Characterization of Flexible Copper Selenide Films on Polyamide Substrate Obtained by SILAR Method—Towards Application in Electronic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Film Preparation
2.2. Characterization of Copper Selenide Films
3. Results
3.1. XRD Characterization of Copper Selenide Thin Films
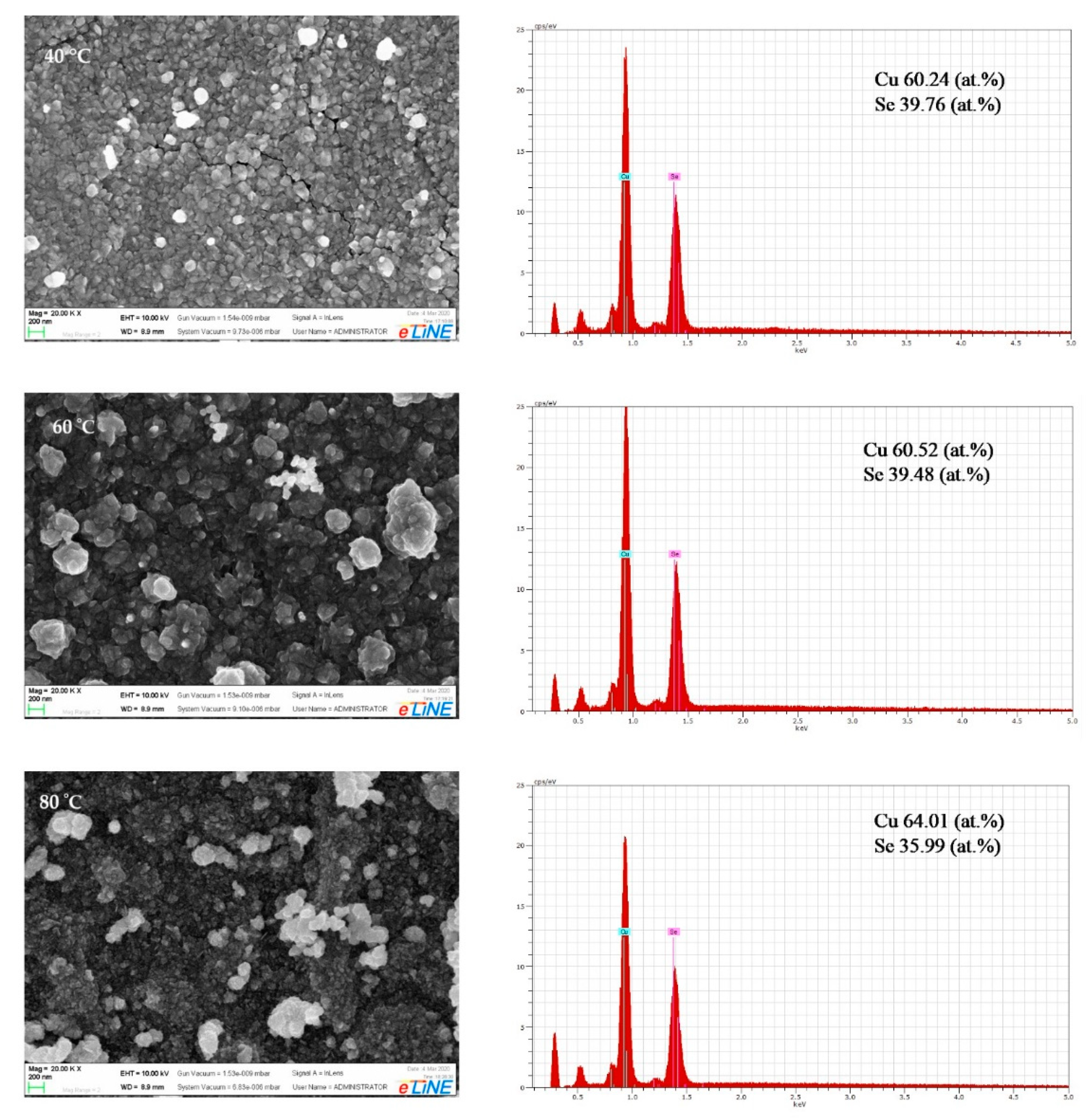
3.2. Scanning Electron Microscopy and Energy Dispersive X-ray Analysis
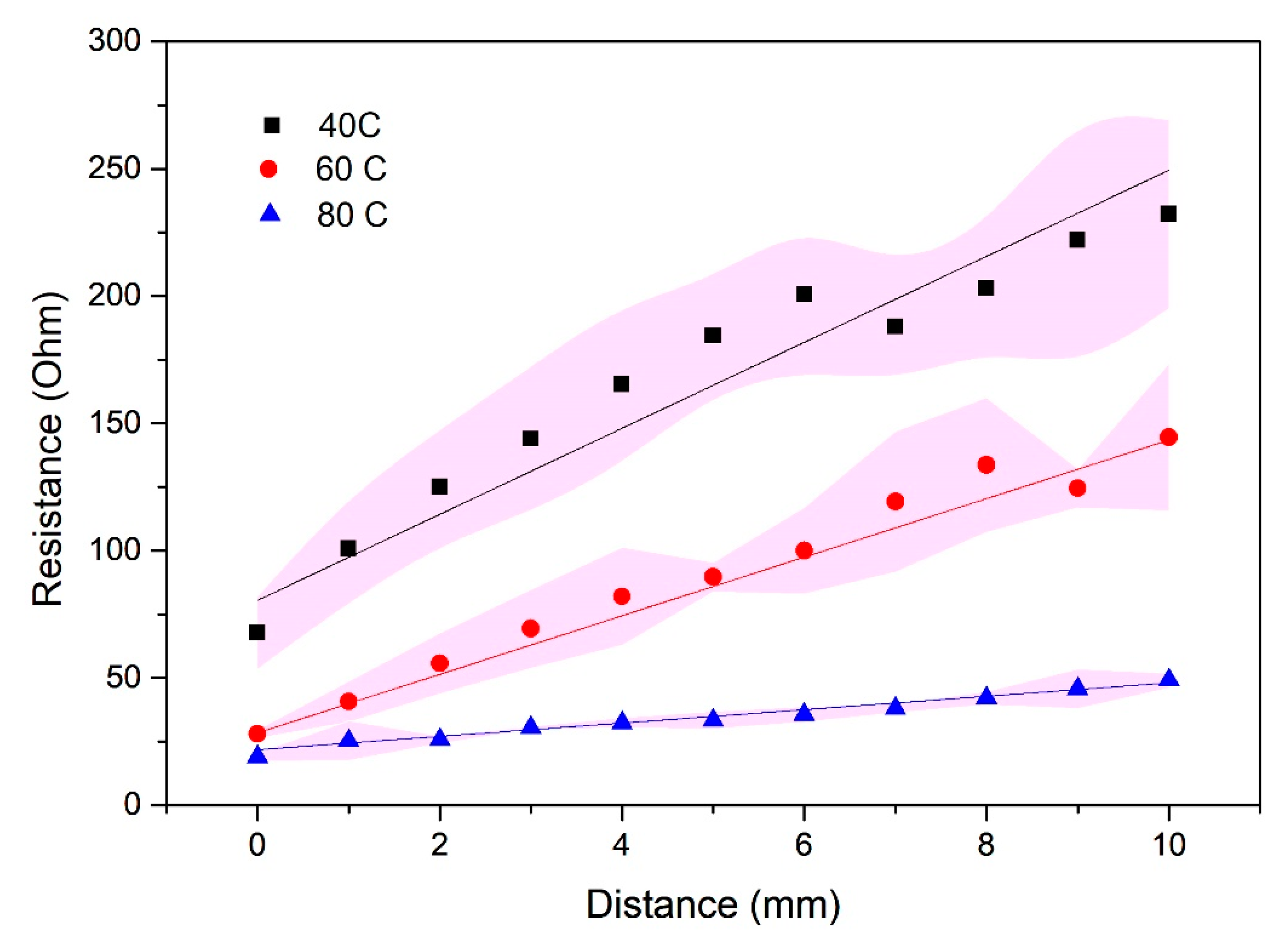
3.3. Measurements of Electrical Resistivity
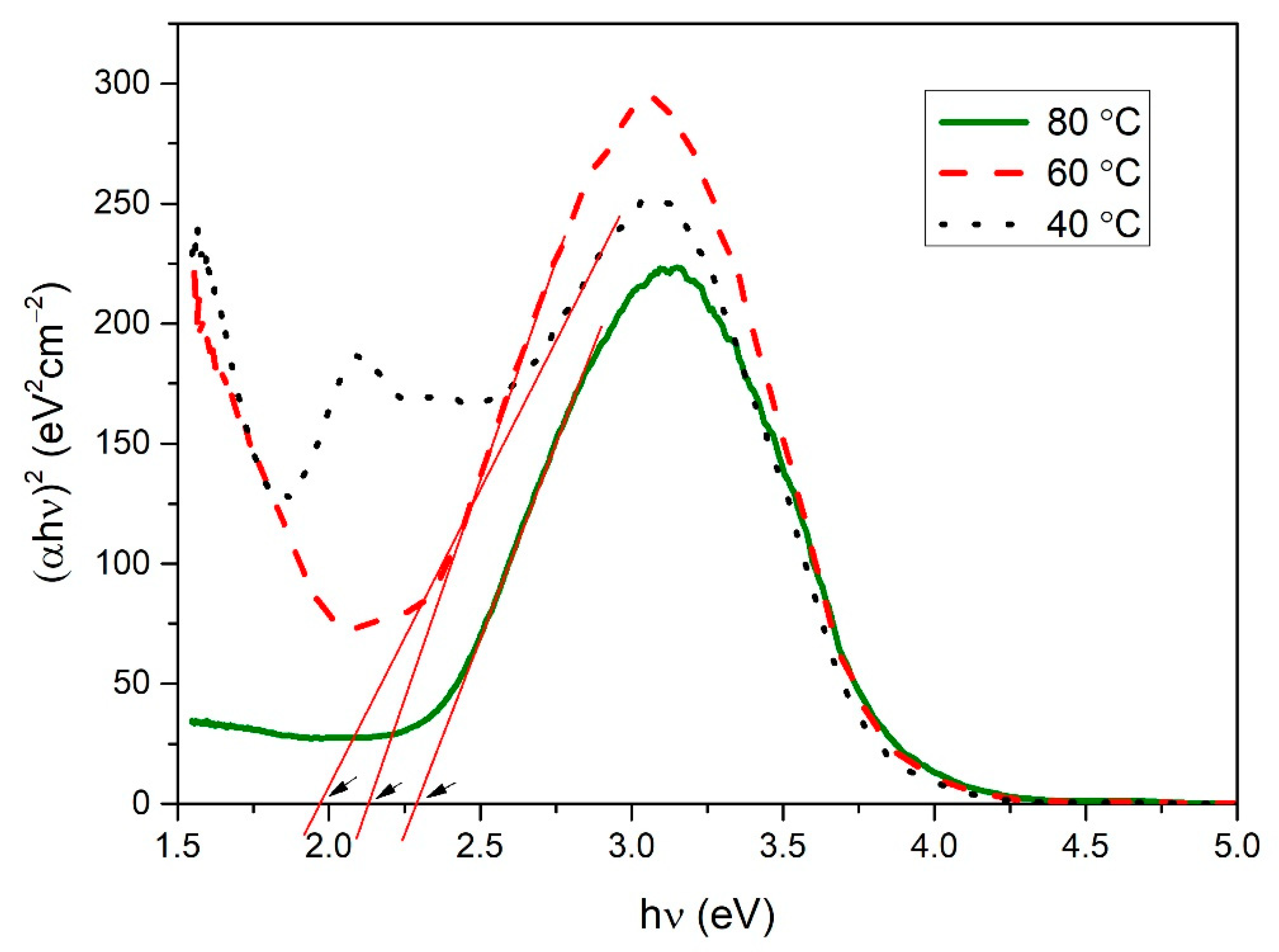
3.4. Optical Analysis of Copper Selenide Thin Films
3.5. Raman Analysis of Copper Selenide Thin Films
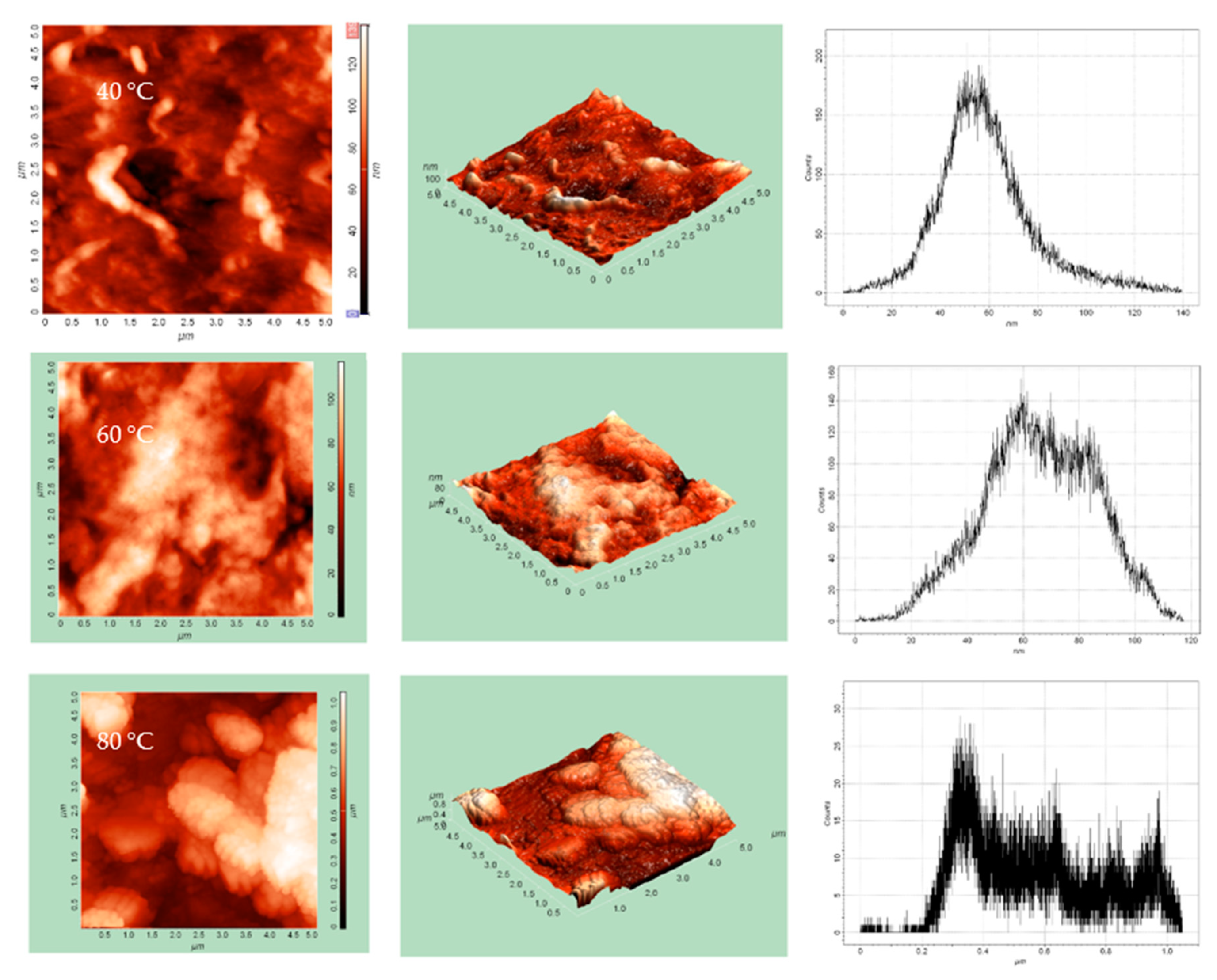
3.6. AFM Analysis of Copper Selenide Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García, V.M.; Nair, P.K.; Nair, M.T.S. Copper Selenide Thin Films by Chemical Bath Deposition. J. Cryst. Growth 1999, 203, 113–124. [Google Scholar] [CrossRef]
- Tyagi, K.; Gahtori, B.; Bathula, S.; Jayasimhadri, M.; Singh, N.K.; Sharma, S.; Haranath, D.; Srivastava, A.K.; Dhar, A. Enhanced Thermoelectric Performance of Spark Plasma Sintered Copper-Deficient Nanostructured Copper Selenide. J. Phys. Chem. Solids 2015, 81, 100–105. [Google Scholar] [CrossRef]
- Thirumavalavan, S.; Mani, K.; Sagadevan, S. Investigation of the Structural, Optical and Electrical Properties of Copper Selenide Thin Films. Mater. Res. 2015, 18, 1000–1007. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Yun, F.F.; Wang, J.; Cheng, Z.; Dou, S.; Wang, J.; Snyder, G.J. The Effects of Te2− and I− Substitutions on the Electronic Structures, Thermoelectric Performance, and Hardness in Melt-Quenched Highly Dense Cu2−xSe. Wiley Online Libr. 2015, 1, 1400015. [Google Scholar] [CrossRef] [Green Version]
- Hussain, R.A.; Hussain, I. Copper Selenide Thin Films from Growth to Applications. Solid State Sci. 2020, 100, 106101. [Google Scholar] [CrossRef]
- Hankare, P.P.; Khomane, A.S.; Chate, P.A.; Rathod, K.C.; Garadkar, K.M. Preparation of Copper Selenide Thin Films by Simple Chemical Route at Low Temperature and Their Characterization. J. Alloy. Compd. 2009, 469, 478–482. [Google Scholar] [CrossRef]
- Silva, S.F.C.; Zanatta, B.S.; Rabelo, A.C.; Bottecchia, O.L.; Tozoni, J.R.; Oliveira, O.N.; Marletta, A. Flexible and Transparent Electrodes of Cu2−XSe with Charge Transport via Direct Tunneling Effect. Adv. Electron. Mater. 2021, 7, 2001189. [Google Scholar] [CrossRef]
- Zyoud, A.; Murtada, K.; Kwon, H.; Choi, H.J.; Kim, T.W.; Helal, M.H.S.; Faroun, M.; Bsharat, H.; Park, D.H.; Hilal, H.S. Copper Selenide Film Electrodes Prepared by Combined Electrochemical/Chemical Bath Depositions with High Photo-Electrochemical Conversion Efficiency and Stability. Solid State Sci. 2018, 75, 53–62. [Google Scholar] [CrossRef]
- Kalenga, M.P.; Govindraju, S.; Airo, M.; Moloto, M.J.; Sikhwivhilu, L.M.; Moloto, N. Fabrication of a Schottky Device Using CuSe Nanoparticles: Colloidal versus Microwave Digestive Synthesis. J. Nanosci. Nanotechnol. 2015, 15, 4480–4486. [Google Scholar] [CrossRef]
- Singh, S.C.; Li, H.; Yao, C.; Zhan, Z.; Yu, W.; Yu, Z.; Guo, C. Structural and Compositional Control in Copper Selenide Nanocrystals for Light-Induced Self-Repairable Electrodes. Nano Energy 2018, 51, 774–785. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Q.; Kang, W.; Huang, X.; Wu, C.; Wang, L.; Luo, L.; Zhang, W.; Lee, C.S. Water Evaporation Induced Conversion of CuSe Nanoflakes to Cu2−XSe Hierarchical Columnar Superstructures for High-Performance Solar Cell Applications. Part. Part. Syst. Charact. 2015, 32, 840–847. [Google Scholar] [CrossRef]
- Zhou, R.; Huang, Y.; Zhou, J.; Niu, H.; Wan, L.; Li, Y.; Xu, J.; Xu, J. Copper Selenide (Cu3Se2 and Cu2−xSe) Thin Films: Electrochemical Deposition and Electrocatalytic Application in Quantum Dot-Sensitized Solar Cells. Dalton Trans. 2018, 47, 16587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, Y. Ratiometry, Wavelength, and Intensity: Triple Signal Readout for Colorimetric Sensing of Mercury Ions by Plasmonic Cu2−xSe Nanoparticles. ACS Sens. 2016, 1, 384–391. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, F.; Wu, Y.; Yang, L.; Zhang, F.; Huang, J. Template Synthesis of Cu2−xSe Nanoboxes and Their Gas Sensing Properties. Cryst. Eng. Comm. 2012, 14, 3528–3533. [Google Scholar] [CrossRef]
- Astam, A.; Akaltun, Y.; Yildirim, M. Conversion of SILAR Deposited Cu3Se2 Thin Films to Cu2−XSe by Annealing. Mater. Lett. 2016, 166, 9–11. [Google Scholar] [CrossRef]
- Bhuse, V.M.; Hankare, P.P.; Garadkar, K.M.; Khomane, A.S. A Simple, Convenient, Low Temperature Route to Grow Polycrystalline Copper Selenide Thin Films. Mater. Chem. Phys. 2003, 80, 82–88. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Wu, Z.; Luo, D.; Yu, H.-Y.; Lu, Z.-H. Review and Perspective of Materials for Flexible Solar Cells. Mater. Rep. Energy 2021, 1, 100001. [Google Scholar] [CrossRef]
- Yoon, H.; Kang, S.M.; Lee, J.K.; Choi, M. Hysteresis-Free Low-Temperature-Processed Planar Perovskite Solar Cells with 19.1% Efficiency. Energy Environ. Sci. 2016, 9, 2262–2266. [Google Scholar] [CrossRef]
- Aernouts, T.; Vanlaeke, P.; Geens, W.; Poortmans, J.; Heremans, P.; Borghs, S.; Mertens, R.; Andriessen, R.; Leenders, L. Printable Anodes for Flexible Organic Solar Cell Modules. Thin Solid Film. 2004, 451–452, 22–25. [Google Scholar] [CrossRef]
- Fonrodona, M.; Escarré, J.; Villar, F.; Soler, D.; Asensi, J.M.; Bertomeu, J.; Andreu, J. PEN as Substrate for New Solar Cell Technologies. Sol. Energy Mater. Sol. Cells 2005, 89, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Gu, Z.G.; Zhang, J. Chiral-Induced Ultrathin Covalent Organic Frameworks Nanosheets with Tunable Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 7245–7252. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abbas, G.; Li, N.; Afzal, A.; Haider, Z.; Ahmed, S.; Xu, X.; Pan, C.; Peng, Z. Significance of Flexible Substrates for Wearable and Implantable Devices: Recent Advances and Perspectives. Adv. Mater. Technol. 2022, 7, 2100773. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, S.; Hu, Q.; Yu, F.; Cheng, Y.; Huang, K.; Yuan, H.; Jiang, J.; Li, W.; Li, J.; et al. Vertical Graphene-Coated Cu Wire for Enhanced Tolerance to High Current Density in Power Transmission. Nano Res. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, C.; Yang, T.; Li, W.; Xu, H.; Sun, Z.; Chen, Y.; Gao, C.; Yang, T.; Li, W.; et al. Research Advances of Ferroelectric Semiconductors of 2D Hybrid Perovskites toward Photoelectronic Applications. Chin. J. Struct. Chem. 2022, 41, 2204001–2204011. [Google Scholar] [CrossRef]
- Chang, H.B.; Liu, J.B.; Dong, Z.; Wang, D.D.; Xin, Y.; Jiang, Z.L.; Tang, S.S. Enhancement of Photocatalytic Degradation of Polyvinyl Chloride Plastic with Fe2O3 Modified AgNbO3 Photocatalyst under Visible-Light Irradiation. Jiegou Huaxue 2021, 40, 1595–1603. [Google Scholar] [CrossRef]
- Vikulov, S.; di Stasio, F.; Ceseracciu, L.; Saldanha, P.L.; Scarpellini, A.; Dang, Z.; Krahne, R.; Manna, L.; Lesnyak, V. Fully Solution-Processed Conductive Films Based on Colloidal Copper Selenide Nanosheets for Flexible Electronics. Adv. Funct. Mater. 2016, 26, 3670–3677. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Hu, J.; Liu, X.; Li, Z.; Ding, Y. Morphology and Mechanical Properties of Polyimide Films: The Effects of UV Irradiation on Microscale Surface. Materials 2017, 10, 1329. [Google Scholar] [CrossRef] [Green Version]
- Parodi, E.; Peters, G.W.M.; Govaert, L.E. Structure-Properties Relations for Polyamide 6, Part 1: Influence of the Thermal History during Compression Moulding on Deformation and Failure Kinetics. Polymers 2018, 10, 710. [Google Scholar] [CrossRef] [Green Version]
- Janickis, V.; Petrašauskiene, N.; Žalenkiene, S.; Morkvenaite-Vilkoňciene, I.; Ramanavǐcius, A. Morphology of CdSe-Based Coatings Formed on Polyamide Substrate. J. Nanosci. Nanotechnol. 2018, 18, 604–613. [Google Scholar] [CrossRef]
- Ivanauskas, R.; Milasiene, D. Fabrication of Polyamide-Ag2Se Composite Films with Controllable Properties by an Adsorption–Diffusion Method. J. Phys. Chem. Solids 2020, 145, 109533. [Google Scholar] [CrossRef]
- Lakshmi, M.; Bindu, K.; Bini, S.; Vijayakumar, K.P.; Sudha Kartha, C.; Abe, T.; Kashiwaba, Y. Chemical Bath Deposition of Different Phases of Copper Selenide Thin Films by Controlling Bath Parameters. Thin Solid Film. 2000, 370, 89–95. [Google Scholar] [CrossRef]
- Xu, S.; Wang, H.; Zhu, J.J.; Chen, H.Y. Sonochemical Synthesis of Copper Selenides Nanocrystals with Different Phases. J. Cryst. Growth 2002, 234, 263–266. [Google Scholar] [CrossRef]
- Li, Y.D.; Fan, P.; Zheng, Z.H.; Luo, J.T.; Liang, G.X.; Guo, S.Z. The Influence of Heat Treatments on the Thermoelectric Properties of Copper Selenide Thin Films Prepared by Ion Beam Sputtering Deposition. J. Alloy. Compd. 2016, 658, 880–884. [Google Scholar] [CrossRef]
- Güzeldir, B.; Sağlam, M. Using Different Chemical Methods for Deposition of Copper Selenide Thin Films and Comparison of Their Characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 111–119. [Google Scholar] [CrossRef]
- Pathan, H.M.; Lokhande, C.D. Deposition of Metal Chalcogenide Thin Films by Successive Ionic Layer Adsorption and Reaction (SILAR) Method. Bull. Mater. Sci. 2004, 27, 85–111. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg Apr, W.L.; H Bragg, B.W.; Professor of Physics, C. The Reflection of X-Rays by Crystals. Proc. R. Soc. London. Ser. A Contain. Pap. A Math. Phys. Character 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-Ray Diffraction Analysis by Williamson-Hall, Halder-Wagner and Size-Strain Plot Methods of CdSe Nanoparticles—A Comparative Study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Ashraf, M.; Akhtar, S.M.J.; Khan, A.F.; Ali, Z.; Qayyum, A. Effect of Annealing on Structural and Optoelectronic Properties of Nanostructured ZnSe Thin Films. J. Alloy. Compd. 2011, 509, 2414–2419. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi (B) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrović, M.; Gilić, M.; Ćirković, J.; Romčević, M.; Romčević, N.; Trajić, J.; Yahia, I. Optical Properties of CuSe Thin Films—Band Gap Determination. Sci. Sinter. 2017, 49, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.S.; Janke, A.; Gohs, U.; Heinrich, G. Electron-Induced Reactive Processing of Polyamide 6/Polypropylene Blends: Morphology and Properties. Eur. Polym. J. 2018, 98, 295–301. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, B.Z. The Effects of Annealing (Solid and Melt) on the Time Evolution of the Polymorphic Structure of Polyamide 6. J. Appl. Polym. Sci. 2010, 115, 1688–1694. [Google Scholar] [CrossRef]
- Minceva-Sukarova, B.; Najdoski, M.; Grozdanov, I.; Chunnilall, C.J. Raman Spectra of Thin Solid Films of Some Metal Sulfides. J. Mol. Struct. 1997, 410–411, 267–270. [Google Scholar] [CrossRef]
- Malavekar, D.B.; Bulakhe, R.N.; Kale, S.B.; Patil, U.M.; In, I.; Lokhande, C.D. Synthesis of Layered Copper Selenide on Reduced Graphene Oxide Sheets via SILAR Method for Flexible Asymmetric Solid-State Supercapacitor. J. Alloy. Compd. 2021, 869, 159198. [Google Scholar] [CrossRef]



| Miller Indices (hkl) | Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PA | 40 °C | 60 °C | 80 °C | ||||||
| (2θ) | d (Å) | (2θ) | d (Å) | (2θ) | d (Å) | (2θ) | d (Å) | ||
| PA | (020γ) | 9.6 | 9.21 | 9.65 | 9.16 | 10.2 | 8.67 | 9.6 | 9.21 |
| (100α) | 20.35 | 4.36 | 20.3 | 4.37 | 20.95 | 4.24 | 20.25 | 4.38 | |
| (001α) | 23.8 | 3.74 | 24 | 3.70 | 24.3 | 3.66 | 23.75 | 3.74 | |
| Cu2−xSe | (111) | – | – | 27.15 | 3.28 | 27.7 | 3.22 | 27.1 | 3.28 |
| PA | (002/202α) | 28.75 | 3.10 | 28.8 | 3.10 | 29.4 | 3.04 | 28.75 | 3.10 |
| Cu2−xSe | (220) | – | – | 44.95 | 2.02 | 45.5 | 1.99 | 44.8 | 2.02 |
| Temperature | 2θ (°) | β (°) | D (nm) | δ·10−3 (nm−2) | ε·10−3 (nm−2) |
|---|---|---|---|---|---|
| 40 °C | 44.95 | 1.30 | 6.61 | 22.89 | 13.72 |
| 60 °C | 45.5 | 0.60 | 14.33 | 4.86 | 6.25 |
| 80 °C | 44.8 | 0.54 | 15.81 | 4.00 | 5.76 |
| Parameters | Temperature | ||
|---|---|---|---|
| 40 °C | 60 °C | 80 °C | |
| Maximum height of peaks, hmax, nm | 1048.41 | 117.32 | 123.16 |
| Average height, hmean, nm | 565.57 | 65.64 | 49.99 |
| Average Roughness, Ra, nm | 194.62 | 16.24 | 13.32 |
| RMS Roughness, Rq, nm | 227.76 | 19.90 | 17.21 |
| Surface skewness, Rsk | 0.42 | −0.13 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubauskas, G.; Gilic, M.; Paluckiene, E.; Mitric, J.; Cirkovic, J.; Ralevic, U.; Usoviene, E.; Griskonis, E.; Petrasauskiene, N. Characterization of Flexible Copper Selenide Films on Polyamide Substrate Obtained by SILAR Method—Towards Application in Electronic Devices. Chemosensors 2022, 10, 313. https://doi.org/10.3390/chemosensors10080313
Jakubauskas G, Gilic M, Paluckiene E, Mitric J, Cirkovic J, Ralevic U, Usoviene E, Griskonis E, Petrasauskiene N. Characterization of Flexible Copper Selenide Films on Polyamide Substrate Obtained by SILAR Method—Towards Application in Electronic Devices. Chemosensors. 2022; 10(8):313. https://doi.org/10.3390/chemosensors10080313
Chicago/Turabian StyleJakubauskas, Gediminas, Martina Gilic, Edita Paluckiene, Jelena Mitric, Jovana Cirkovic, Uros Ralevic, Egle Usoviene, Egidijus Griskonis, and Neringa Petrasauskiene. 2022. "Characterization of Flexible Copper Selenide Films on Polyamide Substrate Obtained by SILAR Method—Towards Application in Electronic Devices" Chemosensors 10, no. 8: 313. https://doi.org/10.3390/chemosensors10080313
APA StyleJakubauskas, G., Gilic, M., Paluckiene, E., Mitric, J., Cirkovic, J., Ralevic, U., Usoviene, E., Griskonis, E., & Petrasauskiene, N. (2022). Characterization of Flexible Copper Selenide Films on Polyamide Substrate Obtained by SILAR Method—Towards Application in Electronic Devices. Chemosensors, 10(8), 313. https://doi.org/10.3390/chemosensors10080313









