Front. Bioeng. Biotechnol. 2022, 10, 969870. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. A superior sensor consisting of porous, Pd nanoparticle–decorated SnO2 nanotubes for the detection of ppb-level hydrogen gas. J. Alloys Compd. 2022, 907, 164459. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hazra, A. Pd functionalized SrTiO3 hollow spheres for humidity-tolerant ethanol sensing. Sens. Actuators B 2022, 372, 132615. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhao, S.; Bai, J.; Qi, Y.; Han, C.; Wei, D. Effect of noble metal elements on ethanol sensing properties of ZnSnO3 nanocubes. J. Alloys Compd. 2021, 887, 161409. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.; Xu, X.; Cao, P.; Liu, W.; Xu, X.; Pei, S. Electrospinning synthesis, crystal structure, and ethylene glycol sensing properties of orthorhombic SmBO3 (B. = Fe, Co) perovskites. J. Alloys Compd. 2021, 876, 160211. [Google Scholar] [CrossRef]
- Yang, T.T.; Ma, S.Y.; Cao, P.F.; Xu, X.L.; Wang, L.; Pei, S.T.; Han, T.; Xu, X.H.; Yun, P.D.; Sheng, H. Synthesis and characterization of ErFeO3 nanoparticles by a hydrothermal method for isopropanol sensing properties. Vacuum 2021, 185, 110005. [Google Scholar] [CrossRef]
- Meng, F.; Hu, J.; Liu, C.; Tan, Y.; Zhang, Y. Highly sensitive and low detection limit of acetone gas sensor based on porous YbFeO3 nanocrystallines. Chem. Phys. Lett. 2021, 780, 138925. [Google Scholar] [CrossRef]
- Aranthady, C.; Jangid, T.; Gupta, K.; Mishra, A.K.; Kaushik, S.D.; Siruguri, V.; Rao, G.M.; Shanbhag, G.V.; Sundaram, N.G. Selective SO2 detection at low concentration by Ca substituted LaFeO3 chemiresistive gas sensor: A comparative study of LaFeO3 pellet vs thin film. Sens. Actuators B 2021, 329, 129211. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Qin, H.; Zhang, H.; Zhang, Z.; Zhou, G.; Gao, C.; Hu, J. CO2 sensing properties and mechanism of PrFeO3 and NdFeO3 thick film sensor. J. Rare Earth 2019, 37, 80–87. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.; Cheng, B.; Cao, Y.; Chen, Y.; Xie, J.; Liu, W.; Gao, C.; Zhou, G.; Hu, J. Pd:LaFe0.9Mg0.1O3: Planar type acetone sensor with high sensitivity. Mater. Sci. Semicond. Process. 2019, 96, 91–98. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, H.; Zhang, H.; Lv, W.; Hu, J. CO2 gas sensors based on Yb1−xCaxFeO3 nanocrystalline powders. J. Rare Earth 2017, 35, 602–609. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, J.; Wang, Y.; Zhang, L.; Zhao, G.; Yang, H.; Wang, H. A portable acetone detector based on SmFeO3 can pre-diagnose diabetes through breath analysis. J. Alloys Compd. 2022, 922, 166160. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.Y.; Xu, X.L.; Xu, X.H.; Pei, S.T.; Tie, Y.; Cao, P.F.; Liu, W.W.; Wang, B.J.; Zhang, R.; et al. Rough SmFeO3 nanofibers as an optimization ethylene glycol gas sensor prepared by electrospinning. Mater. Lett. 2020, 268, 127575. [Google Scholar] [CrossRef]
- Tasaki, T.; Takase, S.; Shimizu, Y. Improvement of sensing performance of impedancemetric C2H2 sensor using SmFeO3 thin-films prepared by a polymer precursor method. Sensors 2019, 19, 773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, J.; Chen, J.; Zhang, L.; Zhang, Y.; Pei, X. Pd-modified SmFeO3 with hollow tubular structure under light shows extremely high acetone gas sensitivity. Rare Met. 2023, 42, 545–557. [Google Scholar] [CrossRef]
- Han, T.; Ma, S.; Yun, P.; Sheng, H.; Xu, X.; Cao, P.; Pei, S.; Alhadi, A. Synthesis and characterization of Ho-doped SmFeO3 nanofibers with enhanced glycol sensing properties. Vacuum 2021, 191, 110378. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.; Zhang, P.; Hu, J. High sensing properties of 3wt% Pd-doped SmFe1-xMgxO3 nanocrystalline powders to acetone vapor with ultralow concentrations under light illumination. ACS Appl. Mater. Interf. 2018, 10, 15558–15564. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qin, H.; Zhang, L.; Hu, J. Ultrasensitive sensing performances to sub-ppb level acetone for Pd-functionalized SmFeO3 packed powder sensors. RSC Adv. 2016, 6, 6967–6974. [Google Scholar]
- Zhang, H.; Qin, H.; Zhang, P.; Chen, Y.; Hu, J. Low concentration acetone gas sensing properties of 3 wt% Pd-doped SmCoxFe1-xO3 nanocrystalline powders under UV light illumination. Sens. Actuators B 2018, 260, 33–41. [Google Scholar] [CrossRef]
- Li, K.; Chen, M.; Rong, Q.; Zhu, Z.; Liu, Q.; Zhang, J. High selectivity methanol sensor based on Co-Fe2O3/SmFeO3 p-n heterojunction composites. J. Alloys Compd. 2018, 765, 193–200. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Acetone sensing behavior of p-SmFeO3/n-ZnO nanocomposite synthesized by thermal treatment method. Sens. Actuators B 2020, 304, 127252. [Google Scholar] [CrossRef]
- Liu, H.; Cao, Y.; Liu, W.; Chen, J.; Hu, J. Gas response of SmFeO3 planar electrode sensor to volatile organic compounds gases under light illumination. Mater. Lett. 2022, 326, 133009. [Google Scholar] [CrossRef]
- Liu, H.; Miao, T.; Liu, W.; Chen, J.; Cheng, B.; Qin, H.; Hu, J. Highly sensitive acetone gas sensor based on YFeO3 planar electrode under multi-wavelength light illumination. Mater. Lett. 2023, 333, 133596. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Song, S.; Tan, M. Structure-controllable synthesis of multiferroic YFeO3 nanopowders and their optical and magnetic properties. Materials 2017, 10, 626. [Google Scholar] [CrossRef]
- Liu, Y.; Kuo, Y.; Liu, W.; Chou, W. Photoelectrocatalytic activity of perovskite YFeO3/carbon fiber composite electrode under visible light irradiation for organic wastewater treatment. J. Taiwan Inst. Chem. Eng. 2021, 128, 227–236. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Huang, H.; Li, Z.; Zou, Z. A novel wide-spectrum response hexagonal YFeO3 photoanode for solar water splitting. RSC Adv. 2017, 7, 18418–18420. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, C.; Jin, K.; Niu, L.; Xing, H.; Luo, B. Dielectric behavior of hexagonal and orthorhombic YFeO3 prepared by modified sol-gel method. J. Electroceram. 2014, 32, 187–191. [Google Scholar] [CrossRef]
- Liu, J.; He, F.; Chen, L.; Qin, X.; Zhao, N.; Huang, Y.; Peng, Y. Novel hexagonal-YFeO3/α-Fe2O3 heterojunction composite nanowires with enhanced visible light photocatalytic activity. Mater. Lett. 2016, 165, 263–266. [Google Scholar] [CrossRef]
- Hu, Q.; Yue, B.; Yang, D.; Zhang, Z.; Wang, Y.; Liu, J. Electrochemical and magnetic properties of electrospun SmFeO3 and SmCoO3 nanofibers. J. Am. Ceram. Soc. 2022, 105, 1149–1158. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, H.; Peng, J.; Duan, Z.; Ma, M.; Xin, X.; Li, W.; Zheng, X. Enhanced humidity sensing properties of SmFeO3-modified MoS2 nanocomposites based on the synergistic effect. Sens. Actuators B 2018, 272, 459–467. [Google Scholar] [CrossRef]
- Alizadeh, A.; Shariatinia, Z. Unveiling the influence of SmFeO3-TiO2 nanocomposites as high performance photoanodes of dye-sensitized solar cells. J. Mol. Liq. 2022, 348, 118070. [Google Scholar] [CrossRef]
- Nand, M.; Tripathi, S.; Rajput, P.; Kumar, M.; Kumar, Y.; Mandal, S.K.; Urkude, R.; Gupta, M.; Dawar, A.; Ojha, S.; et al. Different polymorphs of Y doped HfO2 epitaxial thin films: Insights into structural, electronic and optical properties. J. Alloys Compd. 2022, 928, 167099. [Google Scholar] [CrossRef]
- Cao, E.; Qin, Y.; Cui, T.; Sun, L.; Hao, W.; Zhang, Y. Influence of Na doping on the magnetic properties of LaFeO3 powders and dielectric properties of LaFeO3 ceramics prepared by citric sol-gel method. Ceram. Int. 2017, 43, 7922–7928. [Google Scholar] [CrossRef]
- Hao, P.; Qu, G.; Song, P.; Yang, Z.; Wang, Q. Synthesis of Ba-doped porous LaFeO3 microspheres with perovskite structure for rapid detection of ethanol gas. Rare Met. 2021, 40, 1651–1661. [Google Scholar] [CrossRef]
- Haryadi, H.; Suprayoga, E.; Suhendi, E. An analysis of electronic properties of LaFeO3 using density functional theory with generalized gradient approximation-perdew-burke-ernzerhof method for ethanol gas sensors. Mater. Res. 2022, 25, 554. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, C.; Qin, H.; Hu, J. High-performance acetone gas sensor based on ferrite–DyFeO3. J. Mater. Sci. 2020, 55, 16300–16310. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Shen, X.F.; Wang, T.T.; Jiang, X.H.; Chen, Q.; Qiang, Z.; Yang, H.M.; Chen, H. PrFeO3 hollow nanofibers as a highly efficient gas sensor for acetone detection. Sens. Actuators B 2018, 255, 2546–2554. [Google Scholar] [CrossRef]
- Pei, S.; Ma, S.; Xu, X.; Xu, X.; Almamoun, O. Modulated PrFeO3 by doping Sm3+ for enhanced acetone sensing properties. J. Alloys Compd. 2021, 856, 158274. [Google Scholar] [CrossRef]
- Lou, Z.; Li, Y.; Zhu, L.; Xie, W.; Niu, W.; Song, H.; Ye, Z.; Zhang, S. The crystalline/amorphous contact in Cu2O/Ta2O5 heterostructures: Increasing its sunlight-driven overall water splitting efficiency. J. Mater. Chem. A 2017, 5, 2732–2738. [Google Scholar] [CrossRef]
- Bai, S.; Tian, K.; Han, N.; Guo, J.; Luo, R.; Li, D.; Chen, A. A novel rGO-decorated ZnO/BiVO4 heterojunction for the enhancement of NO2 sensing properties. Inorg. Chem. Front. 2020, 7, 1026–1033. [Google Scholar] [CrossRef]
- Bai, S.; Tian, K.; Meng, J.C.; Zhao, Y.; Sun, J.; Zhang, K.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Reduced graphene oxide decorated SnO2/BiVO4 photoanode for photoelectrochemical water splitting. J. Alloys Compd. 2021, 855, 156780. [Google Scholar] [CrossRef]

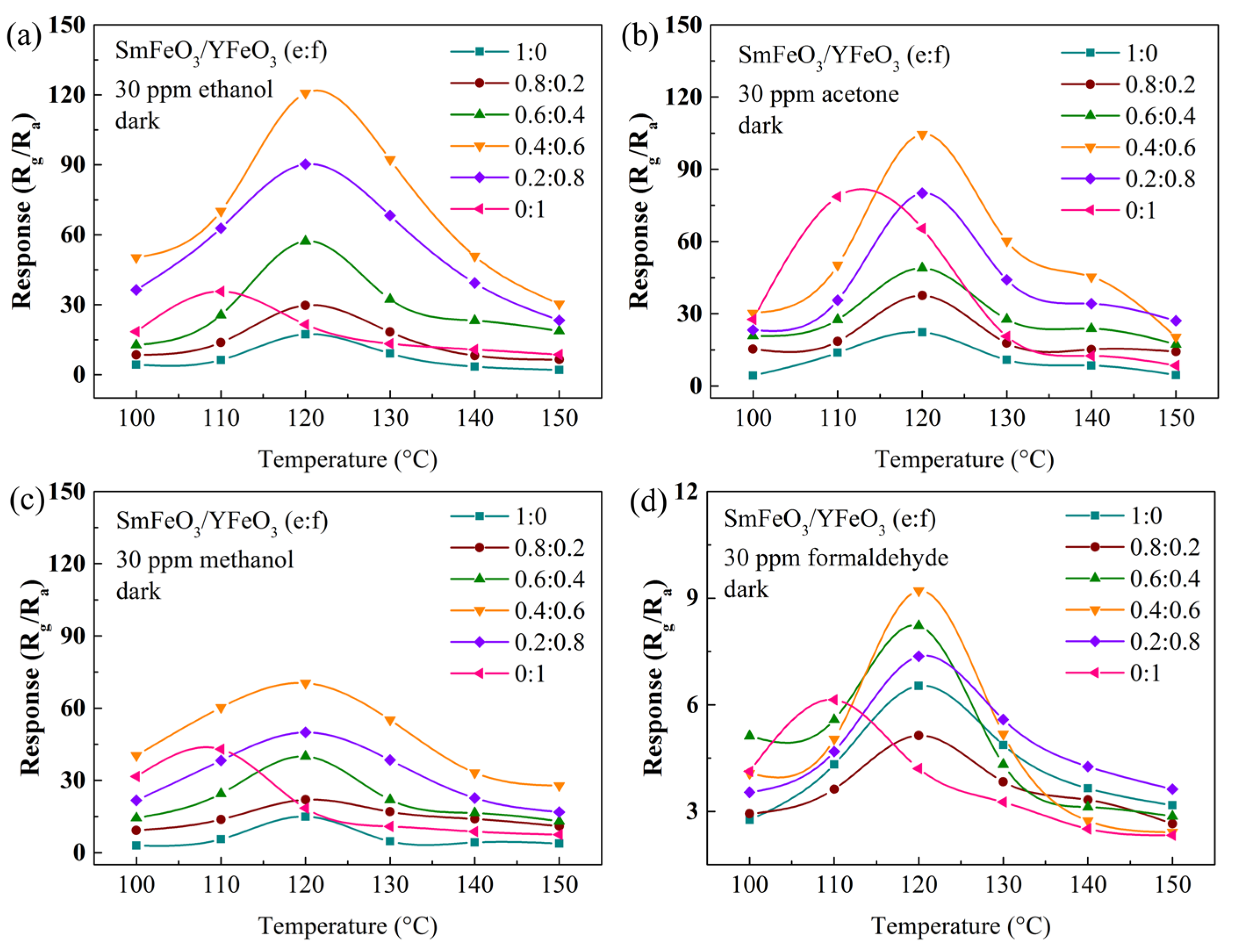
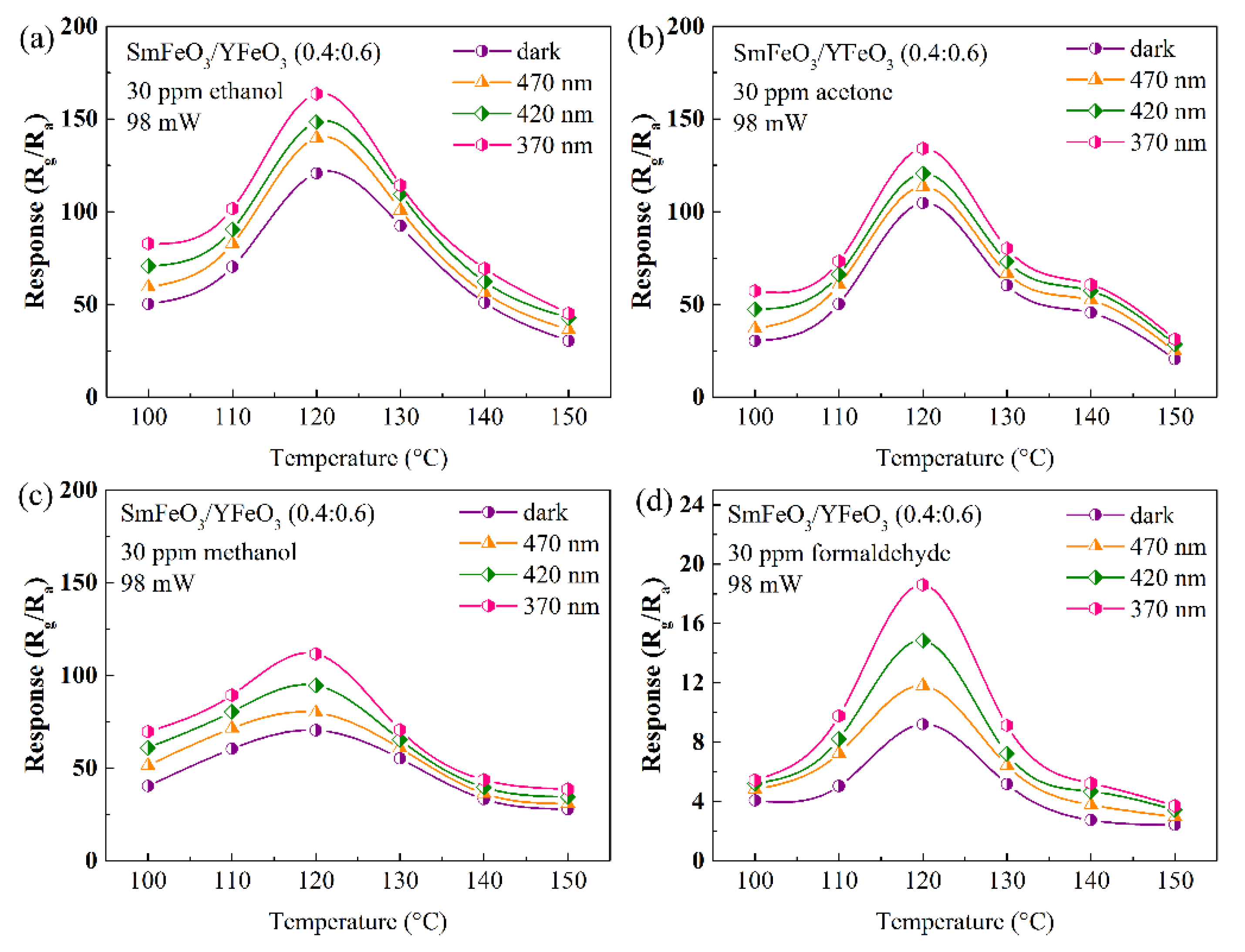
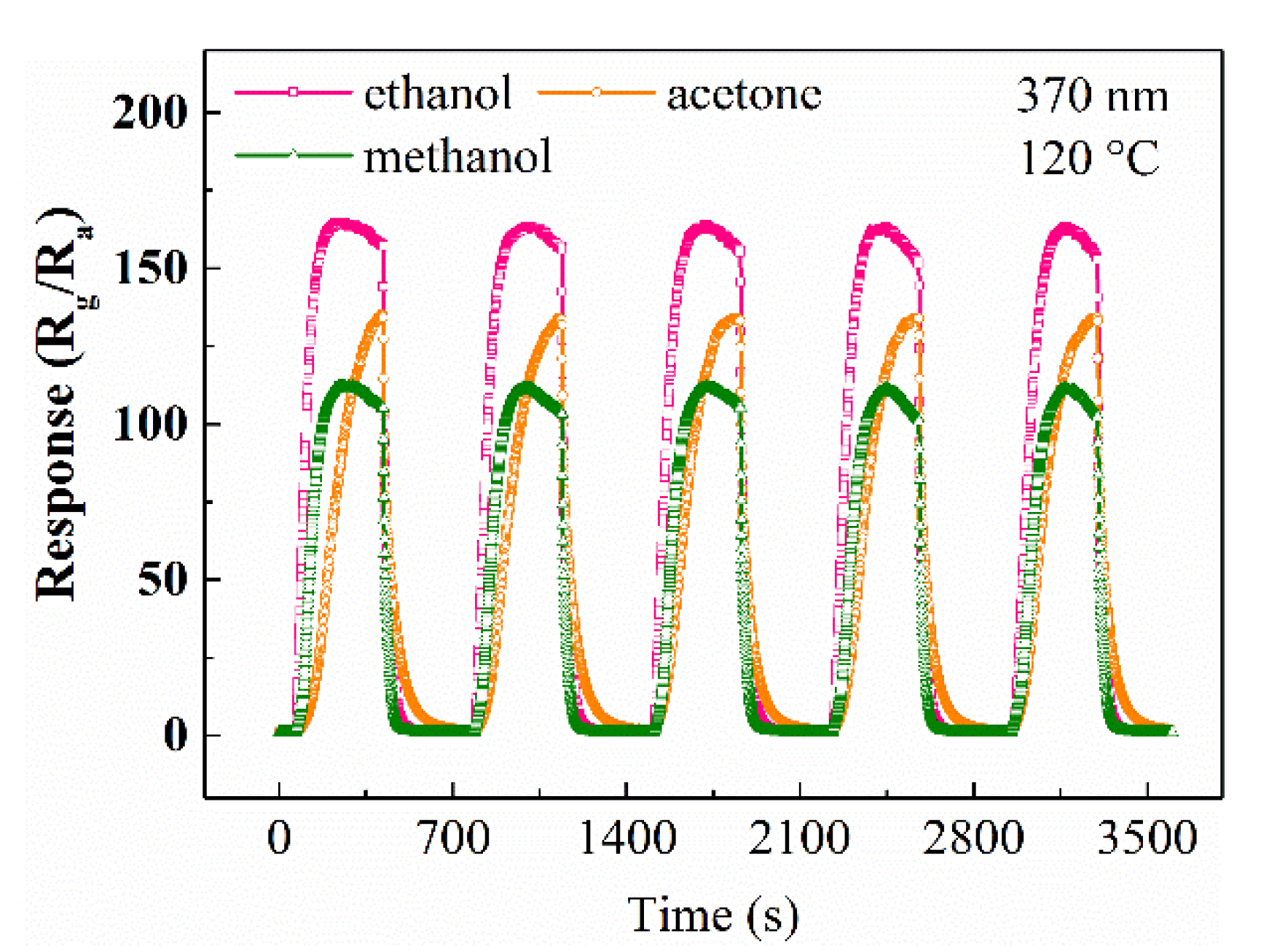
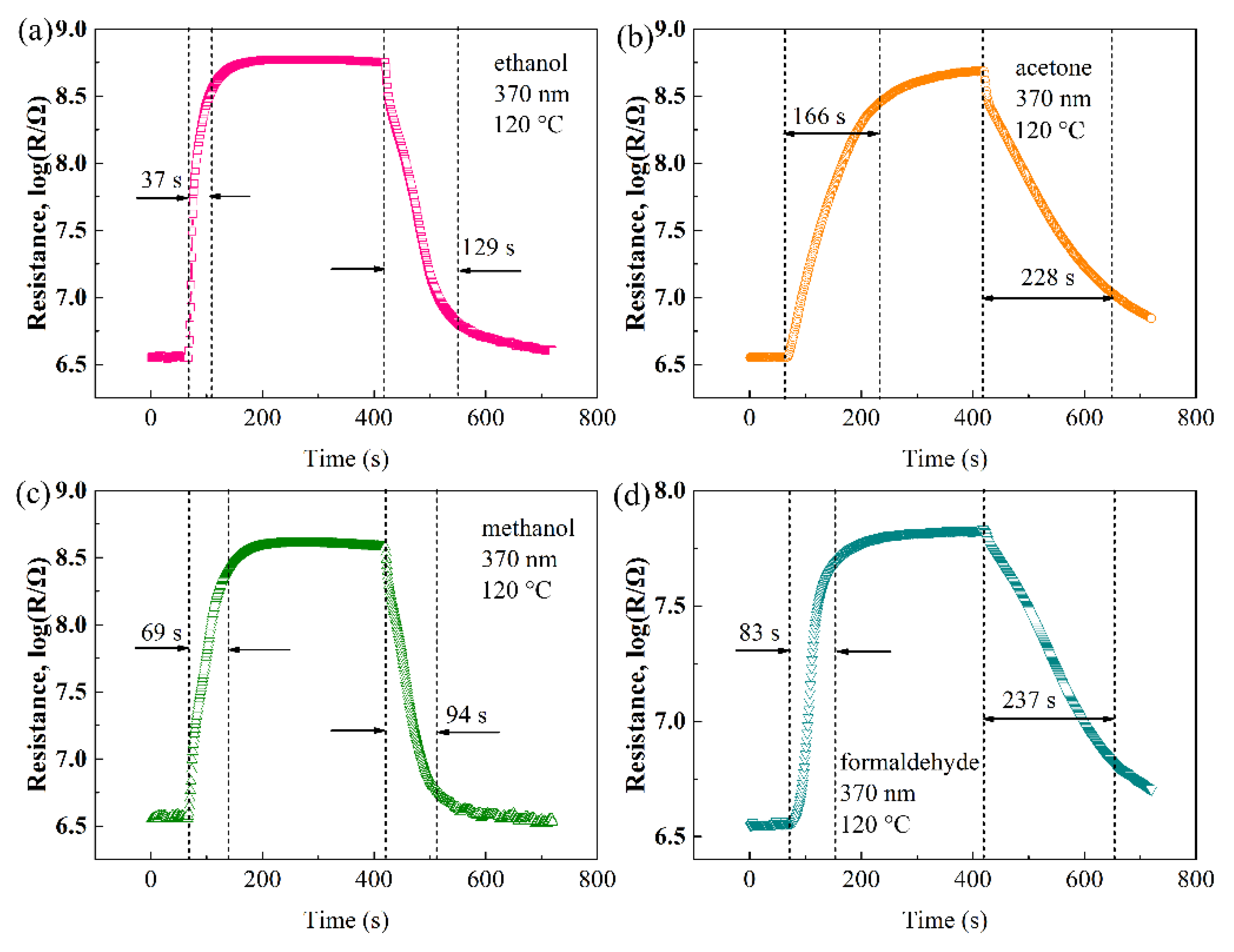

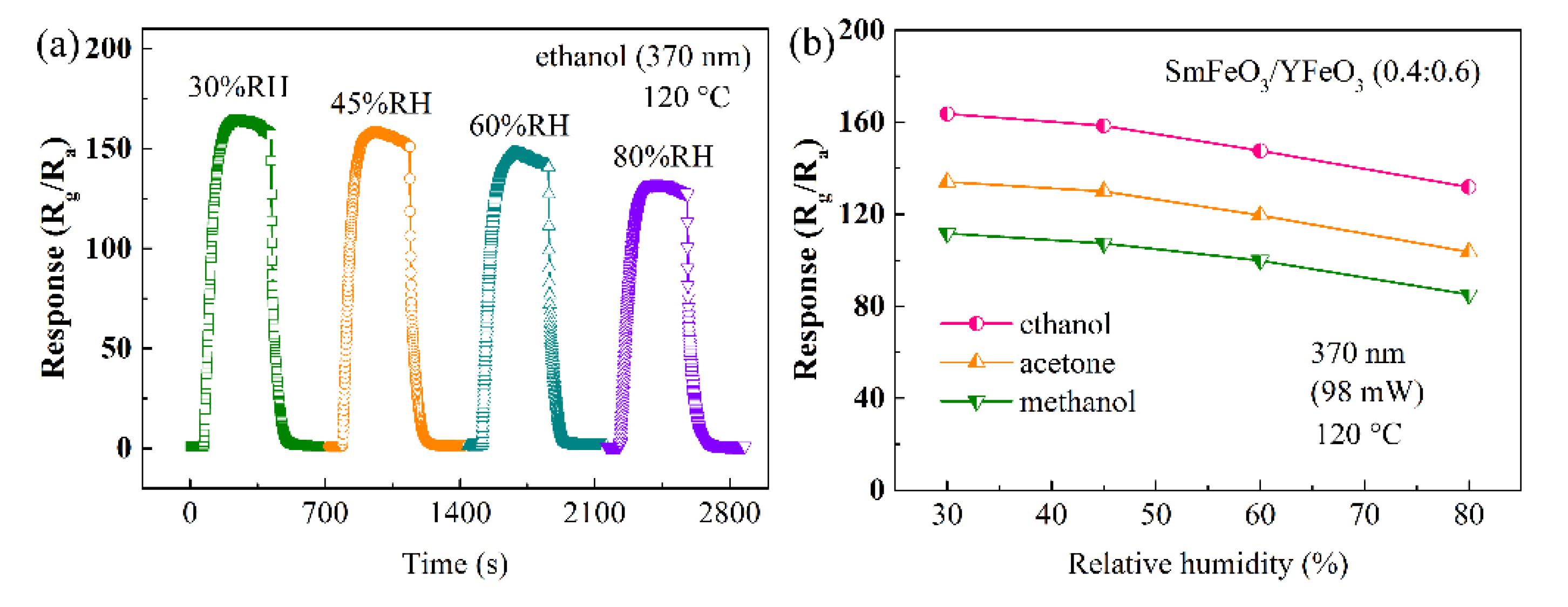
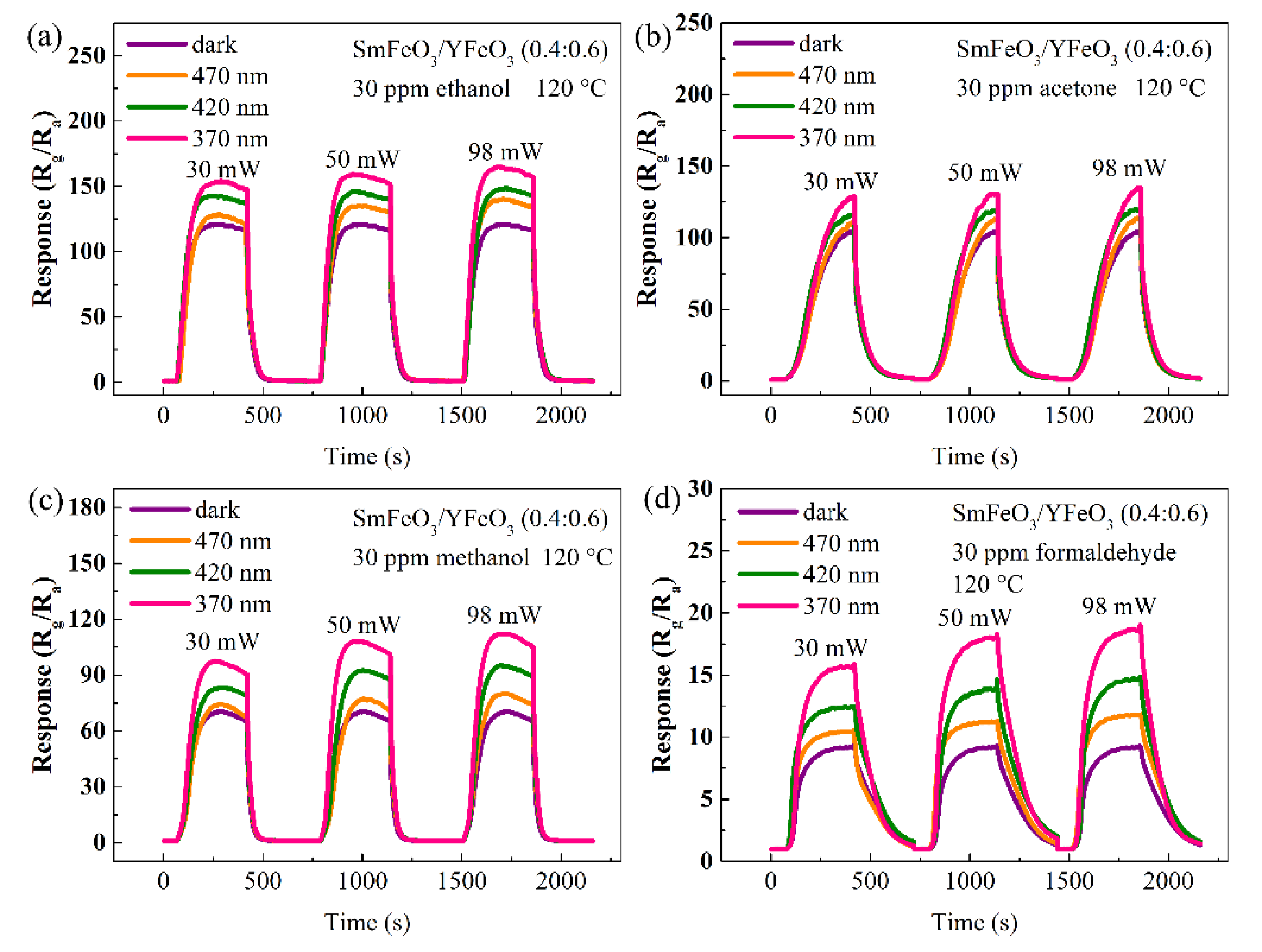
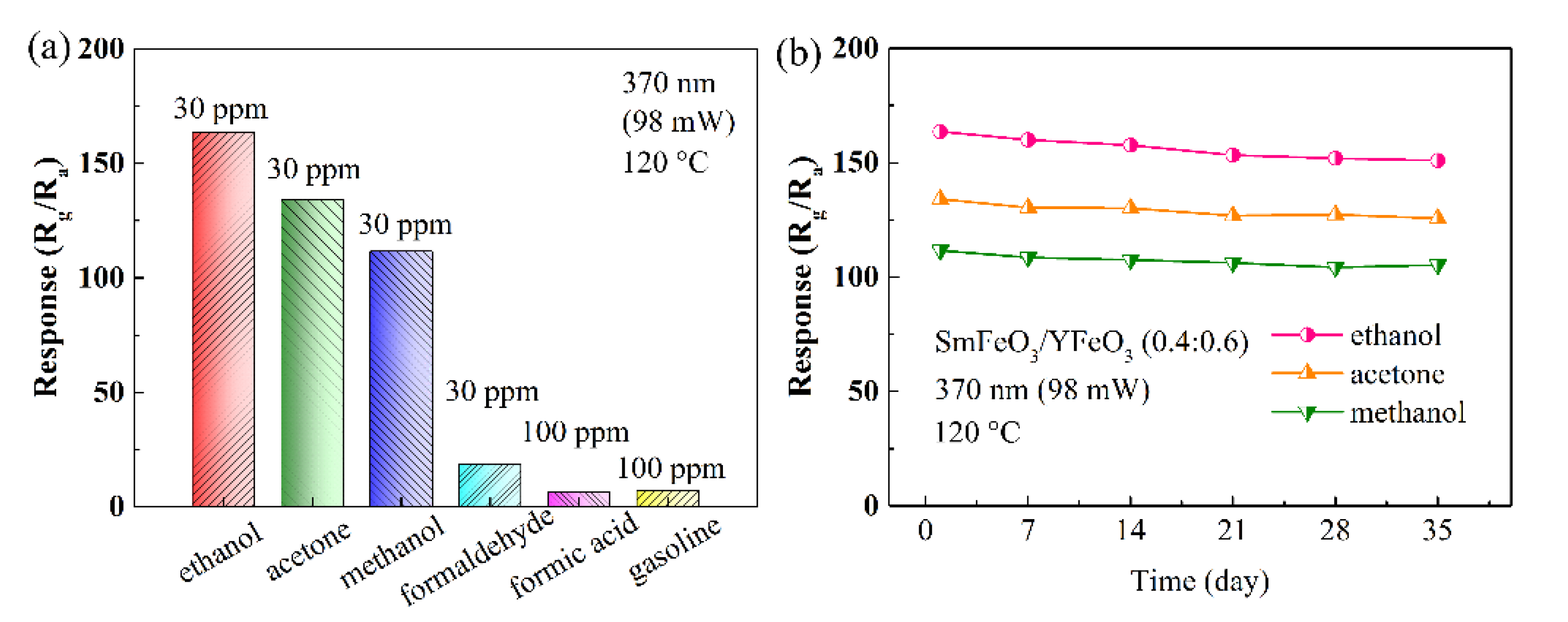

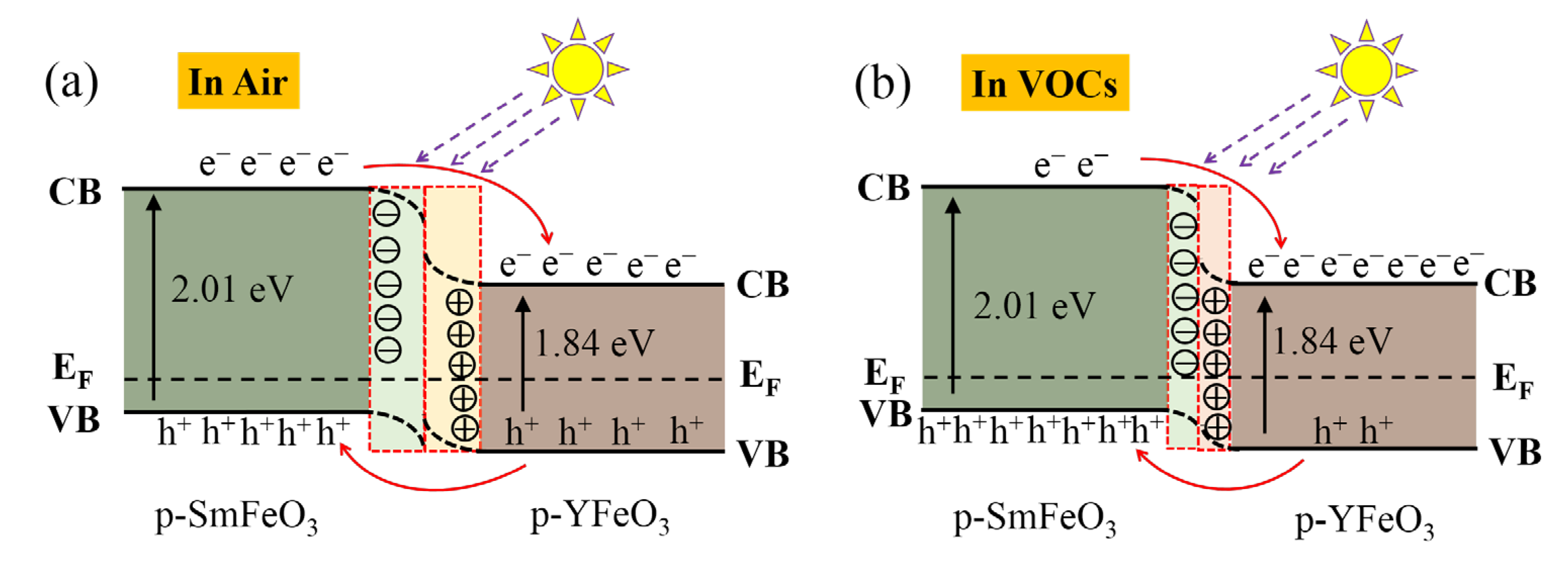
| Materials | Gas | Tem. (°C) | Con. (ppm) | Res. | Refs. |
|---|---|---|---|---|---|
| NdFeO3 | ethanol | 250 | 100 | 150 | [16] |
| La0.98Ba0.02FeO3 | ethanol | 200 | 100 | 79.3 | [51] |
| Au-LaFeO3 | ethanol | 200 | 100 | 44 | [52] |
| SmFeO3 | acetone | 210 | 1 | 5.92 | [29] |
| Pd-SmFeO3 | acetone | 220 | 1 | 10.73 | [32] |
| SmFeO3/ZnO | acetone | 350 | 10 | 45 | [38] |
| YFeO3 | acetone | 110 | 30 | 128.1 | [40] |
| DyFeO3 | acetone | 190 | 2 | 3.81 | [53] |
| PrFeO3 | acetone | 180 | 10 | 6 | [54] |
| Sm-PrFeO3 | acetone | 270 | 50 | 44.94 | [55] |
| Co-Fe2O3/SmFeO3 | methanol | 155 | 5 | 19.7 | [37] |
| SmFeO3 | methanol | 120 | 30 | 26.41 | [39] |
| SmFeO3/YFeO3 | ethanol | 120 | 30 | 163.593 | this work |
| SmFeO3/YFeO3 | acetone | 120 | 30 | 134.023 | this work |
| SmFeO3/YFeO3 | methanol | 120 | 30 | 111.637 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhu, D.; Miao, T.; Liu, W.; Chen, J.; Cheng, B.; Qin, H.; Hu, J. Highly Sensitive p-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds. Chemosensors 2023, 11, 187. https://doi.org/10.3390/chemosensors11030187
Liu H, Zhu D, Miao T, Liu W, Chen J, Cheng B, Qin H, Hu J. Highly Sensitive p-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds. Chemosensors. 2023; 11(3):187. https://doi.org/10.3390/chemosensors11030187
Chicago/Turabian StyleLiu, Huiyang, Denghui Zhu, Tingting Miao, Weikang Liu, Juan Chen, Bin Cheng, Hongwei Qin, and Jifan Hu. 2023. "Highly Sensitive p-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds" Chemosensors 11, no. 3: 187. https://doi.org/10.3390/chemosensors11030187
APA StyleLiu, H., Zhu, D., Miao, T., Liu, W., Chen, J., Cheng, B., Qin, H., & Hu, J. (2023). Highly Sensitive p-SmFeO3/p-YFeO3 Planar-Electrode Sensor for Detection of Volatile Organic Compounds. Chemosensors, 11(3), 187. https://doi.org/10.3390/chemosensors11030187






