Abstract
The Co3O4 hollow dodecahedron (Co3O4@CN) was prepared by calcining ZIF-67 in N2 and then air atmosphere. In the Co3O4@CN compound material, Co3O4 nanoparticles (Co3O4 NPs) are anchored in the carbon skeleton, solving the aggregation problem of Co3O4 NPs. Because Co3O4@CN retains the porous structure of ZIF-67, and the presence of Co2+ and Co3+ favors electron transfer between the enzyme substrates, it has excellent peroxidase-like (POD-like) activity. Enzymatic kinetic tests show that Co3O4@CN has approximately six times more affinity for TMB than HRP and the maximal reaction rate is approximately three times that of HRP. The cholesterol sensor was constructed with Co3O4@CN, and its linear range was 20-1000 μM, and the detection limit was 14.33 μM. The application of Co3O4@CN to the detection of human serum cholesterol will be a valuable research direction.
1. Introduction
The content of cholesterol in the human body accounts for about 0.2% of total weight, mainly in the liver, skin, brain and nerve tissue. It is the precursor of steroid hormones such as vitamin D and cortisol. In the serum of the healthy human body, the total cholesterol content is about 2.86–5.98 mM [1]. Abnormal total cholesterol content may cause coronary heart disease, renal failure, cardiovascular disease and apoplexies [2]. Therefore, the real-time monitoring of cholesterol levels is of great significance to human health. At present, various techniques for detecting cholesterol have been developed, including electrochemical analysis [3,4], colorimetric enzymes [5], chromatography [6], chemiluminescence [7] and fluorescence [8,9], etc. Horseradish peroxidase (HRP) can be used to design a colorimetric method to detect cholesterol. H2O2 is a metabolic by-product of cholesterol. The amount of H2O2 produced is related to the concentration of cholesterol. Horseradish peroxidase can catalyze the in situ generated H2O2 to produce high-active oxygen species (·OH, 1O2, ·O2−, etc.) [10]. These high-active oxygen species can oxidize some organic compounds and make the color change significantly. For example, the colorless 3,3′,5,5′-tetramethylbenzidine dihydrochloride hydrate (TMB) can be oxidized as a blue oxTMB. According to the shade of the color, the content of the H2O2 can be determined, and the content of cholesterol is indirectly determined. However, the natural enzyme HRP has disadvantages such as a complex synthesis process, high cost and easy denaturation, which seriously limit the application of this detection method in the actual situation. Therefore, it is necessary to design and develop an alternative substance to HRP to promote the use of the detection method in the actual situation.
Nanozyme refers to nanomaterials that can catalyze the conversion of the enzyme substrate into corresponding products and follows Michaelis–Menten kinetics [11,12,13,14,15,16]. So far, nanozymes have been widely used in colorimetric sensors because of their advantages such as high stability, easy regulation of activity, easy recycling and low preparation cost [17,18,19,20]. There are many nanomaterials that can simulate the enzyme activity of HRP, including metal (such as Pd nanoparticles [10,21], Au nanoparticles [10,22], Cu nanoparticles [23]), metal oxide (such as Fe3O4 nanoparticles [24], WO3 nanowires [25], porous MnCo2O4 [26]), metal sulfide and metal phosphorus (such as CuS particles [27,28], FeP nanotubes [29]), carbon-based nanomaterials (such as carbon dots [30], graphene [31,32], graphitic carbon nitride [33]), as well as the newly emerged single-atom nanozymes (such as Fe single-atom nanozyme [34], Zn single-atom nanozyme [35]) and so on. Among them, some nanozyme materials have been used to build colorimetric sensors of cholesterol [5,28,36,37]. In 2015, Narsingh et al. built a cholesterol sensor based on the peroxidase-like (POD-like) activity of Au NPs@MoS2 [5]. Subsequently, scientific researchers successively built cholesterol sensors based on the POD-like activity of the CuS@BN nanocomposite [28], MoS2 nanosheets modified by oxidized glutathione (GSSG) [38] and a one-dimensional nuclear shell Fe3O4@C/Ni nanocomposite [36]. These sensors have disadvantages such as a complex preparation process, high cost and high detection limit. Therefore, it is necessary to develop more ideal nanozyme materials to make up for the shortcomings of the existing detection methods.
As we all know, zeolitic imidazolate frameworks (ZIF) belong to metal-organic frameworks (MOF), which have the advantages of a large specific surface area, easy modification, large pores and an adjustable structure. Among the existing ZIF materials, the coordination between metal and organic imidazole esters reaches saturation, which leads to the low catalytic activity of the ZIF materials. In order to improve the catalytic activity of ZIF, researchers have begun to encapsulate an active substance with ZIF [39], or to calcine ZIF at high temperature [23,26,29]. The first strategy requires additional chemicals and increases material costs. Therefore, the high-temperature calcination of ZIF is the most feasible strategy currently. So far, many studies have demonstrated that the high-temperature pyrolysis of ZIF can make the metal active site exposed and improve its enzyme-like activity. Among them, in the process of high-temperature calcination, some metals coordinating with organic ligands in ZIF materials will be converted into metal oxide in situ. After annealing, metal oxide is evenly dispersed in the three-dimensional skeleton. Compared with metal oxides prepared by conventional methods, metal oxides derived from ZIF have the advantages of more active sites and a large specific surface area and can effectively prevent the aggregation of metal oxide nanoparticles, so ZIF-derived metal oxides have higher enzyme-like activity. However, there is still the experimental phenomenon that the skeleton structure of ZIF collapses and the porosity decreases significantly after calcination. Therefore, it is very important to study and improve the experimental conditions of synthesizing and calcining ZIF for improving the phenomenon of the destruction of the ZIF structure.
Here, ZIF-67 was calcined at a high temperature in N2 atmosphere and then air atmosphere to prepare Co3O4 uniformly anchored in a porous dodecahedral carbon skeleton (Co3O4@CN). Compared with Co3O4 NPs prepared by traditional methods, Co3O4@CN has a more excellent POD-like activity. This is because, in the Co3O4@CN composites, Co3O4 NPs are uniformly fixed in the carbon skeleton, which effectively prevents the aggregation of Co3O4 NPs caused by high surface energy. Meanwhile Co3O4@CN retains the porous structure of ZIF-67, which is conducive to the capture of the enzyme substrate. Then, we found that the POD-like activity of Co3O4@CN fits with Michaelis reaction kinetics. Based on the POD-like activity of Co3O4@CN, we constructed H2O2 and cholesterol sensors with detection limits of 67.82 μM and 14.33 μM, respectively. Moreover, the cholesterol sensor has excellent selectivity.
2. Materials and Methods
Reagents. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), 2-methimidazole (C4H6N2), sodium dihydrogen phosphate (NaH2PO4), cholesterol (C27H46O), cholesterol oxidase and glucose (C6H12O6) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Hydrogen peroxide (H2O2, 30 ωt% solution in water), serine (C3H7NO3), threonine (C4H9NO3) and phenol (C6H5OH) were purchased from Aladdin. Methanol (CH3OH) was purchased from Jiangtian Chemical Industry Co., Ltd. Citric acid (C6H8O7) was purchased from Yuanli Chemical Industry Co., Ltd. 3,3′,5,5′-tetramethylbenzidine dihydrochloride hydrate (TMB) and urea (CH4N2O) were purchased from Shanghai Meryer Chemical Technology Co., Ltd. Dimethyl sulfoxide (DMSO) and disodium hydrogen phosphate dihydrate (Na2HPO4·2H2O) were purchased from Kermel Reagent Co., Ltd.
Apparatus. The SEM images were obtained with the SU8000 series scanning electron microscope of Hitachi. The TEM images, HRTEM images, SAED images and elemental mapping were obtained with the JEOL JEM-2100 instrument. The crystalling phase features of the materials were obtained on SmartLab 9KW XRD diffractomete. The XPS curves were obtained with the X-ray Photoelectron Spectrometer (Themor Fisher K-Alpha). The BET test was performed using the ASAP2460 aperture analyzer. All UV visible absorption spectra were recorded with a T6 New Century UV visible spectrophotometer.
Preparation of Co3O4 Hollow Dodecahedron (Co3O4@CN). Totals of 0.546 g of Co(NO3)2·6H2O and 0.616 g of 2-methylimidazole were separately dissolved in 15 mL of methanol. The two solutions were then mixed and sonicated for 15 min. Afterwards, pure ZIF-67 was obtained by washing with methanol 3 times. ZIF-67 was dried in an oven at 60 °C overnight. The ZIF-67 was then calcined at high temperature in the N2 atmosphere. The calcination process was as follows: First, N2 was passed into the tube furnace for 10 min to remove the air. Next, the tube furnace was heated to 350 °C at a heating rate of 2 °C/min and kept for 1 h to obtain porous ZIF-67 (Porous-ZIF-67). After the Porous-ZIF-67 had cooled, the material was calcined in the air atmosphere. The calcination process was as follows: the tube furnace was heated to 300 °C at a heating rate of 2 °C/min and kept for 2 h to obtain a Co3O4 hollow dodecahedron (Co3O4@CN).
Preparation of ZIF-67-Air. Remove the preparation process of calcination in N2; the rest of the preparation process was consistent with the preparation process of Co3O4@CN.
Evaluation of POD-like activity of Co3O4@CN. The exploration process of the enzymatic activity of Co3O4@CN was as follows. Totals of 20 μL of Co3O4@CN (2 mg/mL), 30 μL of TMB (10 mM) and 30 μL of H2O2 (10 M) were sequentially added to buffer solution at pH = 5, and the total volume of the reaction system was 2 mL. The UV-Vis spectral data in the range of 550 to 700 nm were collected by UV-Vis spectrophotometer. This process can be used to analyze the POD-like activity of Co3O4@CN. In addition, the rest of the experimental procedure was the same as above except that H2O2 was not added, which can be used to study the OXD-like activity of Co3O4@CN. Subsequently, Co3O4@CN was replaced by ZIF-67 and Porous-ZIF-67, and the rest of the experimental procedure was consistent with the above procedure to study the POD-like and OXD-like activities of ZIF-67 and Porous-ZIF-67.
Then, the factors affecting the POD-like activity of Co3O4@CN, including pH and temperature, were optimized using the controlled variable method. First, a buffer solution in the pH range of 3 to 9 was prepared with 0.1 M of citric acid and Na2HPO4 solution, and the difference between adjacent pH was 1. At room temperature, 1920 μL of buffer solutions with different pH (3, 4, 5, 6, 7, 8, 9) was taken, and 20 μL of Co3O4@CN (2 mg/mL), 30 μL of TMB (10 mM) and 30 μL of H2O2 (10 M) were added sequentially. Then, the optimal pH obtained was selected and the above experimental steps were followed to optimize the temperature (20 °C, 25 °C, 30 °C, 35 °C, 40 °C, 45 °C, 50 °C, 55 °C, 60 °C). Throughout the optimization process, the absorbance at 652 nm as a function of time was recorded using a UV-Vis spectrophotometer.
Kinetic Analysis of POD-like Co3O4@CN. First, 10 mg/mL of TMB solution was prepared with DMSO, and the rest of the solution was prepared with ultrapure water. A total of 2 mL of buffer solutions of pH = 5 containing different contents (0–20 μg) of Co3O4@CN was prepared. Then, 100 uL of TMB was added. Finally, H2O2 was added so that the final concentration of H2O2 in the mixed solution was 1 M. After adding H2O2, the change of absorbance at 652 nm with time was recorded. Subsequently, without addition of H2O2, the rest of the steps were the same as the above experimental procedure to take the background test.
A total of 10 μg of Co3O4@CN was added to buffer solution of pH = 5. Then, different volumes of TMB (0-50 μL, 10 mg/mL) were added. Finally, H2O2 was added so that the final concentration of H2O2 in the mixed solution was 1 M. The total volume of the mixed solution was 2336 μL during this testing process. The change of absorbance at 652 nm with time was recorded with a UV-visible spectrophotometer.
A total of 10 μg of Co3O4@CN was added to buffer solution of pH = 5. Then, 200 uL of TMB (50 mM) was added. Finally, different volumes of H2O2 (100 mM) were added successively. In this test project, the total volume of the mixed solution was 2000 μL. The change of absorbance at 652 nm with time was recorded.
Visual Detection. Totals of 20 μL of Co3O4@CN (2 mg/mL) and 100 μL of TMB (10 mM) were added to 1860 μL of buffer solution of pH = 5. Then, 20 μL of different concentrations (0.1–10 mM) of H2O2 was added. After incubation for 5 min, the absorption spectrum in the range of 500 to 750 nm was recorded with a UV-Vis spectrophotometer.
A total of 10 mM of PBS buffer solution of pH = 7.4 was prepared. Totals of 60 μL of cholesterol oxidase (1 mg/mL) and 60 uL of cholesterol with different concentrations (0.02–1.0 mM) were added to 240 μL of PBS buffer solution. After incubation for 30 min at 37 °C, 1520 μL of buffer solution of pH = 5, 20 μL of Co3O4@CN (2 mg/mL) and 100 μL of TMB (10 mM) were added in sequence, and the incubation was continued for 30 min at 37 °C. Subsequently, the absorption spectrum in the range of 500 to 750 nm was recorded. A total of 10 mM of glucose, urea, serine, threonine and phenol was prepared with ultrapure water. Cholesterol was replaced with these molecules, and the rest of the experimental procedures were consistent with the above experimental steps to explore the selectivity of the cholesterol sensor.
3. Results and Discussions
Synthesis and structural characterizations of ZIF-67, Porous-ZIF-67 and Co3O4@CN. Figure 1A is the synthesis process of Co3O4@CN. The self-assembly strategy of Co2+ and 2-methylimazole was used under ultrasound conditions to obtain ZIF-67. Then, the Co3O4@CN was obtained by calcining ZIF-67 in N2 and then air atmosphere successively. Then, characterization methods including SEM, TEM, XRD, XPS and BET were used to analyze the structure of Co3O4@CN. Figure 1B and Figure 1C are SEM images of ZIF-67 and Porous-ZIF-67, respectively. ZIF-67 is a rhombic dodecahedron with a good dispersibility and average particle size of 1 μm. Compared with ZIF-67, the appearance and size of the Porous-ZIF-67 has almost no change. Figure 1D is the SEM image of Co3O4@CN. Though Co3O4@CN maintains the appearance of the rhombic dodecahedron, compared to ZIF-67 and Porous-ZIF-67, the appearance of Co3O4@CN has changed greatly. First of all, the surface of the Co3O4@CN rhombic dodecahedron is rough and partial structures collapse regularly. Secondly, the particle size of Co3O4@CN is significantly decreased, with an average particle size of about 700 nm. We infer that the structural collapse and size reduction are mainly due to the decomposition of some organic ligands and surface functional groups during high-temperature calcination. Figure S1 shows that in ZIF-67-Air materials the surface of most of the rhombic dodecahedron is still smooth, and the structure of a small part of the rhombic dodecahedron is completely destroyed. In addition, the average particle size is still 1 μm. These facts show that Co3O4@CN is not obtained by directly calcining ZIF-67 in air atmosphere, and the pretreatment of ZIF-67 in N2 atmosphere is conducive to the successful preparation of Co3O4@CN.
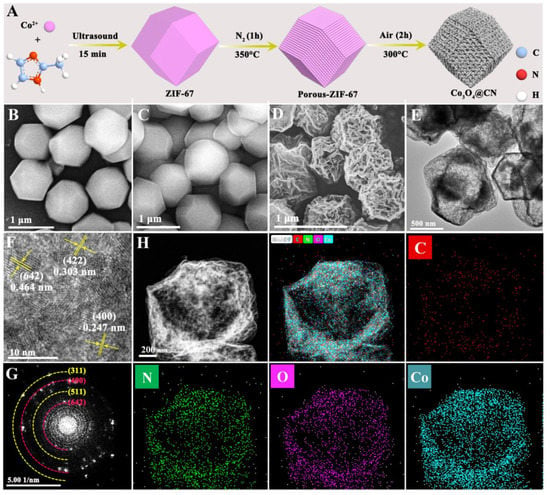
Figure 1.
(A) Synthesis process of Co3O4@CN. SEM images of (B) ZIF-67, (C) Porous-ZIF-67 and (D) Co3O4@CN. (E) TEM image, (F) HRTEM image and (G) SAED image of Co3O4@CN. (H) EDS mapping of Co3O4@CN.
The TEM image (Figure 1E) shows that Co3O4@CN has the appearance of a hollow rhombic dodecahedron, which is caused by the collapse of some of the skeleton during high-temperature calcination. Very obvious lattice stripes can be seen in the HRTEM image of Co3O4@CN (Figure 1F). The lattice spacing is 0.247 nm, 0.464 nm and 0.303 nm, corresponding to the (400), (642) and (422) crystal planes of Co3O4, respectively. From the SAED image (Figure 1G), it can be seen that a large number of diffraction bright spots are arranged into continuous concentric circles, which proves that Co3O4@CN has a polycrystalline structure. In addition, the data analysis shows that the lattice spacing corresponding to the diffraction ring is 0.461 nm, 0.241 nm, 0.321 nm and 0.201 nm, corresponding to (642), (400), (511) and (311) crystal planes of Co3O4, respectively. Figure 1H is the element mapping diagram of Co3O4@CN. The C, N, O and Co elements are evenly distributed in the material, which is beneficial to the Co3O4@CN POD-like activity.
The crystal structure of the intermediate products and Co3O4@CN was analyzed with XRD characterization. In Figure 2A, both ZIF-67 and Porous-ZIF-67 have diffraction peaks of crystal planes such as (011), (002), (112), (022), (013), (222) and (114), which is consistent with the diffraction peak of the XRD of the ZIF-67 crystal that has been reported [40]. In addition, compared with ZIF-67, the diffraction peak of Porous-ZIF-67 is significantly enhanced, indicating that the crystal structure of ZIF-67 is optimized when calcination occurs in the N2 atmosphere. This fact proves that the calcination in N2 can optimize the internal structure of ZIF-67, which can prevent the dodecahedron shape from being damaged when calcination occurs in air and is conducive to the successful preparation of Co3O4@CN. In the XRD spectrum of Co3O4@CN (Figure 2B), the diffraction peaks appear at 36.75°, 55.89°, 59.11° and 65.21°, and the standard card data of the JCPDS: 43-1003 (2θ is 36.845°, 55.655°, 59.353° and 65.231°) match, corresponding to the (311), (422), (511) and (440) crystal planes of Co3O4. The XRD characterization proves after two calcinations the ZIF-67 has successfully transformed into a hollow Co3O4 dodecahedron.
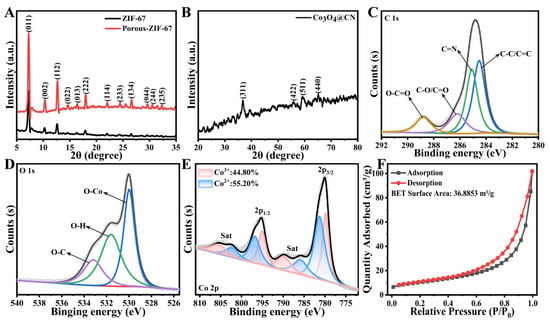
Figure 2.
The XRD spectra of (A) ZIF-67 and Porous-ZIF-67 and (B) Co3O4@CN. High-resolution XPS spectra of (C) C 1s, (D) O1s and (E) Co of Co3O4@CN. (F) The N2 adsorption-desorption isotherm line of Co3O4@CN.
The chemical composition of Co3O4@CN was analyzed with XPS characterization. In the XPS total spectrum of Co3O4@CN (Figure S2), characteristic peaks of C, Co and O elements appeared. There are four peaks in the XPS spectrum of C 1s, corresponding to C-C/C=C (284.5 eV), C=N (285.1 eV), C-O/C=O (286.2 eV) and O-C=O (288.8 eV), respectively (Figure 2C), where C-C/C=C is from the ZIF-67 precursor [41]. The XPS spectrum of the O 1s shows the three types of oxygen species (Figure 2D). One is the oxygen species corresponding to the Co3O4 phase (530.0 eV). The other one indicates that OH exists on the surface of the Co3O4@CN (531.6 eV). Furthermore, the peak at 533.2 eV corresponds to C-O, indicating that the oxygen-containing group binding with C atoms remains in Co3O4@CN [42]. Figure 2E is the high-resolution XPS spectrum of Co 2p in Co3O4@CN. The peaks that appear at 780.10 eV and 795.22 eV correspond to the characteristic peaks of Co 2p3/2 and CO 2p1/2, respectively. Moreover, both peaks can be fitted into the corresponding characteristic peaks of Co3+ and Co2+, indicating there are two valence states of Co elements in Co3O4@CN at the same time [43,44]. In addition, the characteristic satellite peak (sat) of Co3O4 appears in the XPS spectrum of Co 2p. Many studies have shown that the binding energy difference between the satellite peak and 2p orbital peak is very important to determine the valence of the Co element. The satellite peak appears at 787.51 eV, which is 7.41 eV higher than the binding energy of the Co 2p3/2 orbital peak, proving the existence of Co2+. Moreover, another satellite peak appears at 804.31 eV, which is 9.09 eV higher than the binding energy of the Co 2p1/2 orbital peak, proving the existence of Co3+. In summary, the XPS analysis shows that there are Co2+ and Co3+ in Co3O4@CN at the same time. Moreover, the content of Co3+ and Co2+ is 44.80% and 55.20%, respectively. The existence of variable valence metals is conducive to the electron transfer between enzyme substrates. Subsequently, N2 adsorption-desorption characterization was carried out (Figure 2F). The BET surface area of the Co3O4@CN is 36.8853 m2/g, demonstrating that Co3O4@CN inherits part of the pore structure of ZIF-67, which is conducive to the capture of the enzyme substrate.
POD-like activity of Co3O4@CN. First, we evaluated the enzyme-like activity of Co3O4@CN and its intermediate products using TMB and H2O2 as enzyme substrates. From Figure 3A and Figure S3, it can be seen that ZIF-67 and Porous-ZIF-67 cannot catalyze the oxidation of single TMB or the reaction between TMB and H2O2, indicating that the two intermediates do not have OXD-like and POD-like activities. Compared with the control group, Co3O4@CN has a weak catalytic effect on the oxidation of single TMB, but it has a very significant catalytic effect on the reaction of TMB and H2O2, which shows that Co3O4@CN has weak OXD-like activity and excellent POD-like activity. These facts prove that Co3O4 is the main active site, and a large number of pore structures appear after two high-temperature calcinations, which is conducive to the capture of enzyme substrate. In addition, as can be seen from Figure 3B, the absorbance at 652 nm increased significantly after the addition of Co3O4@CN, which further proved that Co3O4@CN has excellent POD-like activity.
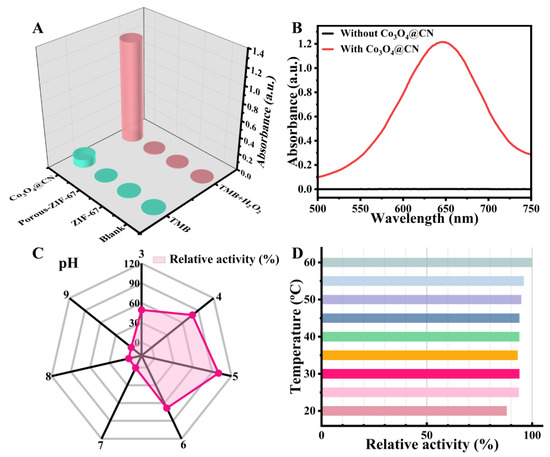
Figure 3.
(A) Absorbance at 652 nm for the different reaction systems. (B) The UV-visible absorption spectra in the range of 500 to 750 nm before and after the addition of Co3O4@CN. (C) Effect of pH on the POD-like activity of Co3O4@CN. (D) Effect of temperature on the POD-like activity of Co3O4@CN.
It is well known that the enzyme-like activity of nanomaterials is related to many factors, including pH and temperature. Here, the control variable method was used to explore the effects of pH and temperature on the POD-like activity of Co3O4@CN. As shown in Figure 3C and Figure S4A, under room temperature conditions, when pH is 5, Co3O4@CN has the highest POD-like activity. Figure 3D and Figure S4B show that when the temperature changes from 20 °C to 60 °C, the relative activity of Co3O4@CN has hardly changed, indicating that the thermal stability of Co3O4@CN is good. Therefore, the optimal conditions for the POD-like activity of Co3O4@CN are as follows: pH, 5; temperature: room temperature.
Kinetic analysis of POD-like activity of Co3O4@CN. To explore the detailed catalytic mechanism of Co3O4@CN, the enzymatic kinetic testing of Co3O4@CN was performed. First, the absorbance of the reaction system as a function of time was measured after the addition of the different contents of Co3O4@CN (Figure S5A). The data analysis results show that the specific activity of the material is 1.781 U mg-1 (Figure S5B). Subsequently, a steady-state kinetic test was conducted on the POD-like activity of Co3O4@CN by changing the concentration of a substrate (TMB or H2O2) while retaining the concentration of another substrate (H2O2 or TMB) unchanged. Figure S5C and Figure S5D describe the relationship between the initial reaction velocity and the concentration of TMB and H2O2, respectively. It can be seen that both curves accord with the Michaelis–Menten curve. The maximum reaction velocity (Vmax) and Michaelis constant (Km) were determined by the Lineweaver−Burk equation. Generally speaking, the Km value can be used to evaluate the affinity of the catalytic material to the enzyme substrate. The smaller the Km value, the higher the affinity of this catalytic material for the enzyme substrate. The data analysis shows that the Km value of the material to TMB is 0.06877 mM and the Vmax is 14.4207 μM min-1, indicating that the affinity of the material for TMB is at least 6 times that of HRP, and the maximum reaction rate is about 3 times that of HRP. Moreover, compared with other peroxidase mimics, the affinity of Co3O4@CN for TMB still has great advantages (Table S1). When H2O2 was used as the substrate, Co3O4@CN had a significantly higher affinity than the single Co3O4 NPs and other peroxidase mimics. These experimental results show that Co3O4@CN has excellent POD-like activity.
Mechanism analysis of POD-like activity of Co3O4@CN. To explore the mechanism by which the Co3O4@CN has excellent POD-like activity, we performed an electron spin resonance test (ESR). In Figure 4A, there is an obvious four-line peak signal with the intensity of 1:2:2:1, which corresponds to the adduct DMPO-·OH generated by the free radical addition reaction of ·OH attacking the C=N+ double bond of DMPO. In addition, there is a six-line peak signal with equal height, which corresponds to the adduct DMPO-·R generated by the free radical addition reaction of ·R attacking the C=N+ double bond of DMPO. There is no obvious signal peak in Figure S6, indicating that DMPO-·O2− is not generated. In summary, Co3O4@CN can catalyze H2O2 to generate OH.
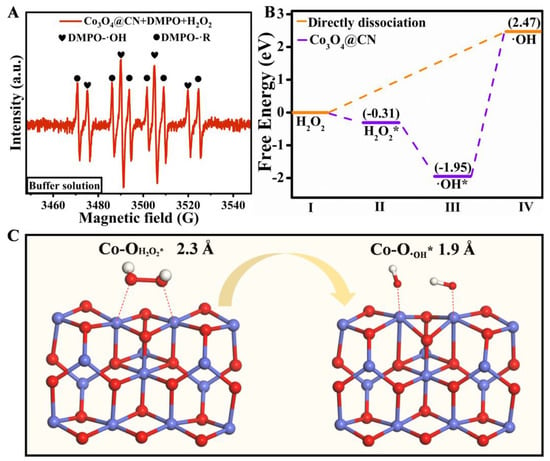
Figure 4.
(A)The ESR signal of Co3O4@CN in the buffer solution. (B)The energy spectrum of the optimal pathway producing ·OH. The (C)DFT calculation of POD-like Activity of Co3O4@CN.
To find out why Co3O4@CN has excellent POD-like activity, we constructed a catalyst model of Co3O4 (311) for the DFT calculations. Figure 4B shows the energy barrier of the direct decomposition of H2O2 to ·OH is 2.47 eV. When there is Co3O4@CN, the adsorption energy of H2O2 is −0.31 eV. Subsequently, H2O2* generates two ·OH* releasing an energy of 1.64 eV. Then, we investigated the effect of Co3O4 (311) on the formation of ·OH from H2O2 at the atomic level (Figure 4C). The Co atoms have an interaction with the O atoms of H2O2, and the distance between Co-OH2O2* is 2.3 Å, making the O-OH2O2* bond easily break to produce ·OH*. The distance between Co-O·OH* is 1.9 Å, and Co atoms can stabilize the generated ·OH*. Therefore, the existence of Co3O4@CN gives the process of H2O2 generating·OH great energy advantages and chemical environment advantages.
Sensors of H2O2 and cholesterol. H2O2 in organisms is involved in many physiological processes, such as energy metabolism, differentiation, proliferation, anti-apoptosis or promoting apoptosis. Here, a simple, rapid and sensitive H2O2 sensor was established based on the POD-like activity of Co3O4@CN. Figure 5B shows that as the H2O2 concentration increases, the absorbance at 652 nm has gradually increased. In addition, from Figure 5C it can be seen that there is a good linear relationship between the absorbance of 652 nm and the concentration of H2O2, and the linear range is 0.1–10 mM (R2 = 0.99576). According to CL = 3δ/slope, the detection limit of the sensor is 67.82 μM.
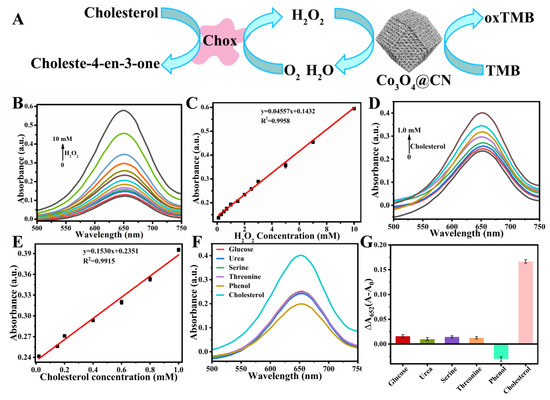
Figure 5.
(A) Schematic diagram of Co3O4@CN-based cholesterol sensor. (B) The UV-visible absorption spectra of the reaction system after the addition of different concentrations of H2O2. (C) Linear relationship between absorbance at 652 nm of reaction system and H2O2 concentration. (D) The UV-visible absorption spectra of the reaction system after the addition of different concentrations of cholesterol. (E) Linear relationship between the absorbance at 652 nm of reaction system and the cholesterol concentration. Selective analysis of the cholesterol sensor. (F) The UV-visible absorption spectra of the reaction system after the addition of the interfering molecules. (G) The variation (ΔA) of absorbance at 652 nm in the reaction system after the addition of the different interfering molecules. A0 and A indicate the absorbance of the reaction system at 652 nm before and after the addition of the interfering molecules, respectively.
Cholesterol is of great significance for human health. It is one of the essential components of the human cell membrane and human hormones. In addition, cholesterol also involves the metabolic process of protein and sugar in the human body. Therefore, the monitoring of cholesterol levels in real time is very important. Based on the excellent POD-like activity of Co3O4@CN, a highly efficient and simple cholesterol sensor was built by establishing a cascade reaction (Figure 5A). Firstly, in PBS buffer solution of pH = 7.4, cholesterol oxidase (Chox) catalyzes the oxidation of cholesterol to produce H2O2. Then, Co3O4@CN catalyzes the oxidation of TMB with the H2O2 generated in situ. As shown in Figure 5D, as the cholesterol concentration increases, the absorbance at 652 nm has gradually increased. Figure 5E indicates that there is a good linear relationship between the absorbance of the 652 nm and the cholesterol concentration, and the linear range is 20–1000 μM (R2=0.9915). According to CL = 3δ/slope, the detection limit of the sensor is 14.33 μM, which is far lower than the normal range of the total cholesterol content in the human serum (2.86–5.98 mM). In addition, in order to verify the feasibility of the sensor in actual serum samples, we selected the interfering molecules in serum samples that may have an impact on the detection of cholesterol and conducted a selective test of the sensor. Cholesterol and phenol have similar functional groups, and the contents of glucose, urea, serine and threonine are high in blood. Figure 5F shows that the absorbance of the reaction system at 652 nm after adding interference molecules is significantly lower than the absorbance after adding cholesterol. It can be seen from Figure 5G that the variation (ΔA) of absorbance of the reaction system at 652 nm after adding cholesterol is much higher than the variation (ΔA) after adding interfering molecules, indicating that the sensor has good specificity for the detection of cholesterol. In summary, the sensor can be applied to the detection of cholesterol content in an actual sample.
4. Conclusions
A Co3O4 hollow dodecahedron was obtained by successively calcining the ZIF-67 in N2 and then air atmosphere. The crystal structure of ZIF-67 can be optimized by pretreatment with N2 atmosphere, which is good for the preparation of Co3O4@CN. In Co3O4@CN materials, Co2+ and Co3+ exist simultaneously and the existence of variable valence metals is conducive to the electron transfer between the enzyme substrates. Compared with the single Co3O4 NPs, Co3O4 NPs in Co3O4@CN are anchored on the carbon skeleton, which prevents the aggregation of Co3O4 NPs caused by high surface energy. Meanwhile, Co3O4@CN retains the mesoporous structure of ZIF-67, which is conducive to capturing the enzyme substrate. Furthermore, the POD-like activity of Co3O4@CN fits with the Michaelis reaction kinetics. Its affinity for TMB is at least 6 times that of HRP, and the maximum reaction rate is about 3 times that of HRP. Based on the POD-like activity of Co3O4@CN, H2O2 and cholesterol sensors were constructed with detection limits of 67.82 μM and 14.33 μM, respectively. Moreover, the cholesterol sensor has excellent selectivity. This is of great significance for the real-time monitoring of the cholesterol content in human serum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11010027/s1, Table S1: Comparing Michaelis constant (Km) and maximum reaction velocity (Vmax) for different peroxidase mimics; Figure S1: SEM images of ZIF-67-Air; Figure S2: XPS total spectra of Co3O4@CN; Figure S3: UV-visible absorption spectra in the range of 500 to 750 nm for the different reaction systems; Figure S4: The absorbance at 652 nm as a function of time at (A) different pH; and (B) different temperature; Figure S5: (A) The absorbance at 652 nm as a function of time after the addition of 0–20 μg of Co3O4@CN. (B) The specifific activity of Co3O4@CN. Steady-state kinetic curve of Co3O4@CN. (C) When the concentration of H2O2 is 1 M, the relationship between the initial reaction velocity and TMB concentration. (D) When the concentration of TMB is 5 mM, the relationship between the initial reaction velocity and H2O2 concentration; Figure S6: The ESR signal of the Co3O4@CN in the methanol.
Author Contributions
M.L. and L.X.: conceptualization, methodology, validation, data curation, soft-ware, investigation and writing original draft; Q.F. and Y.W.: methodology and validation; J.L.: DFT calculation and mechanism proposal; S.Z.: conceptualization, resources, supervision, writing review and editing, project administration and funding acquisition; W.H.: supervision, project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Youth Project of the National Natural Science Foundation of China, grant number 22104109.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Youth Project of the National Natural Science Foundation of China (Grant No. 22104109). We also thank the Haihe Laboratory of Sustainable Chemical Transformations for financial support. Many thanks to Associate Professor Jia Liu for his help in DFT calculation and mechanism proposal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Priyadarshini, E.; Rawat, K. Au@carbon Dot Nanoconjugates as a Dual Mode Enzyme-Free Sensing Platform for Cholesterol. J. Mater. Chem. B 2017, 5, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiong, C.; Xiao, Z.; Ling, L. Colorimetric Detection of Cholesterol with G-Quadruplex-Based DNAzymes and ABTS2−. Anal. Chim. Acta 2012, 724, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xu, H.; Zhang, Z.; et al. AuPt/MOF–Graphene: A Synergistic Catalyst with Surprisingly High Peroxidase-Like Activity and Its Application for H2O2 Detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Han, X.; Lu, Z.; Liu, W.; Zhu, D.; Chao, J.; Fan, C.; Wang, L.; Song, S.; Weng, L.; et al. Facile Synthesis of a MoS2 –Prussian Blue Nanocube Nanohybrid-Based Electrochemical Sensing Platform for Hydrogen Peroxide and Carcinoembryonic Antigen Detection. ACS Appl. Mater. Interfaces 2017, 9, 12773–12781. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Pandey, S.; Bansal, A.; Singh, V.K.; Mukherjee, B.; Saxena, P.S.; Srivastava, A. Different Shades of Cholesterol: Gold Nanoparticles Supported on MoS2 Nanoribbons for Enhanced Colorimetric Sensing of Free Cholesterol. Biosens. Bioelectron. 2015, 74, 207–213. [Google Scholar] [CrossRef]
- Grün, C.H.; Besseau, S. Normal-Phase Liquid Chromatography–Atmospheric-Pressure Photoionization–Mass Spectrometry Analysis of Cholesterol and Phytosterol Oxidation Products. J. Chromatogr. A 2016, 1439, 74–81. [Google Scholar] [CrossRef]
- Yang, D.-P.; Guo, W.; Cai, Z.; Chen, Y.; He, X.; Huang, C.; Zhuang, J.; Jia, N. Highly Sensitive Electrochemiluminescence Biosensor for Cholesterol Detection Based on AgNPs-BSA-MnO2 Nanosheets with Superior Biocompatibility and Synergistic Catalytic Activity. Sens. Actuators B Chem. 2018, 260, 642–649. [Google Scholar] [CrossRef]
- Lu, N.; Wen, Y.; Liu, G.; Ding, L.; Zeng, C.; Aldalbahi, A.; Khan, M.N.; Periyasami, G.; Rahaman, M.; Alrohaili, A.; et al. Multifunctional Yolk–Shell Nanostructure as a Superquencher for Fluorescent Analysis of Potassium Ion Using Guanine-Rich Oligonucleotides. ACS Appl. Mater. Interfaces 2017, 9, 30406–30413. [Google Scholar] [CrossRef]
- Han, T.; Zhu, S.; Wang, S.; Wang, B.; Zhang, X.; Wang, G. Fluorometric Methods for Determination of H2O2, Glucose and Cholesterol by Using MnO2 Nanosheets Modified with 5-Carboxyfluorescein. Microchim Acta 2019, 186, 269. [Google Scholar] [CrossRef]
- Adeniyi, O.; Sicwetsha, S.; Mashazi, P. Nanomagnet-Silica Nanoparticles Decorated with Au@Pd for Enhanced Peroxidase-Like Activity and Colorimetric Glucose Sensing. ACS Appl. Mater. Interfaces 2020, 12, 1973–1987. [Google Scholar] [CrossRef]
- Maynard, A.D. Don’t Define Nanomaterials. Nature 2011, 475, 31. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yan, X.; Fan, K. Structure and Activity of Nanozymes: Inspirations for de Novo Design of Nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes Inspired by Natural Enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A Clear Definition with Fuzzy Edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, T.; Wu, X.; Yang, G. Oxygen Vacancy-Engineered PEGylated MoO3−x Nanoparticles with Superior Sulfite Oxidase Mimetic Activity for Vitamin B1 Detection. Small 2019, 15, 1903153. [Google Scholar] [CrossRef]
- Cheng, N.; Li, J.; Liu, D.; Lin, Y.; Du, D. Single-Atom Nanozyme Based on Nanoengineered Fe–N–C Catalyst with Superior Peroxidase-Like Activity for Ultrasensitive Bioassays. Small 2019, 15, 1901485. [Google Scholar] [CrossRef]
- Darabdhara, G.; Sharma, B.; Das, M.R.; Boukherroub, R.; Szunerits, S. Cu-Ag Bimetallic Nanoparticles on Reduced Graphene Oxide Nanosheets as Peroxidase Mimic for Glucose and Ascorbic Acid Detection. Sens. Actuators B Chem. 2017, 238, 842–851. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Zhang, X.; Shang, D.; Xue, Z.; Shan, D.; Lu, X. Ultratrace Naked-Eye Colorimetric Detection of Hg2+ in Wastewater and Serum Utilizing Mercury-Stimulated Peroxidase Mimetic Activity of Reduced Graphene Oxide-PEI-Pd Nanohybrids. Anal. Chem. 2017, 89, 3538–3544. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Q.; Song, H.; Zhao, X.; Yi, T.; Chen, H.; Chen, X. In Situ Synthesis of Self-Assembled Three-Dimensional Graphene–Magnetic Palladium Nanohybrids with Dual-Enzyme Activity through One-Pot Strategy and Its Application in Glucose Probe. ACS Appl. Mater. Interfaces 2015, 7, 3480–3491. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Takemeura, K.; Li, T.-C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Size-Controlled Preparation of Peroxidase-like Graphene-Gold Nanoparticle Hybrids for the Visible Detection of Norovirus-like Particles. Biosens. Bioelectron. 2017, 87, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, C.; Gao, L.; Li, Q.; Song, Y.; Xu, F.; Wang, T.; Wang, L. Metal-Organic Framework-Derived Copper Nanoparticle@Carbon Nanocomposites as Peroxidase Mimics for Colorimetric Sensing of Ascorbic Acid. Chem. Eur. J. 2014, 20, 16377–16383. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, M.; Cai, B.; Wang, W.; Ye, Z.; Huang, J. 3D Graphene Network@WO3 Nanowire Composites: A Multifunctional Colorimetric and Electrochemical Biosensing Platform. Chem. Commun. 2014, 50, 11135–11138. [Google Scholar] [CrossRef]
- Vetr, F.; Moradi-Shoeili, Z.; Özkar, S. Mesoporous MnCo2O4 with Efficient Peroxidase Mimetic Activity for Detection of H2O2. Inorg. Chem. Commun. 2018, 98, 184–191. [Google Scholar] [CrossRef]
- Xiong, Y.; Su, L.; Yang, H.; Zhang, P.; Ye, F. Fabrication of Copper Sulfide Using a Cu-Based Metal Organic Framework for the Colorimetric Determination and the Efficient Removal of Hg2+ in Aqueous Solutions. New J. Chem. 2015, 39, 9221–9227. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.-N.; Sun, X.-T.; Chen, L.; Xu, Z.-R. Boron Nitride Nanosheet/CuS Nanocomposites as Mimetic Peroxidase for Sensitive Colorimetric Detection of Cholesterol. Sens. Actuators B Chem. 2017, 246, 118–126. [Google Scholar] [CrossRef]
- Yang, W.; Hao, J.; Zhang, Z.; Zhang, B. Metal–Organic Frameworks-Derived Synthesis of Porous FeP Nanocubes: An Effective Peroxidase Mimetic. J. Colloid Interface Sci. 2015, 460, 55–60. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Ahn, H.T.; Chung, M.; Le, X.A.; Saini, D.; Bhati, A.; Sonkar, S.K.; Kim, M.I.; Kim, T. N, S, and P-Co-Doped Carbon Quantum Dots: Intrinsic Peroxidase Activity in a Wide PH Range and Its Antibacterial Applications. ACS Biomater. Sci. Eng. 2020, 6, 5527–5537. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic Peroxidase-like Catalytic Activity of Nitrogen-Doped Graphene Quantum Dots and Their Application in the Colorimetric Detection of H2O2 and Glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 7176–7180. [Google Scholar] [CrossRef]
- Mu, J.; Li, J.; Zhao, X.; Yang, E.-C.; Zhao, X.-J. Cobalt-Doped Graphitic Carbon Nitride with Enhanced Peroxidase-like Activity for Wastewater Treatment. RSC Adv. 2016, 6, 35568–35576. [Google Scholar] [CrossRef]
- Jiao, L.; Wu, J.; Zhong, H.; Zhang, Y.; Xu, W.; Wu, Y.; Chen, Y.; Yan, H.; Zhang, Q.; Gu, W.; et al. Densely Isolated FeN4 Sites for Peroxidase Mimicking. ACS Catal. 2020, 10, 6422–6429. [Google Scholar] [CrossRef]
- Ma, C.; Xu, Y.; Wu, L.; Wang, Q.; Zheng, J.; Ren, G.; Wang, X.; Gao, X.; Zhou, M.; Wang, M.; et al. Guided Synthesis of a Mo/Zn Dual Single-Atom Nanozyme with Synergistic Effect and Peroxidase-like Activity. Angew. Chem. Int. Ed. 2022, 61. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Zeng, C.; Zhou, C.; Li, Q.; Lu, N.; Wang, L. One-Dimensional Synergistic Core–Shell Nanozymes with Superior Peroxidase-like Activity for Ultrasensitive Colorimetric Detection of Blood Cholesterol. ACS Appl. Bio Mater. 2020, 3, 5111–5119. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, Z.; Liu, G.; Lu, H.; Gao, Y.; Liu, F.; Wang, C.; Cui, J.; Lu, G. High-Activity Mo, S Co-Doped Carbon Quantum Dot Nanozyme-Based Cascade Colorimetric Biosensor for Sensitive Detection of Cholesterol. J. Mater. Chem. B 2019, 7, 7042–7051. [Google Scholar] [CrossRef]
- Ma, D.; Yu, J.; Yin, W.; Zhang, X.; Mei, L.; Zu, Y.; An, L.; Gu, Z. Synthesis of Surface-Modification-Oriented Nanosized Molybdenum Disulfide with High Peroxidase-Like Catalytic Activity for H2O2 and Cholesterol Detection. Chem. Eur. J. 2018, 24, 15868–15878. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; He, J.; Guo, W.; Zhou, Z.; Zhang, X.; Nie, S.; Wei, H. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal. Chem. 2016, 88, 5489–5497. [Google Scholar] [CrossRef]
- Guan, W.; Gao, X.; Ji, G.; Xing, Y.; Du, C.; Liu, Z. Fabrication of a Magnetic Nanocomposite Photocatalysts Fe3O4@ZIF-67 for Degradation of Dyes in Water under Visible Light Irradiation. J. Solid State Chem. 2017, 255, 150–156. [Google Scholar] [CrossRef]
- Sun, B.; Yang, S.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H.; Song, X. Fabrication of Molybdenum and Tungsten Oxide, Sulfide, Phosphide (MoxW1-XO2/MoxW1-XS2/MoxW1-XP) Porous Hollow Nano-Octahedrons from Metal-Organic Frameworks Templates as Efficient Hydrogen Evolution Reaction Electrocatalysts. J. Colloid Interface Sci. 2019, 547, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Shao, D.; Wang, W.; Zhang, D.; Li, C. Strongly Coupled Molybdenum Phosphide@phosphorus-Doped Porous Carbon Derived from MOF Used in N2 Electroreduction under Ambient Conditions. Microporous Mesoporous Mater. 2021, 313, 110852. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, X.; Chen, Q.; Li, S.; Cao, H.; Huang, Y. Co3O4/CuO Hollow Nanocage Hybrids with High Oxidase-like Activity for Biosensing of Dopamine. Mater. Sci. Eng. C 2019, 94, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Ding, J.; Xu, Y.; Yang, J.; Xu, Q.; Pang, H. Π-Conjugated Molecule Boosts Metal–Organic Frameworks as Efficient Oxygen Evolution Reaction Catalysts. Small 2018, 14, 1803576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).