The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Ti0.2V1.8AlC MAX-Phase
2.3. Preparation of Ti0.2V1.8C MXene and Its Delamination
2.4. Fabrication of Ti0.2V1.8C MXene-Based Multielectrode Chip
2.5. Quantum-Chemical Calculations
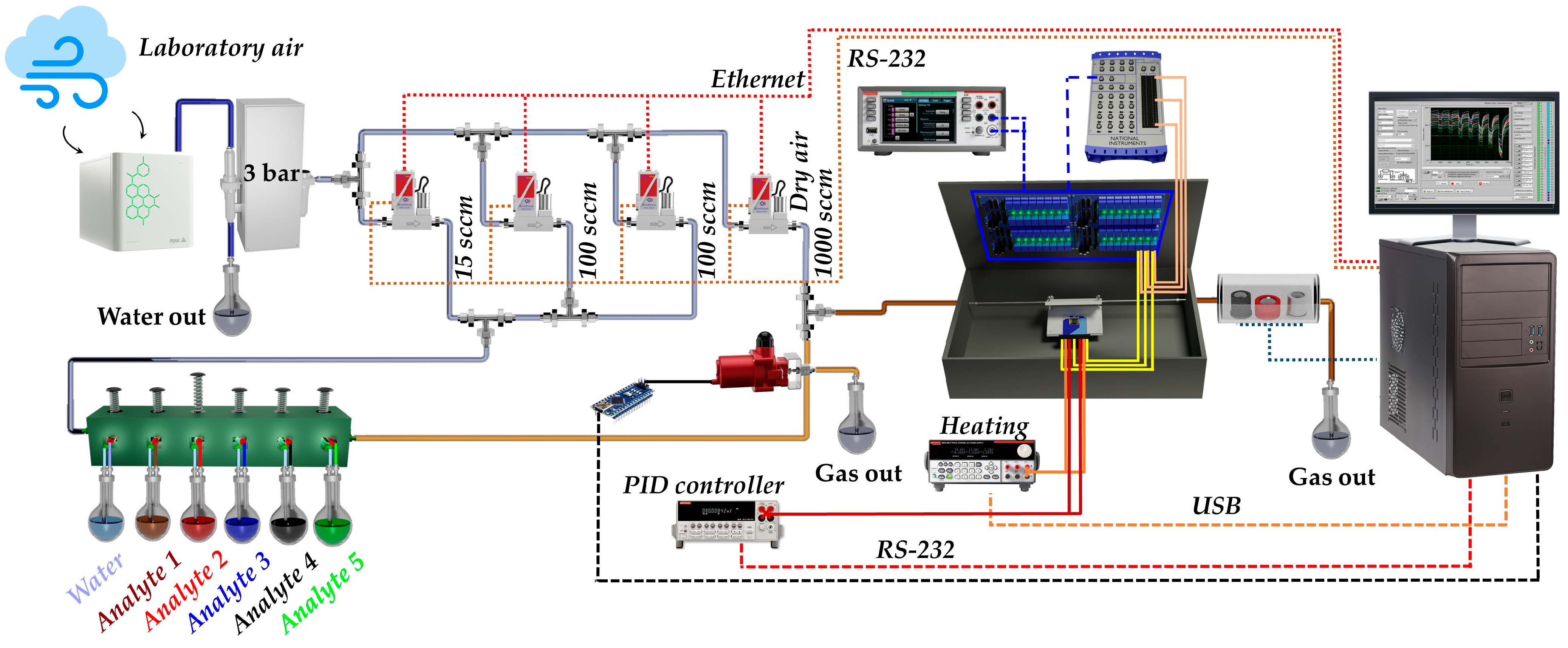
2.6. Instrumentation
3. Results and Discussion
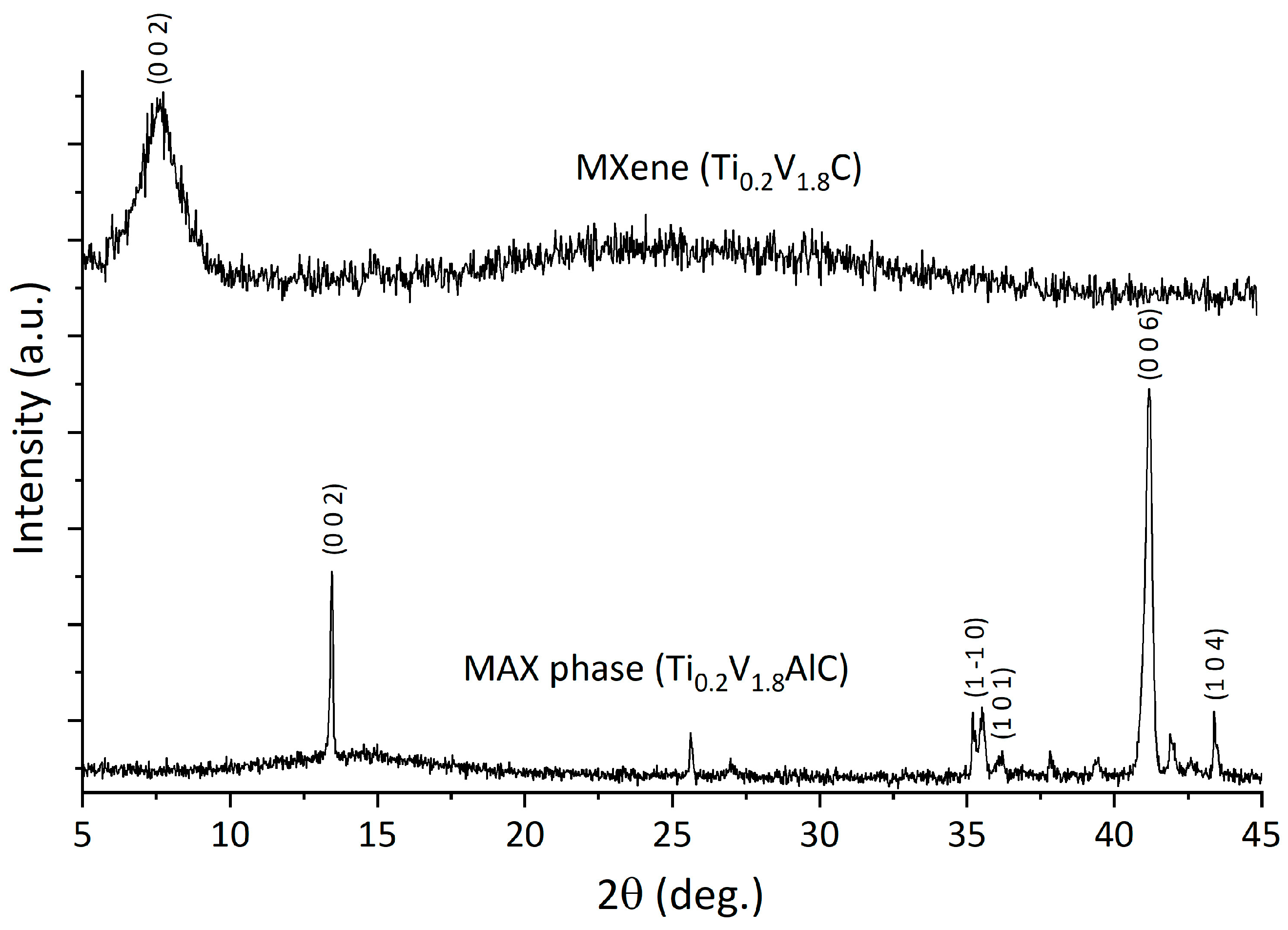
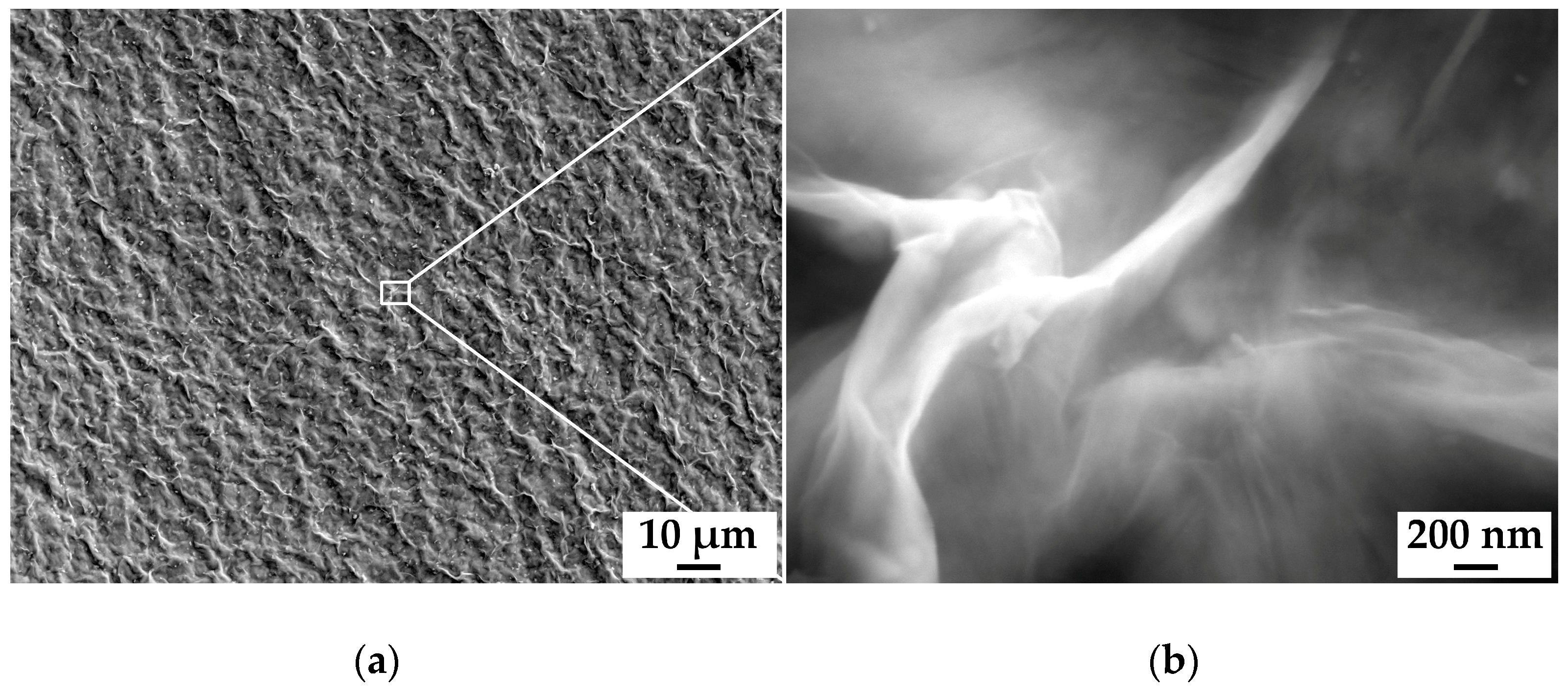
3.1. Characterization of the MAX-Phase and MXene under Study
3.2. Quantum-Chemical Calculations
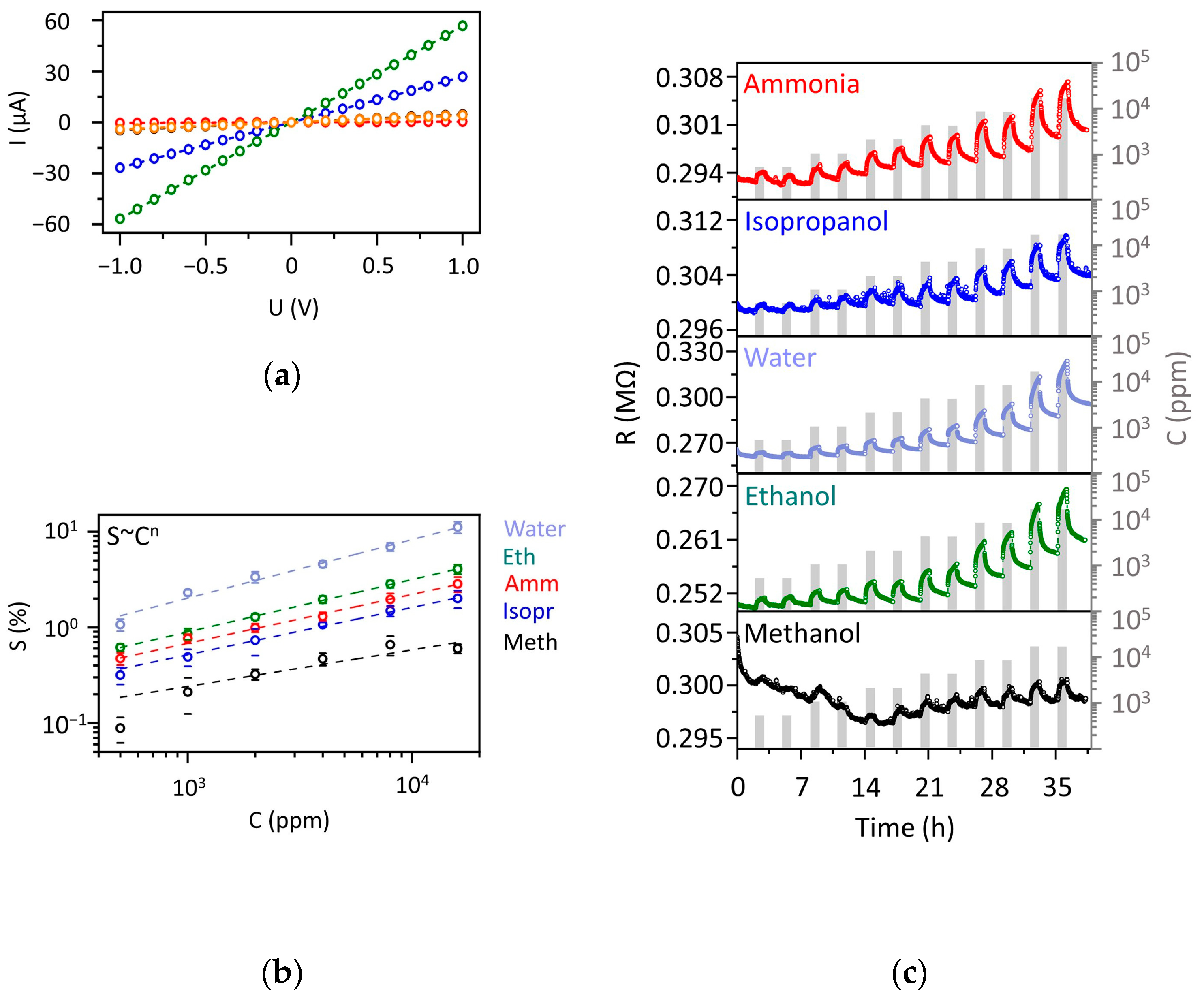
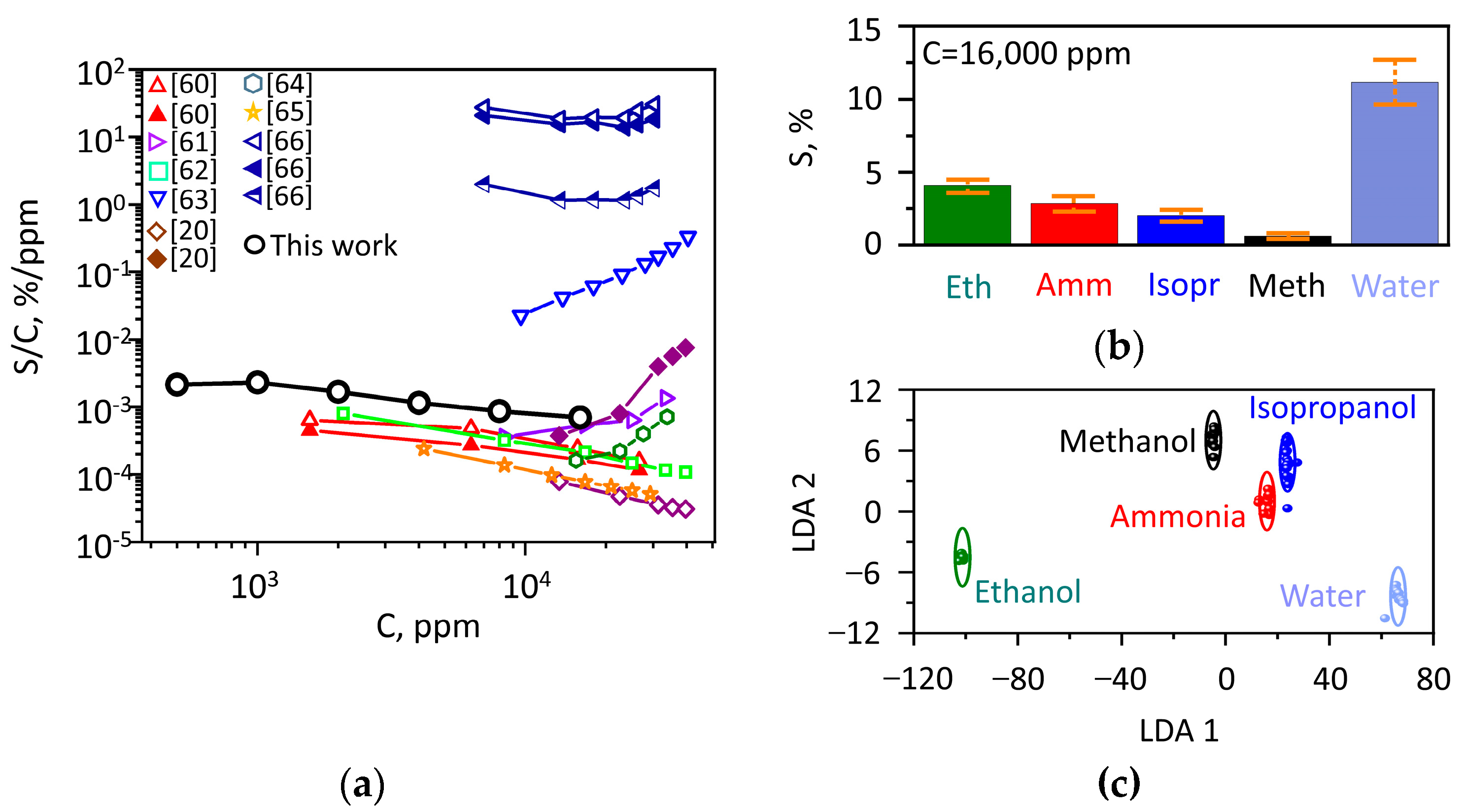
3.3. Chemiresistive Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, C.; Ma, J.; Zou, Y.; Li, G.; Xu, H.; Sysoev, V.V.; Cheng, X.; Deng, Y. Modeling Interfacial Interaction between Gas Molecules and Semiconductor Metal Oxides: A New View Angle on Gas Sensing. Adv. Sci. 2022, 9, 2203594. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Ansari, H.R.; Shahbaz, M.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Metal Oxide Semiconductor Nanostructure Gas Sensors with Different Morphologies. Chemosensors 2022, 10, 289. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Ham, M.H. Materials Acceptable for Gas Sensor Design: Advantages and Limitations. Key Eng. Mater. 2018, 780, 80–89. [Google Scholar] [CrossRef]
- Ou, L.-X.; Liu, M.-Y.; Zhu, L.-Y.; Zhang, D.W.; Lu, H.-L. Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor. Nano-Micro Lett. 2022, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K. Mind the Gap. Nat. Mater. 2007, 6, 720–721. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of Individual Gas Molecules Adsorbed on Graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Dral, A.P.; ten Elshof, J.E. 2D Metal Oxide Nanoflakes for Sensing Applications: Review and Perspective. Sens. Actuators B Chem. 2018, 272, 369–392. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D Materials for Gas Sensing Applications: A Review on Graphene Oxide, MoS2, WS2 and Phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.-J. Two-Dimensional Transition Metal Dichalcogenides and Metal Oxide Hybrids for Gas Sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical Sensing with 2D Materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, I.-D. Recent Developments in 2D Nanomaterials for Chemiresistive-Type Gas Sensors. Electron. Mater. Lett. 2018, 14, 221–260. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Pinna, N.; Zhang, J. Two-Dimensional Nanostructured Materials for Gas Sensing. Adv. Funct. Mater. 2017, 27, 1702168. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S. Gas Sensing in 2D Materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hazra, A. MXene-Based Gas Sensors. J. Mater. Chem. C 2021, 9, 15735–15754. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Chertopalov, S.; Mochalin, V.N. Environment-Sensitive Photoresponse of Spontaneously Partially Oxidized Ti3C2 MXene Thin Films. ACS Nano 2018, 12, 6109–6116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-N.; Zhang, Y.-J.; Duan, Z.-H.; Wang, S.; Liu, C.; Jiang, Y.-D.; Tai, H.-L. A Review on Ti3C2Tx-Based Nanomaterials: Synthesis and Applications in Gas and Humidity Sensors. Rare Met. 2021, 40, 1459–1476. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, A.; Wang, C.; Liu, F.; He, J.; Li, S.; Wang, J.; You, R.; Yan, X.; Sun, P.; et al. Improvement of Gas and Humidity Sensing Properties of Organ-like MXene by Alkaline Treatment. ACS Sens. 2019, 4, 1261–1269. [Google Scholar] [CrossRef]
- Pazniak, H.; Varezhnikov, A.S.; Kolosov, D.A.; Plugin, I.A.; Vito, A.D.; Glukhova, O.E.; Sheverdyaeva, P.M.; Spasova, M.; Kaikov, I.; Kolesnikov, E.A.; et al. 2D Molybdenum Carbide MXenes for Enhanced Selective Detection of Humidity in Air. Adv. Mater. 2021, 33, 2104878. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Liu, W.; Lv, T.; Ma, M.; Liu, Z.; Vasiliev, A.; Li, X. MXene/SnO2 Heterojunction Based Chemical Gas Sensors. Sens. Actuators B Chem. 2021, 329, 129275. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-Dimensional Building Blocks for Future Materials and Devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; VahidMohammadi, A.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Two-Dimensional Vanadium Carbide MXene for Gas Sensors with Ultrahigh Sensitivity Toward Nonpolar Gases. ACS Sens. 2019, 4, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, M.; Li, N.; Wang, W.; Sun, B.; Liu, M.; Zhang, D.; Li, Z.; Wang, C. Vanadium-Doped Tin Oxide Porous Nanofibers: Enhanced Responsivity for Hydrogen Detection. Talanta 2017, 167, 638–644. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Whang, W.-T.; Chen, C.-H. Hollow V2O5 Nanoassemblies for High-Performance Room-Temperature Hydrogen Sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. A Brief History of Electronic Noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Strelcov, V.V.; Kolmakov, A. Multisensor Micro-Arrays Based on Metal Oxide Nanowires for Electronic Nose Applications. In Metal Oxide Nanomaterials for Chemical Sensors; Carpenter, M.A., Mathur, S., Kolmakov, A., Eds.; Springer Science + Business Media: New York, NY, USA, 2013; pp. 425–502. [Google Scholar]
- Fedorov, F.S.; Simonenko, N.P.; Trouillet, V.; Volkov, I.A.; Plugin, I.A.; Rupasov, D.P.; Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Vlasov, I.S.; et al. Microplotter-Printed On-Chip Combinatorial Library of Ink-Derived Multiple Metal Oxides as an “Electronic Olfaction” Unit. ACS Appl. Mater. Interfaces 2020, 12, 56135–56150. [Google Scholar] [CrossRef]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-Based Composites: Synthesis, Properties and Environment-Related Applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic Properties and Applications of MXenes: A Theoretical Review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Anasori, B.; Shi, C.; Moon, E.J.; Xie, Y.; Voigt, C.A.; Kent, P.R.C.; May, S.J.; Billinge, S.J.L.; Barsoum, M.W.; Gogotsi, Y. Control of Electronic Properties of 2D Carbides (MXenes) by Manipulating Their Transition Metal Layers. Nanoscale Horiz. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Khazaei, M.; Mishra, A.; Venkataramanan, N.S.; Singh, A.K.; Yunoki, S. Recent Advances in MXenes: From Fundamentals to Applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 164–178. [Google Scholar] [CrossRef]
- Gao, G.; Ding, G.; Li, J.; Yao, K.; Wu, M.; Qian, M. Monolayer MXenes: Promising Half-Metals and Spin Gapless Semiconductors. Nanoscale 2016, 8, 8986–8994. [Google Scholar] [CrossRef]
- Kumar, H.; Frey, N.C.; Dong, L.; Anasori, B.; Gogotsi, Y.; Shenoy, V.B. Tunable Magnetism and Transport Properties in Nitride MXenes. ACS Nano 2017, 11, 7648–7655. [Google Scholar] [CrossRef]
- Weng, H.; Ranjbar, A.; Liang, Y.; Song, Z.; Khazaei, M.; Yunoki, S.; Arai, M.; Kawazoe, Y.; Fang, Z.; Dai, X. Large-Gap Two-Dimensional Topological Insulator in Oxygen Functionalized MXene. Phys. Rev. B 2015, 92, 075436. [Google Scholar] [CrossRef]
- He, J.; Lyu, P.; Nachtigall, P. New Two-Dimensional Mn-Based MXenes with Room-Temperature Ferromagnetism and Half-Metallicity. J. Mater. Chem. C 2016, 4, 11143–11149. [Google Scholar] [CrossRef]
- Si, C.; Zhou, J.; Sun, Z. Half-Metallic Ferromagnetism and Surface Functionalization-Induced Metal–Insulator Transition in Graphene-like Two-Dimensional Cr2C Crystals. ACS Appl. Mater. Interfaces 2015, 7, 17510–17515. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-H.; Luo, K.; Li, Q.; Huang, Q.; He, J.; Wen, X.; Du, S. Role of the Surface Effect on the Structural, Electronic and Mechanical Properties of the Carbide MXenes. EPL (Europhys. Lett.) 2015, 111, 26007. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Nagornov, I.A.; Simonenko, T.L.; Mokrushin, A.S.; Sevastyanov, V.G.; Kuznetsov, N.T. Synthesis of MAX Phases in the Ti2AlC–V2AlC System as Precursors of Heterometallic MXenes Ti2–xVxC. Russ. J. Inorg. Chem. 2022, 67, 705–714. [Google Scholar] [CrossRef]
- Dash, A.; Vaßen, R.; Guillon, O.; Gonzalez-Julian, J. Molten Salt Shielded Synthesis of Oxidation Prone Materials in Air. Nat. Mater. 2019, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rabchinskii, M.K.; Varezhnikov, A.S.; Sysoev, V.V.; Solomatin, M.A.; Ryzhkov, S.A.; Baidakova, M.V.; Stolyarova, D.Y.; Shnitov, V.V.; Pavlov, S.S.; Kirilenko, D.A.; et al. Hole-Matrixed Carbonylated Graphene: Synthesis, Properties, and Highly-Selective Ammonia Gas Sensing. Carbon 2021, 172, 236–247. [Google Scholar] [CrossRef]
- Fedorov, F.S.; Varezhnikov, A.S.; Kiselev, I.; Kolesnichenko, V.V.; Burmistrov, I.N.; Sommer, M.; Fuchs, D.; Kübel, C.; Gorokhovsky, A.V.; Sysoev, V.V. Potassium Polytitanate Gas-Sensor Study by Impedance Spectroscopy. Anal. Chim. Acta 2015, 897, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.R.; Shukla, V.; Jena, N.K.; Luo, W.; Ahuja, R. Exploring Two-Dimensional M2NS2 (M = Ti, V) MXenes Based Gas Sensors for Air Pollutants. Appl. Mater. Today 2020, 19, 100574. [Google Scholar] [CrossRef]
- Mehdi Aghaei, S.; Aasi, A.; Panchapakesan, B. Experimental and Theoretical Advances in MXene-Based Gas Sensors. ACS Omega 2021, 6, 2450–2461. [Google Scholar] [CrossRef]
- Nahirniak, S.; Saruhan, B. MXene Heterostructures as Perspective Materials for Gas Sensing Applications. Sensors 2022, 22, 972. [Google Scholar] [CrossRef]
- Akkuş, Ü.Ö.; Balcı, E.; Berber, S. Mo2TiC2O2 MXene-based nanoscale pressure sensor. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113762. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA Method for Ab Initio Order- N Materials Simulation. J. Phys. Condens. Matter 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent Developments and Applications. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Johnson, D.D. Modified Broyden’s Method for Accelerating Convergence in Self-Consistent Calculations. Phys. Rev. B 1988, 38, 12807–12813. [Google Scholar] [CrossRef] [PubMed]
- Büttiker, M.; Imry, Y.; Landauer, R.; Pinhas, S. Generalized Many-Channel Conductance Formula with Application to Small Rings. Phys. Rev. B 1985, 31, 6207–6215. [Google Scholar] [CrossRef]
- Lu, H.; Dai, D.; Yang, P.; Li, L. Atomic Orbitals in Molecules: General Electronegativity and Improvement of Mulliken Population Analysis. Phys. Chem. Chem. Phys. 2006, 8, 340–346. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Zhang, Y.; Quan, W.; Chen, X.; Yang, J.; Zeng, M.; Zhou, Z.; Su, Y.; Wei, H.; et al. Fast and Recoverable NO2 Detection Achieved by Assembling ZnO on Ti3C2Tx MXene Nanosheets under UV Illumination at Room Temperature. Nanoscale 2022, 14, 3441–3451. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C. A Contribution on Some Basic Definitions of Sensors Properties. IEEE Sens. J. 2001, 1, 183–190. [Google Scholar] [CrossRef]
- Muckley, E.S.; Naguib, M.; Wang, H.-W.; Vlcek, L.; Osti, N.C.; Sacci, R.L.; Sang, X.; Unocic, R.R.; Xie, Y.; Tyagi, M.; et al. Multimodality of Structural, Electrical, and Gravimetric Responses of Intercalated MXenes to Water. ACS Nano 2017, 11, 11118–11126. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Zhang, H.; Wang, Q.; Guan, F.; Yu, Z. Flexible and Multifunctional Silk Textiles with Biomimetic Leaf-Like MXene/Silver Nanowire Nanostructures for Electromagnetic Interference Shielding, Humidity Monitoring, and Self-Derived Hydrophobicity. Adv. Funct. Mater. 2019, 29, 1905197. [Google Scholar] [CrossRef]
- Muckley, E.S.; Naguib, M.; Ivanov, I.N. Multi-Modal, Ultrasensitive, Wide-Range Humidity Sensing with Ti3C2 Film. Nanoscale 2018, 10, 21689–21695. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, Y.; Zhou, C.; Xiao, Y.; Meng, B.; Wang, Z.; Huang, D.; Xing, C.; Peng, Z. High-Performance Humidity Sensor Based on Urchin-Like Composite of Ti3C2 MXene-Derived TiO2 Nanowires. ACS Appl. Mater. Interfaces 2019, 11, 38116–38125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.-Y.; Tang, C.-Y.; Zha, X.-J.; Liu, Y.; Su, B.-H.; Ke, K.; Bao, R.-Y.; Yang, M.-B.; Yang, W. Smart Ti3C2Tx MXene Fabric with Fast Humidity Response and Joule Heating for Healthcare and Medical Therapy Applications. ACS Nano 2020, 14, 8793–8805. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Patel, A.; Echols, I.; Zhao, X.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Water Sorption in MXene/Polyelectrolyte Multilayers for Ultrafast Humidity Sensing. ACS Appl. Nano Mater. 2019, 2, 948–955. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Fu, C.; Tao, R.; Li, H.; Luo, J. Ultra-Fast and Sensitive Hydrophobic QCM Humidity Sensor by Sulfur Modified Ti3C2Tx MXene. IEEE Sens. J. 2022. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Kiselev, I.; Sysoev, V.; Kaikov, I.; Koronczi, I.; Adil Akai Tegin, R.; Smanalieva, J.; Sommer, M.; Ilicali, C.; Hauptmannl, M. On the Temporal Stability of Analyte Recognition with an E-Nose Based on a Metal Oxide Sensor Array in Practical Applications. Sensors 2018, 18, 550. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of Discrimination Mechanisms in the Mammalian Olfactory System Using a Model Nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Xuan, J.-Y.; Zhang, Q.; Sun, M.-L.; Jia, F.-C.; Wang, X.-M.; Yin, G.-C.; Lu, S.-Y. Strategies and Challenges for Enhancing Performance of MXene-Based Gas Sensors: A Review. Rare Met. 2022, 41, 3976–3999. [Google Scholar] [CrossRef]
- Pazniak, H.; Plugin, I.A.; Loes, M.J.; Inerbaev, T.M.; Burmistrov, I.N.; Gorshenkov, M.; Polcak, J.; Varezhnikov, A.S.; Sommer, M.; Kuznetsov, D.V.; et al. Partially Oxidized Ti3C2Tx MXenes for Fast and Selective Detection of Organic Vapors at Part-per-Million Concentrations. ACS Appl. Nano Mater. 2020, 3, 3195–3204. [Google Scholar] [CrossRef]



| MXene | Analyte | ||||
|---|---|---|---|---|---|
| Methanol | Ethanol | Isopropanol | Ammonia | Water | |
| Ti0.2V1.8C | −0.01e | −0.11e | −0.006e | 0.007e | 0.003e |
| Ti2C | −0.028e | −0.061e | −0.016e | 0.019e | 0.012e |
| V2C | −0.006e | −0.009e | −0.011e | 0.015e | 0.004e |
| MXene | Analyte | ||||
|---|---|---|---|---|---|
| Methanol | Ethanol | Isopropanol | Ammonia | Water | |
| Ti0.2V1.8C | −0.051 | −0.044 | −0.041 | −0.058 | −0.062 |
| Ti2C | −0.101 | −0.128 | −0.112 | −0.118 | −0.082 |
| V2C | −0.084 | −0.098 | −0.073 | −0.106 | −0.063 |
| Analyte | Methanol | Ethanol | Isopropanol | Ammonia | Water |
|---|---|---|---|---|---|
| n | 0.38 ± 0.1 | 0.55 ± 0.01 | 0.49 ± 0.02 | 0.51 ± 0.02 | 0.61 ± 0.03 |
| LOD, ppm | 181 | 24 | 39 | 27 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, N.P.; Glukhova, O.E.; Plugin, I.A.; Kolosov, D.A.; Nagornov, I.A.; Simonenko, T.L.; Varezhnikov, A.S.; Simonenko, E.P.; Sysoev, V.V.; Kuznetsov, N.T. The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs. Chemosensors 2023, 11, 7. https://doi.org/10.3390/chemosensors11010007
Simonenko NP, Glukhova OE, Plugin IA, Kolosov DA, Nagornov IA, Simonenko TL, Varezhnikov AS, Simonenko EP, Sysoev VV, Kuznetsov NT. The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs. Chemosensors. 2023; 11(1):7. https://doi.org/10.3390/chemosensors11010007
Chicago/Turabian StyleSimonenko, Nikolay P., Olga E. Glukhova, Ilya A. Plugin, Dmitry A. Kolosov, Ilya A. Nagornov, Tatiana L. Simonenko, Alexey S. Varezhnikov, Elizaveta P. Simonenko, Victor V. Sysoev, and Nikolay T. Kuznetsov. 2023. "The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs" Chemosensors 11, no. 1: 7. https://doi.org/10.3390/chemosensors11010007
APA StyleSimonenko, N. P., Glukhova, O. E., Plugin, I. A., Kolosov, D. A., Nagornov, I. A., Simonenko, T. L., Varezhnikov, A. S., Simonenko, E. P., Sysoev, V. V., & Kuznetsov, N. T. (2023). The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs. Chemosensors, 11(1), 7. https://doi.org/10.3390/chemosensors11010007









