Applying of C8-BTBT-Based EGOFETs at Different pH Values of the Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
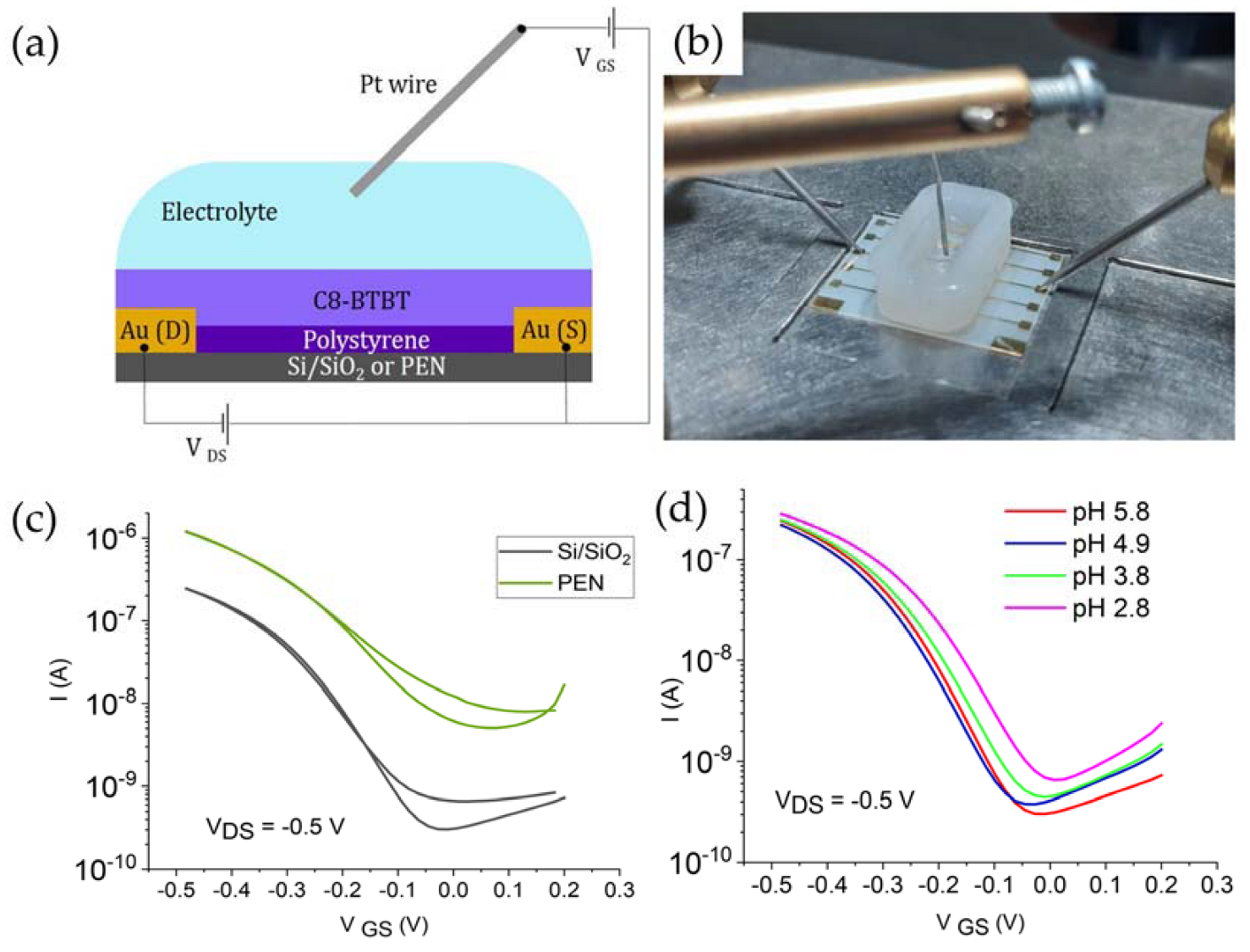
2.2. Fabrication of EGOFET Devices
2.3. Device Characterization
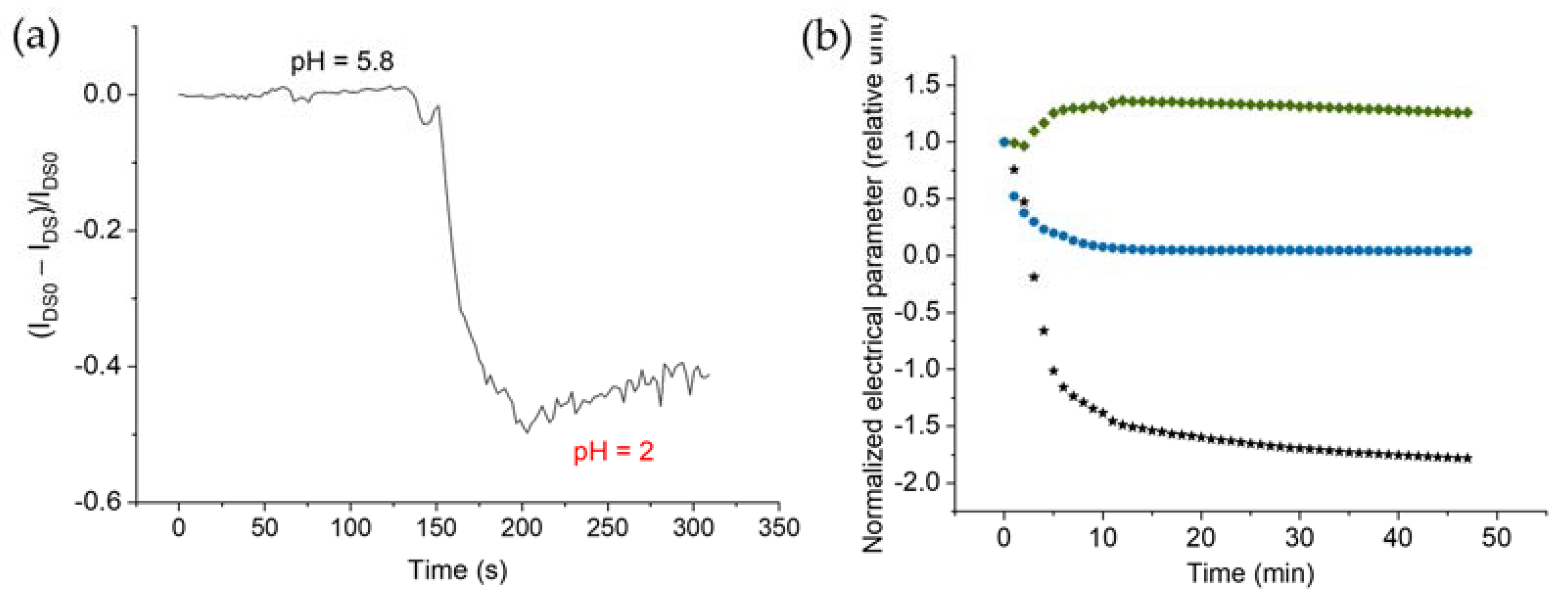
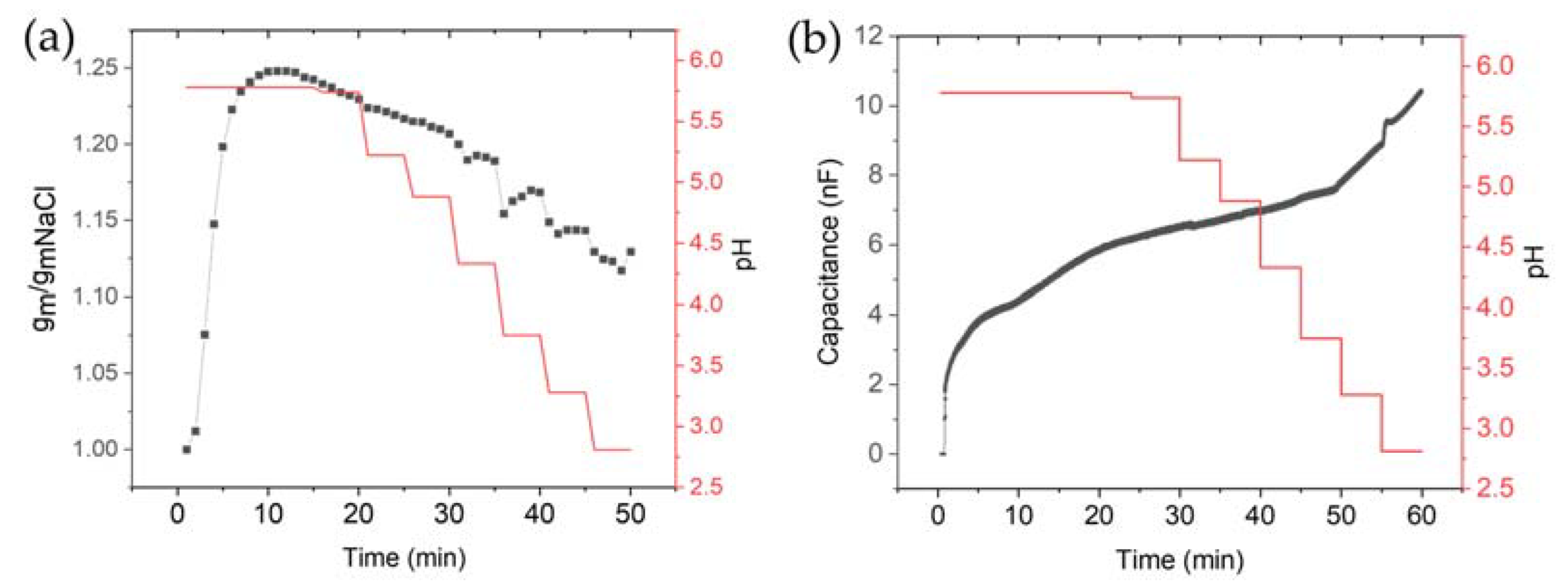
2.4. pH Variation Experiments
2.5. Flow Chamber Development
3. Results and Discussion
3.1. EGOFET Manufacturing Technique
3.2. Electrical Characteristics of the Devices
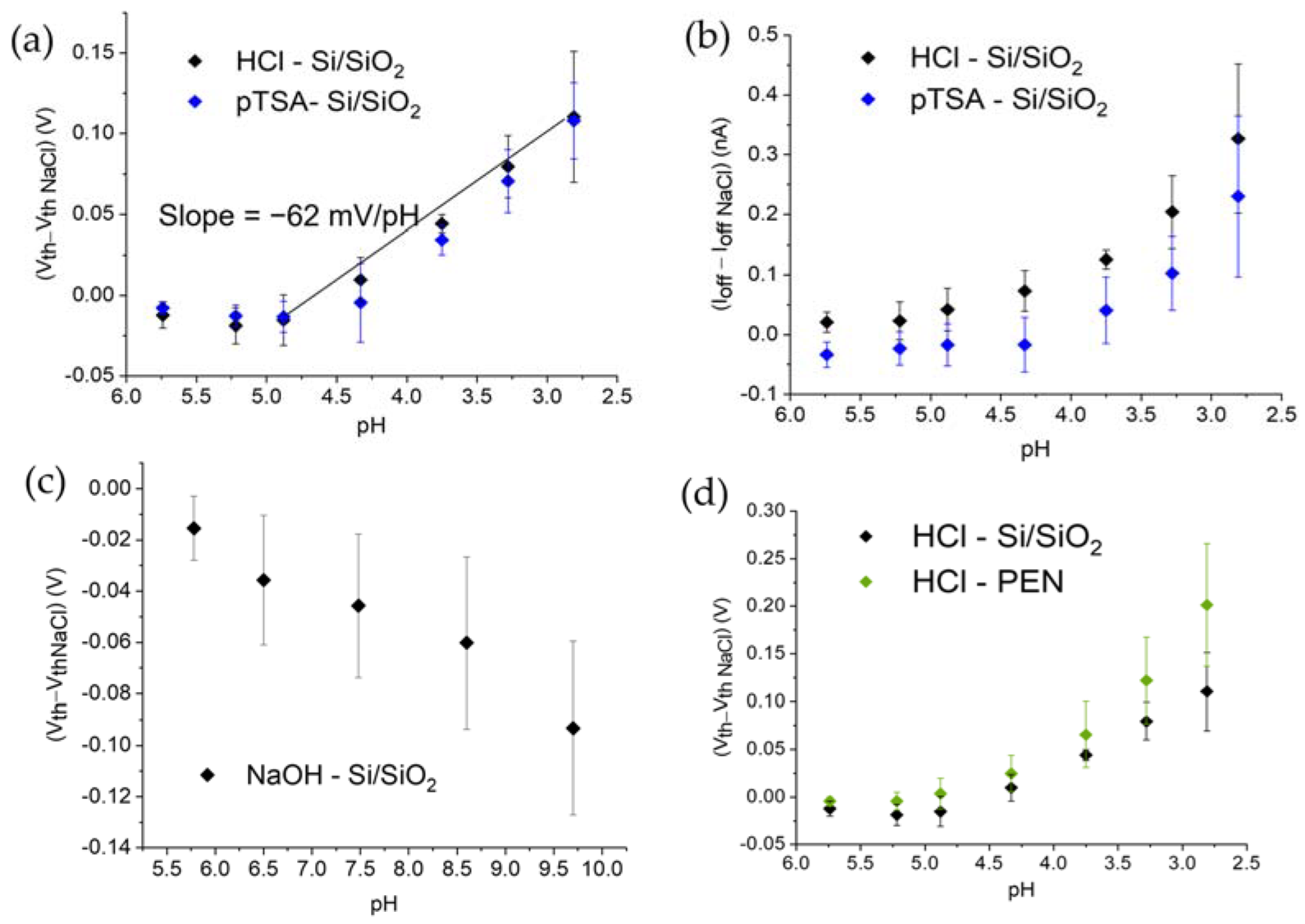
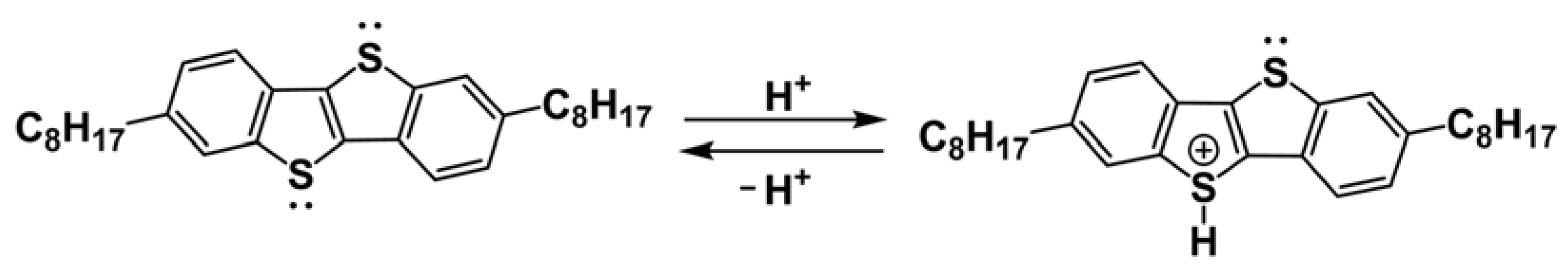
3.3. Effect of HCl and NaOH Adding on the Electrical Characteristics
3.4. Effect of the Substrate Material
3.5. Flow Chamber Development and Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kergoat, L.; Herlogsson, L.; Braga, D.; Piro, B.; Pham, M.C.; Crispin, X.; Berggren, M.; Horowitz, G. A water-gate organic field-effect transistor. Adv. Mater. 2010, 22, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Picca, R.A.; Manoli, K.; Macchia, E.; Sarcina, L.; Di Franco, C.; Cioffi, N.; Blasi, D.; Österbacka, R.; Torricelli, F.; Scamarcio, G.; et al. Ultimately Sensitive Organic Bioelectronic Transistor Sensors by Materials and Device Structure Design. Adv. Funct. Mater. 2020, 30, 1904513. [Google Scholar] [CrossRef]
- Wang, D.; Noël, V.; Piro, B. Electrolytic gated organic field-effect transistors for application in biosensors—A review. Electronics 2016, 5, 9. [Google Scholar] [CrossRef]
- Shaposhnik, P.A.; Zapunidi, S.A.; Shestakov, M.V.; Agina, E.V.; Ponomarenko, S.A. Modern bio and chemical sensors and neuromorphic devices based on organic semiconductors. Russ. Chem. Rev. 2020, 89, 1483–1506. [Google Scholar] [CrossRef]
- Macchia, E.; Romele, P.; Manoli, K.; Ghittorelli, M.; Magliulo, M.; Zsolt, M.K.-V.; Torricelli, F.; Luisa, T. Ultra-sensitive protein detection with organic electrochemical transistors printed on plastic substrate. Flex. Print. Electron. 2018, 034002. [Google Scholar] [CrossRef]
- Macchia, E.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. New trends in single-molecule bioanalytical detection. Anal. Bioanal. Chem. 2020, 412, 5005–5014. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Guo, K.; Wustoni, S.; Koklu, A.; Díaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [Google Scholar] [CrossRef]
- Liu, H.; Yang, A.; Song, J.; Wang, N.; Lam, P.; Li, Y.; Law, H.K.; Yan, F. Ultrafast, sensitive, and portable detection of COVID-19 IgG using flexible organic electrochemical transistors. Sci. Adv. 2021, 7, 8387. [Google Scholar] [CrossRef]
- Desbief, S.; di Lauro, M.; Casalini, S.; Guerin, D.; Tortorella, S.; Barbalinardo, M.; Kyndiah, A.; Murgia, M.; Cramer, T.; Biscarini, F.; et al. Electrolyte-gated organic synapse transistor interfaced with neurons. Org. Electron. 2016, 38, 21–28. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, T.-W.W. Organic Synapses for Neuromorphic Electronics: From Brain-Inspired Computing to Sensorimotor Nervetronics. Acc. Chem. Res. 2019, 52, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, M.; Berto, M.; Giordani, M.; Benaglia, S.; Schweicher, G.; Vuillaume, D.; Bortolotti, C.A.; Geerts, Y.H.; Biscarini, F. Liquid-Gated Organic Electronic Devices Based on High-Performance Solution-Processed Molecular Semiconductor. Adv. Electron. Mater. 2017, 3, 1700159. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, S.M. Improvement in energy consumption and operational stability of electrolyte-gated synapse transistors using atomic-layer-deposited HfO2 thin films. Mater. Sci. Semicond. Process. 2023, 153, 107182. [Google Scholar] [CrossRef]
- Parkula, V.; Maglione, M.S.; Casalini, S.; Zhang, Q.; Greco, P.; Bortolotti, C.A.; Rovira, C.; Mas-Torrent, M.; Biscarini, F. EGOFET Gated by a Molecular Electronic Switch: A Single-Device Memory Cell. Adv. Electron. Mater. 2019, 5, 1800875. [Google Scholar] [CrossRef]
- Seshadri, P.; Manoli, K.; Schneiderhan-Marra, N.; Anthes, U.; Wierzchowiec, P.; Bonrad, K.; Di Franco, C.; Torsi, L. Low-picomolar, label-free procalcitonin analytical detection with an electrolyte-gated organic field-effect transistor based electronic immunosensor. Biosens. Bioelectron. 2018, 104, 113–119. [Google Scholar] [CrossRef]
- Cramer, T.; Kyndiah, A.; Murgia, M.; Leonardi, F.; Casalini, S.; Biscarini, F. Double layer capacitance measured by organic field effect transistor operated in water. Appl. Phys. Lett. 2012, 100, 86. [Google Scholar] [CrossRef]
- Poimanova, E.Y.; Shaposhnik, P.A.; Anisimov, D.S.; Zavyalova, E.G.; Trul, A.A.; Skorotetcky, M.S.; Borshchev, O.V.; Vinnitskiy, D.Z.; Polinskaya, M.S.; Krylov, V.B.; et al. Biorecognition Layer Based On Biotin-Containing [1]Benzothieno[3,2-b][1]benzothiophene Derivative for Biosensing by Electrolyte-Gated Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2022, 14, 16462–16476. [Google Scholar] [CrossRef]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-specific adsorption reduction methods in biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef]
- Nikolka, M.; Simatos, D.; Foudeh, A.; Pfattner, R.; Mcculloch, I.; Bao, Z. Low-voltage, Dual-gate Organic Transistors with High-sensitivity and Stability towards Electrostatic Biosensing. ACS Appl. Mater. Interfaces 2020, 12, 40581–40589. [Google Scholar] [CrossRef]
- Coyle, S.; Morris, D.; Lau, K.; Diamond, D.; Di Francesco, F.; Taccini, N.; Trivella, M.G.; Clinica, F.; Costanzo, D.; Salvo, P. Textile sensors to measure sweat pH and sweat-rate during exercise. In Proceedings of the 3rd International Conference on Pervasive Computing Technologies for Healthcare, London, UK, 1–3 April 2009. [Google Scholar]
- Maalouf, N.M.; Cameron, M.A.; Moe, O.W.; Adams-Huet, B.; Sakhaee, K. Low urine pH: A novel feature of the metabolic syndrome. Clin. J. Am. Soc. Nephrol. 2007, 2, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Manjakkal, L.; Navaraj, W.T.; Lorenzelli, L.; Vinciguerra, V.; Dahiya, R. Stretchable wireless system for sweat pH monitoring. Biosens. Bioelectron. 2018, 107, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Dey, S. A Review on Textile Wastewater Characterization in Bangladesh. Resour. Environ. 2015, 5, 15–44. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Nambiar, S.; Yeow, J.T.W.; Howlader, M.M.R.; Hu, N.X.; Deen, M.J. Polymers and organic materials-based pH sensors for healthcare applications. Prog. Mater. Sci. 2018, 96, 174–216. [Google Scholar] [CrossRef]
- Di Lauro, M.; Casalini, S.; Berto, M.; Campana, A.; Cramer, T.; Murgia, M.; Geoghegan, M.; Bortolotti, C.A.; Biscarini, F. The substrate is a pH-controlled second gate of electrolyte-gated organic field-effect transistor. ACS Appl. Mater. Interfaces 2016, 8, 31783–31790. [Google Scholar] [CrossRef]
- Buth, F.; Kumar, D.; Stutzmann, M.; Garrido, J.A. Electrolyte-gated organic field-effect transistors for sensing applications. Appl. Phys. Lett. 2011, 98, 2009–2012. [Google Scholar] [CrossRef]
- Sinno, H.; Fabiano, S.; Crispin, X.; Berggren, M.; Engquist, I. Bias stress effect in polyelectrolyte-gated organic field-effect transistors. Appl. Phys. Lett. 2013, 102, 113306. [Google Scholar] [CrossRef]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. Highly Soluble [1] Benzothieno [3, 2-b] benzothiophene (BTBT) Derivatives for. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef]
- Zhang, Q.; Leonardi, F.; Casalini, S.; Temiño, I.; Mas-Torrent, M. High performing solution-coated electrolyte-gated organic field-effect transistors for aqueous media operation. Sci. Rep. 2016, 6, 39623. [Google Scholar] [CrossRef]
- Zaumseil, J.; Sirringhaus, H. Electron and ambipolar transport in organic field-effect transistors. Chem. Rev. 2007, 107, 1296–1323. [Google Scholar] [CrossRef]
- Wu, X.; Jia, R.; Jie, J.; Zhang, M.; Pan, J.; Zhang, X.; Zhang, X. Air Effect on the Ideality of p-Type Organic Field-Effect Transistors: A Double-Edged Sword. Adv. Funct. Mater. 2019, 29, 1906653. [Google Scholar] [CrossRef]
- Xie, P.; Liu, T.; Sun, J.; Yang, J. Structures, Properties, and Device Applications for [1]Benzothieno[3,2-b]Benzothiophene Derivatives. Adv. Funct. Mater. 2022, 32, 2200843. [Google Scholar] [CrossRef]
- Shaposhnik, P.A.; Anisimov, D.A.; Trul, A.A.; Agina, E.V.; Ponomarenko, S.A. A Simple Approach to Fabrication of Highly Efficient Electrolyte-Gated Organic Transistors by Phase Microsegregation of 2,7-Dioctyl [1]benzothieno[3,2-b]benzothiophene and Polystyrene Mixtures. Dokl. Phys. Chem. 2021, 496, 20–24. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Temiño, I.; Ocal, C.; Mas-Torrent, M.; Barrena, E. Decoding the Vertical Phase Separation and Its Impact on C8-BTBT/PS Transistor Properties. ACS Appl. Mater. Interfaces 2018, 10, 7296–7303. [Google Scholar] [CrossRef]
- Kyndiah, A.; Leonardi, F.; Tarantino, C.; Cramer, T.; Millan-Solsona, R.; Garreta, E.; Montserrat, N.; Mas-Torrent, M.; Gomila, G. Bioelectronic Recordings of Cardiomyocytes with Accumulation Mode Electrolyte Gated Organic Field Effect Transistors. Biosens. Bioelectron. 2020, 150, 111844. [Google Scholar] [CrossRef] [PubMed]
- Picca, R.A.; Manoli, K.; Macchia, E.; Tricase, A.; Di Franco, C.; Scamarcio, G.; Cioffi, N.; Torsi, L. A Study on the Stability of Water-Gated Organic Field-Effect-Transistors Based on a Commercial p-Type Polymer. Front. Chem. 2019, 7, 667. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst limit in dual-gated Si-nanowire FET sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Spijkman, M.J.; Brondijk, J.J.; Geuns, T.C.T.; Smits, E.C.P.; Cramer, T.; Zerbetto, F.; Stoliar, P.; Biscarini, F.; Blom, P.W.M.; De Leeuw, D.M. Dual-gate organic field-effect transistors as potentiometrie sensors in aqueous solution. Adv. Funct. Mater. 2010, 20, 898–905. [Google Scholar] [CrossRef]
- Jung, S.H.; Seo, Y.M.; Gu, T.; Jang, W.; Kang, S.G.; Hyeon, Y.; Hyun, S.H.; Lee, J.H.; Whang, D. Super-Nernstian pH Sensor Based on Anomalous Charge Transfer Doping of Defect-Engineered Graphene. Nano Lett. 2021, 21, 34–42. [Google Scholar] [CrossRef]
- Cramer, T.; Campana, A.; Leonardi, F.; Casalini, S.; Kyndiah, A.; Murgia, M.; Biscarini, F. Water-gated organic field effect transistors-opportunities for biochemical sensing and extracellular signal transduction. J. Mater. Chem. B 2013, 1, 3728–3741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Babuji, A.; Temiño, I.; Salzillo, T.; D’Amico, F.; Pfattner, R.; Ocal, C.; Barrena, E.; Mas-Torrent, M. Chemical Doping of the Organic Semiconductor C8-BTBT-C8 Using an Aqueous Iodine Solution for Device Mobility Enhancement. Adv. Mater. Technol. 2022, 7, 2101535. [Google Scholar] [CrossRef]
- Völkel, A.R.; Street, R.A.; Knipp, D. Carrier transport and density of state distributions in pentacene transistors. Phys. Rev. B - Condens. Matter Mater. Phys. 2002, 66, 1953361–1953368. [Google Scholar] [CrossRef]
- Poimanova, E.Y.; Shaposhnik, P.A.; Karaman, P.N.; Anisimov, D.S.; Skorotetcky, M.S.; Polinskaya, M.S.; Borshchev, O.V.; Agina, E.V.; Ponomarenko, S.A. Electrolyte-gated organic field-effect transistors based on 2,6-dioctyltetrathienoacene as a convenient platform for fabrication of liquid biosensors. Russ. Chem. Bull. 2022, 71, 2116–2122. [Google Scholar] [CrossRef]
- Li, C.; Duan, L.; Li, H.; Qiu, Y. Universal trap effect in carrier transport of disordered organic semiconductors: Transition from shallow trapping to deep trapping. J. Phys. Chem. C 2014, 118, 10651–10660. [Google Scholar] [CrossRef]

| Substrate | Ion, µA | Ioff, nA | gm, A1/2 V−1(×103) | Vth, mV |
|---|---|---|---|---|
| Si/SiO2 | −0.3 ± 0.1 | −0.3 ± 0.4 | −1.5 ± 0.4 | −130 ± 40 |
| PEN | −1 ± 0.7 | −14 ± 20 | −2.6 ± 1 | −90 ± 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaposhnik, P.A.; Poimanova, E.Y.; Abramov, A.A.; Trul, A.A.; Anisimov, D.S.; Kretova, E.A.; Agina, E.V.; Ponomarenko, S.A. Applying of C8-BTBT-Based EGOFETs at Different pH Values of the Electrolyte. Chemosensors 2023, 11, 74. https://doi.org/10.3390/chemosensors11020074
Shaposhnik PA, Poimanova EY, Abramov AA, Trul AA, Anisimov DS, Kretova EA, Agina EV, Ponomarenko SA. Applying of C8-BTBT-Based EGOFETs at Different pH Values of the Electrolyte. Chemosensors. 2023; 11(2):74. https://doi.org/10.3390/chemosensors11020074
Chicago/Turabian StyleShaposhnik, Polina A., Elena Y. Poimanova, Anton A. Abramov, Askold A. Trul, Daniil S. Anisimov, Elena A. Kretova, Elena V. Agina, and Sergey A. Ponomarenko. 2023. "Applying of C8-BTBT-Based EGOFETs at Different pH Values of the Electrolyte" Chemosensors 11, no. 2: 74. https://doi.org/10.3390/chemosensors11020074
APA StyleShaposhnik, P. A., Poimanova, E. Y., Abramov, A. A., Trul, A. A., Anisimov, D. S., Kretova, E. A., Agina, E. V., & Ponomarenko, S. A. (2023). Applying of C8-BTBT-Based EGOFETs at Different pH Values of the Electrolyte. Chemosensors, 11(2), 74. https://doi.org/10.3390/chemosensors11020074







