Development of a Moisture Pretreatment Device for the Accurate Quantitation of Water-Soluble Volatile Organic Compounds in Air
Abstract
1. Introduction
2. Materials and Methods
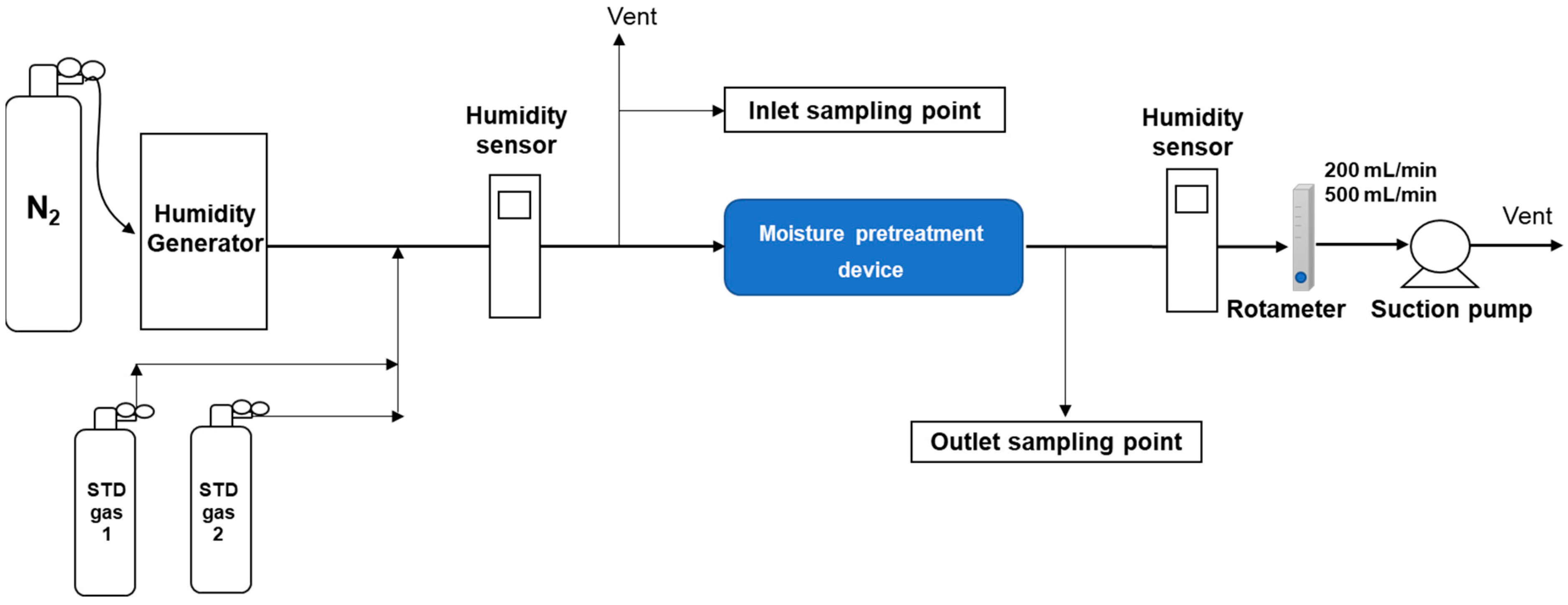
2.1. Apparatus
2.1.1. Principle of Operation of the KPASS–Odor System
2.1.2. Conventional Moisture Pretreatment Devices
2.1.3. VOC Analytical Instruments
2.2. Materials
2.3. Experimental Procedure
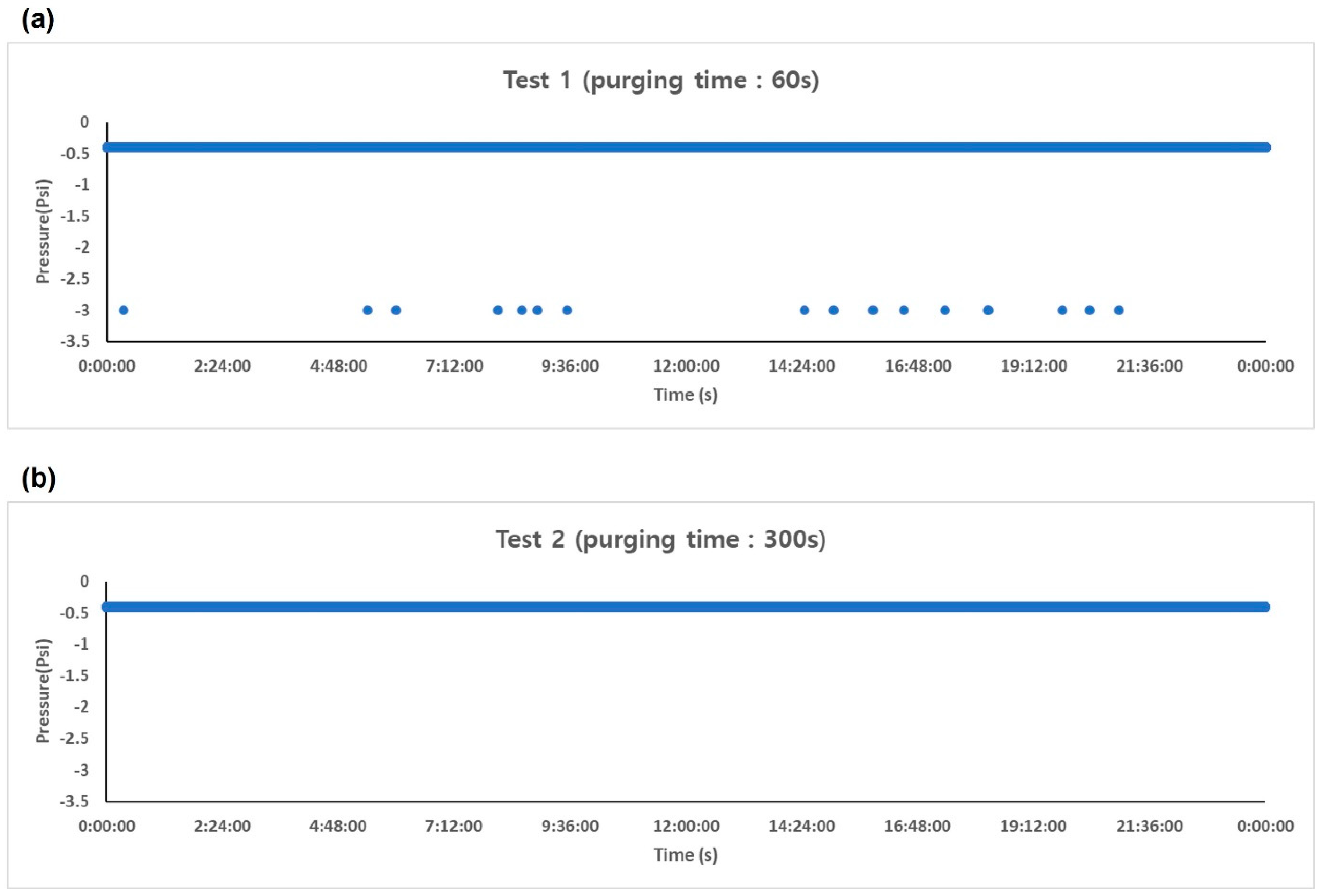
2.3.1. Selection of Optimal Purging Conditions for KPASS–Odor
2.3.2. Investigation of Moisture Removal Efficiencies by Three Moisture Pretreatment Devices
2.3.3. Investigation of VOC Recovery Rates by Three Moisture Pretreatment Devices Using GC/MSD/TD
2.3.4. Comparison of the Effect of KPASS–Odor and the NafionTM Dryer on the Performance of a PID Sensor
3. Results and Discussion
3.1. Selection of Optimal Purging Conditions for KPASS–Odor
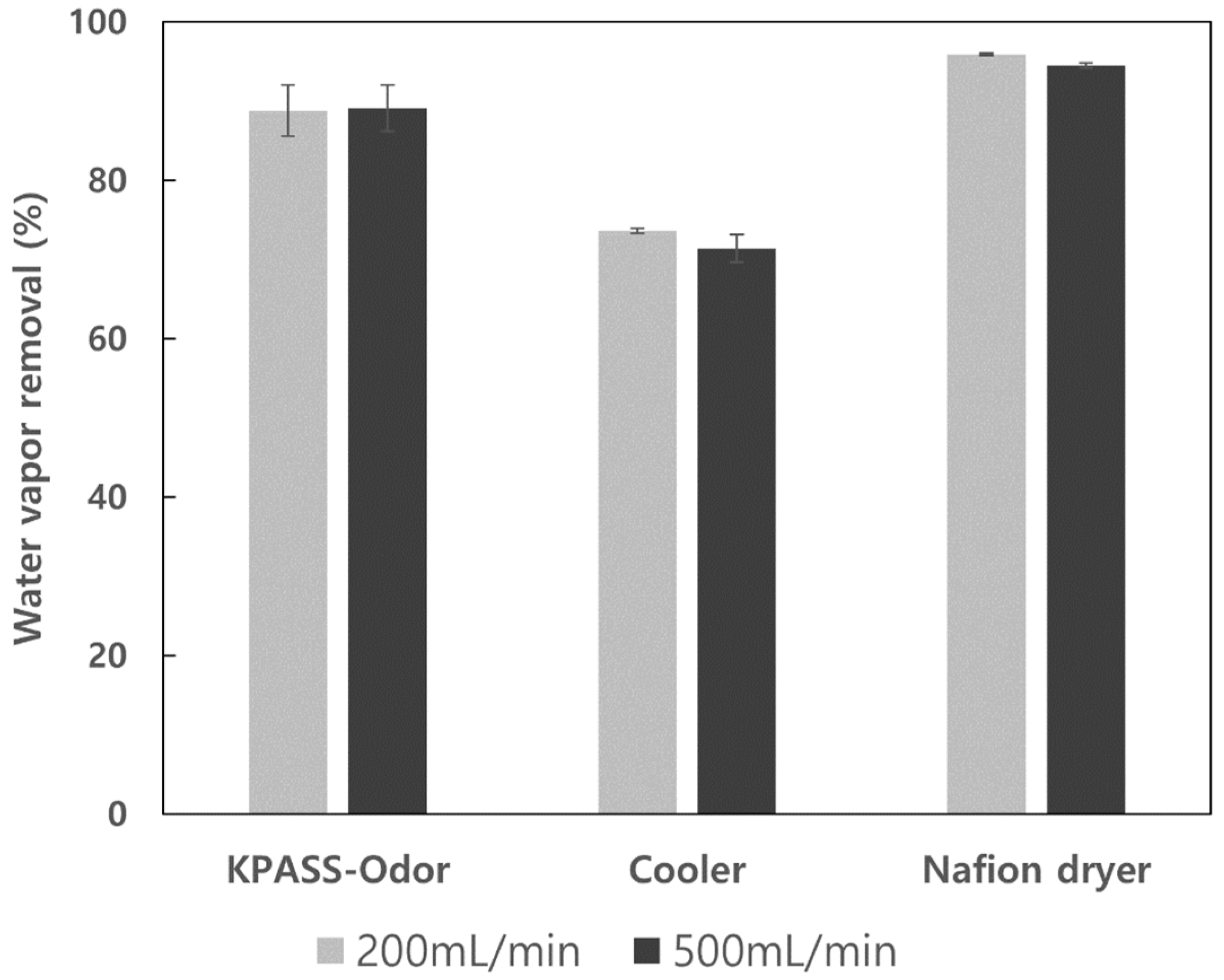
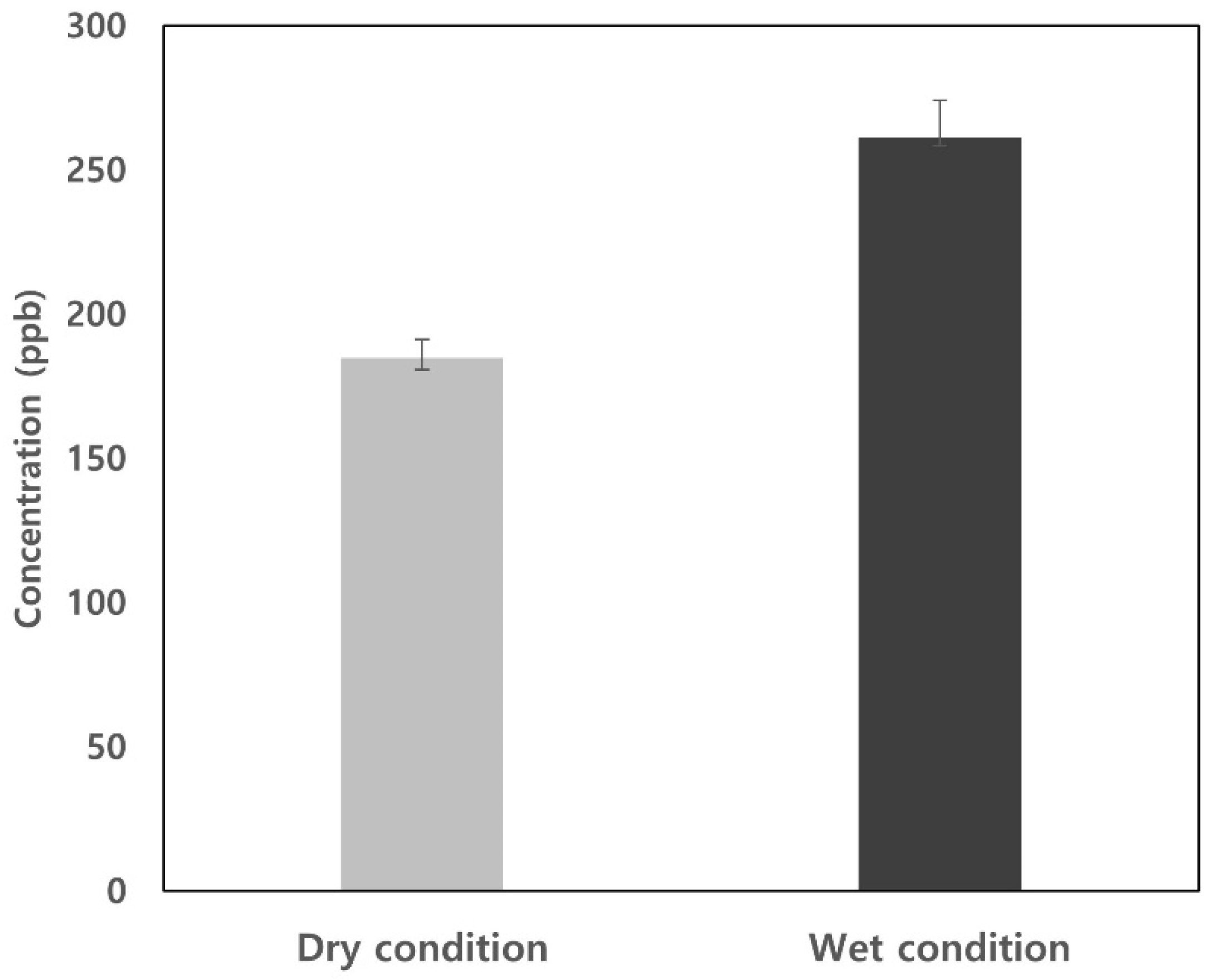
3.2. Moisture Removal Efficiency for Three Moisture Pretreatment Devices
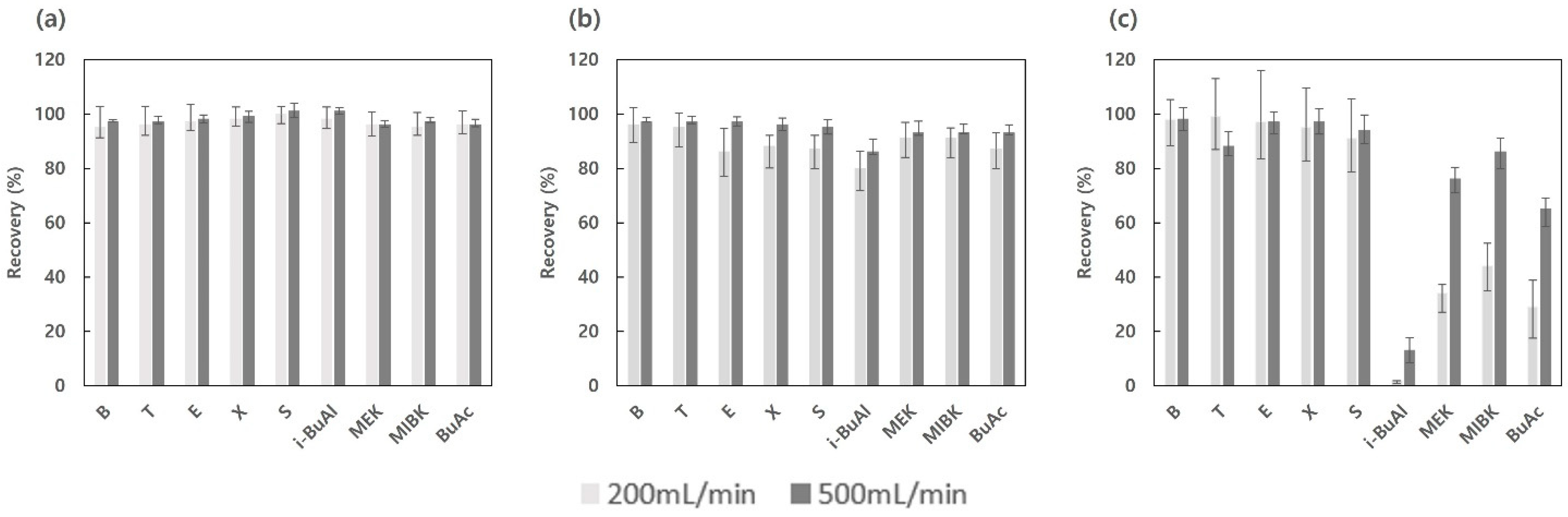
3.3. Investigation of VOC Recovery Rates by Three Moisture Pretreatment Devices Using GC/MSD/TD
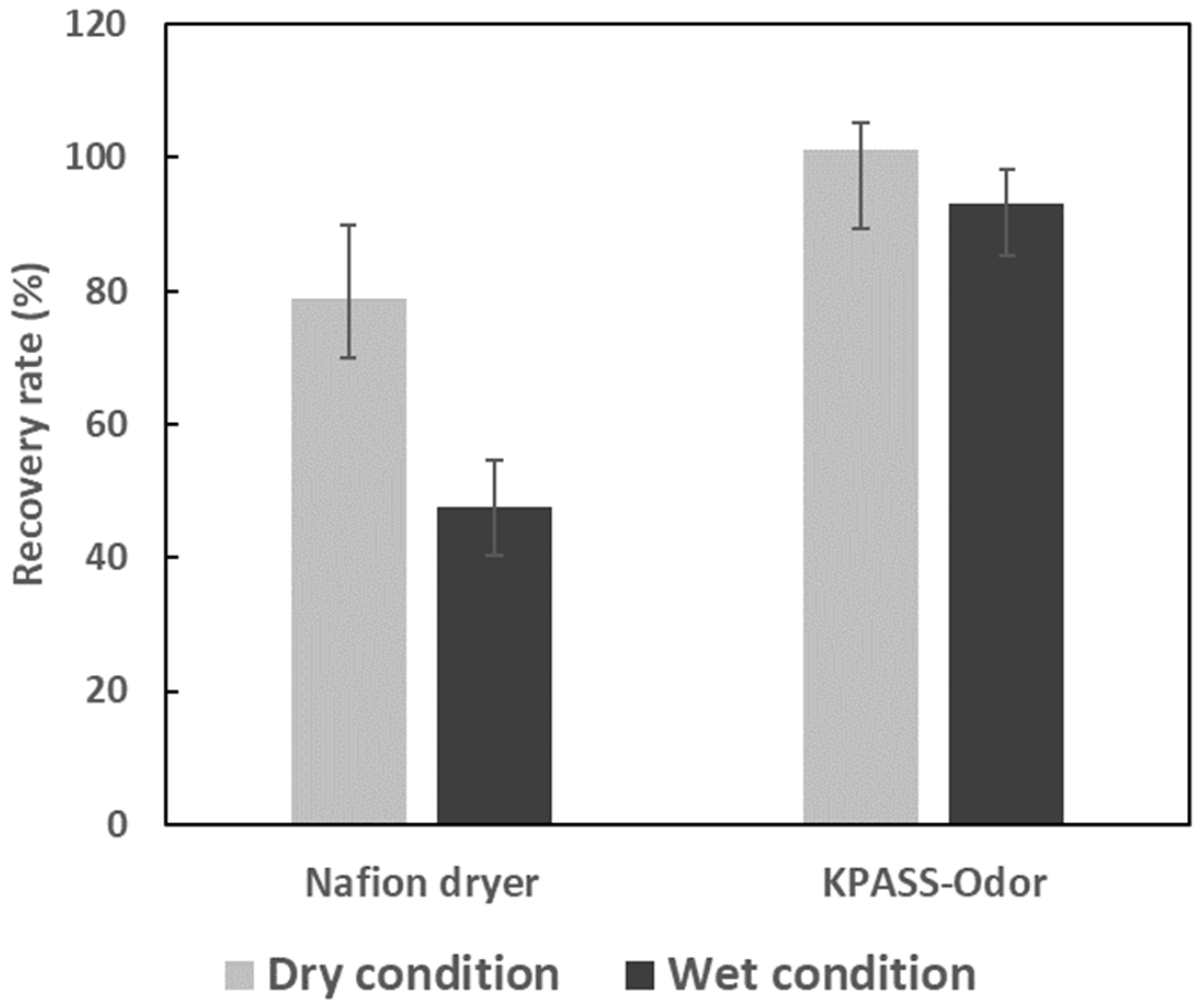
3.3.1. Reproducibility of the VOC Recovery Rate by Three Moisture Pretreatment Devices
3.3.2. Effect of Moisture Pretreatment Devices on the VOC Recovery Rates
3.4. Comparison of the Effect of KPASS–Odor and the NafionTM Dryer on the Performance of a PID Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, P.-H. A Study on Trimethylamine Control Characteristics Using Advanced Oxidizing Technique. Master’s Thesis, Konkuk University, Seoul, Republic of Korea, 2010. [Google Scholar]
- United States Environmental Protection Agency. Technical Overview of Volatile Organic Compounds. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 22 February 2023).
- Park, H.-M.; Lee, G.-B.; Ryu, J.-C.; Agustin, M.R.; Park, I.-Y.; Im, B.-J. Monitoring on Residual Volatile Organic Compounds Remaining in Industrial Waste Water. J. Korean Soc. Environ. Anal. 2003, 6, 169–177. [Google Scholar]
- The Korea Ministry of Environment. Status of Designation of Odor Management Area. Available online: https://www.me.go.kr/home/web/policy_data/read.do?pagerOffset=0&maxPageItems=10&maxIndexPages=10&searchKey=&searchValue=&menuId=10262&orgCd=&condition.orderSeqId=6918&condition.rnSeq=870&condition.deleteYn=N&seq=6919 (accessed on 2 December 2022).
- Badjagbo, K.; Sauvé, S.; Moore, S. Real-Time Continuous Monitoring Methods for Airborne VOCs. TrAC Trends Anal. Chem. 2007, 26, 931–940. [Google Scholar] [CrossRef]
- You, D.-W.; Seon, Y.-S.; Jang, Y.; Bang, J.; Oh, J.-S.; Jung, K.-W. A Portable Gas Chromatograph for Real-Time Monitoring of Aromatic Volatile Organic Compounds in Air Samples. J. Chromatogr. A 2020, 1625, 461267. [Google Scholar] [CrossRef]
- Hori, H.; Ishimatsu, S.; Fueta, Y.; Ishidao, T. Evaluation of a Real-Time Method for Monitoring Volatile Organic Compounds in Indoor Air in a Japanese University. Environ. Health Prev. Med. 2013, 18, 285–292. [Google Scholar] [CrossRef]
- Schütze, A.; Baur, T.; Leidinger, M.; Reimringer, W.; Jung, R.; Conrad, T.; Sauerwald, T. Highly Sensitive and Selective VOC Sensor Systems Based on Semiconductor Gas Sensors: How To? Environments 2017, 4, 20. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Glukhova, O.E.; Plugin, I.A.; Kolosov, D.A.; Nagornov, I.A.; Simonenko, T.L.; Varezhnikov, A.S.; Simonenko, E.P.; Sysoev, V.V.; Kuznetsov, N.T. The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs. Chemosensors 2022, 11, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y. Recent Progress on Anti-Humidity Strategies of Chemiresistive Gas Sensors. Materials 2022, 15, 8728. [Google Scholar] [CrossRef]
- Liu, C.; Duan, Z.; Zhang, B.; Zhao, Y.; Yuan, Z.; Zhang, Y.; Wu, Y.; Jiang, Y.; Tai, H. Local Gaussian Process Regression with Small Sample Data for Temperature and Humidity Compensation of Polyaniline-Cerium Dioxide NH3 Sensor. Sens. Actuators B Chem. 2023, 378, 133113. [Google Scholar] [CrossRef]
- Lee, J.-Y. The Effect of Different Water Pretreatment Systems on the Analysis of Odorous Compounds in Ambient Air. Master’s Thesis, Konkuk University, Seoul, Republic of Korea, 2018. [Google Scholar]
- Kistenev, Y.V.; Kuryak, A.N.; Makogon, M.M.; Ponomarev, Y.N. The System for Dehumidification of Samples in Laser Gas Analysis. Atmos. Ocean. Opt. 2012, 25, 92–95. [Google Scholar] [CrossRef]
- Haberhauer-Troyer, C.; Rosenberg, E.; Grasserbauer, M. Investigation of Membrane Dryers and Evaluation of a New Ozone Scrubbing Material for the Sampling of Organosulphur Compounds in Air. J. Chromatogr. A 1999, 852, 589–595. [Google Scholar] [CrossRef]
- Brown, J. Choosing the Right Adsorbent for Your Thermal Desorption Gas Chromatography Applications. Available online: https://www.sigmaaldrich.com/KR/ko/collections/webinars/w883186467 (accessed on 2 November 2022).
- Lee, M.-D. Review of Odor Measurement and Analysis Method-Standard Method of Odor Compounds. J. Korean Soc. Environ. Eng. 2007, 29, 761–767. [Google Scholar]
- Perma Pure. Available online: http://www.permapure.com (accessed on 2 December 2022).
- The Korea National Institute of Environmental Research. Air Pollution Monitoring Network Installation and Operation Guidelines (2021); The Korea National Institute of Environmental Research: Seoul, Republic of Korea, 2021. [Google Scholar]
- U.S. Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition. Compendium Method TO-17 Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling Onto Sorbent Tubes; Center for Environmental Research: Cincinnati, OH, USA, 1999. [Google Scholar]
- The Korea Ministry of Environment. VOCs—Cold Trap—GC Method—On-Line Monitoring Method; The Korea National Institute of Environmental Research: Incheon, Republic of Korea, 2018. [Google Scholar]
- Gong, Q.; Demerjian, K.L. Hydrocarbon Losses on a Regenerated Nation® Dryer. J. Air Waste Manag. AsSoc. 1995, 45, 490–493. [Google Scholar] [CrossRef]
- Hsu, J.P.; Miller, G.; Moran, V. Analytical Method for Determination of Trace Organics in Gas Samples Collected by Canister. J. Chromatogr. Sci. 1991, 29, 83–88. [Google Scholar] [CrossRef]
- Son, E.-S.; Seo, Y.-K.; Lee, D.-H.; Lee, M.-D.; Han, J.-S.; Baek, S.-O. A Study on the Performance Optimization of a Continuous Monitoring Method for Hazardous VOCs in the Ambient Atmosphere. J. Korean Soc. Atmos. Environ. 2009, 25, 523–538. [Google Scholar] [CrossRef]
- McGlenny, W.A.; Pleil, J.D.; Evans, G.F.; Oliver, K.D.; Holdren, M.W.; Winberry, W.T. Canister-Based Method for Monitoring Toxic VOCs in Ambient Air. J. Air Waste Manag. AsSoc. 1991, 41, 1308–1318. [Google Scholar] [CrossRef]
- Taurková, P.; Svoboda, M.; Musil, S.; Matoušek, T. Loss of Di- and Trimethylarsine on Nafion Membrane Dryers Following Hydride Generation. J. Anal. At. Spectrom. 2011, 26, 220–223. [Google Scholar] [CrossRef]
- Zielinska, B.; Sagebiel, J.C.; Harshfield, G.; Gertler, A.W.; Pierson, W.R. Volatile Organic Compounds up to C20 Emitted from Motor Vehicles; Measurement Methods. Atmos. Environ. 1996, 30, 2269–2286. [Google Scholar] [CrossRef]
- Shaw, S.L. The Production of Non-Methane Hydrocarbons by Marine Plankton; Massachusetts Institute of Technology: Cambridge, MA, USA, 2001. [Google Scholar]
- Baek, S.-O.; Moon, Y.-H. Evaluation of Adsorbent Sampling Methods for Volatile Organic Compounds in Indoor and Outdoor Air. Anal. Sci. Technol. 2004, 17, 496–513. [Google Scholar]
- U.S. Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-14A Determination of Volatile Organic Compounds (VOCs) in Ambient Air Using Specially Prepared Canisters with Subsequent Analysis By Gas Chromatography; Center for Environmental Research: Cincinnati, OH, USA, 1999. [Google Scholar]
- The Korea Ministry of Environment. Toluene, Xylene, Methylethylketone, Methylisobutylketone, Butylacetate, Stylene and i-Butylalcohol—Cold Trap/Thermal Desorption—GC Method; The Korea National Institute of Environmental Research: Incheon, Republic of Korea, 2018. [Google Scholar]
- Yang, J.; Conver, T.S.; Koropchak, J.A.; Leighty, D.A. Use of a Multi-Tube Nafion® Membrane Dryer for Desolvation with Thermospray Sample Introduction to Inductively Coupled Plasma-Atomic Emission Spectrometry. Spectrochim. Acta Part B Spectrosc. 1996, 51, 1491–1503. [Google Scholar] [CrossRef]
- Jahnke, J.A. Continuous Emission Monitoring, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 49–50. [Google Scholar]
- Kim, D.-J.; Dinh, T.-V.; Lee, J.-Y.; Choi, I.-Y.; Son, D.-J.; Kim, I.-Y.; Sunwoo, Y.; Kim, J.-C. Effects of Water Removal Devices on Ambient Inorganic Air Pollutant Measurements. Int. J. Environ. Res. Public Health 2019, 16, 3446. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Dinh, T.-V.; Kim, D.-J.; Choi, I.-Y.; Ahn, J.-W.; Park, S.-Y.; Jung, Y.-J.; Kim, J.-C. Effect of Conventional Water Pretreatment Devices on Polar Compound Analysis. Asian J. Atmos. Environ. 2019, 13, 249–258. [Google Scholar] [CrossRef]
- Wilson, K.L. Water Vapor Interference in the UV Absorption Measurement of Atmospheric Ozone; University of Colorado Boulder: Boulder, CO, USA, 2005. [Google Scholar]
- Son, Y.-S.; Lee, G.; Kim, J.-C.; Han, J.-S. Development of a Pretreatment System for the Analysis of Atmospheric Reduced Sulfur Compounds. Anal. Chem. 2013, 85, 10134–10141. [Google Scholar] [CrossRef]
- Tyrovolas, I.J. Explanation for the Mpemba Effect. J. Mod. Phys. 2017, 08, 2013–2020. [Google Scholar] [CrossRef]
- Sun, C.Q. Mpemba Paradox: Hydrogen Bond Memory and Water-Skin Supersolidity. arXiv 2015, arXiv:1501.00765. [Google Scholar]
- Tao, Y.; Zou, W.; Jia, J.; Li, W.; Cremer, D. Different Ways of Hydrogen Bonding in Water—Why Does Warm Water Freeze Faster than Cold Water? J. Chem. Theory Comput. 2017, 13, 55–76. [Google Scholar] [CrossRef]
- Kim, D.-J. The Effect of Water Pretreatment Device on Environmental Air Pollutants (O3, SO2, CO) Measurements and Analysis. Master’s Thesis, Konkuk University: Seoul, Republic of Korea, 2019. [Google Scholar]
- Gil, H.-N.; Kim, J.-C. The Effect of Hybrid Water Pretreatment Device on the Recovery Rate of HCl from Its Emission Sources. Master’s Thesis, Konkuk University, Seoul, Republic of Korea, 2021. [Google Scholar]
- Giaginis, C.; Tsantili-Kakoulidou, A. Alternative Measures of Lipophilicity: From Octanol–Water Partitioning to IAM Retention. J. Pharm. Sci. 2008, 97, 2984–3004. [Google Scholar] [CrossRef]
- Yun, J.; Cho, K.-S. A Review on the Treatment of Volatile Organic Compounds Using Absorbents. J. Odor. Indoor Environ. 2018, 17, 95–121. [Google Scholar] [CrossRef]
- Bruce, L.J.; Daugulis, A.J. Solvent Selection Strategies for Extractive Biocatalysis. Biotechnol. Prog. 1991, 7, 116–124. [Google Scholar] [CrossRef]
- Vuong, M.-D.; Couvert, A.; Couriol, C.; Amrane, A.; Le Cloirec, P.; Renner, C. Determination of the Henry’s Constant and the Mass Transfer Rate of VOCs in Solvents. Chem. Eng. J. 2009, 150, 426–430. [Google Scholar] [CrossRef]
- Muñoz, R.; Daugulis, A.J.; Hernández, M.; Quijano, G. Recent Advances in Two-Phase Partitioning Bioreactors for the Treatment of Volatile Organic Compounds. Biotechnol. Adv. 2012, 30, 1707–1720. [Google Scholar] [CrossRef]
- The Korea Ministry of Environment. Methods for Determination of Hazardous and Volatile Organic Compounds in Ambient Air—Adsorbent Trap Method; The Korea National Institute of Environmental Research: Incheon, Republic of Korea, 2021. [Google Scholar]
- Ahn, J.-W.; Pandey, S.K.; Kim, K.-H. Comparison of GC-MS Calibration Properties of Volatile Organic Compounds and Relative Quantification Without Calibration Standards. J. Chromatogr. Sci. 2011, 49, 19–28. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-15 Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially-Prepared Canisters and Analyzed by Gas Chromatography/ Mass Spectrometry (GC/MS); Center for Environmental Research: Cincinnati, OH, USA, 1999. [Google Scholar]
- Im, M.; Ju, D.; Kim, H.; Song, K.; Park, K. A Study of Analytical Method for 4 Legally-Designated Compounds [MEK, MIBK, n-Butyl Acetate, i-Butyl Alcohol] in Ambient Air Using On-Line Thermal Desorber with GC/FID. J. Korean Soc. Dor. Res. Eng. 2007, 6, 145–153. [Google Scholar]
- Deming, B.L.; Pagonis, D.; Liu, X.; Day, D.A.; Talukdar, R.; Krechmer, J.E.; de Gouw, J.A.; Jimenez, J.L.; Ziemann, P.J. Measurements of Delays of Gas-Phase Compounds in a Wide Variety of Tubing Materials Due to Gas–Wall Interactions. Atmos. Meas. Tech. 2019, 12, 3453–3461. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Dinh, T.-V.; Kim, D.-J.; Choi, I.-Y.; Ahn, J.-W.; Park, S.-Y.; Jung, Y.-J.; Kim, J.-C. Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds. Appl. Sci. 2019, 9, 4045. [Google Scholar] [CrossRef]
- Dunder, T.A.; Leighty, D.A. Comparison of Thermoelectric and Permeation Dryers for Sulfur Dioxide Removal during Sample Conditioning of Wet Gas Streams; Air and Waste Management Association: Pittsburgh, PA, USA, 1997. [Google Scholar]
- Pyo, S.; Lee, K.; Noh, T.; Jo, E.; Kim, J. Sensitivity Enhancement in Photoionization Detector Using Microelectrodes with Integrated 1D Nanostructures. Sens. Actuators B Chem. 2019, 288, 618–624. [Google Scholar] [CrossRef]
- Xu, W.; Cai, Y.; Gao, S.; Hou, S.; Yang, Y.; Duan, Y.; Fu, Q.; Chen, F.; Wu, J. New Understanding of Miniaturized VOCs Monitoring Device: PID-Type Sensors Performance Evaluations in Ambient Air. Sens. Actuators B Chem. 2021, 330, 129285. [Google Scholar] [CrossRef]
- Khoshakhlagh, A.H.; Golbabaei, F.; Beygzadeh, M.; Carrasco-Marín, F.; Shahtaheri, S.J. Evaluation of Direct Reading Photoionization Detector Performance under Various Operational Parameters. Environ. Health Eng. Manag. 2021, 8, 123–128. [Google Scholar] [CrossRef]



| TD (Unity 2, Markes International, Bridgend, UK) | ||||
| Pre-Desorption | Prepurge time | 1 min | ||
| Sample tube Desorption | Tube hold | 10 min | ||
| Oven temperature | 320 °C | |||
| Split | 1.3:1 | |||
| Cold trap Desorption | Pre-trap fire purge/min | 1 min | ||
| Trap low | −10 °C | |||
| Trap high | 320 °C | |||
| Trap hold | 10 min | |||
| Split | 2.3:1 | |||
| GC (6890, Agilent Technologies, Santa Clara, CA, USA) | ||||
| Column | DB-624 (60 m × 0.32 mm × 1.8 μm, Agilent, USA) | |||
| Oven Condition | Rate | Temp | Hold | |
| Initial | 40 °C | 4 min | ||
| ramp 1 | 10 °C/min | 250 °C | 5 min | |
| Run Time | 30 min | |||
| Carrier | 1.5 mL/min for He gas | |||
| MSD (5975 Agilent technologies, Santa Clara, CA, USA) | ||||
| Source Temperature | 190 °C | |||
| Mass range | 35~350 amu | |||
| Compound | Chemical Formula | Molecular Weight | Mp | Bp | Solubility | Henry’s Constant (H) at 23 °C [44,46,47] | CAS No. | |
|---|---|---|---|---|---|---|---|---|
| Full Name | Abbreviation | (g/g mole) | (°C) | (°C) | (g/100 mL) | (Air–Water Partition, Dimensionless) | ||
| Benzene | B | C6H6 | 78.11 | 6 | 80 | 0.18 | 0.22 | 71-43-2 |
| Toluene | T | C6H5CH3 | 92.14 | −95 | 111 | none | 0.24 | 108-88-3 |
| Ethylbenzene | E | C6H5C2H5 | 106.16 | −95 | 136 | 0.015 | 0.28 | 100-41-4 |
| Xylene | X | C6H4(CH3)2 | 106.16 | −25 | 144 | none | 0.18 | 106-42-3 |
| Styrene | S | C6H5CHCH2 | 104.15 | −30.6 | 145 | 0.03 | 0.10 | 100-42-5 |
| Methyl ethyl ketone | MEK | CH3COCH2CH3 | 72.11 | −86 | 80 | 29 | 2.0 × 10−3 | 78-93-3 |
| Methyl isobutyl ketone | MIBK | CH3COCH2CH(CH3)2 | 100.16 | −84.7 | 117–118 | 1.91 | 8.5 × 10−3 | 108-10-1 |
| Butyl acetate | BuAc | CH3COO(CH2)3CH3 | 116.16 | −78 | 126 | 0.7 | 1.0 × 10−2 | 128-86-4 |
| Isobutyl alcohol | I-BuAl | (CH3)2CHCH2OH | 74.12 | −108 | 108 | 8.7 | 3.1 × 10−4 | 78-83-1 |
| Order | Air Flow | Elapsed Time to Fully Clean |
|---|---|---|
| (L/min) | (min) | |
| 1 | 0.5 | >20:00 |
| 2 | 1 | >20:00 |
| 3 | 2 | >20:00 |
| 4 | 5 | ~10 |
| 5 | 8 | ~8 |
| 6 | 10 | ~7 |
| 7 | 12 | ~5 |
| Purging Time | 60 s | 300 s | ||||
|---|---|---|---|---|---|---|
| Inlet (RH%) | Outlet (RH%) | Removal Efficiency (%) | Inlet (RH%) | Outlet (RH%) | Removal Efficiency (%) | |
| Average | 90.6 | 15 | 83.4 | 89.6 | 11.6 | 87.1 |
| SD | 4.08 | 2.95 | 2.92 | 2.6 | 1.78 | 1.92 |
| N | 720 | 720 | 720 | 720 | 720 | 720 |
| Purging Temperature | 5 °C | 20 °C | 50 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Inlet (RH%) | Outlet (RH%) | Removal Efficiency (%) | Inlet (RH%) | Outlet (RH%) | Removal Efficiency (%) | Inlet (RH%) | Outlet (RH%) | Removal Efficiency (%) |
| Average | 98.1 | 9.69 | 90.1 | 86.0 | 15.3 | 82.3 | 86.2 | 24.4 | 71.7 |
| SD | 0.23 | 0.27 | 1.67 | 0.53 | 1.53 | 0.40 | 1.61 | 2.94 | 0.70 |
| N | 800 | 800 | 800 | 800 | 800 | 800 | 800 | 800 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Dinh, T.-V.; Park, S.-Y.; Choi, I.-Y.; Kim, I.-Y.; Park, B.-G.; Baek, D.-H.; Park, J.-H.; Seo, Y.-B.; Kim, J.-C. Development of a Moisture Pretreatment Device for the Accurate Quantitation of Water-Soluble Volatile Organic Compounds in Air. Chemosensors 2023, 11, 188. https://doi.org/10.3390/chemosensors11030188
Lee S-W, Dinh T-V, Park S-Y, Choi I-Y, Kim I-Y, Park B-G, Baek D-H, Park J-H, Seo Y-B, Kim J-C. Development of a Moisture Pretreatment Device for the Accurate Quantitation of Water-Soluble Volatile Organic Compounds in Air. Chemosensors. 2023; 11(3):188. https://doi.org/10.3390/chemosensors11030188
Chicago/Turabian StyleLee, Sang-Woo, Trieu-Vuong Dinh, Shin-Young Park, In-Young Choi, In-Young Kim, Byeong-Gyu Park, Da-Hyun Baek, Jae-Hyung Park, Ye-Bin Seo, and Jo-Chun Kim. 2023. "Development of a Moisture Pretreatment Device for the Accurate Quantitation of Water-Soluble Volatile Organic Compounds in Air" Chemosensors 11, no. 3: 188. https://doi.org/10.3390/chemosensors11030188
APA StyleLee, S.-W., Dinh, T.-V., Park, S.-Y., Choi, I.-Y., Kim, I.-Y., Park, B.-G., Baek, D.-H., Park, J.-H., Seo, Y.-B., & Kim, J.-C. (2023). Development of a Moisture Pretreatment Device for the Accurate Quantitation of Water-Soluble Volatile Organic Compounds in Air. Chemosensors, 11(3), 188. https://doi.org/10.3390/chemosensors11030188









