Pd-Nanoparticle-Decorated Multilayered MoS2 Sheets for Highly Sensitive Hydrogen Sensing
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials, Chemicals, and Sensor Fabrication
2.1.1. Materials and Sensor Fabrication
2.1.2. Pd-Nanoparticle-Decorated MoS2 Nanosheets Using AACVD Method
2.2. Material Characterization Techniques
2.3. Gas Sensing Measurements
3. Results and Discussions
3.1. Material Characterization
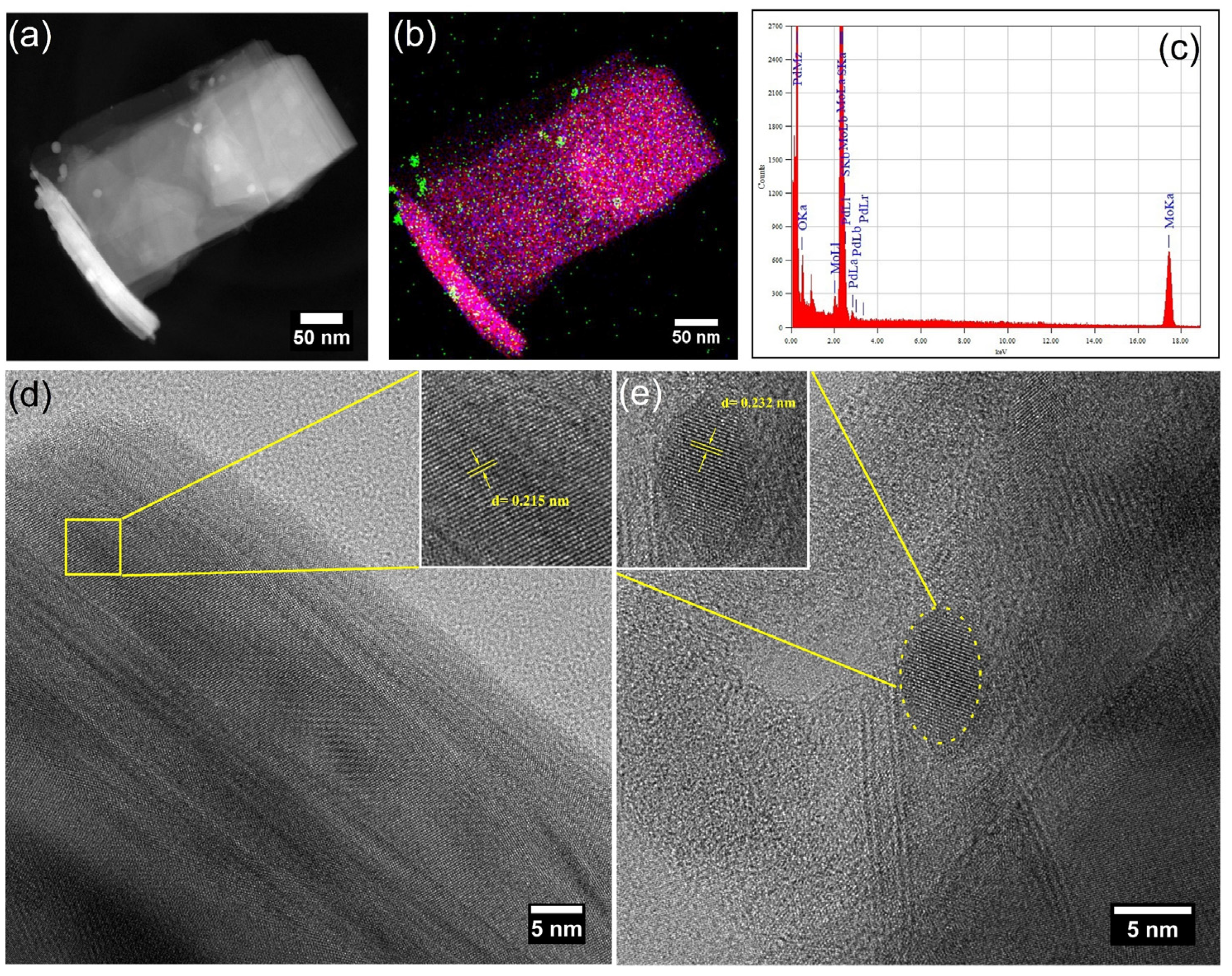
3.1.1. FESEM Analysis
3.1.2. HRTEM Analysis
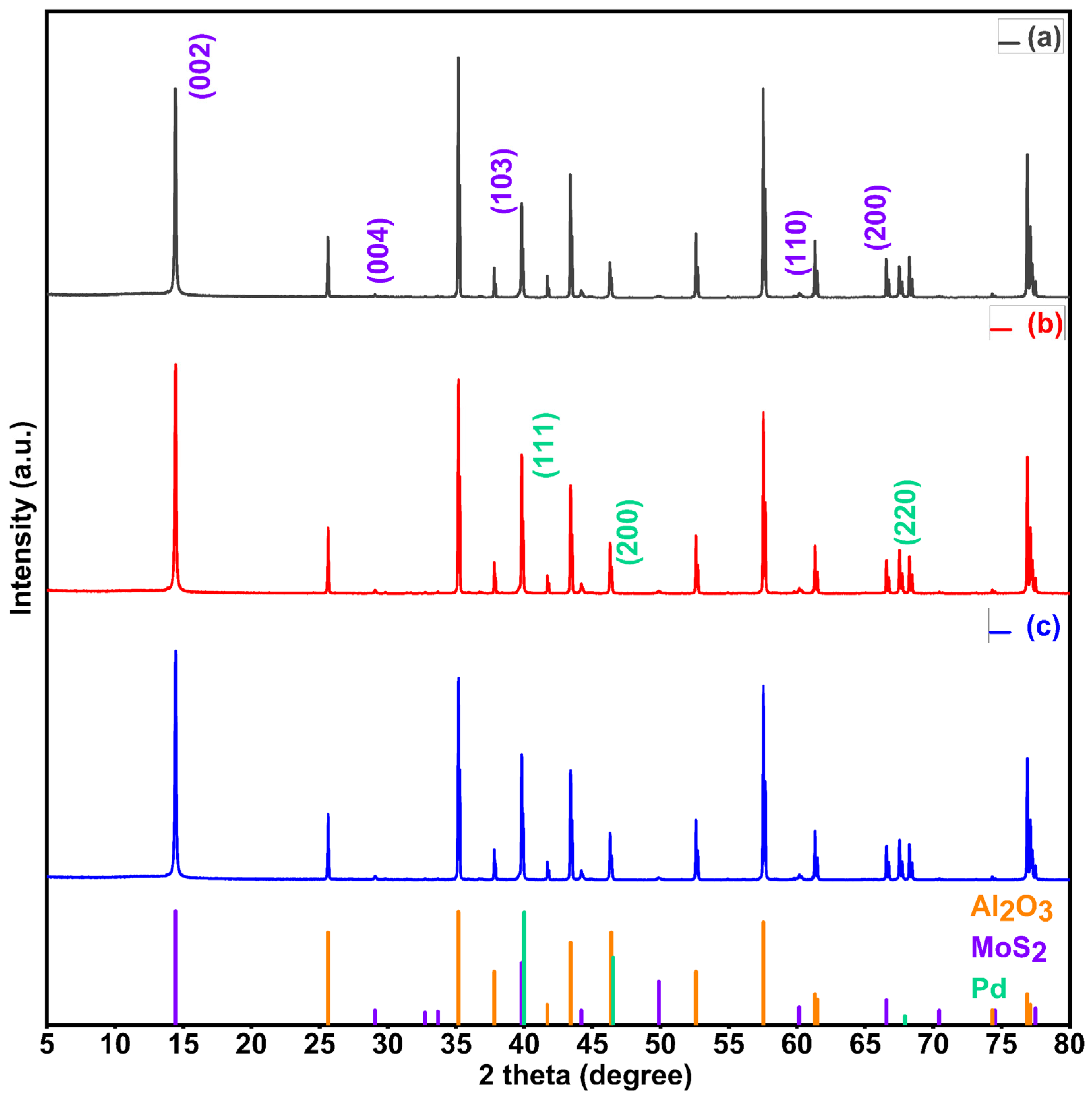
3.1.3. XRD
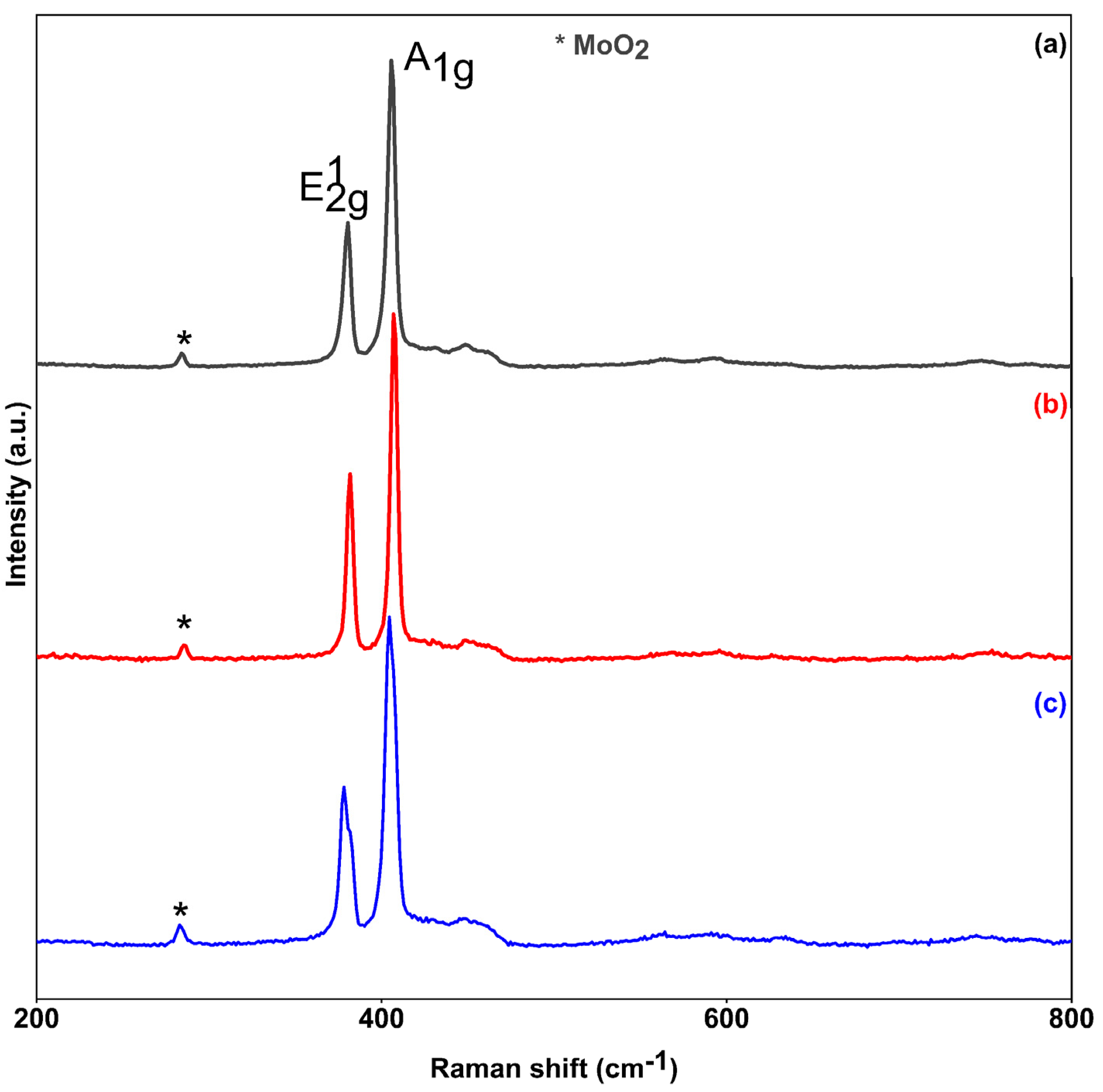
3.1.4. Raman Spectroscopy
3.2. Gas Sensing Results
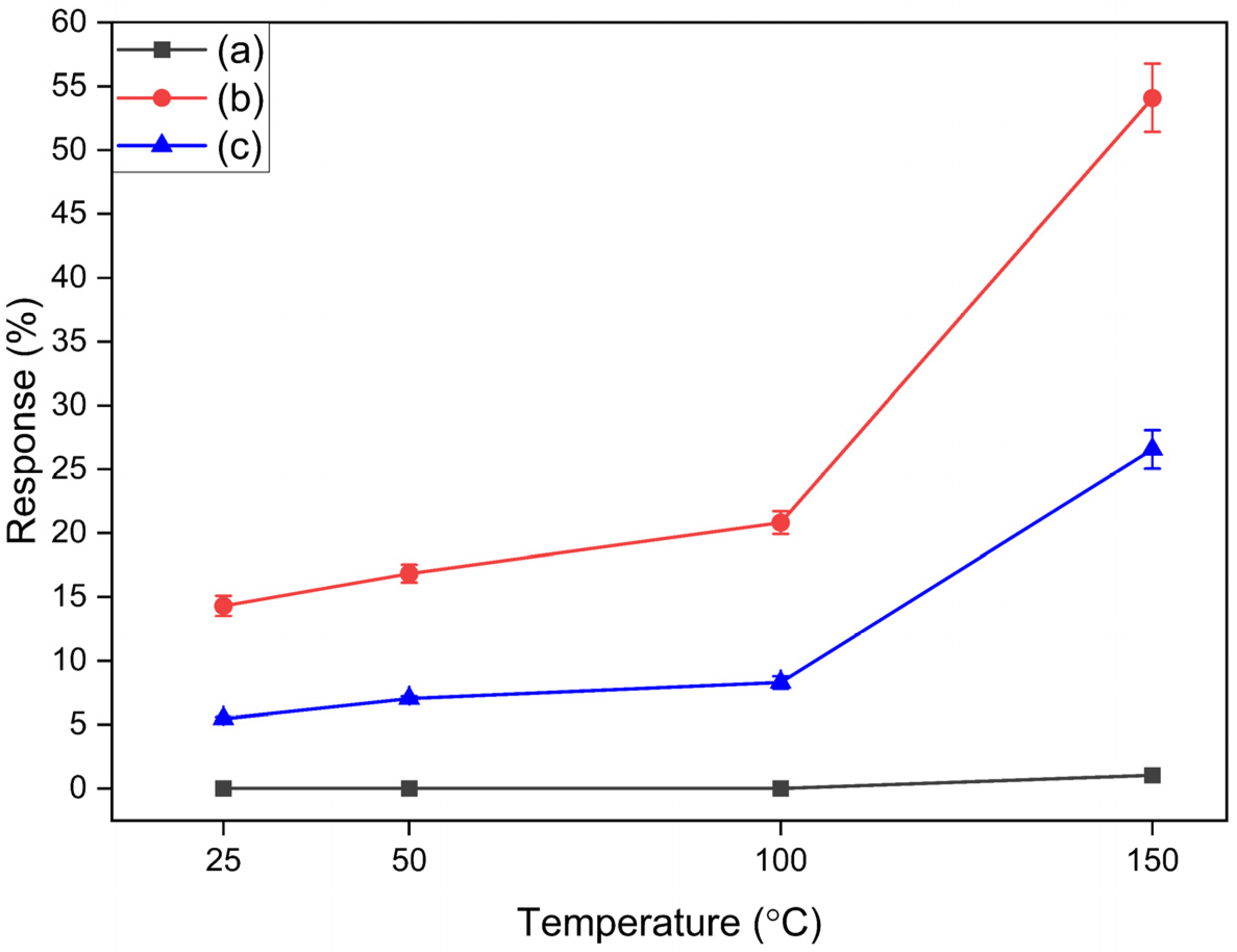
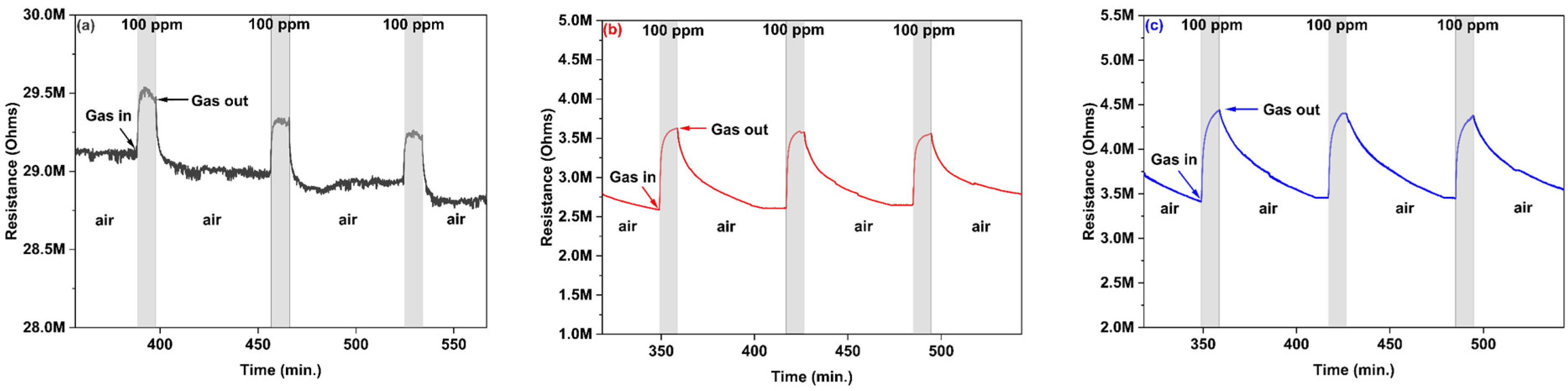
3.2.1. Hydrogen Gas Sensing
3.2.2. Selectivity Test
3.2.3. Hydrogen Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobson, M.Z.; Colella, W.G.; Golden, D.M. Cleaning the Air and Improving Health with Hydrogen Fuel-Cell Vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- van Renssen, S. The hydrogen solution? Nat. Clim. Chang. 2020, 10, 799–801. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Enhanced adsorption sites in monolayer MoS2 pyramid structures for highly sensitive and fast hydrogen sensor. Int. J. Hydrog. Energy 2020, 45, 9268–9277. [Google Scholar] [CrossRef]
- Almaev, A.; Nikolaev, V.; Yakovlev, N.; Butenko, P.; Stepanov, S.; Pechnikov, A.; Scheglov, M.; Chernikov, E. Hydrogen sensors based on Pt/α-Ga2O3:Sn/Pt structures. Sens. Actuators B Chem. 2022, 364. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2010, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-Dimensional Nanostructured Materials for Gas Sensing. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Raza, M.H.; Movlaee, K.; Leonardi, S.G.; Barsan, N.; Neri, G.; Pinna, N. Gas Sensing of NiO-SCCNT Core–Shell Heterostructures: Optimization by Radial Modulation of the Hole-Accumulation Layer. Adv. Funct. Mater. 2019, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2015, 28, 795–831. [Google Scholar] [CrossRef]
- Mai, H.D.; Jeong, S.; Nguyen, T.K.; Youn, J.-S.; Ahn, S.; Park, C.-M.; Jeon, K.-J. Pd Nanocluster/Monolayer MoS2 Heterojunctions for Light-Induced Room-Temperature Hydrogen Sensing. ACS Appl. Mater. Interfaces 2021, 13, 14644–14652. [Google Scholar] [CrossRef]
- Matte, H.S.S.R.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 Analogues of Graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Zhang, X.-Q.; Zhang, W.; Chang, M.-T.; Lin, C.-T.; Chang, K.-D.; Yu, Y.-C.; Wang, J.T.-W.; Chang, C.-S.; Li, L.-J.; et al. Synthesis of Large-Area MoS2Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef]
- Li, W.; Geng, X.; Guo, Y.; Rong, J.; Gong, Y.; Wu, L.; Zhang, X.; Li, P.; Xu, J.; Cheng, G.; et al. Reduced Graphene Oxide Electrically Contacted Graphene Sensor for Highly Sensitive Nitric Oxide Detection. ACS Nano 2011, 5, 6955–6961. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; E Ocola, L.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef]
- Gutés, A.; Hsia, B.; Sussman, A.; Mickelson, W.; Zettl, A.; Carraro, C.; Maboudian, R. Graphene decoration with metal nanoparticles: Towards easy integration for sensing applications. Nanoscale 2011, 4, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Hashtroudi, H.; Yu, A.; Juodkazis, S.; Shafiei, M. Two-Dimensional Dy2O3-Pd-PDA/rGO Heterojunction Nanocomposite: Synergistic Effects of Hybridisation, UV Illumination and Relative Humidity on Hydrogen Gas Sensing. Chemosensors 2022, 10, 78. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Sarkar, D.; Xie, X.; Kang, J.; Zhang, H.; Liu, W.; Navarrete, J.; Moskovits, M.; Banerjee, K. Functionalization of Transition Metal Dichalcogenides with Metallic Nanoparticles: Implications for Doping and Gas-Sensing. Nano Lett. 2015, 15, 2852–2862. [Google Scholar] [CrossRef]
- Xie, X.; Sarkar, D.; Liu, W.; Kang, J.; Marinov, O.; Deen, J.; Banerjee, K.; Engineering, C.; Barbara, S.; States, U. Low-Frequency Noise in Bilayer MoS2. ACS Nano 2014, 8, 5633–5640. [Google Scholar] [CrossRef]
- Siao, M.D.; Shen, W.C.; Chen, R.S.; Chang, Z.W.; Shih, M.C.; Chiu, Y.P.; Cheng, C.-M. Two-dimensional electronic transport and surface electron accumulation in MoS2. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Mennel, L.; Furchi, M.M.; Wachter, S.; Paur, M.; Polyushkin, D.K.; Mueller, T. Optical imaging of strain in two-dimensional crystals. Nat. Commun. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Harivyasi, S.S.; Zojer, E. Controlling the electronic properties of van der Waals heterostructures by applying electrostatic design. 2D Mater. 2018, 5, 035019. [Google Scholar] [CrossRef]
- Liao, F.; Guo, Z.; Wang, Y.; Xie, Y.; Zhang, S.; Sheng, Y.; Tang, H.; Xu, Z.; Riaud, A.; Zhou, P.; et al. High-Performance Logic and Memory Devices Based on a Dual-Gated MoS2 Architecture. ACS Appl. Electron. Mater. 2019, 2, 111–119. [Google Scholar] [CrossRef]
- Yu, S.H.; Lee, Y.B.; Jang, S.K.; Kang, J.; Jeon, J.; Lee, C.G.; Lee, J.Y.; Kim, H.; Hwang, E.; Lee, S.; et al. Dye-Sensitized MoS2 Photodetector with Enhanced Spectral Photoresponse. ACS Nano 2014, 8, 8285–8291. [Google Scholar] [CrossRef]
- Gan, L.-Y.; Zhang, Q.; Cheng, Y.; Schwingenschlögl, U. Photovoltaic Heterojunctions of Fullerenes with MoS2 and WS2 Monolayers. J. Phys. Chem. Lett. 2014, 5, 1445–1449. [Google Scholar] [CrossRef]
- Kuru, C.; Choi, C.; Kargar, A.; Choi, D.; Kim, Y.J.; Liu, C.H.; Yavuz, S.; Jin, S. MoS2 Nanosheet–Pd Nanoparticle Composite for Highly Sensitive Room Temperature Detection of Hydrogen. Adv. Sci. 2015, 2, 1500004. [Google Scholar] [CrossRef]
- Tabata, H.; Matsuyama, H.; Goto, T.; Kubo, O.; Katayama, M. Visible-Light-Activated Response Originating from Carrier-Mobility Modulation of NO2 Gas Sensors Based on MoS2 Monolayers. ACS Nano 2021, 15, 2542–2553. [Google Scholar] [CrossRef]
- Bai, J.; Shen, Y.; Zhao, S.; Chen, Y.; Li, G.; Han, C.; Wei, D.; Yuan, Z.; Meng, F. Flower-like MoS2 hierarchical architectures assembled by 2D nanosheets sensitized with SnO2 quantum dots for high-performance NH3 sensing at room temperature. Sens. Actuators B Chem. 2021, 353, 131191. [Google Scholar] [CrossRef]
- Hou, S.; Pang, R.; Chang, S.; Ye, L.; Xu, J.; Wang, X.-C.; Zhang, Y.; Shang, Y.; Cao, A. Synergistic CNFs/CoS2/MoS2 Flexible Films with Unprecedented Selectivity for NO Gas at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 29778–29786. [Google Scholar] [CrossRef]
- Lee, K.; Gatensby, R.; McEvoy, N.; Hallam, T.; Duesberg, G.S. High-Performance Sensors Based on Molybdenum Disulfide Thin Films. Adv. Mater. 2013, 25, 6699–6702. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of Single- and Multilayer MoS2 Film-Based Field-Effect Transistors for Sensing NO at Room Temperature. Small 2011, 8, 63–67. [Google Scholar] [CrossRef]
- Zhou, Q.; Hong, C.; Yao, Y.; Hussain, S.; Xu, L.; Zhang, Q.; Gui, Y.; Wang, M. Hierarchically MoS2 nanospheres assembled from nanosheets for superior CO gas-sensing properties. Mater. Res. Bull. 2018, 101, 132–139. [Google Scholar] [CrossRef]
- Ozaki, F.; Tanaka, S.; Osada, W.; Mukai, K.; Horio, M.; Koitaya, T.; Yamamoto, S.; Matsuda, I.; Yoshinobu, J. Functionalization of the MoS2 basal plane for activation of molecular hydrogen by Pd deposition. Appl. Surf. Sci. 2022, 593. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Umek, P.; Bittencourt, C.; Colomer, J.F.; Llobet, E. An Ultrasensitive Room-Temperature H2S Gas Sensor Based on 3D Assembly of Cu2O Decorated WS2 Nanomaterial. IEEE Sens. J. 2021, 21, 21212–21220. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; di Maggio, F.; Vallejos, S.; Vilic, T.T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-Assisted CVD-Grown PdO Nanoparticle-Decorated Tungsten Oxide Nanoneedles Extremely Sensitive and Selective to Hydrogen. ACS Appl. Mater. Interfaces 2016, 8, 10413–10421. [Google Scholar] [CrossRef]
- Baek, D.-H.; Kim, J. MoS2 gas sensor functionalized by Pd for the detection of hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Gottam, S.R.; Tsai, C.-T.; Wang, L.-W.; Wang, C.-T.; Lin, C.-C.; Chu, S.-Y. Highly sensitive hydrogen gas sensor based on a MoS2-Pt nanoparticle composite. Appl. Surf. Sci. 2019, 506, 144981. [Google Scholar] [CrossRef]
- Urs, K.M.B.; Katiyar, N.K.; Kumar, R.; Biswas, K.; Singh, A.K.; Tiwary, C.S.; Kamble, V. Multi-component (Ag–Au–Cu–Pd–Pt) alloy nanoparticle-decorated p-type 2D-molybdenum disulfide (MoS2) for enhanced hydrogen sensing. Nanoscale 2020, 12, 11830–11841. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mirzaei, A.; Kim, J.-Y.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Optimization of the surface coverage of metal nanoparticles on nanowires gas sensors to achieve the optimal sensing performance. Sens. Actuators B Chem. 2019, 302, 127196. [Google Scholar] [CrossRef]
- Lee, J.-S.; Katoch, A.; Kim, J.-H.; Kim, S.S. Effect of Au nanoparticle size on the gas-sensing performance of p-CuO nanowires. Sens. Actuators B Chem. 2016, 222, 307–314. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, J.Y.; Choi, S.-W.; Kim, H.S.; Gil Na, H.; Yang, J.C.; Lee, C.; Kim, H.W. Room temperature sensing properties of networked GaN nanowire sensors to hydrogen enhanced by the Ga2Pd5 nanodot functionalization. Int. J. Hydrog. Energy 2011, 36, 2313–2319. [Google Scholar] [CrossRef]
- Stolze, M.; Gogova, D.; Thomas, L.-K. Analogy for the maximum obtainable colouration between electrochromic, gasochromic, and electrocolouration in DC-sputtered thin WO3−y films. Thin Solid Films 2005, 476, 185–189. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Choi, J.-K.; Woo, H.-S.; Kim, S.-J.; Jung, S.-Y.; Seong, T.-Y.; Kim, I.-D.; Lee, J.-H. Facile Control of C2H5OH Sensing Characteristics by Decorating Discrete Ag Nanoclusters on SnO2 Nanowire Networks. ACS Appl. Mater. Interfaces 2011, 3, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Huang, Y.-K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef]
- Eom, T.H.; Cho, S.H.; Suh, J.M.; Kim, T.; Lee, T.H.; Jun, S.E.; Yang, J.W.; Lee, J.; Hong, S.-H.; Jang, H.W. Substantially improved room temperature NO2 sensing in 2-dimensional SnS2 nanoflowers enabled by visible light illumination. J. Mater. Chem. A 2021, 9, 11168–11178. [Google Scholar] [CrossRef]
- Park, J.; Mun, J.; Shin, J.-S.; Kang, S.-W. Highly sensitive two-dimensional MoS2 gas sensor decorated with Pt nanoparticles. R. Soc. Open Sci. 2018, 5, 181462. [Google Scholar] [CrossRef]
- Suh, J.M.; Shim, Y.-S.; Kwon, K.C.; Jeon, J.-M.; Lee, T.H.; Shokouhimehr, M.; Jang, H.W. Pd- and Au-Decorated MoS2 Gas Sensors for Enhanced Selectivity. Electron. Mater. Lett. 2019, 15, 368–376. [Google Scholar] [CrossRef]
- Burman, D.; Raha, H.; Manna, B.; Pramanik, P.; Guha, P.K. Substitutional Doping of MoS2 for Superior Gas-Sensing Applications: A Proof of Concept. ACS Sens. 2021, 6, 3398–3408. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Bao, S.; Wang, M.; Qi, X.; Fan, Z.; Zhang, H. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 2013, 4, 1444. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, T.H.; Park, S.Y.; Eom, T.H.; Cho, I.; Kim, Y.; Kim, C.; A Lee, S.; Choi, M.-J.; Suh, J.M.; et al. Drastic Gas Sensing Selectivity in 2-Dimensional MoS2 Nanoflakes by Noble Metal Decoration. ACS Nano 2023, 17, 4404–4413. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, Y.; Lee, J.; Kim, J.; Shin, J.W.; Sim, S.; Go, D.; Jo, E.; Kye, S.; Kim, J.; et al. Atomic layer deposited Pt nanoparticles on functionalized MoS2 as highly sensitive H2 sensor. Appl. Surf. Sci. 2021, 571, 151256. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Al Youssef, K.; Bittencourt, C.; Güell, F.; Martínez-Alanis, P.R.; Reguant, M.; Llobet, E. PdO and PtO loaded WS2 boosts NO2 gas sensing characteristics at room temperature. Sens. Actuators B Chem. 2022, 364. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Umek, P.; Bittencourt, C.; Sierra-Castillo, A.; Haye, E.; Colomer, J.F.; Llobet, E. CVD growth of self-assembled 2D and 1D WS2 nanomaterials for the ultrasensitive detection of NO2. Sens. Actuators B Chem. 2020, 326, 128813. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Salamone, M.M.; Robertson, A.W.; Nayak, S.; Kim, H.; Tsang, S.C.E.; Pasta, M.; Warner, J.H. Edge-Enriched 2D MoS2 Thin Films Grown by Chemical Vapor Deposition for Enhanced Catalytic Performance. ACS Catal. 2016, 7, 877–886. [Google Scholar] [CrossRef]
- Shomalian, K.; Bagheri-Mohagheghi, M.-M.; Ardyanian, M. Characterization and study of reduction and sulfurization processing in phase transition from molybdenum oxide (MoO2) to molybdenum disulfide (MoS2) chalcogenide semiconductor nanoparticles prepared by one-stage chemical reduction method. Appl. Phys. A 2016, 123, 93. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Alex, C.; Nair, A.N.; John, N.S. Designing Metallic MoO2 Nanostructures on Rigid Substrates for Electrochemical Water Activation. Chem.–A Eur. J. 2018, 24, 18003–18011. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Alagh, A.; Umek, P.; Casanova-Chafer, J.; Bittencourt, C.; Llobet, E. Controlled growth of 3D assemblies of edge enriched multilayer MoS2 nanosheets for dually selective NH3 and NO2 gas sensors. J. Mater. Chem. C 2022, 10, 11027–11039. [Google Scholar] [CrossRef]
- Bendahan, M.; Guérin, J.; Boulmani, R.; Aguir, K. WO3 sensor response according to operating temperature: Experiment and modeling. Sens. Actuators B Chem. 2007, 124, 24–29. [Google Scholar] [CrossRef]
- Park, S.; Garcia-Esparza, A.T.; Abroshan, H.; Abraham, B.; Vinson, J.; Gallo, A.; Nordlund, D.; Park, J.; Kim, T.R.; Vallez, L.; et al. Operando Study of Thermal Oxidation of Monolayer MoS2. Adv. Sci. 2021, 8, 2002768. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Koo, W.-T.; Lee, Y.J.; Kim, Y.H.; Lee, J.; Jang, J.-S.; Yun, H.; Kim, I.-D.; Kim, B.J. Hydrogen Sensors Based on MoS2 Hollow Architectures Assembled by Pickering Emulsion. ACS Nano 2020, 14, 9652–9661. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Czerwosz, E.; Diduszko, R.; Kaminska, A.; Danila, M. Influence of PdHx formation ability on hydrogen sensing properties of palladium-carbonaceous films. Sens. Actuators A Phys. 2013, 203, 434–440. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, W.; Ma, L.; Yu, M.; Ren, X.; He, M.; Polen, S.; Click, K.; Garrett, B.; Lu, J.; et al. Dimeric [Mo2S12]2−Cluster: A Molecular Analogue of MoS2 Edges for Superior Hydrogen-Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 15181–15185. [Google Scholar] [CrossRef]
- Tang, X.; Haddad, P.-A.; Mager, N.; Geng, X.; Reckinger, N.; Hermans, S.; Debliquy, M.; Raskin, J.-P. Chemically deposited palladium nanoparticles on graphene for hydrogen sensor applications. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Jaiswal, J.; Tiwari, P.; Singh, P.; Chandra, R. Fabrication of highly responsive room temperature H2 sensor based on vertically aligned edge-oriented MoS2 nanostructured thin film functionalized by Pd nanoparticles. Sens. Actuators B Chem. 2020, 325. [Google Scholar] [CrossRef]
- Matsunaga, N.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Diffusion equation-based study of thin film semiconductor gas sensor-response transient. Sens. Actuators B Chem. 2002, 83, 216–221. [Google Scholar] [CrossRef]
- Matsunaga, N.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Formulation of gas diffusion dynamics for thin film semiconductor gas sensor based on simple reaction–diffusion equation. Sens. Actuators B Chem. 2003, 96, 226–233. [Google Scholar] [CrossRef]
- Suresh, S.; Urs, K.M.B.; Vasudevan, A.T.; Sriram, S.; Kamble, V.B. Analysis of Unusual and Instantaneous Overshoot of Response Transients in Gas Sensors. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2019, 13. [Google Scholar] [CrossRef]
- Darmadi, I.; Nugroho, F.A.A.; Langhammer, C. High-Performance Nanostructured Palladium-Based Hydrogen Sensors—Current Limitations and Strategies for Their Mitigation. ACS Sens. 2020, 5, 3306–3327. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Xia, Y. Mesoporous MXene/ZnO nanorod hybrids of high surface area for UV-activated NO2 gas sensing in ppb-level. Sens. Actuators B Chem. 2022, 353. [Google Scholar] [CrossRef]
- Rsan, N.B.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Hashtroudi, H.; Atkin, P.; Mackinnon, I.D.R.; Shafiei, M. Low-operating temperature resistive nanostructured hydrogen sensors. Int. J. Hydrog. Energy 2019, 44, 26646–26664. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrog. Energy 2019, 44, 20552–20571. [Google Scholar] [CrossRef]
- Chacko, L.; Massera, E.; Aneesh, P.M. Enhancement in the Selectivity and Sensitivity of Ni and Pd Functionalized MoS2 Toxic Gas Sensors. J. Electrochem. Soc. 2020, 167, 106506. [Google Scholar] [CrossRef]
- Asakuma, Y.; Miyauchi, S.; Yamamoto, T.; Aoki, H.; Miura, T. Numerical analysis of absorbing and desorbing mechanism for the metal hydride by homogenization method. Int. J. Hydrog. Energy 2003, 28, 529–536. [Google Scholar] [CrossRef]





| Sample | Δ = () | ||
|---|---|---|---|
| MoS2 | 381 | 406 | 25 |
| MoS2-Pd_1 | 381 | 407 | 26 |
| MoS2-Pd_2 | 378 | 405 | 27 |
| Gas Sensing Material | Concentration | Response Calculation Formula | Response % | Operating Temperature | Reference |
|---|---|---|---|---|---|
| ALD Pt-decorated MoS2 nanosheets | 1000 ppm | 440 | 250 °C | [53] | |
| Pd nanoclusters–MoS2 heterostructure | 140 ppm | 17 | RT (with light activation) | [9] | |
| Pd-functionalized MoS2 nanosheet | 10,000 ppm | × 100 | 35.3 | RT | [38] |
| Pt-decorated MoS2 hollow structures | 40,000 ppm | × 100 | 11.2 | RT | [63] |
| Pd-functionalized edge-enriched MoS2 | 500 ppm | 33.7 | RT | [67] | |
| Pd-decorated MoS2 | 100 ppm | 55 | 150 °C | This work | |
| 14.9 | RT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, S.B.; Annanouch, F.E.; Llobet, E. Pd-Nanoparticle-Decorated Multilayered MoS2 Sheets for Highly Sensitive Hydrogen Sensing. Chemosensors 2023, 11, 550. https://doi.org/10.3390/chemosensors11110550
Malik SB, Annanouch FE, Llobet E. Pd-Nanoparticle-Decorated Multilayered MoS2 Sheets for Highly Sensitive Hydrogen Sensing. Chemosensors. 2023; 11(11):550. https://doi.org/10.3390/chemosensors11110550
Chicago/Turabian StyleMalik, Shuja Bashir, Fatima Ezahra Annanouch, and Eduard Llobet. 2023. "Pd-Nanoparticle-Decorated Multilayered MoS2 Sheets for Highly Sensitive Hydrogen Sensing" Chemosensors 11, no. 11: 550. https://doi.org/10.3390/chemosensors11110550
APA StyleMalik, S. B., Annanouch, F. E., & Llobet, E. (2023). Pd-Nanoparticle-Decorated Multilayered MoS2 Sheets for Highly Sensitive Hydrogen Sensing. Chemosensors, 11(11), 550. https://doi.org/10.3390/chemosensors11110550







