Multiplexed Detection of Human Papillomavirus Based on AzaBODIPY-Doped Silica-Coated Polystyrene Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
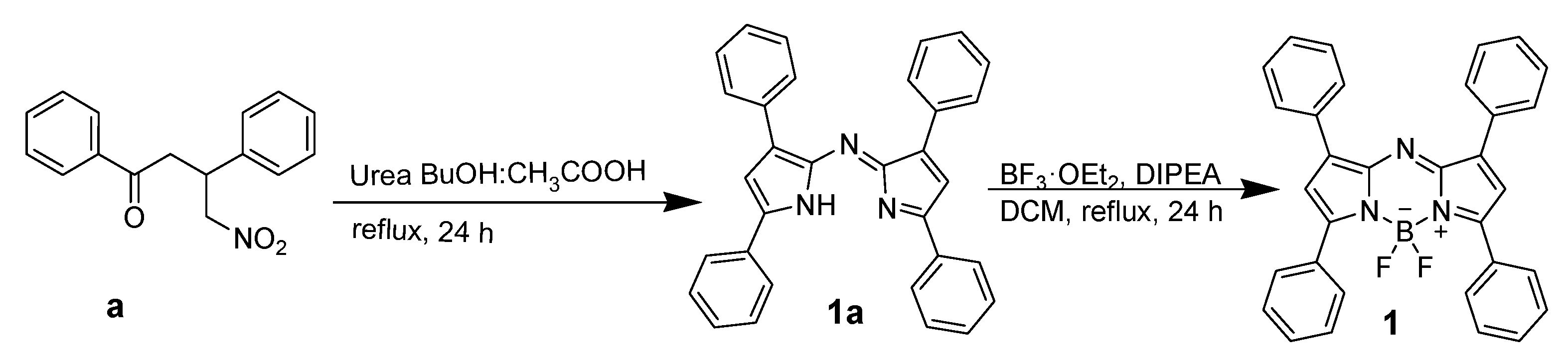
2.3. Synthesis of AzaBODIPY 1
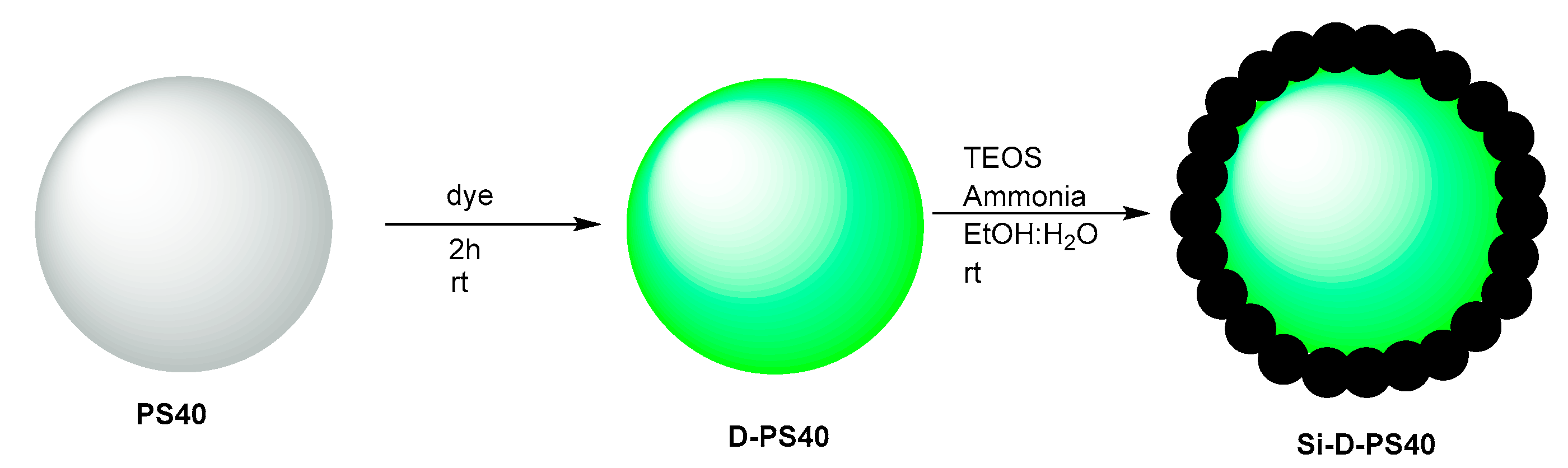
2.4. Preparation of PS40 Particles
2.5. Doping of PS40 Particles
2.6. Coating of PS40 with Silica Shell
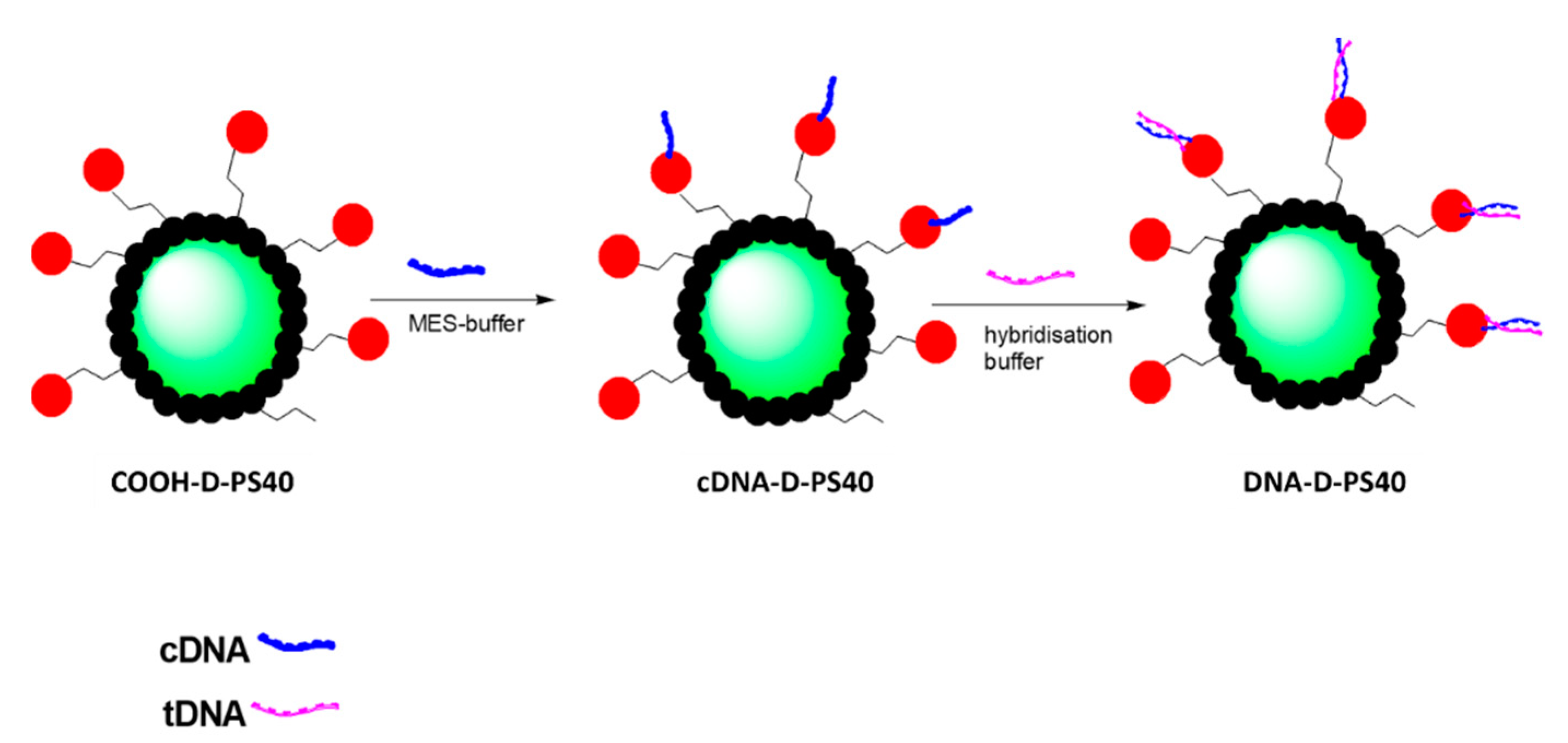
2.7. Functionalization with APTES and COOH Groups
2.8. Coupling with c-HPV and Hybridization Assay
2.9. Ninhydrin Test
3. Results and Discussion
3.1. Synthesis and Characterization

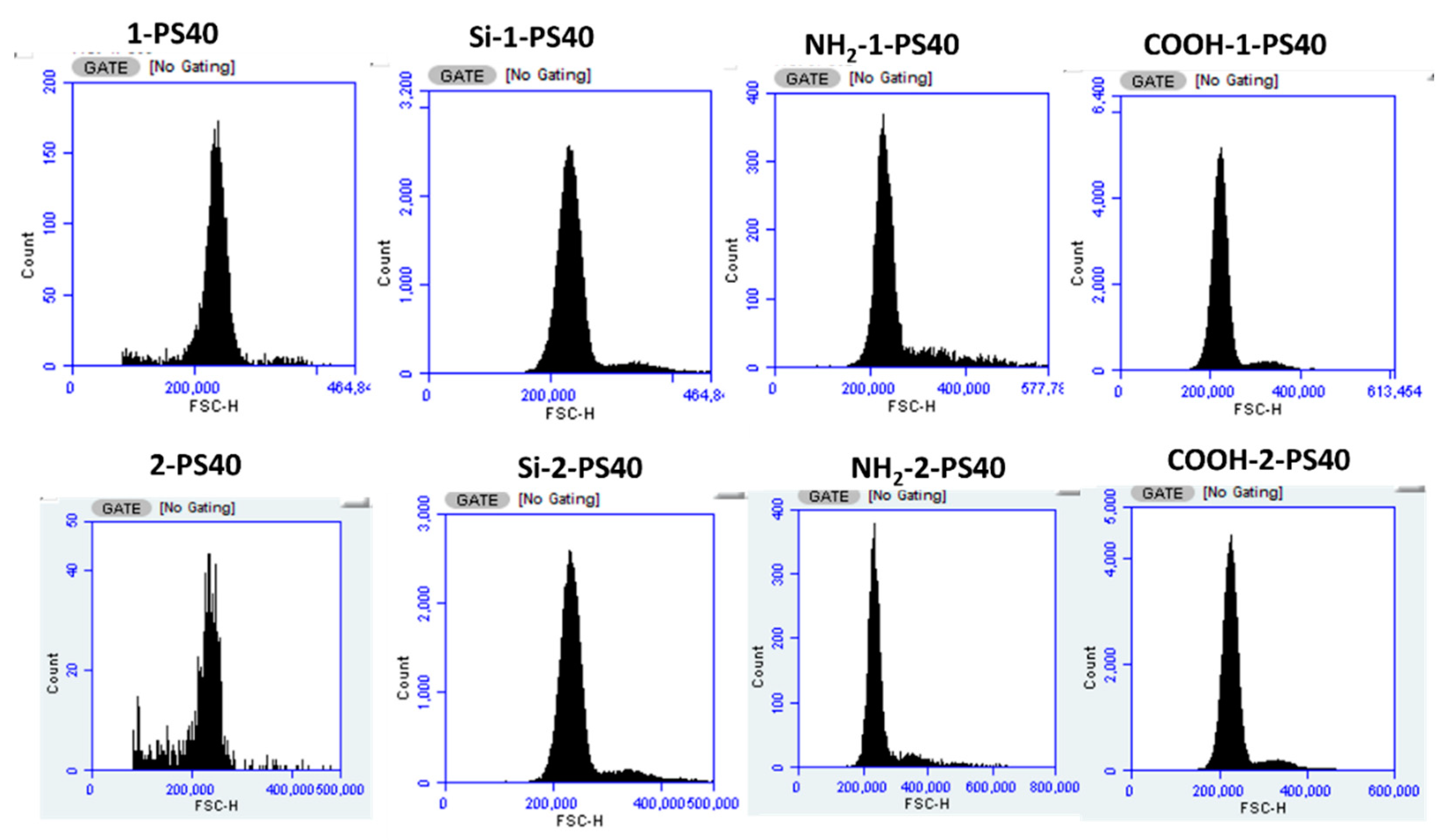
3.2. Characterization of the Particles in Flow Cytometry
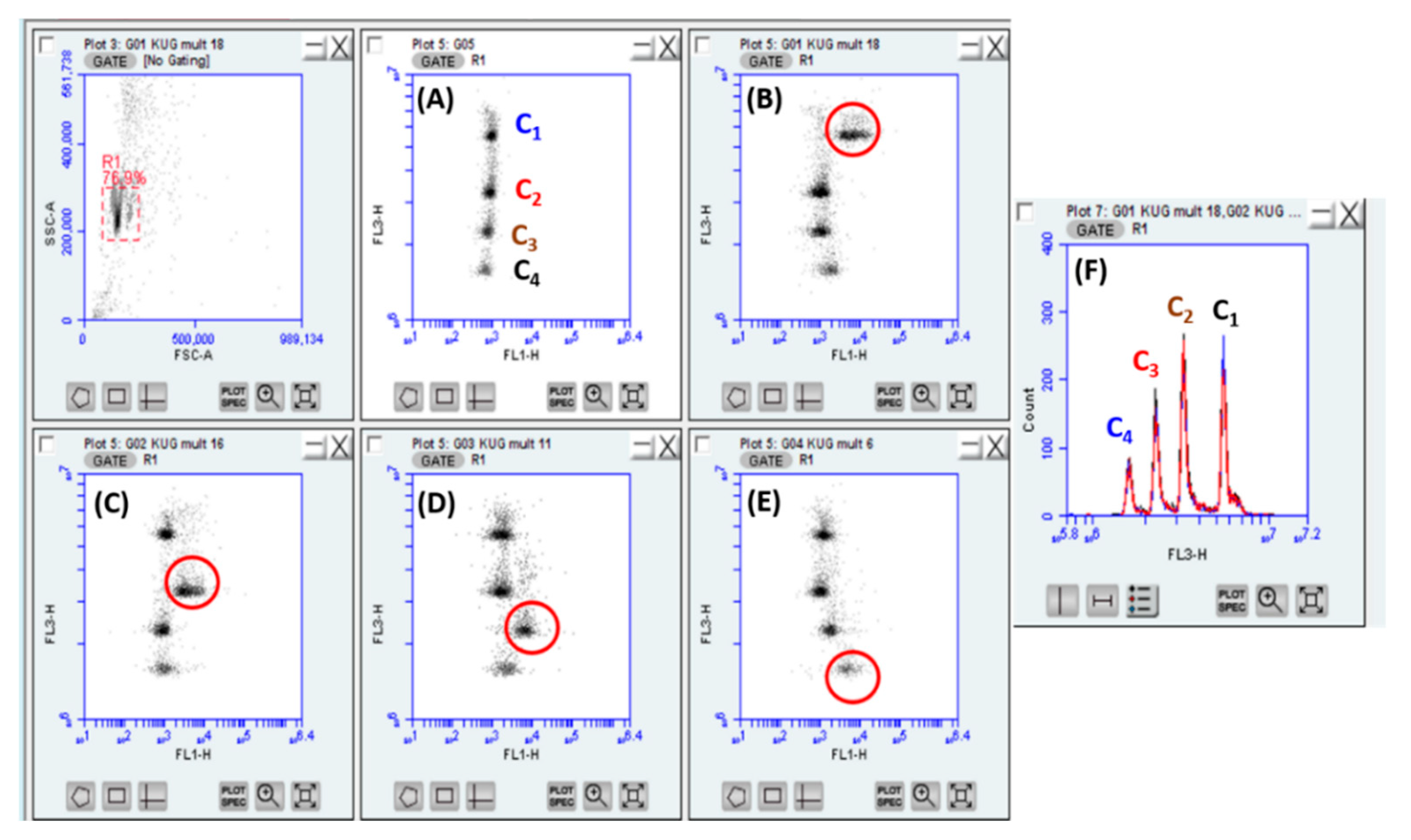
3.3. HPV Detection Using Flow Cytometry
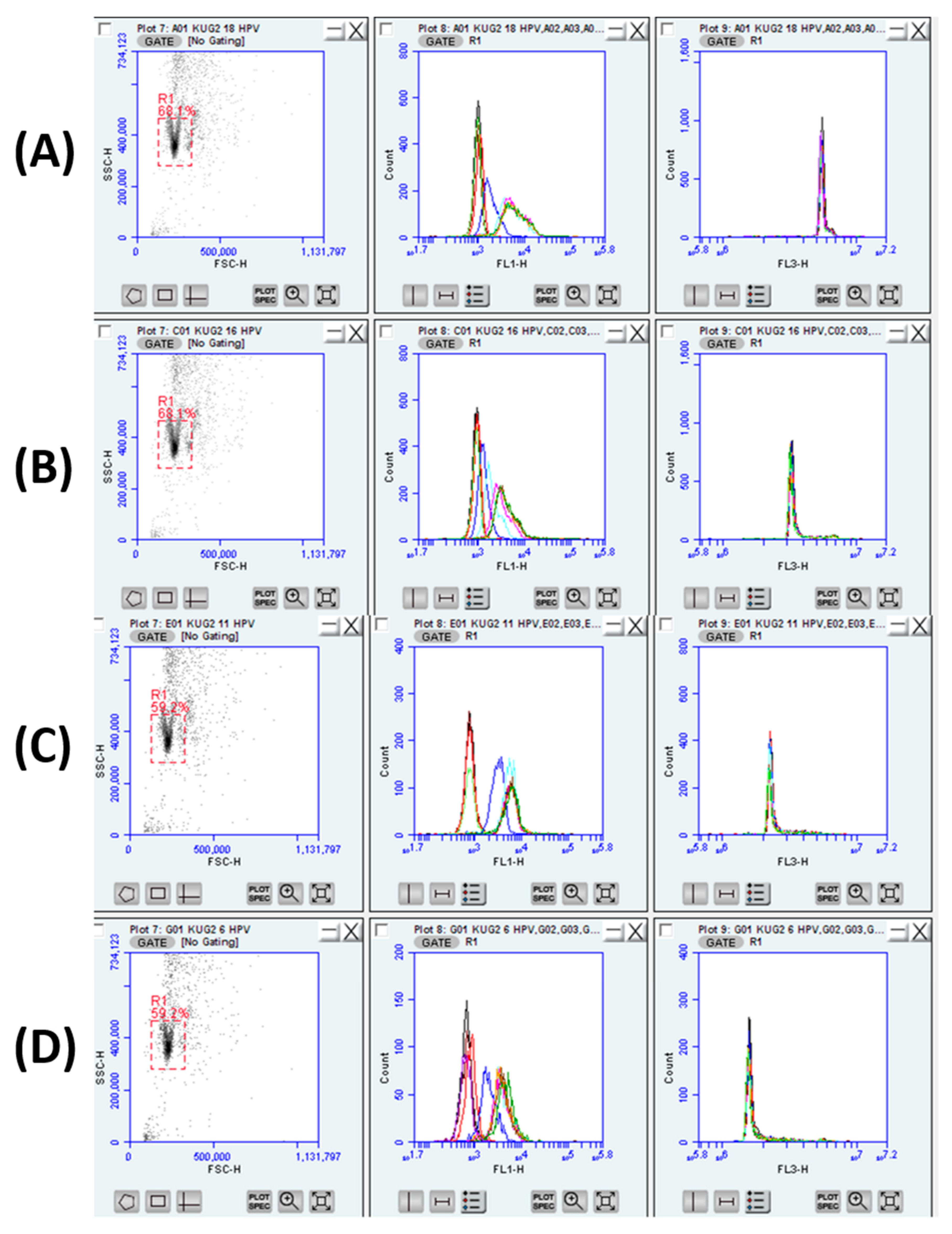
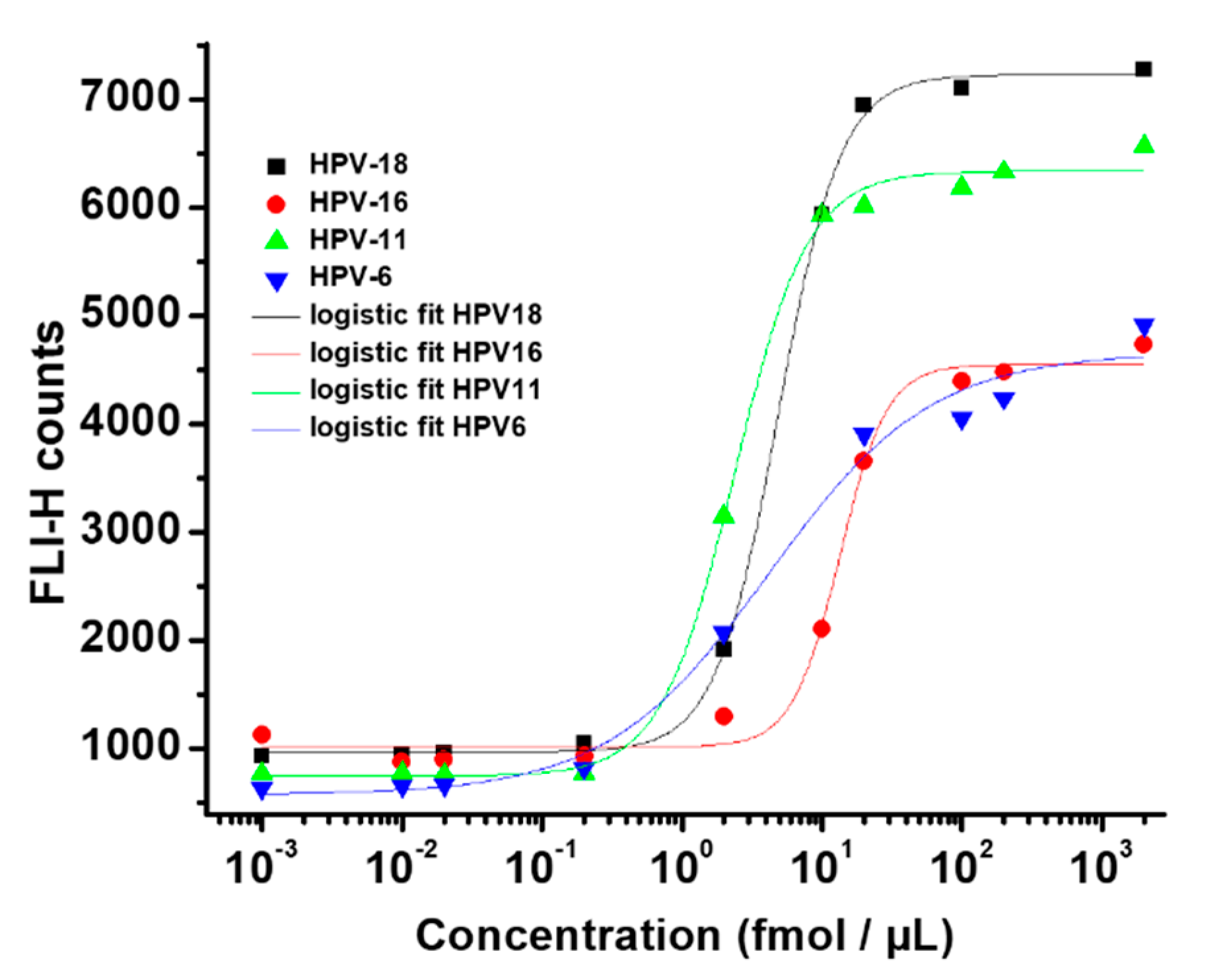
3.4. Hybridization Assays
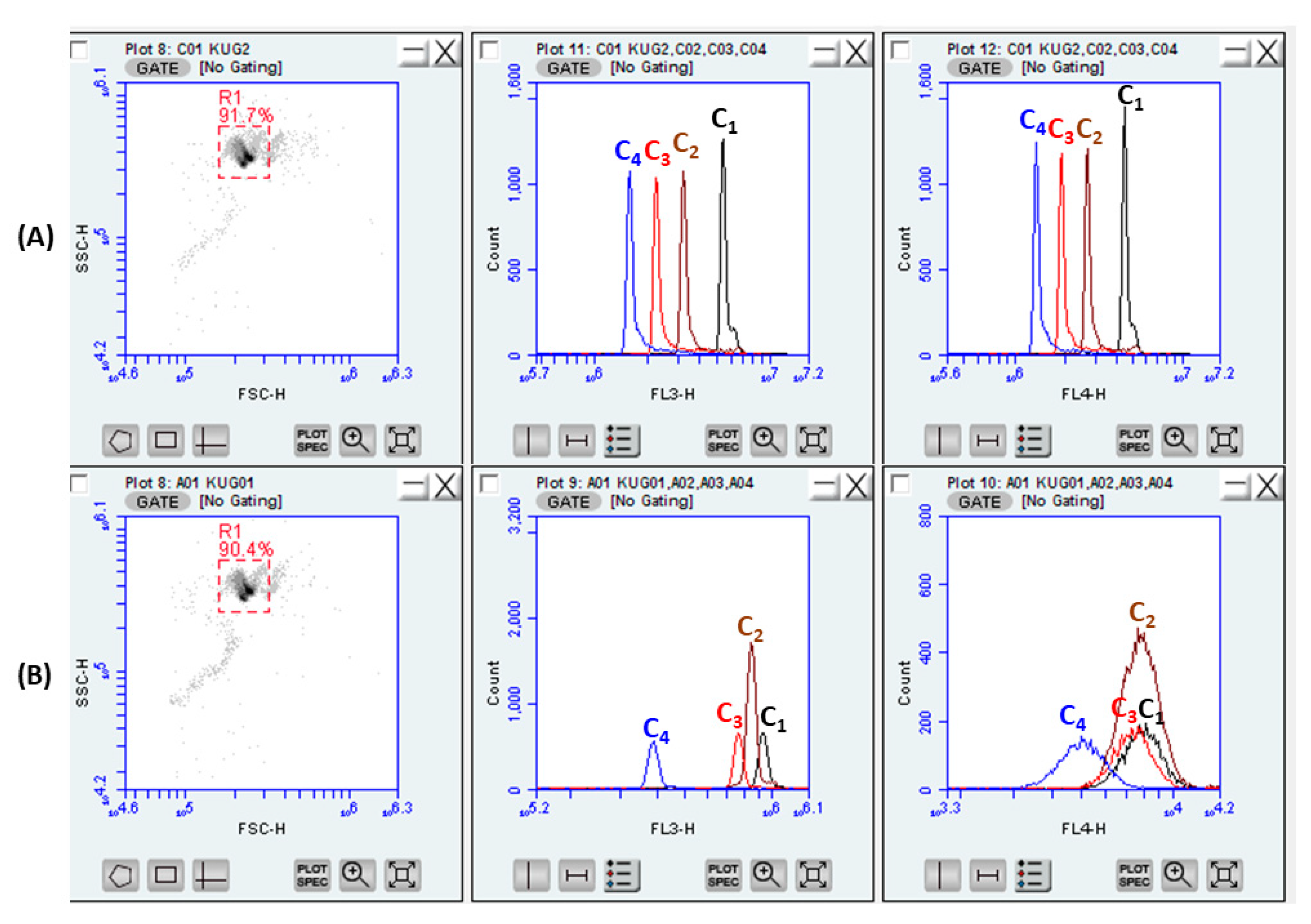
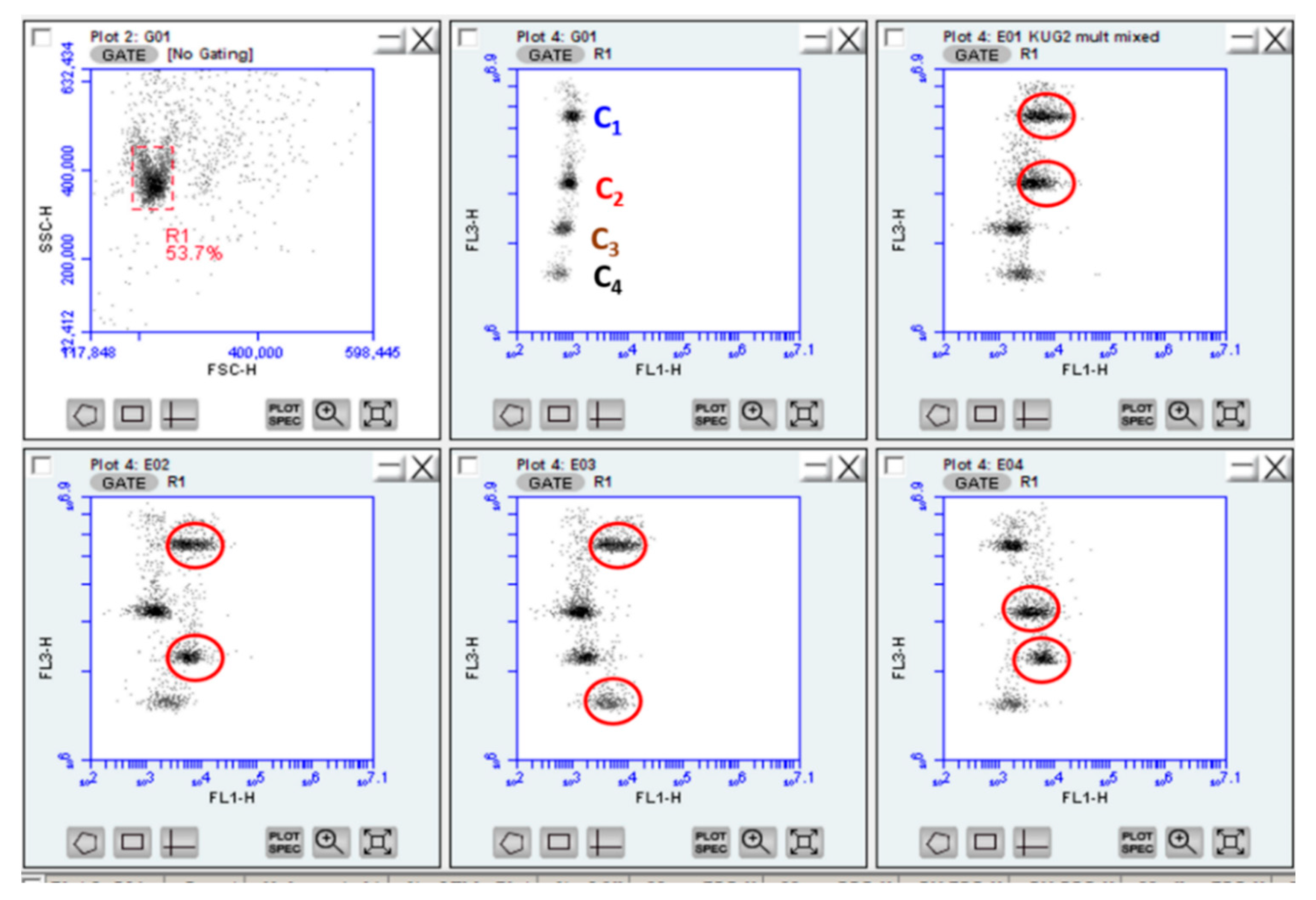
3.5. Multiplexed Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sameiro, M.; Goncalves, T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef]
- Kramer, J.; Kang, R.; Grimm, L.M.; De Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Use of fluorescent probes: Their effect on cell biology and limitations. Anat. Rec. 2012, 295, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Surawski, P.P.T.; Littleton, B.N.; Miller, C.R.; Lawrie, G.A.; Battersby, B.J.; Trau, M. Fluorescent organosilica micro- and nanoparticles with controllable size. J. Colloid Interface Sci. 2007, 310, 144–150. [Google Scholar] [CrossRef]
- Borisov, S.M.; Mayr, T.; Mistlberger, G.; Klimant, I. Dye-Doped Polymeric Particles for Sensing and Imaging. In Advanced Fluorescence Reporters in Chemistry and Biology II: Molecular Constructions, Polymers and Nanoparticles; Demchenko, A.P., Ed.; Springer: Heidelberg, Germany, 2010; pp. 193–228. [Google Scholar] [CrossRef]
- Gubala, V.; Giovannini, G.; Kunc, F.; Monopoli, M.P.; Moore, C.J. Dye-doped silica nanoparticles: Synthesis, surface chemistry and bioapplications. Cancer Nanotechnol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Visaveliya, N.R.; Köhler, J.M. Softness Meets with Brightness: Dye-Doped Multifunctional Fluorescent Polymer Particles via Microfluidics for Labeling. Adv. Opt. Mater. 2021, 9, 2002219. [Google Scholar] [CrossRef]
- Djoba Siawaya, J.F.; Roberts, T.; Babb, C.; Black, G.; Golakai, H.J.; Stanley, K.; Bapela, N.B.; Hoal, E.; Parida, S.; Van Helden, P. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS ONE 2008, 3, e2535. [Google Scholar] [CrossRef] [Green Version]
- Spiro, A.; Lowe, M.; Brown, D. A bead-based method for multiplexed identification and quantitation of DNA sequences using flow cytometry. Appl. Environ. Microbiol. 2000, 66, 4258–4265. [Google Scholar] [CrossRef]
- Sarma, D.; Gawlitza, K.; Rurack, K. Polystyrene Core–Silica Shell Particles with Defined Nanoarchitectures as a Versatile Platform for Suspension Array Technology. Langmuir 2016, 32, 3717–3727. [Google Scholar] [CrossRef]
- Sarma, D.; Carl, P.; Climent, E.; Schneider, R.J.; Rurack, K. Multifunctional polystyrene core/silica shell microparticles with antifouling properties for bead-based multiplexed and quantitative analysis. ACS Appl. Mater. Interfaces 2018, 11, 1321–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiruta, Y.; Nemoto, R.; Kanazawa, H. Design and synthesis of temperature-responsive polymer/silica hybrid nanoparticles and application to thermally controlled cellular uptake. Colloids Surfaces B 2017, 153, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Tobias, C.; Climent, E.; Gawlitza, K.; Rurack, K. Polystyrene microparticles with convergently grown mesoporous silica shells as a promising tool for multiplexed bioanalytical assays. ACS Appl. Mater. Interfaces 2021, 13, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Bravo, I.; Snijders, P.J.; Gissmann, L.; Pawlita, M.; Waterboer, T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006, 44, 504–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Zhan, H.; Fan, L.-F. Preparation of Fluorescence-Encoded Microspheres Based on Hydrophobic Conjugated Polymer–Dye Combination and the Immunoassay. ACS Appl. Bio Mater. 2019, 2, 3009–3018. [Google Scholar] [CrossRef]
- Elshal, M.F.; McCoy, J.P. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Birtwell, S.; Morgan, H. Microparticle encoding technologies for high-throughput multiplexed suspension assays. Integr. Biol. 2009, 1, 345–362. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sklar, L.A. Suspension array technology: Evolution of the flat-array paradigm. Trends Biotechnol. 2002, 20, 9–12. [Google Scholar] [CrossRef]
- Harich, R.; Roger, C.; Garnier, L.; Bienvenu, J.; Fabien, N. Comparison of anti-CCP autoantibodies measurement by ELISA and a bead-based assay in a large patient cohort. Clin. Biochem. 2014, 47, 485–488. [Google Scholar] [CrossRef]
- Fulton, R.J.; McDade, R.L.; Smith, P.L.; Kienker, L.J.; Kettman, J.R. Advanced multiplexed analysis with the FlowMetrixTM system. Clin. Chem. 1997, 43, 1749–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. The pyrrole moiety as a template for COX-1/COX-2 inhibitors. Eur. J. Med. Chem. 2000, 35, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Peseke, K.; Götze, L.; Reinke, H.; Cedeño, Q.A.; Suarez, J.Q.; Andreu, M.G.; Castro, H.V. A Synthesis of Substituted Tetrahydro-2-pyranols. J. Prakt. Chem. 1997, 339, 656–659. [Google Scholar] [CrossRef]
- Gresser, R.; Hartmann, H.; Wrackmeyer, M.; Leo, K.; Riede, M. Synthesis of thiophene-substituted aza-BODIPYs and their optical and electrochemical properties. Tetrahedron 2011, 67, 7148–7155. [Google Scholar] [CrossRef]
- Arroyo, I.J.; Hu, R.; Tang, B.Z.; López, F.I.; Peña-Cabrera, E. 8-Alkenylborondipyrromethene dyes. General synthesis, optical properties, and preliminary study of their reactivity. Tetrahedron 2011, 67, 7244–7250. [Google Scholar] [CrossRef] [Green Version]
- Gresser, R.; Hummert, M.; Hartmann, H.; Leo, K.; Riede, M. Synthesis and characterization of near-infrared absorbing benzannulated aza-BODIPY dyes. Chem. Eur. J. 2011, 17, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Mack, J.; Nyokong, T. Synthesis, characterization and photophysical properties of an acenaphthalene fused-ring-expanded NIR absorbing aza-BODIPY dye. RSC Adv. 2015, 5, 78253–78258. [Google Scholar] [CrossRef]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In vitro demonstration of the heavy-atom effect for photodynamic therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar]
- Soto-Cantu, E.; Cueto, R.; Koch, J.; Russo, P.S. Synthesis and rapid characterization of amine-functionalized silica. Langmuir 2012, 28, 5562–5569. [Google Scholar] [CrossRef]
- Hong, J.; Han, H.; Hong, C.K.; Shim, S.E. A direct preparation of silica shell on polystyrene microspheres prepared by dispersion polymerization with polyvinylpyrrolidone. J. Polym. Sci. A 2008, 46, 2884–2890. [Google Scholar] [CrossRef]
- Sarma, D.; Mielke, J.; Sahre, M.; Beck, U.; Hodoroaba, V.-D.; Rurack, K. TSEM-based contour analysis as a tool for the quantification of the profile roughness of silica shells on polystyrene core particles. Appl. Surf. Sci. 2017, 426, 446–455. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of Red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagorio, M.G.; Román, E.S. How does light scattering affect luminescence? Fluorescence spectra and quantum yields in the solid phase. J. Chem. Educ. 2002, 79, 1362–1367. [Google Scholar] [CrossRef]
- Castillo-Hair, S.M.; Sexton, J.T.; Landry, B.P.; Olson, E.J.; Igoshin, O.A.; Tabor, J.J. FlowCal: A User-Friendly, Open Source Software Tool for Automatically Converting Flow Cytometry Data from Arbitrary to Calibrated Units. ACS Synth. Biol. 2016, 5, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, M.; Williams, L.D. Stoichiometry of formation of Ruhemann’s purple in the ninhydrin reaction. Bioorg. Chem. 1974, 3, 267–280. [Google Scholar] [CrossRef]
- Leng, W.; Chen, M.; Zhou, S.; Wu, L. Capillary force induced formation of monodisperse polystyrene/silica organic− inorganic hybrid hollow spheres. Langmuir 2010, 26, 14271–14275. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Corrie, S.R.; Lawrie, G.A.; Trau, M. Quantitative analysis and characterization of biofunctionalized fluorescent silica particles. Langmuir 2006, 22, 2731–2737. [Google Scholar] [CrossRef]
- Song, X.; Gao, L. Fabrication of hollow hybrid microspheres coated with silica/titania via sol− gel process and enhanced photocatalytic activities. J. Phys. Chem. C 2007, 111, 8180–8187. [Google Scholar] [CrossRef]
- Rollié, S.; Sundmacher, K. Determination of cluster composition in heteroaggregation of binary particle systems by flow cytometry. Langmuir 2008, 24, 13348–13358. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Xu, H.-J.; Xue, Z.-L.; Hoffmann, K.; Shen, Z.; Weller, M.G.; You, X.-Z.; Rurack, K. Phenanthrene-fused boron− dipyrromethenes as bright long-wavelength fluorophores. Org. Lett. 2008, 10, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Behnke, T.; Würth, C.; Hoffmann, K.; Hübner, M.; Panne, U.; Resch-Genger, U. Encapsulation of hydrophobic dyes in polystyrene micro-and nanoparticles via swelling procedures. J. Fluoresc. 2011, 21, 937–944. [Google Scholar] [CrossRef]
- Wittmershaus, B.P.; Baseler, T.T.; Beaumont, G.T.; Zhang, Y.-Z. Excitation energy transfer from polystyrene to dye in 40-nm diameter microspheres. J. Lumin. 2002, 96, 107–118. [Google Scholar] [CrossRef]
- Horejsh, D.; Martini, F.; Poccia, F.; Ippolito, G.; Di Caro, A.; Capobianchi, M.R. A molecular beacon, bead-based assay for the detection of nucleic acids by flow cytometry. Nucleic Acids Res. 2005, 33, e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiollet, S.; Higson, S.; White, N.; Morgan, S.L. Investigation and development of quantum dot-encoded microsphere bioconjugates for DNA detection by flow cytometry. J. Fluoresc. 2012, 22, 685–697. [Google Scholar] [CrossRef] [PubMed]
- AbCam ELISA Kits Specifications. Available online: http://www.abcam.com (accessed on 30 October 2022).
- Momenbeitollahi, N.; Cloet, T.; Li, H. Pushing the detection limits: Strategies towards highly sensitive optical-based protein detection. Anal. Bioanal. Chem. 2021, 413, 5995–6011. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, S.; Negm, O.H.; Hamed, M.R.; Tubby, C.; Todd, I.; Tighe, P.J.; Harrison, T.; Fairclough, L.C. Development and validation of protein microarray technology for simultaneous inflammatory mediator detection in human sera. Mediators Inflamm. 2014, 2014, 820304. [Google Scholar] [CrossRef] [Green Version]
- Haab, B.B.; Dunham, M.J.; Brown, P.O. Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. Genome Biol. 2001, 2, research0004.1. [Google Scholar] [CrossRef]

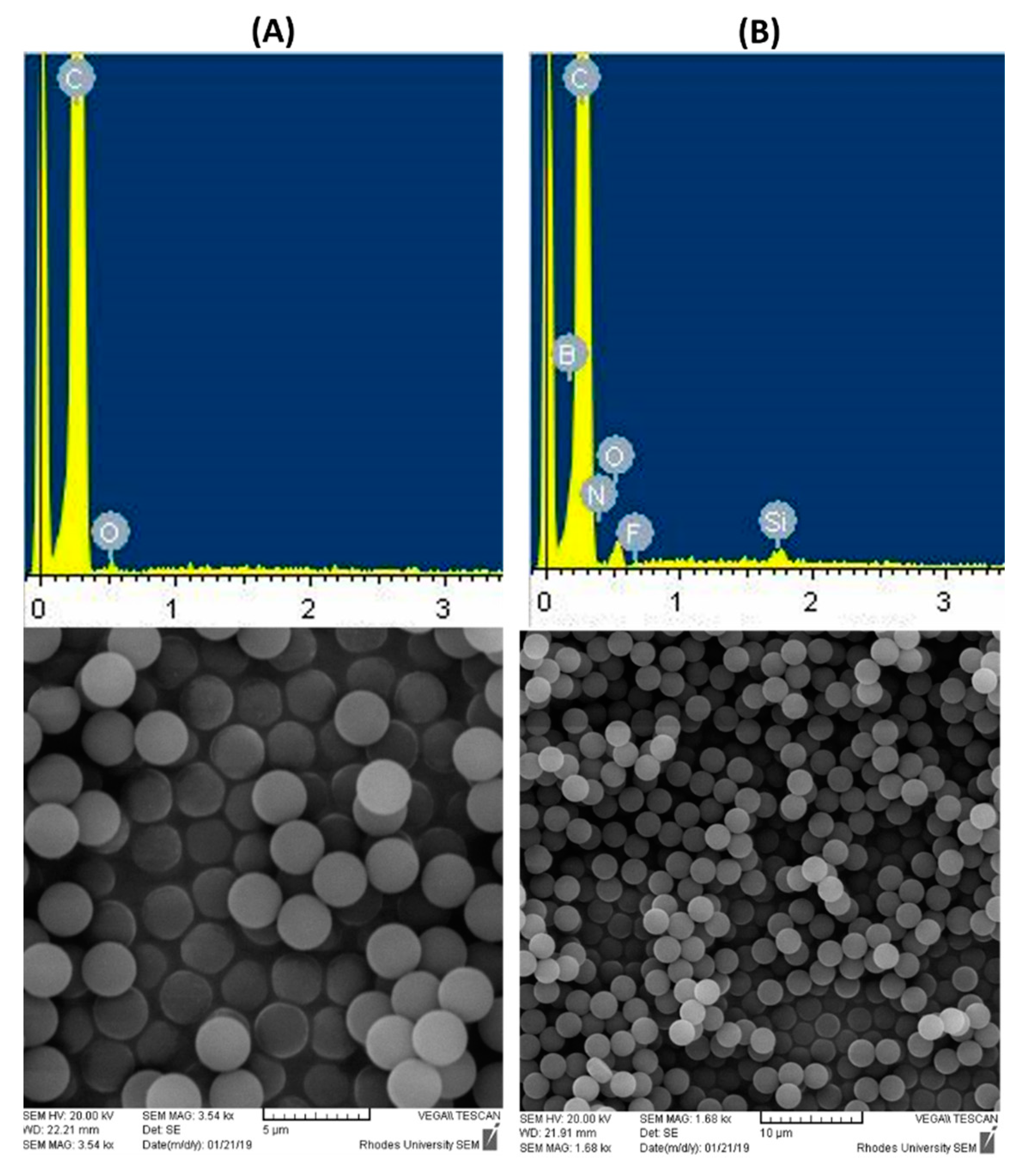
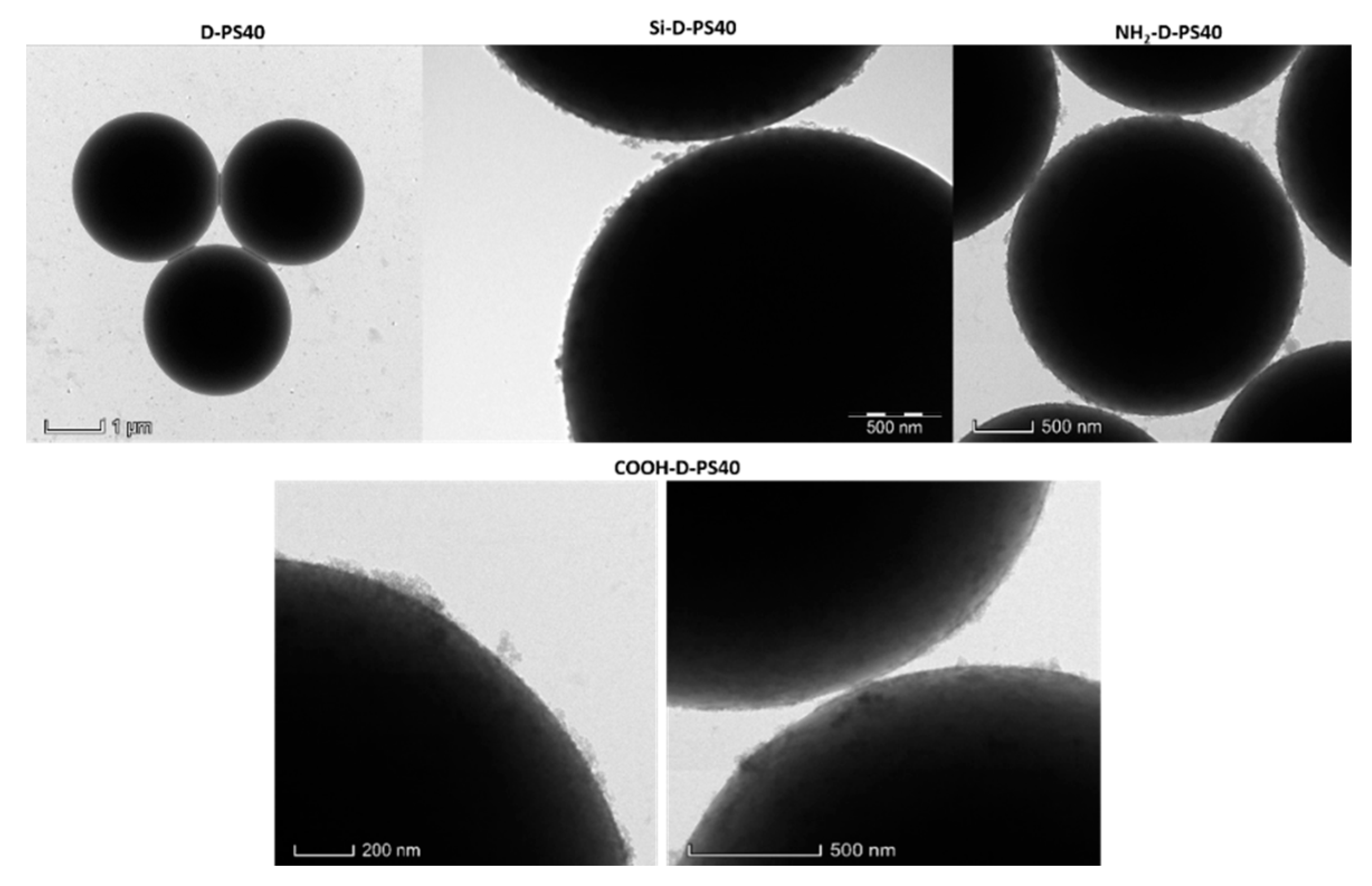
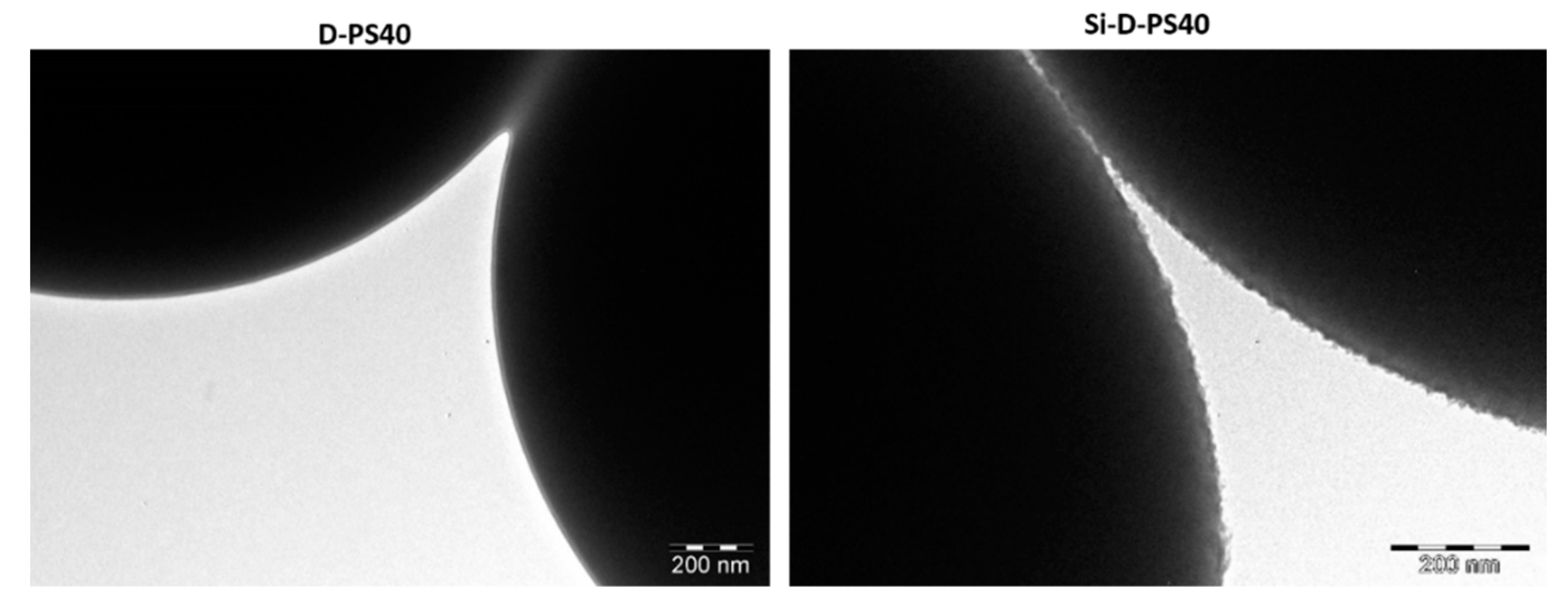
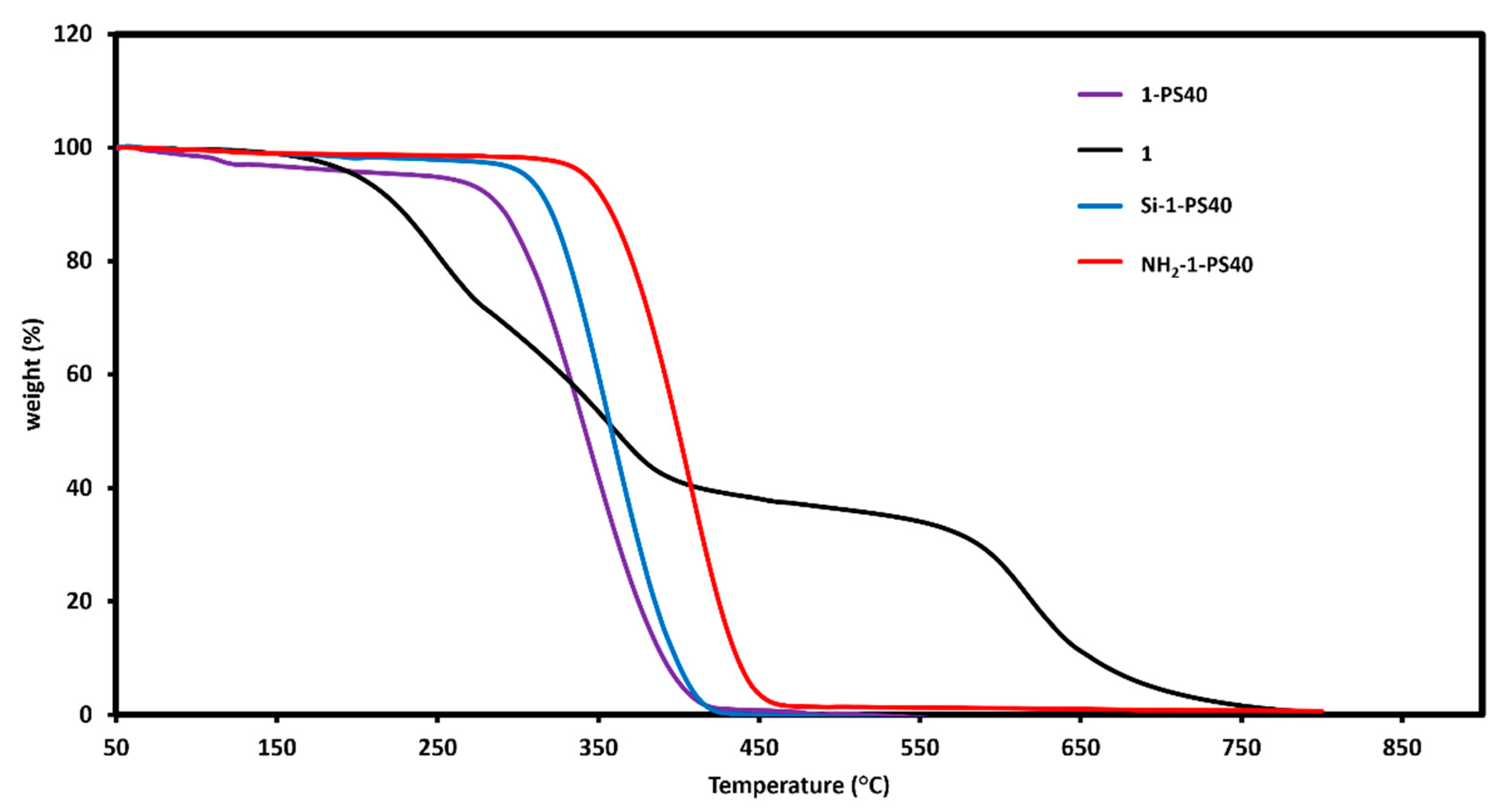




| NH2-D-PS40 | Absorbance | Conc. (mM) | Mass (mg) | Conc. (mM g−1) (×10−5) 1 |
|---|---|---|---|---|
| NH2-1-PS40 | 2.3 × 10−2 | 6.0 × 10−2 | 1 | 6.0 |
| NH2-2-PS40 | 1.3 × 10−2 | 2.4 × 10−2 | 1 | 2.4 |
| NH2-B-PS40 | 5.3 × 10−3 | 2.2 × 10−2 | 1 | 2.2 |
| Mean FSC-H (×105) | Cν FSC-H (%) | Mean SSC-H (×105) | Cν SSC-H (%) | |
|---|---|---|---|---|
| Blank | 2.27 | 7.8 (±0.39) | 3.47 | 11.8 (±0.59) |
| Si-1-PS40 | 2.49 | 8.2 (±0.41) | 4.36 | 11.5 (±0.58) |
| Si-2-PS40 | 2.39 | 11.8 (±0.59) | 3.97 | 11.4 (±0.57) |
| NH2-Blank | 2.62 | 7.4 (±0.37) | 3.86 | 10.7 (±0.53) |
| NH2-1-PS40 | 2.67 | 7.2 (±0.36) | 3.89 | 10.8 (±0.54) |
| NH2-2-PS40 | 2.78 | 6.7 (±0.34) | 3.88 | 10.9 (±0.55) |
| Concentration of Dye Added (mM) | HPV c-DNA Strand Added | Name of Material |
|---|---|---|
| C1 = 14 | HPV-18 | c-DNA18-1-PS40 |
| C2 = 7 | HPV-16 | c-DNA16-1-PS40 |
| C3 = 3.5 | HPV-11 | c-DNA11-1-PS40 |
| C4 = 1.75 | HPV-6 | c-DNA6-1-PS40 |
| C1 = 14 | HPV-18 | c-DNA18-2-PS40 |
| C2 = 7 | HPV-16 | c-DNA16-2-PS40 |
| C3 = 3.5 | HPV-11 | c-DNA11-2-PS40 |
| C4 = 1.75 | HPV-6 | c-DNA6-2-PS40 |
| c-DNA-1-PS40 | HPV18 | HPV16 | HPV11 | HPV6 |
| LOD (fmol µL−1) | 1.47 | 6.64 | 0.78 | 0.83 |
| LOQ (fmol µL−1) | 1.09 | 7.45 | 1.40 | 5.41 |
| Linear range (fmol µL−1) | 2.4−10 | 7.8−23.0 | 1.0−5.5 | 0.66−2.50 |
| c-DNA-2-PS40 | HPV18 | HPV16 | HPV11 | HPV6 |
| LOD (fmol µL−1) | 0.22 | 5.27 | 0.67 | 4.72 |
| LOQ (fmol µL−1) | 3.36 | 4.46 | 3.14 | 9.40 |
| Linear range (fmol µL−1) | 0.99−9.01 | 7.04−17.7 | 0.82−2.07 | 5.4−13.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubheka, G.; Climent, E.; Tobias, C.; Rurack, K.; Mack, J.; Nyokong, T. Multiplexed Detection of Human Papillomavirus Based on AzaBODIPY-Doped Silica-Coated Polystyrene Microparticles. Chemosensors 2023, 11, 1. https://doi.org/10.3390/chemosensors11010001
Kubheka G, Climent E, Tobias C, Rurack K, Mack J, Nyokong T. Multiplexed Detection of Human Papillomavirus Based on AzaBODIPY-Doped Silica-Coated Polystyrene Microparticles. Chemosensors. 2023; 11(1):1. https://doi.org/10.3390/chemosensors11010001
Chicago/Turabian StyleKubheka, Gugu, Estela Climent, Charlie Tobias, Knut Rurack, John Mack, and Tebello Nyokong. 2023. "Multiplexed Detection of Human Papillomavirus Based on AzaBODIPY-Doped Silica-Coated Polystyrene Microparticles" Chemosensors 11, no. 1: 1. https://doi.org/10.3390/chemosensors11010001





