A Silver Nanoparticles-Based Selective and Sensitive Colorimetric Assay for Ciprofloxacin in Biological, Environmental, and Commercial Samples
Abstract
1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. Preparation of PEI-AgNPs
2.3. Applicability of PEI-AgNPs as a Colorimetric Sensing Material for CIP
3. Results and Discussion
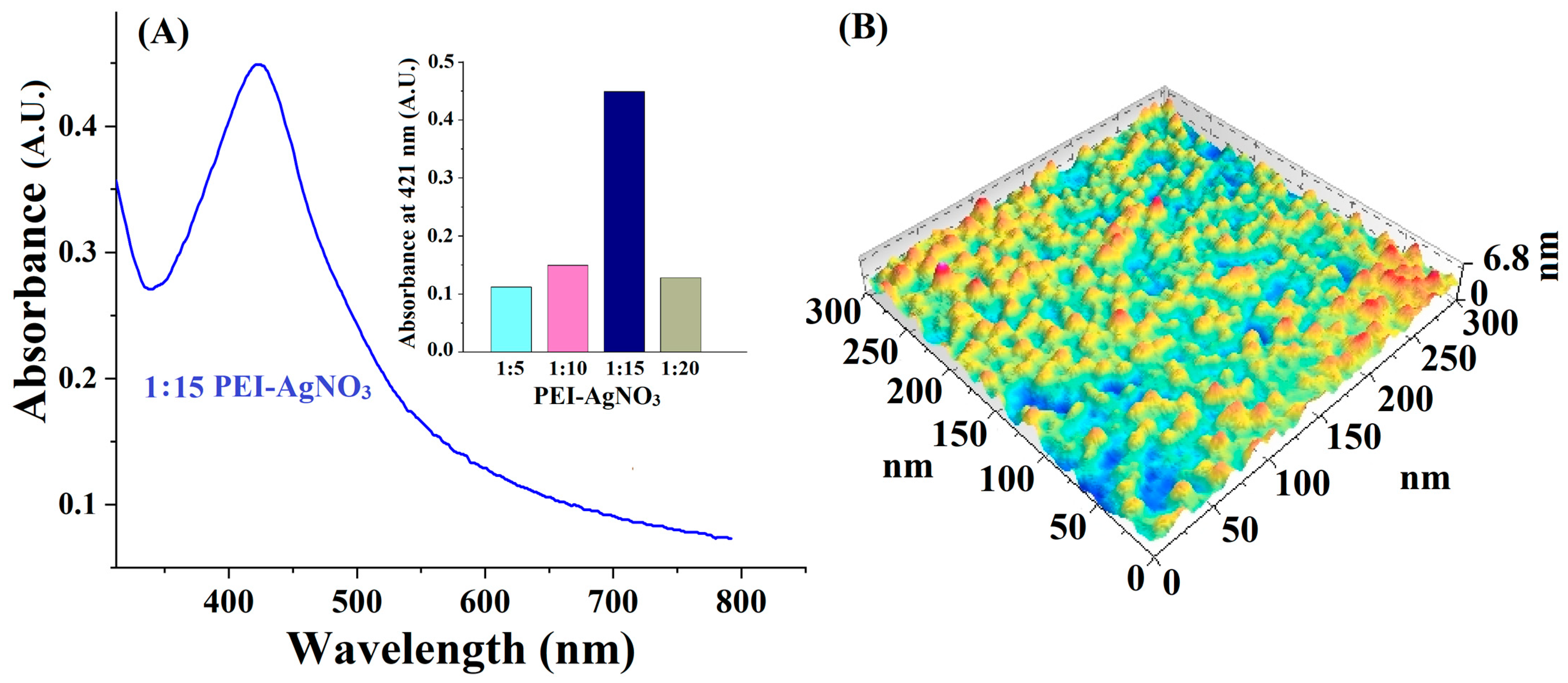
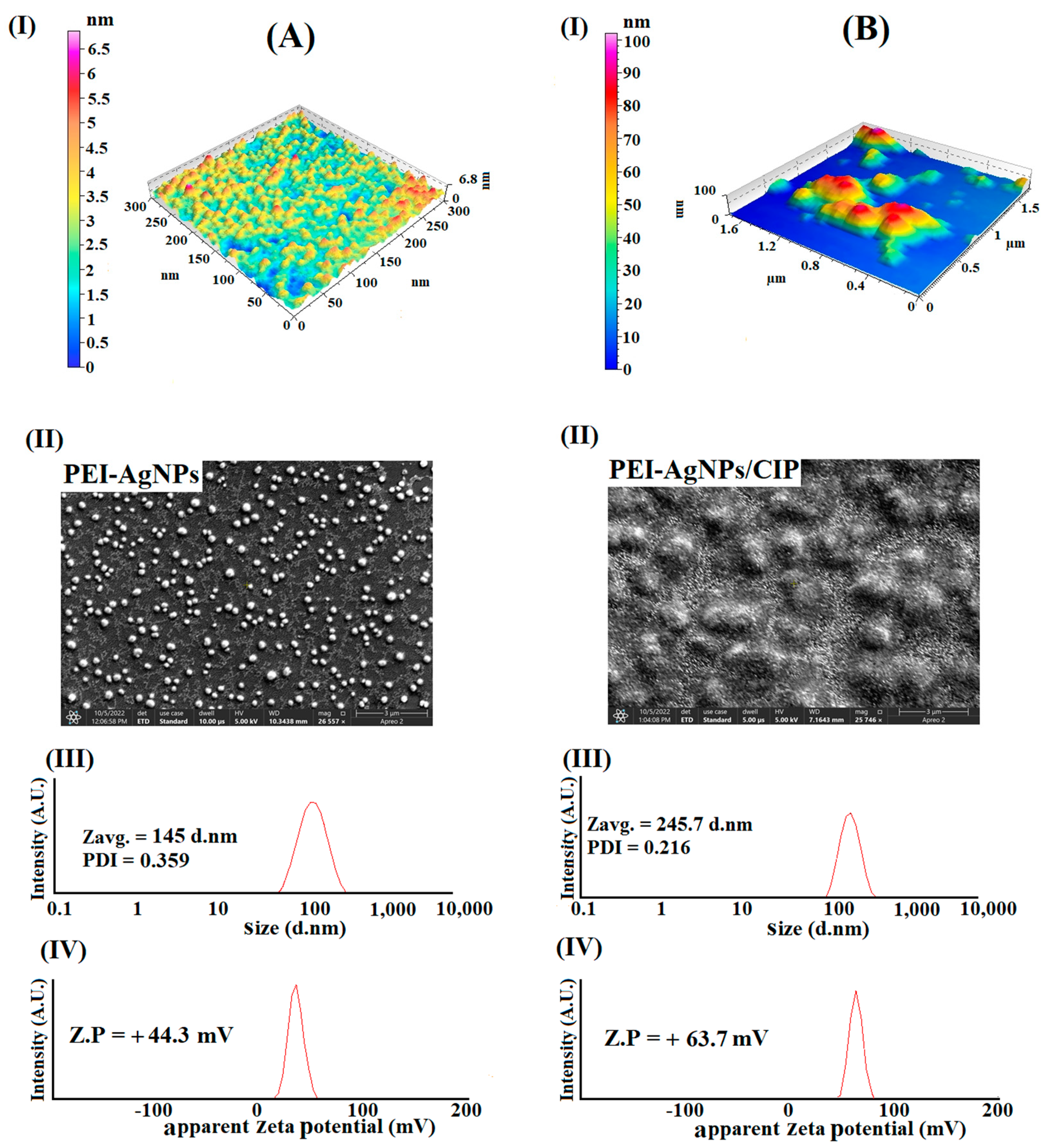
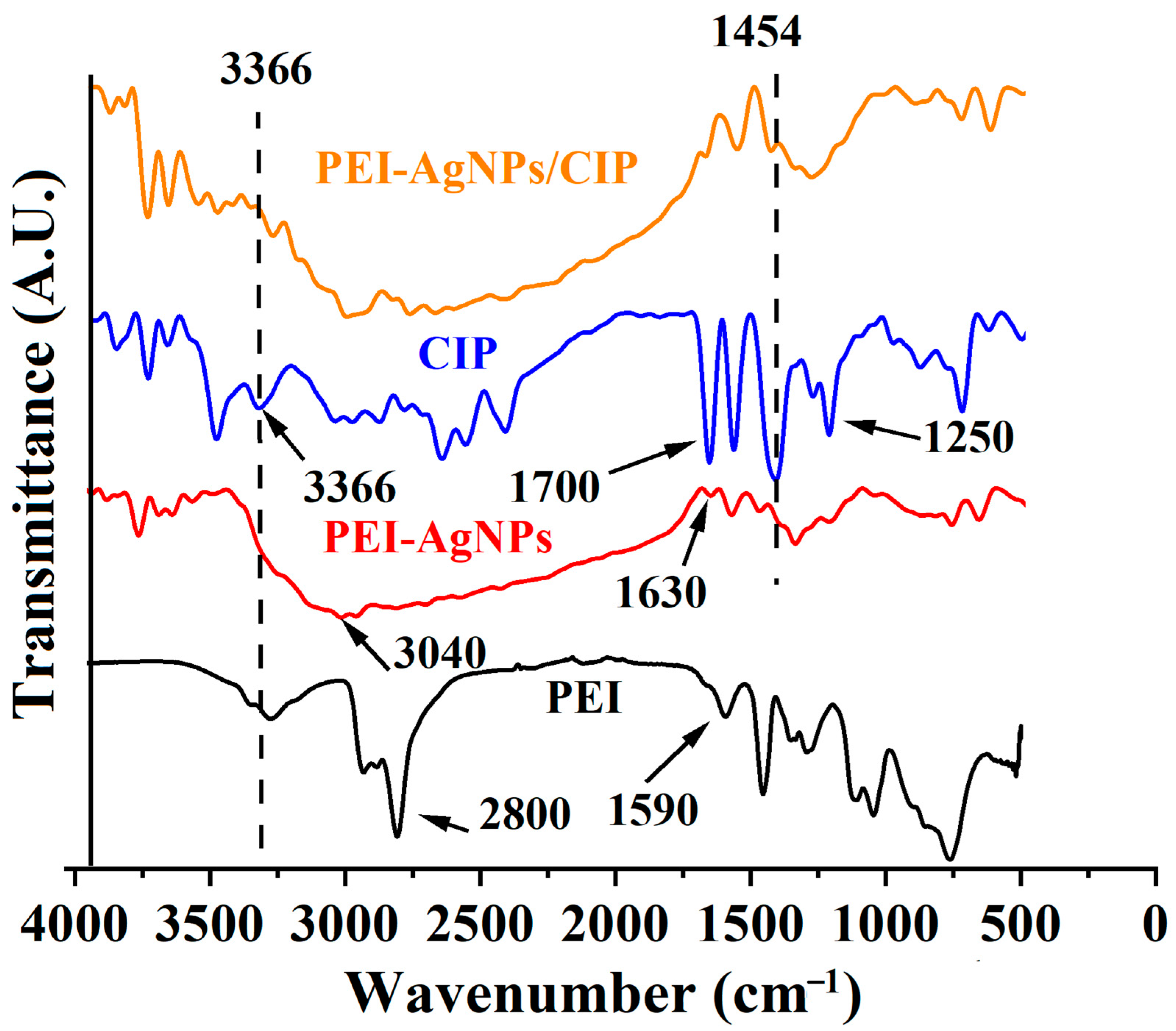
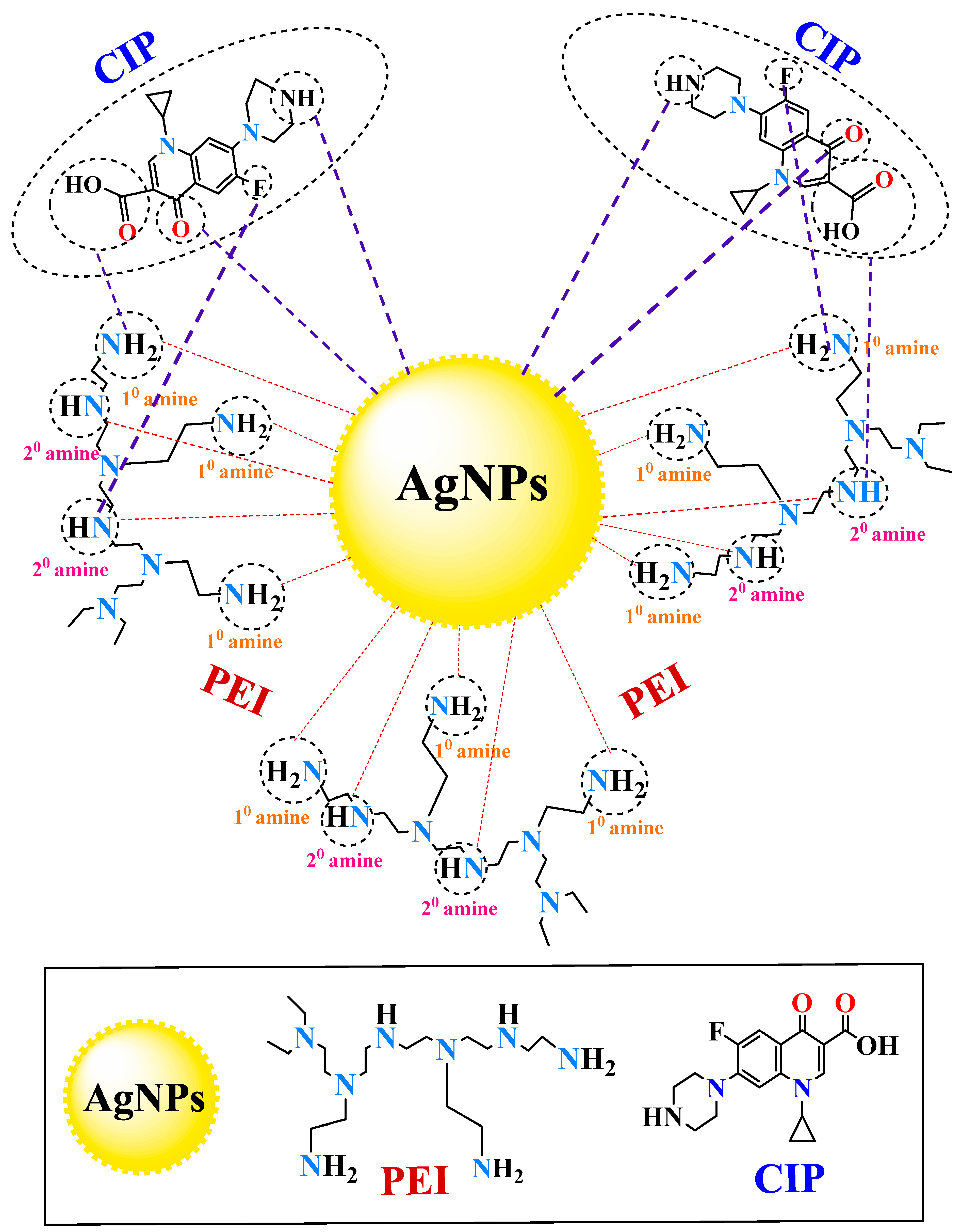
3.1. Preparation and Characterization of PEI-AgNPs
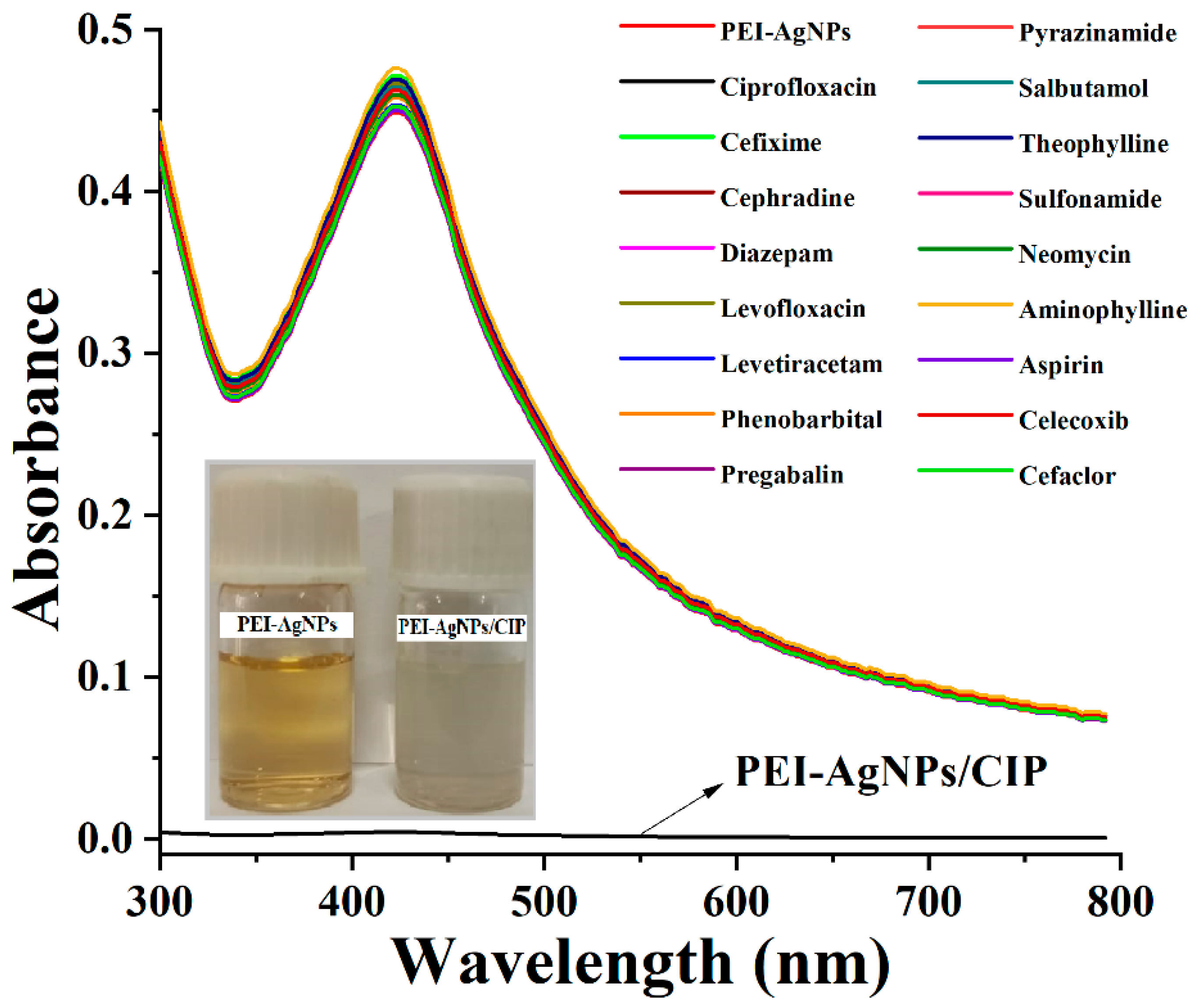
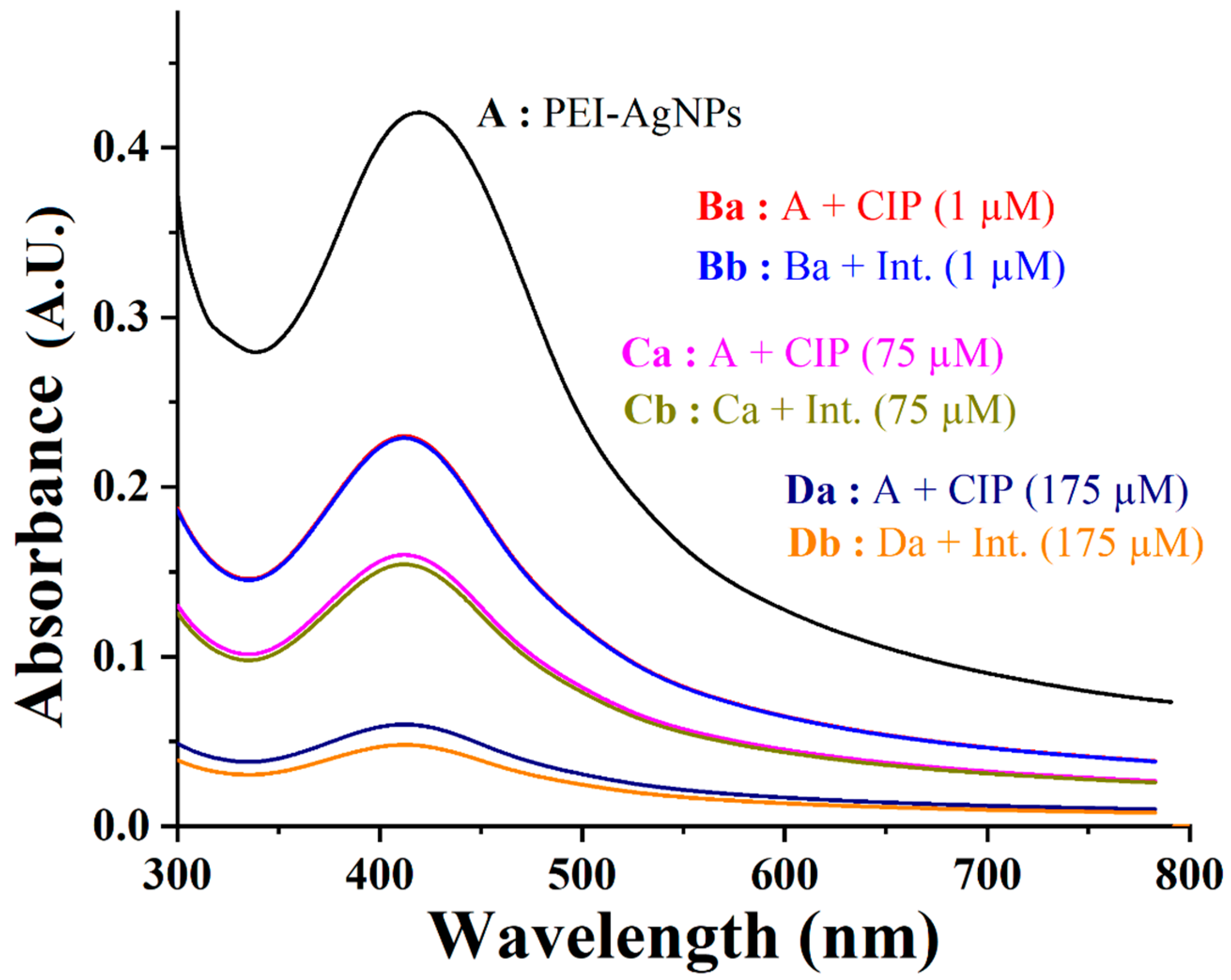
3.2. PEI-AgNPs as a Colorimetric Sensing Material
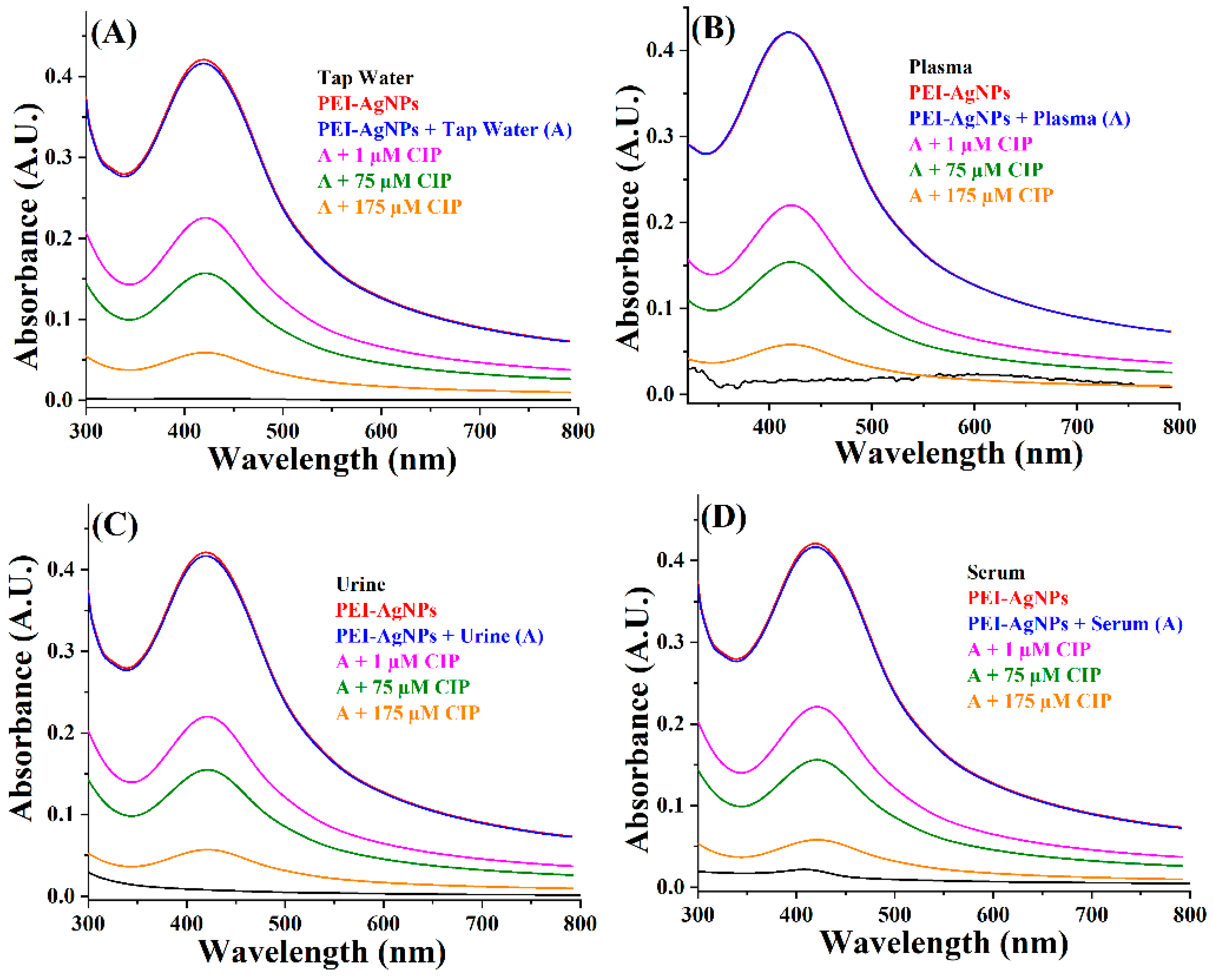
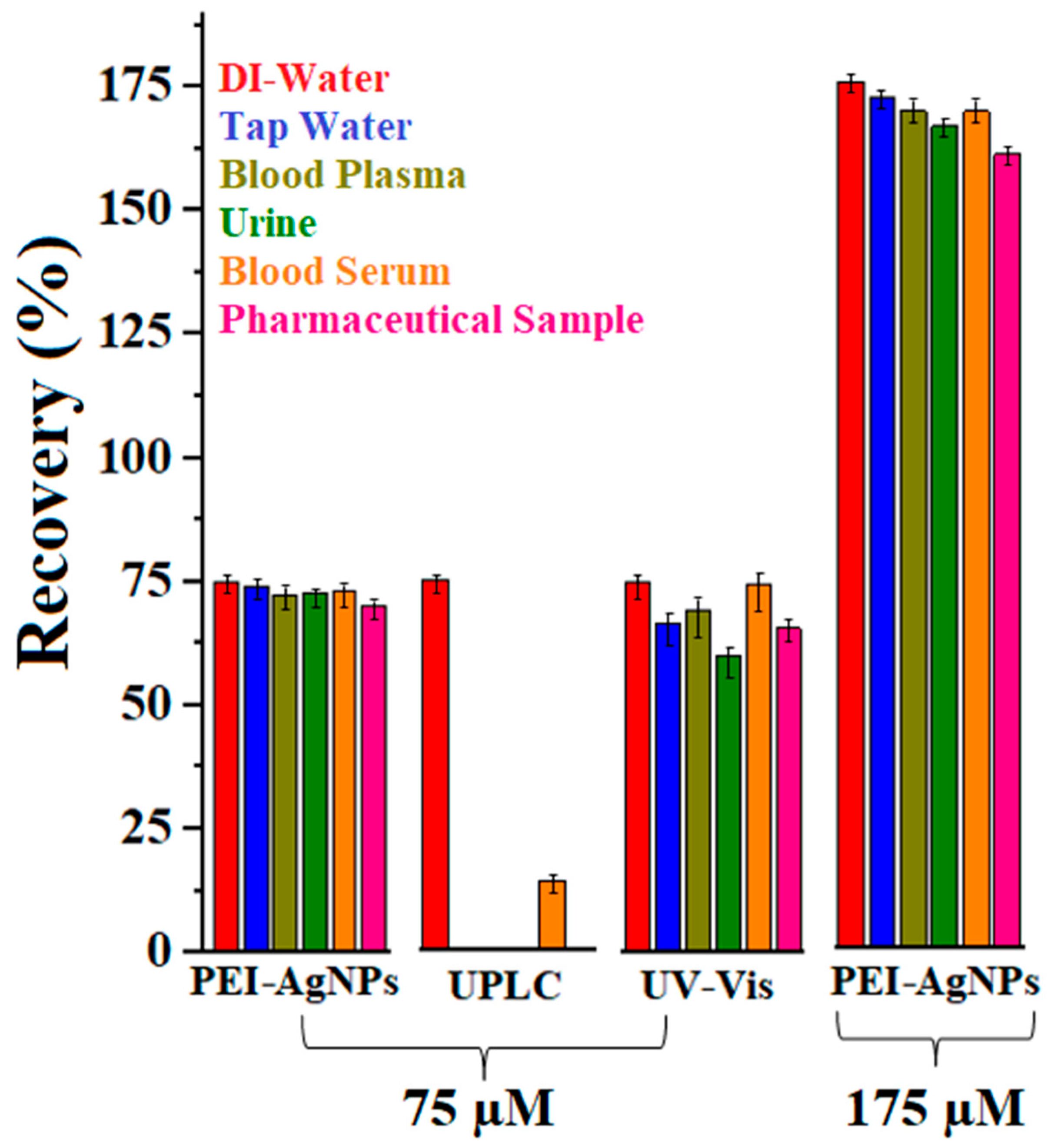
3.3. PEI-AgNPs as Ciprofloxacin Sensing Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Zhang, R.; Li, J.; Cheng, Z.; Luo, C.; Wang, Y.; Yu, K.; Zhang, G. Occurrence and distribution of antibiotics in multiple environmental media of the East River (Dongjiang) catchment, South China. Environ. Sci. Pollut. Res. 2017, 24, 9690–9701. [Google Scholar]
- Paton, J.; Reeves, D. Fluoroquinolone antibiotics. Drugs 1988, 36, 193–228. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Reguera, M.I.; Parras, G.P.; Díaz, A.M. Solid-phase UV spectrophotometric method for determination of ciprofloxacin. Microchem. J. 2004, 77, 79–84. [Google Scholar]
- Meyerhoff, A.; Albrecht, R.; Meyer, J.M.; Dionne, P.; Higgins, K.; Murphy, D. US Food and Drug Administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. Clin. Infect. Dis. 2004, 39, 303–308. [Google Scholar] [PubMed]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enzym. Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar]
- Castro, W.; Navarro, M.; Biot, C. Medicinal potential of ciprofloxacin and its derivatives. Future Med. Chem. 2013, 5, 81–96. [Google Scholar]
- Cao, X.-L.; Zhang, Q.-H.; Pan, X.-H.; Chen, Z.; Lü, J. Mechanochemical synthesis of nano–ciprofloxacin with enhanced antibacterial activity. Inorg. Chem. Commun. 2019, 102, 66–69. [Google Scholar]
- Sha, J.; Lin, H.; Timira, V.; Sui, J. The Construction and Application of Aptamer to Simultaneous Identification of Enrofloxacin and Ciprofloxacin Residues in Fish. Food Anal. Methods 2021, 14, 957–967. [Google Scholar]
- Xiong, J.-Q.; Kurade, M.B.; Kim, J.R.; Roh, H.-S.; Jeon, B.-H. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef]
- Rahimpour, R.; Sabeti, B.; Chekin, F. Electrochemical sensor based on nitrogen doped porous reduced graphene oxide to detection of ciprofloxacin in pharmaceutical samples. Russ. J. Electrochem. 2021, 57, 654–662. [Google Scholar]
- Taghizade, M.; Ebrahimi, M.; Fooladi, E.; Yoosefian, M. Simultaneous spectrophotometric determination of the residual of ciprofloxacin, famotidine, and tramadol using magnetic solid phase extraction coupled with multivariate calibration methods. Microchem. J. 2021, 160, 105627. [Google Scholar]
- Yuan, X.; Lv, W.; Wang, B.; Yan, C.; Ma, Q.; Zheng, B.; Du, J.; Xiao, D. Silicon nanoparticles-based ratiometric fluorescence platform: Real-time visual sensing to ciprofloxacin and Cu2+. Spectrochim. Acta Part A 2021, 253, 119599. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Bernardoni, V.; da Silva, T.M.; Ramos, L.S.; Gomes, M.P.; Assis, D. Development and Validation of a Rapid and Reliable HPLC-FLD Method for the Quantification of Ciprofloxacin and Enrofloxacin Residues in Zea mays. J. Braz. Chem. Soc. 2022, 33, 128–134. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, D.; Mutnuri, S. UPLC–MS/MS method validation of ciprofloxacin in human urine: Application to biodegradability study in microbial fuel cell. Biomed. Chromatogr. 2019, 33, e4392. [Google Scholar]
- Faria, A.F.; de Souza, M.V.; de Oliveira, M.A. Validation of a capillary zone electrophoresis method for the determination of ciprofloxacin, gatifloxacin, moxifloxacin and ofloxacin in pharmaceutical formulations. J. Braz. Chem. Soc. 2008, 19, 389–396. [Google Scholar]
- Ragab, G.H.; Amin, A.S. Atomic absorption spectroscopic, conductometric and colorimetric methods for determination of fluoroquinolone antibiotics using ammonium reineckate ion-pair complex formation. Spectrochim. Acta Part A 2004, 60, 973–978. [Google Scholar]
- Cancino, J.; Razzino, C.A.; Zucolotto, V.; Machado, S.A. The use of mixed self-assembled monolayers as a strategy to improve the efficiency of carbamate detection in environmental monitoring. Electrochim. Acta 2013, 87, 717–723. [Google Scholar]
- Caro, C.; Castillo, P.M.; Klippstein, R.; Pozo, D.; Zaderenko, A.P. Silver nanoparticles: Sensing and imaging applications. Silver Nanoparticles 2010, 201–223. [Google Scholar]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid Interface Sci. Commun. 2020, 35, 100244. [Google Scholar]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and silver nanoparticle-based colorimetric sensors: New trends and applications. Chemosensors 2021, 9, 305. [Google Scholar] [CrossRef]
- Raja, D.A.; Shah, M.R.; Malik, M.I. Polyethyleneimine stabilized silver nanoparticles as an efficient and selective colorimetric assay for promethazine. Anal. Chim. Acta 2022, 1223, 340216. [Google Scholar] [PubMed]
- Fang, R.; Jing, H.; Chai, Z.; Zhao, G.; Stoll, S.; Ren, F.; Liu, F.; Leng, X. Study of the physicochemical properties of the BSA: Flavonoid nanoparticle. Eur. Food Res. Technol. 2011, 233, 275–283. [Google Scholar]
- Jamal, H.S.; Raja, R.; Ahmed, S.W.; Shah, M.R.; Ahmed, S.; Ali, S.A. Simultaneous Colorimetric Sensing of Anion (I−) and Cation (Fe2+) by Protein Functionalized Silver Nanoparticles in Real Samples. J. Clust. Sci. 2022, 33, 1501–1514. [Google Scholar] [CrossRef]
- Xue, Y.; Dong, B.; Liu, X.; Wang, F.; Yang, J.; Liu, D. Using selenium-conjugated polyethylene glycol to enhance the stability of gold nanoparticles in biologically relevant samples. Sci. China Chem. 2019, 62, 280–286. [Google Scholar]
- Rahim, S.; Bhayo, A.M.; Shah, M.R.; Malik, M.I. Star-shaped poly (ethylene oxide)-block-poly (caprolactone) conjugated silver nanoparticles: A colorimetric probe for cephalexin in environmental, biological and pharmaceutical samples. Microchem. J. 2019, 149, 104048. [Google Scholar]
- Rahim, S.; Khalid, S.; Bhanger, M.I.; Shah, M.R.; Malik, M.I. Polystyrene-block-poly (2-vinylpyridine)-conjugated silver nanoparticles as colorimetric sensor for quantitative determination of Cartap in aqueous media and blood plasma. Sens. Actuators B 2018, 259, 878–887. [Google Scholar] [CrossRef]
- Shaban, S.M.; Lee, J.Y.; Kim, D.-H. Dual-surfactant-capped Ag nanoparticles as a highly selective and sensitive colorimetric sensor for citrate detection. ACS Omega 2020, 5, 10696–10703. [Google Scholar]
- Shaban, S.M.; Moon, B.-S.; Pyun, D.-G.; Kim, D.-H. A colorimetric alkaline phosphatase biosensor based on p-aminophenol-mediated growth of silver nanoparticles. Colloids Surf. B 2021, 205, 111835. [Google Scholar]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar]
- Raja, D.A.; Munir, F.; Shah, M.R.; Bhanger, M.I.; Malik, M.I. Colorimetric sensing of cephradine through polypropylene glycol functionalized gold nanoparticles. R. Soc. Open Sci. 2021, 8, 210185. [Google Scholar]
- Raja, D.A.; Musharraf, S.G.; Shah, M.R.; Jabbar, A.; Bhanger, M.I.; Malik, M.I. Poly (propylene glycol) stabilized gold nanoparticles: An efficient colorimetric assay for ceftriaxone. J. Ind. Eng. Chem. 2020, 87, 180–186. [Google Scholar]
- Sarkar, B.; Mondal, P. Colorimetric Sensors of Hg2+ Ion Based on Functionalized Gold and Silver Nanoparticles. J. Water Environ. Nanotechnol. 2021, 6, 22–40. [Google Scholar]
- Rahim, S.; Rauf, A.; Rauf, S.; Shah, M.R.; Malik, M.I. Enhanced electrochemical response of a modified glassy carbon electrode by poly (2-vinlypyridine-b-methyl methacrylate) conjugated gold nanoparticles for detection of nicotine. RSC Adv. 2018, 8, 35776–35786. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandi, M.; Chandhru, M.; Rani, S.K.; Vasimalai, N. Highly selective detection of iodide in biological, food, and environmental samples using polymer-capped silver nanoparticles: Preparation of a paper-based testing kit for on-site monitoring. ACS Omega 2019, 4, 11372–11379. [Google Scholar]
- Jäger, M.; Schubert, S.; Ochrimenko, S.; Fischer, D.; Schubert, U.S. Branched and linear poly (ethylene imine)-based conjugates: Synthetic modification, characterization, and application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef]
- Patnaik, S.; Gupta, K.C. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert Opin. Drug Deliv. 2013, 10, 215–228. [Google Scholar] [CrossRef]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2005, 7, 657–663. [Google Scholar] [CrossRef]
- Kang, J.-H.; Tachibana, Y.; Kamata, W.; Mahara, A.; Harada-Shiba, M.; Yamaoka, T. Liver-targeted siRNA delivery by polyethylenimine (PEI)-pullulan carrier. Bioorg. Med. Chem. 2010, 18, 3946–3950. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.G.; Oh, E.J.; Chung, H.Y.; Han, S.I.; Kim, E.J.; Seo, S.Y.; Do Ghim, H.; Yeum, J.H.; Choi, J.H. Antimicrobial polyethyleneimine-silver nanoparticles in a stable colloidal dispersion. Colloids Surf. B Biointerfaces 2011, 88, 505–511. [Google Scholar] [CrossRef]
- Zhou, T.; Su, Z.; Wang, X.; Luo, M.; Tu, Y.; Yan, J. Fluorescence detections of hydrogen peroxide and glucose with polyethyleneimine-capped silver nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118881. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Khiew, P.-S.; Chiu, W.S.; Tan, Y.-F.; Leong, C.-O. Polyethyleneimine-capped silver nanoparticles as antifouling photocatalyst for wastewater treatment. Mater. Today Proc. 2019, 19, 1497–1506. [Google Scholar]
- Huang, W.; Wang, Y.; Wang, L.; Pan, C.; Shen, G. Colorimetric detection of ciprofloxacin in aqueous solution based on an unmodified aptamer and the aggregation of gold nanoparticles. Anal. Methods 2021, 13, 90–98. [Google Scholar] [PubMed]
- Kobayashi, S.; Hiroishi, K.; Tokunoh, M.; Saegusa, T. Chelating properties of linear and branched poly (ethylenimines). Macromolecules 1987, 20, 1496–1500. [Google Scholar]
- Evanoff Jr, D.D.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667. [Google Scholar] [CrossRef]
- Sarkar, M.; Khandavilli, S.; Panchagnula, R. Development and validation of RP-HPLC and ultraviolet spectrophotometric methods of analysis for the quantitative estimation of antiretroviral drugs in pharmaceutical dosage forms. J. Chromatogr. B 2006, 830, 349–354. [Google Scholar]

| Method | LDR (nM) * | a LOD | b LOD (nM) * | Samples | Remarks | References |
|---|---|---|---|---|---|---|
| Electrochemical sensing (Nitrogen-doped porous RGO) | 1.0 × 102–1.0 × 104 | 39 nM | 39 | Pharmaceutical sample | Expensive instrumentation, high-cost electrodes, trained operators, no interference study, no environmental and biological sample study. | [10] |
| Spectrophotometry (Solid-phase extraction) | 4.5 × 103–90.5 × 103 | 0.005 mg/L | 15.1 | Pharmaceutical sample | Tedious protocol, no naked eye detection, no interference study, no environmental and biological sample study. | [11] |
| Spectrofluorometry | 2.1 × 102–1.3 × 105 | 89 nM | 89 | Pure drug sample | Instrument-based analysis, no naked eye detection, trained personnel, no interference study, no environmental and biological sample study. | [12] |
| HPLC-FLD | 90.5–3.0 × 102 | 16.65 μg/kg | 50.2 | Standard drug sample | Expensive instrumentation, no naked eye detection, trained personnel, no interference study, no environmental and biological sample study. | [13] |
| Colorimetric sensing (aptasensor-based AuNPs) | 20–3.0 × 102 | 0.215 nM | 0.215 | Aqueous sample | Requirement of sequence of CIP–aptamer, no comprehensive interference study, no study in biological sample. | [23,42] |
| UPLC MS/MS | 4.7–3.0 × 102 | 0.76 ng/mL | 2.2 | Human urine sample | Expensive instrumentation, no naked eye detection, trained personnel, no interference study, no environmental and biological sample study. | [14] |
| Capillary Zone Electrophoresis | N/A | 2.72 mg/L | 8209 | Pharmaceutical Sample | Expensive instrumentation, no naked eye detection, trained personnel, no interference study, no environmental and biological sample study. | [15] |
| Atomic absorption spectroscopy | 1.5 × 104–4.2 × 105 | 1.4 µg/mL | 4225 | Pharmaceutical sample | Expensive instrumentation, no naked eye detection, trained personnel, no interference study, no environmental and biological sample study. | [16] |
| Colorimetric sensing (PEI-AgNPs) | 1.0 × 102–2.0 × 105 | 0.038 µM | 38 | Tap Water, Blood Plasma, Serum, Urine, and Pharmaceutical Formulations | Quick and easy method, low-cost, on-site naked eye detection, highly selective and sensitive, reliable quantitation, interferences study, real sample such as biological, environmental, and commercial drug samples | [Present work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aijaz, A.; Raja, D.A.; Khan, F.-A.; Barek, J.; Malik, M.I. A Silver Nanoparticles-Based Selective and Sensitive Colorimetric Assay for Ciprofloxacin in Biological, Environmental, and Commercial Samples. Chemosensors 2023, 11, 91. https://doi.org/10.3390/chemosensors11020091
Aijaz A, Raja DA, Khan F-A, Barek J, Malik MI. A Silver Nanoparticles-Based Selective and Sensitive Colorimetric Assay for Ciprofloxacin in Biological, Environmental, and Commercial Samples. Chemosensors. 2023; 11(2):91. https://doi.org/10.3390/chemosensors11020091
Chicago/Turabian StyleAijaz, Aqsa, Daim Asif Raja, Farooq-Ahmad Khan, Jiri Barek, and Muhammad Imran Malik. 2023. "A Silver Nanoparticles-Based Selective and Sensitive Colorimetric Assay for Ciprofloxacin in Biological, Environmental, and Commercial Samples" Chemosensors 11, no. 2: 91. https://doi.org/10.3390/chemosensors11020091
APA StyleAijaz, A., Raja, D. A., Khan, F.-A., Barek, J., & Malik, M. I. (2023). A Silver Nanoparticles-Based Selective and Sensitive Colorimetric Assay for Ciprofloxacin in Biological, Environmental, and Commercial Samples. Chemosensors, 11(2), 91. https://doi.org/10.3390/chemosensors11020091









