Li6BaLa2Ta2O12 Solid-State Probe for Studying Li Activity in Molten Sn-Li Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis, Sintering and Characterisation of the Lithium Solid-State Electrolyte
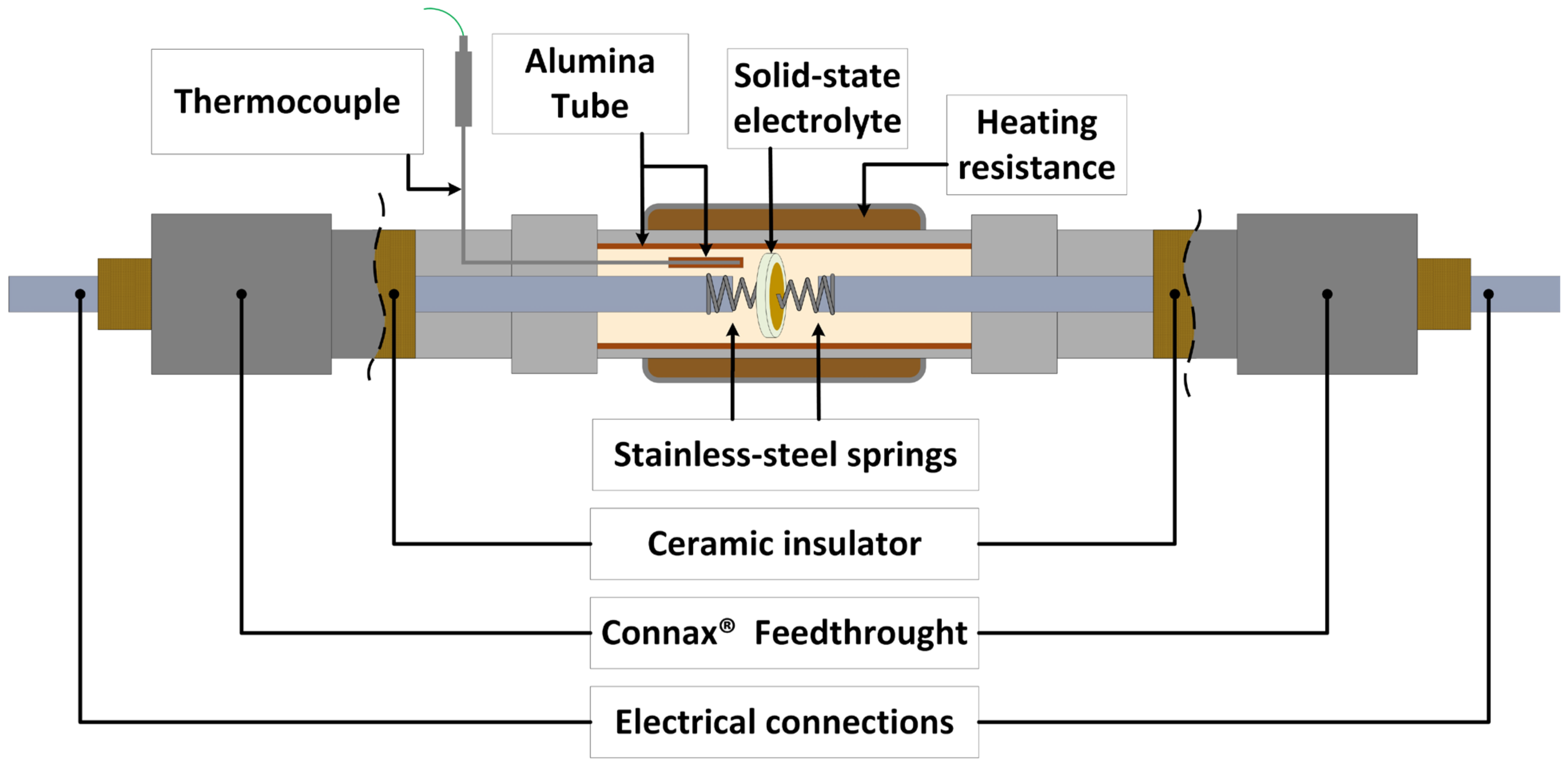
2.2. Sensor Construction
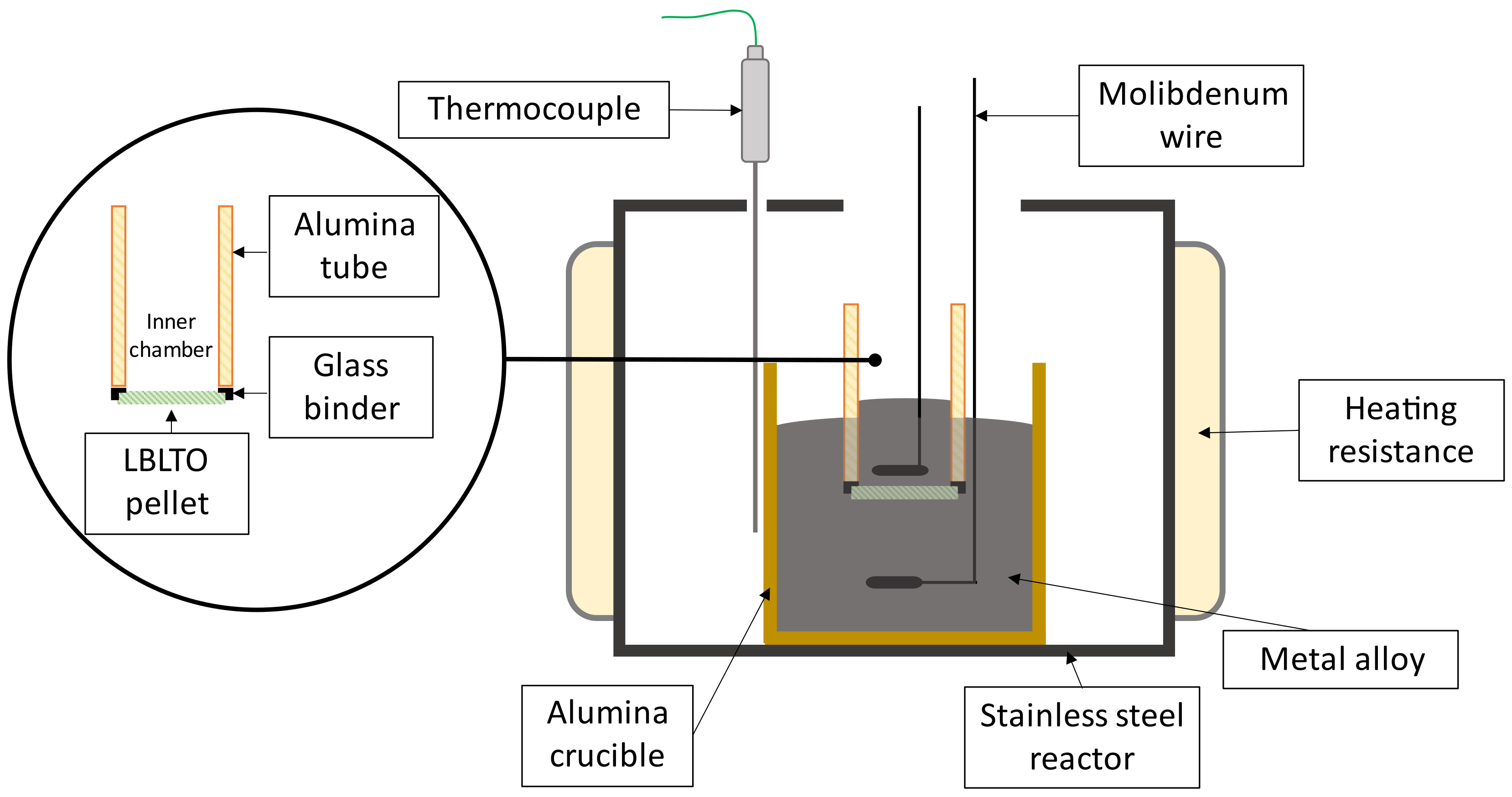
2.3. Synthesis of Sn-Li and Pb-Li Alloys
3. Results and Discussion
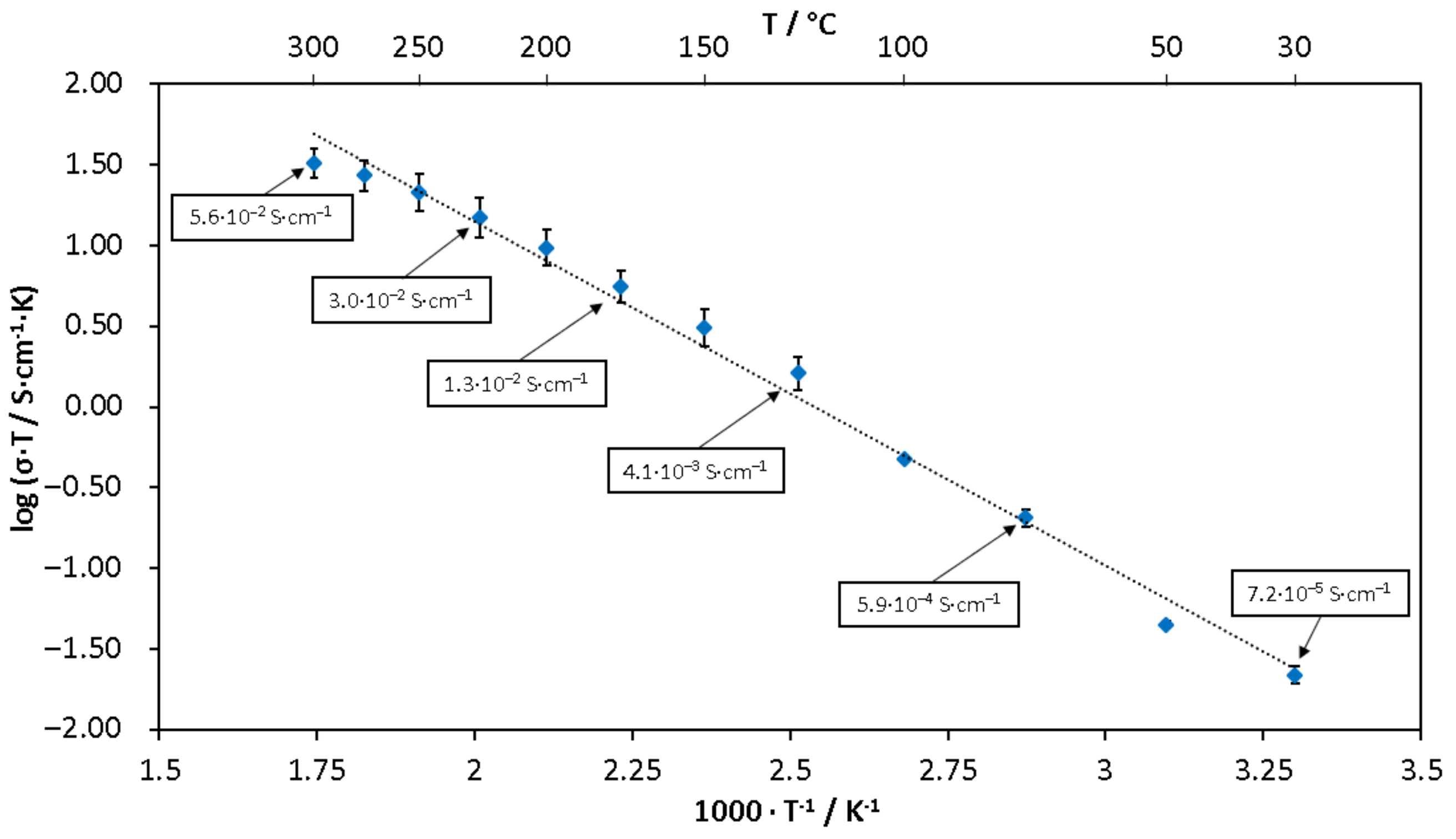
3.1. Ionic Conductivity of LBLTO Solid-State Electrolyte
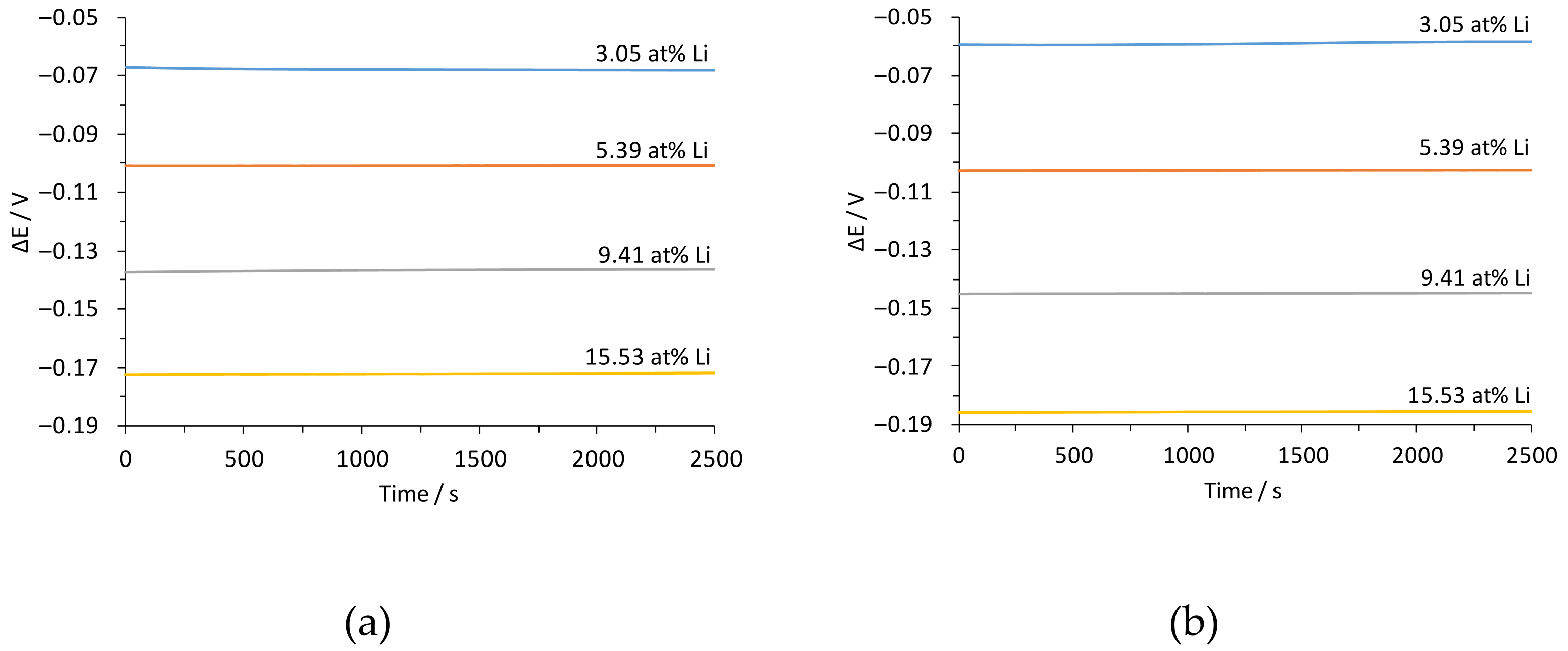
3.2. Performance of the Sensor
3.3. Determination of the Lithium Activity in Sn-3.04Li Alloy
3.4. Determination of Correlations of Lithium Activity in Sn-Li
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Kazem, N.; Hellebrekers, T.; Majidi, C. Soft Multifunctional Composites and Emulsions with Liquid Metals. Adv. Mater. 2017, 29, 1605985. [Google Scholar] [CrossRef] [PubMed]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanesh, K.; Tang, S.-Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-zadeh, K.; Dickey, M.D. Liquid metal enabled microfluidics. Lab. Chip. 2017, 17, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Kanatzidis, M.G.; Pöttgen, R.; Jeitschko, W. The Metal Flux: A Preparative Tool for the Exploration of Intermetallic Compounds. Angew. Chem. Int. Ed. 2005, 44, 6996–7023. [Google Scholar] [CrossRef]
- Sebastian, C.P.; Malliakas, C.D.; Chondroudi, M.; Schellenberg, I.; Rayaprol, S.; Hoffmann, R.-D.; Pöttgen, R.; Kanatzidis, M.G. Indium Flux-Growth of Eu 2 AuGe 3: A New Germanide with an AlB 2 Superstructure. Inorg. Chem. 2010, 49, 9574–9580. [Google Scholar] [CrossRef]
- Bobev, S.; Merz, J.; Lima, A.; Fritsch, V.; Thompson, J.D.; Sarrao, J.L.; Gillessen, M.; Dronskowski, R. Unusual Mn−Mn Spin Coupling in the Polar Intermetallic Compounds CaMn 2 Sb 2 and SrMn 2 Sb 2. Inorg. Chem. 2006, 45, 4047–4054. [Google Scholar] [CrossRef]
- Ladd, C.; So, J.-H.; Muth, J.; Dickey, M.D. 3D Printing of Free Standing Liquid Metal Microstructures. Adv. Mater. 2013, 25, 5081–5085. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Liquid phase 3D printing for quickly manufacturing conductive metal objects with low melting point alloy ink. Sci. China Technol. Sci. 2014, 57, 1721–1728. [Google Scholar] [CrossRef]
- Li, H.; Yin, H.; Wang, K.; Cheng, S.; Jiang, K.; Sadoway, D.R. Liquid Metal Electrodes for Energy Storage Batteries. Adv. Energy Mater. 2016, 6, 1600483. [Google Scholar] [CrossRef]
- Kim, H.; Boysen, D.A.; Newhouse, J.M.; Spatocco, B.L.; Chung, B.; Burke, P.J.; Bradwell, D.J.; Jiang, K.; To-maszowska, A.A.; Wang, K.; et al. Liquid Metal Batteries: Past, Present, and Future. Chem. Rev. 2013, 113, 2075–2099. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, K.; Chung, B.; Ouchi, T.; Burke, P.J.; Boysen, D.A.; Bradwell, D.J.; Kim, H.; Muecke, U.; Sadoway, D.R. Lithium–antimony–lead liquid metal battery for grid-level energy storage. Nature 2014, 514, 348–350. [Google Scholar] [CrossRef]
- Giancarli, L.; Chuyanov, V.; Abdou, M.; Akiba, M.; Hong, B.G.; Lässer, R.; Pan, C.; Strebkov, Y. Breeding Blanket Modules testing in ITER: An international program on the way to DEMO. Fusion Eng. Des. 2006, 81, 393–405. [Google Scholar] [CrossRef]
- Puma, A.L.; Aiello, G.; Gabriel, F.; Laffont, G.; Rampal, G.; Salavy, J.-F. Requirements and proposals for control and monitoring measurements of the HCLL TBM. Fusion Eng. Des. 2010, 85, 1642–1652. [Google Scholar] [CrossRef]
- Abdou, M.; Morley, N.B.; Smolentsev, S.; Ying, A.; Malang, S.; Rowcliffe, A.; Ulrickson, M. Blanket/first wall challenges and required R&D on the pathway to DEMO. Fusion Eng. Des. 2015, 100, 2–43. [Google Scholar] [CrossRef]
- Loureiro, J.P.S.; Tabarés, F.L.; Fernandes, H.; Silva, C.; Gomes, R.; Alves, E.; Mateus, R.; Pereira, T.; Alves, H.; Figueiredo, H. Behavior of liquid Li-Sn alloy as plasma facing material on ISTTOK. Fusion Eng. Des. 2017, 117, 208–211. [Google Scholar] [CrossRef]
- Linke, J.; Du, J.; Loewenhoff, T.; Pintsuk, G.; Spilker, B.; Steudel, I.; Wirtz, M. Challenges for plasma-facing components in nuclear fusion. Matter Radiat. Extrem. 2019, 4, 056201. [Google Scholar] [CrossRef]
- Tabarés, F.L. Present status of liquid metal research for a fusion reactor. Plasma Phys. Control. Fusion 2016, 58, 014014. [Google Scholar] [CrossRef]
- Majeski, R.; Kaita, R.; Boaz, M.; Efthimion, P.; Gray, T.; Jones, B.; Hoffman, D.; Kugel, H.; Menard, J.; Munsat, T.; et al. Testing of liquid lithium limiters in CDX-U. Fusion Eng. Des. 2004, 72, 121–132. [Google Scholar] [CrossRef][Green Version]
- Mazzitelli, G.; Apicella, M.L.; Apruzzese, G.; Crescenzi, F.; Iannone, F.; Maddaluno, G.; Pericoli-Ridolfini, V.; Roccella, S.; Reale, M.; Viola, B.; et al. Experiments on FTU with an actively water cooled liquid lithium limiter. J. Nucl. Materials 2015, 463, 1152–1155. [Google Scholar] [CrossRef]
- Gomes, R.B.; Fernandes, H.; Silva, C.; Sarakovskis, A.; Pereira, T.; Figueiredo, J.; Carvalho, B.; Soares, A.; Varandas, C.; Lielausis, O.; et al. Interaction of a liquid gallium jet with the tokamak ISTTOK edge plasma. Fusion Eng. Des. 2008, 83, 102–111. [Google Scholar] [CrossRef]
- Allain, J.P.; Ruzic, D.N.; Hendricks, M.R. D, He and Li sputtering of liquid eutectic Sn–Li. J. Nucl. Mater. 2001, 290, 33–37. [Google Scholar] [CrossRef]
- Shin, D.J.; Gao, X.; Ueda, S.; Kitamura, S. Measurement of the Activity Coefficients of P and Mn in Carbon-Saturated Fe-P-Mn-C Alloy. Metall. Mater. Trans. B 2019, 50, 825–833. [Google Scholar] [CrossRef]
- Miki, T.; Tsujita, K.; Ban-Ya, S.; Hino, M. Activity measurement of the constituents in molten Fe–B and Fe–B–C alloys. Calphad 2006, 30, 449–454. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connel, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Itoh, S.; Inoue, Y. Activity Measurement of Titanium-Tin Alloys by Knudsen Effusion Method. Mater. Trans. 2011, 52, 704–708. [Google Scholar] [CrossRef]
- Katayama, I.; Zivkovic, D.; Manasijevic, D.; Oishi, T.; Zivkovic, Z.; Iida, T. Activity measurement of Ga in liquid Ga-Pb alloys by EMF method with zirconia solid electrolyte. J. Min. Metall. Sect. B Metall. 2002, 38, 229–236. [Google Scholar] [CrossRef]
- Nel-lo, M.; Colominas, S.; Abellà, J. Lithium conducting ceramics for future electrochemical sensors in molten metals. Fusion Eng. Des. 2019, 146, 1216–1220. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Lithium ion conductivity of Li5+x Ba x La 3-x Ta2O12 (x=0-2) with garnet-related structure in dependence of the barium content. Ionics 2007, 13, 195–203. [Google Scholar] [CrossRef]
- Lopez Maldonado, K.L.; de la Presa, P.; de la Rubia, M.A.; Crespo, P.; de Frutos, J.; Hernando, A.; Matutes Aquino, J.A.; Elizalde Galindo, J.T. Effects of grain boundary width and crystallite size on conductivity and magnetic properties of magnetite nanoparticles. J. Nanoparticle Res. 2014, 16, 2482. [Google Scholar] [CrossRef]
- Narayanan, S.; Hitz, G.T.; Wachsman, E.D.; Thangadurai, V. Effect of Excess Li on the Structural and Electrical Properties of Garnet-Type Li 6 La 3 Ta 1.5 Y 0.5 O 12. J. Electrochem. Soc. 2015, 162, A1772–A1777. [Google Scholar] [CrossRef]
- Braun, P.; Uhlmann, C.; Weber, A.; Störmer, H.; Gerthsen, D.; Ivers-Tiffée, E. Separation of the bulk and grain boundary contributions to the total conductivity of solid lithium-ion conducting electrolytes. J. Electroceramics 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Mori, D.; Sugimoto, K.; Matsuda, Y.; Ohmori, K.; Katsumata, T.; Taminato, S.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Synthesis, Structure and Ionic Conductivity of Garnet Like Lithium Ion Conductor Li 6.25+ x Ga 0.25 La 3-x Sr x Zr 2 O 12. J. Electrochem. Soc. 2019, 166, A5168–A5173. [Google Scholar] [CrossRef]
- Fabry, P.; Khireddine, H.; Cretin, M. Ionic Recognition Using Conducting Ceramics. In Hermodynamic Modeling and Materials Data Engineering; Springer: Berlin/Heidelberg, Germany, 1998; pp. 305–312. [Google Scholar] [CrossRef]
- Hubberstey, P.; Sample, T. Pb-17Li-water interactions: A thermodynamic and experimental characterization. J. Nucl. Mater. 1993, 199, 149–158. [Google Scholar] [CrossRef]


| Temperature/°C | Pb-Li/at% Li | γLi (Sn-3.04Li) |
|---|---|---|
| 400 | 3.05 | 8.3 × 10–5 |
| 5.39 | 8.4 × 10–5 | |
| 9.41 | 8.1 × 10–5 | |
| 15.53 | 7.4 × 10–5 | |
| 500 | 3.05 | 2.6 × 10–4 |
| 5.39 | 2.5 × 10–4 | |
| 9.41 | 2.5 × 10–4 | |
| 15.53 | 2.3 × 10–4 |
| Temperature/°C | γ | Standard Deviation |
|---|---|---|
| 400 | 8.1 × 10–5 | 5 × 10–6 |
| 500 | 2.5 × 10–4 | 1 × 10–5 |
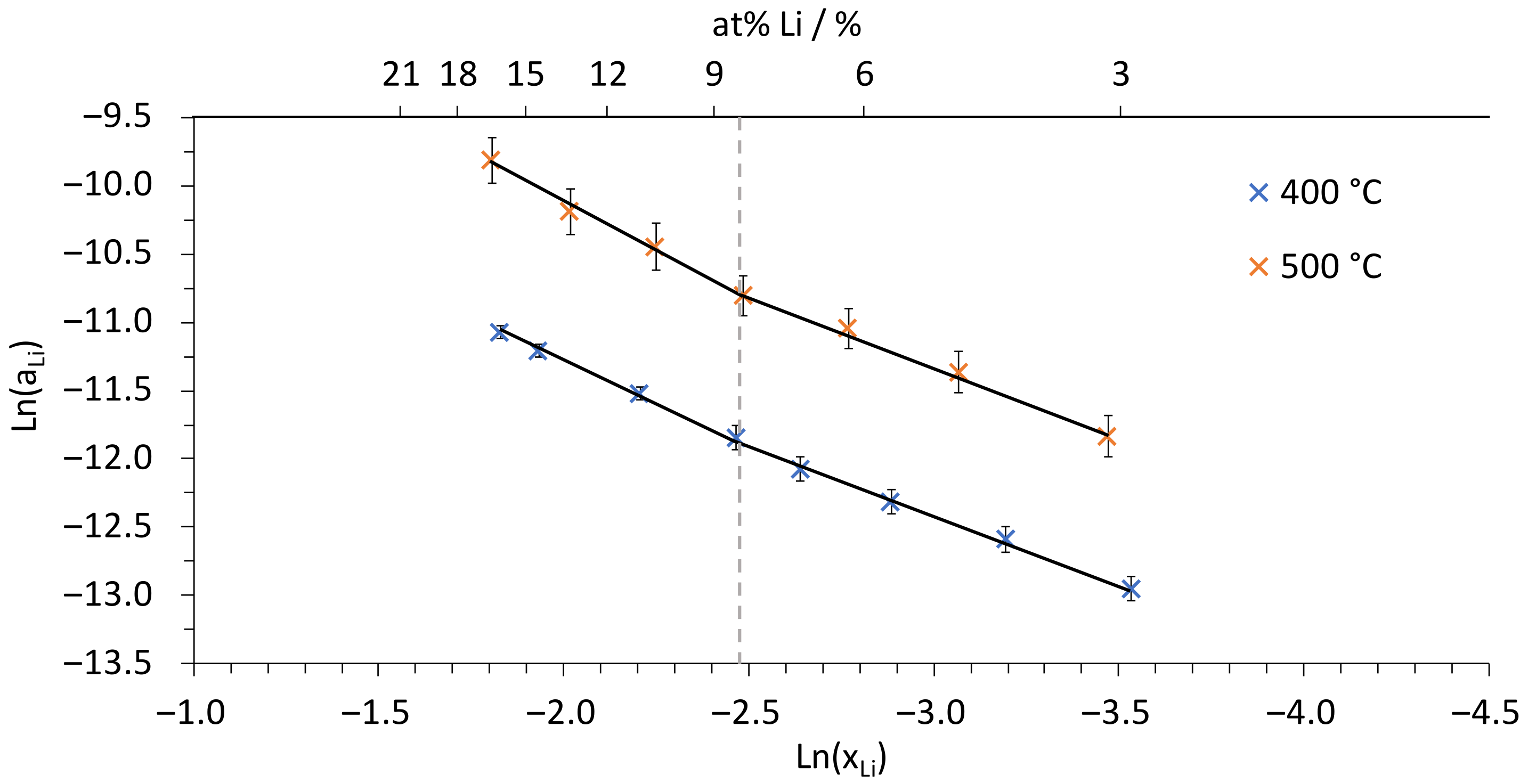
| Temperature/°C | at% Li ≤ 8.5 | at% Li ≥ 8.5 |
|---|---|---|
| 400 | Ln(aLi) = 1.01·ln(xLi) − 9.38 R² = 0.998Uncertainty *: ±7 × 10–7 | Ln(aLi) = 1.20·ln(xLi) − 8.86 R² = 0.999Uncertainty *: ±8 × 10–7 |
| 500 | Ln(aLi) = 1.04·ln(xLi) − 8.20 R² = 0.996Uncertainty *: ±3 × 10–6 | Ln(aLi) = 1.42·ln(xLi) − 7.23 R² = 0.993Uncertainty *: ±1 × 10–5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nel-lo, M.; Lujan, E.; Hinojo, A.; Colominas, S.; Abella, J. Li6BaLa2Ta2O12 Solid-State Probe for Studying Li Activity in Molten Sn-Li Alloys. Chemosensors 2023, 11, 6. https://doi.org/10.3390/chemosensors11010006
Nel-lo M, Lujan E, Hinojo A, Colominas S, Abella J. Li6BaLa2Ta2O12 Solid-State Probe for Studying Li Activity in Molten Sn-Li Alloys. Chemosensors. 2023; 11(1):6. https://doi.org/10.3390/chemosensors11010006
Chicago/Turabian StyleNel-lo, Marc, Enric Lujan, Antonio Hinojo, Sergi Colominas, and Jordi Abella. 2023. "Li6BaLa2Ta2O12 Solid-State Probe for Studying Li Activity in Molten Sn-Li Alloys" Chemosensors 11, no. 1: 6. https://doi.org/10.3390/chemosensors11010006
APA StyleNel-lo, M., Lujan, E., Hinojo, A., Colominas, S., & Abella, J. (2023). Li6BaLa2Ta2O12 Solid-State Probe for Studying Li Activity in Molten Sn-Li Alloys. Chemosensors, 11(1), 6. https://doi.org/10.3390/chemosensors11010006






