Enhancing the Performance of Two Different Commercial CO2 Indicators Using Digital Colourimetric Analysis, DCA
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Lab-Based Drop Check Indicator Solution for Aquaria
2.2.2. Preparation of the Lab-Based Capnography Indicator
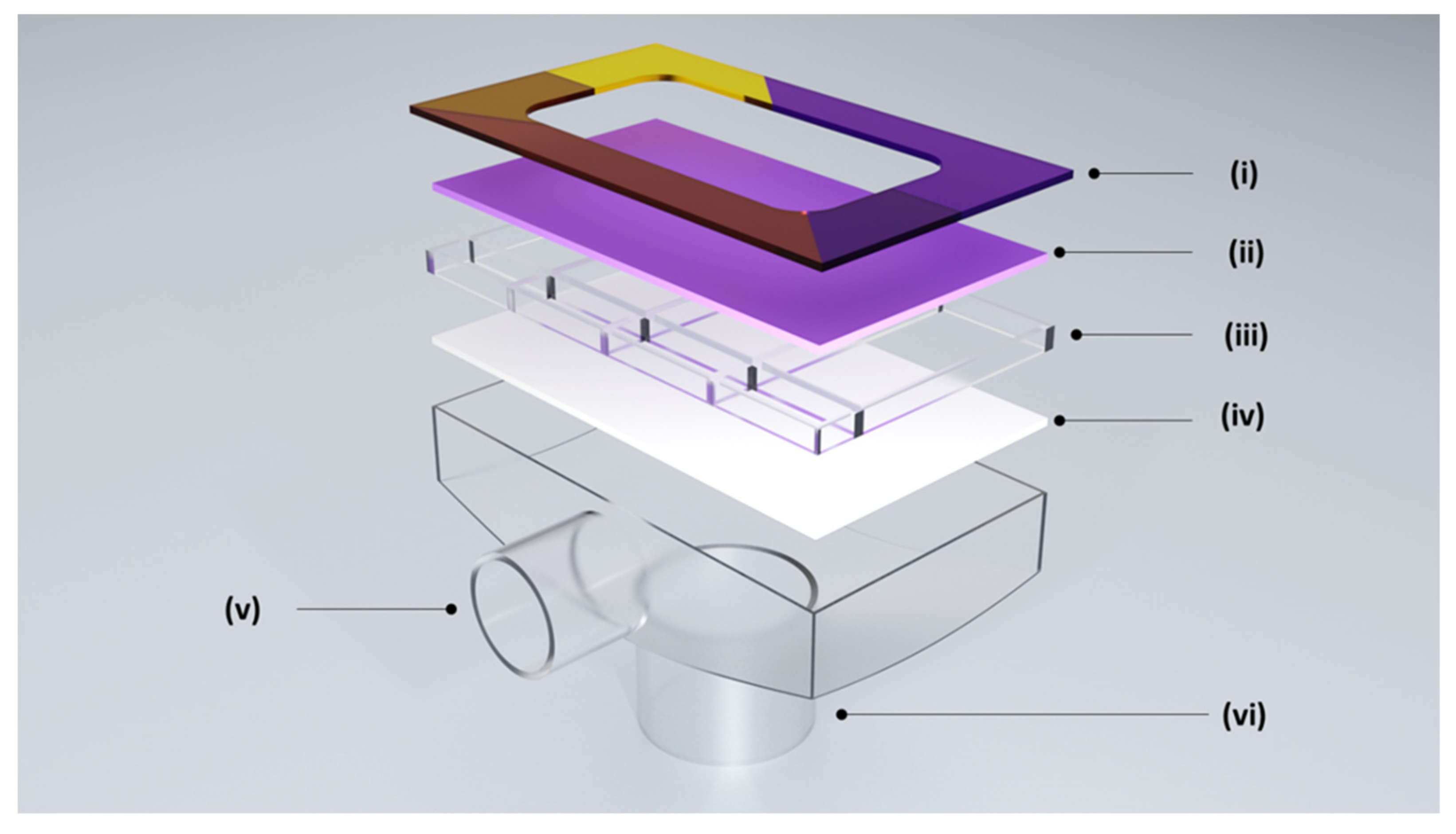
2.2.3. Other Methods
3. Results and Discussion
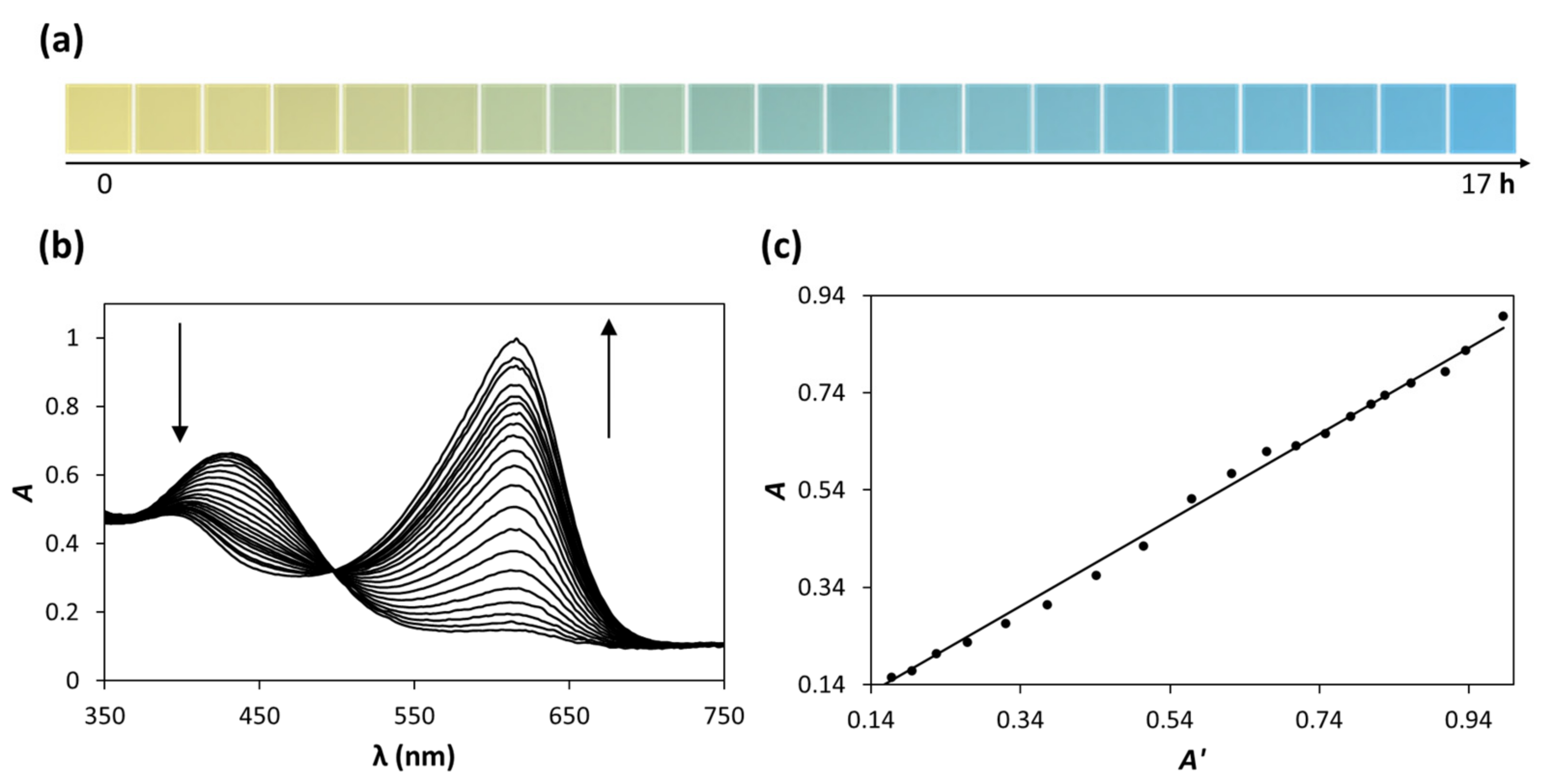
3.1. Colourimetric CO2 Indicators and Apparent Absorbance, A’
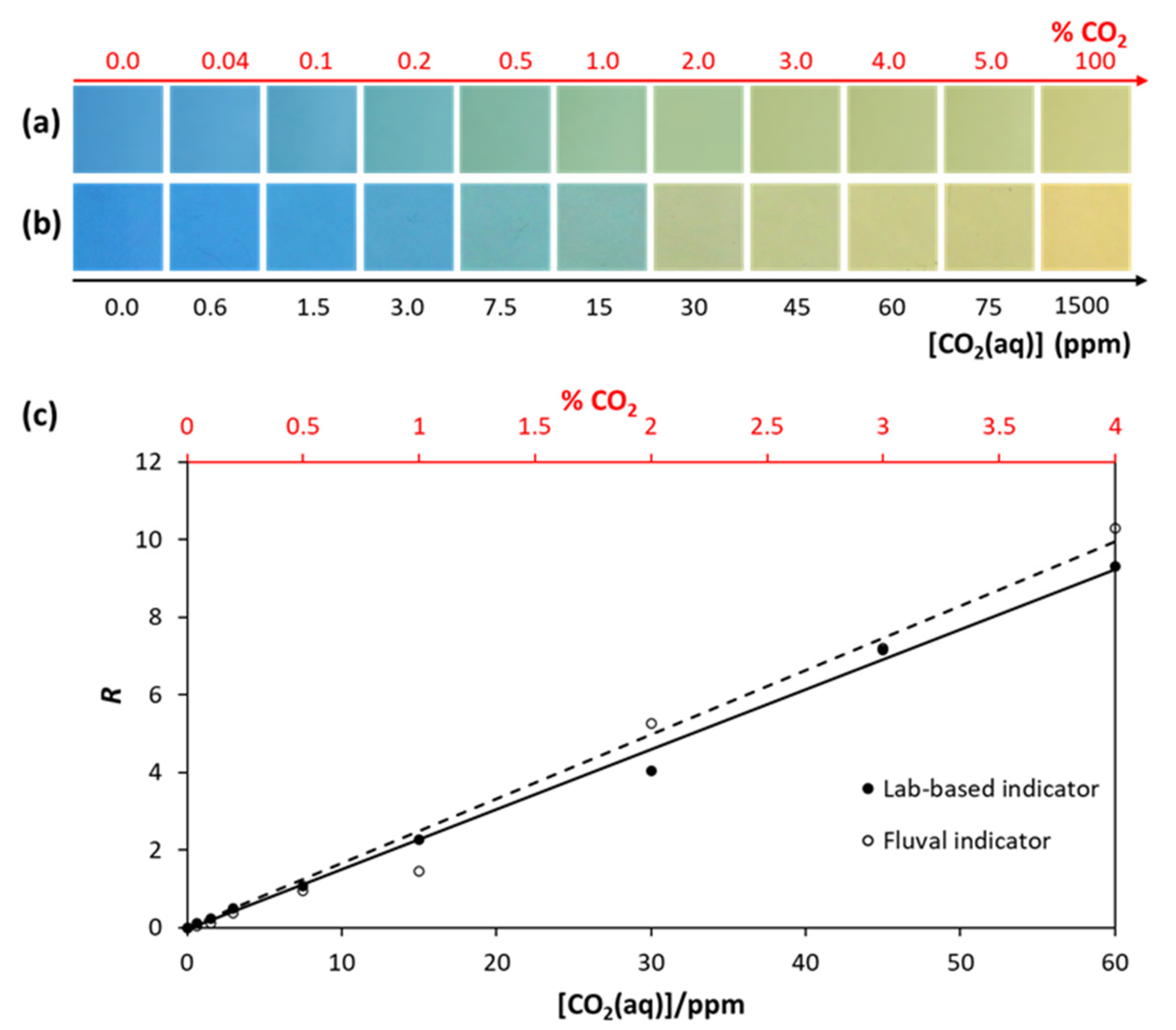
3.2. The Aquarium CO2 Indicator
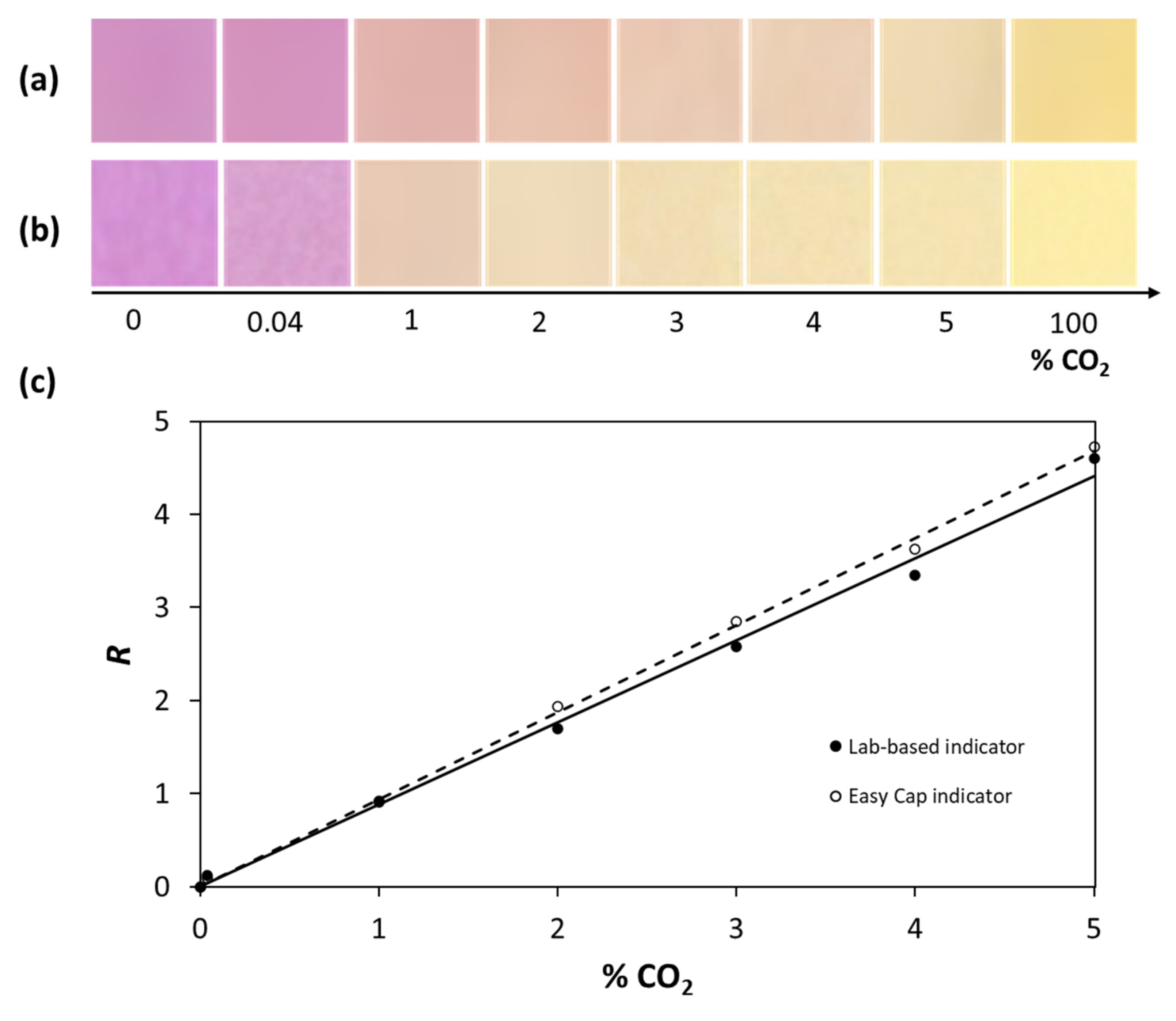
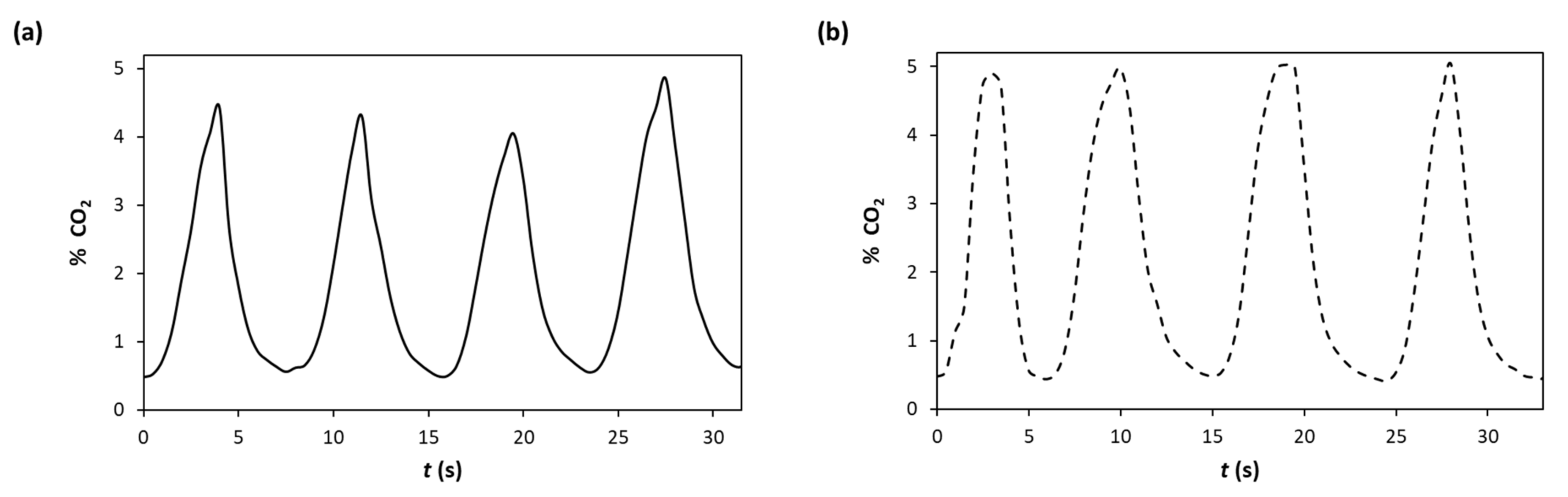
3.3. The Capnography Indicator
3.4. A Simple A′ Calculator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, A. Optical sensors for carbon dioxide and their applications. In Sensors for Environment, Health and Security; Baraton, M.I., Ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Küçükhüseyin, Ö. CO2 monitoring and indoor air quality. REHVA Eur. HVAC J. 2021, 58, 54–59. [Google Scholar]
- The Atmosphere: Getting a Handle on Carbon Dioxide. Available online: https://climate.nasa.gov/news/2915/the-atmosphere-getting-a-handle-on-carbon-dioxide/#:~:text=The%20concentration%20of%20carbon%20dioxide,million%20(ppm)%20and%20rising (accessed on 7 November 2022).
- Falkenberg, L.J.; Bellerby, R.G.J.; Connell, S.D.; Fleming, L.E.; Maycock, B.; Russell, B.D.; Sullivan, F.J.; Dupont, S. Ocean acidification and human health. Int. J. Environ. Res. Public Health 2020, 17, 4563. [Google Scholar] [CrossRef] [PubMed]
- Langford, N.J. Carbon dioxide poisoning. Toxicol. Rev. 2005, 24, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.; Ulrich, G.; Oelßner, W. Carbon Dioxide Sensing, Fundamentals, Principles and Applications; Wiley-VCH: Weinhem, Germany, 2019. [Google Scholar]
- Parry, R.T. Principles and Applications of Modified Atmosphere Packaging of Foods; Springer: Boston, MA, USA, 1993. [Google Scholar]
- Aguayo-Lopez, M.L.; Capitan-Vallvey, L.F.; Fernandez-Ramos, M.D. Optical sensor for carbon dioxide gas determination, characterization and improvements. Talanta 2014, 126, 196–201. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Bradley, A.F. Electrodes for blood pO2 and pCO2 determination. J. Appl. Physiol. 1958, 13, 515–520. [Google Scholar] [CrossRef]
- Mills, A.; Eaton, K. Optical sensors for carbon dioxide: An overview of sensing strategies past and present. Quim. Anal. 2000, 19, 75–86. [Google Scholar]
- Dervieux, E.; Theron, M.; Uhring, W. Carbon dioxide sensing-biomedical applications to human subjects. Sensors 2021, 22, 188. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Yusufu, D.; Mills, A. Spectrophotometric and digital colour colourimetric (DCC) analysis of colour-based indicators. Sens. Actuators B 2018, 273, 1187–1194. [Google Scholar] [CrossRef]
- CO2: Striking the Balance. Available online: https://www.practicalfishkeeping.co.uk/features/co2-striking-the-balance/ (accessed on 7 November 2022).
- Fehder, C.G. Apparatus for Determining Whether Respiratory Gas is Present in a Gaseous Sample. US Patent 1991/ 5179002 A, 25 April 1991. [Google Scholar]
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 7 November 2022).
- Mills, A.; Chang, Q. Tuning colourimetric and fluorimetric gas sensors for carbon dioxide. Anal. Chim. Acta 1994, 285, 113–123. [Google Scholar] [CrossRef]
- Tracheal Intubation. Available online: https://en.wikipedia.org/wiki/Tracheal_intubation (accessed on 7 November 2022).
- Umeda, A.; Ishizaka, M.; Ikeda, A.; Miyagawa, K.; Mochida, A.; Takeda, H.; Takeda, K.; Fukushi, I.; Okada, Y.; Gozal, D. Recent insights into the measurement of carbon dioxide concentrations for clinical practice in respiratory medicine. Sensors 2021, 21, 5636. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Flaherty, T.J.; Alnasleh, B.K. Beyond λmax: Transforming visible spectra into 24-bit color values. J. Chem. Educ. 2007, 84, 1873–1877. [Google Scholar] [CrossRef]
- Ruttanakorn, K.; Phadungcharoen, N.; Laiwattanapaisal, W.; Chinsriwongkul, A.; Rojanarata, T. Smartphone-based technique for the determination of a titration equivalence point from an RGB linear-segment curve with an example application to miniaturized titration of sodium chloride injections. Talanta 2021, 233, 122602. [Google Scholar] [CrossRef]
- Lima, M.J.A.; Sasaki, M.K.; Marinho, O.R.; Freitas, T.A.; Faria, R.C.; Reis, B.F.; Rocha, F.R.P. Spot test for fast determination of hydrogen peroxide as a milk adulterant by smartphone-based digital image colorimetry. Microchem. J. 2020, 157, 105042. [Google Scholar] [CrossRef]
- Firdaus, M.; Aprian, A.; Meileza, N.; Hitsmi, M.; Elvia, R.; Rahmidar, L.; Khaydarov, R. Smartphone coupled with a paper-based colorimetric device for sensitive and portable mercury ion sensing. Chemosensors 2019, 7, 25. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Disposable and low-cost colorimetric sensors for environmental analysis. Int. J. Environ. Res. Public Health 2020, 17, 8331. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Petruci, J.d.S.; Batista, A. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Inamuddin; Bisetty, K. Smartphone based bioanalytical and diagnosis applications: A review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Qian, L.; Cui, Y.; Zheng, X.; Kang, Y.; Fu, X.; Wang, S.; Wang, P.; Wang, D. A hand-held optoelectronic tongue for the identification of heavy-metal ions. Sens. Actuators B 2022, 352, 130971. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, L.T. Colorimetric array indicator for NH3 and CO2 detection. Sens. Actuators B 2018, 255, 3216–3226. [Google Scholar] [CrossRef]
- Araki, H.; Kim, J.; Zhang, S.; Banks, A.; Crawford, K.E.; Sheng, X.; Gutruf, P.; Shi, Y.; Pielak, R.M.; Rogers, J.A. Materials and device designs for an epidermal UV colorimetric dosimeter with near field communication capabilities. Adv. Funct. Mater. 2017, 27, 1604465. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Al Obaidi, A.; Karaca, I.M.; Ayhan, Z.; Haskaraca, G.; Gultekin, E. Fabrication and validation of CO2-sensitive indicator to monitor the freshness of poultry meat. Food Packag. Shelf Life 2022, 34, 100930. [Google Scholar] [CrossRef]
- Choi, I.; Choi, H.; Lee, J.S.; Han, J. Novel color stability and colorimetry-enhanced intelligent CO2 indicators by metal complexation of anthocyanins for monitoring chicken freshness. Food Chem. 2022, 404, 134534. [Google Scholar] [CrossRef]
- Heleine, M.F. Freshwater Aquariums for Dummies; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Farmer, G. Aquascaping: A Step-by-Step Guide to Planting, Styling, and Maintaining Beautiful Aquariums; Skyhorse Publishing: New York, NY, USA, 2020. [Google Scholar]
- Anh, D.H.; Cheunrungsikul, K.; Wichitwechkarn, J.; Surareungchai, W. A colorimetric assay for determination of methyl parathion using recombinant methyl parathion hydrolase. Biotechnol. J. 2011, 6, 565–571. [Google Scholar] [CrossRef]
- Mook, W.G. Environmental Isotopes in the Hydrological Cycle: Principles and Applications; UNESCO and International Atomic Energy Agency: Paris, France, 2000. [Google Scholar]
- Long, B.; Koyfman, A.; Vivirito, M.A. Capnography in the emergency department: A review of uses, waveforms, and limitations. J. Emerg. Med. 2017, 53, 829–842. [Google Scholar] [CrossRef]
- Lederman, D.; Lampotang, S.; Shamir, M.Y. Automatic endotracheal tube position confirmation system based on image classification- a preliminary assessment. Med. Eng. Phys. 2011, 33, 1017–1026. [Google Scholar] [CrossRef]
- Rabitsch, W.; Nikolic, A.; Schellongowski, P.; Kofler, J.; Kraft, P.; Krenn, C.G.; Staudinger, T.; Locker, G.J.; Knöbl, P.; Hofbauer, R.; et al. Evaluation of an End-Tidal Portable ETCO2 Colorimetric Breath Indicator (COLIBRI). Am. J. Emerg. Med. 2004, 22, 4–9. [Google Scholar] [CrossRef]
- Banik, G.D.; Mizaikoff, B. Exhaled breath analysis using cavity-enhanced optical techniques: A review. J. Breath Res. 2020, 14, 043001. [Google Scholar] [CrossRef]
- Vincent, T.A.; Gardner, J.W. A low cost MEMS based NDIR system for the monitoring of carbon dioxide in breath analysis at ppm levels. Sens. Actuators B 2016, 236, 954–964. [Google Scholar] [CrossRef]
- Ward, K.R.; Yealy, D.M. End-tidal carbon dioxide monitoring in emergency medicine, Part 1: Basic principles. Acad. Emerg. Med. 1998, 5, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Anton, W.R.; Gordon, R.W.; Jordan, T.M.; Posner, K.L.; Cheney, F.W. A Disposable End-Tidal CO2 Detector to Verify Endotracheal Intubation. Ann. Emerg. Med. 1991, 20, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Gravenstein, J.S.; Jaffe, M.B.; Paulus, D.A. Capnography, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Mercury Medical StatCO2® CO2 Detector. Available online: https://www.mercurymed.com/product/statco2-mini-statco2-neo-statco2/ (accessed on 7 November 2022).
- MaxCap™ CO2 Indicator. Available online: https://resources.sun-med.com/resource/maxcap-literature-sheet/ (accessed on 7 November 2022).
- FloCap™ CO2 Indicator. Available online: https://resources.sun-med.com/resource/flocap-literature-sheet/ (accessed on 7 November 2022).
- Ventlab STAT Check™ CO2 Indicator. Available online: https://www.buyemp.com/product/ventlab-statcheck-ii-co2-indicator-detection-device/SC200) (accessed on 7 November 2022).
- Nellcor™ Easy Cap II. Available online: https://coastbiomed.com/product/easy-cap-ii-co2-detector (accessed on 7 November 2022).
- How to Read and Interpret End-Tidal Capnography Waveforms. Available online: https://www.jems.com/patient-care/how-to-read-and-interpret-end-tidal-capnography-waveforms/ (accessed on 7 November 2022).
- Capnography Waveform Infographics. Available online: https://ne-np.facebook.com/FOAMfrat/posts/capnography-waveforms-part-2-of-3-of-our-capnography-infographics/3013262415612563/ (accessed on 7 November 2022).
- Mills, A.; Lepre, A.; Wild, L. Breath-by-breath measurement of carbon dioxide using a plastic film optical sensor. Sens. Actuators B 1997, 39, 419–425. [Google Scholar] [CrossRef]
- DCA Tutorial. Available online: https://www.profandrewmills.com/2022/10/dca-tutorial/ (accessed on 7 November 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonnell, L.; Yusufu, D.; O’Rourke, C.; Mills, A. Enhancing the Performance of Two Different Commercial CO2 Indicators Using Digital Colourimetric Analysis, DCA. Chemosensors 2022, 10, 544. https://doi.org/10.3390/chemosensors10120544
McDonnell L, Yusufu D, O’Rourke C, Mills A. Enhancing the Performance of Two Different Commercial CO2 Indicators Using Digital Colourimetric Analysis, DCA. Chemosensors. 2022; 10(12):544. https://doi.org/10.3390/chemosensors10120544
Chicago/Turabian StyleMcDonnell, Lauren, Dilidaer Yusufu, Christopher O’Rourke, and Andrew Mills. 2022. "Enhancing the Performance of Two Different Commercial CO2 Indicators Using Digital Colourimetric Analysis, DCA" Chemosensors 10, no. 12: 544. https://doi.org/10.3390/chemosensors10120544
APA StyleMcDonnell, L., Yusufu, D., O’Rourke, C., & Mills, A. (2022). Enhancing the Performance of Two Different Commercial CO2 Indicators Using Digital Colourimetric Analysis, DCA. Chemosensors, 10(12), 544. https://doi.org/10.3390/chemosensors10120544







