Application of SPR Method as an Approach to Gas Phase Sensing of Volatile Compound Profile in Mezcal Spirits Conferred by Agave Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Mezcal Samples
2.2. Extraction of Volatile Compounds
2.3. Analysis of Volatile Compounds Using GC-MS
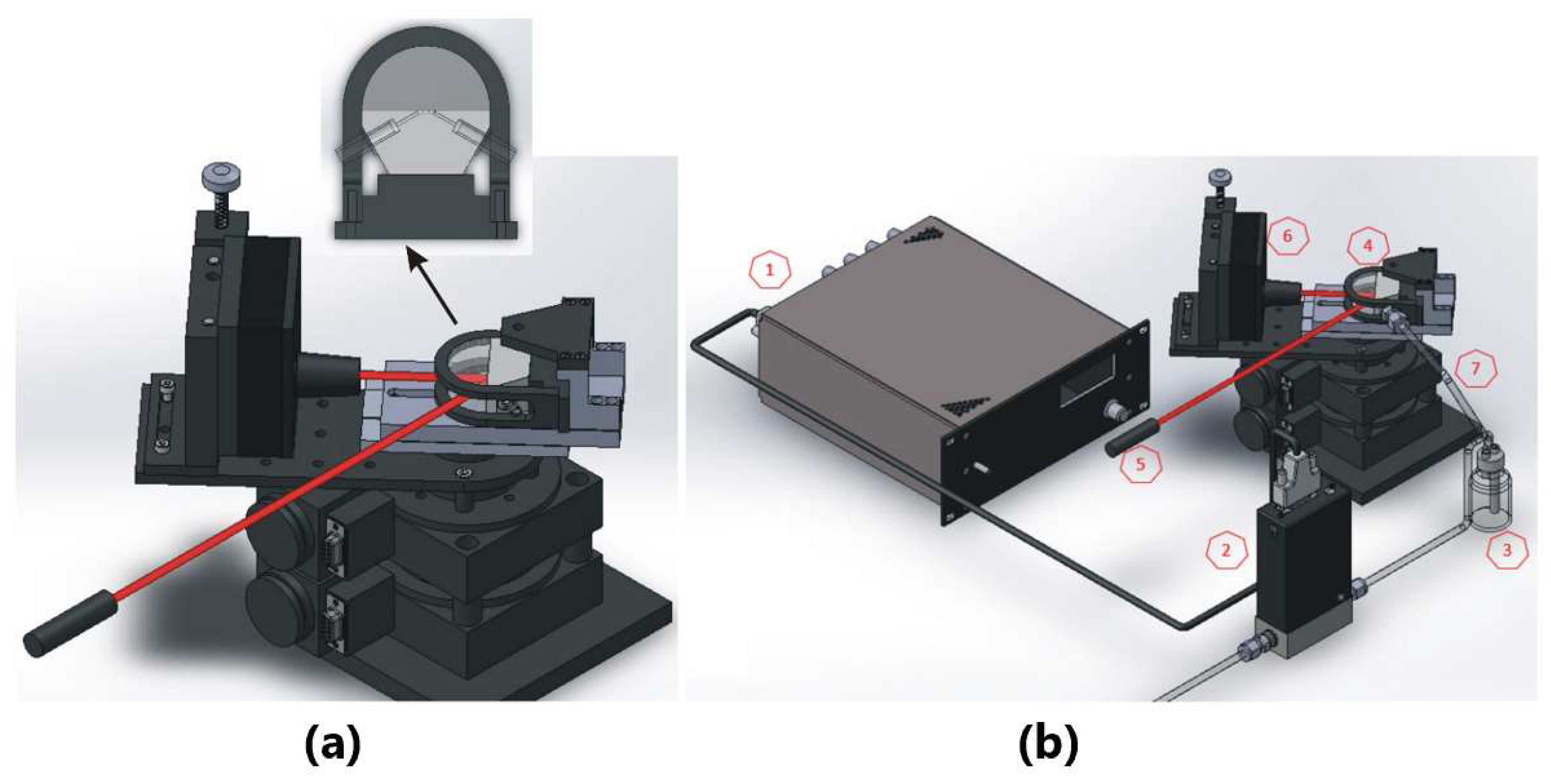
2.4. SPR Measurements with Angular Interrogation
2.5. SPR Measurements at a Fixed Angle
2.6. SPR Theoretical Simulations by Fresnel Equations and the Matrix Method
3. Experimental Results
3.1. Analysis of Volatile Compounds Using GC-MS
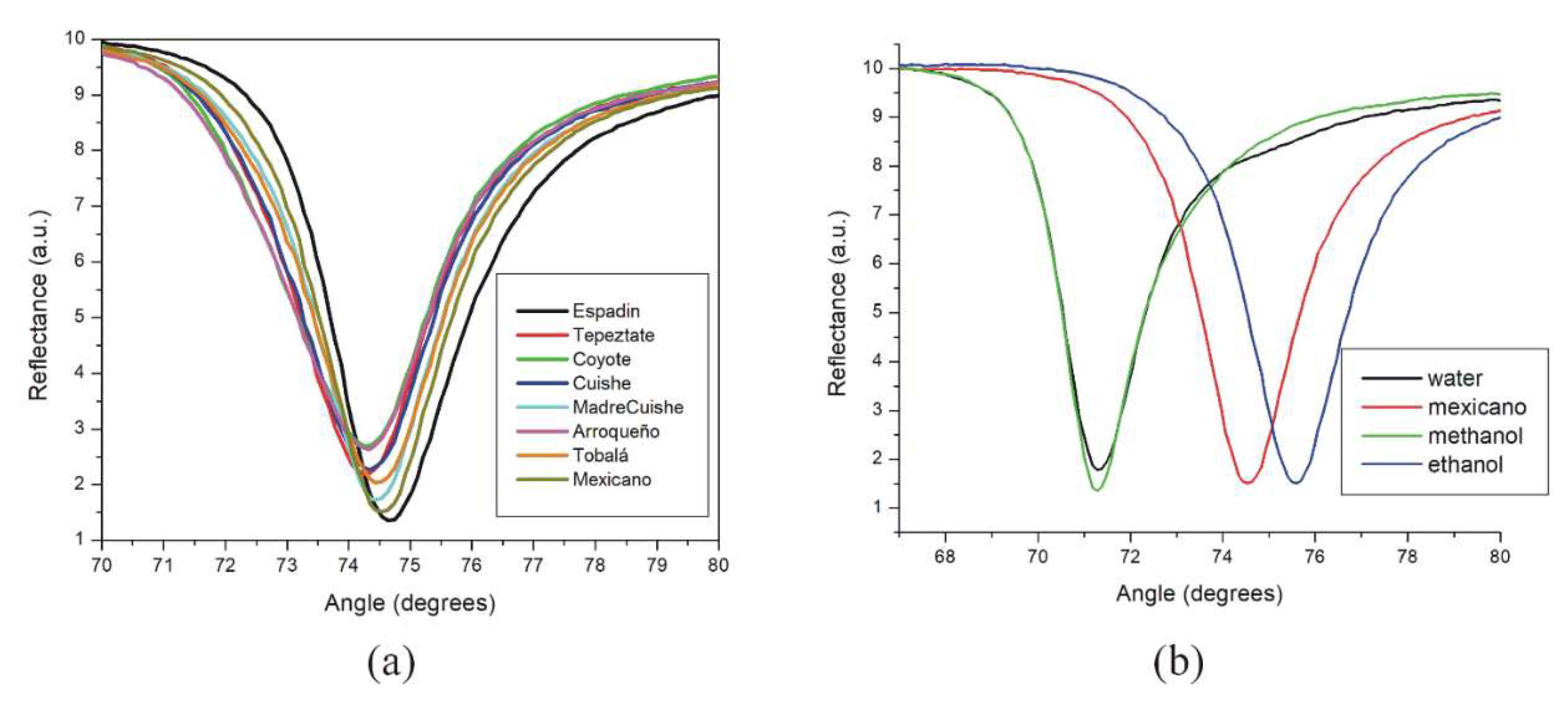
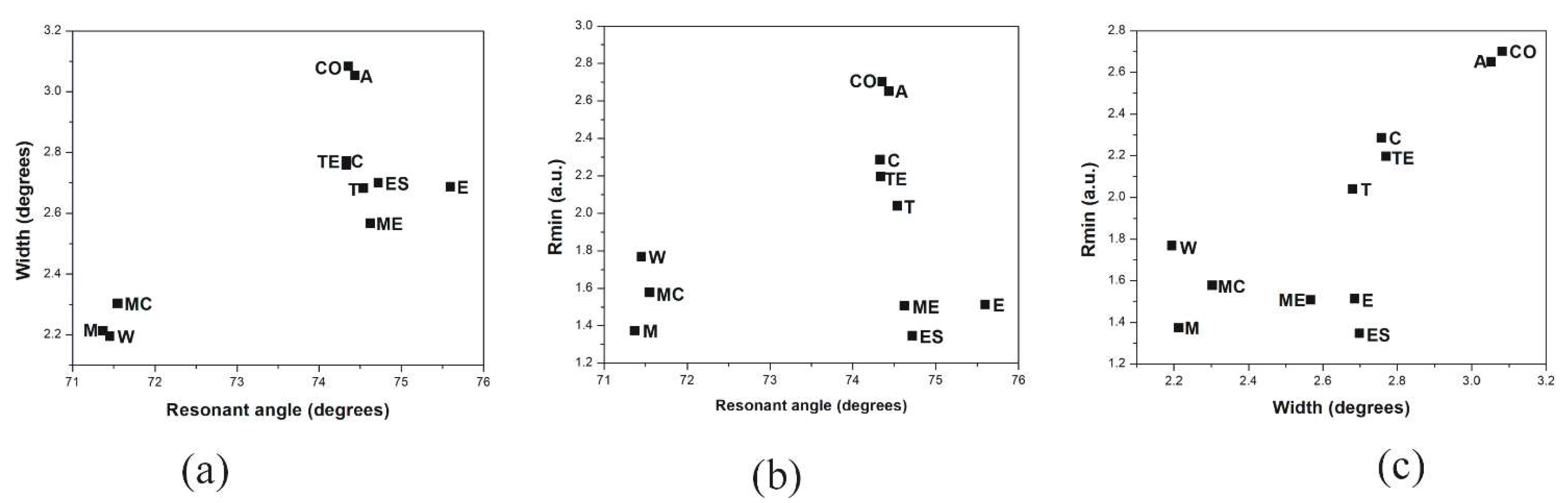
3.2. SPR Measurements with Angular Interrogation
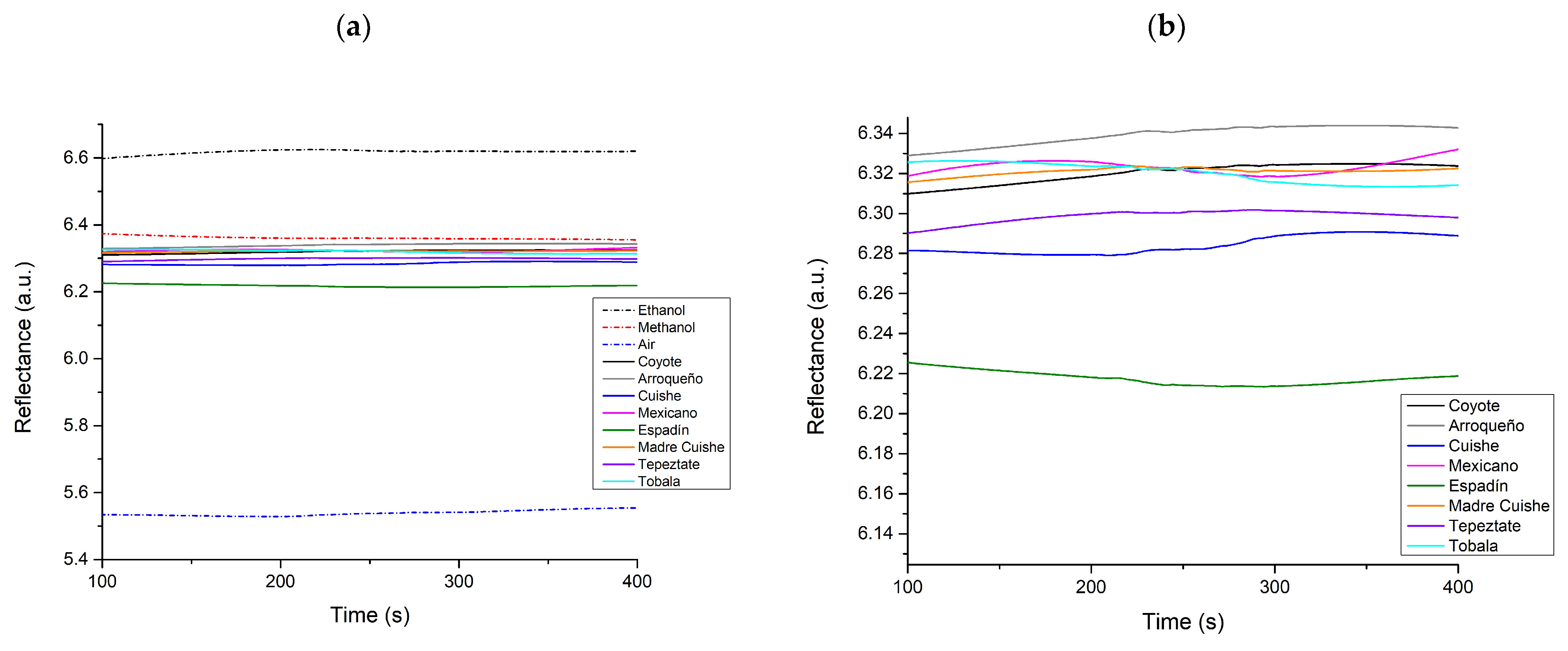
3.3. SPR Measurements at a Fixed Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colunga-GarcíaMarín, P.; Zizumbo-Villarreal, D.; Martínez-Torres, J. Tradiciones en el aprovechamiento de los agaves mexicanos: Una aportación a la protección legal y conservación de su diversidad biológica y cultural. In En lo Ancestral Hay Futuro: Del Tequila, Los Mezcales y Otros Agaves; Colunga-GarcíaMarín, P., Larqué-Saavedra, A., Eguiarte, L.E., Zizumbo-Villarreal, D., Eds.; Cicy-Conacyt-conabio-ine: Yucatán, México, 2007; pp. 229–248. [Google Scholar]
- De León-Rodríguez, A.; González-Hernández, L.; Barba de la Rosa, A.P.; Escalante-Minakata, P.; López, G.M. Characterization of Volatile Compounds of Mezcal, and Ethnic Alcoholic Beverage Obtained from Agave salmiana. J. Agric. Food Chem. 2006, 54, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Lappe-Oliveras, P.; Moreno-Terrazas, R.; Arrizón-Gaviño, J.; Herrera-Suárez, T.; García-Mendoza, A.; Gschaedler-Mathis, A. Yeasts Associated with the Production of Mexican Alcoholic Nondistilled and Distilled Agave Beverages. FEMS Yeast Res. 2008, 8, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Vera-Guzmán, A.M.; Santiago-García, P.A.; López, M.G. Compuestos Volátiles Aromáticos Generados durante la Elaboración de Mezcal de Agave angustifolia y Agave potatorum. Rev. Fitotec. Mex. 2009, 32, 273–279. [Google Scholar] [CrossRef]
- Vera-Guzmán, A.M.; López, M.G.; Hávez-Servia, J.L. Chemical Composition and Volatile Compounds in the Artisanal Fermentation of Mezcal in Oaxaca, Mexico. Afr. J. Biotechnol. 2012, 11, 14344–14353. [Google Scholar] [CrossRef]
- Martell, N.M.A.; Córdova, G.E.E.; López, M.J.; Soto, C.N.O.; López, P.M.G.; Rutiaga, Q.O.M. Effect of Fementation Temperature on Chemical Composition of Mescals Made from Agave duranguensis Juice with Different Native Yeast Genera. Afr. J. Microbiol. Res. 2011, 4, 3669–3676. [Google Scholar]
- Kirchmayr, M.R.; Segura-García, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; De la Rosa, M.; Mathis, A.G. Impact of Environmental Conditions and Process Modifications on Microbial Diversity, Fermentation Efficiency and Chemical Profile during the Fermentation of Mezcal in Oaxaca. LWT Food Sci. Technol. 2017, 79, 160–169. [Google Scholar] [CrossRef]
- Pisarnitskii, A. Formation of wine aroma: Tones and imperfections caused by minor components. Appl. Biochem. Microbiol. 2001, 37, 552–560. [Google Scholar] [CrossRef]
- Cristiani, G.; Monnet, V. Food microorganisms and aromatic ester synthesis. Sci. Aliment. 2001, 21, 211–230. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar]
- Genva, M.; Kenne Kemene, T.; Deleu, M.; Lins, L.; Fauconnier, M.L. Is It Possible to Predict the Odor of a Molecule on the Basis of Its Structure? Int. J. Mol. Sci. 2019, 20, 3018. [Google Scholar] [CrossRef]
- Meilgaard, M. Flavor chemistry of beer: Part I: Flavor interaction between principal volatiles. MBAA Tech. Q. 1975, 12, 107–117. [Google Scholar]
- Vera-Guzmán, A.M.; Guzmán-Gerónimo, R.I.; López, M.G.; Chávez-Servia, J.L. Volatile compound profiles in mezcal spirits as influenced by agave species and production processes. Beverages 2018, 4, 9. [Google Scholar] [CrossRef]
- Ceballos-Magana, S.G.; Jurado, J.M.; Martin, M.J.; Pablos, F. Quantitation of twelve metals in tequila and Mezcal spirits as authenticity parameters. J. Agric. Food Chem. 2009, 57, 1372–1376. [Google Scholar] [CrossRef]
- El Kazzy, M.; Weerakkody, J.S.; Hurot, C.; Mathey, R.; Buhot, A.; Scaramozzino, N.; Hou, Y. An overview of artificial olfaction systems with a focus on surface plasmon resonance for the analysis of volatile organic compounds. Biosensors 2021, 11, 244. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S.A. Review of Gas Sensors Employed in Electronic Nose Applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Bauer-Christoph, C.; Christoph, N.; Aguilar-Cisneros, B.O.; López, M.G.; Richling, E.; Rossmann, A. Authentication of tequila by gas chromatography and stable isotope ratio analyses. Eur. Food Res. Technol. 2003, 217, 438–443. [Google Scholar] [CrossRef]
- Peña-Alvarez, A.; Díaz, L.; Medina, A.; Labastida, C.; Capella, S.; Vera, L.E. Characterization of three agave species by gas chromatography and solid-phase microextraction-gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1027, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Richling, E.; López, M.G.; Frank, W.; Scheier, P. Multivariate analysis of FTIR and ion chromatographic data for the quality control of tequila. J. Agric. Food Chem. 2005, 53, 2151–2157. [Google Scholar] [CrossRef]
- Fraustro-Reyes, C.; Medina-Gutierrez, C.; Sato-Berru, R.; Sahagún, L.R. Qualitative study of etanol content in tequilas by Raman spectroscopy and principal component analysis. Spectroschim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2657–2662. [Google Scholar] [CrossRef]
- Barbosa-García, O.; Ramos-Ortiz Maldonado, J.L.; Pichardo-Molina, J.L.; Meneses-Nava, M.A.; Landgrave, J.E.A. UV–vis absorption spectroscopy and multivariate analysis as a method to discriminate tequila. Spectroschim. Acta Part A Mol. Biomol Spectrosc. 2007, 66, 129–134. [Google Scholar] [CrossRef]
- Brenet, S.; John-Herpin, A.; Gallat, F.-X.; Musnier, B.; Buhot, A.; Herrier, C.; Rousselle, T.; Livache, T.; Hou, Y. Highly-Selective Optoelectronic Nose Based on Surface Plasmon Resonance Imaging for Sensing Volatile Organic Compounds. Anal. Chem. 2018, 90, 9879–9887. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, J.S.; Brenet, S.; Livache, T.; Herrier, C.; Hou, Y.; Buhot, A. Optical Index Prism Sensitivity of Surface Plasmon Resonance Imaging in Gas Phase: Experiment versus Theory. J. Phys. Chem. C. 2020, 124, 3756–3767. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Hurot, C.; Weerakkody, J.S.; Mathey, R.; Buhot, A.; Mascini, M.; Hou, Y.; Compagnone, D. Development of an Optoelectronic Nose Based on Surface Plasmon Resonance Imaging with Peptide and Hairpin DNA for Sensing Volatile Organic Compounds. Sens. Actuators B Chem. 2020, 303, 127188. [Google Scholar] [CrossRef]
- Daly, S.M.; Grassi, M.; Shenoy, D.K.; Ugozzoli, F.; Dalcanale, E. Supramolecular Surface Plasmon Resonance (SPR) Sensors for Organophosphorus Vapor Detection. J. Mater. Chem. 2007, 17, 1809–1818. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lundstrom, I. Biosensing with surface plasmon resonance—How it all started. Biosens. Bioelectron. 1995, 10, 1–9. [Google Scholar] [CrossRef]
- Mezcal Gracias a Dios. Gracias a Dios Mezcal [Internet]. Available online: https://www.thankgad.com/ (accessed on 31 October 2022).
- McPeak, K.M.; Jayanti, S.V.; Kress, S.J.; Meyer, S.; Iotti, S.; Rossinelli, A.; Norris, D.J. Plasmonic Films Can Easely Be Better: Rules and Recipes. ACS Photonics 2015, 2, 326–333. [Google Scholar] [CrossRef]
- Macleod, H.A. Thin Film Optical Filters, 3rd ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 4, 1967p. [Google Scholar]
- Luna-Moreno, D.; Sánchez, Y.E.; de León, Y.P.; Arias, E.N.; Campos, G. Virtual instrumentation in LabVIEW for multiple optical characterizations on the same opto-mechanical system. Optik (Stuttg) 2015, 126, 1923–1929. [Google Scholar] [CrossRef]
- Pronk, J.T.; Steensma, H.Y.; Van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Liu, S.Q.; Quek, A.Y.H. Evaluation of Beer Fermentation with a Novel Yeast Williopsis saturnus. Food Technol. Biotechnol. 2016, 54, 403. [Google Scholar] [CrossRef]
- Arrizon, J.; Gschaedler, A. Increasing fermentation efficiency at high sugar concentrations by supplementing an additional source of nitrogen during the exponential phase of the tequila fermentation process. Can. J. Microbiol. 2002, 48, 965–970. [Google Scholar] [CrossRef]
- Arrizon, J.; Gschaedler, A. Effects of the addition of different nitrogen sources in the tequila fermentation process at high sugar concentration. J. Appl. Microbiol. 2007, 102, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.R.; Watson, D.C. Production of organoleptic compounds. In Yeast Biotechnology; Berry, D.R., Russell, I., Stewart, G.G., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 345–368. [Google Scholar]
- Martínez-Aguilar, J.F.; Peña-Álvarez, A.J. Characterization of Five Typical Agave Plants used to Produce Mezcal through their Simple Lipid Composition Analysis by Gas Chromatography. J. Agric. Food Chem. 2009, 57, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Ding-Wei, H. Approach the angular sensitivity limit in surface plasmon resonance sensors with low index prism and large resonant angle. Opt. Eng. 2010, 49, 054403. [Google Scholar]
- Weast, R.C. Handbook of Chemistry and Physics, 49th ed.; Weast, R.C., Ed.; Chemical Rubber Publishing Company: Cleveland, OH, USA, 1969; p. 423. [Google Scholar]
- Lynch, D.W.; Huttner, W.R. Handbook of Optical Constants of Solids; Palik, E.D., Ed.; Academic Press: New York, NY, USA, 1980; pp. 275–367. [Google Scholar]
- Ekgasit, S.; Tangcharoenbumrungsuk, A.; Yu, F.; Baba, A.; Knoll, W. Resonance shifts in SPR curves of nonabsorbing, weakly absorbing, and strongly absorbing dielectrics. Sens. Actuators B Chem. 2005, 105, 532–541. [Google Scholar] [CrossRef]
- Ortega, J. Densities and refractive indices of pure alcohols as a function of temperature. J. Chem. Eng. Data 1982, 27, 312–317. [Google Scholar] [CrossRef]
- Luna-Moreno, D.; Monzón-Hernández, D.; Noé-Arias, E.; Regalado, L.E. Determination of quality and adulteration of tequila through the use of surface plasmon resonance. Appl. Opt. 2012, 51, 5161–5167. [Google Scholar] [CrossRef]
- Leite, I.; Navarrete, M.C.; Díaz-Herrera, N.; González-Cano, A.; Esteban, Ó. Selectivity of SPR fiber sensors in absorptive media: An experimental evaluation. Sens. Actuators B Chem. 2011, 160, 592–597. [Google Scholar] [CrossRef]
- Panda, A.; Pukhrambam, P.D.; Keiser, G. Performance analysis of graphene-based surface plasmon resonance biosensor for blood glucose and gas detection. Appl. Phys. A 2020, 126, 1–12. [Google Scholar] [CrossRef]
- Gupta, B.D.; Verma, R.K. Surface plasmon resonance-based fiber optic sensors: Principle, probe designs, and some applications. J. Sens. 2009, 2009, 979761. [Google Scholar] [CrossRef]
- Maharana, P.K.; Srivastava, T.; Jha, R. On the performance of highly sensitive and accurate graphene-on-aluminum and silicon-based SPR biosensor for visible and near infrared. Plasmonics 2014, 9, 1113–1120. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jha, R.; Gupta, B.D. Fiber-optic sensors based on surface plasmon resonance: A comprehensive review. IEEE Sens. J. 2007, 7, 1118–1129. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, S.K.; Verma, R.K. An SPR-based sensor with an extremely large dynamic range of refractive index measurements in the visible region. J. Phys. D Appl. Phys. 2015, 48, 435502. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, S.; Chen, H.; Weng, J. Graphene enhances the sensitivity of fiber-optic surface plasmon resonance biosensor. IEEE Sens. J. 2015, 15, 5478–5482. [Google Scholar] [CrossRef]
- Dillon, M.; Zaczek-Moczydlowska, M.A.; Edwards, C.; Turner, A.D.; Miller, P.I.; Moore, H.; McKinney, A.; Lawton, L.; Campbell, K. Current trends and challenges for rapid smart diagnostics at point-of-site testing for marine toxins. Sensors 2021, 21, 2499. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.T. Plasmonic biosensors. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Koyun, A.; Ahlatcolu, E.; Koca, Y.; Kara, S. Biosensors and their principles. In A Roadmap of Biomedical Engineers and Milestones, 1st ed.; Kara, S., Ed.; InTech-Janeza Trdine: Rijeka, Croatia, 2012; pp. 117–142. [Google Scholar]
- Zuppolini, S.; Quero, G.; Consales, M.; Diodato, L.; Vaiano, P.; Venturelli, A.; Santucci, M.; Spyrakis, F.; Costi, M.P.; Giordano, M.; et al. Label-free fiber optic optrode for the detection of class C β-lactamases expressed by drug resistant bacteria. Biomed. Opt. Express 2017, 8, 5191–5205. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
| Agave Specie | A. Karwinskii | A. marmorata | A. potatorum | A. rhodacantha | A. angustifolia | A. americana | |||
|---|---|---|---|---|---|---|---|---|---|
| Agave Ripeness (Years) | |||||||||
| (13) | (13) | (25) | (13) | (10) | (8) | (7) | (15) | ||
| Mezcal Sample | Madre Cuishe | Cuishe | Tepeztate | Tobalá | Mexicano | Espadín | Coyote | Arroqueño | |
| Compounds | |||||||||
| 1-Propanol | 47.85 | 7.98 | 32.86 | 25.95 | 62.03 | 5.88 | 31.82 | 35.5 | |
| Isobutyl alcohol | 2.32 | 16.97 | 4.06 | 7.26 | 2.61 | 8.33 | 8.59 | 4.92 | |
| Cyclopentanone | 0.35 | 0.05 | 0.47 | 0.47 | 0.17 | 0.41 | 0.52 | 0.15 | |
| Isopentyl alcohol | 11.9 | 42.33 | 21.17 | 39.93 | 16.35 | 54.71 | 39.89 | 20.17 | |
| β-Ethoxypropionaldehyde diethyl acetal | 0.1 | 0.25 | 0.38 | 0.44 | 0.3 | 0.14 | 0.26 | 0.65 | |
| Cyclopentanol | 0.18 | 0.19 | 0.16 | 0.12 | 0.29 | ||||
| Cyclopentanol, 2 methyl trans | 0.12 | 0.16 | 0.18 | 0.12 | 0.11 | 0.23 | 0.1 | ||
| Ethyl (S)-lactate | 4.04 | 4.23 | 3.47 | 1.98 | 2.51 | 1.98 | 4.95 | 2.21 | |
| Acetic Acid | 9.84 | 19.66 | 18.22 | 8.56 | 14.29 | 22.06 | 8.6 | 7 | |
| Furfural | 0.39 | 1.25 | 0.32 | 0.2 | 0.28 | 0.28 | 0.3 | 0.18 | |
| Propanoic acid | 0.93 | 0.27 | 1.38 | 0.58 | 0.65 | 0.22 | 0.24 | 0.48 | |
| Isobutyric acid | 0.3 | 0.55 | 0.22 | 0.06 | 0.16 | 0.14 | 0.1 | 0.26 | |
| 2-Furaldehyde, 5-methyl | 0.52 | 0.9 | 0.42 | 0.24 | 0.36 | 1.13 | 0.71 | 0.13 | |
| Isovaleric acid | 0.54 | 0.26 | 0.44 | 0.41 | 0.33 | 0.45 | 0.41 | ||
| α-Terpieol | 0.51 | 0.28 | 0.42 | 0.06 | 0.32 | 0.19 | 1.08 | ||
| Valeric acid, 3 methyl | 0.24 | ||||||||
| Furfuryl alcohol | 0.15 | 0.17 | 0.19 | 0.05 | 0.18 | ||||
| 2-Butanol | 1.12 | 20.08 | 9.33 | 15.69 | 0.81 | 16.65 | |||
| Butanoic acid, ethyl ester | 0.09 | 0.11 | |||||||
| Butanoic acid | 0.2 | 1.76 | 1.73 | 0.4 | |||||
| α-methyl-α-[4-methyl-3-pentenyl] oxiranemethanol | 0.15 | ||||||||
| Terpineol | 0.2 | ||||||||
| Decanoic acid, ethyl ester | 0.23 | ||||||||
| Mezcal Sample | 1-Propanol (%) | Acetic Acid (%) | Propanol/Acetic Acid Ratio |
|---|---|---|---|
| Madre Cuishe | 62.03 | 14.29 | 4.34 |
| Tobala | 47.85 | 9.84 | 4.86 |
| Arroqueño | 35.50 | 7 | 5.07 |
| Tepeztate | 32.86 | 18.22 | 1.80 |
| Coyote | 31.82 | 8.6 | 3.70 |
| Mexicano | 25.95 | 8.56 | 3.03 |
| Cuishe | 7.98 | 19.66 | 0.41 |
| Espadín | 5.88 | 22.06 | 0.27 |
| Mezcal Samples | Resonant Angle (Degrees) | Width (Degrees) | Rmin | Refractive Index (n) |
|---|---|---|---|---|
| Arroqueño (A. americana) | 74.441 | 3.052 | 2.650 | 1.3472 |
| Coyote (A. americana) | 74.357 | 3.082 | 2.70 | 1.3468 |
| Cuishe (A. karwinski) | 74.333 | 2.758 | 2.285 | 1.3474 |
| Madre Cuishe (A. karwinski) | 74.551 | 2.303 | 1.576 | 1.3484 |
| Espadín (A. angustifolia Haw) | 74.723 | 2.699 | 1.345 | 1.3504 |
| Mexicano (A. rhodacantha) | 74.627 | 2.567 | 1.506 | 1.3493 |
| Tepeztate (A. marmorata) | 74.339 | 2.771 | 2.195 | 1.3469 |
| Tobalá (A. potatorum) | 74.543 | 2.681 | 2.039 | 1.3482 |
| Methanol | 71.374 | 2.213 | 1.373 | 1.3284 |
| Distilled water | 71.454 | 2.195 | 1.768 | 1.3293 |
| Ethanol | 75.598 | 2.686 | 1.511 | 1.3556 |
| Type of SPR Sensor | Sensitivity (Degrees/RIU) | Detection Accuracy (Degrees−1) | Reference |
|---|---|---|---|
| Gallium phosphide prism + gold (50 nm) + silicon (9 nm) | 37.08 | 0.225 | [48] |
| N-FK51A prism + gold (55 nm) + graphene (0.34 nm) | 275.15 | 1.41 | [44] |
| Silicon prism + gold (50 nm) | 58 | 1.8 | |
| Silicon prism + silver (50 nm) | 138 | 4.9 | [46] |
| Silicon prism + aluminum (50 nm) | 377 | 23.3 | |
| Fiber optic + gold (40 nm) + graphene (0.34) | 33.98 | 0.298 | [49] |
| FK5 Prism + silver (50 nm) | 164.27 | 0.37 | Present work |
| Sensing Technology | Advantages | Disadvantages | References |
|---|---|---|---|
| Gas chromatography coupled with mass spectrometry | High sensitivity High accuracy High repeatability | Technical expertise required Costly reagents. Time-consuming. Impossibility of in-field detection | [50] |
| Prism-based SPR | Allows label-free detection Highly sensitive to the refractive index of the medium Widely established and commercially available Allows multiplex analysis | Difficulties for miniaturization Only detects refractive index changes close to the metal film surface High requirements for temperature control Difficulties for remote sensing applications. | [51] |
| Fiber Optic-SPR | Label-free detection Ease of miniaturization. Flexible and easy moving Allows remote sensing Low requirements for temperature control Allows multiplex analysis | Complex fabrication and surface functionalization Damage of molecules due to prolonged exposure to incident light Slow response time due to the diffusion effect of analytes. | [52,53] |
| Localized-SPR | Allows multiplex analysis and miniaturization Allows the improvement of the optical properties of the systems by varying the nanoparticles’ size, shape, and composition. Allows the use of wavelengths that do not overlap with the spectral features of strongly absorbing mediums | Only detects refractive index changes at tens of nanometers into the surrounding medium. Detection at the single-molecule level | [45] |
| Electrochemical sensors | Low-cost production of electrodes and microelectronic circuits. Straightforward interface of electronic read-out and processing | Electrical interference effects High effect on sensor’s response due to pH and ionic strength in the sample Increase in the signal-to-noise due to miniaturization Requirement of redox molecules to mediate the electrochemical reactions Fouling effects on the electrodes | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Álvarez, A.; Luna-Moreno, D.; Silva-Hernández, O.; Rodríguez-Delgado, M.M. Application of SPR Method as an Approach to Gas Phase Sensing of Volatile Compound Profile in Mezcal Spirits Conferred by Agave Species. Chemosensors 2023, 11, 70. https://doi.org/10.3390/chemosensors11010070
Sánchez-Álvarez A, Luna-Moreno D, Silva-Hernández O, Rodríguez-Delgado MM. Application of SPR Method as an Approach to Gas Phase Sensing of Volatile Compound Profile in Mezcal Spirits Conferred by Agave Species. Chemosensors. 2023; 11(1):70. https://doi.org/10.3390/chemosensors11010070
Chicago/Turabian StyleSánchez-Álvarez, Araceli, Donato Luna-Moreno, Oscar Silva-Hernández, and Melissa Marlene Rodríguez-Delgado. 2023. "Application of SPR Method as an Approach to Gas Phase Sensing of Volatile Compound Profile in Mezcal Spirits Conferred by Agave Species" Chemosensors 11, no. 1: 70. https://doi.org/10.3390/chemosensors11010070
APA StyleSánchez-Álvarez, A., Luna-Moreno, D., Silva-Hernández, O., & Rodríguez-Delgado, M. M. (2023). Application of SPR Method as an Approach to Gas Phase Sensing of Volatile Compound Profile in Mezcal Spirits Conferred by Agave Species. Chemosensors, 11(1), 70. https://doi.org/10.3390/chemosensors11010070







