Abstract
Well known as the “stress hormone”, cortisol plays an indispensable role in life activities. In the past few decades, accurate information about the intracorporal level of cortisol has been proven to be an important and effective indicator for evaluating physical and mental states and diagnosing a series of pressure-induced diseases. Hence, various rapid and efficient cortisol sensing technologies with high sensitivity and selectivity and low detection limit have been developed. This review examines most recent works and progress in cortisol detection via immunoassay, highlighting the construction of sensitive sensor systems. We aimed to provide a comprehensive description in the aspects of general optical and electrochemical detection methods, novel immunosensing systems, and advanced portable and wearable devices. Outlooks and suggestions for the development of continuous and real-time monitoring techniques and devices were finally provided.
1. Introduction
Cortisol is a glucocorticoid released by the hypothalamic-pituitary-adrenal (HPA) system [1], which plays a significant role in physiological activities. Specifically, different life processes, such as immune and inflammatory adjustment [2], protein synthesis [3], and glucose and pressure control [4], rely on cortisol. Normally, the secretion of cortisol in vivo follows the regularity with daily time [5,6] The amount of cortisol secreted increases in the morning and gradually reaches the highest level in approximately half an hour after waking up (cortisol awakening response) [7]. Subsequently, the level and rate of release drop and reach the lowest values in midnight.
After being released from HPA, cortisol enters the blood circulatory system; hence, it can be found in many different parts of the body, as plasma, serum, saliva, sweat, urine, and interstitial fluid [8,9,10] and hair [11]. The blood has the highest level, which can reach 2–25 g/mL from midnight to morning [1]. Two types of cortisol are found in the blood: corticosterone-binding-globulin inactive type and free active type [12]. The amount of free type cortisol is considerably lower, approximately 1/10 of the inactive type. In addition, the active type cortisol can generate stress response by binding with a glucocorticoid receptor and plays a role as indicator to reflect the stressed condition. Through diffusion process, free cortisol access other parts of the body. The concentrations in other body fluids are much lower than the concentration in the blood, which is usually in the ng/mL level [13,14]. Despite that body fluids have different cortisol concentrations, a closed relationship can be found between these concentrations [12,15], accounting for the diversity of detection methods.
When the body is stimulated by a change in the external environment, the normal release of cortisol is disrupted. For example, in an emergency situation, the sense of tension and stress results in an immediate release of a large amount of cortisol from HPA system. This feature is the reason that cortisol is often called the “stress hormone”. Apparently, the flight-or-fight response triggered by instantaneous cortisol increment can effectively provide protection against accidents. However, if cortisol content maintains an abnormal level in a long period, it will harm physical and psychological health, particularly causing depression [16], fatigue [17], obesity [18], and high blood glucose and pressure [4]. Moreover, a serious illness may occur, such as Cushing syndrome [19], Addison syndrome [20], cardiovascular issues [21], and autism [22]. Hence, tools that can monitor cortisol level is of great importance as it may have practical applications in disease diagnosis and therapy and facilitate scientific progress and pathological investigation.
Fortunately, the simplicity and validity of sampling step derived from a wide distribution and high correlation among distributed regions facilitate the manufacture of various cortisol sensing techniques and tools. In the past, cortisol level was commonly tested in the laboratories or medical institutions with specialized equipment. Liquid chromatography (LC) is mostly employed laboratory analysis for its broad detective range and high accuracy in the early stage [23].
Developments in immunology and biology have allowed the assessment of cortisol level through radioimmunoassay (RIA) [24] and enzyme-linked immunosorbent assay (ELISA) [25]. These technologies can realize simple detection and do not depend on professional operators and instruments. However, the level of radioactivity in RIA is a serious safety hazard, and this technology is gradually abandoned and replaced by optical detection techniques [26,27]. ELISA, with high selectivity and sensitivity because of the utilization of enzymes, has become widely approved and successfully commercialized cortisol detection method. Developments in various disciplines, such as material, electronics, physics and chemistry, have largely contributed to the improvement of sensing performance in nonenzymatic systems, such as electrocatalysis-based cortisol sensors [28,29,30,31,32]. Obviously, the achievement of detection is not the only property focused at this stage. Practicality (lifetime and stability), portability, and wearability of sensors have also become essential requirements in cortisol monitoring.
In this review, we summarize recent progress in cortisol sensing via immunoassay technology from many aspects (including sensing techniques, systems, and devices), highlighting the construction of novel immunosensor systems. Challenges, suggestions, and outlooks for the development of continuous cortisol monitoring techniques were discussed, with the wish to obtain a simple, convenient, and practical real-time cortisol monitoring device in future.
2. Fundamental Detection Methods
Apart from LC, RIA, and ELISA, detection methods based on electrochemistry [33] and optics [34] have been developed and validated in the past years. Owing to their low costs and simplicity, these methods have attracted considerable interest, and substantial progress has been made in the field of detection.
2.1. Optical Detection
2.1.1. Colorimetric Method
The colorimetric method is an efficient and simple optical technology for cortisol detection. Color change might have different causes, and one of these causes relies on the addition of various chromogens, which react with targets. The color of a chromogen changes with cortisol concentration. Tu [35] reported that four different species (sulfuric acid, Porter–Silber reagent, Prussian blue, and bule tetrazolium) generated different colors upon cortisol addition. Additionally, the most apparent color change can be observed when tetrazolium is used.
Although this analytical technology is simple, it causes chemical pollution. Another colorimetric approach is based on color change generated by the aggregation of nanoparticles. For instance, when a cortisol molecule is decorated with gold nanoparticles and captured in a particular region through specific binding, the accumulation of gold nanoparticles produces a conspicuous red color, which is closely related to captured cortisol level. With this approach, colorimetric sensors, such as lateral flow assay strips, have been produced [36,37].
2.1.2. Fluorescence Analytical Method
Through a comparable principle, fluorescence labels can be introduced into a system and connected to cortisol molecules instead of gold nanoparticles [38]. When labeled targets are captured, fluorescence response can be correlated to target concentration. As fluorescence labels can be cheap, this analysis method can largely reduce test cost. However, the utilization of labeled target species depends on external test solution for the addition of labeled particles. This dependence can be dramatically depleted by means of the employment of surface plasmon resonance (SPR) technique [39,40].
2.1.3. SPR-Based Method
When incident light reaches the surfaces of some metals (such as Ag and Au), it interacts with the electrons of the metal atoms at the surfaces, generating a special optics phenomenon, which is called surface plasmon resonance (SPR). Change in the environment of a surface affects the path of light, therefore some researchers have exploited this effect in designing biosensors [41,42,43]. While cortisol binds with a functionalized surface, refractive index (RI) or resonance wavelength changes [44]. In this method, cortisol level is related directly to the refractive property of a surface material, and introducing external labeled-molecules is unnecessary. In addition, different surface modification methods and various recognition sites might result in large variations in sensitivity among SPR sensors [45].
Additionally, SPR can enhance optic response in fluorescence or Raman spectrum and can vastly improve sensor performance. As shown in Figure 1a [34], thousandfold intensity increment can be observed with SPR effect, compared with the increment observed in conventional fluorophores (CW800) and quantum dots. Moreover, this plasmonic fluorescence method can be validated by ELISA and exhibit a low limit of detection (LOD).
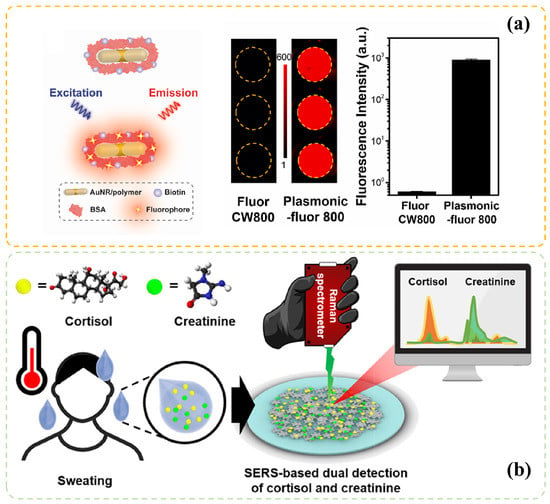
Figure 1.
SPR enhanced sensing technologies: (a) Fluorescence intensity comparison between Plasmonic-fluor 800 and conventional fluorophore CW800, adapted with permission from Ref. [34] Copyright 2022, Elsevier B.V. (b) The illustration of SERs through a portable hand-held Raman spectrometer for the simultaneous detection of cortisol and creatinine in sweat, reprinted with permission from Ref. [46] Copyright 2021, American Chemical Society.
Surface-enhanced Raman spectroscopy is another technology that exploits the SPR effect and can be utilized in molecule detection [46,47]. Different from the fluorescence method, Raman spectroscopy focuses on molecular structures and particular Raman active modes, which account for the excellent selectivity of targets (as shown in Figure 1b). Hence, many additional modified steps, such as fluorophores labeling or antibody functionalization can be omitted [48].
2.2. Electrochemical Detection
When the applied potential in electrolyte solutions is sufficient for the oxidation or reduction reaction of the internal species, electron transfer between particles and electrodes can produce a current alteration in this electrochemical system. The amount of transfer electrons in this process depends on the concentration of reacted particles, so the response current intensity can indicate the concentration of a specific molecule. This kind of sensing method is called electrochemical detection, and the devices used is electrochemical sensors.
2.2.1. Voltammetry Method
When an electrochemistry system is indistinct, cyclic voltammetry (CV) is generally the first employed technique in obtaining information of the occurring electrochemical redox reaction [49,50]. A potential that changes linearly and circularly with time is applied, and the response current is recorded. When the changeable voltage reaches the reaction point, a current variation appears at the corresponding voltage position. Subsequently, a figure that contains the correlation between voltage and response current can be depicted.
Cortisol is relatively electro-inactive, and thus the response current is difficult to obtain, unless deliberate and customized electrode is used [28,32]. Hence, in most cortisol electrochemical systems, indirect sensing protocol coupled with immunoassay technique is employed [51]. Specifically, cortisol recognition elements are pre-immobilized at surfaces, and redox probes with high electro-activity are usually used. After cortisol binds with recognition components, the resulting alterations in spatial environments at electrode surfaces lead to variable response signals that can be related to the concentrations of cortisol in the sample solutions.
Owing to non-Faraday processes, such as the charging effect of electric double layer, the redox signal might be covered, thus limiting trace detection. Hence, the pulse technique was introduced, which generate a series of pulse-based methods, such as square-wave voltammetry (SWV) [51] and differential pulse voltammetry (DPV) [52,53]. Repeated and instantaneous oscillation in voltage extremely weakens the impact of the charging effect, thereby efficiently improving sensing performance [52].
2.2.2. Impedance Method
Apart from voltammetry technologies, electrochemical impedance spectroscopy (EIS) is another popular technology that employs AC signals in the design of cortisol sensors [54,55]. In this method, variation in impedance of the system is emphasized instead of response current. However, unlike the utilization of fixed ac potential in pulse voltammetry techniques, a changeable AC signal frequency is applied in EIS. This method facilitates the simultaneous acquisition of information of separated mass transfer and charge transfer process in different frequency regions. Some experimental parameters, such as the external addition of BSA, the concentrations of antibodies, and the incubation time in sample solutions, can play an important role in the analytical performance of sensors based on EIS technology [56].
2.2.3. Amperometric Method
All electrochemical methods contain a break between tests because of the utilization of changeable applied voltage. Compared with them, a constant potential is used in chronoamperometry, which can continuously record current variations [32]. This method facilitates the design of real-time monitoring systems. However, owing to the constant redox reaction at electrode surfaces, absolute anti-poisoning ability is required in the practical application, which is difficult to achieve in cortisol immunosensing. Therefore, the approach to construct a practical real-time monitoring system is still under investigation.
2.2.4. Other Methods
Some researchers add redox probes with luminescence, such as the combination of [Ru(bpy)] and tripropylamine, generating an optics-electrochemical coupled detection method, called electrochemiluminescence (ECL). The ECL method is relatively mature to date and is usually applied in validating developed sensors [57].
In addition, quartz crystal micro balance (QCM) method is utilized in cortisol detection. Owing to the response of QCM originates from the pressure at the surface of quartz crystal, it is easily influenced by the nonspecific bindings and environmental changes. Hence, Ito et al. [58] reported a twin QCM sensor for cortisol detection. In their sensing system, two channels were set up. One channel was decorated with a cortisol–antibody complex, whereas the other channel was coated by an immunoassay stabilizer and acted as the reference sensor. Environmental impacts were mitigated, and noise level was dramatically decreased.
In summary, developments in various disciplines have contributed to the success of cortisol detection technologies and sensing systems. We listed the specific analytical performance of some interesting works on optical (Table 1) and electrochemical cortisol detection (Table 2), respectively, aiming to provide an initial picture of this field.

Table 1.
Construction and detailed analytical performance (detection range and LOD) of recent cortisol detection system through various optical sensing technologies a.

Table 2.
Construction and detailed analytical performance (detection range and LOD) of recent cortisol detection system through various electrochemical sensing technologies a.
3. Developed Immunosensor Systems
In the previous section, two general types of detection methods are briefly introduced. The emphasis of this section will focus on recently developed immunosensing technologies and the construction of these immunosensor systems. As the recognition element is usually the most important and distinctive part in an immunosensor system, related studies were classified according to recognition elements: antibody, aptamer, and molecularly imprinted polymer film.
3.1. Antibody Based Recognition and Sensing
Owing to the extraordinary selectivity of specific binding between an antibody and an antigen, antibody-based sensing becomes a well-known and common technique in the field of detection for different kinds of targets, such as various molecules, nucleic acid, and proteins [90]. One of the classical antibody-modified sensors is constructed on the basis of competition coupled with label species. A system consisting of biotinylated competitors that can bind with cortisol antibodies and as-prepared calcium nanoflowers was reported by Yang [91]. In their work, calcium ion (Ca), horseradish peroxidase (HRP), -amylase, and streptavidin were decorated into nanoflowers, which can consequently realize the detection of cortisol through detecting glucose. Thus, cortisol level in a range of 0.33–1000 ng/mL (with a LOD of 98.5 pg/mL) can be determined with a blood glucose meter. An analogous competition system can be seen in the research of Rebeca [15]. Compared with Yang’s work, Rebeca et al. designed a simpler sensing system with a competitor of HRP-labeled cortisol. Furthermore, a sensor for the simultaneous detection of multiple targets can be accomplished [92].
Compared with a competition system, a noncompetition type can be built without dependence on the addition of an external competitor, leading to a more convenient sensing protocol. Dong et al. [93] discovered a Quenchbody (Q-body) probe made from protein M in Mycoplasma genitalium. This probe can be tagged in many kinds of antibodies and can exhibit a fluorescence property. While targets bind to Q-body-labeled antibodies, variations in fluorescence intensities can provide information on the targets’ concentrations. With this labeled antibody, biomarkers, such as thyroxine and cortisol in nM level can be detected.
As the binding between an antibody and an antigen can alter spatial occupancy and molecular motion, antibodies are usually used in electrochemical technologies for specific detection without a labeling step. High sensor performance can be achieved by using a large number of antibodies and stable electrodes with high conductivity. These parameters mainly depend on surface area and electrode composition, which are closely related to the material and preparation process. Hence, many studies on antibody-based electrochemical sensors are focused on optimizing electrodes. Materials with different conductive sites, including noble metals [74], metal composites [94] and nonmetallic conductors [95] have been investigated.
Liu et al. [74] reported a AuNPs/MWCNTs/PDMS electrode for the electrochemical detection of cortisol in sweat. The utilization of MWCNTs can support a large surface area for adequate antibody modification, and Au nanoparticles improve electrode conductivity. The result showed an efficient sensing in a range of 1 fg/mL–1 g/mL (R = 0.995) and LOD of 0.3 fg/mL, which is in good agreement with the values obtained using commercially chemiluminescence immunoassay. Moreover, they demonstrated that a PDMS substrate has potential applications in wearable device fabrication. Two-dimensional (2D) materials, such as TiCT MXene nanosheets, have been explored for the application in wearable cortisol sensors by Nah [84]. They developed an electrochemical impedimetric antibody sensor based on TiCT MXene/LBG (laser-burned graphene)/PDMS electrode. Additionally, as another well-known 2D material, graphene oxide has attracted interest as a material for functionalized electrodes, due to its high surface area and outstanding electric property [95].
A typical antibody-based sensing process usually consists of an incubation step for the binding of antigens and antibodies, and this step results in a delayed readout and might cause errors in interpreting the real situation of biomarkers, especially cortisol, which changes over time. Hence, faster detection is required. Nanowell array electrodes can facilitate rapid detection because of the reduced mass transfer limitation, and AC signals can accelerate antigen-antibody binding. Mahmoodi et al. [85] incorporated these effects and developed a biochip sensor that can display a response within minutes. The sensor achieved detection in a range of 1–15 g/dL and had an LOD of 0.5 g/dL, which can be validated by standard ELISA.
Although many meaningful and impressive antibody-based studies have been carried out, constructing continuous monitoring systems, which are essential for evaluating physical conditions, still remains difficult. Smeden et al. [96,97] have focused on solving this problem. In their recent study, a novel technique named biosensing by particle mobility was introduced. This sensor was built according to the measured frequencies of antibody-modified molecule binding and unbinding events (Figure 2a) with free cortisol or immobilized cortisol analog. While the cortisol level in a solution changes, variations in bound and unbound states occur, resulting in a frequency fluctuation (defined as activity in Figure 2b). In order to observe this fluctuation in frequency, the antibody-modified molecule is limited in the vicinity of cortisol analogs with free Brownian motion. In addition, an elaborate selection of antibody and optimization of density was considered. With this tool, a continuous cortisol detection over multiple hours can be achieved in microdialysis-sampled plasma (Figure 2c). Despite requiring further reduction in response time, the technology still exhibited large potential in real-time cortisol monitoring.
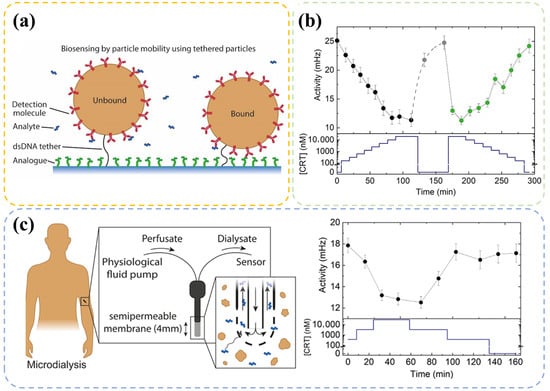
Figure 2.
(a) Illustration of bound and unbound state of antibody-modified particles in the Biosensing by Particle Mobility (BPM) technology. A particle that binds with free cortisol in a sample solution belongs to the unbound state. If the particle binds with an immobilized cortisol analog at the surface, then it belongs to the bound state. (b) Continuous cortisol sensing with different cortisol concentrations (CRT). The bottom part shows the cortisol concentration in the sample solution over time, and the top part shows the corresponding activity. (c) Sketch of use of a microdialysis probe and experimental results. Panels are adapted from Ref. [96] Copyright 2022, The Authors (Prins, M. W.).
3.2. Aptamer Based Recognition and Sensing
Since the first cortisol aptamer sequence was reported by Martin et al. [98] in 2014, studies on the use of aptamers in cortisol detection have increased. Aptamers have many advantages over antibodies, such as in vitro synthesis, small spatial occupancy, and high affinity. Thus, aptamers have become popular receptors in the design of biosensors systems. Recent efficient aptamer sequences are listed in the Table 3. Based on different sensing mechanism, various aptasensors have been developed.

Table 3.
Recent employed aptamer sequences for cortisol capture.
An aptamer–antibody sandwich sensor with synergistic signal amplification system for salivary cortisol level was proposed by Huang et al. [76]. The sensor achieved an ultrasensitive outcome in a range of 0.1 pg/mL–10 ng/mL (LOD of 0.09 pg/mL). When cortisol was present in the system, it combined with the aptamer at the electrode surface and antibody-AuNP conjugates in the solution, forming a barrier layer to the redox species and reducing DPV current. However, the sandwich-type sensor had drawbacks, such as requiring external addition (antibody-AuNPs conjugates), which limited its application outside the laboratory.
A simpler design was proposed by Fashtali et al. [77], which can be used without antibody-AuNP conjugates. The conformational variations of the aptamer after binding with cortisol changed the charge transfer process of probes and reduced the DPV current. This can be a promotion to the sandwich-type aptasensor. However, the selectivity data were not satisfied enough in this study, probably due to the utilization of an aptamer sequence with lower selectivity [100].
Apart from sensors built based on the inhibiting effect (signal decreases with increasing target), sensors with promoting effect (signal increases with increasing targets) have been reported. Singh et al. [78] tagged an aptamer with methylene blue (MB) and immobilized it at the surface of a gold electrode. After capturing cortisol, MB at the end of aptamer moved toward the electrode, thereby increasing the DPV current. A reagent-free sensing system was constructed, and a detection at the nM level was obtained. This aptasensor showed promise in developing portable and wearable devices.
Apart from current detection, electrochemical impedance is a popular method used in combination with aptamers. Pusomjit et al. [86] reported a disposable sensor that connected an aptamer to an electrode via magnetic beads for cortisol detection in spiked artificial human sweat. Consequently, a linear range of 0.1–100 ng/mL and LOD of 2.1 pg/mL were achieved.
Optical aptasensors are also investigated in the field of cortisol sensing. After a target binds with an aptamer, optical properties, such as fluorescence, reflectivity, and absorbance, vary from those in the original case, indicating a strong correlation between response signal variation and target concentration.
Noble metals, especially gold, possess an obvious SPR effect and are thus widely applied to optical sensor systems. Localized SPR (LSPR) sensors have advantages over traditional SPR sensors and many applications due to the involvement of nanoparticles [104]. An LSPR aptasensor was presented by Jo et al. [68] to detect cortisol level in saliva. After optimizing the particle size, they found that an extinction peak shift in wavelength occurred while cortisol was captured by the aptamer-modified gold nanoparticles and achieved a detection range of 0.1–1000 nM. Additionally, a comparison between antibody and aptamer was included to exhibit improvement in sensitivity after the use of the aptamer. Wu et al. [67] focused on another aspect. They constructed a AuNP-based aptasensor and correlated UV-vis absorbance intensity with the cortisol concentration. Consequently, they obtained similar sensing performance.
Lateral flow assay (LFA) is commonly used in colorimetric sensing. It exploits the changeable visible color of gold, which depends on the aggregation situation of Au nanoparticles. Dalirirad et al. [60] reported an LFA cortisol sensor and achieved detection at the ng/mL level, which was validated by ELISA. To obtain optimal performance, they considered many factors, such as aptamer loading density, aptamer-to-DNA ratio, and AuNP concentration.
Except Au particles, silver is also a common additive in optical aptasensors. The aggregation state of some materials, such as Ag nanoclusters, shows higher luminescence efficiency, which is known as aggregation-induced emission (AIE). In the recent work of Moghadam [63], Ag nanoclusters were prepared and conjugated at both 3 and 5 ends of a cortisol aptamer. The AIE and self-assemble induced emission were applied during aptamer spatial structure alteration. The aim was to improve response signal and promote sensitivity in the saliva test. Consequently, a cortisol linear detection range of 1–900 nM and LOD of 1 nM were achieved.
Apart from emission enhancement, sensor system that exploits fluorescence quenching phenomenon was proposed. Liu et al. [62] discovered that the fluorescence of quantum dots (QDs) can be quenched by hormones positioned nearby. This finding inspired them to develop a QD-based cortisol aptasensor, which can associate fluorescence quenching efficiency with cortisol concentration.
Zhang et al. [99] constructed a sensing system via fluorescence quenching but in a different way to examine targets. A covered-tetrahedron nucleic acid tweezer containing three fluorescence-quenching molecules (FAM-BHQ1, ROX-BHQ2, and Cy5-BHQ2) conjugated at the end of different DNA strands was fabricated. The molecules were hybridized to the same outer DNA strand. Initially, fluorescence was suppressed due to the quenching particles. After cDNA was released from aptamer modified magnetic nanoparticles due to the competition reaction between the target and aptamer and bound with the outer DNA strand, a fluorescence recovery can occur because of the configuration variation. Using an exponential amplification reaction technique, they successfully developed a sensitive (LOD in pM level) detection strategy for three kinds of targets in serum samples (testosterone, cortisol, and creatine kinase isoenzymes) for accurate fatigue diagnosis.
3.3. MIP Based Recognition and Sensing
Molecularly imprinted polymer (MIP) can specifically recognize detection targets. Different from antibody or aptamer, MIP is usually prepared directly at electrode surfaces through the electropolymerization of various organic monomers. In a general fabrication process, targets and monomers are copolymerized for the preparation of specific films with unique spaces or microstructures. After the elution of targets introduced from preparation process in the film, objects in the sample solution can reload into the film and change the physicochemical properties. Hence, MIP is commonly named as “artificial antibody” and exhibits high selectivity.
Yeasmin et al. [81] reported a nano gold-doped MIP film based on poly-o-PD (poly-o-phenylenediamine) to detect trace levels of cortisol in saliva. The in situ reduction and co-deposition of gold nanoparticles in MIP promoted polymerization in the film and facilitated charge transfer process, thereby improving detection response and sensitivity. Through DPV analysis, they found that response current can be related to cortisol concentration in a linear range of 1 pM–500 nM and LOD of approximately 200 fM. Similarly, Duan et al. [79] developed a phenylenediamine MIP film for cortisol sensing. However, in their study, a nitrogen-doped bamboo-like carbon nanotube with nickel nanocluster material was used for the modification of charge transfer property. A linear detection range of 10 fM–1 nM and LOD of 2.37 fM were achieved via DPV in salivary sensing.
MIPs have also been utilized for cortisol detection in other applications, for example, cortisol level determination in wastewater. Cui et al. [80] presented a poly-dopamine/electro-reduced graphene oxide-based MIP sensor. A high correlation coefficient (R = 0.9908) was achieved between DPV current and cortisol concentration at the 1 nM–50 M range. However, the selectivity of the material was not as good as that of a phenylenediamine-based film. Obviously, the prepared sensor was unable to support a result with enough reliability if progesterone was present in the sample solution.
An external redox probe, such as hexacyanoferrate ([Fe(CN)]), is required, given that a sensing mechanism is based on change in redox response signal. Apparently, this feature impedes the development of mobilized device. Some researchers tried to simplify the MIP sensing system by directly embedding a redox active species in the film and eliminating added external label. Goyal et al. [72] designed a polypyrrole-based mixed MIP systems, including internally installed hexacyanoferrate redox probe, reduced graphene oxide, and -cyclodextrin (-CD). In this composite film, -CD served as an inclusion site for free cortisol. After a 10 min incubation step in solution, the CV response current of this film loaded with cortisol decreased with the level of solution concentration compared with the initial pristine situation. Consequently, a detection across seven orders of magnitude (from 5 pg/mL to 5000 ng/mL; R = 0.995) with sensitivity of 8.8 A log(ng/mL) cm and LOD of 19.3 pM was reported. Although -CD in the composite membrane was unable to effectively distinguish cortisol from a series of species with analogous structures, such as testosterone and progesterone, the design of internal redox probe contributed to the development of point-of-care devices.
Tang et al. [88] successfully developed a touch-based disposable MIP electrode with an internal Prussian blue probe. The electrode can be assembled in a portable or wearable device for sweat cortisol detection at the fingertip. This sensing system is illustrated in Figure 3. A highly permeable porous hydrogel (Figure 3c) can largely reduce the incubation time, which is more representative of the real situation as the cortisol level in body changes over time. Using this sensor, their team carried out a series of cortisol fluctuation experiments, such as circadian cycle (shown in Figure 3d) and cold-pressor test, and confirmed its validity and feasibility for semi-continuous monitoring using other immunoassay methods.
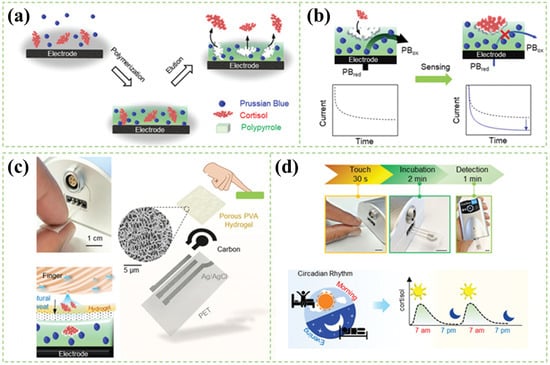
Figure 3.
Schematic illustration of the MIP-based sensor for rapid and stressless cortisol sensing. (a) Synthesis of the MIP layer for cortisol sensing. (b) The sensing mechanism of an MIP. (c) The construction of touch-based fingertip cortisol sensor and its sensing mechanism, where the cortisol from the accumulated finger sweat diffuses through the hydrogel onto the MIP electrode. (d) Illustration showing the fluctuation of cortisol through the circadian cycle. Panels are reprinted with permission from Ref. [88] Copyright 1999–2022, John Wiley & Sons, Inc.
Apart from focusing on the correlation between response current and cortisol concentration, some studies have focused on changes in other electric properties in the sensing system, such as capacitance [73] or impedance [82].
While targets fill the specific space of an MIP film, variations in optical properties occur. These differences have facilitated many optical detection technologies. Fan et al. [69] fabricated a methacrylic acid-based MIP membrane copolymerized with silver nanoparticles. The surface-enhanced Raman effect introduced by Ag nanoparticles improved the resolution of sensors, leading to a detection range of 100 nM–1 mM. Additionally, they evaluated the cortisol adsorption behavior in the MIP membrane via adsorption isotherm models and demonstrated that the process can be ascribed to Freundlich isotherm model, as indicated by the high fitting correlation coefficient (R = 0.9910).
Similarly, Villa et al. [105] prepared a methacrylic acid MIP film but at the surface of FeO nanoparticles. They found that a Langmuir–Freundlich model is more suitable to describing the adsorption process in a system. Different adsorption mechanisms occur even in a polymer composed of the same organic monomer, indicating a strong relationship between preparation parameters and sensing performance.
Advancements in computer science have produces powerful tools for exploring the intrinsic rules of experiment data. In a recent work of Dykstra [106], machine learning technique was used for the quantitative description of the impacts of synthesis parameters on corresponding performance. Five factors: pyrrole (SP1) and cortisol (SP2) concentration, CV cycles of electropolymerization (SP3), overoxidation (SP4), and CV scan rate of electropolymerization (SP5) were evaluated with a Gaussian process surrogate model. In the Sobel index assessment method, SP1, SP3, and SP4 were much more significant than SP2 and SP4. Based on the results, an optimal preparation setup was obtained with the sensitivity of 9.47 A/logC, which was higher than all of used data. Obviously, this interdisciplinary study combined with machine learning technique can largely reduce time and experiment cost, thus greatly benefitting material synthesis and sensor design.
4. Advanced Immunosensing Devices
4.1. Portable Devices
With the development of integrated circuit and micro-electronics, the electrochemical station can be fabricated into a miniaturization device, which offers much more mobility than ever [88]. In addition, sensors based on optic property variation, such as LFA, have also attracted considerable interest. LFA is a rapid paper-based diagnostic technology, which has already been commercially available for the detection of many targets [107,108]. A typical LFA setup consists of six parts: sample pad, conjugate pad, adsorbent pad, test line, control line, and membrane (Figure 4a). According to response mechanism (competition binding reaction or antibody-antigen-antibody binding reaction), it can be classified into competitive and sandwich types.
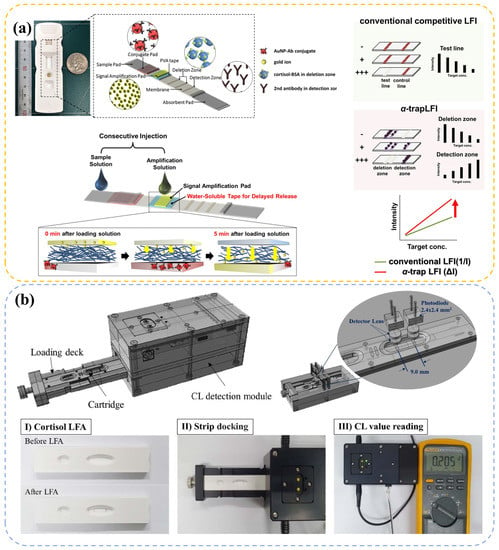
Figure 4.
Figures of portable LFA-based cortisol detection devices. (a) Schematic illustration of the new -trapLFI sensor, the signal amplification mechanism and a comparison with conventional LFA, reprinted from Ref. [36] Copyright 2021, The Authors (Kim, M. G.). (b) Illustration of portable fluorescence immunoassay detector for serum cortisol detection, adapted from Ref. [37] Copyright 2021, The Authors (Lee, M. H.).
In a competitive type LFA test, cortisol sample (usually sweat or saliva) is dropped at a sample pad. Driven by the capillary effect, the solution flows from the sample pad to the adsorbent pad. While passing through the conjugate pad, the solution mixes with external labeled molecules, such as antibody-modified gold particles. If the cortisol level in the sample is extremely low, abundant gold particles bind with cortisol–BSA species immobilized in the test line, exhibiting a distinct color. When the concentration level in the sample increases, it ends up with more dissociative cortisol–Au compound, leading to a decrement in number of bonds in the test line due to the competition reaction. Hence, concentration can be related to test line color intensity, or the test line/control line intensity ratio. Panfilova [61] compared the data obtained from this method with data from ELISA. They demonstrated the validity and used the method to monitor the cortisol levels of 10 participants for a month. As a result, they found a relatively complex correlation between cortisol level and subjectively perceived emotional stress.
An advanced device named as -trapLFI was developed by Oh et al. [36] to improve sensitivity via an automatically implemented signal amplification setup. In the sensitive LFA-based device in Figure 4a, a water-soluble PVA tape was installed in the signal amplification pad, which postponed the entrance time of amplification solution (Au ion in this study) for about 5 min. When the sample solution flowed and reacted at the deletion and detection zone, subsequent gold ion accumulated in the vicinity of Au nanoparticles captured at the deletion and detection zone, improving the signal. With this PVA setup, a more automatic test process with high detection performance was obtained. Compared with ELISA, this method exhibited good correlation (R = 0.90) in human salivary cortisol test.
Unlike the competitive type, cortisol antibodies were immobilized in the test line instead of cortisol–BSA in the sandwich type, and thus the intensity of the test line signal increased with cortisol level [37]. However, owing to the requirement of formation of sandwich type structure, which is not easy in the detection of small biomolecules with limited binding space [108,109], the sandwich type seems to be not as popular as the competitive type in the cortisol detection.
To acquire a highly precision data in LFA, optical instruments are usually used. Thus, the development of portable optical devices for testing LFA signals is of great significance. In the recent work of Kim et al. [37], a sandwich-type chemiluminescence-based LFA portable platform was presented (Figure 4b), which can be applied to the detection of clinical serum sample and was validated by a conventional instrument (Cobas-8000) with a correlation coefficient (R) of 0.96. Park et al. [109] offered a simpler LFA protocol named quick light normalization exam (qLiNE), which can collect LFA information via a pervasive smartphone. According to Figure 5a, a reference card in qLiNE for calibration (coordinate setting, illumination correction, color adjustment, and line scan) and a software for the processing of LFA strip images were included. They found that this technology accomplished a cortisol detection in saliva, which can be validated by ELISA (R = 0.95). Moreover, the results showed no relationship between intensity and light environment (Figure 5b,c) or camera position (Figure 5d). They were planning to introduce a deep neural network in their future design for correction algorithm improvement. This work elucidates a more convenient and powerful development of LFA in cortisol detection with the combination of advanced computer science and technology.
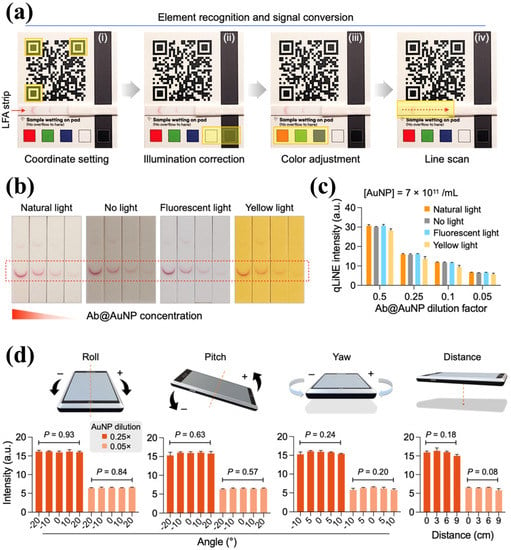
Figure 5.
Image correction with quick light normalization exam (qLiNE). (a) The entire qLiNE operation sequence, including coordinate setting, illumination correction, color adjustment and line scan. (b) Strips images with decreasing amount of membrane-bound Ab@AuNPs (from left to right) under different environmental conditions: natural light, no light, fluorescent light, and yellow light, and (c) the corresponding qLiNE intensity results, which exhibited stable signal under different illumination conditions. (d) The results of corrected intensity in qLiNE with different angles and distances of camera, which were statistically identical regardless of the camera positions. The figure is used from Ref. [109] Copyright 2022, The Authors (Shin, I. S. and Lee, H.).
4.2. Wearable Devices
Paper-based tests are powerful portable detection methods [38] but are commonly disposable. Thus, they are unsuitable for massive and continuous detection tasks. Microfluidic devices, which possess minimized scales with microchannels with sizes at the m level, can achieve duplicate detection with a thimbleful body fluid (an illustration of microfluidic sensor can be found in Figure 6a,b). Hence, microfluidic-based device is recommended for a person who needs to check his or her cortisol level continually, and considerable research on wearable microfluidics chips has been carried out.
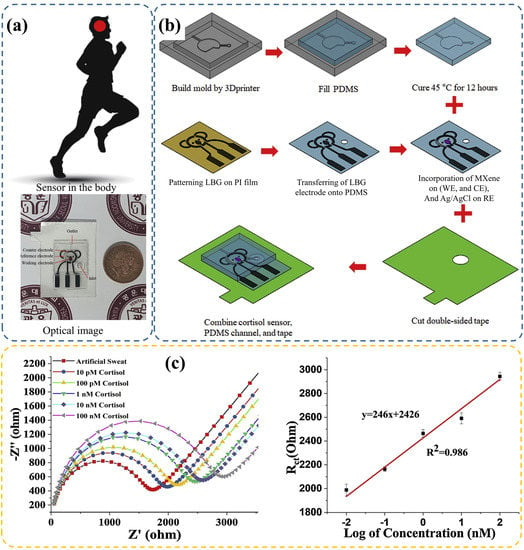
Figure 6.
(a) Digital image of the wearable patch, and (b) the fabrication process of the microfluidic based electrochemical sensing chip. (c) Cortisol biomarker detection using EIS method and corresponding linearity results. Panels are reprinted with permission from Ref. [84] Copyright 2020, Elsevier B. V.
Weng et al. [64] developed an aptamer-modified wearable microfluidic patch and a portable fluorescence detector transformed by a smartphone for sweat cortisol detection. Consequently, a detection range of 10–1000 ng/mL and LOD of 6.76 ng/mL were achieved. Obviously, a dark box is usually required to eliminate environmental implications in optics intensity-based sensors [64], unless an additional calibration card is applied [109]. Compared with these sensors, electrochemical sensors exhibit better anti-interference performance to external environments. Nah et al. [84] reported a wearable microfluidic patch fabricated with PDMS. According to variation in impedance after cortisol binding with immobilized antibody, a linear range of 0.01–100 nM and LOD of 88 pM were obtained (Figure 6c). This finding indicated practicality in the sweat cortisol test.
Naik [89] proposed a polyimide and hydrophilic polyethylene-made microfluidic device. With an antibody-derived inkjet-printed graphene working electrode, similar sensing performance was acquired via the chronoamperometric method. However, in this study, a redox probe [Fe(CN)] was manually added. This procedure presents some safety issues. To solve this problem, Lee [87] presented a wearable lab-on-a-patch, which contained an internal one-touch operation of reagent (redox mediator) delivery setup. Special design of the channel and unique valves between each region prevented the backflow of different solutions and generated a directional flow, which efficiently protected the skin from added chemicals. Consequently, a cortisol detection range of 1 pg/mL–1 g/mL and sensitivity of 0.273 Ohm/(ng mL) were obtained. The patch was successfully attached to a human forehead for sweat cortisol measurement during exercise. According to the results, cortisol level obtained from this device was approximate to that obtained from a commercial ELISA kit, demonstrating the validity and practicality of the device.
Apart from microfluidics technology, transistors are widely applied to the design of wearable devices due to their simple and microminiaturization construction and high sensor performance. Some interesting transistor-based wearable cortisol sensors are provided in pictured in Figure 7. Janardhanan et al. [110] presented a technology to construct an organic electrochemical transistor through a simple template-free electropolymerization, featuring nanotube structure in the channel for detecting cortisol in sweat. To facilitate conjugation with cortisol antibodies, EDOT-COOH and EDOT-EG3 were introduced into the system. They found that devices with nanotube embedding possessed more immobilized antibodies than those without any nanostructure topology, and achieved a sensing range of 1 fg/mL–1 g/mL and LOD of 0.0088 fg/mL (R = 0.9566). They demonstrated that their sensor is a promising component for wearable devices in terms of stability, reproducibility, and selectivity.
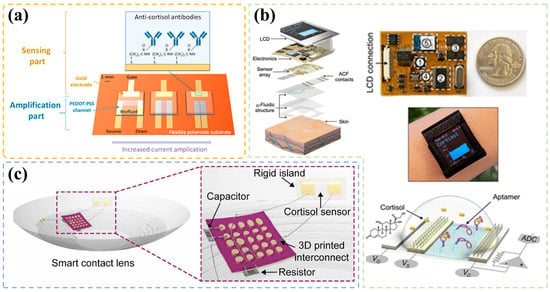
Figure 7.
(a) Schematic of the organic transistor structure, including antibody-coated gate electrodes and the organic PEDOT: PSS channel for current amplification, reprinted with permission from Ref. [113] Copyright 2022, American Chemical Society. (b) An image of the construction of multichannel cortisol biosensing smartwatch realized by an aptamer-FET (field effect transistor) sensing system, reprinted from Ref. [102] Copyright 2022, The Authors (Andrews, A. M. & Emaminejad, S.). (c) Schematic of the packaged smart contact lens integrated with three-dimensional printed stretchable interconnects and transistor-based cortisol sensor located on the rigid island, reprinted from Ref. [112] Copyright 2020, The Authors (Park, J. U., Lee, H. H. & Cheon, J.).
Similarly, Park et al. [111] reported another approach to optimize the conjugation in recognition process. MoS multiple-layer material with nanopores was utilized instead of an organic layer in the transistor system. Compared with pristine MoS, MoS modified via nanopatterning process exhibited superior performance in terms of sensitivity and selectivity of cortisol sensing due to the optimization of aptamer conjugation. Consequently, a steep linear detection range of 10 to 10 g/mL was achieved.
Apart from structure design, research has been focused on exploring novel recognition materials. Wang et al. [102] exploited a novel cortisol aptamer, aiming to improve detection range (M–mM level in wearable sensor systems). The smartwatch in Figure 7b was developed for sweat cortisol detection and was based on the aptamer-field effect transistor, which is detectable in a concentration of 1 pM. In a real trier social stress test, they found the trend of sweat cortisol level determined by their device was in good agreement with that of salivary level quantified by a standard laboratory method. This finding supported the validity and practicability of this sensor. Except sweat monitoring, researchers have developed other techniques for cortisol level assessment, such as through tears. Ku et al. [112] fabricated an integrated and soft lens (Figure 7c) based on an antibody-modified graphene field-effect transistor, which can reach a detection limit of 10 pg/mL. Low LOD and high biocompatibility assure its applicability for tears test.
Modification in the layer connecting the source and drain in a transistor has already been proven to be an efficient approach for building a sensing system due to the properties change of channel. However, the antigen-antibody reaction process reduces the lifetime of the layer very quickly. Hence, some researchers have focused on modifying gate electrode (usually a very common metal material), as it is shown in Figure 7a. This method can simplify the modification and regeneration process as the gate electrode is easy to replace.
A typical illustration of this modification was provided by Demuru [113]. Their team developed a wearable cortisol OECT sensor with an antibody-coated gate electrode. When integrated into a microfluidic system, the microdevice collected sweat and detected cortisol directly while it was stuck on the skin. They explored the influence of PEDOT:PSS layer geometry on the response signal and found that the one with short channel (W/L = 12.5) displayed the best amplification result. A sensing range of 1–1000 nM and a high sensitivity of 50 A/dec were obtained. In addition, 5 min of incubation was needed for real sweat detection.
5. Conclusions and Future Perspective
An accurate information of cortisol level can efficiently assess the physical and mental conditions and can be a crucial index in medical diagnosis for several diseases. This review examined recent advances in cortisol immunosensing technologies, including optics-/electrochemical-based technologies. Over the past years, various optical and electrochemical immunosensors with different bio-recognition elements (antibody, aptamer, and MIP film), have been developed for the rapid, sensitive, and quantitative detection of cortisol in body fluids, especially saliva, sweat, and serum, due to the simple sampling process. Moreover, advanced electronic techniques and microfluidics have led to great progress in the fabrication of portable and wearable devices and offered a more convenient and effective approaches for self-estimation or medical surveillance.
Despite the numerous works on the development of immuno-biosensors, continuous and real-time cortisol monitoring has not been achieved yet. Many challenges and obstacles persist. Continuous detection is expected to offer a precise and subtle information about the body state and thus motivates many researchers. This goal requires not only instant and unremitting but also permanently stable response signals, which raises the demands in the design of sensing system. Obviously, success cannot be separated from the development of material science, surface chemistry, and biochemistry or the understanding of fundamental sensing theory.
As this field is gradually entering a new term of continuous and real-time monitoring, we are expecting that some more efforts can be made for a further breakthrough, and the outlooks are as follows.
- 1.
- According to the analysis mentioned above, the property of binding site is the most significant factor that limits the realization of real-time monitoring with immunosensors. At first, irreversible binding between the recognition sites with cortisol or other molecules will cause an unpredictable response, limiting the lifetime of a sensor after long-term usage. Apparently, strong binding might not be suitable for the design of continuous sensing systems. However, if bonding is too weak, a long incubation time will be required for equilibrium process. In addition, this will contradict real-time monitoring. Moreover, if an MIP system is utilized, as the spatial structure in the organic film is the source of specific binding, the structure stability should be considered. Hence, a meticulous design or a novel system with long-term stability should be developed.
- 2.
- As it can be seen in Table 1 and Table 2, the correlation between signal and concentration is fickle and puzzling. The signal can be either linearly related to concentration, the logarithm of concentration, or other forms of concentration in different works. Clearly, the correlation should depend on the sensing mechanism of a particular system, but studies on such mechanism are rare. This might cause a lack of uniformity in cortisol detection and a difficulty to summarize the difference between various studies. The incomprehension of fundamental principle in sensing might lead to a misinterpretation of obtained data under actual conditions and can cause serious misdiagnosis. Thus, this aspect should be considered carefully in future studies.
- 3.
- Apart from immunosensing methods, some nonimmune electrochemical sensing methods for cortisol detection have gradually emerged. Compared with immunosensing, immune recognition elements are eliminated in this technique, and the current change originated from the redox reaction of C=O and C-OH group is directly measured. Thus, this method has high selectivity for cortisol detection. Although the response signal might not be as obvious as that in immunosensors, this technique has already exhibited potential in real-time cortisol monitoring owing to the developments in high-performance electrode materials and electrochemistry.
Author Contributions
Writing—original draft preparation, Y.Z.; writing—literature collection, Y.Z. and Q.L.; writing—review and editing, C.Z., W.C., L.M. and Z.L.; supervision, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 61771493), the Natural Science Foundation of Hunan Province of China (No. 2022JJ30754), Hunan Provincial Innovation Foundation for Postgraduate (No. CX20220105), and the Fundamental Research Funds for the Central Universities of Central South University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zea, M.; Bellagambi, F.G.; Halima, H.B.; Zine, N.; Jaffrezic-Renault, N.; Villa, R.; Gabriel, G.; Errachid, A. Electrochemical sensors for cortisol detections: Almost there. TrAC Trends Anal. Chem. 2020, 132, 116058. [Google Scholar] [CrossRef]
- Yeager, M.P.; Pioli, P.A.; Collins, J.; Barr, F.; Metzler, S.; Sites, B.D.; Guyre, P.M. Glucocorticoids enhance the in vivo migratory response of human monocytes. Brain, Behav. Immun. 2016, 54, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kidson, C. Cortisol in the regulation of RNA and protein synthesis. Nature 1967, 213, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Da Silva, E.V.F.; Cândido, N.B.; Nóbrega, A.S.; De Medeiros, R.A.; Sumida, D.H.; Chiba, F.Y.; Dos Santos, D.M. Evaluation of the level of cortisol, capillary blood glucose, and blood pressure in response to anxiety of patients rehabilitated with complete dentures. BMC Oral Health 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Schmidt-Reinwald, A.; Pruessner, J.; Hellhammer, D.; Federenko, I.; Rohleder, N.; Schürmeyer, T.; Kirschbaum, C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999, 64, 1653–1660. [Google Scholar] [CrossRef]
- Ganguly, A.; Lin, K.C.; Muthukumar, S.; Prasad, S. Autonomous, real-time monitoring electrochemical aptasensor for circadian tracking of cortisol hormone in sub-microliter volumes of passively eluted human sweat. ACS Sens. 2020, 6, 63–72. [Google Scholar] [CrossRef]
- Grosser, L.; Knayfati, S.; Yates, C.; Dorrian, J.; Banks, S. Cortisol and shiftwork: A scoping review. Sleep Med. Rev. 2022, 64, 101581. [Google Scholar] [CrossRef]
- Arya, S.K.; Chornokur, G.; Venugopal, M.; Bhansali, S. Dithiobis (succinimidyl propionate) modified gold microarray electrode based electrochemical immunosensor for ultrasensitive detection of cortisol. Biosens. Bioelectron. 2010, 25, 2296–2301. [Google Scholar] [CrossRef]
- Gaudl, A.; Kratzsch, J.; Bae, Y.J.; Kiess, W.; Thiery, J.; Ceglarek, U. Liquid chromatography quadrupole linear ion trap mass spectrometry for quantitative steroid hormone analysis in plasma, urine, saliva and hair. J. Chromatogr. A 2016, 1464, 64–71. [Google Scholar] [CrossRef]
- Ray, P.; Steckl, A.J. Label-free optical detection of multiple biomarkers in sweat, plasma, urine, and saliva. ACS Sens. 2019, 4, 1346–1357. [Google Scholar] [CrossRef]
- Quinete, N.; Bertram, J.; Reska, M.; Lang, J.; Kraus, T. Highly selective and automated online SPE LC–MS3 method for determination of cortisol and cortisone in human hair as biomarker for stress related diseases. Talanta 2015, 134, 310–316. [Google Scholar] [CrossRef]
- Estrada-Y-Martin, R.M.; Orlander, P.R. Salivary cortisol can replace free serum cortisol measurements in patients with septic shock. Chest 2011, 140, 1216–1222. [Google Scholar] [CrossRef]
- Villa, J.E.; Garcia, I.; de Aberasturi, D.J.; Pavlov, V.; Sotomayor, M.D.; Liz-Marzán, L.M. SERS-based immunoassay for monitoring cortisol-related disorders. Biosens. Bioelectron. 2020, 165, 112418. [Google Scholar] [CrossRef]
- Mohammad-Andashti, P.; Ramezani, Z.; Zare-Shahabadi, V.; Torabi, P. Rapid and green synthesis of highly luminescent MoS2 quantum dots via microwave exfoliation of MoS2 powder and its application as a fluorescence probe for cortisol detection in human saliva. Colloids Surf. A Physicochem. Eng. 2022, 647, 129048. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Shin, J.; Kim, S.; Yoon, T.; Joo, C.; Jung, H.I. Smart Fatigue Phone: Real-time estimation of driver fatigue using smartphone-based cortisol detection. Biosens. Bioelectron. 2019, 136, 106–111. [Google Scholar] [CrossRef]
- Tomiyama, A.J. Stress and obesity. Annu. Rev. Psychol. 2019, 70, 703–718. [Google Scholar] [CrossRef]
- Newell-Price, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Ten, S.; New, M.; Maclaren, N. Addison’s disease 2001. J. Clin. Endocrinol. Metab. 2001, 86, 2909–2922. [Google Scholar]
- Mohd Azmi, N.A.S.; Juliana, N.; Azmani, S.; Mohd Effendy, N.; Abu, I.F.; Mohd Fahmi Teng, N.I.; Das, S. Cortisol on circadian rhythm and its effect on cardiovascular system. Int. J. Environ. Res. Public Health 2021, 18, 676. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kabra, P.M.; Tsai, L.; Marton, L. Improved liquid-chromatographic method for determination of serum cortisol. Clin. Chem. 1979, 25, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Noguchi, M.; Kitamura, T.; Shima, S. Liquid chromatography and radioimmunoassay compared for determination of cortisol and corticosterone in plasma after a dexamethasone suppression test. Clin. Chem. 1987, 33, 1639–1642. [Google Scholar] [CrossRef]
- Kämäräinen, S.; Mäki, M.; Tolonen, T.; Palleschi, G.; Virtanen, V.; Micheli, L.; Sesay, A.M. Disposable electrochemical immunosensor for cortisol determination in human saliva. Talanta 2018, 188, 50–57. [Google Scholar] [CrossRef]
- Small, B.C.; Davis, K.B. Validation of a time-resolved fluoroimmunoassay for measuring plasma cortisol in channel catfish Ictalurus punctatus. J. World Aquac. Soc. 2002, 33, 184–187. [Google Scholar] [CrossRef]
- Franco-Martinez, L.; Tvarijonaviciute, A.; Martinez-Subiela, S.; Teles, M.; Tort, L. Chemiluminescent assay as an alternative to radioimmunoassay for the measurement of cortisol in plasma and skin mucus of Oncorhynchus mykiss. Ecol. Indic. 2019, 98, 634–640. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Rana, A.R.S. A comparison of edge-and basal-plane pyrolytic graphite electrodes towards the sensitive determination of hydrocortisone. Talanta 2010, 83, 149–155. [Google Scholar] [CrossRef]
- Sonawane, A.; Mujawar, M.A.; Bhansali, S. Atmospheric plasma treatment enhances the biosensing properties of graphene oxide-silver nanoparticle composite. J. Electrochem. Soc. 2019, 166, B3084. [Google Scholar] [CrossRef]
- Rison, S.; Rajeev, R.; Bhat, V.S.; Mathews, A.T.; Varghese, A.; Hegde, G. Non-enzymatic electrochemical determination of salivary cortisol using ZnO-graphene nanocomposites. RSC Adv. 2021, 11, 37877–37885. [Google Scholar] [CrossRef]
- Sharma, N.; Reddy, A.S.; Yun, K. Electrochemical detection of hydrocortisone using green-synthesized cobalt oxide nanoparticles with nafion-modified glassy carbon electrode. Chemosphere 2021, 282, 131029. [Google Scholar] [CrossRef]
- Gevaerd, A.; Watanabe, E.; Belli, C.; Marcolino-Junior, L.; Bergamini, M. A complete lab-made point of care device for non-immunological electrochemical determination of cortisol levels in salivary samples. Sens. Actuators B Chem. 2021, 332, 129532. [Google Scholar] [CrossRef]
- Urizar, G.G., Jr.; Hernandez, H.S.; Rayo, J.; Bhansali, S. Validation of an electrochemical sensor to detect cortisol responses to the trier social stress test. Neurobiol. Stress 2020, 13, 100263. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Q.; Seth, A.; Kolla, S.; Luan, J.; Jiang, Q.; Rathi, P.; Gupta, P.; Morrissey, J.J.; Naik, R.R.; et al. Plasmonically-enhanced competitive assay for ultrasensitive and multiplexed detection of small molecules. Biosens. Bioelectron. 2022, 200, 113918. [Google Scholar] [CrossRef]
- Tu, E.; Pearlmutter, P.; Tiangco, M.; Derose, G.; Begdache, L.; Koh, A. Comparison of colorimetric analyses to determine cortisol in human sweat. ACS Omega 2020, 5, 8211–8218. [Google Scholar] [CrossRef]
- Oh, H.K.; Kim, K.; Park, J.; Jang, H.; Kim, M.G. Advanced trap lateral flow immunoassay sensor for the detection of cortisol in human bodily fluids. Sci. Rep. 2021, 11, 22580. [Google Scholar] [CrossRef]
- Kim, H.T.; Jin, E.; Lee, M.H. Portable chemiluminescence-based lateral flow assay platform for the detection of cortisol in human serum. Biosensors 2021, 11, 191. [Google Scholar] [CrossRef]
- Vinitha, T.; Ghosh, S.; Milleman, A.; Nguyen, T.; Ahn, C.H. A new polymer lab-on-a-chip (LOC) based on a microfluidic capillary flow assay (MCFA) for detecting unbound cortisol in saliva. Lab A Chip 2020, 20, 1961–1974. [Google Scholar]
- Soares, M.S.; Silva, L.C.; Vidal, M.; Loyez, M.; Fac ao, M.; Caucheteur, C.; Segatto, M.E.; Costa, F.M.; Leit ao, C.; Pereira, S.O.; et al. Label-free plasmonic immunosensor for cortisol detection in a D-shaped optical fiber. Biomed. Opt. Express 2022, 13, 3259–3274. [Google Scholar] [CrossRef]
- Leit ao, C.; Pereira, S.O.; Alberto, N.; Lobry, M.; Loyez, M.; Costa, F.M.; Pinto, J.L.; Caucheteur, C.; Marques, C. Cortisol in-fiber ultrasensitive plasmonic immunosensing. IEEE Sens. J. 2020, 21, 3028–3034. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Lowe, T.E.; Ingram, J.R. Rapid ultrasensitive measurement of salivary cortisol using nano-linker chemistry coupled with surface plasmon resonance detection. Analyst 2009, 134, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Mazzarino, M.; Botrè, F.; Mazzei, F. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal. Bioanal. Chem. 2009, 394, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Huang, Z.; Kiritoshi, T.; Onodera, T.; Toko, K. Development of indirect competitive immuno-assay method using SPR detection for rapid and highly sensitive measurement of salivary cortisol levels. Front. Bioeng. Biotechnol. 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Leit ao, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021, 29, e00587. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Cui, D. Surface plasmon resonance immunoassay for cortisol determination with a self-assembling denaturalised bovine serum albumin layer on surface plasmon resonance chip. Micro Nano Lett. 2016, 11, 20–23. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Lee, J.; Lee, T.; Yun, J.; Lee, G.; Hong, Y. Hand-held Raman spectrometer-based dual detection of creatinine and cortisol in human sweat using silver nanoflakes. Anal. Chem. 2021, 93, 14996–15004. [Google Scholar] [CrossRef]
- Moore, T.J.; Sharma, B. Direct surface enhanced Raman spectroscopic detection of cortisol at physiological concentrations. Anal. Chem. 2019, 92, 2052–2057. [Google Scholar] [CrossRef]
- Oktavius, A.K.; Gu, Q.; Wihardjo, N.; Winata, O.; Sunanto, S.W.; Li, J.; Gao, P. Fully-conformable porous polyethylene nanofilm sweat sensor for sports fatigue. IEEE Sens. J. 2021, 21, 8861–8867. [Google Scholar] [CrossRef]
- Manickam, P.; Madhu, S.; Fernandez, R.E.; Viswanathan, C.; Bhansali, S. Fabric based wearable biosensor for continuous monitoring of steroids. ECS Trans. 2017, 77, 1841. [Google Scholar] [CrossRef]
- Manickam, P.; Fernandez, R.E.; Umasankar, Y.; Gurusamy, M.; Arizaleta, F.; Urizar, G.; Bhansali, S. Salivary cortisol analysis using metalloporphyrins and multi-walled carbon nanotubes nanocomposite functionalized electrodes. Sens. Actuators B Chem. 2018, 274, 47–53. [Google Scholar] [CrossRef]
- Nong, C.; Yang, B.; Li, X.; Feng, S.; Cui, H. An ultrasensitive electrochemical immunosensor based on in-situ growth of CuWO4 nanoparticles on MoS2 and chitosan-gold nanoparticles for cortisol detection. Microchem. J. 2022, 179, 107434. [Google Scholar] [CrossRef]
- Chavan, S.G.; Yagati, A.K.; Koyappayil, A.; Go, A.; Yeon, S.; Lee, M.H. Recombinant histidine-tagged nano-protein-based highly sensitive electro-sensing device for salivary cortisol. Bioelectrochemistry 2022, 144, 108046. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Ding, Y.; Liu, H.; Cui, H. Molecularly imprinted electrochemical aptasensor based on functionalized graphene and nitrogen-doped carbon quantum dots for trace cortisol assay. Analyst 2022, 147, 744–752. [Google Scholar] [CrossRef]
- Pali, M.; Jagannath, B.; Lin, K.C.; Upasham, S.; Sankhalab, D.; Upashama, S.; Muthukumar, S.; Prasad, S. CATCH (Cortisol Apta WATCH):‘Bio-mimic alarm’to track Anxiety, Stress, Immunity in human sweat. Electrochim. Acta 2021, 390, 138834. [Google Scholar] [CrossRef]
- Roushani, M.; Hosseini, H.; Hajinia, Z.; Rahmati, Z. Rationally designed of hollow nitrogen doped carbon nanotubes double shelled with hierarchical nickel hydroxide nanosheet as a high performance surface substrate for cortisol aptasensing. Electrochim. Acta 2021, 388, 138608. [Google Scholar] [CrossRef]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz-Duval, A.S.; Misra, S.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2019, 144, 1448–1457. [Google Scholar] [CrossRef]
- Mori, M.; Aoyagi, K.; Tomoda, T.; Ishikawara, F.; Sakamoto, S.; Myochin, H.; Kuga, M.; Kozaki, D.; Ohshima, N.; Izumi, T.; et al. Simultaneous capillary electrophoresis of anions and cations in a single injection using an anion exchanger-modified capillary for determination of salivary ions in combination with statistical analyses. J. Chromatogr. A 2021, 1635, 461647. [Google Scholar] [CrossRef]
- Ito, T.; Aoki, N.; Kaneko, S.; Suzuki, K. Highly sensitive and rapid sequential cortisol detection using twin sensor QCM. Anal. Methods 2014, 6, 7469–7474. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, J.; Hur, H.; Oh, S.; Lee, H.H. Highly sensitive colorimetric assay of cortisol using cortisol antibody and aptamer sandwich assay. Biosensors 2021, 11, 163. [Google Scholar] [CrossRef]
- Dalirirad, S.; Han, D.; Steckl, A.J. Aptamer-based lateral flow biosensor for rapid detection of salivary cortisol. ACS Omega 2020, 5, 32890–32898. [Google Scholar] [CrossRef]
- Panfilova, E. Development of a prototype lateral flow immunoassay of cortisol in saliva for daily monitoring of stress. Biosensors 2021, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, B.; Tanyi, E.K.; Yeasmin, S.; Cheng, L.J. Label-free sensitive detection of steroid hormone cortisol based on target-induced fluorescence quenching of quantum dots. Langmuir 2020, 36, 7781–7788. [Google Scholar] [CrossRef]
- Moghadam, F.M.; Bigdeli, M.; Tamayol, A.; Shin, S.R. TISS nanobiosensor for salivary cortisol measurement by aptamer Ag nanocluster SAIE supraparticle structure. Sens. Actuators B Chem. 2021, 344, 130160. [Google Scholar] [CrossRef]
- Weng, X.; Fu, Z.; Zhang, C.; Jiang, W.; Jiang, H. A portable 3D microfluidic origami biosensor for cortisol detection in human sweat. Anal. Chem. 2022, 94, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Singh, A.K.; Das, P.; Jana, P.; Kanvah, S.; Bhaktha BN, S.; Ramamurthy, S.S. Superior resonant nanocavities engineering on the photonic crystal-coupled emission platform for the detection of femtomolar iodide and zeptomolar cortisol. ACS Appl. Mater. Interfaces 2020, 12, 34323–34336. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Saylan, Y.; Göktürk, I.; Yılmaz, F.; Denizli, A. Selective amplification of plasmonic sensor signal for cortisol detection using gold nanoparticles. Biosensors 2022, 12, 482. [Google Scholar] [CrossRef]
- Wu, T.; Ding, L.; Zhang, Y.; Fang, W. A simple cortisol biosensor based on AuNPs-DNA aptamer conjugate. IEEE Sens. J. 2022, 22, 12485–12492. [Google Scholar] [CrossRef]
- Jo, S.; Lee, W.; Park, J.; Kim, W.; Kim, W.; Lee, G.; Lee, H.J.; Hong, J.; Park, J. Localized surface plasmon resonance aptasensor for the highly sensitive direct detection of cortisol in human saliva. Sens. Actuators B Chem. 2020, 304, 127424. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Z.; Zhang, Y.; Song, Y.; Yang, H.; Wang, F. Molecularly imprinted Monolithic column-based SERS sensor for selective detection of cortisol in dog saliva. Talanta 2022, 249, 123609. [Google Scholar] [CrossRef]
- Mugo, S.M.; Alberkant, J.; Bernstein, N.; Zenkina, O.V. Flexible electrochemical aptasensor for cortisol detection in human sweat. Anal. Methods 2021, 13, 4169–4173. [Google Scholar] [CrossRef]
- Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci. Rep. 2019, 9, 403. [Google Scholar] [CrossRef]
- Goyal, A.; Sakata, T. Development of a redox-label-doped molecularly imprinted polymer on β-cyclodextrin/reduced graphene oxide for electrochemical detection of a stress biomarker. ACS Omega 2022, 7, 33491–33499. [Google Scholar] [CrossRef]
- Mugo, S.M.; Alberkant, J. Flexible molecularly imprinted electrochemical sensor for cortisol monitoring in sweat. Anal. Bioanal. Chem. 2020, 412, 1825–1833. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, W.; Tian, L.; Su, M.; Jiang, M.; Li, J.; Gu, H.; Yu, C. Preparation of nanostructured PDMS film as flexible immunosensor for cortisol analysis in human sweat. Anal. Chim. Acta 2021, 1184, 339010. [Google Scholar] [CrossRef]
- Liu, J.; Xu, N.; Men, H.; Li, S.; Lu, Y.; Low, S.S.; Li, X.; Zhu, L.; Cheng, C.; Xu, G.; et al. Salivary cortisol determination on smartphone-based differential pulse voltammetry system. Sensors 2020, 20, 1422. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, H.; Ye, H.; Chen, Z.; Jaffrezic-Renault, N.; Guo, Z. An ultrasensitive aptamer–antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens. Bioelectron. 2021, 190, 113451. [Google Scholar] [CrossRef]
- Rezapoor-Fashtali, Z.; Ganjali, M.R.; Faridbod, F. A novel electrochemical aptasensor based on a new platform of Samarium molybdate flower-like nanoparticles@ Poly(pyrrole) for non-invasive determination of saliva cortisol. Biosensors 2022, 12, 720. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Sveiven, M.; Hall, D.A. Cortisol detection in undiluted human serum using a sensitive electrochemical structure-switching aptamer over an antifouling nanocomposite layer. ACS Omega 2021, 6, 27888–27897. [Google Scholar] [CrossRef]
- Duan, D.; Lu, H.; Li, L.; Ding, Y.; Ma, G. A molecularly imprinted electrochemical sensors based on bamboo-like carbon nanotubes loaded with nickel nanoclusters for highly selective detection of cortisol. Microchem. J. 2022, 175, 107231. [Google Scholar] [CrossRef]
- Cui, X.; Han, J.; Chen, G.; Wang, L.; Luo, Z.; Chang, C.; Zhang, J.; Fu, Q. Development of a highly sensitive imprinted electrochemical sensor for the detection of hydrocortisone in wastewater. J. Electrochem. Soc. 2021, 168, 057508. [Google Scholar] [CrossRef]
- Yeasmin, S.; Wu, B.; Liu, Y.; Ullah, A.; Cheng, L.J. Nano gold-doped molecularly imprinted electrochemical sensor for rapid and ultrasensitive cortisol detection. Biosens. Bioelectron. 2022, 206, 114142. [Google Scholar] [CrossRef] [PubMed]
- Uygun, H.D.E.; Uygun, Z.O.; Canbay, E.; Sağın, F.G.; Sezer, E. Non-invasive cortisol detection in saliva by using molecularly cortisol imprinted fullerene-acrylamide modified screen printed electrodes. Talanta 2020, 206, 120225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kannan, P.; Natarajan, B.; Maiyalagan, T.; Subramanian, P.; Jiang, Z.; Mao, S. MnO2 cacti-like nanostructured platform powers the enhanced electrochemical immunobiosensing of cortisol. Sens. Actuators B Chem. 2020, 317, 128134. [Google Scholar] [CrossRef]
- San Nah, J.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar]
- Mahmoodi, S.R.; Xie, P.; Zachs, D.P.; Peterson, E.J.; Graham, R.S.; Kaiser, C.R.; Lim, H.H.; Allen, M.G.; Javanmard, M. Single-step label-free nanowell immunoassay accurately quantifies serum stress hormones within minutes. Sci. Adv. 2021, 7, eabf4401. [Google Scholar] [CrossRef]
- Pusomjit, P.; Teengam, P.; Thepsuparungsikul, N.; Sanongkiet, S.; Chailapakul, O. Impedimetric determination of cortisol using screen-printed electrode with aptamer-modified magnetic beads. Microchim. Acta 2021, 188, 1–8. [Google Scholar] [CrossRef]
- Lee, H.B.; Meeseepong, M.; Trung, T.Q.; Kim, B.Y.; Lee, N.E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 2020, 156, 112133. [Google Scholar] [CrossRef]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.M.; Teymourian, H.; Wang, J. Touch-based stressless cortisol sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef]
- Naik, A.R.; Zhou, Y.; Dey, A.A.; Arellano, D.L.G.; Okoroanyanwu, U.; Secor, E.B.; Hersam, M.C.; Morse, J.; Rothstein, J.P.; Carter, K.R.; et al. Printed microfluidic sweat sensing platform for cortisol and glucose detection. Lab A Chip 2022, 22, 156–169. [Google Scholar] [CrossRef]
- Wang, X.; Walt, D.R. Simultaneous detection of small molecules, proteins and microRNAs using single molecule arrays. Chem. Sci. 2020, 11, 7896–7903. [Google Scholar] [CrossRef]
- Yang, S.; Dai, F.; Lu, L.; Yin, M.; Xue, L.; Feng, W.; Li, B.; Jiao, J.; Chen, Q. All-in-one calcium nanoflowers for dual outputs biosensor: A simultaneous strategy for depression drug evaluation and non-invasive stress assessment. Biosens. Bioelectron. 2022, 216, 114655. [Google Scholar] [CrossRef]
- Vargas, E.; Povedano, E.; Krishnan, S.; Teymourian, H.; Tehrani, F.; Campuzano, S.; Dassau, E.; Wang, J. Simultaneous cortisol/insulin microchip detection using dual enzyme tagging. Biosens. Bioelectron. 2020, 167, 112512. [Google Scholar] [CrossRef]
- Dong, J.; Miyake, C.; Yasuda, T.; Oyama, H.; Morita, I.; Tsukahara, T.; Takahashi, M.; Jeong, H.J.; Kitaguchi, T.; Kobayashi, N.; et al. PM Q-probe: A fluorescent binding protein that converts many antibodies to a fluorescent biosensor. Biosens. Bioelectron. 2020, 165, 112425. [Google Scholar] [CrossRef]
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO nanorod integrated flexible carbon fibers for sweat cortisol detection. ACS Appl. Electron. Mater. 2020, 2, 499–509. [Google Scholar] [CrossRef]
- Santiago, E.; Poudyal, S.S.; Shin, S.Y.; Yoon, H.J. Graphene oxide functionalized biosensor for detection of stress-related biomarkers. Sensors 2022, 22, 558. [Google Scholar] [CrossRef]
- van Smeden, L.; Saris, A.; Sergelen, K.; de Jong, A.M.; Yan, J.; Prins, M.W. Reversible immunosensor for the continuous monitoring of cortisol in blood plasma sampled with microdialysis. ACS Sens. 2022, 7, 3041–3048. [Google Scholar] [CrossRef]
- Buskermolen, A.D.; Lin, Y.T.; van Smeden, L.; van Haaften, R.B.; Yan, J.; Sergelen, K.; de Jong, A.M.; Prins, M.W. Continuous biomarker monitoring with single molecule resolution by measuring free particle motion. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Martin, J.A.; Chávez, J.L.; Chushak, Y.; Chapleau, R.R.; Hagen, J.; Kelley-Loughnane, N. Tunable stringency aptamer selection and gold nanoparticle assay for detection of cortisol. Anal. Bioanal. Chem. 2014, 406, 4637–4647. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Peng, Y.; Li, S.; Han, D.; Ren, S.u.; Qin, K.; Li, S.; Han, T.; Wang, Y.; et al. Exploring the performance of multi-channel tetrahedral nucleic acid tweezers platforms for efficient and sensitive biosensing. Chem. Eng. J. 2022, 448, 137635. [Google Scholar] [CrossRef]
- Karuppaiah, G.; Velayutham, J.; Hansda, S.; Narayana, N.; Bhansali, S.; Manickam, P. Towards the development of reagent-free and reusable electrochemical aptamer-based cortisol sensor. Bioelectrochemistry 2022, 145, 108098. [Google Scholar] [CrossRef]
- Yuan, Y.; Bali, A.; White, R.J.; Heikenfeld, J. Solution-phase electrochemical aptamer-based sensors. IEEE Trans. Biomed. Eng. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, C.; Wang, Z.; Yang, K.A.; Cheng, X.; Liu, W.; Yu, W.; Lin, S.; Zhao, Y.; Cheung, K.M.; et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022, 8, eabk0967. [Google Scholar] [CrossRef]
- Hayashi, H.; Toyama, R.; Takibuchi, R.; Hideshima, S.; Kuroiwa, S.; Kaneko, N.; Horii, K.; Ohashi, K.; Momma, T.; Osaka, T. Immobilization of target-bound aptamer on field effect transistor biosensor to improve sensitivity for detection of uncharged cortisol. Electrochemistry 2021, 89, 134–137. [Google Scholar] [CrossRef]
- Jeon, J.; Uthaman, S.; Lee, J.; Hwang, H.; Kim, G.; Yoo, P.J.; Hammock, B.D.; Kim, C.S.; Park, Y.S.; Park, I.K. In-direct localized surface plasmon resonance (LSPR)-based nanosensors for highly sensitive and rapid detection of cortisol. Sens. Actuators B Chem. 2018, 266, 710–716. [Google Scholar] [CrossRef]
- Villa, J.E.; Khan, S.; Neres, L.; Sotomayor, M.D. Preparation of a magnetic molecularly imprinted polymer for non-invasive determination of cortisol. J. Polym. Res. 2021, 28, 1–9. [Google Scholar] [CrossRef]
- Dykstra, G.; Reynolds, B.; Smith, R.; Zhou, K.; Liu, Y. Electropolymerized molecularly imprinted polymer synthesis guided by an integrated data-driven framework for cortisol detection. ACS Appl. Mater. Interfaces 2022, 14, 25972–25983. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. Ultrasensitive detection of seventeen chemicals simultaneously using paper-based sensors. Mater. Chem. Front. 2018, 2, 1900–1910. [Google Scholar] [CrossRef]
- Khelifa, L.; Hu, Y.; Jiang, N.; Yetisen, A.K. Lateral flow assays for hormone detection. Lab A Chip 2022, 22, 2451–2475. [Google Scholar] [CrossRef]
- Park, J.H.; Park, E.K.; Cho, Y.K.; Shin, I.S.; Lee, H. Normalizing the optical signal enables robust assays with lateral flow biosensors. ACS Omega 2022, 7, 17723–17731. [Google Scholar] [CrossRef]
- Aerathupalathu Janardhanan, J.; Chen, Y.L.; Liu, C.T.; Tseng, H.S.; Wu, P.I.; She, J.W.; Hsiao, Y.S.; Yu, H.h. Sensitive detection of sweat cortisol using an organic electrochemical transistor featuring nanostructured Poly(3, 4-Ethylenedioxythiophene) derivatives in the channel layer. Anal. Chem. 2022, 94, 7584–7593. [Google Scholar] [CrossRef]
- Park, H.; Baek, S.; Sen, A.; Jung, B.; Shim, J.; Park, Y.C.; Lee, L.P.; Kim, Y.J.; Kim, S. Ultrasensitive and selective field-effect transistor-based biosensor created by rings of MoS2 nanopores. ACS Nano 2021, 16, 1826–1835. [Google Scholar] [CrossRef]
- Ku, M.; Kim, J.; Won, J.E.; Kang, W.; Park, Y.G.; Park, J.; Lee, J.H.; Cheon, J.; Lee, H.H.; Park, J.U. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 2020, 6, eabb2891. [Google Scholar] [CrossRef]
- Demuru, S.; Kim, J.; El Chazli, M.; Bruce, S.; Dupertuis, M.; Binz, P.A.; Saubade, M.; Lafaye, C.; Briand, D. Antibody-coated wearable organic electrochemical transistors for cortisol detection in human sweat. ACS Sens. 2022, 7, 2721–2731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).