Macrocyclic Compounds Comprising Tris(3-Aminopropyl)Amine Units and Fluorophore Moieties: Synthesis and Spectroscopic Studies in the Presence of Metal Salts
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
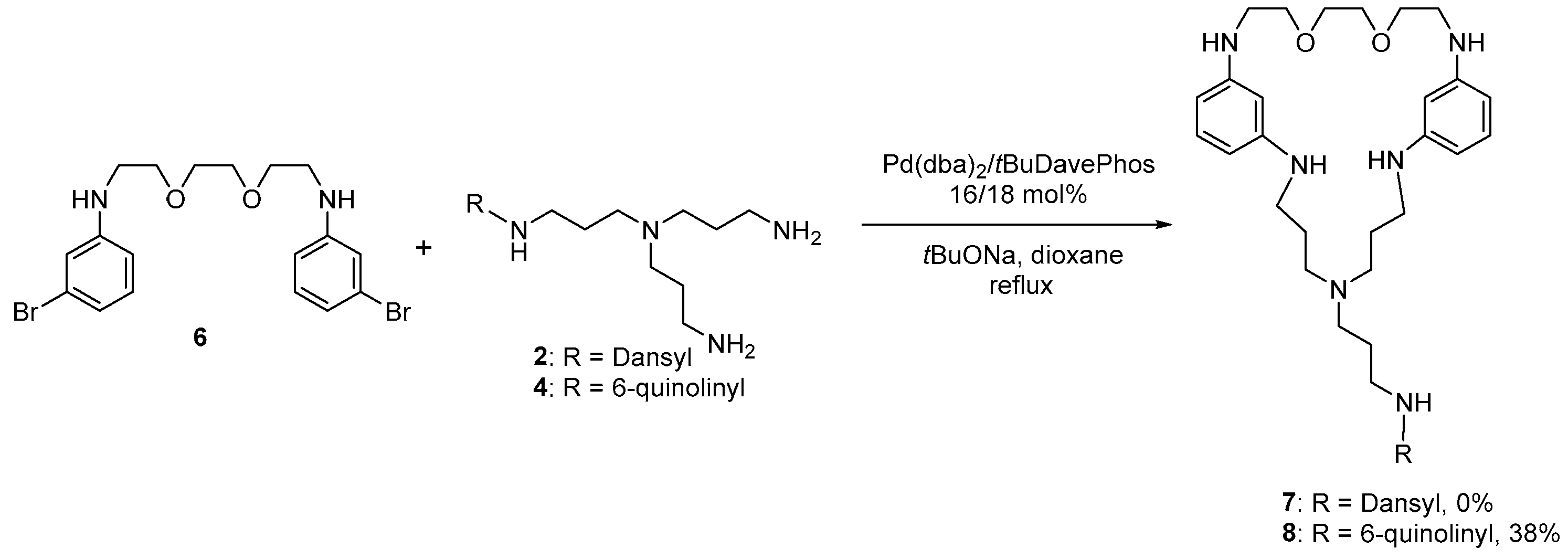
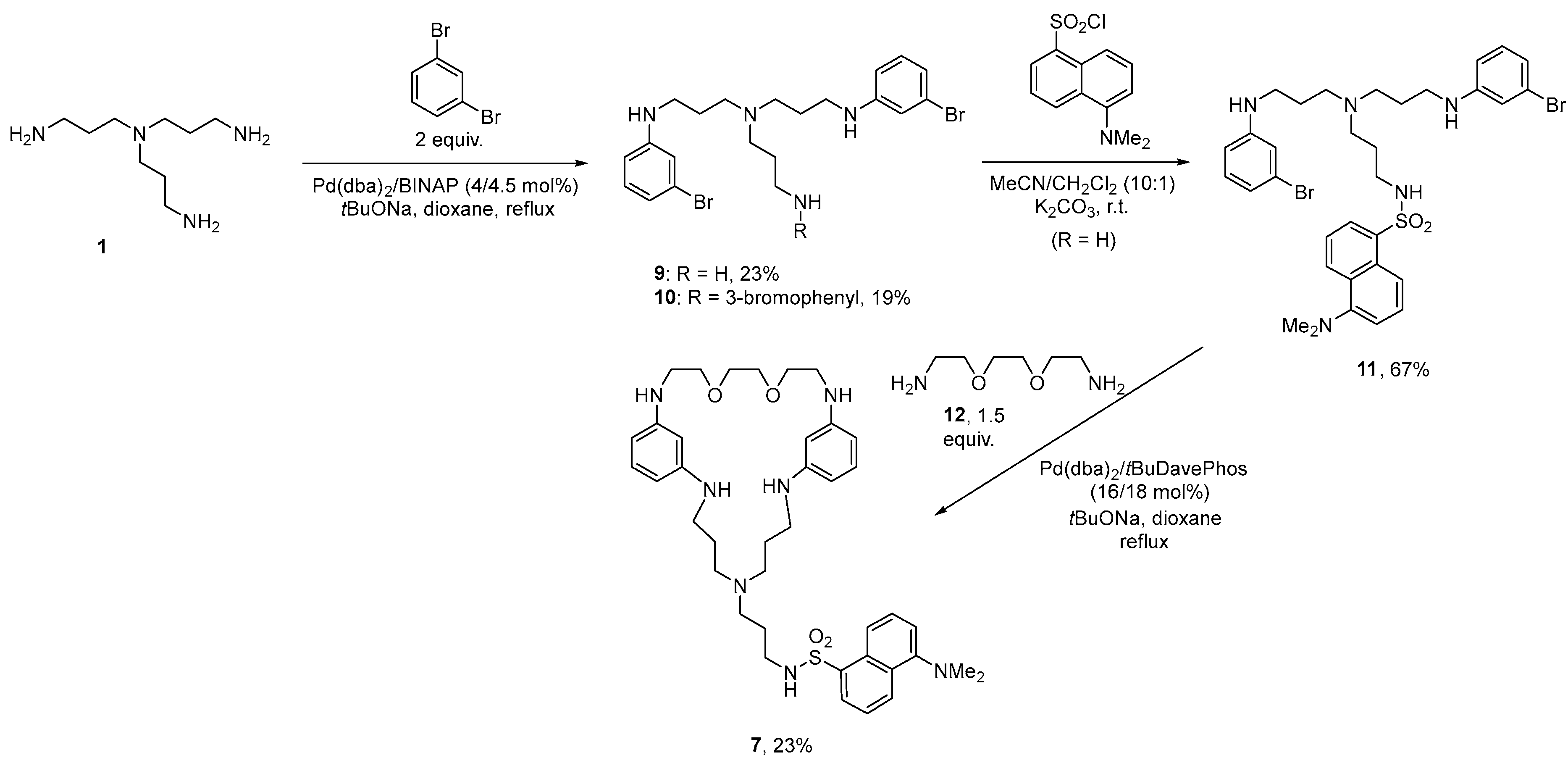
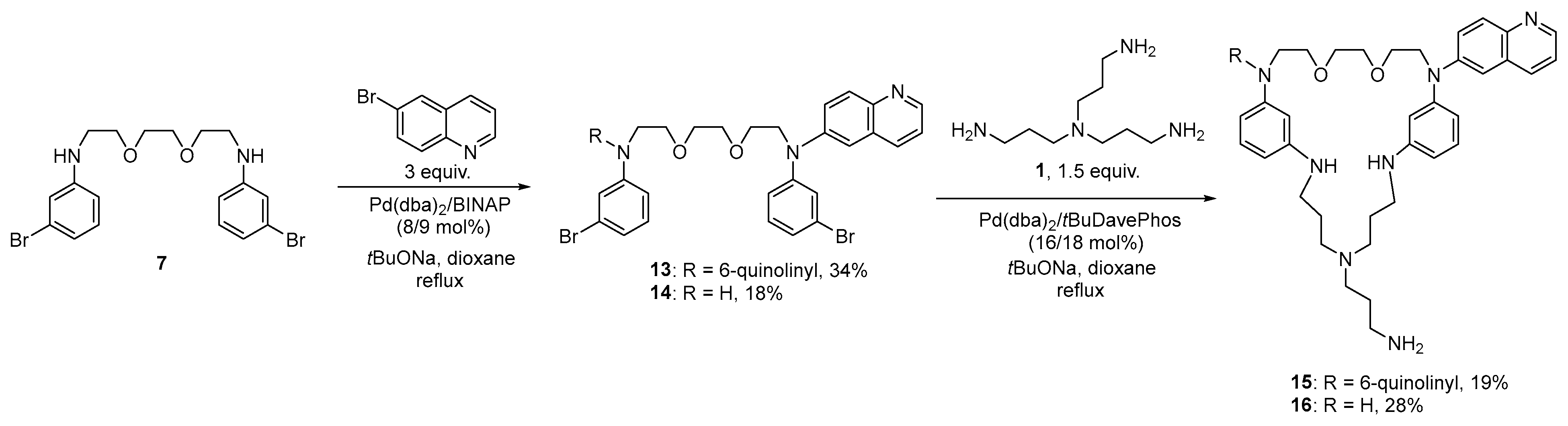
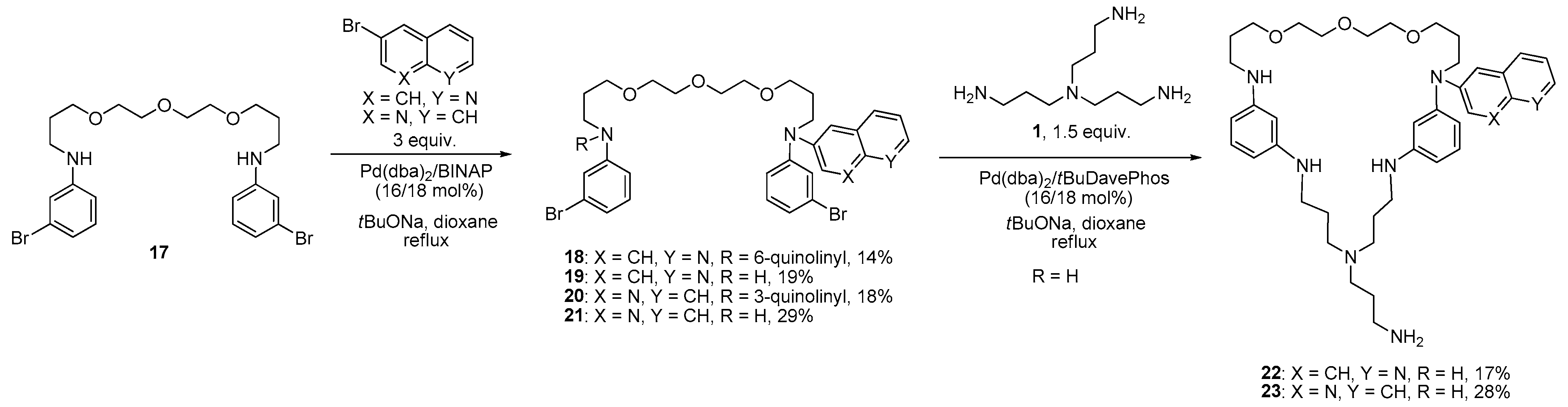
3.1. Synthesis of the Macrocycles Comprising a Sructural Unit of Tris(3-Aminopropyl)Amine
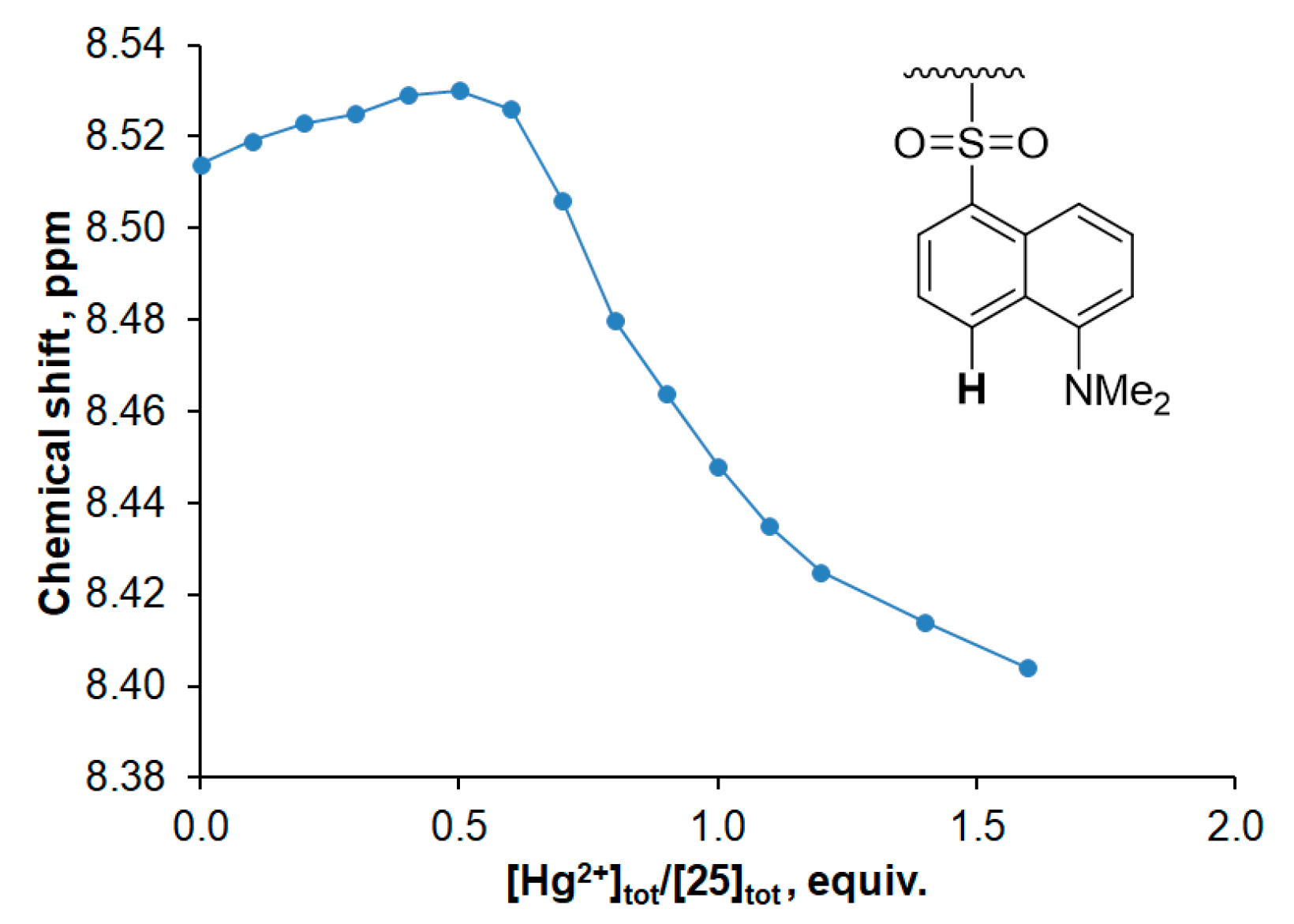
3.2. Spectroscopic Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fantozzi, N.; Pétuya, R.; Insuasty, A.; Long, A.; Lefevre, S.; Schmitt, A.; Robert, V.; Dutasta, J.-P.; Baraille, I.; Guy, L.; et al. A new fluorescent hemicryptophane for acetylcholine recognition with an unusual recognition mode. New J. Chem. 2020, 44, 11853–11860. [Google Scholar]
- Domínguez, M.; Blandez, J.F.; Lozano-Torres, B.; de la Torre, C.; Licchelli, M.; Mangano, C.; Amendola, V.; Sancenón, F.; Martínez-Máñez, R. A Nanoprobe based on gated mesoporous silica nanoparticles for the selective and sensitive detection of benzene metabolite t,t-muconic acid in urine. Chem. Eur. J. 2021, 27, 1306–1310. [Google Scholar] [PubMed]
- Li, C.; Manick, A.D.; Dutasta, J.P.; Bugaut, X.; Chatelet, B.; Martinez, A. Frustrated behavior of Lewis/Brønsted pairs inside molecular cages. Org. Chem. Front. 2022, 9, 1826–1836. [Google Scholar]
- Sarkar, S.; Sarkar, P.; Ghosh, P. Heteroditopic macrobicyclic molecular vessels for single step aerial oxidative transformation of primary alcohol appended cross azobenzenes. J. Org. Chem. 2021, 86, 6648–6664. [Google Scholar]
- Li, C.; Manick, A.-D.; Jean, M.; Albalat, M.; Vanthuyne, N.; Dutasta, J.-P.; Bugaut, X.; Chatelet, B.; Martinez, A. Hemicryptophane cages with a C1-symmetric cyclotriveratrylene unit. J. Org. Chem. 2021, 86, 15055–15062. [Google Scholar] [CrossRef]
- Paz Clares, M.; Aguilar, J.; Aucejo, R.; Lodeiro, C.; Albelda, M.T.; Pina, F.; Lima, J.C.; Parola, A.J.; Pina, J.; Seixas De Melo, J.; et al. Synthesis and H+, Cu2+, and Zn2+ coordination behavior of a bis(fluorophoric) bibrachial lariat aza-crown. Inorg. Chem. 2004, 43, 6114–6122. [Google Scholar]
- Castillo, C.E.; Algarra, A.G.; Ferrer, A.; Angeles Máñez, M.; Basallote, M.G.; Paz Clares, M.; Soriano, C.; Teresa Albelda, M.; García-España, E. Equilibrium and kinetics studies on bibrachial lariat aza-crown/Cu(II) systems reveal different behavior associated with small changes in the structure. Inorg. Chim. Acta 2014, 417, 246–257. [Google Scholar]
- Hamacek, J.; Elhabiri, M.; Le Guennic, B.; Shanzer, A.; Albrecht-Gary, A.-M. Metal-mediated interactions in homo- and heterobimetallic edifices with lanthanides: A study in solution. Eur. J. Inorg. Chem. 2022, 2022, e202200235. [Google Scholar]
- Godart, E.; Long, A.; Rosas, R.; Lemercier, G.; Jean, M.; Leclerc, S.; Bouguet-Bonnet, S.; Godfrin, C.; Chapellet, L.L.; Dutasta, J.P.; et al. High-relaxivity Gd(III)-hemicryptophane complex. Org. Lett. 2019, 21, 1999–2003. [Google Scholar]
- Johnston, H.M.; Freire, D.M.; Mantsorov, C.; Jamison, N.; Green, K.N. Manganese (III/IV) μ-oxo dimers and manganese (III) monomers with tetraaza macrocyclic ligands and historically relevant open-chain ligands. Eur. J. Inorg. Chem. 2022, 2022, e202200039. [Google Scholar] [CrossRef]
- Massoud, S.S.; Mautner, F.A.; Vicente, R.; Gallo, A.A.; Ducasse, E. Dinuclear and Polynuclear Bridged Azido–Nickel(II) Complexes: Synthesis, Structure Determination, and Magnetic Properties. Eur. J. Inorg. Chem. 2007, 2007, 1091–1102. [Google Scholar] [CrossRef]
- Golbedaghi, R.; Moradi, S.; Salehzadeh, S.; Blackman, A.G. Some metal complexes of three new potentially heptadentate (N4O3) tripodal Schiff base ligands; synthesis, characterizatin and X-ray crystal structure of a novel eight coordinate Gd(III) complex. J. Mol. Struct. 2016, 1108, 727–734. [Google Scholar] [CrossRef]
- Salehzadeh, S.; Golbedaghi, R.; Rakhtshah, J.; Adams, H. A new series of manganese(II) complexes of three fully condensed Schiff base ligands derived from some symmetrical and asymmetrical tripodal tetraamines and 2-pyridinecarboxyaldehyde. J. Mol. Struct. 2021, 1245, 130982. [Google Scholar] [CrossRef]
- Salehzadeh, S.; Golbedaghi, R.; Adams, H. Nickel(II) complexes of two potentially heptadentate(N7) Tripodal Schiff-base ligands; X-ray crystal structure and theoretical studies. J. Mol. Struct. 2022, 1247, 131359. [Google Scholar] [CrossRef]
- Emami Khansari, M.; Johnson, C.R.; Basaran, I.; Nafis, A.; Wang, J.; Leszczynski, J.; Hossain, M.A. Synthesis and anion binding studies of tris(3-aminopropyl)amine-based tripodal urea and thiourea receptors: Proton transfer-induced selectivity for hydrogen sulfate over sulfate. RSC Adv. 2015, 5, 17606–17614. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; et al. Design, synthesis and antimalarial activity of novel bis{N-[(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef]
- Guillon, J.; Cohen, A.; Das, R.N.; Boudot, C.; Gueddouda, N.M.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Tisnerat, C.; et al. Design, synthesis, and antiprotozoal evaluation of new 2,9-bis[(substituted-aminomethyl)phenyl]-1,10-phenanthroline derivatives. Chem. Biol. Drug Des. 2018, 91, 974–995. [Google Scholar]
- Murugesan, K.; Jeyasingh, V.; Lakshminarayanan, S.; Narayanan, S.; Piramuthu, L. Traditional hydrogen bonding donors controlled colorimetric selective anion sensing in tripodal receptors: First-naked-eye detection of cyanide by a tripodal receptor via fluoride displacement assay. Spectrochim. Acta Part A 2019, 223, 117238. [Google Scholar] [CrossRef]
- Jeyasingh, V.; Murugesan, K.; Lakshminarayanan, S.; Selvapalam, N.; Das, G.; Piramuthu, L. Selective colorimetric sensing and perfect linear recognition of azide: Formation of Cu-azide-Cu cascade complex within the cavity of cryptand. Spectrochim. Acta Part A 2020, 240, 118550. [Google Scholar] [CrossRef]
- Jeyasingh, V.; Murugesan, K.; Lakshminarayanan, S.; Selvapalam, N.; Das, G.; Enoch, I.V.M.V.; Piramuthu, L. Most efficient tris(3-aminopropyl) amine based electron deficient tripodal receptor for azide. J. Fluoresc. 2020, 30, 291–300. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Zhuo, Y.; Chai, Y.; Yuan, R. Self-enhanced electrochemiluminescence nanorods of tris(bipyridine) ruthenium(II) derivative and its sensing application for detection of N-Acetyl-β-d-glucosaminidase. Anal. Chem. 2016, 88, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Louka, F.R.; Haq, S.J.; Guidry, H.R.; Williams, B.R.; Henary, M.M.; Fischer, R.C.; Torvisco, A.; Massoud, S.S.; Mautner, F.A. Polynuclear and coordination polymers of copper(II) complexes assembled by flexible polyamines and bridging rigid N-heterocyclic multicarboxylates. Inorg. Chim. Acta 2020, 500, 119240. [Google Scholar] [CrossRef]
- Chernikova, E.A.; Glukhov, L.M.; Kustov, L.M.; Krasovsky, V.G. Adsorbents of SO2 based on amine-modified porous materials. Russ. Chem. Bull. 2015, 64, 2958–2962. [Google Scholar] [CrossRef]
- Lyu, H.; Li, H.; Hanikel, N.; Wang, K.; Yaghi, O.M. Covalent organic frameworks for carbon dioxide capture from air. J. Am. Chem. Soc. 2022, 144, 12989–12995. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.J.; Niederegger, L.; Hess, C.R. Neighbouring effects on catalytic epoxidation by Fe-cyclam in M2-PDIxCy complexes. Dalton Trans. 2020, 49, 17642–17648. [Google Scholar] [CrossRef]
- Medina-Molner, A.; Rohner, M.; Pandiarajan, D.; Spingler, B. Mono- and dinuclear metal complexes containing the 1,5,9-triazacyclododecane ([12]aneN3) unit and their interaction with DNA. Dalton Trans. 2015, 44, 3664–3672. [Google Scholar] [CrossRef]
- Chand, D.K.; Bharadwaj, P.K. Heteroditopic cryptands of tunable cavity size: Imposition of distorted geometry onto copper(II) and nickel(II) and molecular recognition of water molecules. Inorg. Chem. 1998, 37, 5050–5055. [Google Scholar] [CrossRef]
- Krzystof, E.; Bordunov, A.V.; Bradshow, J.S. Synthesis of hexaazacryptands containing furan and benzene groups in the bridging arms. J. Heterocycl. Chem. 1998, 35, 169–171. [Google Scholar]
- Yoon, J.; Ohler, N.E.; Vance, D.H.; Aumiller, W.D.; Czarnik, A.W. A fluorescent chemosensor signalling only Hg(II) and Cu(II) in water. Tetrahedron Lett. 1997, 38, 3845–3848. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Averin, A.D.; Bessmertnykh, A.G.; Denat, F.; Guilard, R. Palladium-catalyzed amination in the synthesis of polyazamacrocycles. Russ. J. Org. Chem. 2010, 46, 946–967. [Google Scholar] [CrossRef]
- Averin, A.D.; Beletskaya, I.P. Synthesis of polymacrocyclic compounds via Pd-catalyzed amination and evaluation of their derivatives as metal detectors. Pure Appl. Chem. 2019, 91, 633–651. [Google Scholar] [CrossRef]
- Averin, A.D.; Grigorova, O.K.; Malysheva, A.S.; Shaferov, A.V.; Beletskaya, I.P. Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors. Pure Appl. Chem. 2020, 92, 1367–1386. [Google Scholar] [CrossRef]
- Ukai, T.; Kawazura, H.; Ishii, Y.; Bonnet, J.J.; Ibers, J.A. Chemistry of dibenzylideneacetone-palladium(0) complexes: I. Novel tris(dibenzylideneacetone)dipalladium(solvent) complexes and their reactions with quinones. J. Organomet. Chem. 1974, 65, 253–266. [Google Scholar] [CrossRef]
- Averin, A.D.; Shukhaev, A.V.; Golub, S.L.; Buryak, A.K.; Beletskaya, I.P. Palladium-catalyzed amination in the synthesis of polyazamacrocycles containing a 1,3-disubstiituted benzene moiety. Synthesis 2007, 2007, 2995–3012. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Hynes, M.J. EQNMR: A Computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. J. Chem. Soc. Dalton Trans. 1993, 1993, 311–312. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]




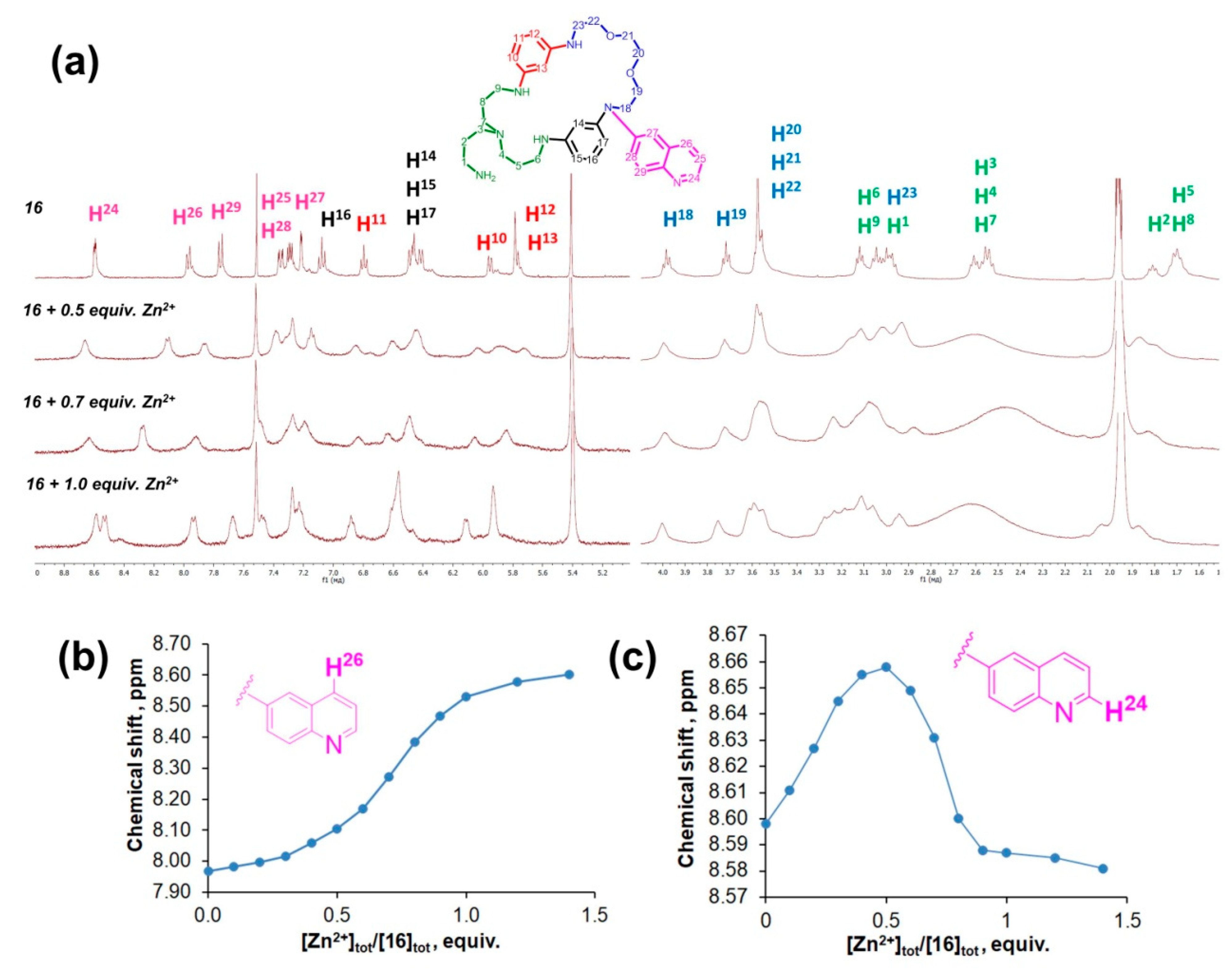

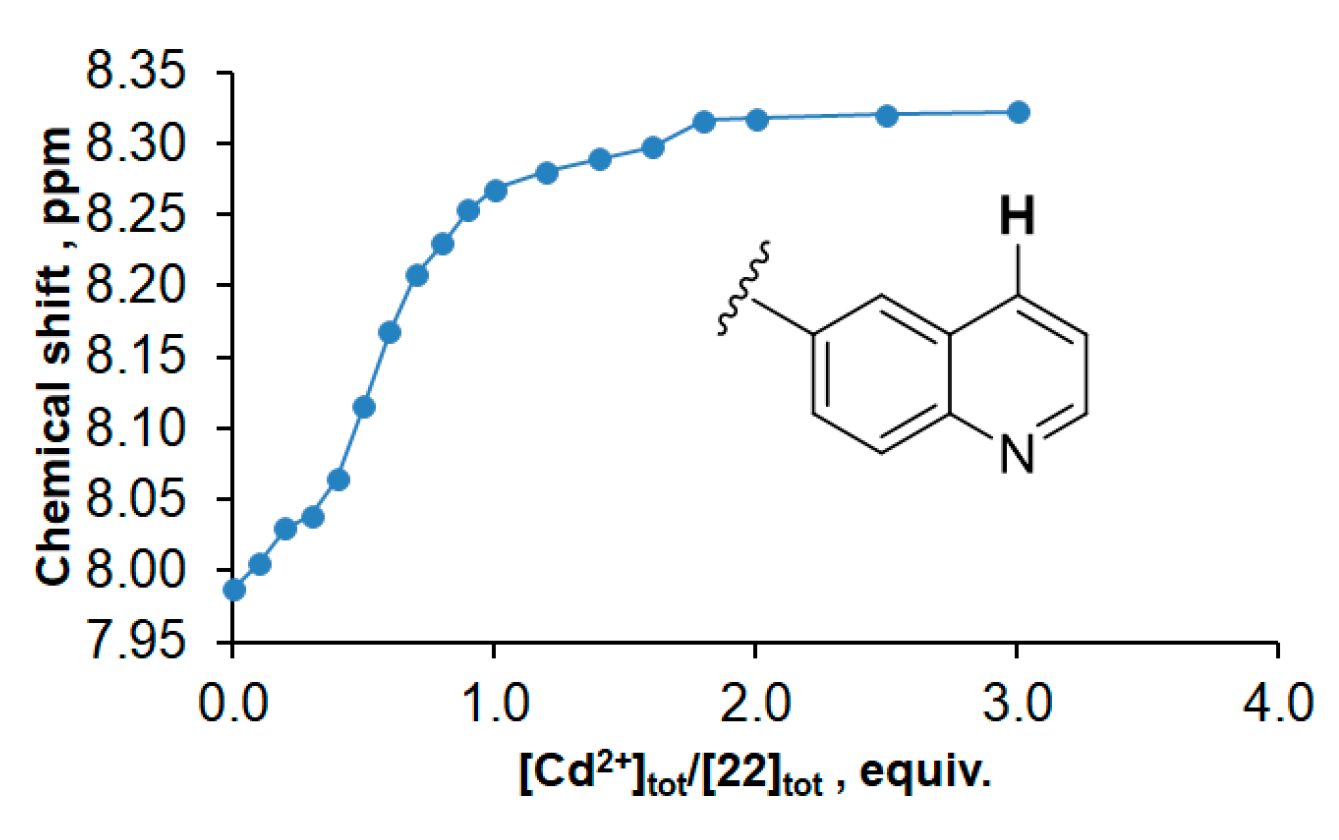
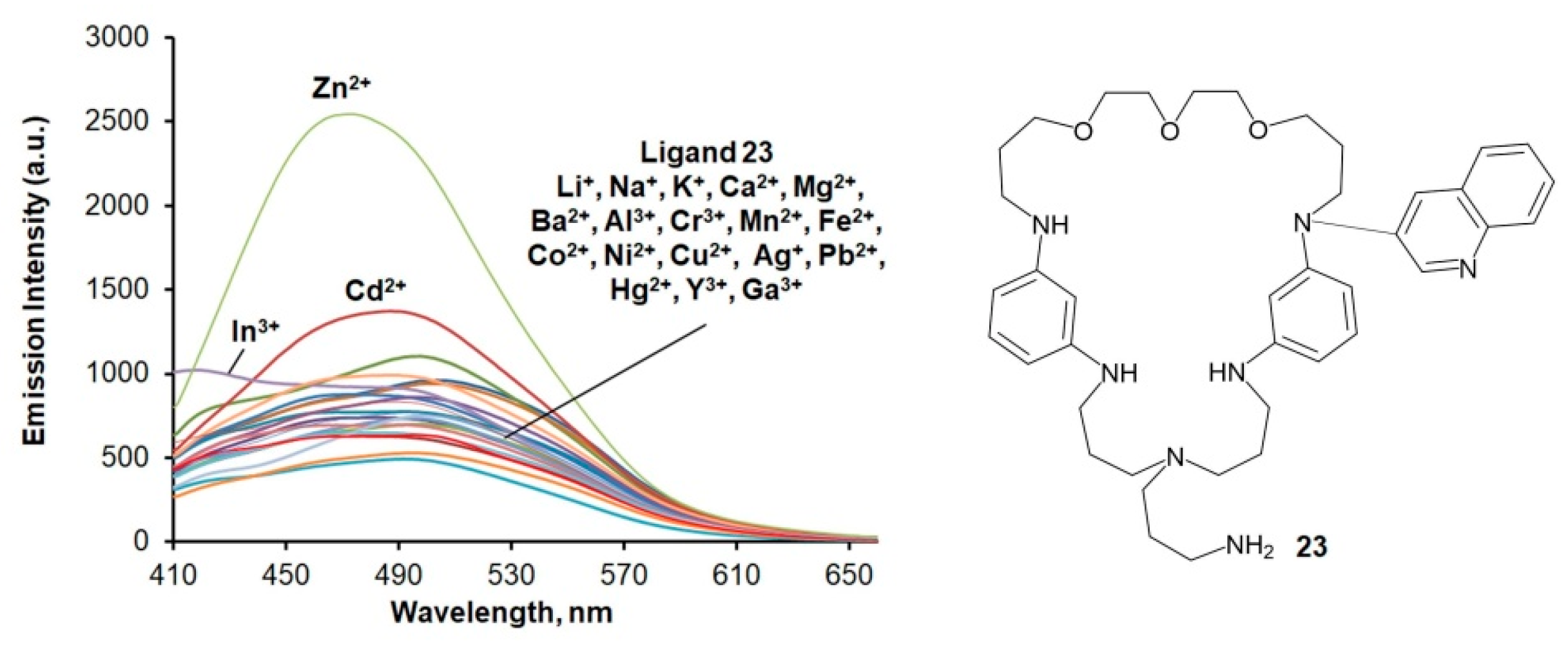
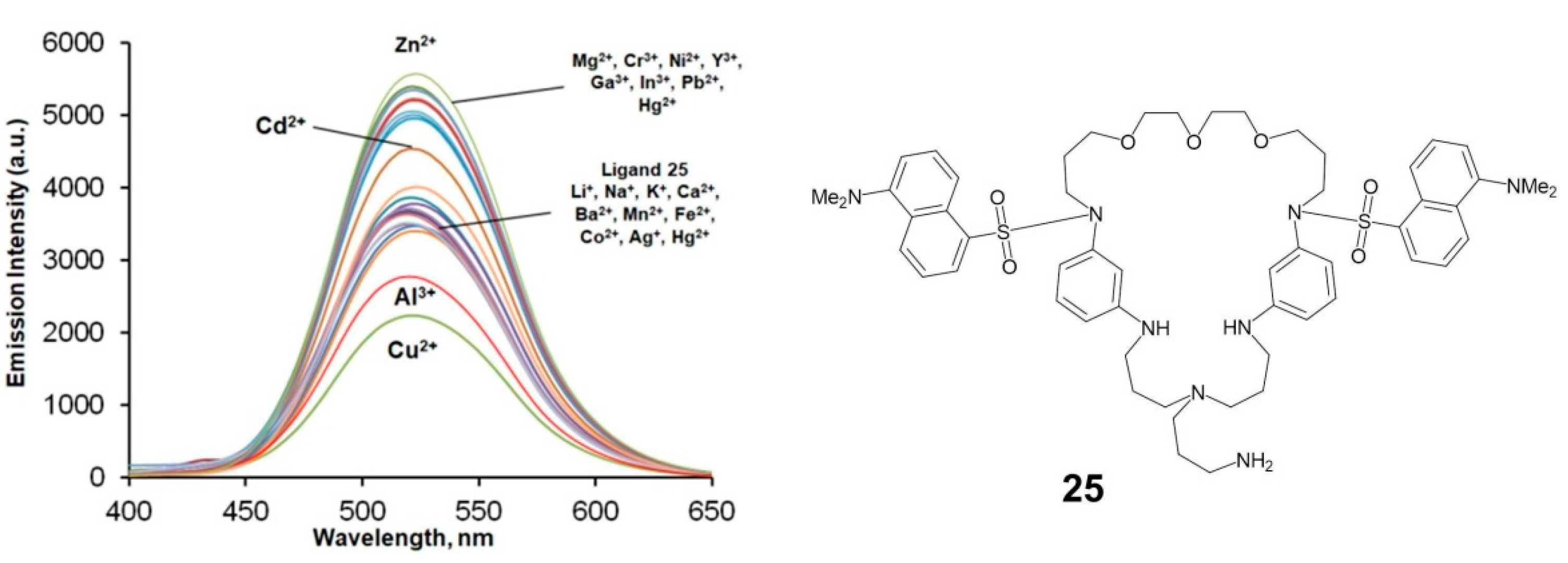
| Macrocycle | Concentration, μM | λabs, nm | lgε | λex, nm | λem, nm |
|---|---|---|---|---|---|
| 7 | 4.8 | 305 | 4.47 | 340 | 508 |
| 340 | 4.20 | ||||
| 8 | 23.0 | 300 | 4.08 | 365 | 430 |
| 365 | 3.66 | ||||
| 16 | 17.5 | 308 | 4.19 | 370 | 443 |
| 373 | 3.80 | ||||
| 22 | 14.3 | 309 | 4.28 | 370 | 485 |
| 374 | 3.86 | ||||
| 23 | 21.2 | 306 | 4.03 | 370 | 510 |
| 370 | 3.68 | ||||
| 25 | 12.3 | 313 | 3.96 | 340 | 516 |
| 342 | 3.93 |
| Macrocycle | Metal | Complex | lgβ | Method |
|---|---|---|---|---|
| 16 | Pb(II) | L4M | 17.5(3) | Spectrophotometry |
| L2M | 10.27(1) | Spectrophotometry | ||
| LM | 5.05(1) | Spectrophotometry | ||
| Zn(II) | L2M | 11.88(3) | Spectrophotometry | |
| LM | 8.31(1) | Spectrophotometry | ||
| LM2 | 12.2(3) | Spectrophotometry | ||
| 22 | Cu(II) | LM | 8.45(8) | Spectrophotometry |
| LM2 | 16.1(1) | Spectrophotometry | ||
| LM3 | 21.1(2) | Spectrophotometry | ||
| Zn(II) | L4M | 28.2(1) | Spectrofluorometry | |
| L2M | 14.69(7) | Spectrofluorometry | ||
| Cd(II) | L2M | 2.7(3) | NMR (EQNMR) | |
| LM | 3.1(2) | NMR (EQNMR) | ||
| 23 | Zn(II) | L4M | 21.34(5) | Spectrophotometry |
| L2M | 11.46(2) | Spectrophotometry | ||
| Hg(II) | L4M | 22.94(8) | Spectrophotometry | |
| L2M | 13.23(7) | Spectrophotometric | ||
| LM | 7.80(5) | Spectrophotometric | ||
| LM2 | 12.3(1) | Spectrophotometric | ||
| 25 | Zn(II) | L4M | 25.3(1) | Spectrophotometric |
| L2M | 13.49(5) | Spectrophotometric | ||
| Pb(II) | L3M | 19.48(5) | Spectrophotometric | |
| LM | 7.74(2) | Spectrophotometric | ||
| LM2 | 12.1(1) | Spectrophotometric | ||
| Hg(II) | L2M | 2.5(2) | NMR (BindFit) | |
| LM | 3.8(2) | NMR (BindFit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuliukhina, D.S.; Chernichenko, N.M.; Averin, A.D.; Abel, A.S.; Maloshitskaya, O.A.; Beletskaya, I.P. Macrocyclic Compounds Comprising Tris(3-Aminopropyl)Amine Units and Fluorophore Moieties: Synthesis and Spectroscopic Studies in the Presence of Metal Salts. Chemosensors 2023, 11, 186. https://doi.org/10.3390/chemosensors11030186
Kuliukhina DS, Chernichenko NM, Averin AD, Abel AS, Maloshitskaya OA, Beletskaya IP. Macrocyclic Compounds Comprising Tris(3-Aminopropyl)Amine Units and Fluorophore Moieties: Synthesis and Spectroscopic Studies in the Presence of Metal Salts. Chemosensors. 2023; 11(3):186. https://doi.org/10.3390/chemosensors11030186
Chicago/Turabian StyleKuliukhina, Daria S., Nataliya M. Chernichenko, Alexei D. Averin, Anton S. Abel, Olga A. Maloshitskaya, and Irina P. Beletskaya. 2023. "Macrocyclic Compounds Comprising Tris(3-Aminopropyl)Amine Units and Fluorophore Moieties: Synthesis and Spectroscopic Studies in the Presence of Metal Salts" Chemosensors 11, no. 3: 186. https://doi.org/10.3390/chemosensors11030186
APA StyleKuliukhina, D. S., Chernichenko, N. M., Averin, A. D., Abel, A. S., Maloshitskaya, O. A., & Beletskaya, I. P. (2023). Macrocyclic Compounds Comprising Tris(3-Aminopropyl)Amine Units and Fluorophore Moieties: Synthesis and Spectroscopic Studies in the Presence of Metal Salts. Chemosensors, 11(3), 186. https://doi.org/10.3390/chemosensors11030186










