Nanogel for Selective Recognition of Nanoparticles in Water Samples
Abstract
1. Introduction
1.1. Nanoparticles (NPs)
1.2. Imprinted Hydrogel
1.3. Quartz Crystal Microbalance (QCM)
2. Materials and Methods
2.1. Preparation of Polymerization Mixture
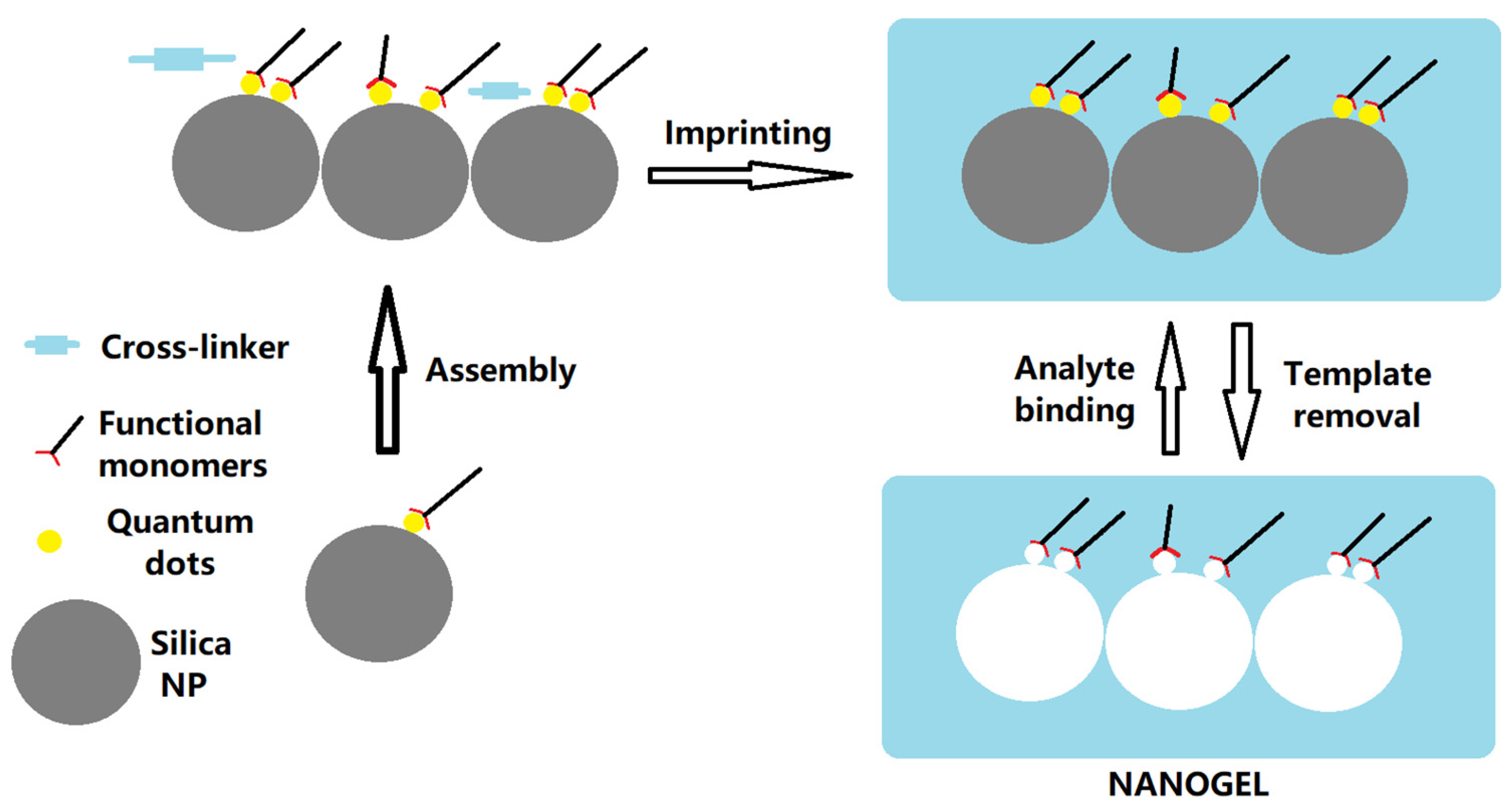
2.2. Synthesis of Nanogel
2.3. Preparation of QCM Sensor Chip
2.4. Preparation of Sample Solutions
2.5. Regeneration
2.6. Use of QCM
2.7. Characterization of Nanoparticles
2.8. Characterization of Nanogel
3. Results and Discussion
3.1. Characterization of Nanogel
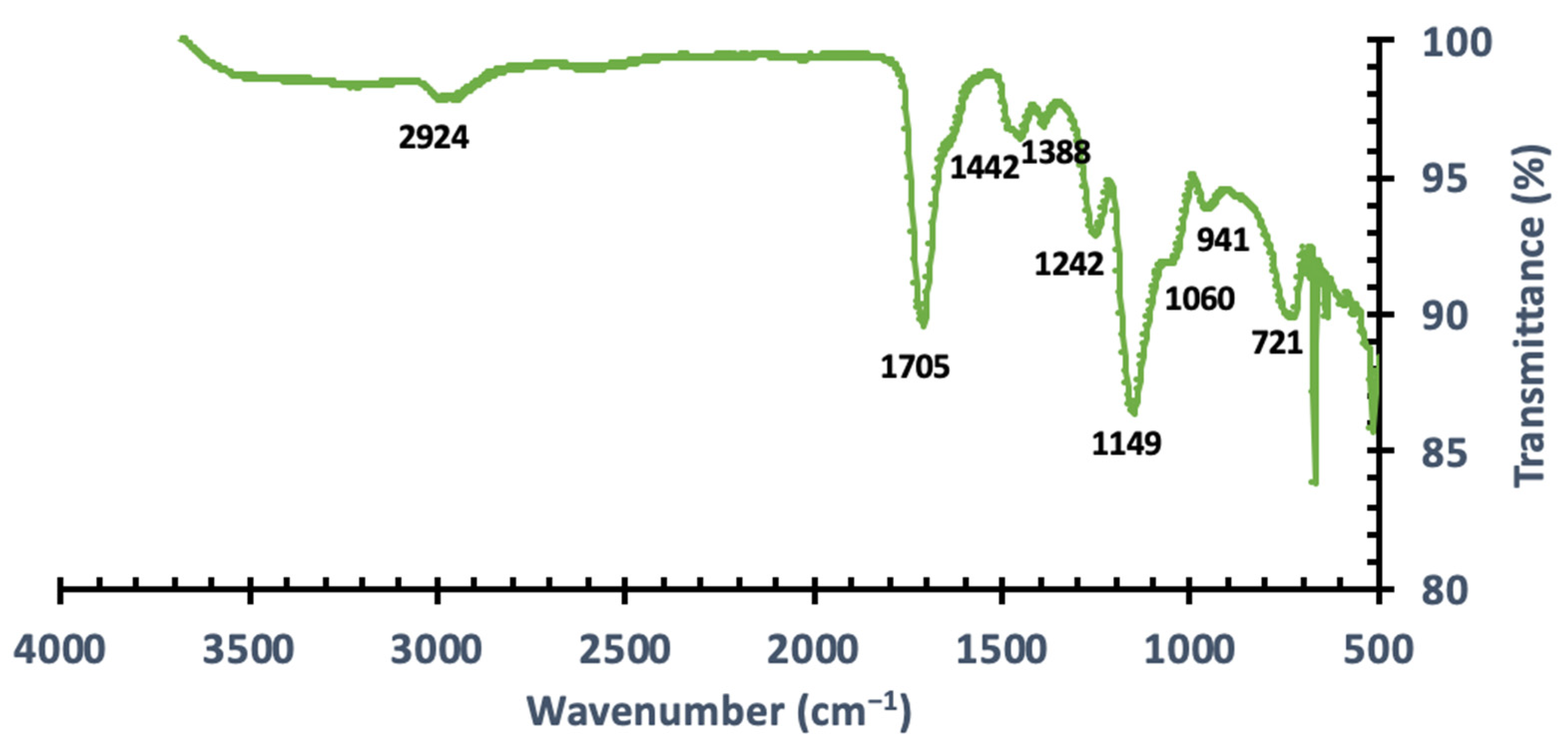
3.1.1. Fourier Transform Infrared (FT-IR) Spectroscopy
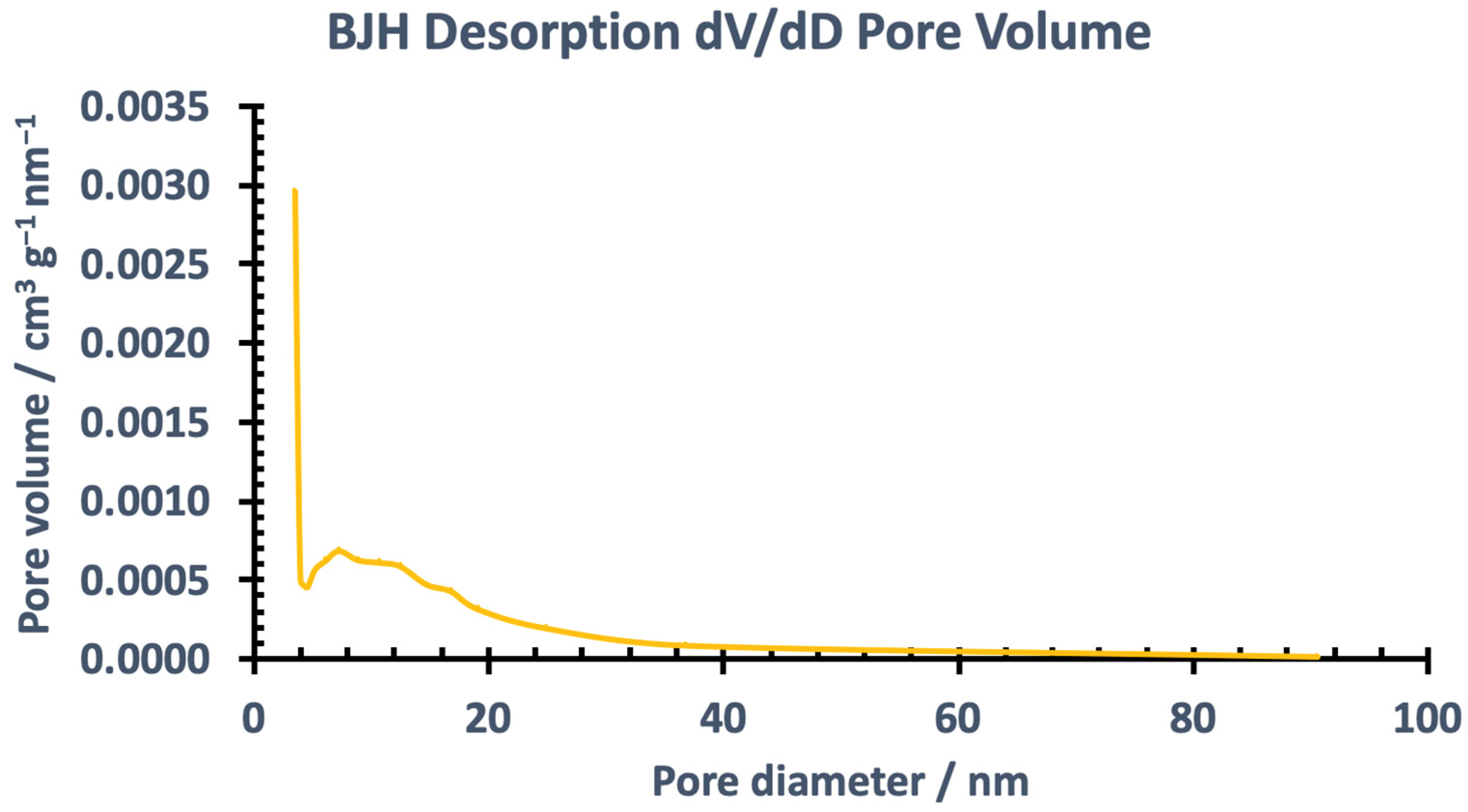
3.1.2. Brunauer-Emmett-Teller (BET) Analysis
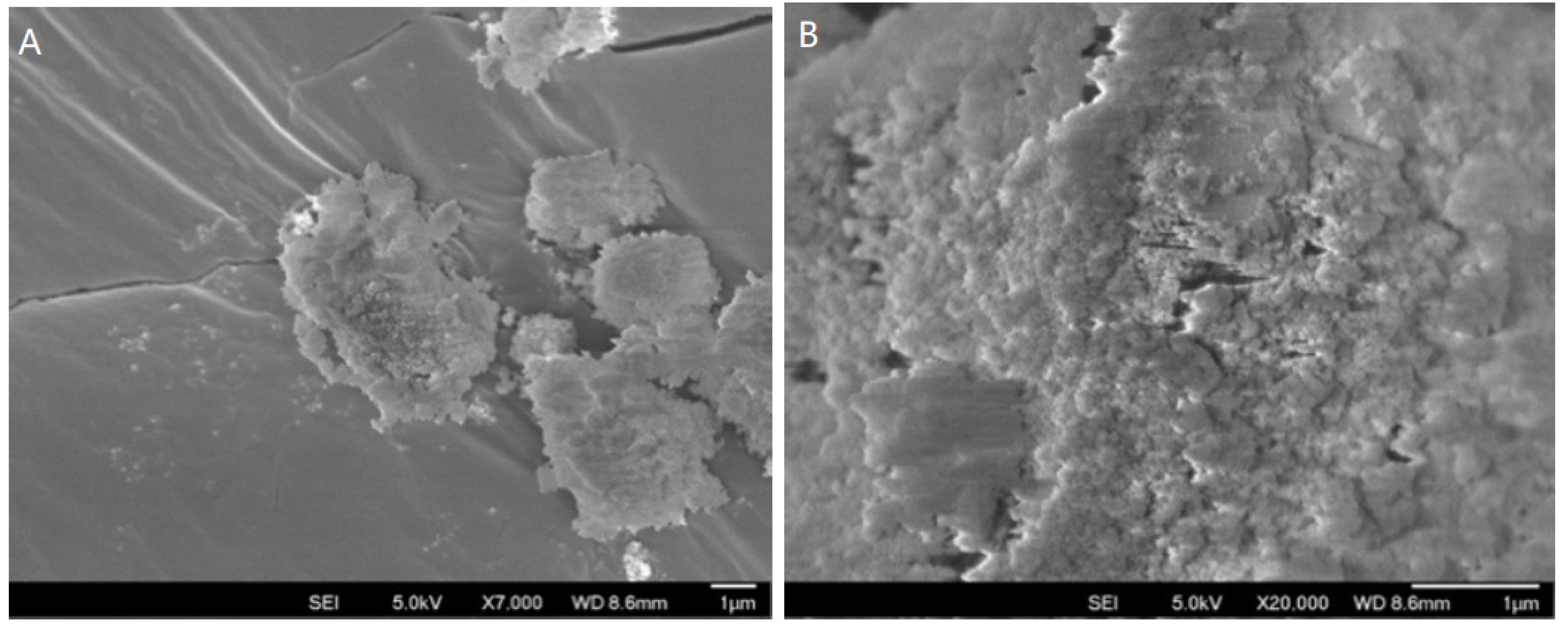
3.1.3. SEM Analysis
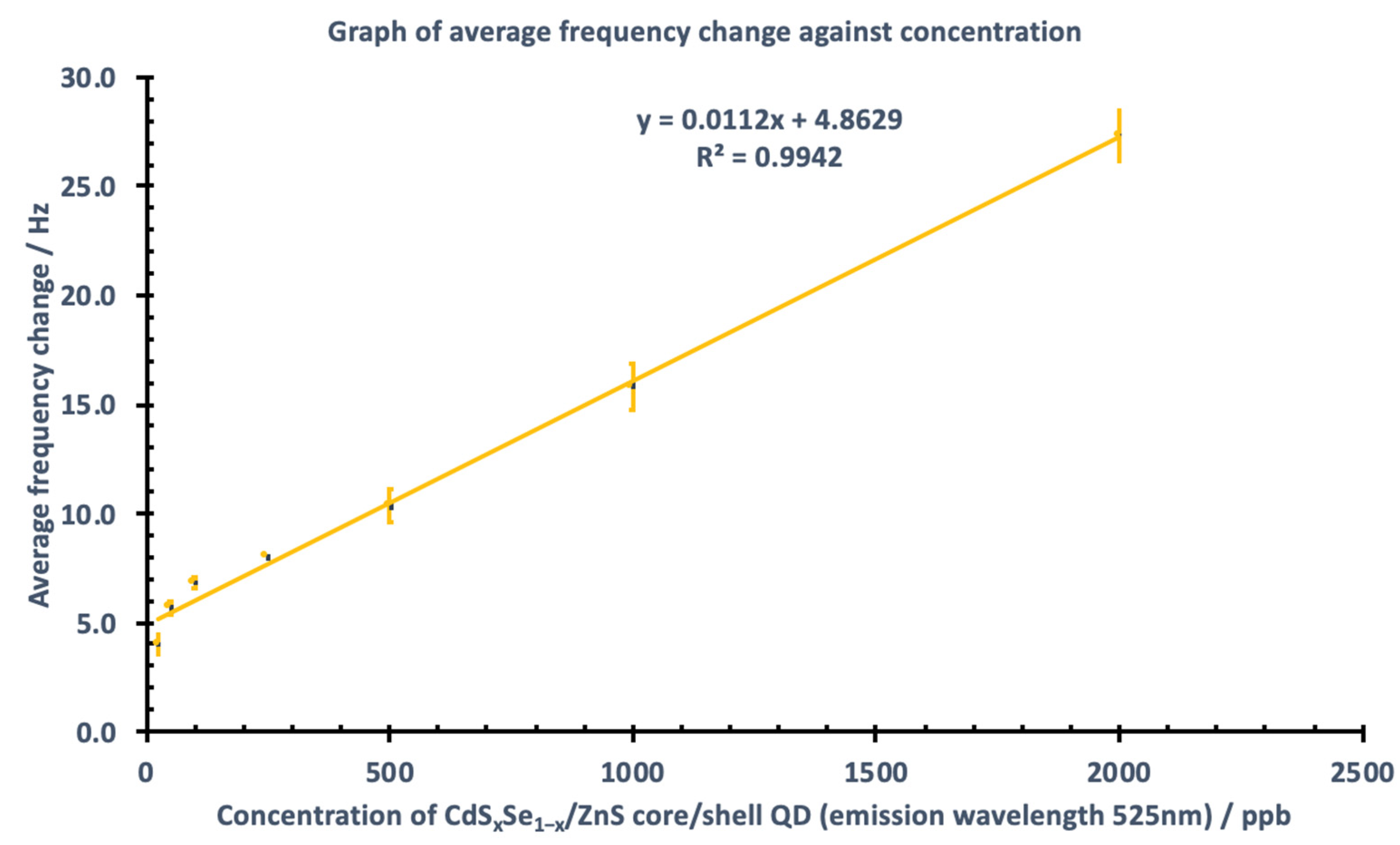
3.2. Sensitivity, Linearity, Limit of Detection (LOD), and Limit of Quantification (LOQ)
3.3. Selectivity Study
Comparison to Non-Imprinted Hydrogel
3.4. Properties of Size and Surface Characteristics
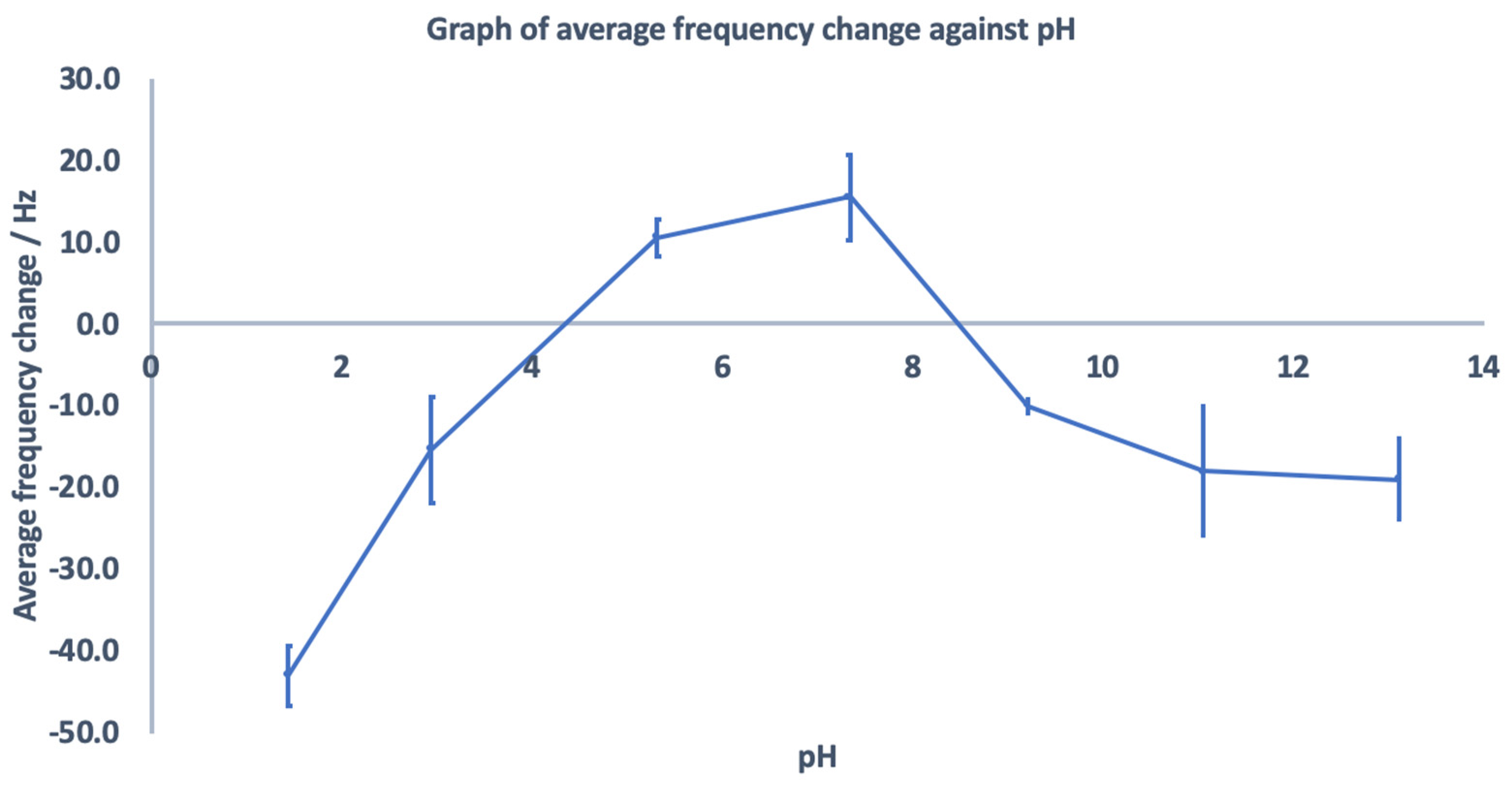
3.5. Effect of pH
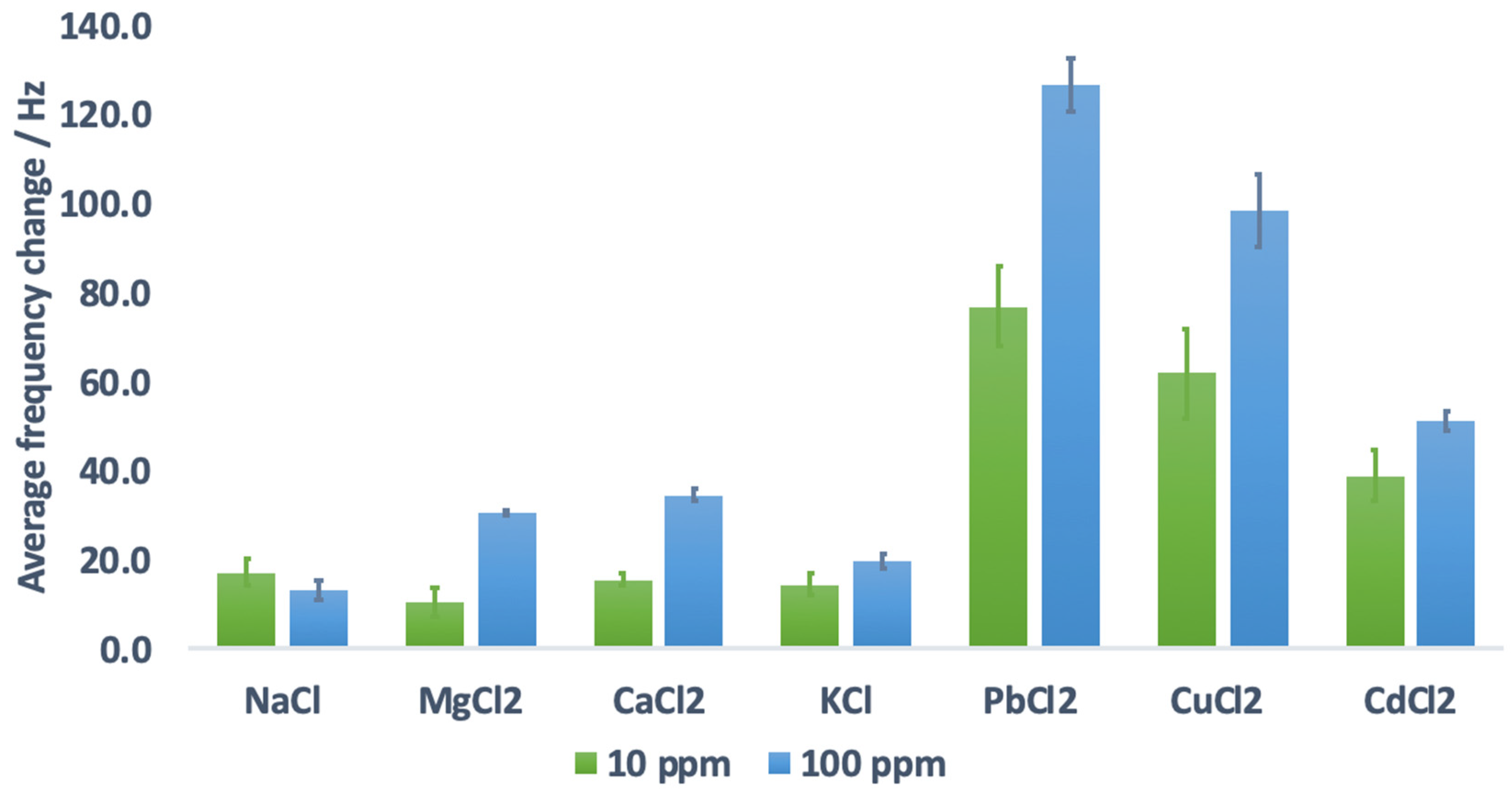
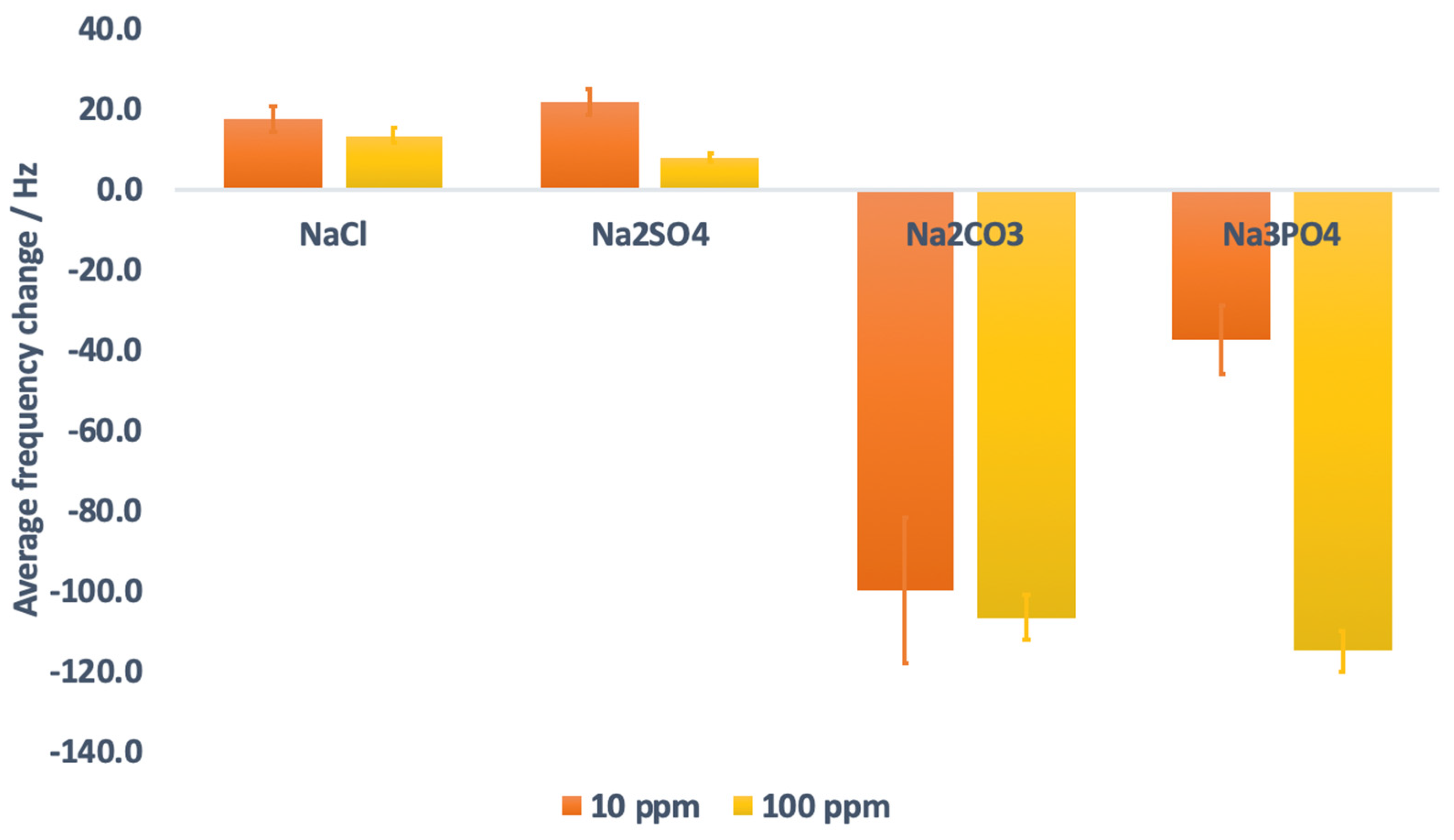
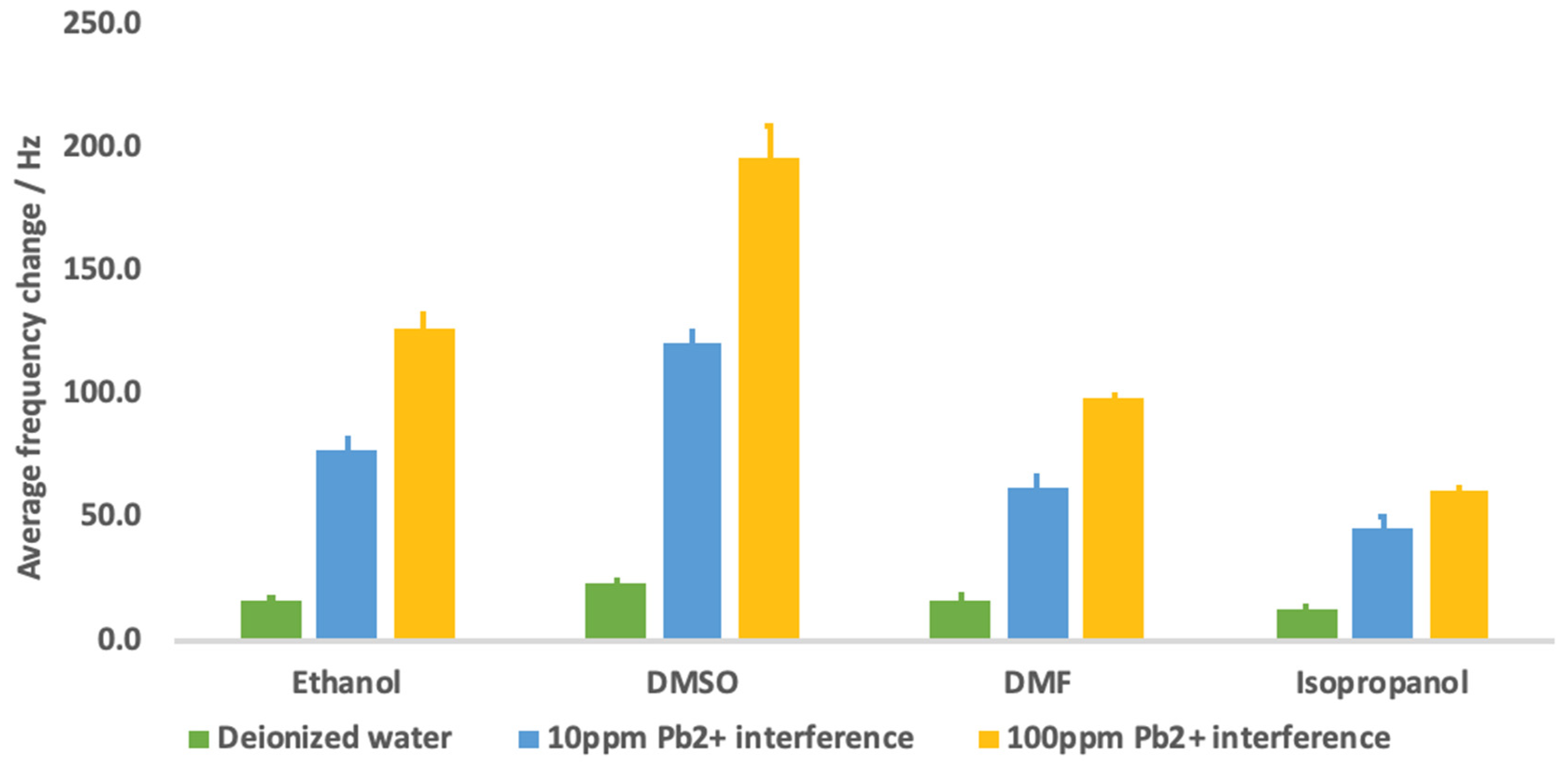
3.6. Effect of Ionic Solutions (Potential Matrix in Real Sample Analysis)
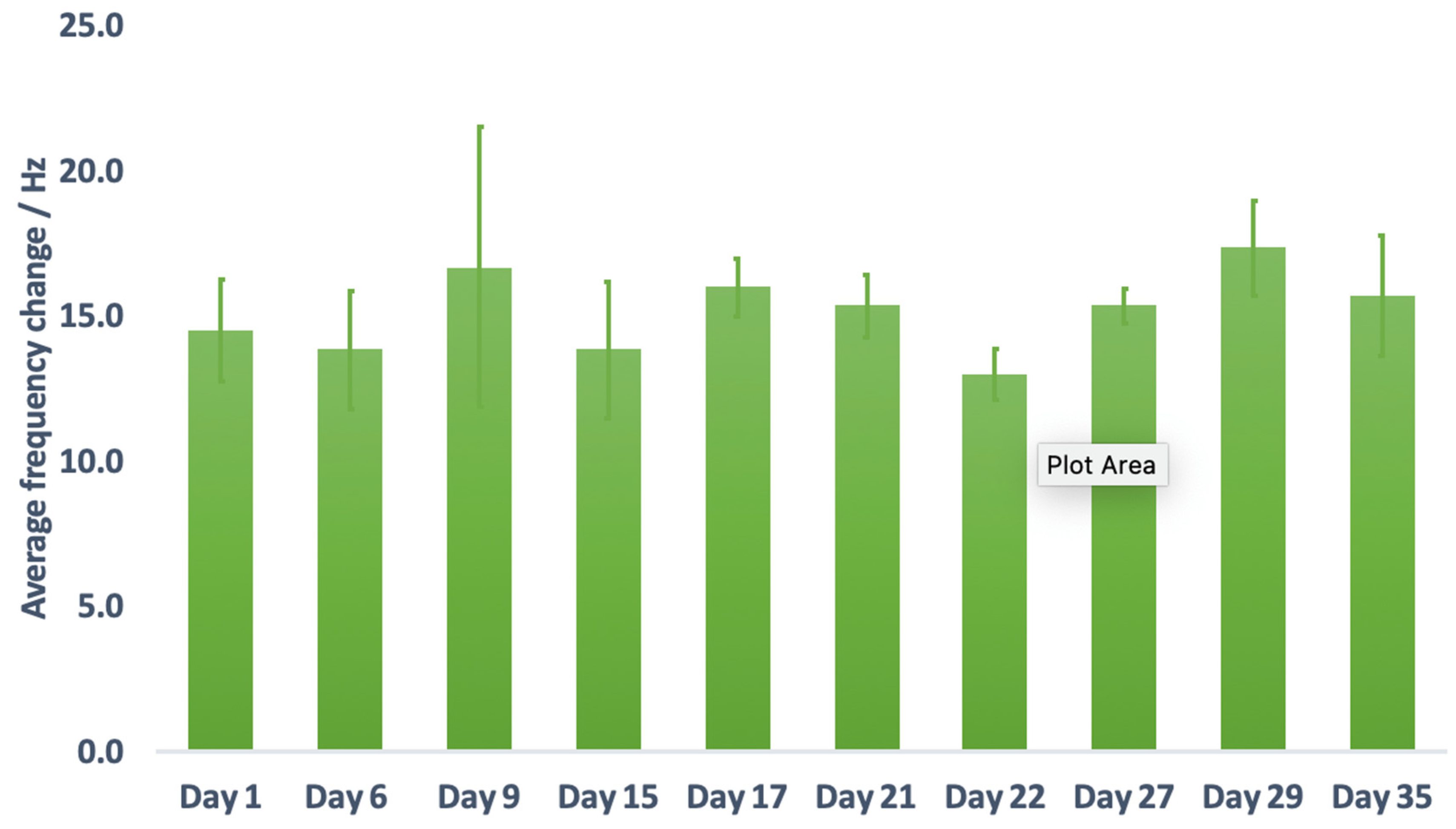
3.7. Repeatability Study
3.8. Reproducibility Study
3.9. Stability Study
3.10. Evaluation of Effect of Solvent Used for Synthesis of Nanogel
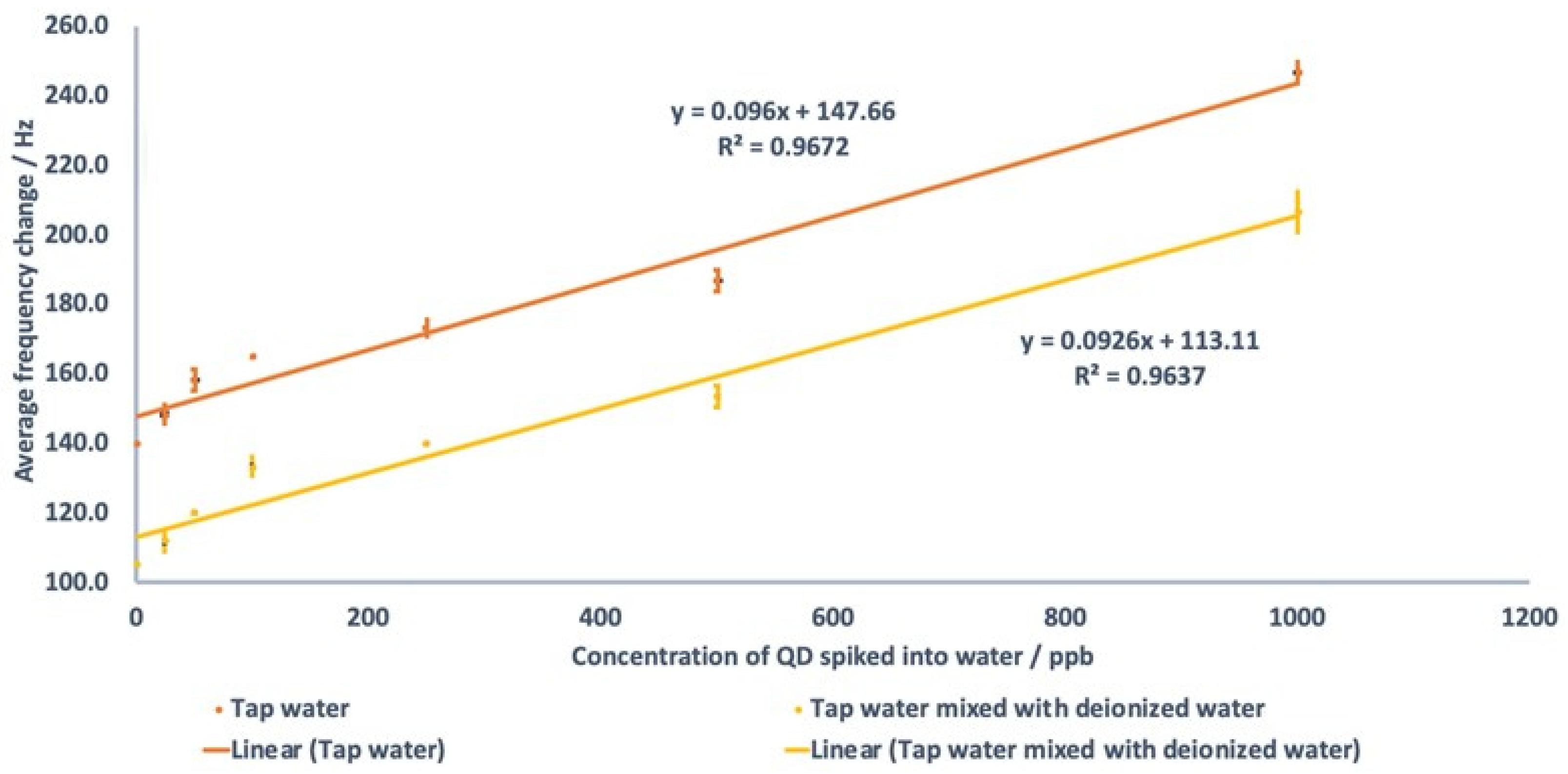
3.11. Use of Nanogel on Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leopold, K.; Philippe, A.; Wörle, K.; Schaumann, G.E. Analytical Strategies to the Determination of Metal-Containing Nanoparticles in Environmental Waters. TrAC Trends Anal. Chem. 2016, 84, 107–120. [Google Scholar] [CrossRef]
- Hong, N.H. Introduction to Nanomaterials: Basic Properties, Synthesis, and Characterization. In Nano-Sized Multifunctional Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar]
- Jha, M. Current Trends in Industrial Scale Synthesis of Quantum Dots and Its Application in Electronics. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–385. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shankar, S.; Ravindra, S.; VeenuJoshi, P.; Kumar Jain, V.K.; Vyas, A. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Liang Hu, L.; Zhong, H.; He, Z. Toxicity evaluation of cadmium-containing quantum dots: A review of optimizing physicochemical properties to diminish toxicity. Colloids Surf. B Biointerfaces 2021, 200, 111609. [Google Scholar]
- Wang, Z.; Tang, M. The cytotoxicity of core-shell or non-shell structure quantum dots and reflection on environmental friendly: A review. Environ. Res. 2021, 194, 110593. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Kraus-Ophir, S.; Witt, J.; Wittstock, G.; Mandler, D. Nanoparticle-Imprinted Polymers for Size-Selective Recognition of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 294–298. [Google Scholar] [CrossRef]
- Gam-Derouich, S.; Bourdillon, C.; Lakhdar Chaouche, S.; Coolen, L.; Maître, A.; Mangeney, C.; Schwob, C. Imprinted Photonic Hydrogels for the Size-and Shell-selective Recognition of Nanoparticles. Angew. Chem. Weinheim Bergstr. Ger. 2017, 129, 9842–9846. [Google Scholar] [CrossRef]
- Sobiech, M.; Lulinski, P.; Wieczorek, P.P.; Marc, M. Quantum and carbon dots conjugated molecularly imprinted polymers as advanced nanomaterials for selective recognition of analytes in environmental, food and biomedical applications. TrAC Trends Anal. Chem. 2021, 142, 116306. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly imprinted polymers (MIPs) combined with nanomaterials as electrochemical sensing applications for environmental pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Janczura, M.; Lulinski, P.; Sobiech, M. Imprinting Technology for Effective Sorbent Fabrication: Current State-of-Art and Future Prospects. Materials 2021, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.G.; Benhawy, A.H.; Khalifa, R.M.; El Nashar, R.; Trojanowicz, M. Application of Molecularly Imprinted Polymers in the Analysis of Waters and Wastewaters. Molecules 2021, 26, 6515. [Google Scholar] [CrossRef] [PubMed]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94. [Google Scholar] [CrossRef] [PubMed]
- Orbay, S.; Kocaturk, O.; Sanyal, R.; Sanjal, A. Molecularly Imprinted Polymer-Coated Inorganic Nanoparticles: Fabrication and Biomedical Applications. Micromachines 2022, 13, 1464. [Google Scholar] [CrossRef]
- Haupt, K.; Rangel, P.X.M.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Turiel, E.; Esteban, A.M. Molecularly Imprinted Polymers. In Solid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 215–233. [Google Scholar]
- Hu, X.; An, Q.; Li, G.; Tao, S.; Liu, J. Imprinted Photonic Polymers for Chiral Recognition. Angew. Chem. Int. Ed. 2006, 45, 8145–8148. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent Advances in the Synthesis of Smart Hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Asadi, H.; Khoee, S. Nanogels: Chemical Approaches to Preparation. In Handbook of Encapsulation and Controlled Release; CRC Press: Iran, 2015; Chapter 54; pp. 1271–1309. Available online: https://books.google.com.hk/books?hl=zh-CN&lr=&id=pY7wCgAAQBAJ&oi=fnd&pg=PP1&dq=22.%09Asadi,+H.%3B+Khoee,+S.+Nanogels:+Chemical+Approaches+to+Preparation.+In+Handbook+of+Encapsulation+and+Controlled+Release%3B+CRC+Press:+Iran,+2015%3B+Chapter+54,+pp.+1271%E2%80%931309.&ots=OqnLGh8l5p&sig=8qCo2Dky6EJjE66GN9GYwI3HyFI&redir_esc=y#v=onepage&q&f=false (accessed on 1 December 2022).
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Murthy, N.S.; Damodaran, V.B.; Lee, S.H.; Hwang, A.S.; Sung, H.-J. Characterization of Thin Films for Biomedical Applications. In Thin Film Coatings for Biomaterials and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–115. [Google Scholar]
- Vogt, B.D.; Lin, E.K.; Wu, W.-L.; White, C.C. Effect of Film Thickness on the Validity of the Sauerbrey Equation for Hydrated Polyelectrolyte Films. J. Phys. Chem. B 2004, 108, 12685–12690. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman Spectroscopic Features of Plant Cuticles: A Review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Zhang, W.; Pan, J.; Cai, J. Enlargement of Diatom Frustules Pores by Hydrofluoric Acid Etching at Room Temperature. J. Mater. Sci. 2011, 46, 5665–5671. [Google Scholar] [CrossRef]
- Al Kobaisi, M. Rapid On-Site Monitoring of Pesticide Residues with MIP Sensors. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2007. [Google Scholar]
- Kim, I.; Taghavy, A.; DiCarlo, D.; Huh, C. Aggregation of Silica Nanoparticles and Its Impact on Particle Mobility under High-Salinity Conditions. J. Pet. Sci. Eng. 2015, 133, 376–383. [Google Scholar] [CrossRef]
- Pan, G.; Guo, Q.; Cao, C.; Yang, H.; Li, B. Thermo-responsive molecularly imprinted nanogels for specific recognition and controlled release of proteins. Soft Matter 2013, 9, 3840–3850. [Google Scholar] [CrossRef]
- Rosillo-Lopez, M.; Salzmann, C.G. Highly efficient heavy-metal extraction from water with carboxylated graphene nanoflakes. RSC Adv. 2018, 8, 11043–11050. [Google Scholar] [CrossRef]
- Grochowska, J. Assessment of Water Buffer Capacity of Two Morphometrically Different, Degraded, Urban Lakes. Water 2020, 12, 1512. [Google Scholar] [CrossRef]
- Neal, J.N.; Wesolowski, D.J.; Henderson, D.; Wu, J. Ion Distribution and Selectivity of Ionic Liquids in Microporous Electrodes. J. Chem. Phys. 2017, 146, 174701. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Maelaningsih, F.S.; Apriliandi, F.; Sabarudin, A. Synthesis and Characterisation of a Monolithic Imprinted Column Using a Methacrylic Acid Monomer with Porogen Propanol for Atenolol Analysis. J. Anal. Methods Chem. 2020, 2020, 3027618. [Google Scholar] [CrossRef]
- Lofgreen, J.E.; Ozin, G.A. Controlling Morphology and Porosity to Improve Performance of Molecularly Imprinted Sol-Gel Silica. Chem. Soc. Rev. 2014, 43, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, S. Rapid synthesis of macroporous graphene oxide/poly (acrylic acid-co-acrylamide) nanocomposite hydrogels with pH-sensitive behavior by frontal polymerization. RSC Adv. 2016, 6, 33426–33432. [Google Scholar] [CrossRef]
- Yan, H.; Row, K. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Azoulay, A.; Garzon, P.; Eisenberg, M.J. Comparison of the Mineral Content of Tap Water and Bottled Waters. J. Gen. Intern. Med. 2001, 16, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-Crystal Colloidal Multilayers of Controlled Thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]



| Basis of Comparison | Literature and Previously Published Studies | Nanogel for Detection of Nanoparticles in Water Samples |
|---|---|---|
| Type of hydrogel | Hydrogels can be responsive to different stimuli, such as temperature, pH, light, electrical conductivity, enzymes, etc. [21]. | The nanogel in our study has been imprinted using a particular template of quantum dots, allowing it to have specific binding sites to quantum dots and nanoparticles. It functions responsively, similar to that of an enzyme stimulus, where the enzyme binds to the substrate via the lock and key hypothesis. |
| Method of synthesis | Synthetic strategies for the preparation of nanogels can be mainly divided into two different categories, that involving the formation of nanogels using preformed polymers versus the other entailing the formation of nanogels via the direct polymerization of monomers [22]. | The nanogel in our study was formed via the direct polymerization of monomers in the presence of cross-linkers. |
| Main advantage of hydrogel usage | Hydrogels have the diversity of creating various adsorbents suitable for capturing potential pollutants [23]. | With a different template used during the imprinting process, the nanogel will be able to detect different forms of nanoparticles and allow the quantification to be done with the quartz crystal microbalance. |
| Linear Range/ppb | Sx,y | LOD/ppb | LOQ/ppb |
|---|---|---|---|
| 25–2000 | 0.67482 | 198.83 | 602.53 |
| Nanoparticles | Functional Groups | Emission λ (nm) | Size/nm | Zeta Potential/mV | Detection by NANOGEL |
|---|---|---|---|---|---|
| CdSxSe1−x/ZnS core/shell QDs | carboxyl | 490 | 6.284 | 3.71 | Yes |
| CdSxSe1−x/ZnS core/shell QDs * | carboxyl | 525 | 5.524 | −1.35 | Yes |
| CdSxSe1−x/ZnS core/shell QDs | carboxyl | 575 | 9.537 | −9.35 | Yes |
| CdSxSe1−x/ZnS core/shell QDs | carboxyl | 630 | 7.726 | −23.1 | Yes |
| CdSxSe1−x/ZnS core/shell QDs | carboxyl | 665 | 11.49 | −25.1 | Yes |
| CdTe core-type QDs | carboxyl | 510 | 12.45 | −27.5 | Yes |
| CdTe core-type QDs | carboxyl | 570 | 13.65 | −44.0 | Yes |
| CdTe core-type QDs | carboxyl | 610 | 10.60 | −47.6 | Yes |
| CdTe core-type QDs | carboxyl | 770 | 16.00 | −49.2 | Yes |
| Gold NPs | carboxyl | NE ** | 8.576 | −14.2 | Yes |
| Gold NPs | carboxyl | NE ** | 10.38 | −14.6 | Yes |
| Gold NPs | carboxyl | NE ** | 12.16 | −12.0 | Yes |
| Gold NPs | carboxyl | NE ** | 36.18 | −1.15 | No |
| Carbon dots | carboxyl | NE ** | 0.7363 | −9.70 | Yes |
| Carbon dots | carboxyl and amine | NE ** | 1.185 | 3.28 | No |
| CdSe/ZnS core/shell QDs | amine | NE ** | 15.50 | −61.6 | No |
| CdSe/ZnS core/shell QDs | alkyl | NE ** | 2.052 | −26.1 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, Y.Y.; Lin, X.H.; Li, S.F.Y. Nanogel for Selective Recognition of Nanoparticles in Water Samples. Chemosensors 2023, 11, 72. https://doi.org/10.3390/chemosensors11010072
Tay YY, Lin XH, Li SFY. Nanogel for Selective Recognition of Nanoparticles in Water Samples. Chemosensors. 2023; 11(1):72. https://doi.org/10.3390/chemosensors11010072
Chicago/Turabian StyleTay, Yong Ying, Xuan Hao Lin, and Sam Fong Yau Li. 2023. "Nanogel for Selective Recognition of Nanoparticles in Water Samples" Chemosensors 11, no. 1: 72. https://doi.org/10.3390/chemosensors11010072
APA StyleTay, Y. Y., Lin, X. H., & Li, S. F. Y. (2023). Nanogel for Selective Recognition of Nanoparticles in Water Samples. Chemosensors, 11(1), 72. https://doi.org/10.3390/chemosensors11010072









