Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Variants Preparation
- -
- octanoic acid—in doses of 10, 20 and 30 mg L−1;
- -
- decanoic acid—in doses of 10, 20 and 30 mg L−1;
- -
- octanoic and decanoic acid combinations—10 mg L−1 and 15 mg L−1 of each;
- -
- SO2—60 mg L−1 for samples with fatty acids and 120 mg L−1 for control samples;
- -
- 0.6 g L−1 bentonite was added in each sample, including the control.
2.2. Wine Variants Analyses
3. Results
3.1. Compounds Identified in Tămâioasă Românească Wines by GC e-Nose
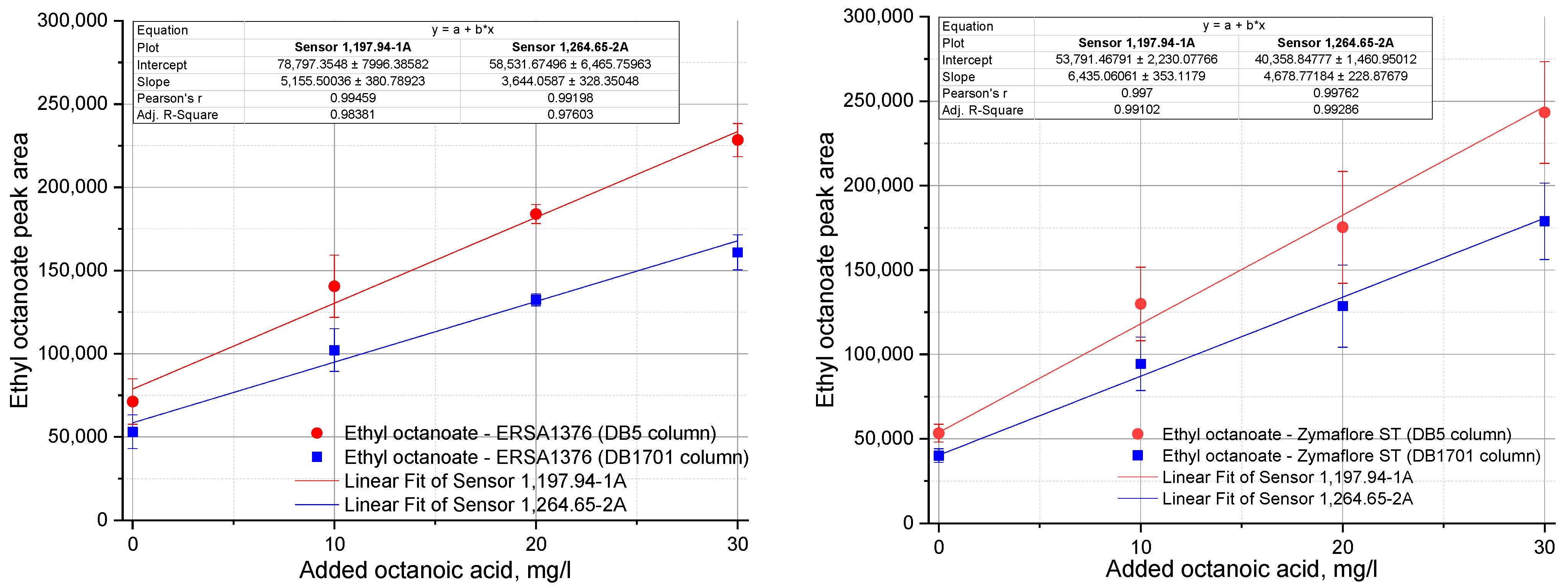
3.2. Influence of ERSA Yeast on the Volatile Compounds of Tămâioasă Românească Wines Treated with Octanoic or Decanoic Acid
3.3. Discrimination Analysis Performed by the e-Nose for Samples Treated with Various Doses of Octanoic and Decanoic Acid to Stop Fermentation
3.3.1. Discrimination of Samples Considered Altogether, Irrespective of the Yeast Used for Fermentation
3.3.2. Discrimination of Samples Fermented with One Type of Yeast (ERSA or ST)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Taylor, S.L.; Higley, N.A.; Bush, R.K. Sulfites in Foods: Uses, Analytical Methods, Residues, Fate, Exposure Assessment, Metabolism, Toxicity, and Hypersensitivity. In Advances in Food Research; Chichester, C.O., Mrak, E.M., Schweigert, B.S., Eds.; Academic Press: Cambridge, MA, USA, 1986; Volume 30, pp. 1–76. [Google Scholar] [CrossRef]
- Csutoras, C.; Bakos-Barczi, N.; Burkus, B. Medium chain fatty acids and fatty acid esters as potential markers of alcoholic fermentation of white wines. Acta Aliment. 2022, 51, 33–42. [Google Scholar] [CrossRef]
- Novak, M.; Strehaiano, P.; Moreno, M.; Goma, G. Alcoholic fermentation: On the inhibitory effect of ethanol. Biotechnol. Bioeng. 1981, 23, 201–211. [Google Scholar] [CrossRef]
- Lafon-Lafourcade, S.; Geneix, C.; Ribereau-Gayon, P. Inhibition of alcoholic fermentation of grape must by fatty acids produced by yeasts and their elimination by yeast ghosts. Appl. Environ. Microbiol. 1984, 47, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.A.; Rosa, M.F.; Correia, I.S.; Novais, J.M. Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl. Environ. Microbiol. 1989, 55, 21–28. [Google Scholar] [CrossRef]
- Licek, J.; Baron, M.; Sochor, J.; Kumsta, M.; Mlcek, J. Observation of residues content after application of a medium-chain fatty acids mixture at the end of alcoholic fermentation. Fermentation 2022, 8, 105. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM fragrance ingredient safety assessment, octanoic acid, CAS Registry Number 124-07-2. Food Chem. Toxicol. 2020, 138 (Suppl. S1), 111271. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM fragrance ingredient safety assessment, decanoic acid, CAS Registry Number 334-48-5. Food Chem. Toxicol. 2020, 144 (Suppl. S1), 111465. [Google Scholar] [CrossRef]
- Baroň, M.; Kumšta, M.; Prokeš, K.; Tomášková, L.; Tomková, M. The inhibition of Saccharomyces cerevisiae population during alcoholic fermentation of grape must by octanoic, decanoic and dodecanoic acid mixture. BIO Web Conf. 2017, 9, 02025. [Google Scholar] [CrossRef]
- Licek, J.; Baron, M.; Sochor, J. Comparison of MCFA and other methods of terminating alcohol fermentation and their influence on the content of carbonyl compounds in wine. Molecules 2020, 25, 5737. [Google Scholar] [CrossRef]
- Antoce, A.O.; Cojocaru, G.A. Evaluation by Flash GC Electronic Nose of the Effect of Combinations of Yeasts and Nutrients on the Aromatic Profiles of Feteasca Regala Wines after Two Years of Storage. Fermentation 2021, 7, 223. [Google Scholar] [CrossRef]
- Cojocaru, G.A.; Antoce, A.O. Influence of glutathione and ascorbic acid treatments during vinification of Feteasca Regala variety and their antioxidant effect on volatile profile. Biosensors 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Antoce, O.A.; Namolosanu, C.I. Rapid and precise discrimination of wines by means of an electronic nose based on gas-chromatography. Rev. Chim. 2011, 62, 593–595. [Google Scholar]
- Strani, L.; D’Alessandro, A.; Ballestrieri, D.; Durante, C.; Cocchi, M. Fast GC E-Nose and Chemometrics for the Rapid Assessment of Basil Aroma. Chemosensors 2022, 10, 105. [Google Scholar] [CrossRef]
- Yimenu, S.M.; Kim, J.Y.; Kim, B.S. Prediction of egg freshness during storage using electronic nose. Poult. Sci. 2017, 96, 3733–3746. [Google Scholar] [CrossRef] [PubMed]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Legras, J.L.; Erny, C.; Le Jeune, C.; Lollier, M.; Adolphe, Y.; Demuyter, C.; Delobel, P.; Blondin, B.; Karst, F. Activation of two different resistance mechanisms in Saccharomyces cerevisiae upon exposure to octanoic and decanoic acids. Appl. Environ. Microbiol. 2010, 76, 7526–7535. [Google Scholar] [CrossRef]
- Antoce, O.A.; Antoce, V.; Takahashi, K.; Pomohaci, N.; Nămoloşanu, I. A calorimetric method applied to the study of yeast growth inhibition by alcohols and organic acids. Am. J. Enol. Vitic. 1997, 48, 413–422. [Google Scholar] [CrossRef]
- Antoce, O.A.; Antoce, V.; Takahashi, K. Inhibitory effects of decanoic acid on yeast growth at various pHs and ethanol concentrations. Biocontrol Sci. 1998, 2, 7–15. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Espínola-Espínola, V.; López-Solís, R. Wine pH Prevails over Buffering Capacity of Human Saliva. J. Agric. Food Chem. 2016, 64, 8154–8159. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.; Hofmeyr, J.H.S. Effects of ethanol, octanoic and decanoic acids on fermentation and the passive influx of protons through the plasma membrane of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 656–663. [Google Scholar] [CrossRef]
- Sa-Correia, I.; Salgueiro, S.P.; Viegas, C.A.; Novais, J.M. Leakage induced by ethanol, octanoic and decanoic acids in Saccharomyces cerevisiae. Yeast 1989, 5, S123–S127. [Google Scholar]
- Viegas, C.A.; Sa-Correia, I. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J. Gen. Microbiol. 1991, 137, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.A.; Sa-Correia, I.; Novais, J.M. Synergistic inhibition of the growth of Saccharomyces bayanus by ethanol and octanoic or decanoic acids. Biotechnol. Lett. 1985, 7, 611–614. [Google Scholar] [CrossRef]
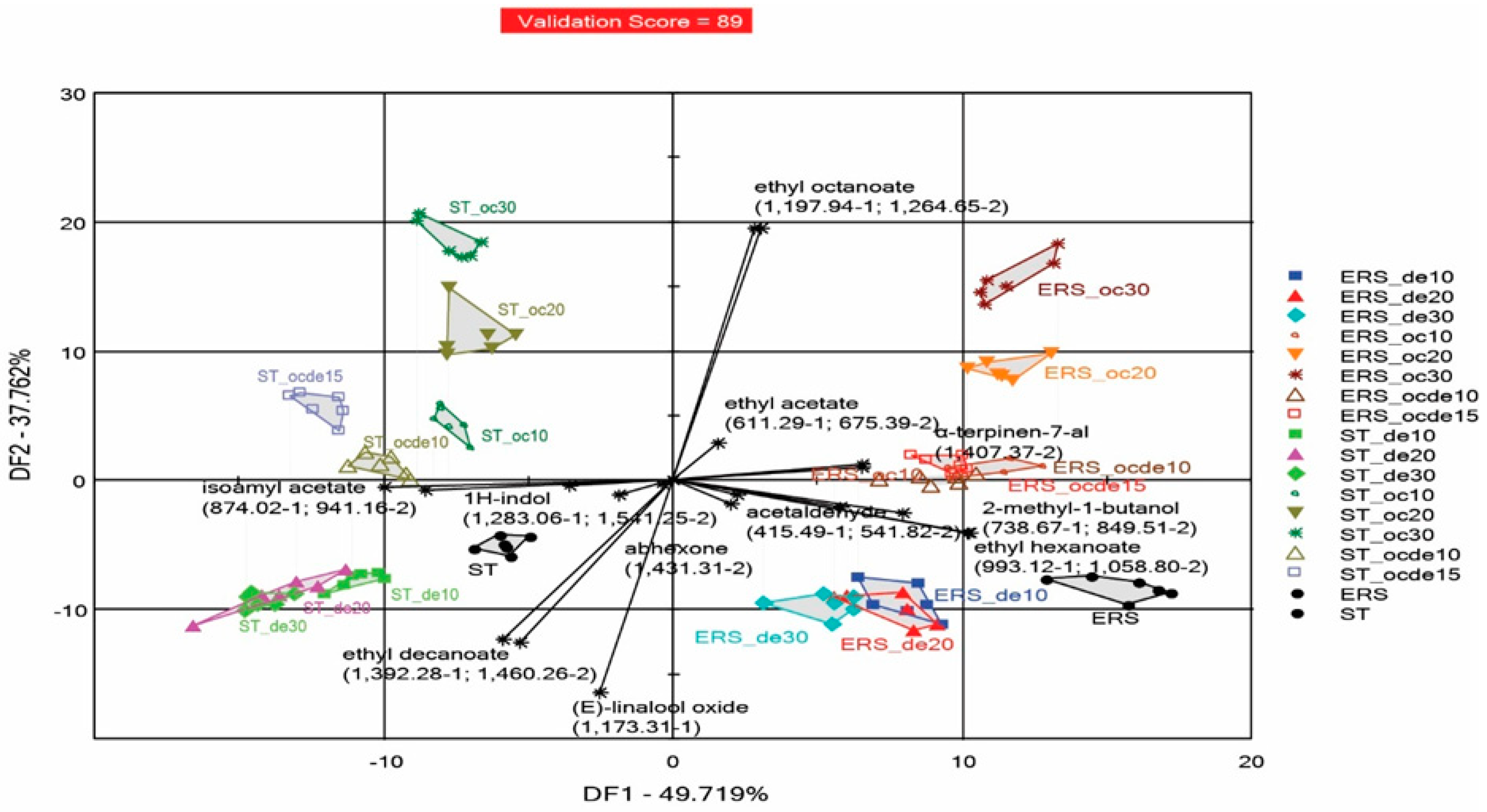
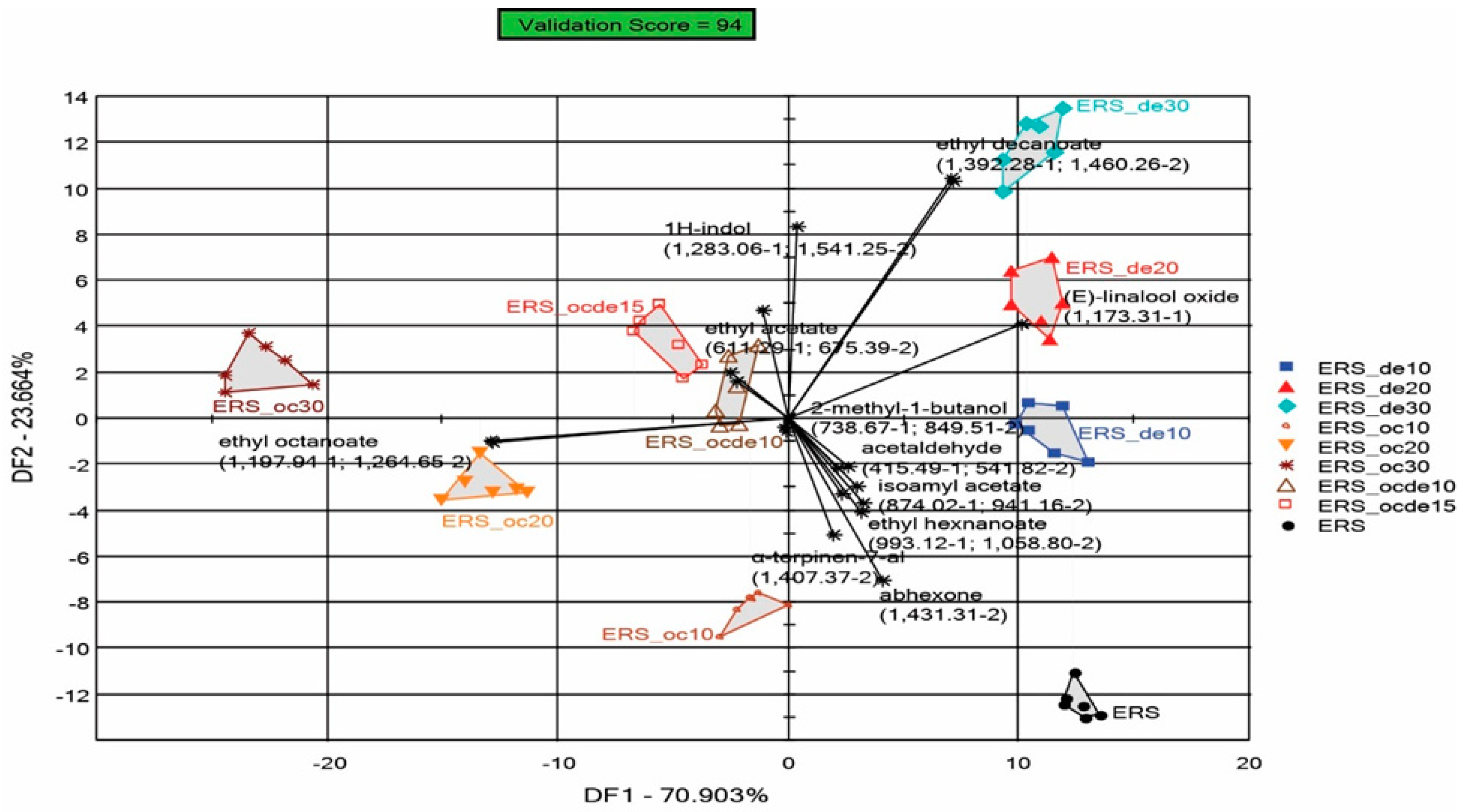
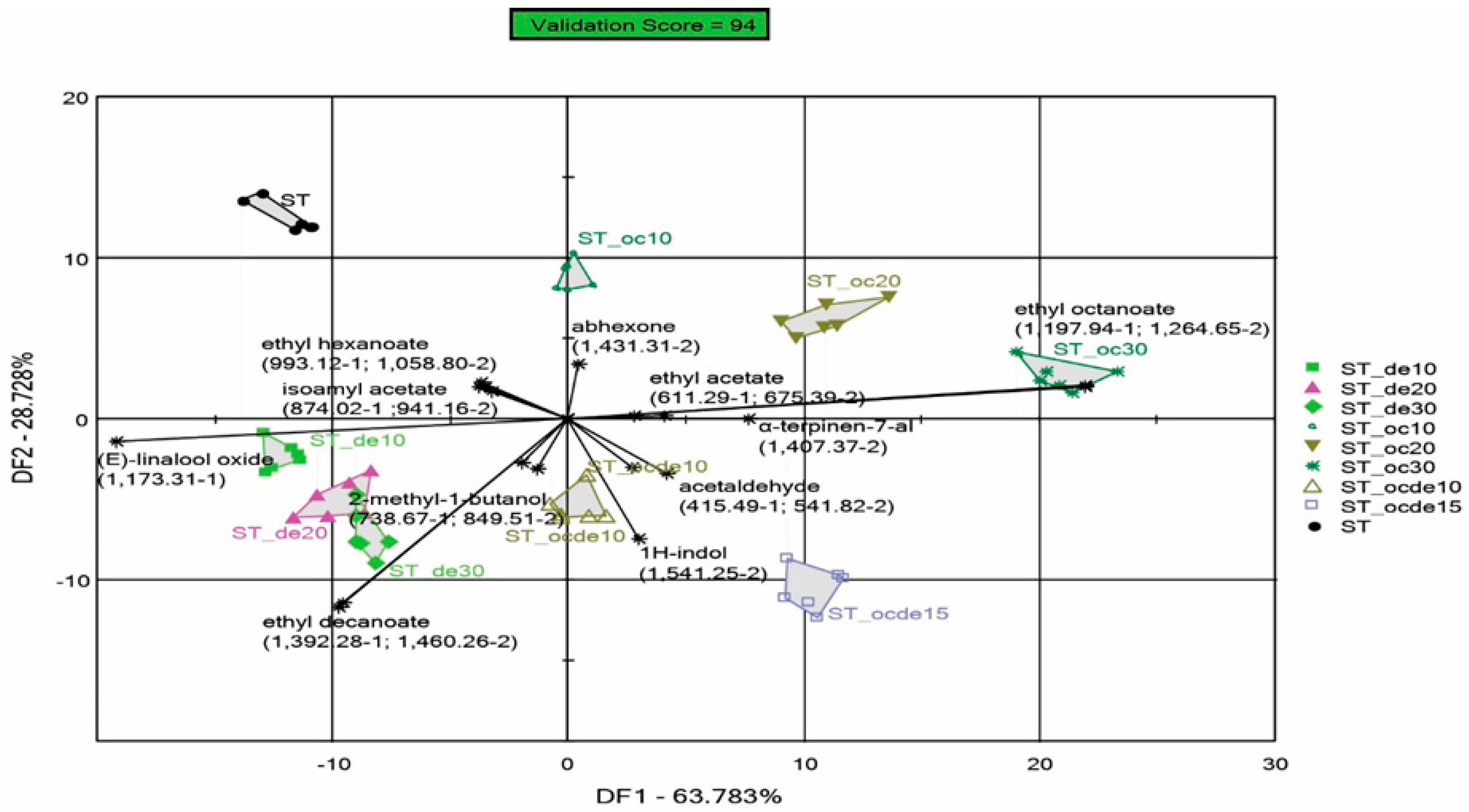
| Wine Samples Fermented with ERSA Yeast | Wine Samples Fermented with ST Yeast | Octanoic Acid mg L−1 | Decanoic Acid mg L−1 | SO2 mg L−1 |
|---|---|---|---|---|
| ERSA_0 | ST_0 | - | - | 120 |
| ERSA_oc10 | ST_oc10 | 10 | - | 60 |
| ERSA_oc20 | ST_oc20 | 20 | - | 60 |
| ERSA_oc30 | ST_oc30 | 30 | - | 60 |
| ERSA_de10 | ST_de10 | - | 10 | 60 |
| ERSA_de20 | ST_de20 | - | 20 | 60 |
| ERSA_de30 | ST_de30 | - | 30 | 60 |
| ERSA_ocde10 | ST_ocde10 | 10 | 10 | 60 |
| ERSA_ocde15 | ST_ocde15 | 15 | 15 | 60 |
| Compound | Column DB5 RT (s) | Sensors on DB5 (Peak Area) | Column DB1701 RT (s) | Sensors on DB1701 (Peak Area) | Sensory Attributes |
|---|---|---|---|---|---|
| Aldehydes | |||||
| acetaldehyde | 3.13 | 415.49-1-A | 3.86 | 541.82-2-A | pungent, ethereal |
| 2-methyl-butanal | 6.23 | 646.08-1-A | 7.10 | 730.27-2-A | nut, caramel, sweet |
| 2-phenyl-acetaldehyde | 19.15 | 1026.76-1-A | 24.19 | 1176.08-2-A | honey, sweet, rose, herbaceous, floral |
| (e)-2-undecenal | 31.84 | 1361.38-1-A | 36.45 | 1507.84-2-A | geranium, metallic, pungent, sweet, herbaceous, fruity, fatty |
| Alcohols | |||||
| 2-methyl-1-butanol | 7.97 | 738.67-1-A | 11.16 | 849.51-2-A | malt, wine, ethereal, fusel alcohols, fatty |
| 2,3-butanediol | 8.94 | 768.28-1-A | 16.07 | 971.57-2-A | fruits |
| 2-phenyl-ethanol | 21.78 | 1088.98-1-A | 28.09 | 1279.81-2-A | floral, rose, honey, sweet, spicy |
| Ethyl esters | |||||
| ethyl butanoate | 9.86 | 796.22-1-A | 11.57 | 860.18-2-A | banana, ethereal, pineapple |
| ethyl-2-methyl-butanoate | 11.84 | 846.48-1-A | 13.51 | 909.56-2-A | green apple, plum |
| ethyl hexanoate | 17.8 | 993.12-1-A | 19.60 | 1058.80-2-A | apple, banana, wine, pineapple |
| ethyl octanoate | 25.77 | 1197.94-1-A | 27.50 | 1264.65-2-A | pear, pineapple, floral, apricot |
| ethyl decanoate | 32.97 | 1392.28-1-A | 34.70 | 1460.26-2-A | grape, pear, oily, sweet, waxy, fruity, apple, soapy, winey |
| Acetate esters | |||||
| ethyl acetate | 4.76 | 611.29-1-A | 5.71 | 675.39-2-A | ethereal, aniseed, pineapple |
| isoamyl acetate | 12.94 | 874.02-1-A | 14.81 | 941.16-2-A | banana, pear |
| cis-3-hexenyl acetate | 18.27 | 1004.67-1-A | 20.29 | 1076.04-2-A | herbaceous, banana, vegetable |
| 2-phenylethyl acetate | 27.80 | 1253.13-1A | 30.28 | 1339.07-2-A | fruity, sweet |
| Terpenes | |||||
| β-myrcene | 17.10 | 976.36-1-A | 17.85 | 1015.20-2-A | sweet, fruity, spice, woody, metallic |
| cis-β-ocimene | 19.76 | 1042.09-1-A | 22.56 | 1133.75-2-A | citrus, herbal |
| β-linalool | 22.35 | 1107.22-1-A | 24.69 | 1189.38-2-A | citrus, floral, sweet |
| nerol oxide | 23.88 | 1147.51-1-A | 30.92 | 1356.24-2-A | sweet, fruity, floral, rose |
| (e)-linalool oxide | 24.63 | 1173.31-1-A | - | - | floral |
| trans-geraniol | 27.47 | 1242.49-1-A | 31.93 | 1378.40-2-A | sweet, apple, apricot, berries, rose |
| limonen-1,2-epoxide | 23.49 | 1137.14-1-A | 25.92 | 1221.66-2-A | sweet, fruity, spicy, woody, metallic |
| α-terpinen-7-al * | - | - | 32.78 | 1407.37-2-A | fat, spice |
| Heterocyclic compounds | |||||
| abhexone ** | - | - | 33.45 | 1431.31-2-A | curry |
| 1H-indol | 28.94 | 1283.06-1-A | 37.55 | 1541.25-2-A | sweet, burnt, floral, jasmine, earthy |
| Peak Area/Compound * | ERS | ERS_oc10 | ERS_oc20 | ERS_oc30 | ERS_de10 | ERS_de20 | ERS_de30 | ERS_ocde10 | ERS_ocde15 |
|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | |||||||||
| 415.49-1 | 72,627 ± 7300 a | 72,157 ± 3424 a | 70,593 ± 1169 a | 70,331 ± 4569 a | 73,524 ± 2915 a | 72,952 ± 5438 a | 69,304 ± 3392 a | 69,955 ± 2440 a | 71,461 ± 4647 a |
| 541.82-2-A | 61,410 ± 6777 a | 60,160 ± 2272 a | 58,534 ± 1277 a | 58,911 ± 3451 a | 61,650 ± 2444 a | 61,080 ± 4198 a | 58,142 ± 2865 a | 59,126 ± 2031 a | 60,563 ± 3886 a |
| Ethyl acetate | |||||||||
| 611.29-1 | 115,742 ± 6491 a | 117,375 ± 6851 a | 120,215 ± 2789 a | 118,769 ± 5080 a | 112,220 ± 11,834 a | 118,661 ± 6686 a | 119,235 ± 4301 a | 121,163 ± 5373 a | 115,826 ± 5595 a |
| 675.39-2-A | 83,543 ± 5404 a | 83,693 ± 5752 a | 86,814 ± 1789 a | 86,370 ± 4473 a | 80,768 ± 9799 a | 85,279 ± 5306 a | 86,397 ± 3045 a | 88,513 ± 3904 a | 83,960 ± 4177 a |
| 2-Methylbutanal | |||||||||
| 646.08-1 | 572 ± 182 a | 704 ± 114 a | 637 ± 146 a | 574 ± 122 a | 562 ± 192 a | 539 ± 107 a | 529 ± 107 a | 605 ± 160 a | 624 ± 191 a |
| 730.27-2-A | 9672 ± 849 a | 9562 ± 323 a | 9376 ± 263 a | 9409 ± 342 a | 9421 ± 400 a | 9623 ± 267 a | 9354 ± 440 a | 9575 ± 410 a | 9683 ± 356 a |
| 2-Methyl-1-butanol | |||||||||
| 738.67-1 | 162,167 ± 13,867 a | 163,996 ± 6739 a | 162,758 ± 5876 a | 161,097 ± 10,036 a | 162,962 ± 7171 a | 164,218 ± 8921 a | 159,802 ± 8889 a | 161,917 ± 7062 a | 166,926 ± 6017 a |
| 849.51-2-A | 125,821 ± 10,438 a | 126,833 ± 5224 a | 126,318 ± 4719 a | 124,693 ± 7558 a | 125,635 ± 4788 a | 127,350 ± 6798 a | 124,073 ± 6921 a | 125,375 ± 4629 a | 130,309 ± 5313 a |
| 2,3-Butanediol | |||||||||
| 768.28-1 | 1841 ± 288 a | 2059 ± 158 a | 1970 ± 165 a | 2001 ± 266 a | 1949 ± 169 a | 1959 ± 275 a | 1936 ± 135 a | 1937 ± 241 a | 2069 ± 250 a |
| 971.57-2-A | 2487 ± 366 a | 2523 ± 409 a | 2252 ± 131 a | 2303 ± 286 a | 2474 ± 485 a | 2397 ± 280 a | 2289 ± 171 a | 2314 ± 176 a | 2546 ± 204 a |
| Ethyl butanoate | |||||||||
| 796.22-1 | 5879 ± 639 a | 6030 ± 337 a | 5794 ± 203 a | 5762 ± 451 a | 5865 ± 226 a | 5897 ± 531 a | 5631 ± 182 a | 5674 ± 303 a | 5890 ± 252 a |
| 860.18-2-A | 5373 ± 573 a | 5364 ± 161 a | 5146 ± 140 a | 5162 ± 259 a | 5324 ± 337 a | 5402 ± 225 a | 5066 ± 258 a | 5218 ± 272 a | 5342 ± 341 a |
| Ethyl 2-Methylbutanoate | |||||||||
| 846.48-1-A | 670 ± 247 a | 757 ± 234 a | 643 ± 157 a | 690 ± 203 a | 710 ± 183 a | 685 ± 181 a | 677 ± 102 a | 656 ± 235 a | 715 ± 160 a |
| 909.56-2-A | 705 ± 125 ab | 621 ± 61 ab | 569 ± 36 b | 597 ± 99 b | 577 ± 152 b | 661 ± 49 ab | 630 ± 79 ab | 717 ± 100 ab | 776 ± 94 a |
| Isoamyl acetate | |||||||||
| 874.02-1-A | 72,352 ± 6235 a | 72,544 ± 3861 a | 69,411 ± 2062 a | 68,603 ± 4693 a | 70,707 ± 3031 a | 72,287 ± 5827 a | 68,079 ± 3376 a | 67,802 ± 2575 a | 70,896 ± 3898 a |
| 941.16-2-A | 54,005 ± 4386 a | 53,579 ± 1888 a | 51,668 ± 1421 a | 50,819 ± 3326 a | 52,484 ± 1691 a | 53,943 ± 3714 a | 51,033 ± 3243 a | 50,889 ± 1912 a | 52,947 ± 2999 a |
| β-Myrcene | |||||||||
| 976.36-1-A | 761 ± 0 a | 657 ± 120 a | 608 ± 96 a | 550 ± 62 a | 668 ± 75 a | 549 ± 18 a | 573 ± 82 a | 542 ± 168 a | 655 ± 76 a |
| 1015.20-2-A | 1128 ± 326 a | 979 ± 213 a | 894 ± 86 a | 941 ± 168 a | 1005 ± 266 a | 1016 ± 120 a | 1012 ± 170 a | 1092 ± 199 a | 1179 ± 207 a |
| Ethyl hexanoate | |||||||||
| 993.12-1-A | 41,346 ± 5969 a | 38,497 ± 3382 a | 36,251 ± 972 a | 36,564 ± 4520 a | 38,987 ± 4042 a | 39,295 ± 5302 a | 35,670 ± 2238 a | 35,523 ± 1519 a | 38,586 ± 3583 a |
| 1058.80-2-A | 30,982 ± 3963 a | 28,826 ± 2273 a | 27,234 ± 656 a | 27,375 ± 3178 a | 29,089 ± 3001 a | 29,164 ± 3698 a | 26,698 ± 1769 a | 26,649 ± 1052 a | 28,876 ± 2681 a |
| Cis-3-hexenyl acetate | |||||||||
| 1004.67-1-A | 8155 ± 1261 a | 8042 ± 768 a | 7476 ± 199 a | 7486 ± 1001 a | 8096 ± 855 a | 8027 ± 1104 a | 7234 ± 504 a | 7209 ± 473 a | 7945 ± 665 a |
| 1076.04-2-A | 7695 ± 1166 a | 7280 ± 593 a | 6801 ± 176 a | 6862 ± 853 a | 7343 ± 842 a | 7324 ± 840 a | 6709 ± 466 a | 6806 ± 376 a | 7386 ± 719 a |
| 2-Phenylacetaldehyde | |||||||||
| 1026.76-1-A | 1634 ± 378 a | 1773 ± 229 a | 1599 ± 180 a | 1609 ± 261 a | 1767 ± 131 a | 1713 ± 179 a | 1615 ± 147 a | 1585 ± 272 a | 1724 ± 162 a |
| 1176.08-2-A | 288 ± 104 a | 200 ± 91 a | 166 ± 37 a | 142 ± 72 a | 224 ± 119 a | 183 ± 43 a | 203 ± 68 a | 216 ± 87 a | 236 ± 91 a |
| cis-β-Ocimene | |||||||||
| 1042.09-1-A | 644 ± 191 a | 765 ± 173 a | 676 ± 137 a | 692 ± 166 a | 758 ± 87 a | 719 ± 132 a | 707 ± 102 a | 714 ± 170 a | 782 ± 88 a |
| 1,133.75-2-A | 562 ± 113 a | 386 ± 119 ab | 351 ± 38 b | 324 ± 73 b | 368 ± 150 b | 353 ± 84 b | 392 ± 78 ab | 422 ± 95 ab | 450 ± 81 ab |
| 2-Phenylethanol | |||||||||
| 1088.98-1-A | 1057 ± 415 a | 1425 ± 197 a | 1266 ± 180 a | 1283 ± 225 a | 1357 ± 115 a | 1237 ± 152 a | 1196 ± 130 a | 1219 ± 225 a | 1303 ± 107 a |
| 1279.81-2-A | 1670 ± 254 a | 1656 ± 198 a | 1639 ± 101 a | 1351 ± 663 a | 1544 ± 265 a | 1416 ± 185 a | 1382 ± 155 a | 1528 ± 137 a | 1791 ± 189 a |
| β-Linalool | |||||||||
| 1107.22-1-A | 1959 ± 995 a | 2255 ± 292 a | 2013 ± 138 a | 1938 ± 64 a | 2187 ± 254 a | 2044 ± 360 a | 1940 ± 227 a | 1961 ± 292 a | 2362 ± 333 a |
| 1189.38-2-A | 1577 ± 295 a | 1393 ± 247 a | 1377 ± 149 a | 1292 ± 125 a | 1279 ± 263 a | 1193 ± 109 a | 1229 ± 154 a | 1357 ± 216 a | 1396 ± 213 a |
| Limonene-1,2-epoxide | |||||||||
| 1137.14-1-A | 226 ± 137 a | 292 ± 58 a | 220 ± 59 a | 166 ± 15 a | 281 ± 53 a | 227 ± 49 a | 227 ± 52 a | 198 ± 115 a | 230 ± 73 a |
| 1221.66-2-A | 0 ± 0 a | 133 ± 52 a | 88 ± 16 a | 106 ± 39 a | 143 ± 53 a | 135 ± 43 a | 52 ± 0 a | 142 ± 36 a | 136 ± 62 a |
| Nerol oxide | |||||||||
| 1147.51-1-A | 455 ± 179 a | 536 ± 131 a | 491 ± 108 a | 486 ± 60 a | 467 ± 99 a | 423 ± 53 a | 480 ± 97 a | 476 ± 198 a | 557 ± 109 a |
| 1356.24-2-A | 196 ± 118 a | 165 ± 65 a | 124 ± 27 a | 170 ± 54 a | 166 ± 58 a | 223 ± 27 a | 181 ± 63 a | 169 ± 79 a | 218 ± 78 a |
| (E)-Linalool oxide | |||||||||
| 1173.31-1-A | 980 ± 309 cd | 804 ± 177 cde | 378 ± 80 ef | 315 ± 75 f | 1529 ± 211 ab | 1147 ± 444 bc | 1884 ± 185 a | 539 ± 337 def | 366 ± 49 ef |
| Ethyl octanoate | |||||||||
| 1197.94-1-A | 71,285 ± 13,666 d | 140,537 ± 18,627 c | 183,934 ± 5719 b | 245,027 ± 43,655 a | 73,573 ± 10,399 d | 71,003 ± 12,528 d | 64,707 ± 5177 d | 121,240 ± 6544 c | 143,604 ± 16,296 c |
| 1264.65-2-A | 53,094 ± 10,103 d | 102,170 ± 12,833 c | 132,385 ± 3664 b | 177,575 ± 33,812 a | 54,366 ± 7752 d | 51,881 ± 8292 d | 48,002 ± 3589 d | 87,525 ± 4553 c | 103,766 ± 12,322 c |
| trans-Geraniol | |||||||||
| 1242.49-1-A | 240 ± 185 b | 461 ± 93 ab | 488 ± 87 a | 431 ± 133 ab | 428 ± 63 ab | 367 ± 66 ab | 407 ± 76 ab | 359 ± 198 ab | 445 ± 90 ab |
| 1378.40-2-A | 639 ± 79 a | 425 ± 150 b | 404 ± 96 b | 336 ± 131 b | 453 ± 135 ab | 345 ± 61 b | 396 ± 77 b | 398 ± 112 b | 432 ± 93 b |
| 2-Phenylethyl acetate | |||||||||
| 1253.13-1-A | 1194 ± 708 b | 1889 ± 225 a | 1847 ± 199 ab | 1634 ± 293 ab | 2069 ± 321 a | 1691 ± 188 ab | 1705 ± 154 ab | 1504 ± 540 ab | 1762 ± 150 ab |
| 1339.07-2-A | 1067 ± 220 ab | 885 ± 271 b | 1095 ± 154 ab | 1291 ± 446 ab | 1256 ± 347 ab | 1345 ± 305 ab | 1534 ± 195 a | 1168 ± 178 ab | 1411 ± 213 a |
| 1H-indole | |||||||||
| 1283.06-1-A | 380 ± 298 b | 1191 ± 410 a | 1159 ± 277 a | 886 ± 214 ab | 1129 ± 324 a | 1176 ± 241 a | 1129 ± 186 a | 825 ± 495 ab | 1169 ± 257 a |
| 1541.25-2-A | 587 ± 173 cd | 301 ± 278 d | 702 ± 249 bcd | 759 ± 348 abc | 725 ± 162 abcd | 777 ± 163 abc | 1134 ± 154 a | 795 ± 251 abc | 1125 ± 209 ab |
| (E)-2-Undecenal | |||||||||
| 1361.38-1-A | 169 ± 149 d | 296 ± 125 cd | 452 ± 119 bc | 531 ± 225 bc | 373 ± 96 cd | 493 ± 121 bc | 673 ± 91 ab | 552 ± 200 bc | 837 ± 55 a |
| 1507.84-2-A | 614 ± 94 ab | 521 ± 218 ab | 656 ± 148 a | 729 ± 264 a | 518 ± 120 ab | 351 ± 125 b | 457 ± 100 ab | 575 ± 167 ab | 675 ± 143 a |
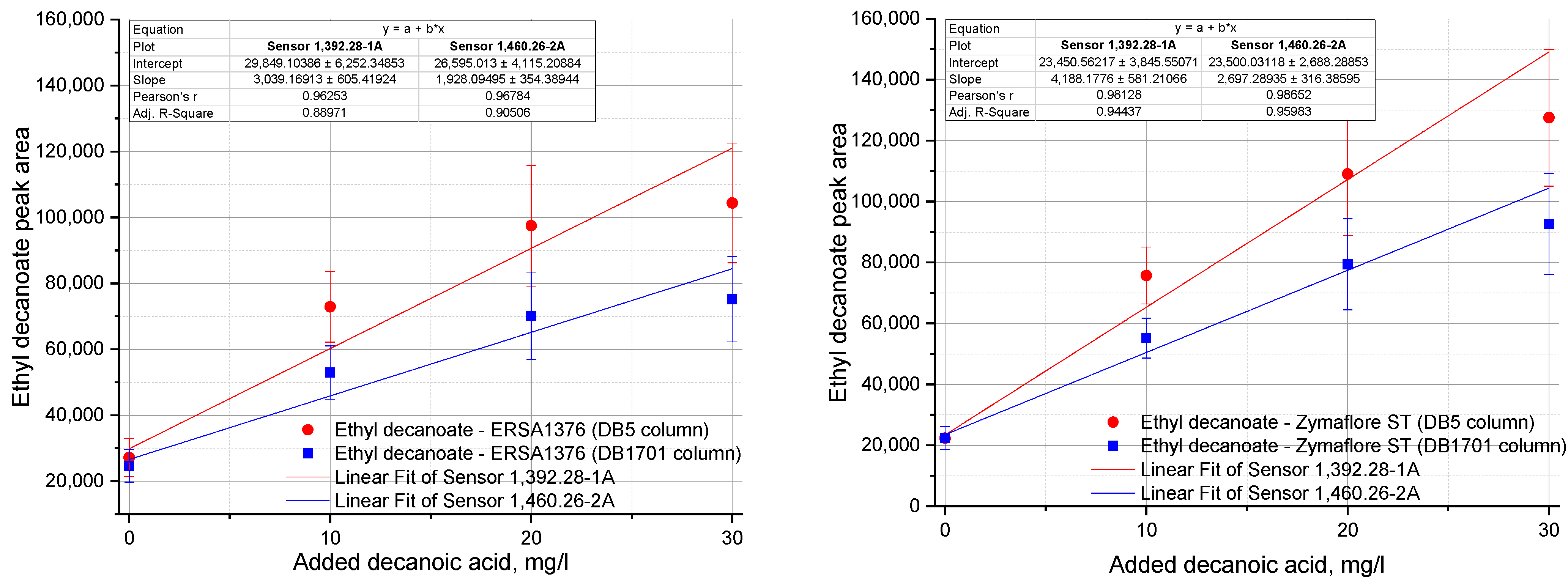
| Ethyl decanoate | |||||||||
| 1392.28-1-A | 27,190 ± 5754 c | 28,524 ± 7012 c | 26,090 ± 2906 c | 29,330 ± 9233 c | 72,926 ± 10,727 b | 97,502 ± 18,319 a | 104,400 ± 18,163 a | 65,583 ± 6806 b | 72,961 ± 9533 B |
| 1460.26-2-A | 24,616 ± 4933 c | 23,995 ± 4034 c | 22,721 ± 2217 c | 24,617 ± 4572 c | 52,950 ± 8103 b | 70,150 ± 13,245 a | 75,229 ± 12,953 a | 47,774 ± 4823 b | 52,972 ± 7148 b |
| α.-Terpinen-7-al | |||||||||
| 1407.37-2-A | 385 ± 103 a | 225 ± 105 ab | 231 ± 86 ab | 185 ± 129 b | 224 ± 80 ab | 164 ± 64 b | 201 ± 66 b | 202 ± 94 b | 237 ± 76 ab |
| Abhexone | |||||||||
| 1431.31-2-A | 709 ± 124 a | 393 ± 215 b | 386 ± 162 b | 227 ± 185 b | 468 ± 155 ab | 241 ± 91 b | 310 ± 130 b | 342 ± 137 b | 395 ± 131 b |
| Peak Area/Compound * | ST | ST_oc10 | ST_oc20 | ST_oc30 | ST_de10 | ST_de20 | ST_de30 | ST_ocde10 | ST_ocde15 |
|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | |||||||||
| 415.49-1 | 66,323 ± 5163 a | 69,854 ± 5079 a | 69,884 ± 6581 a | 71,488 ± 3658 a | 71,125 ± 4688 a | 71,761 ± 4787 a | 69,472 ± 2697 a | 71,321 ± 3019 a | 70,153 ± 1367 a |
| 541.82-2-A | 56,199 ± 4107 a | 59,623 ± 4374 a | 60,106 ± 5730 a | 61,349 ± 3315 a | 60,388 ± 3837 a | 61,488 ± 3592 a | 59,228 ± 3224 a | 61,118 ± 3158 a | 60,523 ± 850 a |
| Ethyl acetate | |||||||||
| 611.29-1 | 111,999 ± 10,461 a | 110,119 ± 5613 a | 113,674 ± 5694 a | 114,301 ± 4325 a | 105,371 ± 11,220 a | 113,362 ± 4425 a | 115,287 ± 3848 a | 111,644 ± 7355 a | 110,074 ± 5497 a |
| 675.39-2-A | 84,110 ± 7345 a | 82,734 ± 4649 a | 85,688 ± 4527 a | 86,356 ± 3276 a | 77,796 ± 9180 a | 85,257 ± 3574 a | 86,628 ± 3142 a | 83,783 ± 5798 a | 83,191 ± 4389 a |
| 2-Methylbutanal | |||||||||
| 646.08-1 | 426 ± 163 a | 500 ± 104 a | 427 ± 81 a | 528 ± 152 a | 614 ± 109 a | 522 ± 144 a | 670 ± 127 a | 515 ± 181 a | 553 ± 187 a |
| 730.27-2-A | 7644 ± 544 a | 7941 ± 358 a | 7936 ± 568 a | 8091 ± 414 a | 7974 ± 355 a | 8165 ± 234 a | 8025 ± 501 a | 8046 ± 205 a | 7950 ± 281 a |
| 2-Methyl-1-butanol | |||||||||
| 738.67-1 | 147,793 ± 10,556 a | 154,833 ± 10,939 a | 148,307 ± 11,337 a | 152,818 ± 10,351 a | 155,016 ± 8510 a | 156,039 ± 9457 a | 155,245 ± 7482 a | 154,468 ± 6279 a | 152,308 ± 3550 a |
| 849.51-2-A | 116,655 ± 7801 a | 121,411 ± 8786 a | 117,431 ± 9029 a | 120,754 ± 8438 a | 121,178 ± 7639 a | 123,167 ± 7374 a | 123,206 ± 7051 a | 122,724 ± 4501 a | 120,511 ± 2890 a |
| 2,3-Butanediol | |||||||||
| 768.28-1 | 1626 ± 231 a | 1745 ± 161 a | 1651 ± 148 a | 1781 ± 136 a | 1836 ± 71 a | 1829 ± 217 a | 1916 ± 121 a | 1814 ± 185 a | 1971 ± 252 a |
| 971.57-2-A | 2181 ± 182 a | 2320 ± 282 a | 2190 ± 312 a | 2208 ± 256 a | 2362 ± 266 a | 2285 ± 233 a | 2201 ± 233 a | 2296 ± 237 a | 2270 ± 58 a |
| Ethyl butanoate | |||||||||
| 796.22-1 | 5518 ± 420 a | 5846 ± 450 a | 5642 ± 432 a | 5843 ± 420 a | 5726 ± 348 a | 5891 ± 445 a | 5841 ± 211 a | 5810 ± 267 a | 5519 ± 240 a |
| 860.18-2-A | 5121 ± 348 a | 5327 ± 327 a | 5260 ± 446 a | 5336 ± 343 a | 5267 ± 423 a | 5426 ± 237 a | 5301 ± 339 a | 5378 ± 291 a | 5129 ± 241 a |
| Ethyl 2-Methylbutanoate | |||||||||
| 846.48-1-A | 721 ± 76 a | 746 ± 38 a | 737 ± 47 a | 830 ± 127 a | 827 ± 142 a | 799 ± 123 a | 879 ± 159 a | 790 ± 125 a | 831 ± 160 a |
| 909.56-2-A | 783 ± 96 a | 762 ± 70 a | 813 ± 106 a | 828 ± 96 a | 835 ± 151 a | 849 ± 50 a | 824 ± 134 a | 859 ± 88 a | 904 ± 83 a |
| Isoamyl acetate | |||||||||
| 874.02-1-A | 75,984 ± 4944 a | 79,100 ± 7034 a | 75,201 ± 6669 a | 77,132 ± 7160 a | 76,972 ± 4478 a | 78,791 ± 5628 a | 76,919 ± 3660 a | 77,859 ± 4546 a | 70,454 ± 2678 a |
| 941.16-2-A | 57,751 ± 3825 a | 59,939 ± 5119 a | 57,272 ± 4964 a | 58,625 ± 5438 a | 58,085 ± 3684 a | 60,121 ± 4857 a | 58,360 ± 2772 a | 58,877 ± 3438 a | 53,536 ± 2080 a |
| β-Myrcene | |||||||||
| 976.36-1-A | 658 ± 0 a | 636 ± 68 a | 579 ± 78 a | 637 ± 93 a | 683 ± 98 a | 627 ± 78 a | 640 ± 1 10 a | 635 ± 81 a | 585 ± 148 a |
| 1015.20-2-A | 1125 ± 233 a | 1208 ± 125 a | 1166 ± 245 a | 1137 ± 140 a | 1217 ± 211 a | 1190 ± 111 a | 1095 ± 241 a | 1213 ± 138 a | 1136 ± 170 a |
| Ethyl hexanoate | |||||||||
| 1058.80-2-A | 24,550 ± 1882 a | 25,678 ± 3297 a | 24,034 ± 3239 a | 24,866 ± 3023 a | 25,317 ± 2067 a | 25,298 ± 2618 a | 24,217 ± 1643 a | 25,377 ± 2403 a | 21,972 ± 832 a |
| Cis-3-hexenyl acetate | |||||||||
| 1004.67-1-A | 7672 ± 709 a | 8115 ± 1084 a | 7512 ± 999 a | 7737 ± 1022 a | 7975 ± 656 a | 7832 ± 903 a | 7453 ± 519 a | 7772 ± 662 a | 6608 ± 363 a |
| 1076.04-2-A | 7185 ± 653 a | 7568 ± 941 a | 7037 ± 1004 a | 7243 ± 868 a | 7469 ± 700 a | 7381 ± 751 a | 7019 ± 652 a | 7441 ± 722 a | 6432 ± 315 a |
| 2-Phenylacetaldehyde | |||||||||
| 1026.76-1-A | 1840 ± 249 ab | 1891 ± 104 a | 1710 ± 157 ab | 1767 ± 186 ab | 1781 ± 148 ab | 1828 ± 152 ab | 1757 ± 207 ab | 1768 ± 127 ab | 1515 ± 216 b |
| 1176.08-2-A | 207 ± 84 a | 232 ± 51 a | 209 ± 74 a | 188 ± 74 a | 251 ± 89 a | 234 ± 59 a | 208 ± 105 a | 245 ± 59 a | 213 ± 72 a |
| cis-β-Ocimene | |||||||||
| 1042.09-1-A | 739 ± 150 a | 835 ± 52 a | 750 ± 114 a | 796 ± 92 a | 816 ± 103 a | 814 ± 82 a | 804 ± 137 a | 802 ± 89 a | 701 ± 114 a |
| 1133.75-2-A | 400 ± 85 b | 391 ± 43 b | 417 ± 66 ab | 402 ± 93 b | 448 ± 88 ab | 445 ± 51 ab | 444 ± 113 ab | 486 ± 57 ab | 560 ± 120 a |
| 2-Phenylethanol | |||||||||
| 1088.98-1-A | 1193 ± 128 a | 1325 ± 59 a | 1306 ± 154 a | 1388 ± 133 a | 1284 ± 127 a | 1272 ± 112 a | 1255 ± 156 a | 1238 ± 121 a | 1188 ± 216 a |
| 1279.81-2-A | 1630 ± 173 a | 1941 ± 326 a | 186 9 ± 386 a | 2030 ± 305 a | 1718 ± 211 a | 1648 ± 238 a | 1577 ± 334 a | 1934 ± 224 a | 2025 ± 339 a |
| β-Linalool | |||||||||
| 1107.22-1-A | 2328 ± 249 ab | 2432 ± 305 ab | 2313 ± 366 ab | 2175 ± 385 b | 2347 ± 240 ab | 2274 ± 305 ab | 2242 ± 361 ab | 2486 ± 330 ab | 2857 ± 388 a |
| 1189.38-2-A | 1120 ± 184 a | 1315 ± 121 a | 1324 ± 143 a | 1285 ± 308 a | 1284 ± 216 a | 1258 ± 154 a | 1153 ± 235 a | 1366 ± 136 a | 1403 ± 180 a |
| Limonene-1,2-epoxide | |||||||||
| 1137.14-1-A | 211 ± 77 a | 234 ± 18 a | 188 ± 46 a | 176 ± 49 a | 240 ± 54 a | 210 ± 64 a | 230 ± 89 a | 210 ± 58 a | 182 ± 77 a |
| 1221.66-2-A | 0 ± 0 - | 121 ± 49 - | 102 ± 51 - | 161 ± 60 - | 98 ± 35 - | 117 ± 50 - | 145 ± 0 - | 153 ± 51 - | 149 ± 52 - |
| Nerol oxide | |||||||||
| 1147.51-1-A | 491 ± 112 a | 497 ± 36 a | 538 ± 71 a | 623 ± 82 a | 565 ± 67 a | 551 ± 93 a | 634 ± 133 a | 620 ± 92 a | 713 ± 274 a |
| 1356.24-2-A | 262 ± 96 a | 155 ± 42 a | 141 ± 64 a | 145 ± 96 a | 0 ± 0 a | 187 ± 57 a | 204 ± 78 a | 223 ± 36 a | 250 ± 71 a |
| (E)-Linalool oxide | |||||||||
| 1173.31-1-A | 1296 ± 230 a | 723 ± 336 b | 302 ± 33 b | 313 ± 65 b | 1304 ± 186 a | 1523 ± 288 a | 1742 ± 199 a | 599 ± 470 b | 306 ± 50 b |
| Ethyl octanoate | |||||||||
| 1197.94-1-A | 53,385 ± 5333 d | 129,918 ± 2183 c | 175,314 ± 33,106 b | 243,330 ± 30,130 a | 60,200 ± 6905 d | 57,131 ± 8451 d | 53,848 ± 5173 d | 123,993 ± 15,280 c | 137,807 ± 3202 c |
| 1264.65-2-A | 40,095 ± 4020 d | 94,435 ± 15,863 c | 128,602 ± 24,295 b | 178,860 ± 22,634 a | 44,669 ± 5099 d | 42,868 ± 6192 d | 40,595 ± 3980 d | 91,417 ± 11,194 c | 102,126 ± 3520 c |
| trans-Geraniol | |||||||||
| 1242.49-1-A | 293 ± 176 a | 352 ± 19 a | 347 ± 56 a | 388 ± 106 a | 371 ± 147 a | 304 ± 61 a | 357 ± 96 a | 380 ± 53 a | 373 ± 152 a |
| 1378.40-2-A | 447 ± 108 a | 509 ± 83 a | 444 ± 102 a | 447 ± 126 a | 446 ± 75 a | 395 ± 101 a | 364 ± 130 a | 476 ± 52 a | 465 ± 68 a |
| 2-Phenylethyl acetate | |||||||||
| 1253.13-1-A | 1263 ± 210 b | 1616 ± 96 ab | 1506 ± 141 ab | 1572 ± 237 ab | 1593 ± 204 ab | 1555 ± 123 ab | 1630 ± 208 a | 1626 ± 124 a | 1482 ± 270 ab |
| 1339.07-2-A | 1248 ± 210 a | 947 ± 159 a | 1022 ± 262 a | 1315 ± 440 a | 1094 ± 116 a | 1260 ± 270 a | 1408 ± 311 a | 1288 ± 183 a | 1294 ± 205 a |
| 1H-indole | |||||||||
| 1283.06-1-A | 510 ± 195 b | 973 ± 196 ab | 839 ± 273 ab | 1048 ± 357 ab | 971 ± 253 ab | 1146 ± 466 a | 1479 ± 525 a | 1375 ± 308 a | 1273 ± 453 a |
| 1541.25-2-A | 684 ± 107 c | 619 ± 196 c | 577 ± 191 c | 748 ± 137 abc | 682 ± 140 c | 726 ± 190 bc | 802 ± 269 abc | 1027 ± 117 ab | 1069 ± 156 a |
| (E)-2-Undecenal | |||||||||
| 1361.38-1-A | 572 ± 156 ab | 437 ± 44 b | 373 ± 127 b | 524 ± 159 b | 416 ± 131 b | 446 ± 93 b | 617 ± 187 ab | 737 ± 155 a | 735 ± 139 a |
| 1507.84-2-A | 395 ± 126 cd | 580 ± 134 bcd | 658 ± 145 ab | 859 ± 143 a | 360 ± 144 d | 393 ± 156 cd | 359 ± 179 d | 638 ± 39 abc | 768 ± 72 ab |
| Ethyl decanoate | |||||||||
| 1392.28-1-A | 22,344 ± 3694 c | 24,711 ± 4347 c | 23,607 ± 4337 c | 25,987 ± 1720 c | 75,717 ± 9326 b | 109,065 ± 20,283 a | 127,502 ± 22,464 a | 74,607 ± 9252 b | 83,732 ± 4696 b |
| 1460.26-2-A | 22,424 ± 3758 c | 24,842 ± 4229 c | 23,574 ± 4248 c | 25,518 ± 1808 c | 55,160 ± 6529 b | 79,389 ± 14,933 a | 92,663 ± 16,640 a | 54,676 ± 6636 b | 61,401 ± 3125 b |
| α-Terpinen-7-al | |||||||||
| 1407.37-2-A | 178 ± 130 a | 163 ± 46 a | 195 ± 24 a | 189 ± 91 a | 150 ± 23 a | 163 ± 34 a | 190 ± 46 a | 199 ± 47 a | 213 ± 55 a |
| Abhexone | |||||||||
| 1431.31-2-A | 472 ± 218 a | 464 ± 133 a | 345 ± 125 a | 368 ± 158 a | 402 ± 158 a | 306 ± 161 a | 229 ± 173 a | 434 ± 61 a | 405 ± 77 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baniţă, C.; Antoce, O.A.; Cojocaru, G.A. Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors 2023, 11, 98. https://doi.org/10.3390/chemosensors11020098
Baniţă C, Antoce OA, Cojocaru GA. Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors. 2023; 11(2):98. https://doi.org/10.3390/chemosensors11020098
Chicago/Turabian StyleBaniţă, Cornel, Oana Arina Antoce, and George Adrian Cojocaru. 2023. "Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines" Chemosensors 11, no. 2: 98. https://doi.org/10.3390/chemosensors11020098
APA StyleBaniţă, C., Antoce, O. A., & Cojocaru, G. A. (2023). Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors, 11(2), 98. https://doi.org/10.3390/chemosensors11020098







