An NIR Emissive Donor-π-Acceptor Dicyanomethylene-4H-Pyran Derivative as a Fluorescent Chemosensor System towards Copper (II) Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of 2,6-dimethyl-3,5-diphenyl-4H-pyran-4-one (1)
2.1.2. Synthesis of 2-(2,6-dimethyl-3,5-diphenyl-4H-pyran-4-ylidene) Malononitrile (2)
2.1.3. Synthesis of 2-(2,6-bis((E)-4-((2-(bis(pyridin-2-ylmethyl)amino)ethyl)(methyl)amino) styryl)-3,5-diphenyl-4H-pyran-4-ylidene)malononitrile (4)
2.2. UV-Vis and Fluorescence Measurements
3. Results and Discussion
3.1. Synthesis
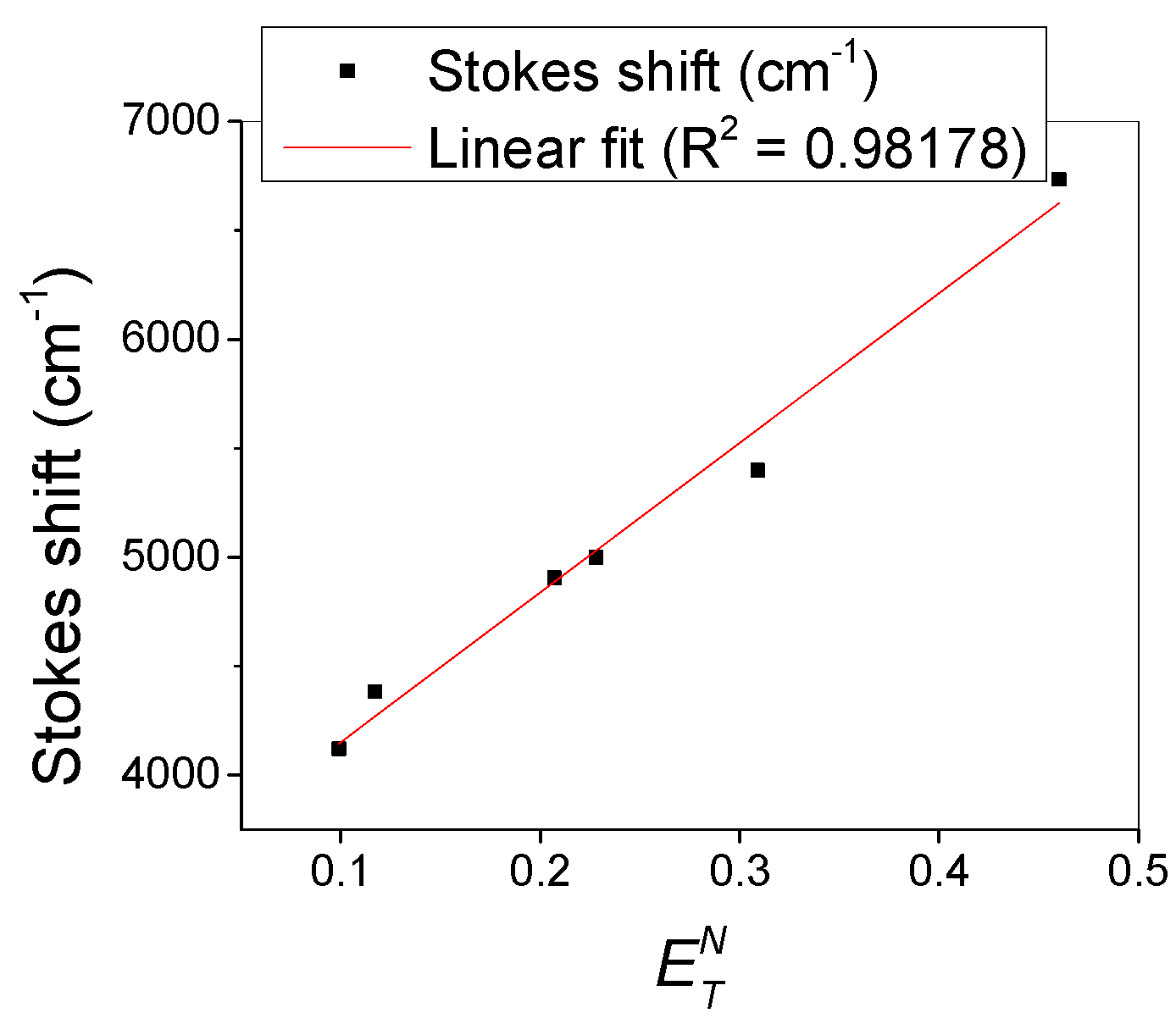
3.2. Photophysical Characterization
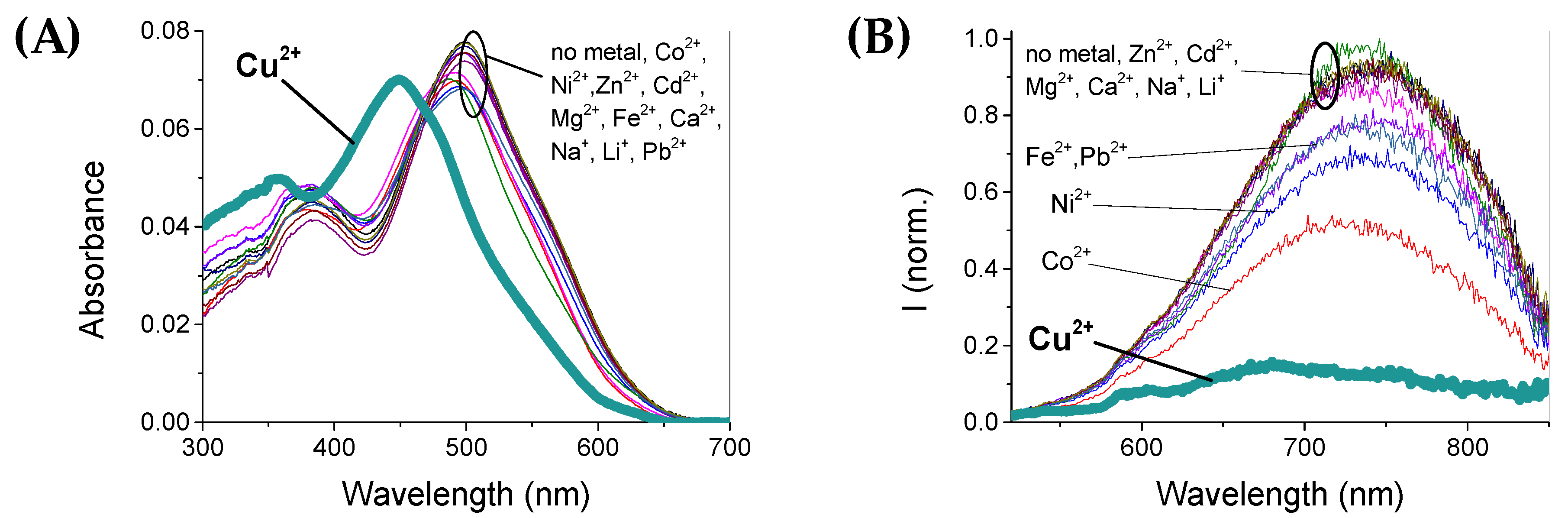
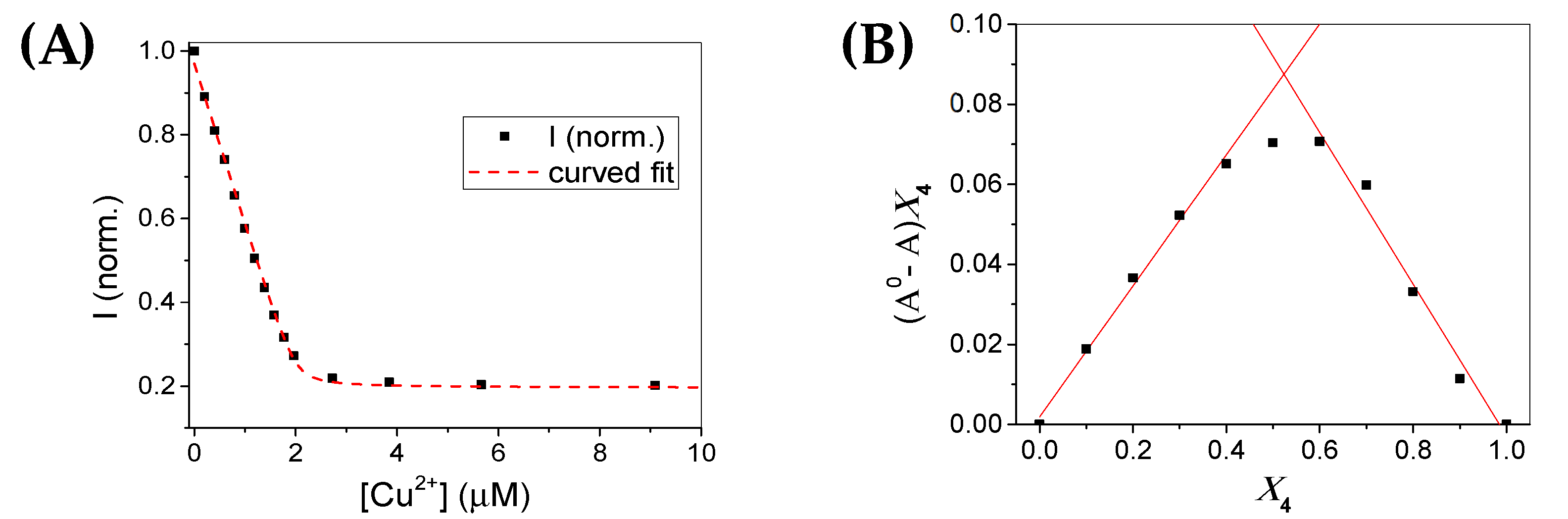
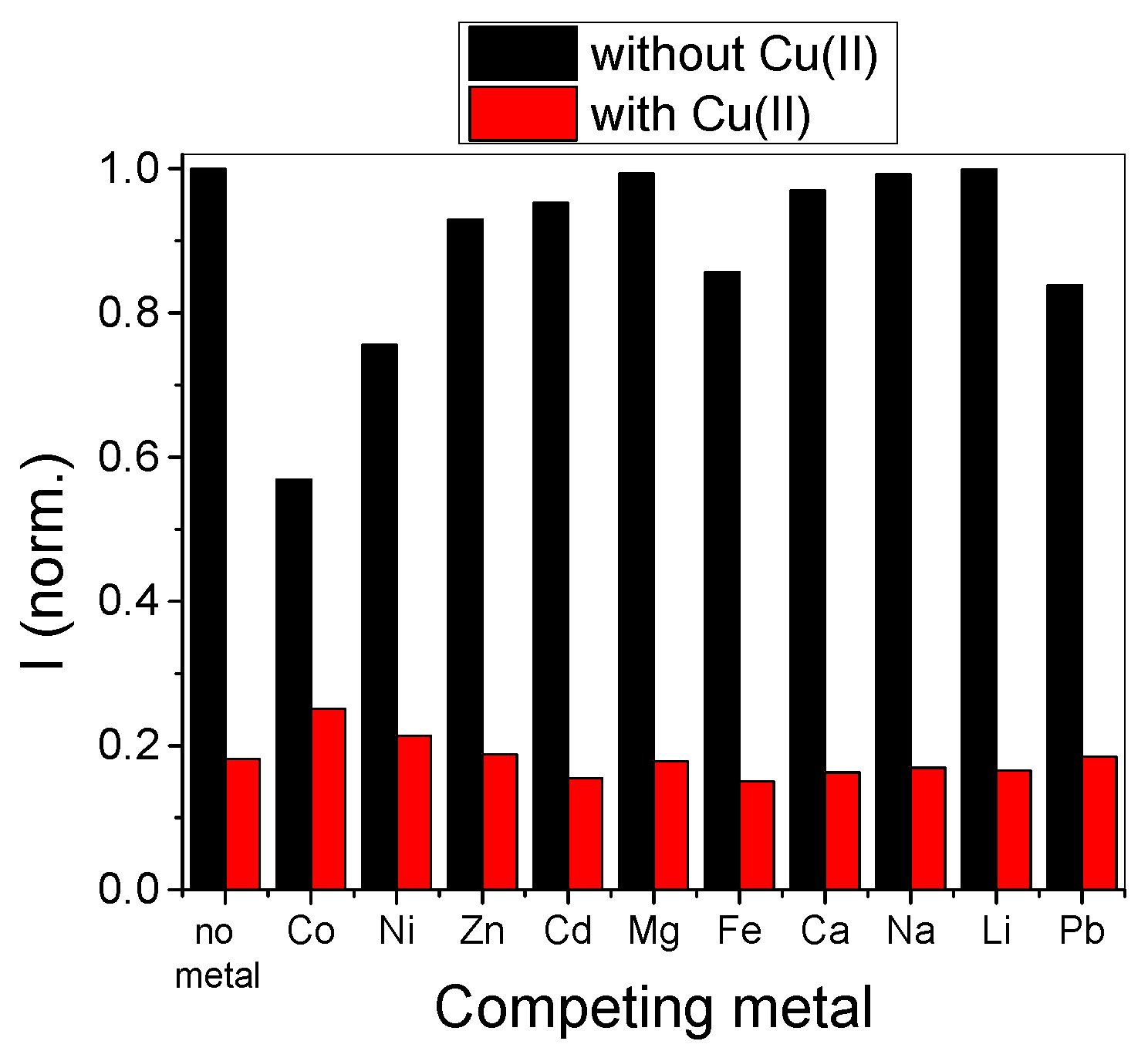
3.3. Metal Sensitivity/Selectivity
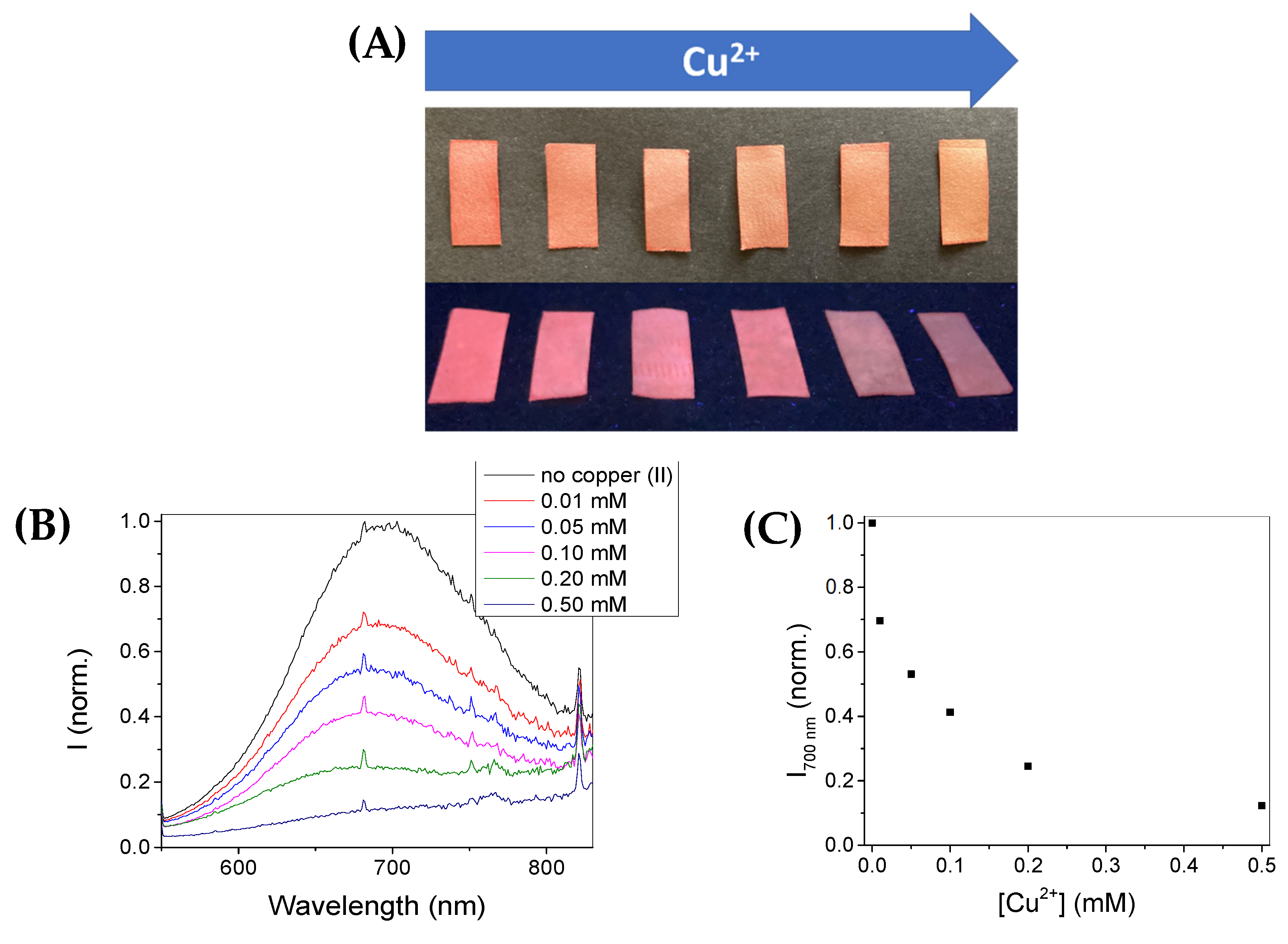
3.4. Paper Test-Strips for Cu2+ Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Yoon, J. Recent Progress on Fluorescent Chemosensors for Metal Ions. Inorg. Chim. Acta 2012, 381, 2–14. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Gerd, M.; Andrea, S.; Lars, H.; Dirk, B.; Thomas, R.; Colin, L.M.; Konrad, B. The Amyloid Precursor Protein of Alzheimer’s Disease in the Reduction of Copper (II) to Copper (I). Science 1996, 271, 1406–1409. [Google Scholar]
- Lutsenko, S. Atp7b-/- Mice as a Model for Studies of Wilson’s Disease. Biochem. Soc. Trans. 2008, 36, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Liu, Y.; Lim, M.H. Untangling Amyloid- β, Tau, and Metals in Alzheimer’s Disease. ACS Chem. Biol. 2013, 8, 856–865. [Google Scholar]
- Liu, X.W.; Xiao, Y.; Zhang, S.B.; Lu, J.L. A Selective Luminescent Sensor for the Detection of Copper (II) Ions Based on a Ruthenium Complex Containing DPA Moiety. Inorg. Chem. Commun. 2017, 84, 56–58. [Google Scholar] [CrossRef]
- Arora, A.; Kaushal, J.; Kumar, A.; Kumar, P.; Kumar, S. Ruthenium(II)-Polypyridyl-Based Sensor Bearing a DPA Unit for Selective Detection of Cu(II) Ion in Aqueous Medium. ChemistrySelect 2019, 4, 6140–6147. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Zhu, Z.; Li, Y.; Zhao, Y.; Liu, X.; Zhang, H. Two New Phosphorescent Ir(III) Complexes as Efficient Selective Sensors for the Cu2+ Ion. Dye. Pigment. 2019, 161, 252–260. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Zhang, W.; Zhou, Y.; Zhao, B.; Li, X. A Colorimetric Probe for Copper(II) Ion Based on 4-Amino-1,8-Naphthalimide. Inorg. Chim. Acta 2012, 381, 111–116. [Google Scholar] [CrossRef]
- Lee, Y.H.; Verwilst, P.; Park, N.; Lee, J.H.; Kim, J.S. Organelle-Selective Di-(2-Picolyl)Amine-Appended Water-Soluble Fluorescent Sensors for Cu(II): Synthesis, Photophysical and in Vitro Studies. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 109–116. [Google Scholar] [CrossRef]
- Moro, A.J.; Santos, M.; Outis, M.; Mateus, P.; Pereira, P.M. Selective Coordination of Cu2+ and Subsequent Anion Detection Based on a Naphthalimide-Triazine(DPA)2 Chemosensor. Biosensors 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.J.; Moreira, T.; Rodríguez, L.; Moro, A.J. Chalcone-Based Fluorescent Chemosensors as New Tools for Detecting Cu2+ Ions. Dye. Pigment. 2022, 197, 109845. [Google Scholar] [CrossRef]
- Huang, J.; Wang, B.; Ye, J.; Liu, B.; Qiu, H.; Yin, S. A Highly Copper-Selective Ratiometric Fluorescent Sensor Based on BODIPY. Chin. J. Chem. 2012, 30, 1857–1861. [Google Scholar] [CrossRef]
- Hafuka, A.; Satoh, H.; Yamada, K.; Takahashi, M.; Okabe, S. 3-[Bis(Pyridin-2-Ylmethyl)Amino]-5-(4-Carboxyphenyl)- BODIPY as Ratiometric Fluorescent Sensor for Cu2+. Materials 2018, 11, 814. [Google Scholar] [CrossRef] [Green Version]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically Diverse Small Molecule Fluorescent Chemosensors for Copper Ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, Y.; Jia, C.; Ji, M. Development and Application of Several Fluorescent Probes in near Infrared Region. Dye. Pigment. 2021, 190, 109284. [Google Scholar] [CrossRef]
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and Opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Tachapermpon, Y.; Thavornpradit, S.; Charoenpanich, A.; Sirirak, J.; Burgess, K.; Wanichacheva, N. Near-Infrared Aza-BODIPY Fluorescent Probe for Selective Cu2+ Detection and Its Potential in Living Cell Imaging. Dalton Trans. 2017, 46, 16251–16256. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Z.; Zhu, W.; Xie, Y.; Tian, H. A Colorimetric and Fluorescent Turn-on Sensor for Pyrophosphate Anion Based on a Dicyanomethylene-4H-Chromene Framework. Chem. Commun. 2008, 5143–5145. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Li, G.; Xiao, H.; Lei, Y.; Huang, X.; Chen, J.; Wu, H.; Ding, J.; Cheng, Y. Aggregation-Induced Fluorescence Emission Properties of Dicyanomethylene-1,4-Dihydropyridine Derivatives. J. Phys. Chem. C 2015, 119, 6737–6748. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, Y.; Liu, M.; Li, F.; Xiao, H.; Chen, J.; Huang, X.; Gao, W.; Wu, H.; Cheng, Y. The Effect of: N-Alkyl Chain Length on the Photophysical Properties of Indene-1,3-Dionemethylene-1,4-Dihydropyridine Derivatives. J. Mater. Chem. C 2016, 4, 5970–5980. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, M.; Lei, Y.; Xie, Y.; Liu, M.; Zhou, Y.; Gao, W.; Huang, X.; Wu, H. An Unexpected 4,5-Diphenyl-2,7-Naphthyridine Derivative with Aggregation-Induced Emission and Mechanofluorochromic Properties Obtained from a 3,5-Diphenyl-4H-Pyran Derivative. Chem.–Asian J. 2020, 15, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Küster, F.W.; Thiel, A. Tabelle per le Analisi Chimiche e Chimico-Fisiche, 14th ed.; Hoepli: Milano, Italy, 1985. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. International Union of Pure Appl. Chem. Compendium of Chemical Terminology; Blackwell Science: West Sussex, UK, 1997. [Google Scholar]
- Drake, J.M.; Lesiecki, M.L.; Camaioni, D.M. Photophysics and Cis-Trans Isomerization of DCM. Chem. Phys. Lett. 1985, 113, 530–534. [Google Scholar] [CrossRef]
- Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Reichardt, C.; Harbusch-Görnert, E. Über Pyridinium-N-phenolat-Betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, X. Erweiterung, Korrektur und Neudefinition der ET-Lösungsmittelpolaritätsskala mit Hilfe eines lipophilen penta-tert-butyl-substituierten Pyridinium-N-phenolat-Betainfarbstoffes. Liebigs Ann. Chem. 1983, 1983, 721–896. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Serpa, C.; Pereira, M.M.; Seixas De Melo, J.; Arnaut, L.G.; Formosinho, S.J. Intramolecular Charge Transfer of P-(Dimethylamino)Benzethyne: A Case of Nonfluorescent ICT State. J. Phys. Chem. A 2001, 105, 10025–10030. [Google Scholar] [CrossRef] [Green Version]
- Moro, A.J.; Cywinski, P.J.; Körsten, S.; Mohr, G.J. An ATP Fluorescent Chemosensor Based on a Zn(II)-Complexed Dipicolylamine Receptor Coupled with a Naphthalimide Chromophore. Chem. Commun. 2010, 46, 1085–1087. [Google Scholar] [CrossRef]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef] [Green Version]
- Alcock-Earley, B.; Garry, L.; McCarthy, C.; Breslin, C.; Rooney, D. The Protection of Water Resources: Developing Novel Sensor Materials; Environmental Protection Agency (EPA): Washington, DC, USA, 2015. [Google Scholar]
- Hadži Jordanov, S.; Maletić, M.; Dimitrov, A.; Slavkov, D.; Paunović, P. Waste Waters from Copper Ores Mining/Flotation in’Bučbim’ Mine: Characterization and Remediation. Desalination 2007, 213, 65–71. [Google Scholar] [CrossRef]
- Santoro, S.; Estay, H.; Avci, A.H.; Pugliese, L.; Ruby-Figueroa, R.; Garcia, A.; Aquino, M.; Nasirov, S.; Straface, S.; Curcio, E. Membrane Technology for a Sustainable Copper Mining Industry: The Chilean Paradigm. Clean. Eng. Technol. 2021, 2, 100091. [Google Scholar] [CrossRef]



| Compound | Conditions/ Solvents | Type of Response | Fluor. QY | Binding Constant | LOD | Ref. |
|---|---|---|---|---|---|---|
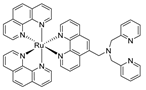 | 10 mM HEPES (pH = 7.2) | Luminescent | - | - | 155 nM | [7] |
 | 10 mM HEPES (pH = 7.2) | Colorimetric/ luminescent | - | 1.14 × 105 M−1 | 18.9 nM | [8] |
 | methanol | Luminescent | 0.062 (in CH2Cl2) | - | 22.3 nM | [9] |
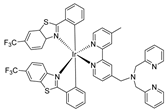 | methanol | Luminescent | 0.301 (in CH2Cl2) | - | 11.5 nM | [9] |
 | Acetonitrile-water (8:2, v/v) | Colorimetric/ luminescent | - | 4.5 × 104 M−1 | - | [10] |
 | 10 mM HEPES (pH = 7.2) | Luminescent | 0.190 | 3.3 × 105 M−1 | 2.09 μM | [12] |
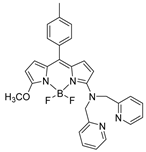 | acetonitrile | Colorimetric/ luminescent | 0.008 | 1.28 × 105 M−1 | 250 nM | [14] |
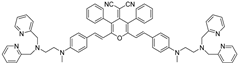 | MeOH-10 mM HEPES (pH = 7.1) (1:1, v/v) | Colorimetric/ luminescent | 0.008 | 7.45 × 107 M−1 | 90.1 nM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karkosik, A.; Moro, A.J. An NIR Emissive Donor-π-Acceptor Dicyanomethylene-4H-Pyran Derivative as a Fluorescent Chemosensor System towards Copper (II) Detection. Chemosensors 2022, 10, 343. https://doi.org/10.3390/chemosensors10080343
Karkosik A, Moro AJ. An NIR Emissive Donor-π-Acceptor Dicyanomethylene-4H-Pyran Derivative as a Fluorescent Chemosensor System towards Copper (II) Detection. Chemosensors. 2022; 10(8):343. https://doi.org/10.3390/chemosensors10080343
Chicago/Turabian StyleKarkosik, Agata, and Artur J. Moro. 2022. "An NIR Emissive Donor-π-Acceptor Dicyanomethylene-4H-Pyran Derivative as a Fluorescent Chemosensor System towards Copper (II) Detection" Chemosensors 10, no. 8: 343. https://doi.org/10.3390/chemosensors10080343
APA StyleKarkosik, A., & Moro, A. J. (2022). An NIR Emissive Donor-π-Acceptor Dicyanomethylene-4H-Pyran Derivative as a Fluorescent Chemosensor System towards Copper (II) Detection. Chemosensors, 10(8), 343. https://doi.org/10.3390/chemosensors10080343







