Asymmetric Pt(II)-Porphyrin Incorporated in a PVC Ion-Selective Membrane for the Potentiometric Detection of Citrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
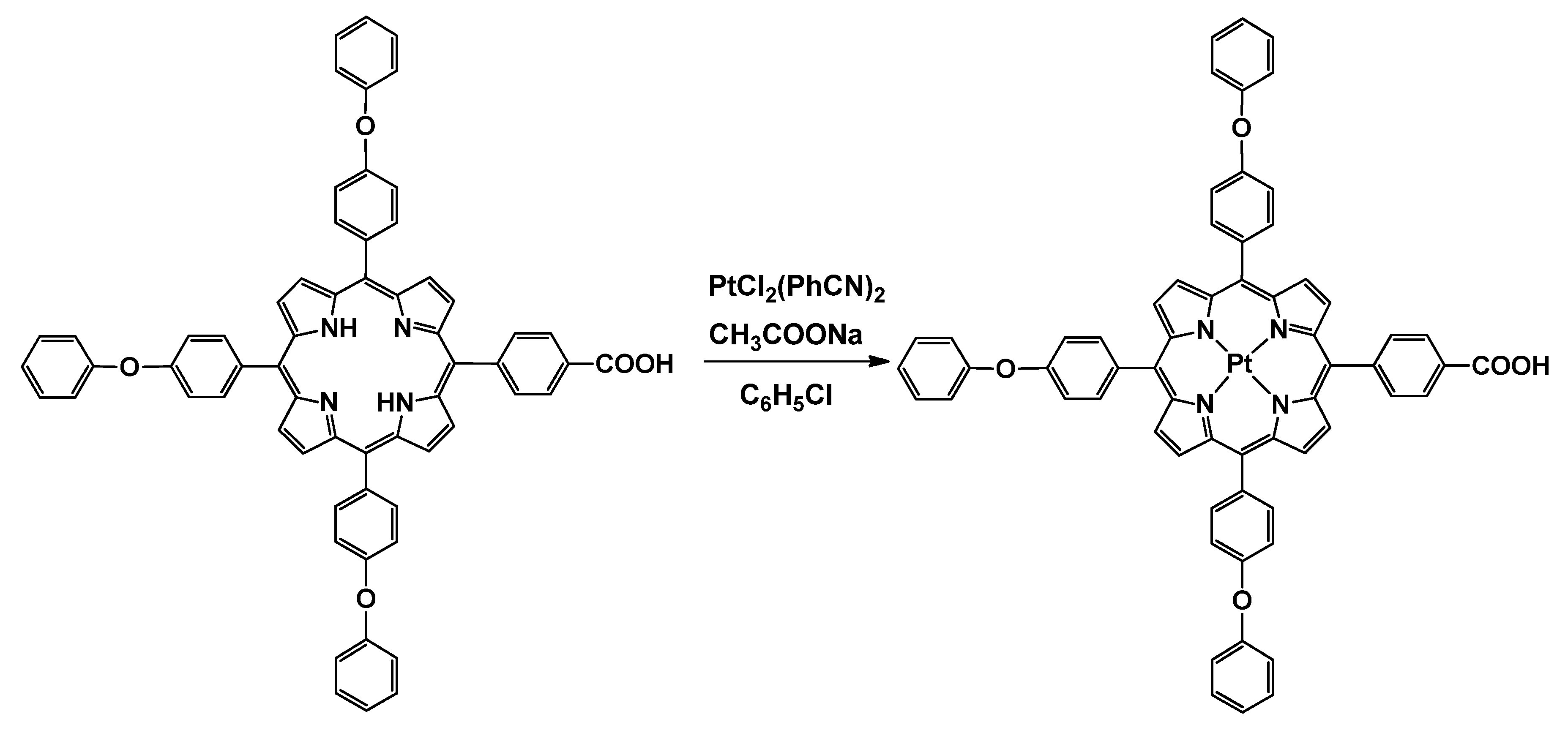
2.3. Synthesis of Pt(II)-5-(4-Carboxyphenyl)-10,15,20-tris(4-phenoxyphenyl)-porphyrin (Pt(II)-COOH-TPOPP)
2.4. Electrode Membrane Preparation and Measurements
3. Results and Discussion
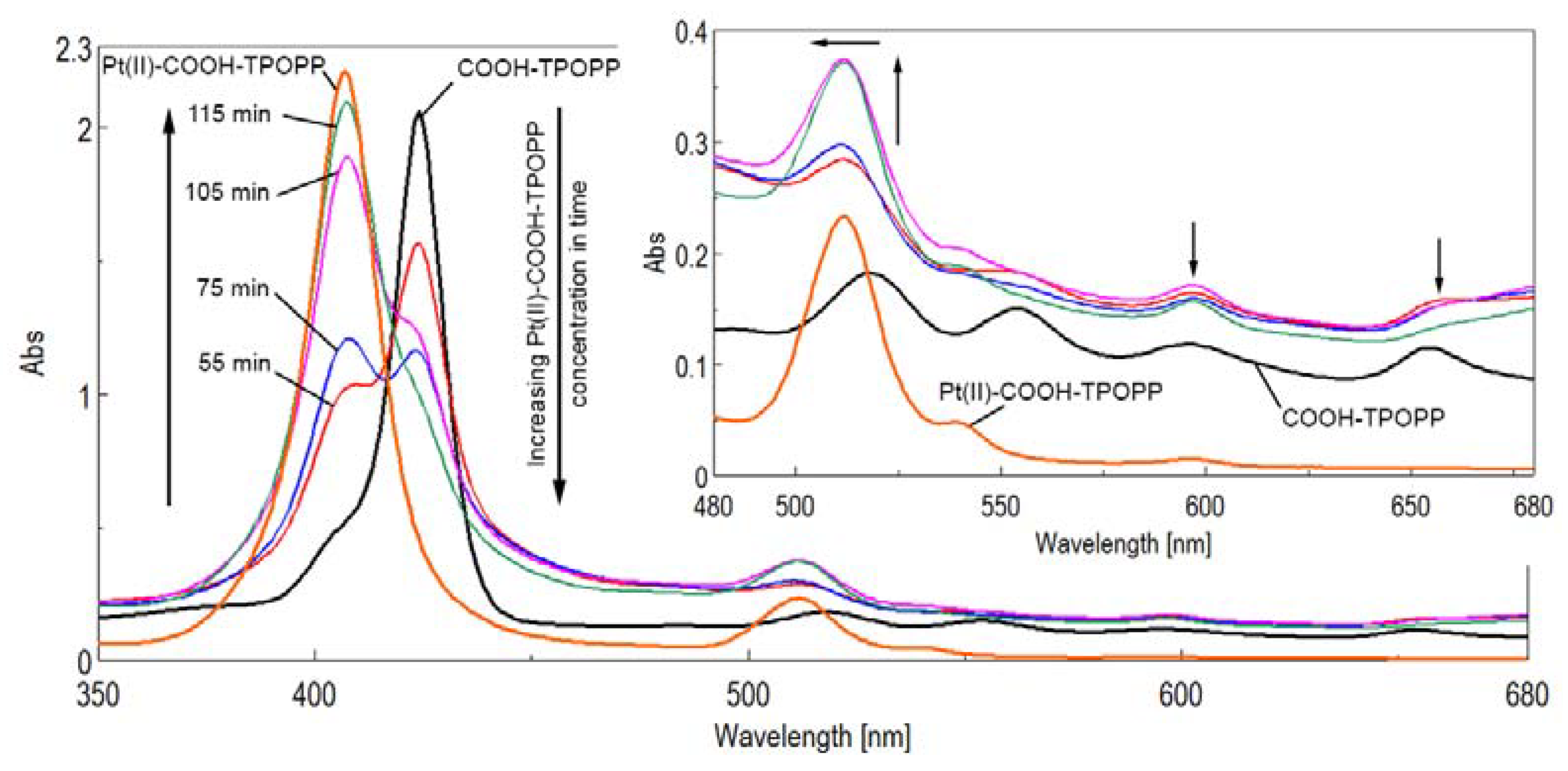
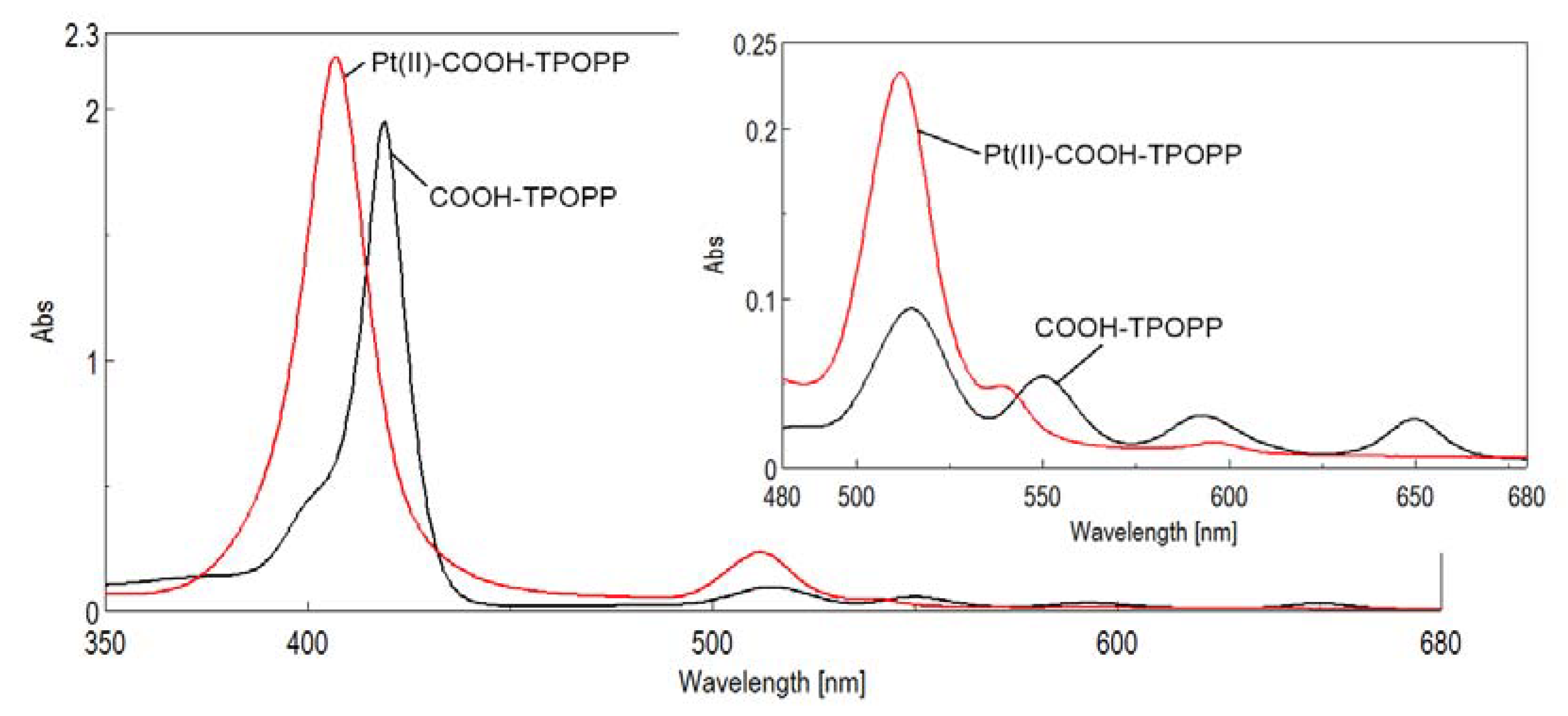
3.1. UV–Vis Monitoring of the Pt(II)-Carboxy-phenyl-tris-(phenoxy-phenyl)porphyrin Synthesis
3.2. Nuclear Magnetic Resonance Characterization
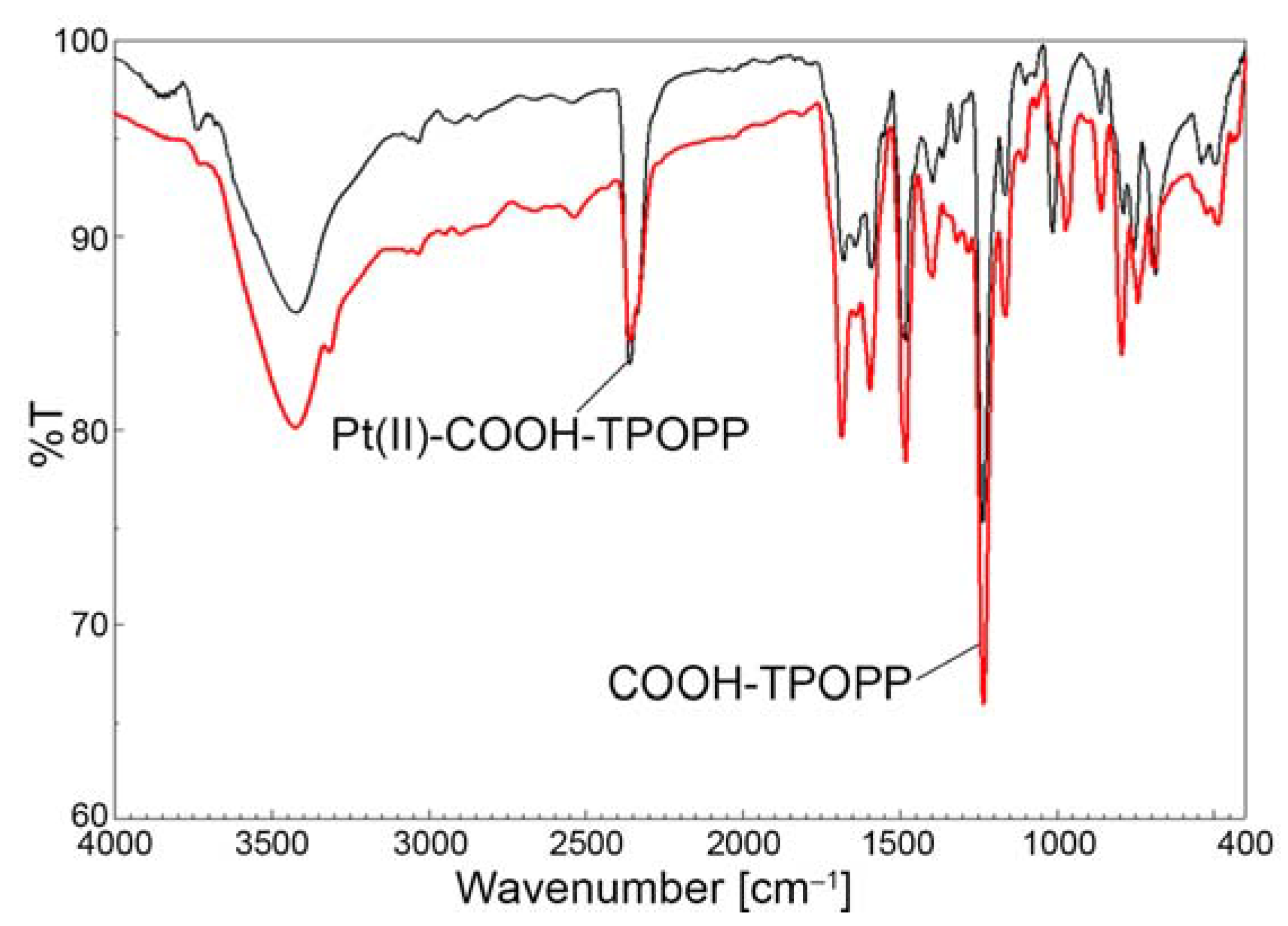
3.3. Comparative FT-IR-Analysis
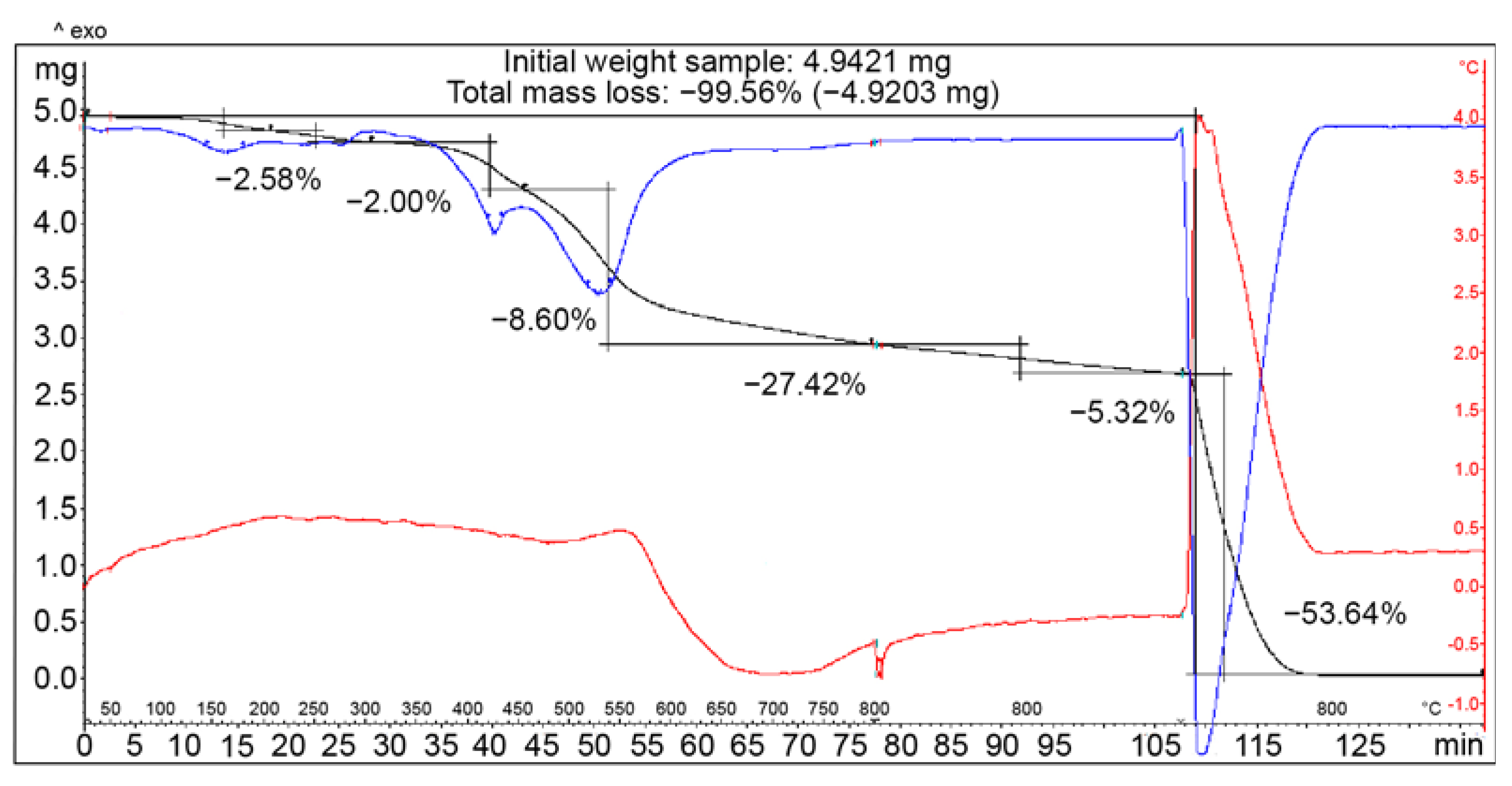
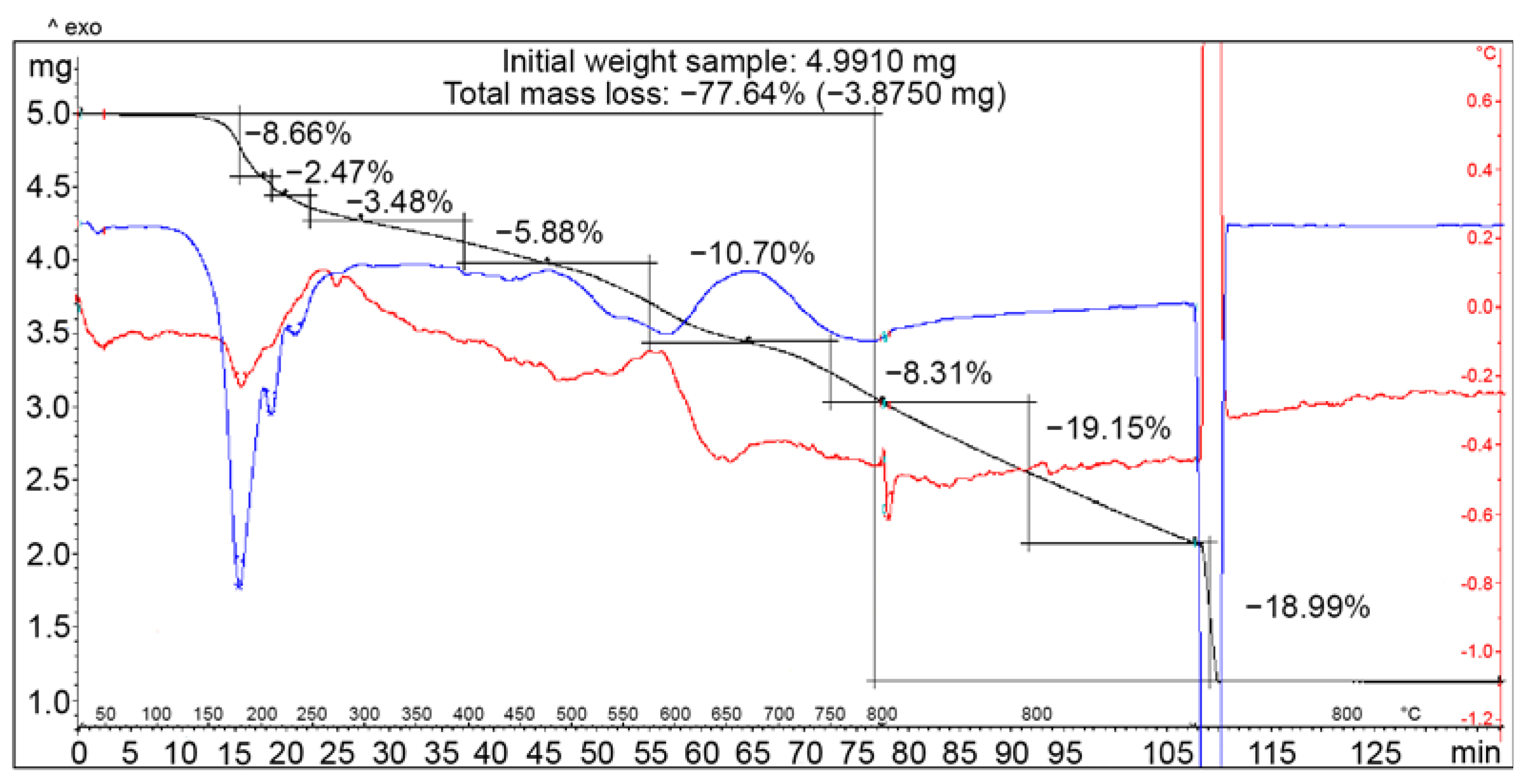
3.4. Thermogravimetric Analysis of COOH-TPOPP and Pt(II)-COOH-TPOPP
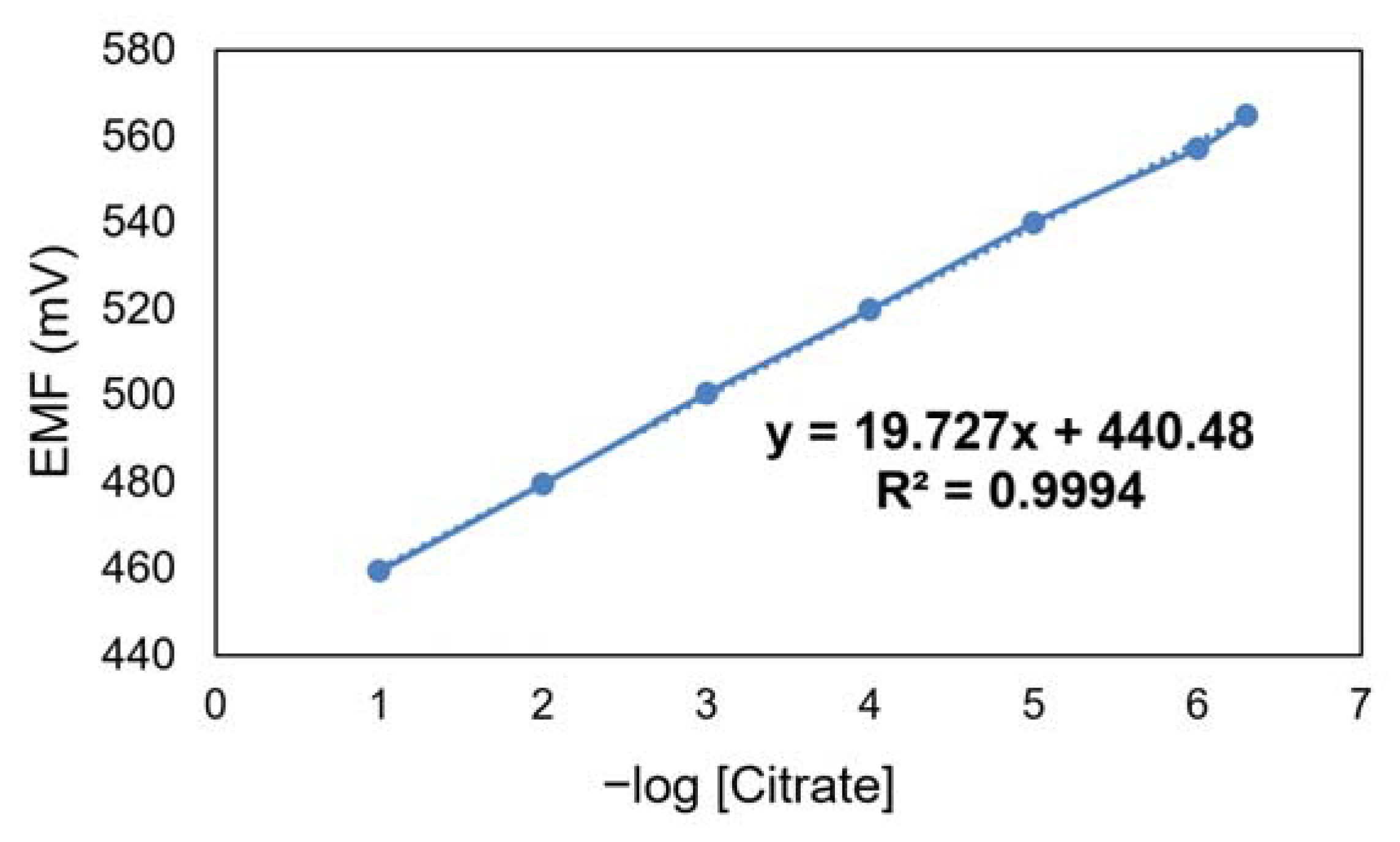
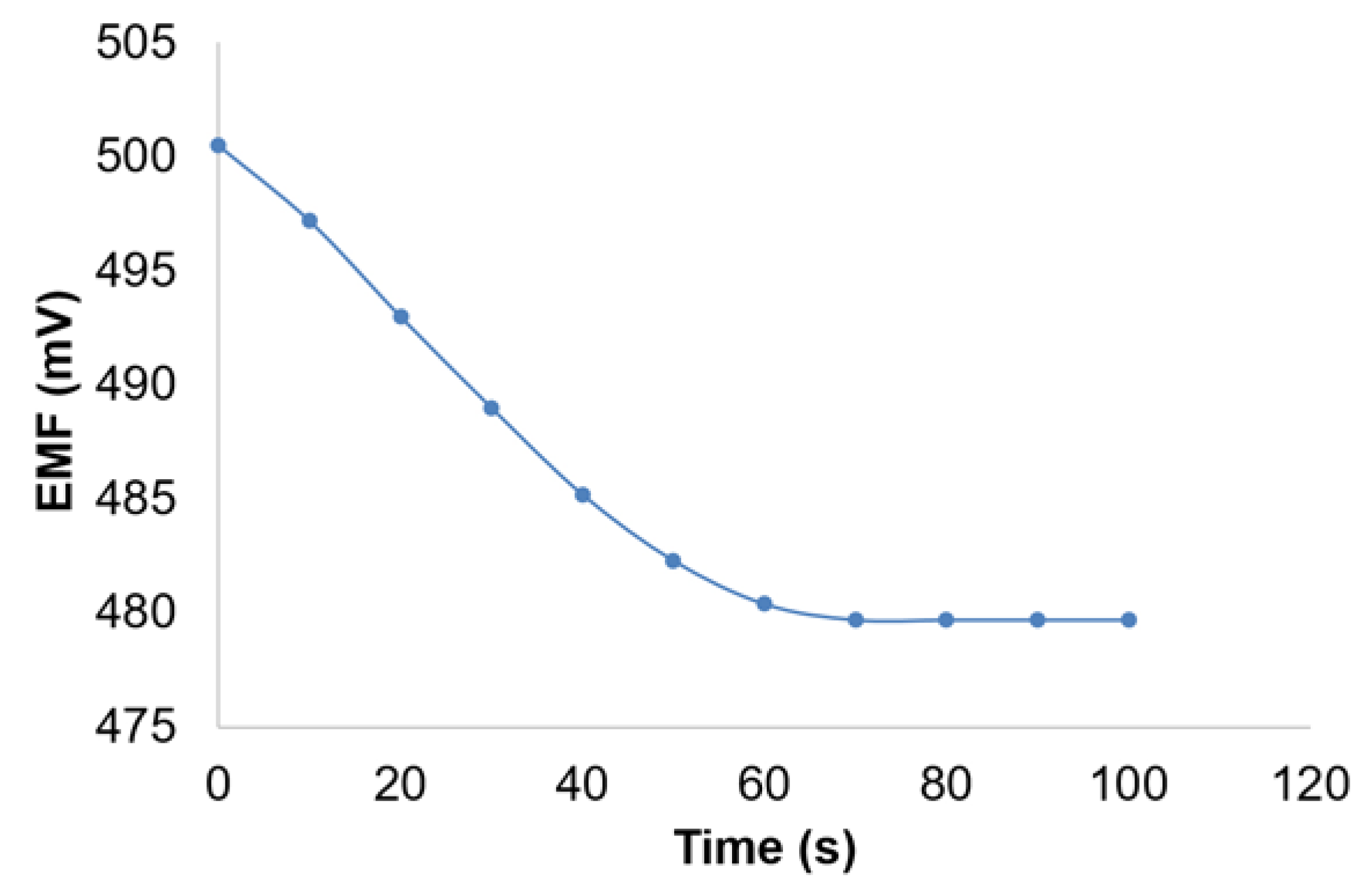
3.5. Potentiometric Detection
3.6. Presumed Mechanism of Citrate Recognition
- -
- Interactions between two opposite electrical charges (positively charged platinum coordinated in the porphyrin ring and negatively charged oxygen belonging to the carboxylate group contained in the citrate anion, depicted in yellow in Figure 10);
- -
- π-anion interactions between π-electrons of the extended aromatic heterocycle belonging to the porphyrin and the negatively charged oxygen from the carboxylate ion (represented in orange in Figure 10);
- -
- π–π interaction between the non-participating electron pairs present both in the porphyrin macrocycle and in the carbonyl oxygen from citrate (green lines in Figure 10).
3.7. Analytical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Templeton, H.L.; Sommer, H.H. The Use of Citric Acid and Sodium Citrate in Starter Cultures. J. Dairy Sci. 1929, 12, 21–36. [Google Scholar] [CrossRef]
- Viegas, O.J.; Ravindran, R.S.; Shumacker, C.A. Gastric fluid pH in patients receiving sodium citrate. Anesth. Analg. 1981, 60, 521–523. Available online: http://www.anesthesia-analgesia.org/cgi/content/abstract/60/7/521 (accessed on 30 January 2023). [CrossRef] [PubMed]
- Weiner, I.D.; Verlander, J.W.; Wingo, C.S. Renal Acidification Mechanisms. In Core Concepts in the Disorders of Fluid, Electrolytes and Acid-Base Balance; Mount, D., Sayegh, M., Singh, A., Eds.; Springer: Boston, MA, USA, 2013; pp. 203–233. [Google Scholar] [CrossRef]
- Borrego Utiel, F.J.; Herrera Contreras, I.; Merino García, E.M.; Camacho Reina, M.V.; Morina Dominguez, C.; Ocana Perez, E. Urinary citrate as a marker of renal function in patients with autosomal dominant polycystic kidney disease. Int. Urol. Nephrol. 2022, 54, 873–881. [Google Scholar] [CrossRef]
- de Sequera, P.; Pérez-García, R.; Molina, M.; Álvarez-Fernández, G.; Muñoz-González, R.I.; Mérida, E.; Camba, M.J.; Blázquez, L.A.; Alcaide, M.P.; Echarri, R. Advantages of the use of citrate over acetate as a stabilizer in hemodialysis fluid: A randomized ABC-treat study. Nefrologia 2022, 42, 327–337. [Google Scholar] [CrossRef]
- Schneider, A.G.; Journois, D.; Rimmelé, T. Complications of regional citrate anticoagulation: Accumulation or overload? Crit. Care. 2017, 21, 281. [Google Scholar] [CrossRef]
- Thanapongsatorn, P.; Chaijamorn, W.; Sirivongrangson, P.; Tachaboon, S.; Peerapornratana, S.; Lumlertgul, N.; Lucksiri, A.; Srisawat, N. Citrate pharmacokinetics in critically ill liver failure patients receiving CRRT. Sci. Rep. 2022, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, S.; Osouli-Bostanabad, K.; Nazemiyeh, H.; Javadzadeh, Y.; Parvizpur, A.; Barzegar-Jalali, M.; Adibkia, K. A comparative study of eco-friendly silver nanoparticles synthesis using Prunus domestica plum extract and sodium citrate reducing agents. Adv. Powder Technol. 2020, 31, 169–180. [Google Scholar] [CrossRef]
- Grys, D.B.; de Nijs, B.; Salmon, A.R.; Huang, J.; Wang, W.; Chen, W.H.; Scherman, O.A.; Baumberg, J.J. Citrate Coordination and Bridging of Gold Nanoparticles: The Role of Gold Adatoms in AuNP Ageing. ACS Nano. 2020, 14, 8689–8696. [Google Scholar] [CrossRef] [PubMed]
- Ally, N.; Hendricks, N.; Gumbi, B. A Colorimetric Detection of Noradrenaline in Wastewater Using Citrate-Capped Colloidal Gold Nanoparticles Probe. Colloids Interfaces 2022, 6, 61. [Google Scholar] [CrossRef]
- Ali, M.; Khalid, M.A.U.; Shah, I.; Kim, S.W.; Kim, Y.S.; Lim, J.H.; Choi, K.H. Paper-based selective and quantitative detection of uric acid using citrate-capped Pt nanoparticles (PtNPs) as a colorimetric sensing probe through a simple and remote-based device. New J. Chem. 2019, 43, 7636–7645. [Google Scholar] [CrossRef]
- DeBorba, B.M.; Rohrer, J.S.; Bhattacharyya, L. Development and validation of an assay for citric acid/citrate and phosphate in pharmaceutical dosage forms using ion chromatography with suppressed conductivity detection. J. Pharm. Biomed. Anal. 2004, 36, 517–524. [Google Scholar] [CrossRef]
- Luque-Peréz, E.; Ríos, A.; Valcárcel, M. Flow-injection spectrophotometric determination of citric acid in beverages based on a photochemical reaction. Anal. Chim. Acta 1998, 366, 231–240. [Google Scholar] [CrossRef]
- Morales, M.L.; Ferreira, R.; González, A.G.; Troncoso, A.M. Simultaneous determination of organic acids and sweeteners in soft drinks by ion-exclusion HPLC. J. Sep. Sci. 2001, 24, 879–884. [Google Scholar] [CrossRef]
- Muthusamy, S.; Zhu, D.; Rajalakshmi, K.; Zhu, W.; Wang, S.; Lee, K.-B.; Zhao, L. Successive Detection of Zinc Ion and Citrate Using a Schiff Base Chemosensor for Enhanced Prostate Cancer Diagnosis in Biosystems. ACS Appl. Bio. Mater. 2021, 4, 1932–1941. [Google Scholar] [CrossRef]
- Liu, C.; Hang, Y.; Jiang, T.; Yang, J.; Zhang, X.; Hua, J. A light-up fluorescent probe for citrate detection based on bispyridinum amides with aggregation-induced emission feature. Talanta 2018, 178, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, K.; Deng, T.; Muthusamy, S.; Xie, M.; Xie, J.; Lee, K.-B.; Xu, Y. Prostate cancer biomarker citrate detection using triaminoguanidinium carbon dots, its applications in live cells and human urine samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 268, 120622. [Google Scholar] [CrossRef]
- Shaban, S.M.; Lee, J.Y.; Kim, D.-H. Dual-Surfactant-Capped Ag Nanoparticles as a Highly Selective and Sensitive Colorimetric Sensor for Citrate Detection. ACS Omega 2020, 5, 10696–10703. [Google Scholar] [CrossRef]
- Fukushima, Y.; Aikawa, S. Colorimetric chemosensor based on a Ni2+ complex of a pyridylazo dye for detection of citrate in aqueous solution. Tetrahedron Lett. 2020, 61, 151681. [Google Scholar] [CrossRef]
- Deng, X.; Shen, Y.; Liu, B.; Song, Z.; He, X.; Zhang, Q.; Ling, D.; Liu, D.; Wei, D. Terahertz Metamaterial Sensor for Sensitive Detection of Citrate Salt Solutions. Biosensors 2022, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Wester, N.; Mynttinen, E.; Etula, J.; Lilius, T.; Kalso, E.; Mikladal, B.F.; Zhang, Q.; Jiang, H.; Sainio, S.; Nordlund, D.; et al. Single-Walled Carbon Nanotube Network Electrodes for the Detection of Fentanyl Citrate. ACS Appl. Nano Mater. 2020, 3, 1203–1212. [Google Scholar] [CrossRef]
- Ozbek, O.; Isildak, O.; Berkel, C. The use of porphyrins in potentiometric sensors as ionophores. J. Incl. Phenom. Macrocycl. Chem. 2020, 98, 1–9. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, E.; Pica, E.M.; Cosma, V.; Bizerea, O.; Mihailescu, G.; Olenic, L. Free Base Porphyrins as Ionophores for Heavy Metal Sensors. Sensors 2008, 8, 4995–5004. [Google Scholar] [CrossRef]
- Vlascici, D.; Popa, I.; Chiriac, V.A.; Fagadar-Cosma, G.; Popovici, H.; Fagadar-Cosma, E. Potentiometric detection and removal of copper using porphyrins. Chem. Cent. J. 2013, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Taranu, B.O.; Vlascici, D.; Sebarchevici, I.; Fagadar-Cosma, E. The aggregation behavior of an A3B free base porphyrin and its application as chromium(III)-selective membrane sensor. Stud. Univ. Babes-Bolyai Chem. LXI 2016, 1, 199–212. [Google Scholar]
- Bodedla, G.B.; Dong, Y.; Tang, G.; Zhao, J.; Zhang, F.; Zhu, X.; Wong, W.-Y. Long-lived excited states of platinum(II)-porphyrins for highly efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2022, 10, 13402–13409. [Google Scholar] [CrossRef]
- Orfanos, E.; Ladomenou, K.; Angaridis, P.A.; Papadopoulos, T.; Charalambidis, G.; Vasilopoulou, M.; Coutsolelos, A.G. A stable platinum porphyrin based photocatalyst for hydrogen production under visible light in water. Sustain. Energy Fuels 2022, 6, 5072–5076. [Google Scholar] [CrossRef]
- Wang, X.; Peng, H.; Cheng, K.; Liu, X.; Liu, Y.; Yang, W. Two-photon oxygen nanosensors based on a conjugated fluorescent polymer doped with platinum porphyrins. Methods Appl. Fluoresc. 2018, 6, 035008. [Google Scholar] [CrossRef] [PubMed]
- Garai, A.; Villa, M.; Marchini, M.; Patra, S.K.; Pain, T.; Mondal, S.; Ceroni, P.; Kar, S. Synthesis, Structure, Photophysics, and Singlet Oxygen Sensitization by a Platinum(II) Complex of Meso-Tetra-Acenaphthyl Porphyrin. Eur. J. Inorg. Chem. 2021, 39, 4089–4095. [Google Scholar] [CrossRef]
- Subedi, D.R.; Reid, R.; D’Souza, P.F.; Nesterov, V.N.; D’Souza, F. Singlet Oxygen Generation in Peripherally Modified Platinum and Palladium Porphyrins: Effect of Triplet Excited State Lifetimes and meso-Substituents on 1O2 Quantum Yields. ChemPlusChem 2022, 87, e202200010. [Google Scholar] [CrossRef]
- Yao, B.; He, Y.; Wang, S.; Sun, H.; Liu, X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. Int. J. Mol. Sci. 2022, 23, 6036. [Google Scholar] [CrossRef]
- Yang, M.; Deng, J.; Guo, D.; Zhang, J.; Yang, L.; Wu, F. A folate-conjugated platinum porphyrin complex as a new cancer-targeting photosensitizer for photodynamic therapy. Org. Biomol. Chem. 2019, 17, 5367–5374. [Google Scholar] [CrossRef]
- Gao, Y.; Piradi, V.; Zhu, X.; So, S.K. Palladium(II) and Platinum(II) Porphyrin Donors for Organic Photovoltaics. ACS Appl. Energy Mater. 2022, 5, 4916–4925. [Google Scholar] [CrossRef]
- Fratilescu, I.; Lascu, A.; Taranu, B.O.; Epuran, C.; Birdeanu, M.; Macsim, A.-M.; Tanasa, E.; Vasile, E.; Fagadar-Cosma, E. One A3B Porphyrin Structure—Three Successful Applications. Nanomaterials 2022, 12, 1930. [Google Scholar] [CrossRef]
- Lvova, L.; Verrelli, G.; Stefanelli, M.; Nardis, S.; Di Natale, C.; D’Amico, A.; Makarychev-Mikhailov, S.; Paolesse, R. Platinum porphyrins as ionophores in polymeric membrane electrodes. Analyst 2011, 136, 4966–4976. [Google Scholar] [CrossRef] [PubMed]
- Lvova, L.; Paolesse, R.; Di Natale, C.; D’Amico, A. Detection of alcohols in beverages: An application of porphyrin-based Electronic tongue. Sens. Actuators B Chem. 2006, 118, 439–447. [Google Scholar] [CrossRef]
- Vlascici, D.; Plesu, N.; Fagadar-Cosma, G.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric Sensors for Iodide and Bromide Based on Pt(II)-Porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Plesu, N.; Lascu, A.; Anghel, D.; Cazacu, M.; Ianasi, C.; Fagadar-Cosma, G.; Fratilescu, I.; Epuran, C. Novel Platinum-Porphyrin as Sensing Compound for Efficient Fluorescent and Electrochemical Detection of H2O2. Chemosensors 2020, 8, 29. [Google Scholar] [CrossRef]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef] [PubMed]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Augusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef]
- Yamashita, K.; Katsumata, N.; Tomita, S.; Fuwa, M.; Fujimaki, K.; Yoda, T.; Hirano, D.; Sugiura, K. Facile and practical synthesis of platinum(II) porphyrins under mild conditions. Chem. Lett. 2015, 44, 492–494. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.-F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-Ray structure elucidation of a Pt-metalloporphyrin and its application for obtaining sensitive AuNPs-plasmonic hybrids capable to detect triiodide anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef] [PubMed]
- Fringu, I.; Lascu, A.; Macsim, A.M.; Fratilescu, I.; Epuran, C.; Birdeanu, M.; Fagadar-Cosma, E. Pt(II)-A2B2 metalloporphyrin-AuNPS hybrid material suitable for optical detection of 1-anthraquinonsulfonic acid. Chem. Pap. 2022, 76, 2513–2527. [Google Scholar] [CrossRef]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part, I. Inorganic cations. Pure Appl. Chem. 2002, 74, 923–994. [Google Scholar] [CrossRef]
- Öztürk, N.; Çırak, Ç.; Bahçeli, S. FT-IR Spectroscopic Study of 1,5-Pentanedithiol and 1,6-Hexanedithiol Adsorbed on NaA, CaA and NaY Zeolites. Z. Naturforsch. A. 2014, 60, 633–636. [Google Scholar] [CrossRef]
- Wen, P.; Gong, P.; Mi, Y.; Wang, J.; Yang, S. Scalable fabrication of high quality graphene by exfoliation of edge sulfonated graphite for supercapacitor application. RSC Adv. 2014, 4, 35914–35918. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhu, W.; Wang, Q.; Yang, Y.; Zhou, H.; Zhang, F.; Zhou, L.; Razal, J.M.; Wallace, G.G.; Chen, J. Metal porphyrin intercalated reduced graphene oxide nanocomposite utilized for electrocatalytic oxygen reduction. Green Energy Environ. 2017, 2, 285–293. [Google Scholar] [CrossRef]
- Chen, B.-K.; Su, C.-T.; Tseng, M.-C.; Tsay, S.-Y. Preparation of Polyetherimide Nanocomposites with Improved Thermal, Mechanical and Dielectric Properties. Polym. Bull. 2006, 57, 671–681. [Google Scholar] [CrossRef]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2018, 306, 268–275. [Google Scholar] [CrossRef]
- Wei, X.; Du, X.; Chen, D.; Chen, Z. Thermal analysis study of 5,10,15,20-tetrakis (methoxyphenyl) porphyrins and their nickel complexes. Thermochim. Acta 2006, 440, 181–187. [Google Scholar] [CrossRef]
- Elghamry, I.; Ibrahim, S.S.; Abdelsalam, M.; Al-Faiyz, Y.; Al-Qadri, M. Synthesis, Thermal Stability and Electrocatalytic Activities of meso-tetrakis (5-bromothiophen-2-yl) Porphyrin and Its Cobalt and Copper Complexes. Int. J. Electrochem. Sci. 2018, 13, 10233–10246. [Google Scholar] [CrossRef]
- Pinto, V.H.A.; CarvalhoDa-Silva, D.; Santos, J.L.M.S.; Weitner, T.; Fonseca, M.G.; Yoshida, M.I.; Idemori, Y.M.; Batinić-Haberle, I.; Rebouças, J.S. Thermal stability of the prototypical Mn porphyrin-based superoxide dismutase mimic and potent oxidative-stress redox modulator Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin chloride, MnTE-2-PyP5+. J. Pharm. Biomed. Anal. 2013, 25, 29–34. [Google Scholar] [CrossRef]
- Yajima, T.; Maccarrone, G.; Takani, M.; Contino, A.; Arena, G.; Takamido, R.; Hanaki, M.; Funahashi, Y.; Odani, A.; Yamauchi, O. Combined effects of electrostatic and pi-pi stacking interactions: Selective binding of nucleotides and aromatic carboxylates by platinum(II)-aromatic ligand complexes. Chemistry 2003, 9, 3341–3352. [Google Scholar] [CrossRef]
- Dimitrijević, B.P.; Borozan, S.Z.; Stojanović, S.Đ. π–π and cation–π interactions in protein–porphyrin complex crystal structures. RSC Adv. 2012, 2, 12963. [Google Scholar] [CrossRef]
- Almeida, S.A.A.; Heitor, A.M.; Montenegro, M.C.B.S.M.; Sales, M.G.F. Sulfadiazine-selective determination in aquaculture environment: Selective potentiometric transduction by neutral or charged ionophores. Talanta 2011, 85, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.G.; Araújo, A.N.; Couto, C.M.C.M.; Montenegro, M.C.B.S.M. Potentiometric behaviour of ion selective electrodes based on iron porphyrins: The influence of porphyrin substituents on the response properties and analytical determination of diclofenac in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2006, 42, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, M.; Nezamzadeh-Ejhieh, A. A novel citrate selective electrode based on surfactant modified nano-clinoptilolite. Food Chem. 2015, 172, 794–801. [Google Scholar] [CrossRef]
- Nooredeen, N.M.; Abd El-Ghaffar, M.A.; Darwish, W.M.; Elshereafy, E.; Radwan, A.A.; Abbas, M.N. Graphene oxide with covalently attached zinc monoamino-phthalocyanine coated graphite electrode as a potentiometric platform for citrate sensing in pharmaceutical preparations. J Solid State Electrochem. 2015, 19, 2141–2154. [Google Scholar] [CrossRef]
- Ribeiro, C.M.F.; Matos, C.D.; Sales, M.G.F.; Vaz, M.C.V.F. Citrate selective electrodes for the flow injection analysis of soft drinks, beers and pharmaceutical products. Anal. Chim. Acta 2002, 471, 41–49. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hoseynidokht, F.; Zare, H.R.; Vafazadeh, R. Development of a Polymeric Membrane Electrode for Determination of Citrate Ions by Potentiometric Technique. Anal. Bioanal. Electrochem. 2018, 10, 1093–1107. [Google Scholar]

| Fluorophore | Concentration Domain (mol/L)/Medium | LOD (mol/L) | Ref. |
|---|---|---|---|
| Zn-triamino-guandine-thiophene (TAT) | 0−1.8 × 10−6 In prostate cancer cells | 10.8 × 10−9 | [15] |
| Tetraphnyl- ethylene covalently linked to bipyridinium amide | 1–5 × 10−6 Artificial urine | 1.0 × 10−7 | [16] |
| Triaminoguanidine carbon dots | 0−650 × 10−9 Bioimaging in MCF-7 cells (breast epithelial cancer cells) | 4 × 10−9 | [17] |
| Membrane Composition. | Sensor 1 | Sensor 2 | Sensor 3 | |
|---|---|---|---|---|
| Ionophore | 1 | 1 | 1 | |
| Plasticizer | DOS | 66 | ||
| DOP | 66 | |||
| NPOE | 66 | |||
| PVC | 33 | 33 | 33 | |
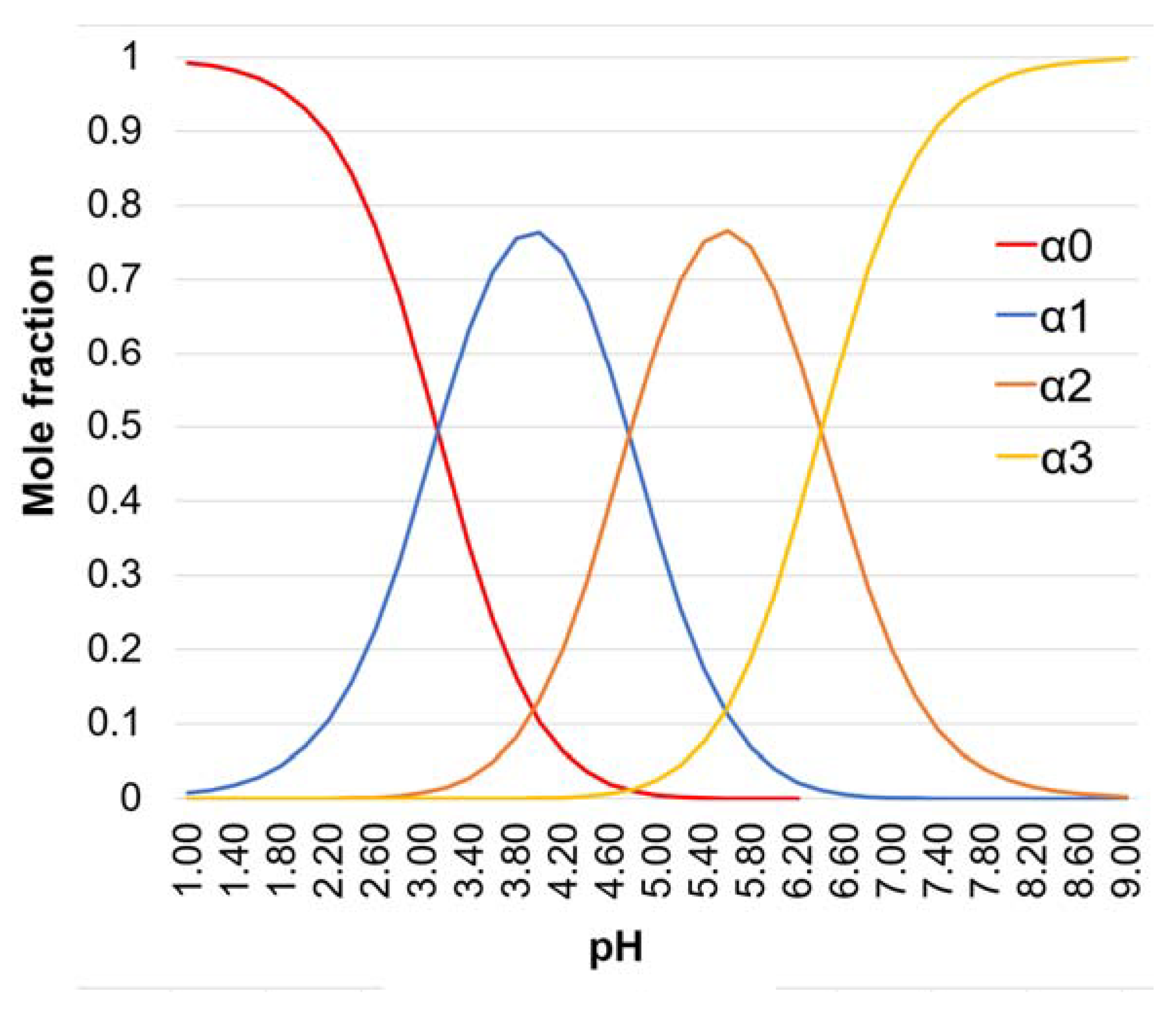
| pH | H3Cit | H2Cit- | HCit2- | Cit3- |
|---|---|---|---|---|
| 5.50 | - | 1.46 | 7.93 | 1 |
| 8.00 | - | - | 1 | 39.8 |
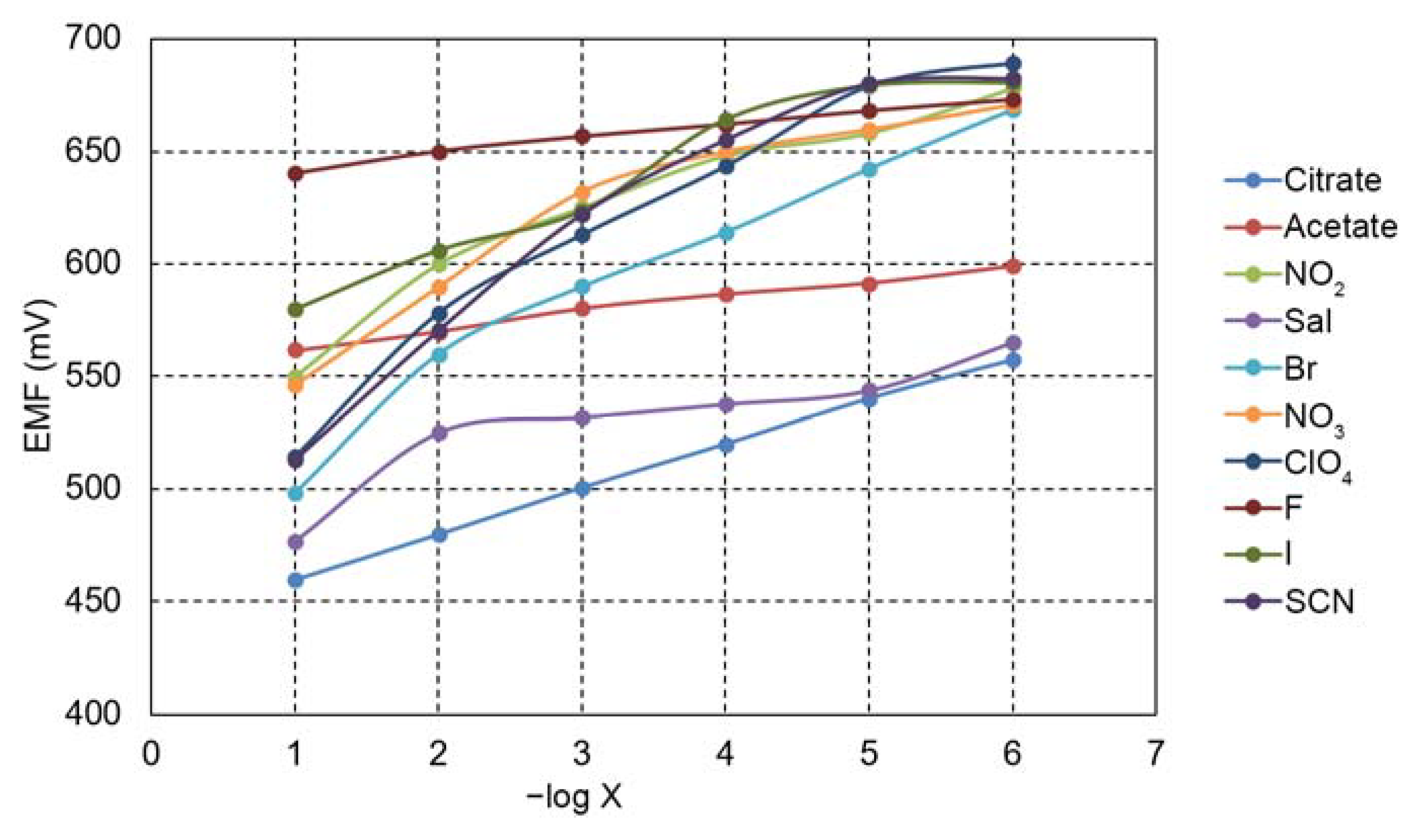
| Anion | log KCitr, X |
|---|---|
| Ac − | −(0.68 ± 0.02) |
| −(2.10 ± 0.04) | |
| Sal− | −(0.20 ± 0.01) |
| Br− | -(0.40 ± 0.02) |
| −(1.59 ± 0.04) | |
| F− | −(4.64 ± 0.05) |
| I− | −(2.41 ± 0.05) |
| SCN− | −(0.60 ± 0.02) |
| −(1.00 ± 0.04) |
| Synthetic Sample | Amount (mg) | Found by Sensor (mg ± S a) |
|---|---|---|
| Citrate | 200 | 198 ± 0.5 |
| Citrate | 600 | 597 ± 0.6 |
| Sample | Amount (mg) | Found by Sensor (mg) | Found by Titration (mg) |
|---|---|---|---|
| Magnesium citrate (Gym Beam) | 500 | 498 ± 0.9 | 492 ± 2.0 |
| Calcium citrate (Now) | 800 | 795 ± 2.0 | 790 ± 3.0 |
| Magnesium citrate (Solgar) tablets | 200 | 197 ± 0.5 | 195 ± 2.0 |
| Sensitive Material/Type of Electrode | Polymeric Membrane/Plasticizer | Membrane Composition (wt%) | Detection Domain (mol/L)/Medium | LOD (mol/L) | Ref. |
|---|---|---|---|---|---|
| Nano-clinoptilolite particles stabilized with hexadecyltrimethyl ammonium (SMZ) | Polyvinyl chloride (PVC) /dioctyl phthalate (DOP) | SMZ/PVC/DOP = 10:30:60 | 5.0 × 10-5–5.0 × 10-2Pharmaceutical tablets | 1.3 × 10-5 | [58] |
| Graphene functionalized with zinc monoamino- phthalocyanine (ZnMAPc-G) | PVC/ dioctyl sebacate (DOS) | PVC/DOS/ZnMAPc-G = 31.1: 58.9:10 | 8 × 10−7–1 × 10−2 Pharmaceuticals | 5 × 10−7 | [59] |
| Bis(triphenyl-phosphoran- iylden) ammonium chloride (BTPPIA) | PVC/ bis (2-ethylhexyl) sebacate (bEHS)/ p-tert-octylphenol -(TOP) | BTPPIA/bEHS/ TOP/PVC= 1.3:60.6:6.5: 31.6 | 1.0 × 10−4 to 1.0 × 10−2 Soft drinks and pharmaceuticals | 5 × 10−5 | [60] |
| Mono- and di-nuclear copper(II) complex with salicylaldehyde-semicarbazone ligand (CuLBr) | PVC/ DOP, Trioctylmethyl ammonium chloride (MTOAC) | PVC/DOP/ CuLBr/MTOAC = 30.3: 62: 4: 3.7 | 1.0 × 10−7−1.0 × 10−1In juices | 6.3 × 10−8 | [61] |
| Pt(II)-5-(4-carboxyphenyl)-10,15,20-tris(4-phenoxyphenyl) -porphyrin (Pt(II)-COOH-TPOPP) | PVC/ dioctyl sebacate (DOS) | Pt(II)-COOH-TPOPP/PVC/DOS = 1:33:66 | 5.0 × 10−7–1.0 × 10−1Synthetic samples and food supplements | 3 × 10−7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlascici, D.; Lascu, A.; Fratilescu, I.; Anghel, D.; Epuran, C.; Birdeanu, M.; Chiriac, V.; Fagadar-Cosma, E. Asymmetric Pt(II)-Porphyrin Incorporated in a PVC Ion-Selective Membrane for the Potentiometric Detection of Citrate. Chemosensors 2023, 11, 108. https://doi.org/10.3390/chemosensors11020108
Vlascici D, Lascu A, Fratilescu I, Anghel D, Epuran C, Birdeanu M, Chiriac V, Fagadar-Cosma E. Asymmetric Pt(II)-Porphyrin Incorporated in a PVC Ion-Selective Membrane for the Potentiometric Detection of Citrate. Chemosensors. 2023; 11(2):108. https://doi.org/10.3390/chemosensors11020108
Chicago/Turabian StyleVlascici, Dana, Anca Lascu, Ion Fratilescu, Diana Anghel, Camelia Epuran, Mihaela Birdeanu, Vlad Chiriac, and Eugenia Fagadar-Cosma. 2023. "Asymmetric Pt(II)-Porphyrin Incorporated in a PVC Ion-Selective Membrane for the Potentiometric Detection of Citrate" Chemosensors 11, no. 2: 108. https://doi.org/10.3390/chemosensors11020108
APA StyleVlascici, D., Lascu, A., Fratilescu, I., Anghel, D., Epuran, C., Birdeanu, M., Chiriac, V., & Fagadar-Cosma, E. (2023). Asymmetric Pt(II)-Porphyrin Incorporated in a PVC Ion-Selective Membrane for the Potentiometric Detection of Citrate. Chemosensors, 11(2), 108. https://doi.org/10.3390/chemosensors11020108







