Fluorescence Modulation by Amines: Mechanistic Insights into Twisted Intramolecular Charge Transfer (TICT) and Beyond
Abstract
1. Introduction
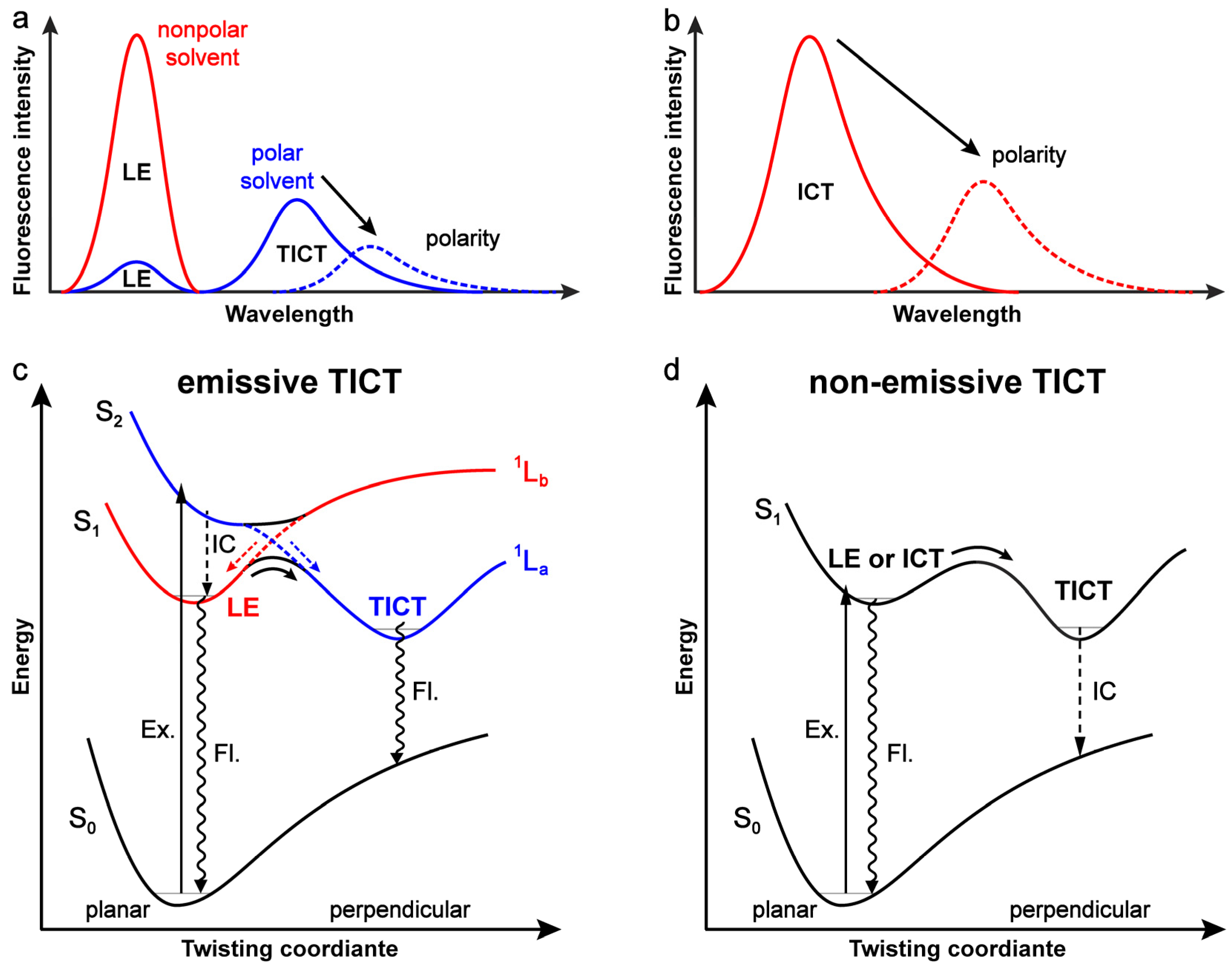
2. Insights into the Chromophores with an Emissive TICT State
2.1. Evidence for TICT
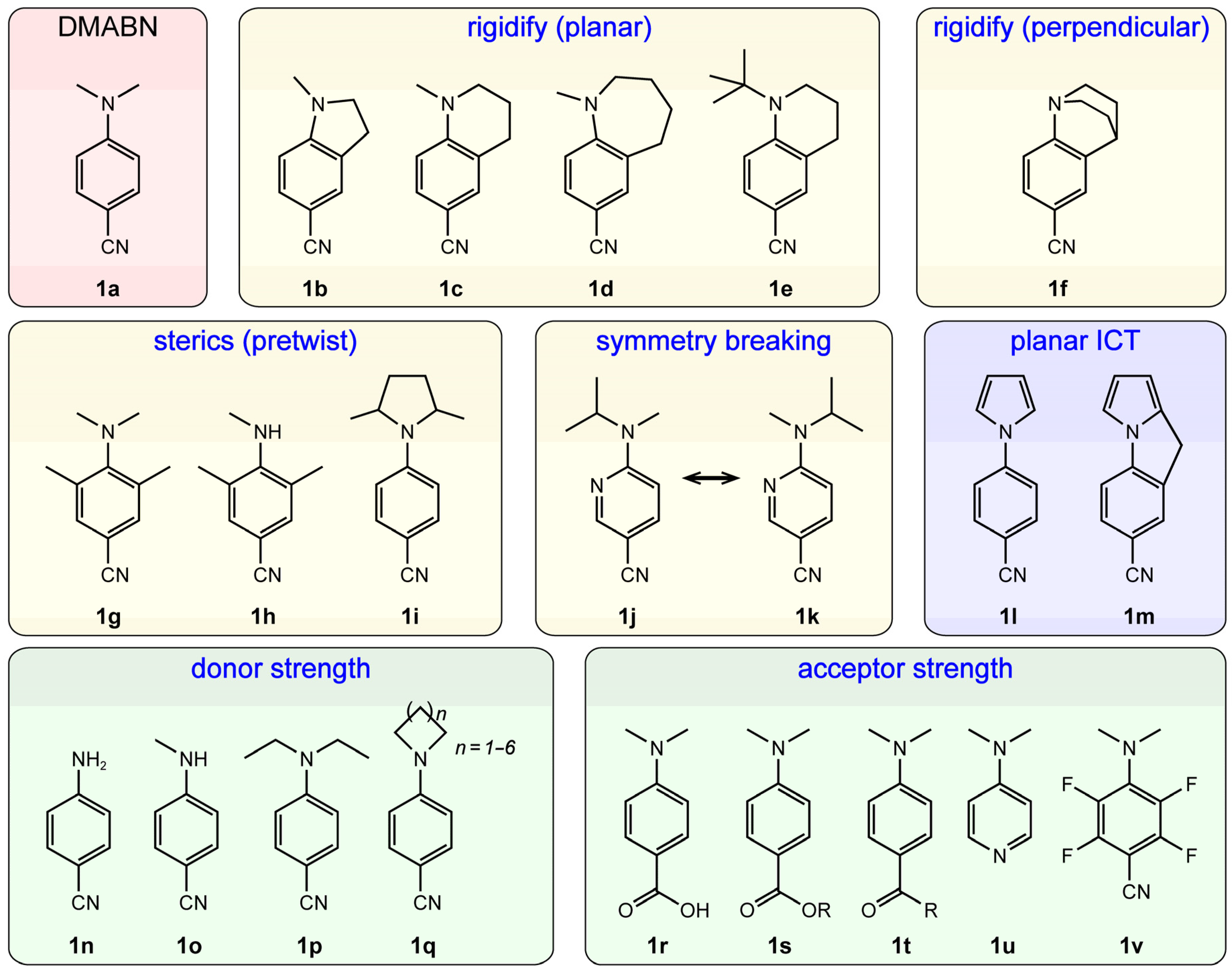
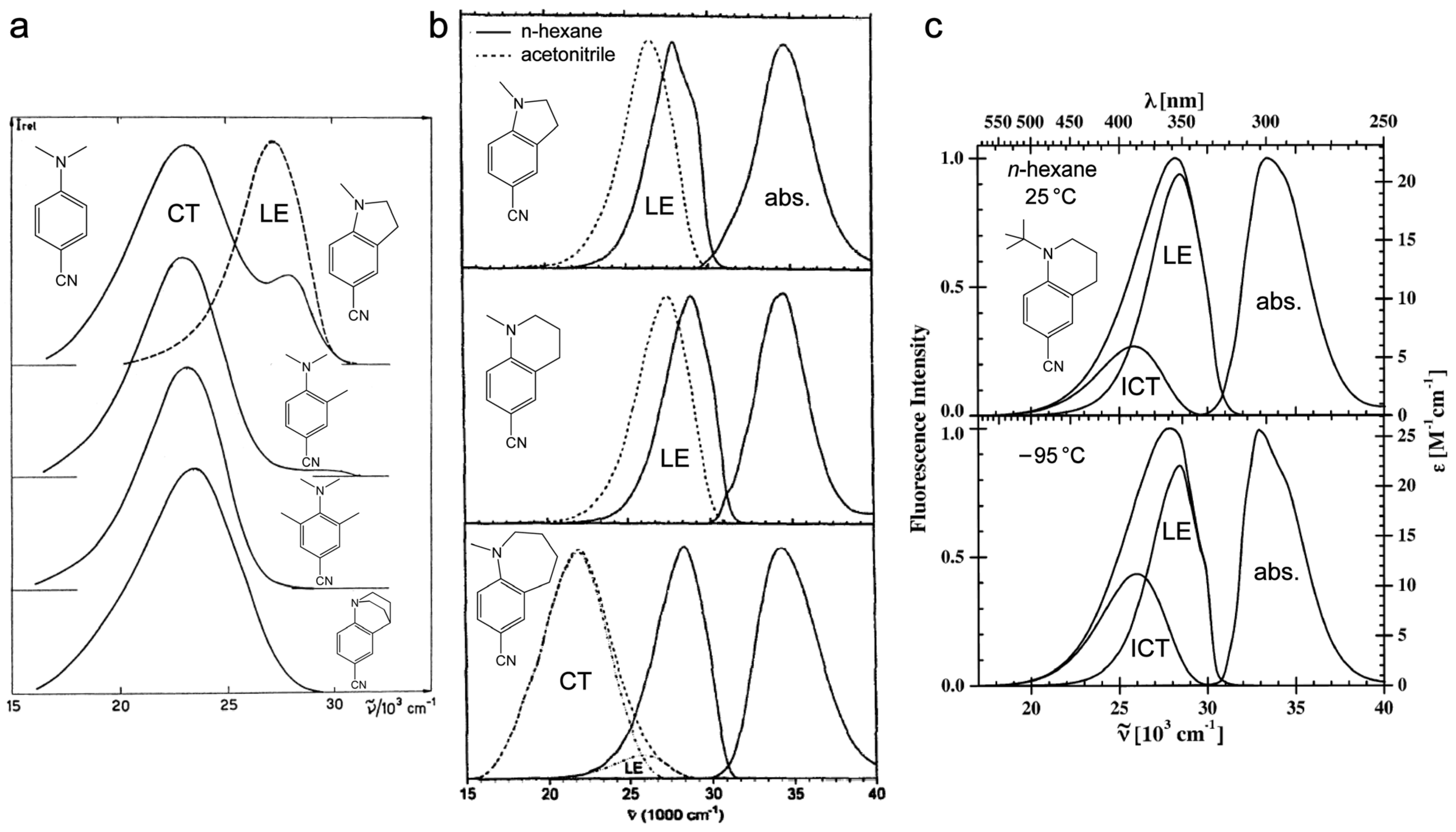
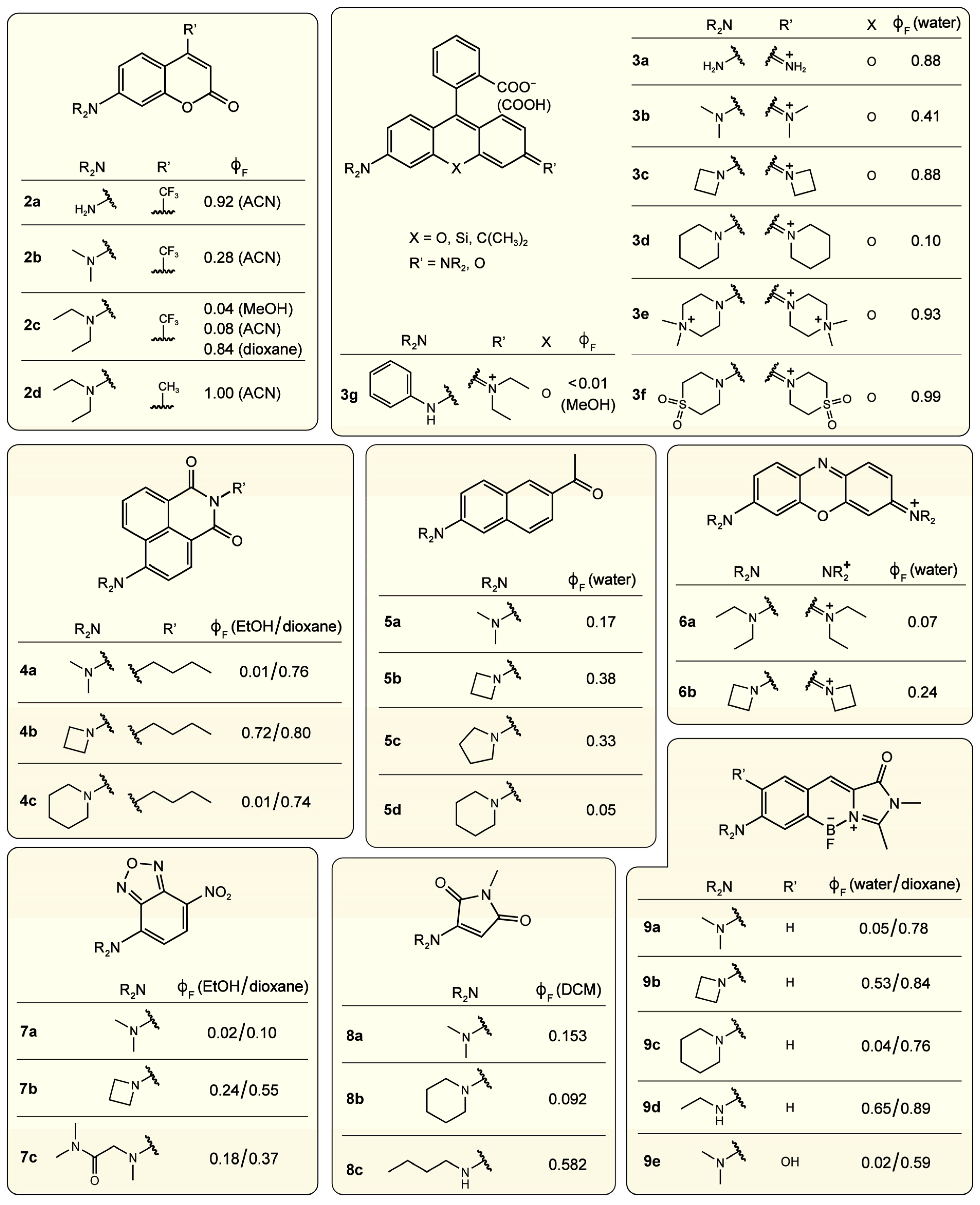
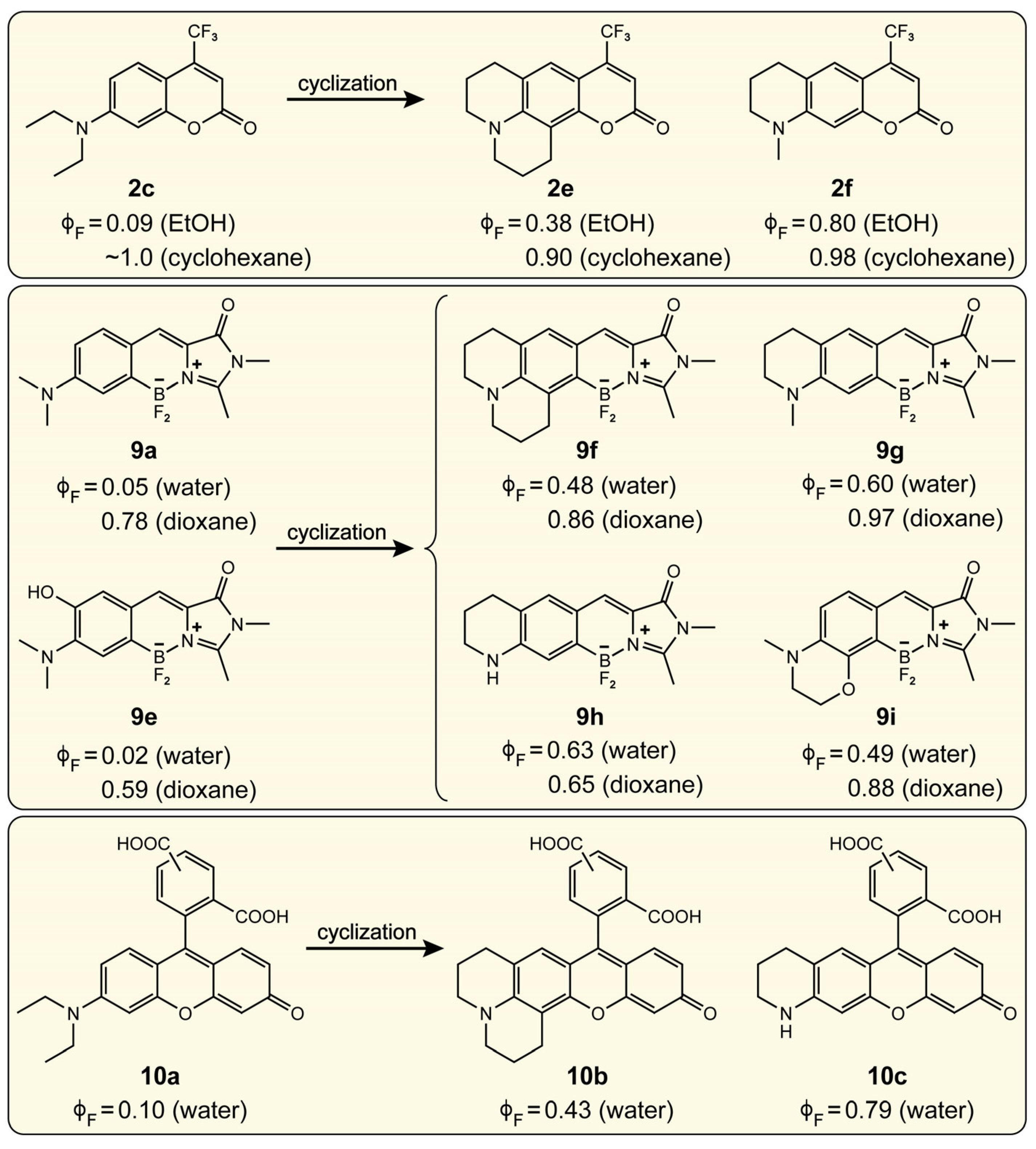
2.1.1. Strategic Modifications of Chemical Structure
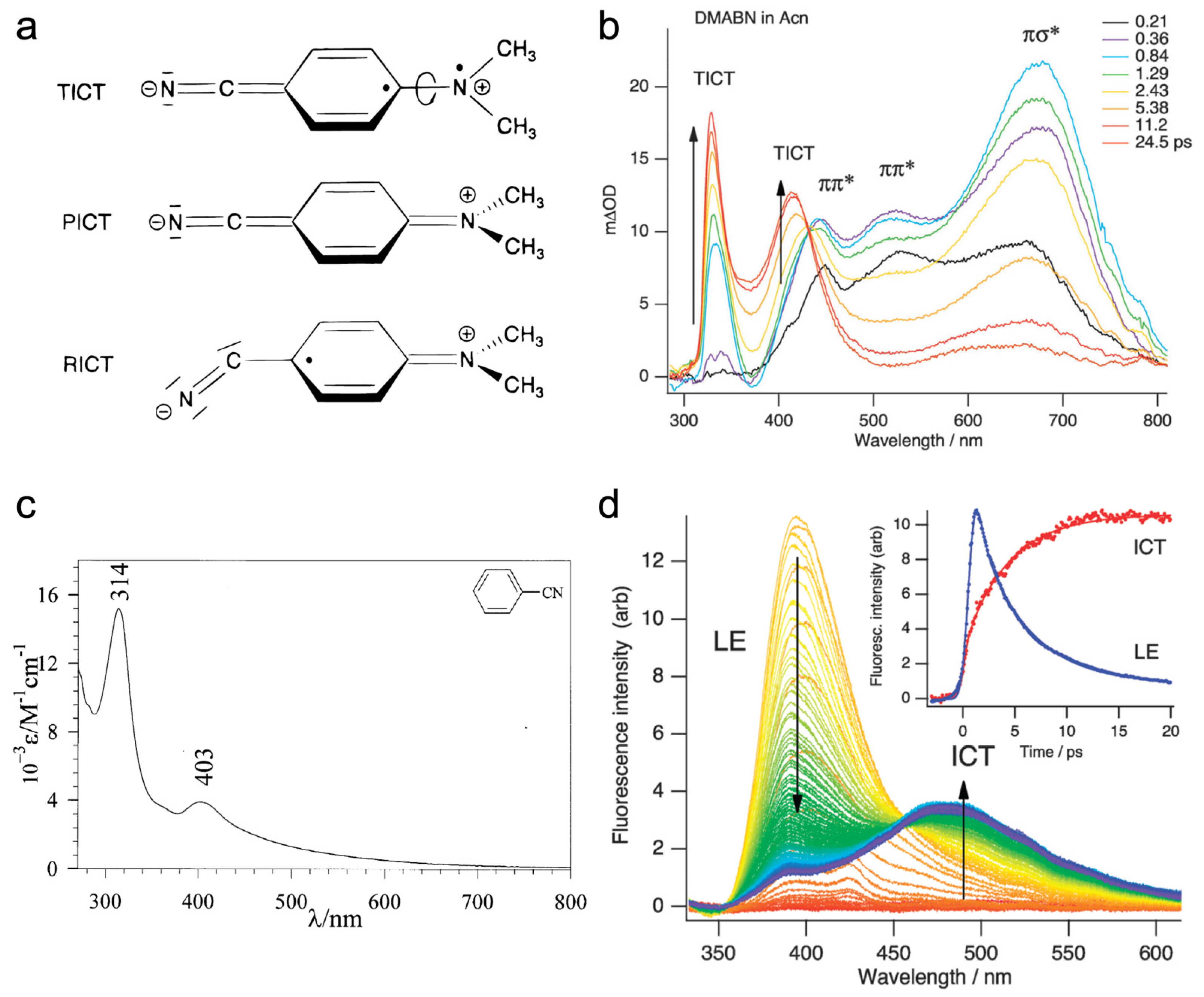
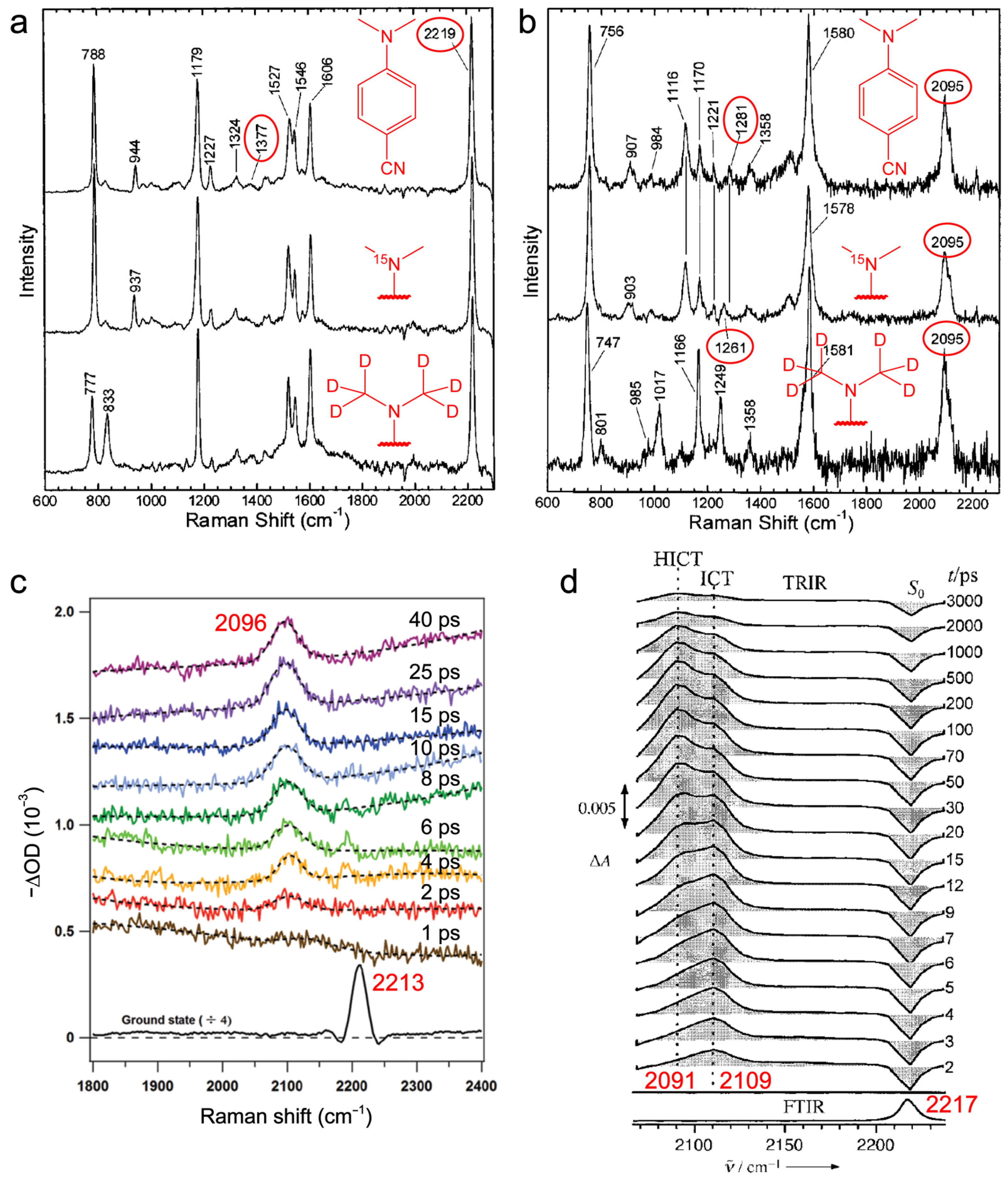
2.1.2. Time-Resolved Electronic and Vibrational Spectroscopies
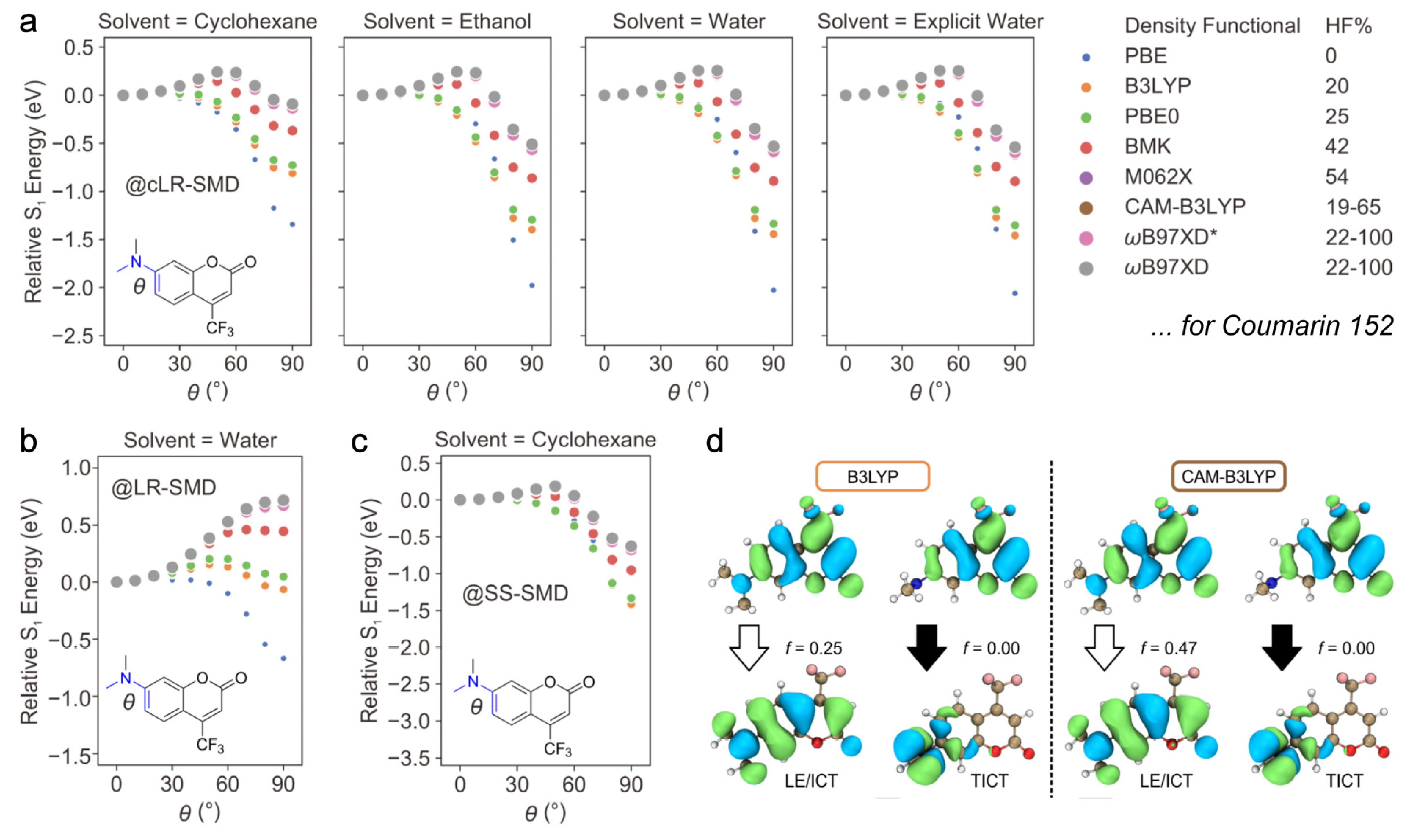
2.2. Potential Energy Surface for TICT in DMABN
3. Insights into Chromophores with a Non-Emissive TICT State
3.1. Rationalization of TICT
3.2. Comparison with TICT in DMABN
4. Factors Affecting TICT
4.1. Donor and Acceptor Strengths
4.2. Steric Hindrance and Structural Constraint
4.3. Solvent Polarity and Viscosity
4.4. Site Specificity of Donor and Acceptor
5. Brief Insights into Other Fluorescence Quenching Mechanisms
5.1. Intersystem Crossing
5.2. Energy Gap Law
5.3. Solvent-Assisted Electronic-to-Vibrational FRET
5.4. H-Bonding-Induced Fluorescence Quenching
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef]
- Xiao, H.; Li, P.; Tang, B. Recent progresses in fluorescent probes for detection of polarity. Coord. Chem. Rev. 2021, 427, 213582. [Google Scholar] [CrossRef]
- Qin, X.; Yang, X.; Du, L.; Li, M. Polarity-based fluorescence probes: Properties and applications. RSC Med. Chem. 2021, 12, 1826–1838. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G.-i. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chi, W.; Qiao, Q.; Tan, D.; Xu, Z.; Liu, X. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: From mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev. 2021, 50, 12656–12678. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, R.; Batsanov, A.S.; Pander, P.; Hsu, Y.-T.; Chi, Z.; Dias, F.B.; Bryce, M.R. Intramolecular charge transfer controls switching between room temperature phosphorescence and thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 2018, 57, 16407–16411. [Google Scholar] [CrossRef] [PubMed]
- Haberhauer, G.; Gleiter, R.; Burkhart, C. Planarized intramolecular charge transfer: A concept for fluorophores with both large Stokes shifts and high fluorescence quantum yields. Chem. Eur. J. 2016, 22, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Dereka, B.; Svechkarev, D.; Rosspeintner, A.; Tromayer, M.; Liska, R.; Mohs, A.M.; Vauthey, E. Direct observation of a photochemical alkyne–allene reaction and of a twisted and rehybridized intramolecular charge-transfer state in a donor–acceptor dyad. J. Am. Chem. Soc. 2017, 139, 16885–16893. [Google Scholar] [CrossRef]
- Stallhofer, K.; Nuber, M.; Schüppel, F.; Thumser, S.; Iglev, H.; de Vivie-Riedle, R.; Zinth, W.; Dube, H. Electronic and geometric characterization of TICT formation in hemithioindigo photoswitches by picosecond infrared spectroscopy. J. Phys. Chem. A 2021, 125, 4390–4400. [Google Scholar] [CrossRef] [PubMed]
- Rout, Y.; Montanari, C.; Pasciucco, E.; Misra, R.; Carlotti, B. Tuning the fluorescence and the intramolecular charge transfer of phenothiazine dipolar and quadrupolar derivatives by oxygen functionalization. J. Am. Chem. Soc. 2021, 143, 9933–9943. [Google Scholar] [CrossRef] [PubMed]
- Lippert, E.; Lüder, W.; Moll, F.; Nägele, W.; Boos, H.; Prigge, H.; Seibold–Blankenstein, I. Umwandlung von elektronenanregungsenergie. Angew. Chem. 1961, 73, 695–706. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; von der Haar, T.; Hebecker, A.; Leinhos, U.; Kuhnle, W. Intramolecular charge transfer in aminobenzonitriles: Requirements for dual fluorescence. Pure Appl. Chem. 1993, 65, 1745–1750. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Grobys, M.; von der Haar, T.; Hebecker, A.; Il’ichev, Y.V.; Morawski, O.; Rückert, I.; Kühnle, W. Photo-induced intramolecular charge transfer and internal conversion in molecules with a small energy gap between S1 and S2. Dynamics and structure. J. Photochem. Photobiol. A 1997, 105, 373–383. [Google Scholar] [CrossRef]
- Gorse, A.-D.; Pesquer, M. Intramolecular charge transfer excited state relaxation processes in para-substituted N,N-dimethylaniline: A theoretical study including solvent effects. J. Phys. Chem. 1995, 99, 4039–4049. [Google Scholar] [CrossRef]
- Parusel, A.B.J.; Köhler, G.; Grimme, S. Density functional study of excited charge transfer state formation in 4-(N,N-dimethylamino)benzonitrile. J. Phys. Chem. A 1998, 102, 6297–6306. [Google Scholar] [CrossRef]
- Sudholt, W.; Sobolewski, A.L.; Domcke, W. Ab initio study of the amino group twisting and wagging reaction paths in the intramolecular charge transfer of 4-(N,N-dimethylamino)benzonitrile. Chem. Phys. 1999, 240, 9–18. [Google Scholar] [CrossRef]
- Sobolewski, A.L.; Domcke, W. Charge transfer in aminobenzonitriles: Do they twist? Chem. Phys. Lett. 1996, 250, 428–436. [Google Scholar] [CrossRef]
- Sobolewski, A.L.; Domcke, W. Promotion of intramolecular charge transfer in dimethylamino derivatives: Twisting versus acceptor-group rehybridization. Chem. Phys. Lett. 1996, 259, 119–127. [Google Scholar] [CrossRef]
- Dreyer, J.; Kummrow, A. Shedding light on excited-state structures by theoretical analysis of femtosecond transient infrared spectra: Intramolecular charge transfer in 4-(dimethylamino)benzonitrile. J. Am. Chem. Soc. 2000, 122, 2577–2585. [Google Scholar] [CrossRef]
- Yoshihara, T.; Druzhinin, S.I.; Zachariasse, K.A. Fast intramolecular charge transfer with a planar rigidized electron donor/acceptor molecule. J. Am. Chem. Soc. 2004, 126, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, Z.; Zhang, Q. Computational characterization of low-lying states and intramolecular charge transfers in N-phenylpyrrole and the planar-rigidized fluorazene. J. Phys. Chem. A 2006, 110, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.; Zilberg, S.; Haas, Y. The electronic origin of the dual fluorescence in donor−acceptor substituted benzene derivatives. J. Am. Chem. Soc. 2006, 128, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Druzhinin, S.I.; Kovalenko, S.A.; Senyushkina, T.; Zachariasse, K.A. Dynamics of ultrafast intramolecular charge transfer with 1-tert-butyl-6-cyano-1,2,3,4-tetrahydroquinoline (NTC6) in n-hexane and acetonitrile. J. Phys. Chem. A 2007, 111, 12878–12890. [Google Scholar] [CrossRef] [PubMed]
- Hättig, C.; Hellweg, A.; Köhn, A. Intramolecular charge-transfer mechanism in quinolidines: The role of the amino twist angle. J. Am. Chem. Soc. 2006, 128, 15672–15682. [Google Scholar] [CrossRef]
- Galievsky, V.A.; Druzhinin, S.I.; Demeter, A.; Jiang, Y.-B.; Kovalenko, S.A.; Pérez Lustres, L.; Venugopal, K.; Ernsting, N.P.; Allonas, X.; Noltemeyer, M.; et al. Ultrafast intramolecular charge transfer and internal conversion with tetrafluoro-aminobenzonitriles. ChemPhysChem 2005, 6, 2307–2323. [Google Scholar] [CrossRef]
- Jones, G., II; Jackson, W.R.; Choi, C.Y.; Bergmark, W.R. Solvent effects on emission yield and lifetime for coumarin laser dyes. Requirements for a rotatory decay mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar] [CrossRef]
- Vogel, M.; Rettig, W.; Sens, R.; Drexhage, K.H. Structural relaxation of rhodamine dyes with different N-substitution patterns: A study of fluorescence decay times and quantum yields. Chem. Phys. Lett. 1988, 147, 452–460. [Google Scholar] [CrossRef]
- Peng, T.; Yang, D. Construction of a library of rhodol fluorophores for developing new fluorescent probes. Org. Lett. 2010, 12, 496–499. [Google Scholar] [CrossRef]
- Saha, S.; Samanta, A. Influence of the structure of the amino group and polarity of the medium on the photophysical behavior of 4-amino-1,8-naphthalimide derivatives. J. Phys. Chem. A 2002, 106, 4763–4771. [Google Scholar] [CrossRef]
- Nad, S.; Kumbhakar, M.; Pal, H. Photophysical properties of coumarin-152 and coumarin-481 dyes: Unusual behavior in nonpolar and in higher polarity solvents. J. Phys. Chem. A 2003, 107, 4808–4816. [Google Scholar] [CrossRef]
- Soujanya, T.; Fessenden, R.W.; Samanta, A. Role of nonfluorescent twisted intramolecular charge transfer state on the photophysical behavior of aminophthalimide dyes. J. Phys. Chem. 1996, 100, 3507–3512. [Google Scholar] [CrossRef]
- Baranov, M.S.; Solntsev, K.M.; Baleeva, N.S.; Mishin, A.S.; Lukyanov, S.A.; Lukyanov, K.A.; Yampolsky, I.V. Red-shifted fluorescent aminated derivatives of a conformationally locked GFP chromophore. Chem. Eur. J. 2014, 20, 13234–13241. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Boulanger, S.A.; Sokolov, A.I.; Baranov, M.S.; Fang, C. A novel dialkylamino GFP chromophore as an environment-polarity sensor reveals the role of twisted intramolecular charge transfer. Chemosensors 2021, 9, 234. [Google Scholar] [CrossRef]
- Chi, W.; Qiao, Q.; Wang, C.; Zheng, J.; Zhou, W.; Xu, N.; Wu, X.; Jiang, X.; Tan, D.; Xu, Z.; et al. Descriptor ΔGc-o enables the quantitative design of spontaneously blinking rhodamines for live-cell super-resolution imaging. Angew. Chem. Int. Ed. 2020, 59, 20215–20223. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, W.; Wang, C.; Zheng, Y.; Chi, W.; Liu, X.; Huang, Z.; Li, X.; Xiao, Y. Quaternary piperazine-substituted rhodamines with enhanced brightness for super-resolution imaging. J. Am. Chem. Soc. 2019, 141, 14491–14495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Ren, T.-B.; D’Este, E.; Xiong, M.; Xiong, B.; Johnsson, K.; Zhang, X.-B.; Wang, L.; Yuan, L. A synergistic strategy to develop photostable and bright dyes with long Stokes shift for nanoscopy. Nat. Commun. 2022, 13, 2264. [Google Scholar] [CrossRef]
- Rotkiewicz, K.; Grellmann, K.H.; Grabowski, Z.R. Reinterpretation of the anomalous fluorescense of p-N,N-dimethylamino-benzonitrile. Chem. Phys. Lett. 1973, 19, 315–318. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Siemiarczuk, A. Dual fluorescence of donor-acceptor molecules and the twisted intramolecular charge transfer (TICT) states. J. Lumin. 1979, 18-19, 420–424. [Google Scholar] [CrossRef]
- Demeter, A.; Druzhinin, S.; George, M.; Haselbach, E.; Roulin, J.-L.; Zachariasse, K.A. Dual fluorescence and fast intramolecular charge transfer with 4-(diisopropylamino)benzonitrile in alkane solvents. Chem. Phys. Lett. 2000, 323, 351–360. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Druzhinin, S.I.; Bosch, W.; Machinek, R. Intramolecular charge transfer with the planarized 4-aminobenzonitrile 1-tert-butyl-6-cyano-1,2,3,4-tetrahydroquinoline (NTC6). J. Am. Chem. Soc. 2004, 126, 1705–1715. [Google Scholar] [CrossRef]
- Rotkiewicz, K.; Rubaszewska, W. Intramolecular charge transfer state and unusual fluorescence from an upper excited singlet of a nonplanar derivative of p-cyano-N, N-dimethylaniline. J. Lumin. 1982, 27, 221–230. [Google Scholar] [CrossRef]
- Rotkiewicz, K.; Rubaszewska, W.x. Intramolecular electron-transfer excited state in 6-cyanobenzquinuclidine. Chem. Phys. Lett. 1980, 70, 444–448. [Google Scholar] [CrossRef]
- Köhler, G.; Rechthaler, K.; Grabner, G.; Luboradzki, R.; Suwińska, K.; Rotkiewicz, K. Structure of cage amines as models for twisted intramolecular charge-transfer states. J. Phys. Chem. A 1997, 101, 8518–8525. [Google Scholar] [CrossRef]
- Bulliard, C.; Allan, M.; Wirtz, G.; Haselbach, E.; Zachariasse, K.A.; Detzer, N.; Grimme, S. Electron energy loss and DFT/SCI study of the singlet and triplet excited states of aminobenzonitriles and benzoquinuclidines: Role of the amino group twist angle. J. Phys. Chem. A 1999, 103, 7766–7772. [Google Scholar] [CrossRef]
- Rettig, W.; Gleiter, R. Dependence of intramolecular rotation in p-cyano-N,N-dialkylanilines on the twist angle. A fluorescence, UV absorption, and photoelectron spectroscopic study. J. Phys. Chem. 1985, 89, 4676–4680. [Google Scholar] [CrossRef]
- Dobkowski, J.; Michl, J.; Waluk, J. Electronic spectroscopy and photophysics of 2-(N-methyl-N-isopropylamino)-5-cyanopyridine and related compounds. Phys. Chem. Chem. Phys. 2003, 5, 1027–1031. [Google Scholar] [CrossRef]
- Dobkowski, J.; Wójcik, J.; Koźmiński, W.; Kołos, R.; Waluk, J.; Michl, J. An experimental test of C−N bond twisting in the TICT state: Syn−anti photoisomerization in 2-(N-methyl-N-isopropylamino)-5-cyanopyridine. J. Am. Chem. Soc. 2002, 124, 2406–2407. [Google Scholar] [CrossRef]
- Cornelissen-Gude, C.; Rettig, W. Dual fluorescence and multiple charge transfer nature in derivatives of N-pyrrolobenzonitrile. J. Phys. Chem. A 1998, 102, 7754–7760. [Google Scholar] [CrossRef]
- Bohnwagner, M.V.; Burghardt, I.; Dreuw, A. Solvent polarity tunes the barrier height for twisted intramolecular charge transfer in N-pyrrolobenzonitrile (PBN). J. Phys. Chem. A 2016, 120, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Schuddeboom, W.; Jonker, S.A.; Warman, J.M.; Leinhos, U.; Kuehnle, W.; Zachariasse, K.A. Excited-state dipole moments of dual fluorescent 4-(dialkylamino)benzonitriles: Influence of alkyl chain length and effective solvent polarity. J. Phys. Chem. 1992, 96, 10809–10819. [Google Scholar] [CrossRef]
- von Der Haar, T.; Hebecker, A.; Il’Ichev, Y.; Jiang, Y.-B.; Kühnle, W.; Zachariasse, K.A. Excited-state intramolecular charge transfer in donor/acceptor-substituted aromatic hydrocarbons and in biaryls. The significance of the redox potentials of the D/A subsystems. Recl. Trav. Chim. Pays-Bas 1995, 114, 430–442. [Google Scholar] [CrossRef]
- Cowley, D.J.; Peoples, A.H. Rotational isomerism and dual luminescence in dipolar dialkylamino-compounds. J. Chem. Soc., Chem. Commun. 1977, 352–353. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Dobkowski, J.; Kühnle, W. Model compounds in study of the photophysical behaviour of carbonyl derivatives of N,N-dimethylaniline. J. Mol. Struct. 1984, 114, 93–100. [Google Scholar] [CrossRef]
- Herbich, J.; Waluk, J. Excited charge transfer states in 4-aminopyrimidines, 4-(dimethylanilino)pyrimidine and 4-(dimethylamino)pyridne. Chem. Phys. 1994, 188, 247–265. [Google Scholar] [CrossRef]
- Gustavsson, T.; Coto, P.B.; Serrano-Andrés, L.; Fujiwara, T.; Lim, E.C. Do fluorescence and transient absorption probe the same intramolecular charge transfer state of 4-(dimethylamino)benzonitrile? J. Chem. Phys. 2009, 131, 031101. [Google Scholar] [CrossRef]
- Fujiwara, T.; Lee, J.-K.; Zgierski, M.Z.; Lim, E.C. Photophysical and spectroscopic manifestations of the low-lying πσ* state of 4-(dimethylamino)benzethyne: Solvent-polarity dependence of fluorescence and excited-state absorptions. Phys. Chem. Chem. Phys. 2009, 11, 2475–2479. [Google Scholar] [CrossRef]
- Coto, P.B.; Serrano-Andrés, L.; Gustavsson, T.; Fujiwara, T.; Lim, E.C. Intramolecular charge transfer and dual fluorescence of 4-(dimethylamino)benzonitrile: Ultrafast branching followed by a two-fold decay mechanism. Phys. Chem. Chem. Phys. 2011, 13, 15182–15188. [Google Scholar] [CrossRef]
- Fujiwara, T.; Zgierski, M.Z.; Lim, E.C. The role of the πσ* state in intramolecular charge transfer of 4-(dimethylamino)benzonitrile. Phys. Chem. Chem. Phys. 2011, 13, 6779–6783. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Druzhinin, S.I.; Kovalenko, S.A.; Senyushkina, T. Intramolecular charge transfer of 4-(dimethylamino)benzonitrile probed by time-resolved fluorescence and transient absorption: No evidence for two ICT states and a πσ* reaction intermediate. J. Chem. Phys. 2009, 131, 224313. [Google Scholar] [CrossRef] [PubMed]
- Fdez. Galván, I.; Martín, M.E.; Aguilar, M.A. On the absorption properties of the excited states of DMABN. Chem. Phys. Lett. 2010, 499, 100–102. [Google Scholar] [CrossRef]
- Pedersen, S.U.; Bo Christensen, T.; Thomasen, T.; Daasbjerg, K. New methods for the accurate determination of extinction and diffusion coefficients of aromatic and heteroaromatic radical anions in N,N-dimethylformamide. J. Electroanal. Chem. 1998, 454, 123–143. [Google Scholar] [CrossRef]
- Okada, T.; Uesugi, M.; Köhler, G.; Rechthaler, K.; Rotkiewicz, K.; Rettig, W.; Grabner, G. Time-resolved spectroscopy of DMABN and its cage derivatives 6-cyanobenzquinuclidine (CBQ) and benzquinuclidine (BQ). Chem. Phys. 1999, 241, 327–337. [Google Scholar] [CrossRef]
- Chutny, B.; Swallow, A.J. Aromatic anions and free radicals in the pulse radiolysis of aqueous solutions of benzonitrile. Trans. Faraday Soc. 1970, 66, 2847–2854. [Google Scholar] [CrossRef]
- Kwok, W.M.; Ma, C.; Matousek, P.; Parker, A.W.; Phillips, D.; Toner, W.T.; Towrie, M.; Umapathy, S. A determination of the structure of the intramolecular charge transfer state of 4-dimethylaminobenzonitrile (DMABN) by time-resolved resonance Raman spectroscopy. J. Phys. Chem. A 2001, 105, 984–990. [Google Scholar] [CrossRef]
- Rhinehart, J.M.; Challa, J.R.; McCamant, D.W. Multimode charge-transfer dynamics of 4-(dimethylamino)benzonitrile probed with ultraviolet femtosecond stimulated Raman spectroscopy. J. Phys. Chem. B 2012, 116, 10522–10534. [Google Scholar] [CrossRef]
- Kwok, W.-M.; George, M.W.; Grills, D.C.; Ma, C.; Matousek, P.; Parker, A.W.; Phillips, D.; Toner, W.T.; Towrie, M. Direct observation of a hydrogen-bonded charge-transfer state of 4-dimethylaminobenzonitrile in methanol by time-resolved IR spectroscopy. Angew. Chem. Int. Ed. 2003, 42, 1826–1830. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hamaguchi, H.-o. Structure of the twisted-intramolecular-charge-transfer excited singlet and triplet states of 4-(dimethylamino)benzonitrile as studied by nanosecond time-resolved infrared spectroscopy. J. Phys. Chem. 1995, 99, 7875–7877. [Google Scholar] [CrossRef]
- Kwok, W.M.; Ma, C.; George, M.W.; Grills, D.C.; Matousek, P.; Parker, A.W.; Phillips, D.; Toner, W.T.; Towrie, M. Solvent effects on the charge transfer excited states of 4-dimethylaminobenzonitrile (DMABN) and 4-dimethylamino-3,5-dimethylbenzonitrile (TMABN) studied by time-resolved infrared spectroscopy: A direct observation of hydrogen bonding interactions. Photochem. Photobiol. Sci. 2007, 6, 987–994. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Han, K.-L. Time-dependent density functional theory study on hydrogen-bonded intramolecular charge-transfer excited state of 4-dimethylamino-benzonitrile in methanol. J. Comput. Chem. 2008, 29, 2010–2017. [Google Scholar] [CrossRef]
- Juchnovski, I.; Tsvetanov, C.; P anayotov, I. IR-spektren der anion-radikale von aromatischen mononitrilen und elektronenübergänge zwischen anion-radikalen und neutralen molekülen. Monatsh. Chem. 1969, 100, 1980–1992. [Google Scholar] [CrossRef]
- Juchnovski, I.N.; Binev, I.G. Relation between the electronic structure and infrared frequencies of the cyano-group in alkali-metal complexes of some substituted benzonitriles. J. Mol. Struct. 1971, 7, 490–494. [Google Scholar] [CrossRef]
- Sobolewski, A.L.; Sudholt, W.; Domcke, W. Ab initio investigation of reaction pathways for intramolecular charge transfer in dimethylanilino derivatives. J. Phys. Chem. A 1998, 102, 2716–2722. [Google Scholar] [CrossRef]
- Serrano-Andres, L.; Merchan, M.; Roos, B.O.; Lindh, R. Theoretical study of the internal charge transfer in aminobenzonitriles. J. Am. Chem. Soc. 1995, 117, 3189–3204. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Photoinduced intramolecular charge transfer in 4-(dimethyl)aminobenzonitrile—A theoretical perspective. J. Am. Chem. Soc. 2004, 126, 1277–1284. [Google Scholar] [CrossRef]
- Parusel, A.B.J. A DFT/MRCI study on the excited state charge transfer states of N-pyrrolobenzene, N-pyrrolobenzonitrile and 4-N,N-dimethylaminobenzonitrile. Phys. Chem. Chem. Phys. 2000, 2, 5545–5552. [Google Scholar] [CrossRef]
- Fuß, W.; Pushpa, K.K.; Rettig, W.; Schmid, W.E.; Trushin, S.A. Ultrafast charge transfer via a conical intersection in dimethylaminobenzonitrile. Photochem. Photobiol. Sci. 2002, 1, 255–262. [Google Scholar] [CrossRef]
- Druzhinin, S.I.; Ernsting, N.P.; Kovalenko, S.A.; Lustres, L.P.; Senyushkina, T.A.; Zachariasse, K.A. Dynamics of ultrafast intramolecular charge transfer with 4-(dimethylamino)benzonitrile in acetonitrile. J. Phys. Chem. A 2006, 110, 2955–2969. [Google Scholar] [CrossRef]
- Druzhinin, S.I.; Galievsky, V.A.; Demeter, A.; Kovalenko, S.A.; Senyushkina, T.; Dubbaka, S.R.; Knochel, P.; Mayer, P.; Grosse, C.; Stalke, D.; et al. Two-state intramolecular charge transfer (ICT) with 3,5-dimethyl-4-(dimethylamino)benzonitrile (MMD) and its meta-isomer mMMD. Ground state amino twist not essential for ICT. J. Phys. Chem. A 2015, 119, 11820–11836. [Google Scholar] [CrossRef]
- Dahl, K.; Biswas, R.; Ito, N.; Maroncelli, M. Solvent dependence of the spectra and kinetics of excited-state charge transfer in three (alkylamino)benzonitriles. J. Phys. Chem. B 2005, 109, 1563–1585. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Reguero, M.; Boggio-Pasqua, M.; Robb, M.A. Intramolecular charge transfer in 4-aminobenzonitriles does not necessarily need the twist. J. Am. Chem. Soc. 2005, 127, 7119–7129. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiao, Q.; Tian, W.; Liu, W.; Chen, J.; Lang, M.J.; Xu, Z. Aziridinyl fluorophores demonstrate bright fluorescence and superior photostability by effectively inhibiting twisted intramolecular charge transfer. J. Am. Chem. Soc. 2016, 138, 6960–6963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiao, Q.; Chi, W.; Chen, J.; Liu, W.; Tan, D.; McKechnie, S.; Lyu, D.; Jiang, X.-F.; Zhou, W.; et al. Quantitative design of bright fluorophores and AIEgens by the accurate prediction of twisted intramolecular charge transfer (TICT). Angew. Chem. Int. Ed. 2020, 59, 10160–10172. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yao, L.; Luo, L.; Wang, H.-X.; Yang, Z.; Wang, Z.; Ai, S.-L.; Zhang, Y.; Zou, Q.-C.; Zhang, H.-L. Alkylaminomaleimide fluorophores: Synthesis via air oxidation and emission modulation by twisted intramolecular charge transfer. Org. Chem. Front. 2021, 8, 239–248. [Google Scholar] [CrossRef]
- Hoelzel, C.A.; Hu, H.; Wolstenholme, C.H.; Karim, B.A.; Munson, K.T.; Jung, K.H.; Zhang, H.; Liu, Y.; Yennawar, H.P.; Asbury, J.B.; et al. A general strategy to enhance donor-acceptor molecules using solvent-excluding substituents. Angew. Chem. Int. Ed. 2020, 59, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.; Fang, C. Polarity-dependent twisted intramolecular charge transfer in diethylamino coumarin revealed by ultrafast spectroscopy. Chemosensors 2022, 10, 411. [Google Scholar] [CrossRef]
- Baleeva, N.S.; Zaitseva, S.O.; Gorbachev, D.A.; Smirnov, A.Y.; Zagudaylova, M.B.; Baranov, M.S. The role of N-substituents in radiationless deactivation of aminated derivatives of a locked GFP chromophore. Eur. J. Org. Chem. 2017, 2017, 5219–5224. [Google Scholar] [CrossRef]
- Whitaker, J.E.; Haugland, R.P.; Ryan, D.; Hewitt, P.C.; Haugland, R.P.; Prendergast, F.G. Fluorescent rhodol derivatives: Versatile, photostable labels and tracers. Anal. Biochem. 1992, 207, 267–279. [Google Scholar] [CrossRef]
- Pedone, A. Role of solvent on charge transfer in 7-aminocoumarin dyes: New hints from TD-CAM-B3LYP and state specific PCM calculations. J. Chem. Theory Comput. 2013, 9, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Rettig, W. Charge separation in excited states of decoupled systems—TICT compounds and implications regarding the development of new laser dyes and the primary process of vision and photosynthesis. Angew. Chem. Int. Ed. 1986, 25, 971–988. [Google Scholar] [CrossRef]
- Hinckley, D.A.; Seybold, P.G.; Borris, D.P. Solvatochromism and thermochromism of rhodamine solutions. Spectrochim. Acta A 1986, 42, 747–754. [Google Scholar] [CrossRef]
- Rettig, W. Photoinduced charge separation via twisted intramolecular charge transfer states. In Electron Transfer I; Springer: Berlin, Germany, 1994; Volume 169, pp. 253–299. [Google Scholar]
- Farrell, P.G.; Newton, J. Ionization potentials of aromatic amines. J. Phys. Chem. 1965, 69, 3506–3509. [Google Scholar] [CrossRef]
- Aue, D.H.; Webb, H.M.; Bowers, M.T. Quantitative proton affinities, ionization potentials, and hydrogen affinities of alkylamines. J. Am. Chem. Soc. 1976, 98, 311–317. [Google Scholar] [CrossRef]
- Hanaoka, K.; Iwaki, S.; Yagi, K.; Myochin, T.; Ikeno, T.; Ohno, H.; Sasaki, E.; Komatsu, T.; Ueno, T.; Uchigashima, M.; et al. General design strategy to precisely control the emission of fluorophores via a twisted intramolecular charge transfer (TICT) process. J. Am. Chem. Soc. 2022, 144, 19778–19790. [Google Scholar] [CrossRef]
- Bassolino, G.; Nançoz, C.; Thiel, Z.; Bois, E.; Vauthey, E.; Rivera-Fuentes, P. Photolabile coumarins with improved efficiency through azetidinyl substitution. Chem. Sci. 2018, 9, 387–391. [Google Scholar] [CrossRef]
- Lv, X.; Gao, C.; Han, T.; Shi, H.; Guo, W. Improving the quantum yields of fluorophores by inhibiting twisted intramolecular charge transfer using electron-withdrawing group-functionalized piperidine auxochromes. Chem. Commun. 2020, 56, 715–718. [Google Scholar] [CrossRef]
- Lv, X.; Han, T.; Wu, Y.; Zhang, B.; Guo, W. Improving the fluorescence brightness of distyryl Bodipys by inhibiting the twisted intramolecular charge transfer excited state. Chem. Commun. 2021, 57, 9744–9747. [Google Scholar] [CrossRef]
- Grimm, J.B.; Muthusamy, A.K.; Liang, Y.; Brown, T.A.; Lemon, W.C.; Patel, R.; Lu, R.; Macklin, J.J.; Keller, P.J.; Ji, N.; et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 2017, 14, 987–994. [Google Scholar] [CrossRef]
- Rettig, W.; Wermuth, G.; Lippert, E. Photophysical primary processes in solutions of p-substituted dialkylanilines. Ber. Bunsen-Ges. Phys. Chem. 1979, 83, 692–697. [Google Scholar] [CrossRef]
- Abedi, S.A.A.; Chi, W.; Tan, D.; Shen, T.; Wang, C.; Ang, E.C.X.; Tan, C.-H.; Anariba, F.; Liu, X. Restriction of twisted intramolecular charge transfer enables the aggregation-induced emission of 1-(N,N-dialkylamino)-naphthalene derivatives. J. Phys. Chem. A 2021, 125, 8397–8403. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. Effects of solvent polarity and solvent viscosity on the fluorescent properties of molecular rotors and related probes. Bioorg. Chem. 2005, 33, 415–425. [Google Scholar] [CrossRef]
- Chen, C.; Tachibana, S.R.; Baleeva, N.S.; Myasnyanko, I.N.; Bogdanov, A.M.; Gavrikov, A.S.; Mishin, A.S.; Malyshevskaya, K.K.; Baranov, M.S.; Fang, C. Developing bright green fluorescent protein (GFP)-like fluorogens for live-cell imaging with nonpolar protein−chromophore interactions. Chem. Eur. J. 2021, 27, 8946–8950. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Dahiya, P.; Kumbhakar, M.; Maity, D.K.; Mukherjee, T.; Mittal, J.P.; Tripathi, A.B.R.; Chattopadhyay, N.; Pal, H. Photophysical properties of 2-amino-9,10-anthraquinone: Evidence for structural changes in the molecule with solvent polarity. Photochem. Photobiol. Sci. 2005, 4, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Satpati, A.K.; Kumbhakar, M.; Nath, S.; Pal, H. Photophysical properties of coumarin-7 dye: Role of twisted intramolecular charge transfer state in high polarity protic solvents. Photochem. Photobiol. 2009, 85, 119–129. [Google Scholar] [CrossRef]
- Rettig, W.; Klock, A. Intramolecular fluorescence quenching in aminocoumarines. Identification of an excited state with full charge separation. Can. J. Chem. 1985, 63, 1649–1653. [Google Scholar] [CrossRef]
- Rettig, W.; Bliss, B.; Dirnberger, K. Pseudo-Jahn–Teller and TICT-models: A photophysical comparison of meta- and para-DMABN derivatives. Chem. Phys. Lett. 1999, 305, 8–14. [Google Scholar] [CrossRef]
- Zachariasse, K.A. Comment on “Pseudo-Jahn–Teller and TICT-models: A photophysical comparison of meta- and para-DMABN derivatives” [Chem. Phys. Lett. 305 (1999) 8]: The PICT model for dual fluorescence of aminobenzonitriles. Chem. Phys. Lett. 2000, 320, 8–13. [Google Scholar] [CrossRef]
- Oshima, J.; Shiobara, S.; Naoumi, H.; Kaneko, S.; Yoshihara, T.; Mishra, A.K.; Tobita, S. Extreme fluorescence sensitivity of some aniline derivatives to aqueous and nonaqueous environments: Mechanistic study and its implication as a fluorescent probe. J. Phys. Chem. A 2006, 110, 4629–4637. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and fluorogenic dyes as environment-sensitive probes: Design and biological applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Chatterjee, T.; Das, A.; Mandal, S.; Sen, A.; Ta, M.; Mandal, P.K. Meta-fluors—A unique way to create a 200 Da ultrasmall fluorophore emitting in red with intense Stokes/solvatochromic shift: Imaging subcellular nanopolarity in live stem cells. J. Phys. Chem. C 2019, 123, 24786–24792. [Google Scholar] [CrossRef]
- Chen, C.; Baranov, M.S.; Zhu, L.; Baleeva, N.S.; Smirnov, A.Y.; Zaitseva, S.O.; Yampolsky, I.V.; Solntsev, K.M.; Fang, C. Designing redder and brighter fluorophores by synergistic tuning of ground and excited states. Chem. Commun. 2019, 55, 2537–2540. [Google Scholar] [CrossRef]
- Chen, C.; Fang, C. Devising efficient red-shifting strategies for bioimaging: A generalizable donor-acceptor fluorophore prototype. Chem. Asian J. 2020, 15, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.; Nad, S.; Kumbhakar, M. Photophysical properties of Coumarin-120: Unusual behavior in nonpolar solvents. J. Chem. Phys. 2003, 119, 443–452. [Google Scholar] [CrossRef]
- Bozkurt, E.; Onganer, Y. Photophysical features of Coumarin 120 in reverse micelles. J. Mol. Struct. 2018, 1173, 490–497. [Google Scholar] [CrossRef]
- Lopez Arbeloa, T.; Lopez Arbeloa, F.; Tapia, M.J.; Lopez Arbeloa, I. Hydrogen-bonding effect on the photophysical properties of 7-aminocoumarin derivatives. J. Phys. Chem. 1993, 97, 4704–4707. [Google Scholar] [CrossRef]
- Krystkowiak, E.; Dobek, K.; Burdziński, G.; Maciejewski, A. Radiationless deactivation of 6-aminocoumarin from the S1-ICT state in nonspecifically interacting solvents. Photochem. Photobiol. Sci. 2012, 11, 1322–1330. [Google Scholar] [CrossRef]
- Liu, X.; Cole, J.M.; Xu, Z. Substantial intramolecular charge transfer induces long emission wavelengths and mega Stokes shifts in 6-aminocoumarins. J. Phys. Chem. C 2017, 121, 13274–13279. [Google Scholar] [CrossRef]
- Krystkowiak, E.; Dobek, K.; Maciejewski, A. An intermolecular hydrogen-bonding effect on spectral and photophysical properties of 6-aminocoumarin in protic solvents. Photochem. Photobiol. Sci. 2013, 12, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Krystkowiak, E.; Dobek, K.; Maciejewski, A. Deactivation of 6-aminocoumarin intramolecular charge transfer excited state through hydrogen bonding. Int. J. Mol. Sci. 2014, 15, 16628–16648. [Google Scholar] [CrossRef]
- Ayuk, A.A.; Rettig, W.; Lippert, E. Temperature and viscosity effects on an excited state equilibrium as revealed from the dual fluorescence of very dilute solutions of 1-dimethylamino-4-cyanonaphthalene. Ber. Bunsen-Ges. Phys. Chem. 1981, 85, 553–555. [Google Scholar] [CrossRef]
- Davis, B.N.; Abelt, C.J. Synthesis and photophysical properties of models for twisted PRODAN and dimethylaminonaphthonitrile. J. Phys. Chem. A 2005, 109, 1295–1298. [Google Scholar] [CrossRef]
- Chen, T.; Lee, S.W.; Abelt, C.J. 1,5-prodan emits from a planar intramolecular charge-transfer excited state. ACS Omega 2018, 3, 4816–4823. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.S.; Nagirimadugu, N.V.; Abelt, C.J. Fluorescence quenching of carbonyl-twisted 5-acyl-1-dimethylaminonaphthalenes by alcohols. ACS Omega 2019, 4, 14067–14073. [Google Scholar] [CrossRef]
- Lum, K.; Zielinski, S.M.; Abelt, C.J. Dansyl emits from a PICT excited state. J. Phys. Chem. A 2021, 125, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Lobo, B.C.; Abelt, C.J. Does PRODAN possess a planar or twisted charge-transfer excited state? Photophysical properties of two PRODAN derivatives. J. Phys. Chem. A 2003, 107, 10938–10943. [Google Scholar] [CrossRef]
- Everett, R.K.; Nguyen, H.A.A.; Abelt, C.J. Does PRODAN possess an O-TICT excited state? Synthesis and properties of two constrained derivatives. J. Phys. Chem. A 2010, 114, 4946–4950. [Google Scholar] [CrossRef]
- Boulanger, S.A.; Chen, C.; Myasnyanko, I.N.; Sokolov, A.I.; Baranov, M.S.; Fang, C. Excited-state dynamics of a meta-dimethylamino locked GFP chromophore as a fluorescence turn-on water sensor. Photochem. Photobiol. 2022, 98, 311–324. [Google Scholar] [CrossRef]
- Chi, W.; Qiao, Q.; Lee, R.; Liu, W.; Teo, Y.S.; Gu, D.; Lang, M.J.; Chang, Y.-T.; Xu, Z.; Liu, X. A photoexcitation-induced twisted intramolecular charge shuttle. Angew. Chem. Int. Ed. 2019, 58, 7073–7077. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Chen, J.; Liu, W.; Wang, C.; Qi, Q.; Qiao, Q.; Tan, T.M.; Xiong, K.; Liu, X.; Kang, K.; et al. A general descriptor ΔE enables the quantitative development of luminescent materials based on photoinduced electron transfer. J. Am. Chem. Soc. 2020, 142, 6777–6785. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Bednar, R.M.; Rozanov, N.D.; Hemshorn, M.L.; Mehl, R.A.; Fang, C. Rational design for high bioorthogonal fluorogenicity of tetrazine-encoded green fluorescent proteins. Nat. Sci. 2022, 2, e20220028. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Ling, J.; de Silva, A.P. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef]
- Escudero, D. Revising intramolecular photoinduced electron transfer (PET) from first-principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence on/off switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef]
- de Silva, A.P. Luminescent photoinduced electron transfer (PET) molecules for sensing and logic operations. J. Phys. Chem. Lett. 2011, 2, 2865–2871. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef]
- Jiao, G.-S.; Thoresen, L.H.; Burgess, K. Fluorescent, through-bond energy transfer cassettes for labeling multiple biological molecules in one experiment. J. Am. Chem. Soc. 2003, 125, 14668–14669. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, W.; Sung, J.; Kim, E.; Park, S.B. Monochromophoric design strategy for tetrazine-based colorful bioorthogonal probes with a single fluorescent core skeleton. J. Am. Chem. Soc. 2018, 140, 974–983. [Google Scholar] [CrossRef]
- Köhler, G.; Grabner, G.; Rotkiewicz, K. Nonradiative deactivation and triplet states in donor-aryl-acceptor compounds (dialkylaminobenzonitriles). Chem. Phys. 1993, 173, 275–290. [Google Scholar] [CrossRef]
- Zachariasse, K.A.; Grobys, M.; von der Haar, T.; Hebecker, A.; Il’ichev, Y.V.; Jiang, Y.B.; Morawski, O.; Kühnle, W. Intramolecular charge transfer in the excited state. Kinetics and configurational changes. J. Photochem. Photobiol. A 1996, 102, 59–70. [Google Scholar] [CrossRef]
- Suzuki, K.; Demeter, A.; Kühnle, W.; Tauer, E.; Zachariasse, K.A.; Tobita, S.; Shizuka, H. Internal conversion in 4-substituted 1-naphthylamines. Influence of the electron donor/acceptor substituent character. Phys. Chem. Chem. Phys. 2000, 2, 981–991. [Google Scholar] [CrossRef]
- Penfold, T.J.; Gindensperger, E.; Daniel, C.; Marian, C.M. Spin-vibronic mechanism for intersystem crossing. Chem. Rev. 2018, 118, 6975–7025. [Google Scholar] [CrossRef]
- Marian, C.M. Understanding and controlling intersystem crossing in molecules. Annu. Rev. Phys. Chem. 2021, 72, 617–640. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, S.K.; Ramamurthy, V.; Sen, P. Vibration-assisted intersystem crossing in the ultrafast excited-state relaxation dynamics of halocoumarins. J. Phys. Chem. A 2022, 126, 1475–1485. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 1970, 18, 145–164. [Google Scholar] [CrossRef]
- Robinson, G.W.; Frosch, R.P. Electronic excitation transfer and relaxation. J. Chem. Phys. 1963, 38, 1187–1203. [Google Scholar] [CrossRef]
- Siebrand, W. Radiationless transitions in polyatomic molecules. I. Calculation of Franck–Condon factors. J. Chem. Phys. 1967, 46, 440–447. [Google Scholar] [CrossRef]
- Freed, K.F.; Jortner, J. Multiphonon processes in the nonradiative decay of large molecules. J. Chem. Phys. 1970, 52, 6272–6291. [Google Scholar] [CrossRef]
- Lin, S.H.; Bersohn, R. Effect of partial deuteration and temperature on triplet-state lifetimes. J. Chem. Phys. 1968, 48, 2732–2736. [Google Scholar] [CrossRef]
- Kellmann, A. Intersystem crossing and internal conversion quantum yields of acridine in polar and nonpolar solvents. J. Phys. Chem. 1977, 81, 1195–1198. [Google Scholar] [CrossRef]
- Ermolaev, V.L. The influence of deuteration of complex organic molecules on their fluorescence quantum yield (a review). Opt. Spectrosc. 2016, 121, 567–584. [Google Scholar] [CrossRef]
- Erker, C.; Basché, T. The energy gap law at work: Emission yield and rate fluctuations of single NIR emitters. J. Am. Chem. Soc. 2022, 144, 14053–14056. [Google Scholar] [CrossRef] [PubMed]
- Caspar, J.V.; Kober, E.M.; Sullivan, B.P.; Meyer, T.J. Application of the energy gap law to the decay of charge-transfer excited states. J. Am. Chem. Soc. 1982, 104, 630–632. [Google Scholar] [CrossRef]
- Wang, C.; Otto, S.; Dorn, M.; Kreidt, E.; Lebon, J.; Sršan, L.; Di Martino-Fumo, P.; Gerhards, M.; Resch-Genger, U.; Seitz, M.; et al. Deuterated molecular Ruby with record luminescence quantum yield. Angew. Chem. Int. Ed. 2018, 57, 1112–1116. [Google Scholar] [CrossRef]
- Avouris, P.; Gelbart, W.M.; El-Sayed, M.A. Nonradiative electronic relaxation under collision-free conditions. Chem. Rev. 1977, 77, 793–833. [Google Scholar] [CrossRef]
- Kusinski, M.; Nagesh, J.; Gladkikh, M.; Izmaylov, A.F.; Jockusch, R.A. Deuterium isotope effect in fluorescence of gaseous oxazine dyes. Phys. Chem. Chem. Phys. 2019, 21, 5759–5770. [Google Scholar] [CrossRef]
- Inoue, H.; Hida, M.; Nakashima, N.; Yoshihara, K. Picosecond fluorescence lifetimes of anthraquinone derivatives. Radiationless deactivation via intra- and intermolecular hydrogen bonds. J. Phys. Chem. 1982, 86, 3184–3188. [Google Scholar] [CrossRef]
- Magde, D.; Rojas, G.E.; Seybold, P.G. Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem. Photobiol. 1999, 70, 737–744. [Google Scholar] [CrossRef]
- Das, S.; Datta, A.; Bhattacharyya, K. Deuterium isotope effect on 4-aminophthalimide in neat water and reverse micelles. J. Phys. Chem. A 1997, 101, 3299–3304. [Google Scholar] [CrossRef]
- Aharoni, A.; Oron, D.; Banin, U.; Rabani, E.; Jortner, J. Long-range electronic-to-vibrational energy transfer from nanocrystals to their surrounding matrix environment. Phys. Rev. Lett. 2008, 100, 057404. [Google Scholar] [CrossRef] [PubMed]
- Yatsuhashi, T.; Nakajima, Y.; Shimada, T.; Inoue, H. Photophysical properties of intramolecular charge-transfer excited singlet state of aminofluorenone derivatives. J. Phys. Chem. A 1998, 102, 3018–3024. [Google Scholar] [CrossRef]
- Lee, S.F.; Vérolet, Q.; Fürstenberg, A. Improved super-resolution microscopy with oxazine fluorophores in heavy water. Angew. Chem. Int. Ed. 2013, 52, 8948–8951. [Google Scholar] [CrossRef]
- Klehs, K.; Spahn, C.; Endesfelder, U.; Lee, S.F.; Fürstenberg, A.; Heilemann, M. Increasing the brightness of cyanine fluorophores for single-molecule and superresolution imaging. ChemPhysChem 2014, 15, 637–641. [Google Scholar] [CrossRef]
- Maillard, J.; Klehs, K.; Rumble, C.; Vauthey, E.; Heilemann, M.; Fürstenberg, A. Universal quenching of common fluorescent probes by water and alcohols. Chem. Sci. 2021, 12, 1352–1362. [Google Scholar] [CrossRef]
- Braun, C.L.; Smirnov, S.N. Why is water blue? J. Chem. Edu. 1993, 70, 612–614. [Google Scholar] [CrossRef]
- Fita, P.; Fedoseeva, M.; Vauthey, E. Ultrafast excited-state dynamics of Eosin B: A potential probe of the hydrogen-bonding properties of the environment. J. Phys. Chem. A 2011, 115, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-J.; Han, K.-L. Hydrogen bonding in the electronic excited state. Acc. Chem. Res. 2012, 45, 404–413. [Google Scholar] [CrossRef]
- Waluk, J. Hydrogen-bonding-induced phenomena in bifunctional heteroazaaromatics. Acc. Chem. Res. 2003, 36, 832–838. [Google Scholar] [CrossRef]
- Oshima, J.; Yoshihara, T.; Tobita, S. Water-induced fluorescence quenching of mono- and dicyanoanilines. Chem. Phys. Lett. 2006, 423, 306–311. [Google Scholar] [CrossRef]
- Biczók, L.; Bérces, T.; Linschitz, H. Quenching processes in hydrogen-bonded pairs: Interactions of excited fluorenone with alcohols and phenols. J. Am. Chem. Soc. 1997, 119, 11071–11077. [Google Scholar] [CrossRef]
- Krystkowiak, E.; Dobek, K.; Maciejewski, A. Origin of the strong effect of protic solvents on the emission spectra, quantum yield of fluorescence and fluorescence lifetime of 4-aminophthalimide: Role of hydrogen bonds in deactivation of S1-4-aminophthalimide. J. Photochem. Photobiol. A 2006, 184, 250–264. [Google Scholar] [CrossRef]
- Sobolewski, A.L.; Domcke, W. Computational studies of the photophysics of hydrogen-bonded molecular systems. J. Phys. Chem. A 2007, 111, 11725–11735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-J.; Han, K.-L. Ultrafast hydrogen bond strengthening of the photoexcited fluorenone in alcohols for facilitating the fluorescence quenching. J. Phys. Chem. A 2007, 111, 9218–9223. [Google Scholar] [CrossRef]
- Huang, G.-J.; Ho, J.-H.; Prabhakar, C.; Liu, Y.-H.; Peng, S.-M.; Yang, J.-S. Site-selective hydrogen-bonding-induced fluorescence quenching of highly solvatofluorochromic GFP-like chromophores. Org. Lett. 2012, 14, 5034–5037. [Google Scholar] [CrossRef]
- Yatsuhashi, T.; Inoue, H. Molecular mechanism of radiationless deactivation of aminoanthraquinones through intermolecular hydrogen-bonding interaction with alcohols and hydroperoxides. J. Phys. Chem. A 1997, 101, 8166–8173. [Google Scholar] [CrossRef]
- Dereka, B.; Vauthey, E. Direct local solvent probing by transient infrared spectroscopy reveals the mechanism of hydrogen-bond induced nonradiative deactivation. Chem. Sci. 2017, 8, 5057–5066. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Han, K.-L. Role of intramolecular and intermolecular hydrogen bonding in both singlet and triplet excited states of aminofluorenones on internal conversion, intersystem crossing, and twisted intramolecular charge transfer. J. Phys. Chem. A 2009, 113, 14329–14335. [Google Scholar] [CrossRef]


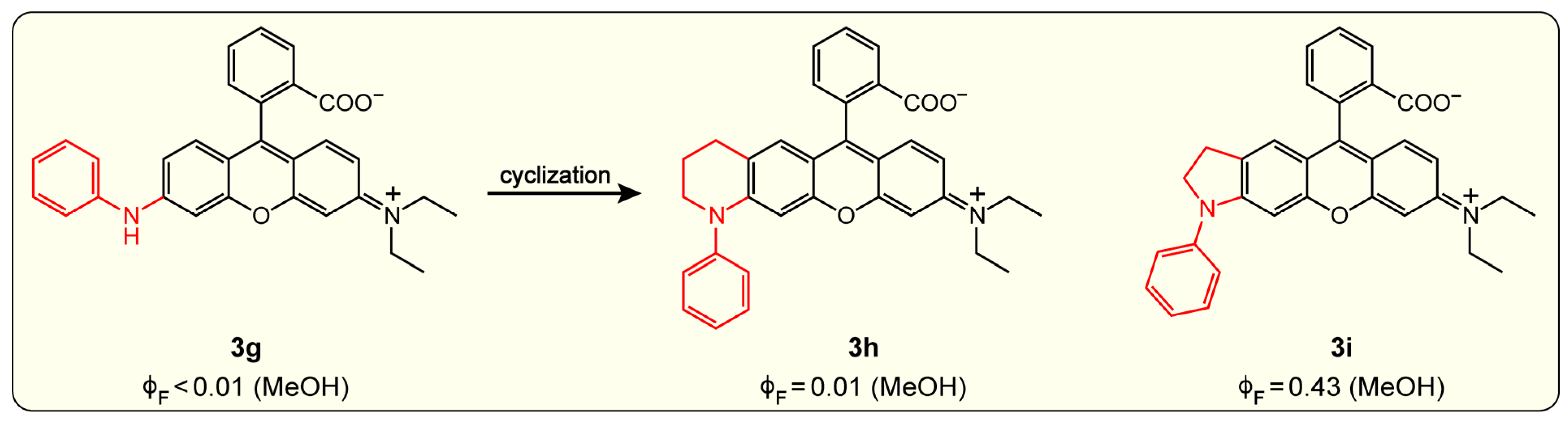
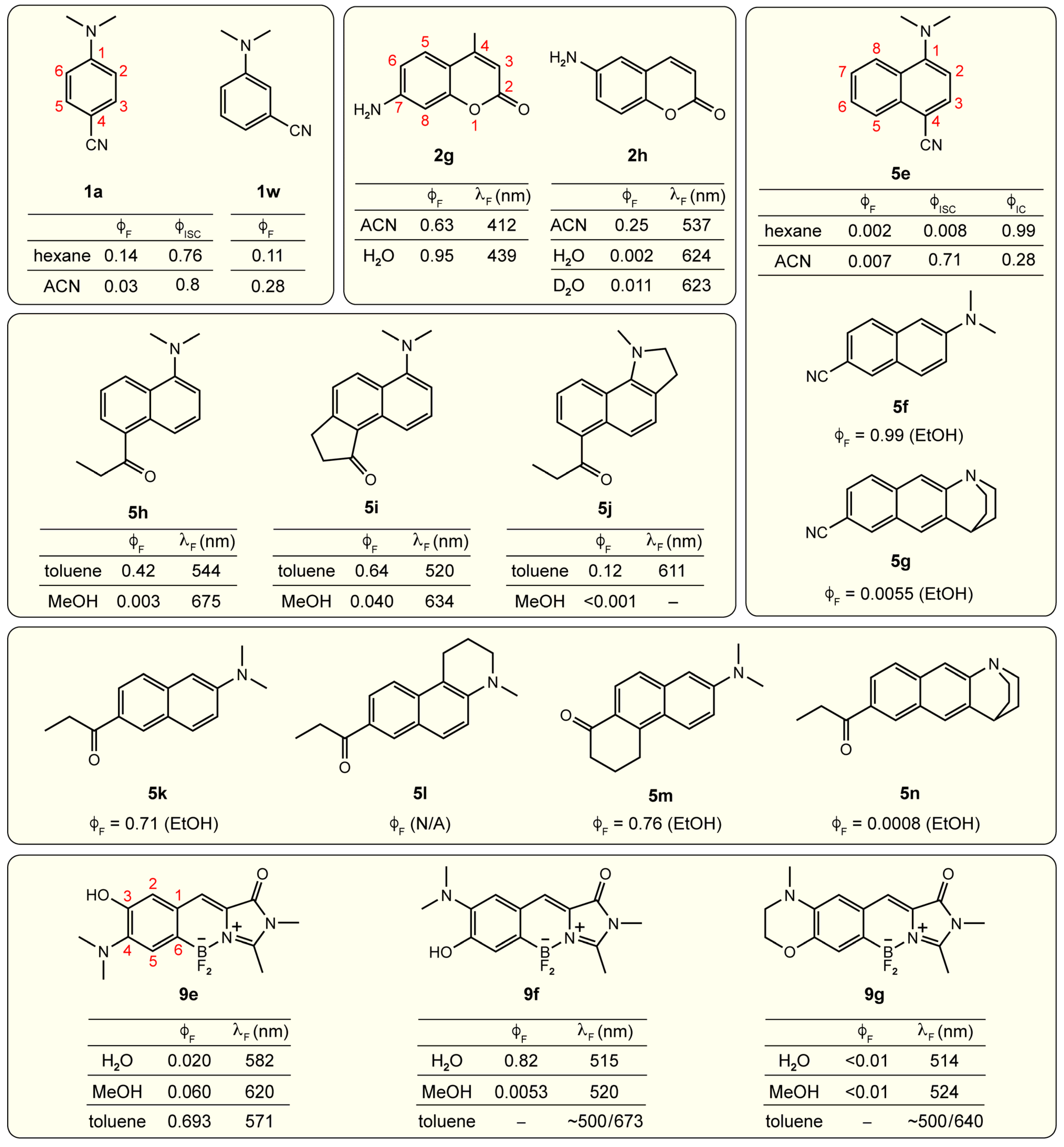
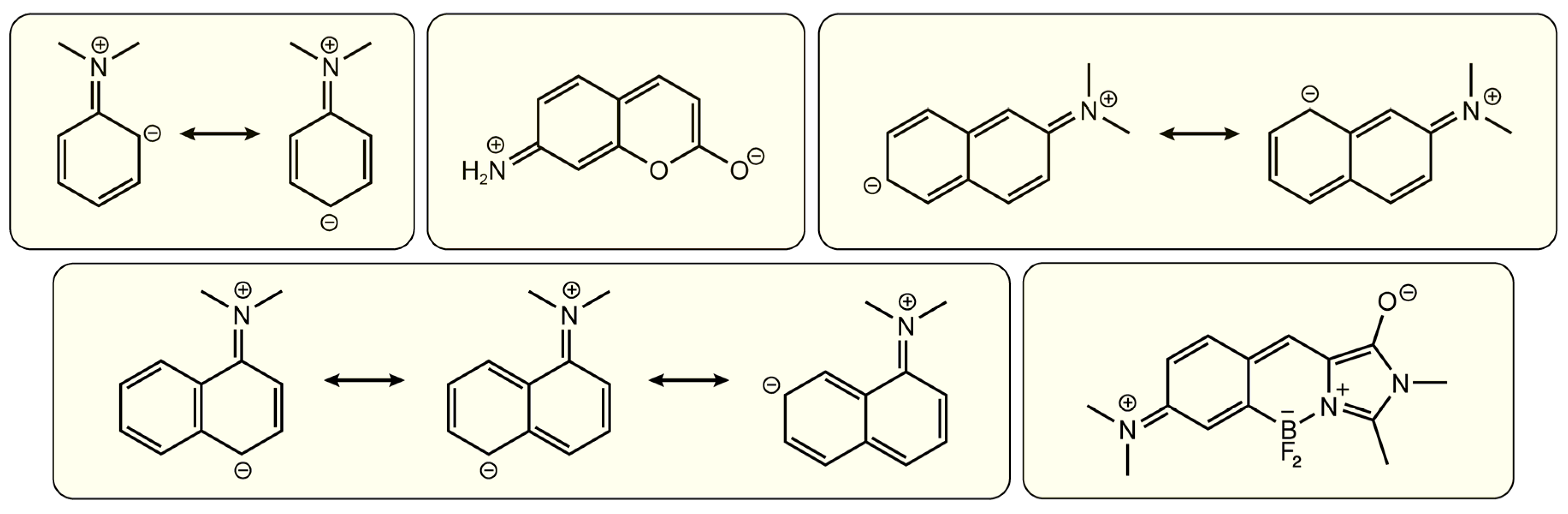
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Fang, C. Fluorescence Modulation by Amines: Mechanistic Insights into Twisted Intramolecular Charge Transfer (TICT) and Beyond. Chemosensors 2023, 11, 87. https://doi.org/10.3390/chemosensors11020087
Chen C, Fang C. Fluorescence Modulation by Amines: Mechanistic Insights into Twisted Intramolecular Charge Transfer (TICT) and Beyond. Chemosensors. 2023; 11(2):87. https://doi.org/10.3390/chemosensors11020087
Chicago/Turabian StyleChen, Cheng, and Chong Fang. 2023. "Fluorescence Modulation by Amines: Mechanistic Insights into Twisted Intramolecular Charge Transfer (TICT) and Beyond" Chemosensors 11, no. 2: 87. https://doi.org/10.3390/chemosensors11020087
APA StyleChen, C., & Fang, C. (2023). Fluorescence Modulation by Amines: Mechanistic Insights into Twisted Intramolecular Charge Transfer (TICT) and Beyond. Chemosensors, 11(2), 87. https://doi.org/10.3390/chemosensors11020087




