Abstract
Pressure ulcers (PUs) are a serious global health challenge, affecting a large section of the population and putting immense pressure on healthcare systems. Sensor-based diagnostic tools and monitoring systems have emerged as a potential non-invasive solution to reduce the occurrence of new cases of PUs and promise a significant reduction in treatment expenditure and time. In this endeavour, the present manuscript reviews the advancements made in the last decade in the development and commercial adoption of different sensor systems for PU-associated chronic wound management. Different types of smart sensor systems have been developed in which pressure, chemical, and optical sensors have witnessed a lot of interest and significant advancement among research communities and industries alike. These sensors utilize a host of nanomaterial-based sensing materials, flexible support, diverse transducing modes, and different device designs to achieve high sensitivity and selectivity for skin pressure, temperature, humidity, and biomarkers released from the wound. Some of these sensor’s array-based electronic skin (e-skin) has reached the stage of commercialization and is being used in commercial products, such as smart bandages, shoes, watches, and mattress among others. Nonetheless, further innovations are necessary in the direction of associating multiple types of sensor arrays, particularly pressure and chemical sensor-based e-skins in a microsystem for performing real-time assessment of all the critical wound parameters.
1. Introduction
With the ageing of the global population, especially in developed countries, and the rise in the sedentary lifestyle because of the technological revolution, new health challenges are emerging. One of the most evident outcomes is pressure ulcers (PUs) or bedsores, whose spread has become pervasive in recent years and put a tremendous burden on public healthcare. PUs are a type of chronic wound which takes an exceedingly long time, spanning from a few weeks to years, to heal compared with acute wounds, which heal rather fast and in a time-bound manner [1]. PUs originate mainly from lack of mobility in the body parts. Prolonged immobility causes the accumulation of external pressure on the soft subcutaneous tissues, resulting in their swelling, which progressively takes the form of an ulcer. According to European Pressure Ulcers Advisories and Prevention (EPUAP) guidelines [2], the growth of PUs has been divided into four different phases, as depicted in Figure 1. The first phase comprises inflammation of the skin, which takes place because of the blockage of lymph fluid’s smooth movement, owing to the compression of the blood vessels. It causes building up of the fluid and the associated waste in the interstitial space between the tissues, resulting in its swelling. At this stage, the skin does not rupture and is characterized by the appearance of redness. As the skin compression continues, the PU passes into the second stage, in which the superficial skin is broken, and the underlayer dermis or epidermis is exposed. With the further continuation of skin pressure, the PU grows in the third stage. In this stage, the skin is fully broken, and the subcutaneous fat layer is exposed and removed. In the fourth and final stage, the PU expands further to reach the underlying bones, muscles, and tendons, which is the most severe state of the ulcer. In this stage, an ulcer may also expand through, undermining and tunneling to the deeper tissues and the fat layers. The final stage of a PU is prone to transcend into secondary and more serious infections which may be life-threatening if left untreated.
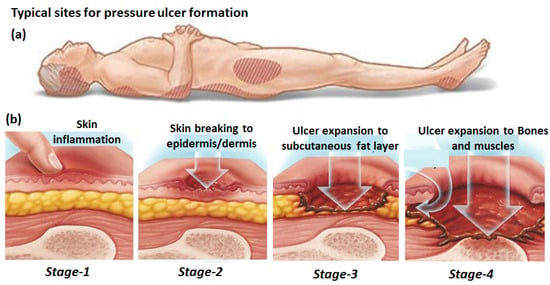
Figure 1.
Potential position of the body prone to pressure ulcer development (a) and four different stages of pressure ulcer formation (b). Adapted from [3].
Other than the extrinsic factors causing continuous pressure build-up, consequently causing different phases of PU development, there are some additional intrinsic factors. They include diabetes, obesity, spinal disorders, malnutrition, renal diseases, and frequent smoking, which further amplify the severity of PUs. Indeed, people with diabetes and spinal disorders form the largest group of PU patients [4,5]. Among the different risk groups, nursing home patients account for the largest share of PUs, which is attributed to a combination of extrinsic and intrinsic factors [6]. The global prevalence of PUs is huge, and it is estimated that around 1–2% of the populations in developed countries experience the occurrence of PUs in their lifetimes [7]. With the ageing of the global population and the rise of morbidities such as diabetes, this proportion is expected to rise sharply. The prevalence of PUs among hospitalized adults in different countries was systematically studied recently, which reported that around 12.8% of patients suffered from PUs [8]. Among this group, the highest proportion was recorded in Europe and North America [9] (Figure 2a), comprising more than 75% of the cases of PUs. Such wide-scale prevalence of PUs and its long recovery periods has seriously affected public healthcare systems across the globe, putting immense burden on the medical staffs and healthcare budgets [10]. In a recent comprehensive survey, it was reported that the costs of PU treatment per patient varied between EUR 1.71 and 470.49 every day, depending on the stage of PU and labor costs. Such a staggering cost, combined with the huge number of patients and longer treatment period, resulted in an annual financial burden of USD 25 billion in the USA, USD 13 billion in Europe, USD 9 billion in Asia, and USD 3.5 billion in Australia (Figure 2b) [11].
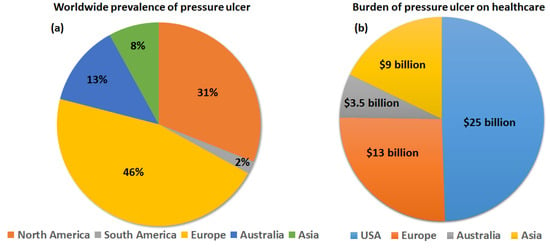
Figure 2.
Representation of the worldwide prevalence (a) and financial burden (b) of PUs.
Such a huge medical expenditure is partly attributed to the current methods of chronic wound management, mainly involving visual inspection and diagnostic dressing. The former approach is based on regular assessment of the skin’s surface around the wound and tracking the wound’s healing kinetics. However, this requires in-depth clinical experiences, which take many years to accumulate and are unable to provide information about dead and infected tissues below the skin, which are mainly responsible for the stagnation of the wound-healing process. The other approach involves wound-healing dressing. The traditional dressing mainly involves applying gauze, plaster, and bandages to the wounds, which are made up of cotton-based natural fibers as well as synthetic fibers (rayon and polyesters). These dressings provide some level of deterrence against bacterial infection, as they can absorb wound exudates and fluid. However, in an open wound with a lot of fluid generation, such dressings require frequent changing to avoid healthy tissue maceration, which adds to the nursing time and thus generates higher treatment costs. Moreover, these dressings absorb the moisture content of the wound, which retards its healing process and causes stiff adherence of the dressing materials to the wound, which results in pain while removing the dressing.
To overcome the limitations of the traditional wound dressing, different modern dressings have been developed [12] which promote healing of the wound in addition to external covering. These dressings are made up of synthetic polymers, which can be passive, interactive, or bioactive to the wound’s surface. Semi-permeable film and foam-based dressings have shown advantages in highly exudating wounds because of their excellent absorption efficiency. Moreover, they maintain the required moisture level around the wound and promote granulation of new tissues around the wound by permeating water vapor, O2, and CO2. However, they need to be regularly changed and are not suitable for dry wounds. Hydrogels and hydrocolloid-based dressings are hydrophilic in nature and are suitable for dry chronic wounds. They maintain the necessary moisture level near the wound site for the germination of new tissues and contain antibacterial membranes to prevent infections. These dressings are applied these days in treating foot ulcers. Seaweed-derived alginate dressings have drawn attention recently for treating moderately and highly exudating wounds. The alginates exchange ions with the blood to form a protective coating around the wound and minimize bacterial infections. Biomaterial-based wound dressings are another emerging strategy, as they are biocompatible, biodegradable, non-toxic, and promote natural wound healing. These dressings favor collagen synthesis and thus accelerate the granulation of new tissues at the wound sites. Aside from these, tissue-engineered artificial skin substitutes, medicated dressings, and composite dressings are some recently reported methods for chronic wound management which are claimed to be highly promising for the fast healing of wounds. Nanomaterials are also gaining significant relevance in wound dressing development [13], but such dressings require significant cost reduction to reach successful commercialization.
Because of the huge expenditure and human-intensive nursing associated with PU treatment, focus is now shifting to preventing the occurrence of PUs or detecting them in the early stages, which is much cheaper [14]. Moreover, it improves the quality of life of the patients, as they do not have to undergo the extended painful situation encountered in the advanced stages of PUs. The key strategy that is being adopted for this purpose is the development of miniaturized monitoring platforms [15]. These platforms can provide a real-time evolution of physical and chemical analytes, such as the pressure, pH, ions, chemical compounds, and gases in the wound or in the anticipated zone of wound formation. Moreover, by integrating wireless data transmission systems and artificial intelligence to these monitoring platforms, they can further reduce the workload on healthcare infrastructures. Considering the requirement of real-time monitoring of different physio-chemical parameters, the interest in sensor development is tremendously increasing in the area of chronic wound management. A recent review by Walia et al. [16] presented an advancement in the pressure-monitoring sensor systems and highlighted the efficiency of these monitoring systems in preventing chronic wound occurrence (88%). The development of different biological or chemical sensors to detect biochemical species in wound exudates, such as uric acid, protease, pH, moisture, and temperature, was also recently discussed [17,18]. The utilization of biopolymeric materials in sensor development for monitoring the wound-healing process was explored, which highlighted the faster healing of the wounds by strengthening the natural antibodies of patients [19]. The potential application of wearable electrochemical sensors in this domain was explored [20], highlighting major advances in the non-invasive monitoring of the chemical constituents in sweat, tears, or saliva. Trung et al. [21] also focused on the development of flexible and stretchable physical sensor-integrated platforms for wearable human activity monitoring and personal healthcare. Swisher et al. [22] developed a flexible electronic device which is able to map damaged tissue in a non-invasive way by pressure measurement.
From this quick overview on the current non-invasive methods for monitoring chronic wounds, it is evident that sensors are highly promising in smart chronic wound management strategies. Although some reviews have reported the advancements made in the last decade in wearable sensor technologies, an intercomparison of different sensing methods and the advancements made in the integration of these sensors into marketable technologies for wound management are missing points. In this endeavour, the objective of this review is to present a non-exhaustive overview of the recently developed sensing techniques utilizing different types of transducing modes. The focus area would be to assess the progress made in the last decade in pressure sensors, volatile organic compound (VOC) chemical sensors, and optical analysis. Finally, the status of technological integration of these sensors into commercialized products will be briefly reviewed.
2. Chronic Wound Monitoring
2.1. Pressure Monitoring
To reduce the occurrence of PUs, a common prevention approach is to reduce the prolonged exposure of targeted subjects to extended and extreme pressure. Currently, this method is implemented only on a subjective basis at a predefined schedule. In this approach, the actual pressure on human tissues is continuously monitored and compared to a reference threshold. Any excess pressure from this threshold is given as a warning signal from the sensor. The desired range of pressure measurements can vary depending on the wound type and its healing or infection status. However, a broad range from 1.5 kPa to 5.5 kPa was reported for different types of wounds [23]. Moreover, a resolution of 50 Pa is desired to accommodate different scenarios of wound healing or infection during the monitoring process [24]. These sensors are often integrated in flexible and stretchable electronic devices in combination with artificial intelligence, mimicking the human curvilinear tissues, and they are often termed as electronic skin (e-skin) [25]. Here, a review on recent advancements in e-skin and its potential for wound monitoring by pressure, strain, shear force, and twist deformation measurements is discussed.
2.1.1. Different Transduction Mechanisms
To measure the parameter of pressure variation, sensors based on different types of transducer have been developed, in which the piezoresistive [26], capacitive [27], and piezoelectric [28] types are worth mentioning. Piezoresistive sensors are extensively used because of their simple design and working mechanism, in which an external force application induces a change in the resistance that can be detected by an electrical measurement set-up.
For instance, while using a conductive nanocomposite as a sensing material, which has also been extensively used in such sensors’ development, resistance changes are encountered at the contact point of an interconnected conductive network when external pressure is applied [26]. A mechanistic scheme is depicted in Figure 3a, exhibiting a conducting network formed by the percolation of nanofiller material within an insulating polymer matrix. Charge conduction takes place by electron tunneling at the nanodomain junction of the nanofiller material. When such materials are used as a sensing coating for a piezoresistive pressure sensor, under the state of depression (without any external pressure imposition), there is an optimum nanoscale distance between the nanofiller particles, resulting in a steady state resistance. However, under the state of compression (imposition of external pressure), the nanoscale distance between the nanofiller particles is reduced, facilitating the electron tunneling process and thus decreasing the resistance value from the steady state level. These sensors are highly sensitive, owing to large change in the tunneling resistance with a small variation in the nanoscale distance, resulting from external pressure application. Previously, different types of filler materials, such as metallic particles [29], carbon nanoparticles (CNPs) [30], carbon nanotubes (CNTs) [31], and graphene [32], have been investigated to enhance the mechanical and electrical properties of these nanocomposites. Park et al. [33] reported highly sensitive piezoresistive sensors based on CNT-filled elastomer composites which works on the principle of electron tunneling resistance variations between CNT strands with external stress. Bao et al. [34] developed a pressure sensor array based on elastic hollow sphere conductive polymers, exhibiting a fast response and operating in a low-pressure regime (as low as 1 Pa). Tung et al. [26] reported a simple, scalable, and low-cost method of piezoresistive sensor development by dispersing reduced graphene oxide (rGO) into a poly(epoxide) matrix (EP), demonstrating high sensing performances. Aside from that, for carbon-based materials, Gong et al. reported on a highly sensitive wearable piezoresistive sensor for measuring the resistances between gold nanowire and interdigitated electrode arrays on an elastic substrate [35], and it was capable of measuring the pressure in a range of 13–50,000 Pa.
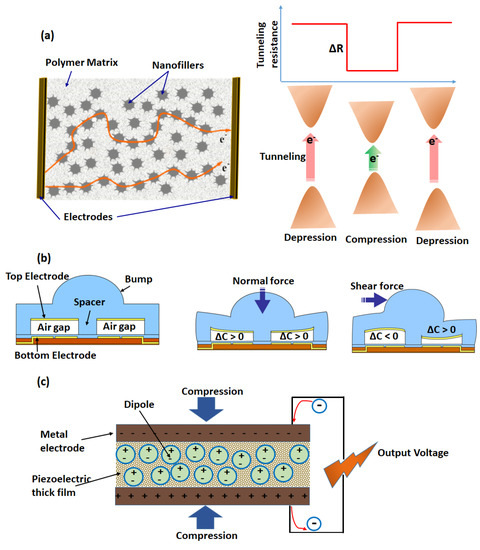
Figure 3.
Schematic illustrations of three common transduction methods for pressure sensing based on piezoresistive (a), capacitive (b), and piezoelectric types (c). (b) is adapted from [36].
Capacitive sensors were widely used to measure different forces resulting from pressure or shearing. The left image panel of Figure 3b depicts the sensor components, consisting of two parallel plates separated by an air gap, a spacer, and a bump. Upon application of a normal force on the bump, the air gap is reduced between the plates, and consequently, the capacitance is increased [36]. On the other hand, when a shear force is applied (right image panel of Figure 3b), the air gap is increased between the plates close to the point of application on the bump, while the air gap is decreased between the plates away from the bump. Accordingly, the capacitance changes in the opposite way. Aside from a low-power consumption and the capability to precisely modify the device’s design, the key advantage of these sensors is their high strain sensitivity with a static force [37]. By using CNT electrodes on an elastic substrate, Bao et al. developed an array of transparent and stretchable capacitive sensors showing high sensitivity to both pressure and strain [38]. With the rapid development of flexible field effect transistors, capacitive tactile sensors with variable effective dielectric constants have attracted great interest [39]. Recently, Wang et al. [40] presented a high-resolution, pressure-sensitive, flexible, and durable triboelectric sensor matrix that can visualize touch actions or track motion trajectories in real time. The structure of the typical transistor usually consists of a gate electrode, source and drain electrodes, a semiconductor, and a gate dielectric. Taking advantage of the microstructural air gaps in the elastic dielectric layer, the capacitive transistor displayed high sensitivity and accurately measured the distribution of pressure over a large area [41]. To impart stretchable functions, highly stretchable capacitive sensors with silver nanowire electrodes were also studied [42]. It was found that the compressibility of the capacitive sensor could be enhanced due to the air gaps between the silver nanowires.
Piezoelectricity is characterized by the variation of electrical properties in certain materials under the application of an external mechanical force. The origin of such a property is attributed to electrical dipole moments arranged in an organized fashion (Figure 3c). Because of the strain-dependent charge change, piezoelectric materials have typically been used for sensor applications. Dipole moments could be produced either by porous electrets with long-lasting positive carriers in the pores [43] or through the deformation of oriented non-centrosymmetric crystal structures [44]. The change in electrical signals via piezoelectric materials was used to detect mechanical stresses with high sensitivity, fast response, and a high piezoelectric coefficient (d33). Piezoelectric inorganic materials typically exhibit high d33 values but low flexibility, while piezoelectric polymers display the opposite behavior. For obtaining a high d33 with flexible piezoelectric pressure sensors, piezoelectric inorganic thin films [45] and piezoelectric polymers [46] were studied. For example, Dagdeviren et al. presented piezoelectric transducers based on thin films of PZT on elastomer substrates, exhibiting low hysteresis measurements of pressure on the skin with high levels of sensitivity [47]. Oriented piezoelectric nanowires (NWs) and nanobelts (NBs) with intrinsically high piezoelectricity and good mechanical stability are of great interest for developing high-resolution sensing arrays for e-skin. For example, Wang et al. mapped the pressure distribution by flexible sensor arrays based on oriented ZnO NW with high sensitivity and a high spatial resolution, which were also compatible for integration with human skin [48].
2.1.2. Application for Wound Monitoring
The application of pressure sensors in wound monitoring and healing was demonstrated by using them as “in-shoe systems” [49]. These sensors were used to evaluate plantar pressures by measuring the force distribution over the sole of the foot [50]. Such sensors are effective in preventing foot ulcers, which are commonly found among diabetic persons. However, they are less convenient for the prevention of other pressure ulcers, owing to their ability to evaluate only the plantar pressure. The current development status of such sensor systems is mainly limited to laboratory-scale use due to their high level of complexity in metrological analysis, mechanical susceptibility, and relatively high cost. To overcome these limitations, Shu et al. [51] presented a light textile-based sensor array incorporated in a soft polymeric support which was durable and highly sensitive to pressure. The acquired data were wirelessly transmitted to a remote receiver through a Bluetooth interface. The experimental results showed that this system had stable performances during both static and dynamic measurements. To further improve the sensitivity, Gerlach et al. [52] developed a low-cost, flexible pressure sensor system suitable for everyday use with a simple metrological complexity that can be used to prevent pressure ulcers. They used a piezoresistive sensor system based on MWCNT-PDMS composite connected to screen-printed silver electrodes inside a running shoe. Six single MWCNT-PDMS pressure sensors were situated on characteristic points of the insole to detect the unhealthy rollover patterns. As a result, this device showed great potential for plantar pressure measurement on a human foot inside a shoe during walking. The development of artificial skin based on pressure sensor arrays on a flexible substrate has attracted great interest in the past decade owing to their inherent advantages, such as a higher sensitivity, response speed, and resolution of tactile mapping [40,53]. However, these sensor arrays were only sensitive to spatial pressure and were not stretchable enough. To overcome these limitations, other strategies were explored, such as the use of intrinsically stretchable materials and new architecture design in mechanical sensors [27,54]. The summary of these sensors’ performances is listed in Table 1. In this direction, Suh et al. [55] developed a skin-attachable strain gauge sensor which could detect pressure, shear, and torsion thanks to the nanoscale mechanical interlocking between metal-coated nanofibers. By sandwiching a porous PDMS layer and air gap between two stretchable carbon nanotube film electrodes, Bao et al. [56] reported on a stretchable mechanical sensor which was capable of sensing pressure, lateral strain, and flexion (Figure 4). This device exhibited high sensitivity to pressure (average and maximum of 0.7 kPa−1 and 1.5 kPa−1, respectively, in the pressure region < 1 kPa).

Table 1.
Summary of sensors able to detect several sensitive factors.
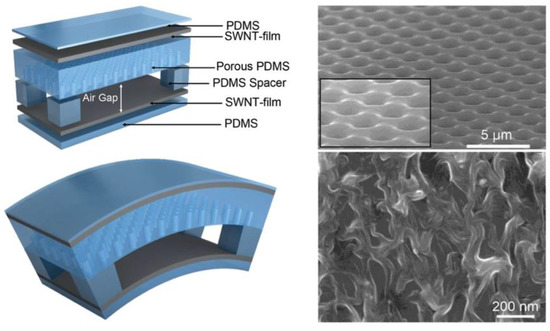
Figure 4.
Scheme of the flexible device fabricated using different deformation modes. Device architecture of a stretchable tactile electronic skin based on porous PDMS and air gap as a dielectric layer and SWNT film as electrodes. SEM images of PDMS (top right) and SWNT film (bottom right). Reproduced with permission [56].
Despite the progress made in detecting multiple mechanical forces using a single sensor unit, stretchable and multi-force sensor arrays have not been developed yet. The main challenge of fabricating such sensor arrays is the lack of robustness and reproducibility because of the poor mechanical strength of the conducting adhesive used for interconnects and electrical wiring. Although the artificial skin based on silicon demonstrated high performance, it is not widely used for real applications because of its complex and expensive large-area fabrication. Aside from this, a new type of piezoresistive sensor has attracted great attention, in which biodegradable fiber films with rough surfaces serve as the skeleton materials and flexible supporting substrates [62]. In the recent report by Ge et al., it was presented that the mechanical stresses induced by normal pressure, lateral strain, and flexion can be simultaneously mapped and quantified by a low-cost electronic fabric with stretchable sensor arrays [62]. In the fabric, intertwined composite fibers with piezoresistive rubber are the shell-sensing elements, and the helical silver nanowire network is the stretchable and highly conductive core electrode (Figure 5a,b). The fabric sensor arrays displayed multiple force sensitivities and allowed stretching at the system level, owing to the fibrous architecture of the sensor unit and the coaxial structure of the stretchable sensor electrode. During the measurements, the pressure sensitivity was noted to be more than 50% of its original value, while the tensile strain of the sensor electrodes increased up to 100% (Figure 5c). Bhang et al. reported on a piezoelectric dermal patch for wound healing [64]. Here, zinc oxide nanorods were used as a piezoelectric material, which generated a piezoelectric-induced electric field upon mechanical deformation around the wound sites. The in situ generated electric field promotes wound healing through improved cellular metabolism and protein synthesis. A recent study by Rajala et al. reported on an in-sole sensor system based on a piezoelectric poly(vinylidene fluoride) film [63] for the measurement of planter pressure distribution in shoes. In this study, sensors were placed at eight different locations in shoes and could sensitively detect pressure in the range of 58–486 kPa. This sensor system is highly promising for monitoring foot ulcers among diabetic patients.
Yi et al. reported on a highly sensitive, 3D-printed, and self-powered pressure sensor based on a triboelectric nanogenerator [65]. In the sensor platform, an MXene with negative triboelectric properties was coupled with a layered substrate possessing positive triboelectric properties and high stretchability. The sensor demonstrated a sensitivity of ca. 6 kPa−1 with a detection limit of ca. 9 Pa. Deng et al. reported on an integrated microsystem equipped with a triboelectric-electromagnetic nanogenerator [66] powering the microelectronic components which could sensitively detect pressure. Jose et al. developed a printed and stretchable thermoelectric sensor patch [67] for simultaneous measurement of the strain and temperature. The sensor was prepared using a PEDOT:PSS ink printed on a thermoplastic substrate. The sensor could sensitively detect the temperature and strain in vivo on the mouse wound. Wen et al. reported on a wearable thermoelectric generator in the dual chain configuration [68] which was screen printed on a polymer support. In the set-up, silk fibroin was used as a functional coating to simultaneously detect the water vapor and temperature.
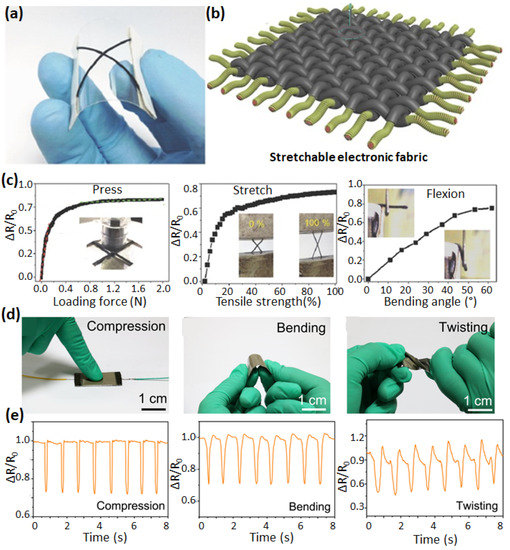

Figure 5.
Optical image (a) and scheme (b) of Ag nanowire sensor unit on a flexible polyethylene terephthalate (PET) substrate. The variations in Ag nanowire’s flexible sensor resistance are associated with different modes of shape deformation under press, stretching, and flexion (c). Adapted with permission [62]. Photographs of different deformation modes (compression, bending, and twisting) of stretchable sensor based on conductive cotton fiber decorated with rGO and AgNW networks (d). The associated change in the resistances is shown in the image panel below (e). Adapted with permission [69].
Recently, Wei et al. presented a bio-based flexible piezoresistive sensor based on the “dynamic bridging effect” of silver nanowires (AgNWs) toward reduced graphene oxide (rGO) [69]. Thanks to the “dynamic bridging effect”, this sensor can detect various mechanical forces (such as compression, bending, and twisting) (Figure 5d,e). The sensor showed high sensitivity (5.8 kPa−1), fast response and relaxation properties (29.5 ms and 15.6 ms, respectively), an ultralow detection limit (0.125 Pa), and excellent stability (>10,000 loading/unloading cycles). According to the conformal contact with the surface, pressure sensors are usually sensitive and spatially accurate. However, they are also sensitive to mechanical deformation (bending). Indeed, the normal pressure cannot be detected independent from the mechanical stress when a soft object is pressed by another soft object. To reduce the bending sensitivity, Lee et al. [70] presented a transparent and bending-insensitive pressure sensor fabricated using composite NANOFIBERS which exhibited an extremely small sensitivity to bending-induced strain and measured only the normal pressure, even under high bending levels.
Recent efforts to develop sensor-laden bionic systems have utilized pressure, strain, and temperature sensors, but their stretchability, detection ranges, and spatiotemporal resolutions are still limited [71]. Chortos et al. [72] reviewed the materials and devices designed for mimicking the skin’s ability to sense and generate biomimetic signals. To make a multi-sensitivity platform, Kim et al. successfully developed a stretchable prosthetic skin based on single-crystalline silicon nanoribbon (Figure 6) [73]. It included different sensor arrays to identify the strain, pressure, temperature, and humidity as well as stretchable multi-electrode arrays for nerve stimulation. This design strategy provides a high mechanical reliability and spatiotemporal sensitivity. The sensing capability of skin moisture and body temperature regulation was imitated by the integration of stretchable humidity sensors and heaters, respectively. Nassar et al. introduced a “paper skin” with simultaneous real-time sensing capability for pressure, temperature, humidity, proximity, pH, and flow [74] (Figure 6). In this work, off-the-shelf inexpensive household elements such as aluminium foil, scotch tapes, sticky notes, napkins, and sponges were used. Additionally, the proximity and motion features obtained in this work suggest the possibility for paper-based touchless motion systems, bringing the user-to-computer interface experience to a new level. Therefore, paper skin could be applicable for wound monitoring as an affordable, all-in-one flexible sensing platform, where the essential requirements are sensing diversity, surface adaptability, and large-area mapping. Other than developing physical sensors, Gillard et al. reported on a simulation of a human buttock, taking into account the variations in the muscle stiffness and blood reperfusion upon external pressure application [75]. The method can predict the temporal distribution of pressure and is promising for early detection of PUs.
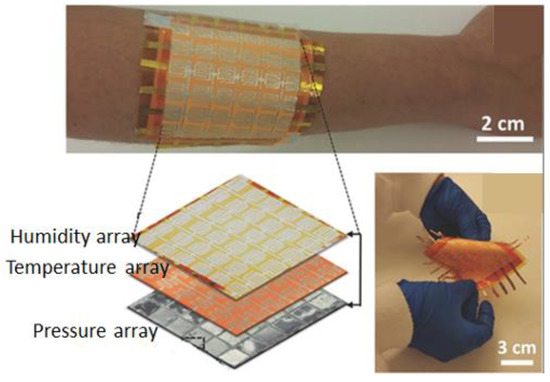
Figure 6.
Digital photograph of paper-based electronic skin, consisting of a sensor array of 6×6 sensors wrapped around the hand. An enlarged scheme of the stacked sensor arrays in the e-skin is shown in the bottom left image, while a digital photograph of the flexible temperature sensor array is shown in the bottom right image. Reproduced with permission [74].
2.2. VOC Chemical Monitoring
2.2.1. VOC Biomarkers in a Chronic Wound
Volatile organic compounds (VOCs) is a generic term used to classify a wide range of organic molecules with a boiling point of Tb ≤ 250 °C. It includes the family of alcohols, aldehydes, ketones, isocyanates, sulphides, and hydrocarbons. The human body emits VOCs through a host of endogenous and exogenous processes. Until now, the emission of VOCs has been detected through skin (sebum, sweat, skin emanations, and hair), breath, serum, urine, saliva, cerebrospinal fluid, feces, breast, milk, semen, amniotic fluid and tissue homogenates [76]. The emitted VOC changes with the body’s metabolic or hormonal state. For example, the metabolic changes associated with diabetes cause the release of acetone in the breath [77]. Variations in the external environment also cause changes in the VOC emission patterns. For instance, in the case of wound formation and healing, colonization of different bacterial and fungal species on the wound surface prompts the release of different VOCs, as depicted in Figure 7a. These VOC biomarkers have a long list [78,79], among which some commonly observed compounds are organic acids (n-hexanoic, 2-methylhexanoic, n-octanoic, n-decanoic, n-undecanoic), carbonyls (γ-C8-lactone, γ-C9-lactone, γ-C10-lactone), and alcohol (phenol, tetradecanol, n-hexadecanol) [80]. The concentration profiles of these VOCs are very different from one another and are not uniform in each patient. They also depend on the nature of the wound’s exudation and the healing state of the wound. In many in vivo studies near the wound sites, these were estimated in a range from ppm to ppt [81]. Thus, these compounds can act as biomarkers to predict microbial infection in wounds and can provide the status of the wound healing. Even before wound formation in PUs, the different stages of skin compression and specific sites of compression result in the release of VOC mixtures, corresponding to different stages of PU growth. This was demonstrated by recent GC-MS analysis coupled with e-nose performed on an air mixture sampled at different compressed skin sites from a healthy patient and a chronic wound patient [82] (Figure 7b). Analysis of the data by principal component analysis (PCA) revealed that the healthy patient exhibited a different mixture and concentration of the VOCs released than the patient inflicted with a chronic wound (Figure 7c).
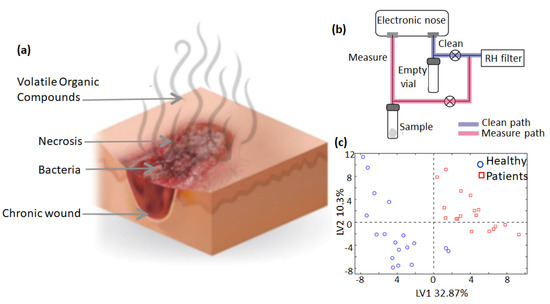
Figure 7.
Scheme of VOC emission from a bacterial infection in a chronic wound (a). Depiction of an analytical workstation based on electronic nose for profiling VOC gases (b) and PCA distribution of different VOC gases sampled in a healthy and an ill person (c). Reproduced with permission [82].
Apart from the underlying causative factors that result in the formation of pressure ulcers, another important aspect in their chronicity is the concurrent bacterial colonization or infection of the ulcerated area. Wounds are usually colonized by the commensal skin flora, but pathogenic bacterial species are also commonly involved [83]. Colonization of a wound is not itself a barrier to wound healing. However, repeated infections cause increased levels of pro-inflammatory cytokines and matrix metallopeptidases, which hamper the natural wound healing process. These changes have been hypothesized to be the underlying causes of wound chronicity. Through GC-MS analysis, Shirasu et al. identified a sulfur-containing odor released from wounds [84]. Romanelli et al. reported on polymer-based chemoresistors mounted in an e-nose, which was used for selective quantification of wound odors [85]. Yusuf et al. highlighted the importance of early detection of infection in a diabetic foot ulcer with an e-nose [86]. While using the e-nose in wound monitoring, the discrimination of signals is important to achieve for the selective response of each VOC. This was highlighted in the recent work of Yan et al. [87]. They applied the PCA method to accurately classify the signal pattern of six sensors toward different VOCs in the wound. In a recent review, Ousey et al. suggested that the detection of wound smells by e-nose technology can be useful in the early identification of a wound [88]. Akhmetova et al. reviewed the recent advancement in charcoal adsorbate-based wound dressing, which controls the bad odor in chronic wounds, and performed passive sampling of the VOCs released during the healing process for further analysis with an e-nose [89].
2.2.2. Sampling of VOCs at Chronic Wound Sites
The current strategy of chemical monitoring of VOCs in wounds is based on sampling followed by analysis using chromatography or sensors. There are various methods of sampling in which a widely used approach is solid phase microextraction (SPME), which is used for chromatographic analysis. In this approach, biomarkers are absorbed on solid absorbent columns and then subsequently desorbed using thermal desorption for GC-MS analysis. Different steps of the SPME method have been depicted in Figure 8a. The VOC samples are mainly collected from the hand, foot, hip, and armpit areas. For this, SPME fibers are kept in contact with the skin area for proper absorption. For freely moving areas such as the fingers, VOCs can be collected in a sampling jar or bag under dry nitrogen, and then VOCs can be absorbed through SPME, or in some cases (when Nalophan bags are used for sampling), bags can be directly used for GC-MS analysis. Other methods include the usage of skin patches, flexible PDMS films, poly(ester), and cotton gauge pads, as shown in Figure 8a. These patches can be put in direct contact with the skin for absorption, and VOCs can be extracted directly by thermal desorption or solvent extraction [90]. The VOC biomarkers in such sampled air are present at concentrations as low as parts per million to parts per trillion (ppm–ppt; v/v) [91]. Therefore, the preconcentration of the VOCs is performed during sampling at first in order to enhance the detection limit. Moreover, samples collected from the skin may contain water vapor, which needs to be removed before analysis. Other than this, cryo-focusing techniques have been developed to increase the efficiency of sorptive material and collect samples from very low concentrations. The water molecules can also be eliminated during analysis by using separation membrane dryers. Thomas et al. used PDMS skin sampling patches (20 mm × 15 mm × 0.45 mm) to collect biomarkers from the foot wounds of patients. The patch was attached using a cotton wool patch around the wound for 30 min. Subsequently, it was cleaned, thermally desorbed, and analyzed by GC-MS [92]. Dutkiewicz et al. used hydrogel-based micropatches embedded inside a PTFE film. These were attached to the skin with medical adhesive tape. Due to the hydrophilic nature of hydrogels, they have a good ability to sample hydrophilic skin biomarkers. Hydrogel micropatches were made from Agarose, a homopolymer derived from red algae [93]. Jiang et al. used a PDMS membrane which was sandwiched between stainless steel mesh pieces, as shown in Figure 8b. This assembly was covered with aluminium foil using surgery tape to protect it from contamination. The stainless steel mesh was very thin and flexible, and thus it was suitable for skin application [94]. Another procedure has been developed by Riazanskaia et al. for skin VOC sampling. The skin was cleaned with distilled water and dried with cotton. A PDMS-based skin patch was placed on the skin area. Then, it was covered with a cotton pad and fixed with microporous tape. After a specified time of sampling, the patches were taken out and immediately stored at 4 °C inside an airtight container. These stored samples were taken out just before analysis [95].
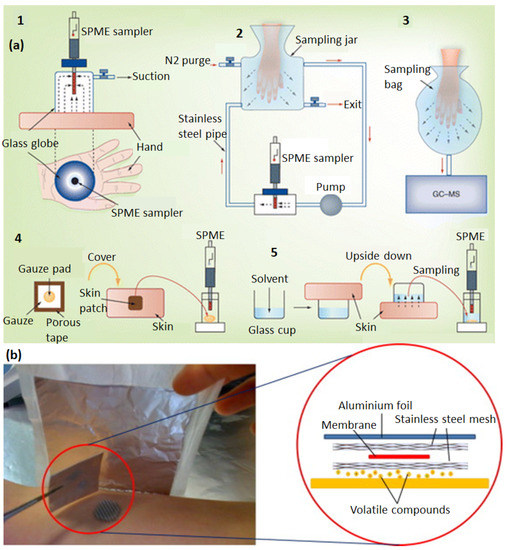
Figure 8.
Different methods used for VOC sampling on skin (a), including SPME in sealed glass container (1), SPME in fluidic chambers (2), sampling bags (3), sampling using a skin patch (4), and sampling in liquid using glass cups (5) Reproduced with permission [90]. (b) A scheme of a skin patch for VOC sampling based on a sandwiched membrane between a porous stainless steel mesh. Reproduced with permission [94].
Tran et al. [96] proposed a combined non-invasive technique based on the monitoring of both pressure and VOC vapors with quantum resistive sensors (QRSs) for anticipating the development of chronic wounds. The first step consists of preparing quantum resistive pressure sensors (pQRSs) in order to monitor the pressure distribution on the skin, since it is known to be one of the important factors influencing bedsore development. Notably, a linear and tuneable response in a wide range of measurements is expected, and a series of pQRS was prepared by a spraying method through layer-by-layer coating of a nanocomposite suspension of thermoplastic poly-(urethane) (TPU) filled with an optimized assembly of nanocarbons made of CNTs and pristine graphene (pG). The use of two conducting components in the nanofiller facilitated getting rid of the typical double-peak response usually obtained with single-conducting component nanofillers when the compression strain switches to lateral stretching due to POISSON’s effect. High sensitivity, a large linearity range, and long-term stability of these sensors were obtained. The second step takes advantage of the ability of quantum resistive sensors (vQRSs) to detect VOC biomarkers associated with PU formation. For vQRS optimization, the Flory-Huggins intermolecular interaction parameter χ12 was used to predict the different polymer nanocomposite structure deemed specific to a VOC detection. Subsequently, the vQRSs were mounted into an array (e-nose) for analysis of the VOCs released from the PU [97,98]. The vQRSs were exposed to concentrations ranging from ppm to ppb of three VOC biomarkers and water vapor to assess their selectivity and sensitivity [99]. The preliminary results exhibited that the e-nose was able to discriminate between artificial vapor cocktails made of different proportions of biomarkers and water molecules. It was shown that the VOCs released from the patches applied on the skin could be discriminated with the e-nose [96].
2.3. Optical Monitoring
2.3.1. Image Analysis
Imaging analysis is a non-invasive diagnosis approach for chronic wound monitoring and is less expensive than pressure monitoring and VOC chemical sensing-based methods. In this approach, images of the wound site are at first captured using a digital camera and then further analyzed with image processing algorithms (segmentation, edge detection, color processing, active contour, texture features, and volumetric information) to demarcate the wound region [100]. However, due to the increased melanin content, the detection of stage 1 PUs is difficult in darker skin, and imaging must be aided by visual inspection. Rajendran et al. [101] described an image enhancement procedure that improves the ability to detect PUs when applied to the color images of ulcer sites. Wannous et al. [102] presented an automatic wound assessment using a simple digital camera by designing a complete 3D and color wound assessment tool. Digital image-based and computer-aided segmentation have also been explored to improve the accuracy of wound assessment [103]. For developing an advanced methodology of image analysis, Papazoglou et al. [104] calculated the wound surface area of both animal and human model patients. In this work, a standard low-cost point-and-shoot camera equipped with a polarizing filter was used to reduce light reflection for photographing in the dark with a flash camera. A MatLab® program identified a wound area and a non-wound area using grayscale images derived from as-taken wound images. Consequently, the wound area was identified with high precision using the boundary detection method. Moreover, it gave a consistent trend of wound size change over time, which can be further associated with the healing status of the wound. Dhane et al. proposed a wound area detection method using a handheld optical camera [105]. This strategy involved the spectral approach for clustering based on the affinity matrix. The method was robust and demonstrated accurate measurement of the ulcer’s perimeter and healing progression. Although digital cameras are convenient in wound measurements and assessments, their efficiency is limited in assessing heterogeneous wounds and in poor lighting conditions. Moreover, they are unable to provide rapid, real-time analytical information as obtained from pressure and VOC monitoring. The use of digital photography evidenced advanced wound imaging by coupling it with the photoluminescence spectroscopy. Meier et al. [106] used a digital camera fitted with a 405-nm LED excitation ring to capture a 2-dimensional image of the pH and partial pressure of oxygen (pO2) within wounds (Figure 9). In this work, they measured the luminescence lifetime of oxygen and fluorescein isothiocyanate (FITC) with pH-dependent luminophore sensors, which were based on poly(styrene-co-acrylonitrile) particles loaded with palladium(II)-porphyrin complexes. The mechanism for oxygen sensing was based on the oxygen-dependent quenching of the luminescence lifetime of the immobilized porphyrin. When a thin poly(urethane) film containing particles loaded with the sensors and a reference diphenyl anthracene dye were placed on acute and chronic wounds, an image map showing pO2 and pH were obtained using the digital camera (Figure 9b).
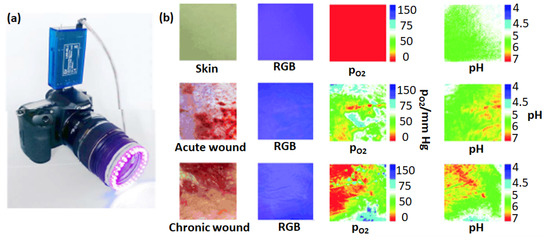
Figure 9.
A digital camera fitted with a 405 nm-LED light and an emission filter for photographing wounds covered with a sensor film (a). Images of skin, acute wounds, chronic wounds, visible light pictures, and pO2 and pH maps comparing acute and chronic wounds (b). Reproduced with permission [106].
In this method, the red, green, and blue (RGB) channels of the camera were separated, and the output from different probes was recorded onto each channel. The emission of the Pt (II) probe, which was dependent on the pO2, and the luminescence of the FITC, which was a probe for the pH, were recorded on the red and green channels, respectively. These recordings were then referenced to diphenyl anthracene dye in the blue channel. In the oxygen and pH maps of the chronic wound, the upper left corner is an area with a low oxygen concentration and high pH, possibly indicative of inflammation, which was absent in the acute wound.
2.3.2. Spectroscopic Analysis
Since near-infrared (NIR) light shows weak absorption but a high scattering by tissue, photonics-based instruments employing NIR have been clinically adopted and are emerging technologies. In this method, the light could access tissue layers deeper than ultraviolet, visible, and infrared radiation [107]. The deeper penetration of NIR light is the key basis of its extensive use for the assessment of wounds. In this section, recent NIR-based techniques employed for wound assessment have been reviewed. In fact, wound management could be influenced by various systemic risk factors including age, stress, diabetes, obesity, alcohol consumption, smoking, nutrition, and local factors. To determine wound outcomes and appropriately protect wounds, adequate perfusion of the wound with oxygenated blood is the key local factor [108]. Papazoglou et al. described a model of the expected optical changes to be observed during wound healing for the in vivo assessment of diabetic foot ulcers [109]. Notably, oxyhemoglobin and deoxyhemoglobin are the primary chromophores in the near-infrared wavelength region (650 nm–850 nm) in a living tissue, and the absorption of light in the NIR spectrum is relatively low compared with the visible and infrared wavelengths. Therefore, it would be possible to predict wound healing in diabetic foot ulcers earlier and with greater accuracy than existing clinical methods by measuring the oxygenated hemoglobin, deoxygenated hemoglobin, and blood volume with diffuse NIR technology [110,111]. Although this technology is highly promising, the clinical utility of NIR spectroscopy (NIRS) has been limited by large inter-patient and intra-patient variability. For addressing the inter-patient variability problem, Van Haren et al. reported on proof-of-concept studies, in which the bilateral NIRS could detect vascular injury by continuous monitoring of the bilateral limbs [112]. Most patients were relatively young and hemodynamically stable. In real conditions, changes in oxygen saturation may be difficult to interpret in patients in shock or in an elderly population, where differences may reflect chronic peripheral vascular disease rather than vascular trauma. Aside from hemoglobin, indocyanine green (ICG) has recently drawn significant interest in wound perfusion by angiographic methods [113,114]. It is a water-soluble tricarbocyanine (anhydro-3,3,3′,3′-tetramethyl-1-1′-di-(4-sulfobutyl)-4,5,4′,5-dibenzoindotricya-nine hydroxide sodium salt) dye with both lipophilic and hydrophilic characteristics. In these methods, a contrast agent is employed, and its distribution and transit time in the vascular bed of interest are monitored. Consequently, it provides an image of the lumen of larger vessels and tracks the transportation of blood to the capillary bed. Figure 10 describes how to distinguish the areas of a pedicle skin flap with inadequate perfusion by using ICG in-flow kinetics. In Figure 10a, it is evident that the florescence intensity decreased in the region with lower blood perfusion. The left panel shows the fluorescence intensity captured by a video frame rate camera measuring the first-pass transition of the dye after administering a tablet of ICG. The different colors and contrasts in the image are associated with the levels of blood perfusion in the different zones of the tissue.
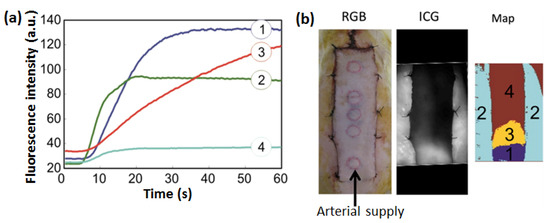
Figure 10.
The evolution of fluorescence intensity over time in four different zones of a pedicle skin flap having different levels of blood perfusion (a). The first panel in (b) shows a digital image of a skin flap used for florescence imaging. The second panel in (b) indicates the distribution of total florescence intensity of ICG in the different zones of the skin flap. The right panel showing a color map is associated with the four regions (labelled 1 to 4 in the map) of the tissues where the fluorescence spectra in (a) were recorded. Reproduced with permission [115].
In this example, different areas of the flap and native tissue resulted in four distinct in-flow kinetic profiles, and perfusion to the distal portion of the flap was compromised. In the healthy individuals, the ICG decreased from 18% to 24% in the vascular compartment per minute, with a half-life of 2–4 min. Notably, the serum should remain at no more than 4% of the initial concentration of the dye after 20 min [116]. It was possible to visualize perfusion to the wound with a useful ICG flow and by repeated injection and perfusion measurements, which can rapidly form the blood stream. However, because of the necessity of dye injection, ICG fluorescence angiography shows a considerable weakness when monitoring wounds with this technology.
3. Chronic Wound Management
3.1. Management with Smart Bandages
Ideally, a wound management plan can be launched only once the etiology and the status of the wound have been established. This can involve swiping, cleaning, and dressing the wound and covering it with a bandage. However, this process usually takes days to months and is expensive. Therefore, rapid, specific, and quantitative assessments that can be completed during a standard medical consultation are needed to manage wounds. Smart dressings have the potential to accelerate the diagnosis of wounds by using biosensors either incorporated into the wound dressing or used near it to produce a rapid result. Smart dressing readouts may also be used to not only monitor wound healing but also ensure that potentially problematic wounds will be immediately warned about at the earliest stage. In recent years, such types of smart bandages have been commercialized. For instance, “ACTICOAT” is an antimicrobial barrier dressing developed by Smith & NephewTM that claims to reduce infection symptoms within 2 weeks and requires fewer dressing changes. By using the silver technology, ACTICOAT is effective against a very broad spectrum of pathogens, thus reducing the risk of repetitive infections in chronic wounds. Mepilex Border Sacrum introduced by Mölnlycke health care limited is also a promising bandage in wound management. It effectively absorbs and retains exudate and maintains a moist wound environment. In this product, a special layer, called Safetac®, ensures that the dressing can be changed without damaging the wound or surrounding skin or exposing the patient to additional pain because it seals the wound edges, preventing the exudate from leaking onto the surrounding skin, which minimizes the risk for maceration. During wear time, Mepilex Border Sacrum can redistribute shear forces, friction, and pressure for hospital-acquired PU patients. The retention, spreading, and absorption layers are necessary to dissipate shear and redistribute pressure, which reduces the risk of breakdowns, allowing one to reposition the dressing after skin assessment. The DuoDerm® Extra Thin Dressing developed by ConvaTec Inc. has been designed to reduce the risk of further skin degradation due to friction. It can be used as a primary hydrocolloid dressing for drying in mildly exuding wounds. In this product, a thin poly-(urethane) film provides a bacterial and viral barrier when the dressing remains intact and without leakage. Moreover, the polymeric film provides a waterproof barrier over the dressing. In addition, it can be used to manage the early stages of PUs (stage 1 and stage 2). Aside from that, there are several products that can be used for a “smart dressing” purpose, such as Aquacel (Ag hydrofiber dressing with silver), Allevyn (adhesive), Kendall AMD (antimicrobial foam dressing), Mepilex (border), Nu-Derm (alginate or hydrocolloid), Nu-Gel (wound dressing), Promogran Silvercel (hydro-alginate), WoundcheckTM, and Topper 8 Santyl (ointment). Among this non-exhaustive list, WoundcheckTM has a protease status that is among the best point-of-care (POC) devices for evaluating elevated protease activity. It is well-suited to wound management because it is a simple, rapid point-of-care test for the qualitative assessment of human neutrophil-derived inflammatory protease activity, using fluid from chronic wounds collected on swabs. The test takes approximately 15 min per sample to complete and does not require the use of special equipment, and all reagents are provided as ready to use and can be stored at room temperature.
3.2. Management with Sensors
3.2.1. Management by Monitoring Applied Forces
As discussed previously, pressure sensors hold great promise in wound monitoring and thus can be an integral part of the wound management plan. However, only a few of such sensors have attained the level of commercial maturity due to the difficulty of measuring the force applied on the skin accurately. For wound monitoring, pressure mapping technology has been used to visualize the contact pressure distribution between the human body and a supporting surface, such as a seat or a mattress. X3 sensors developed by X Sensors, BodiTrak’s smart bed pressure mapping systems by Vista Medical Ltd., and the ConformatTM system by Tekscan, Inc. are the most common pressure mapping products for wound monitoring, as shown in Figure 11a–c, respectively. The X3 medical mattress system contains several thousand sensing points with a resolution of 1.27, cm and its working range is from 1.3 to 27 kPa. These pressure mapping systems are simple to set up, easy to use, and include responsive technical support. The mattress can be connected with any Windows computer or Android tablet through a suitable software interface. Interestingly, they allow the inspection of real-time pressure data beneath the patient, facilitating the identification of high-pressure areas and providing objective information regarding support surface effectiveness and proper pressure relief. As a result, they have been widely used in hospitals, medical institutions, and healthcare centers.
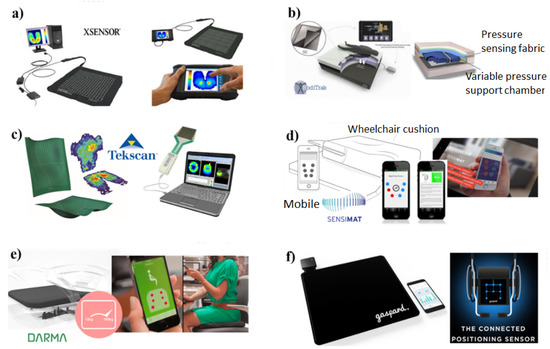
Figure 11.
A few examples of commercialized pressure sensors currently used for wound monitoring: (a) X3 sensor system (https://www.xsensor.com, accessed on 1 June 2022), (b) BodiTrak’s smart bed pressure mapping system (https://www.boditrak.com, accessed on 1 June 2022), (c) the Conformat™ system (https://www.tekscan.com, accessed on 1 June 2022), (d) Sensimat for wheelchairs (https://www.sensimatsystems.com, accessed on 1 June 2022), (e) Darma Pro (https://www.darmo.co, accessed on 1 June 2022), and (f) Gaspard wheelchair mat (https://www.mistergaspard.com, accessed on 1 June 2022).
Sensimat, as shown in Figure 11d and designed by a team at University of Toronto, reduced the frequency of PUs in wheelchair users. Sensimat’s proprietary pressure risk algorithm monitors pressure in the user’s seat and sends this data to the user’s mobile device. Darma Pro (Figure 11e), developed by Darma Inc., is capable of tracking the stress level in the skin and thus helps the user to better understand and improve his or her health status by sending poor posture alerts and stretching guides. The Gaspard wheelchair mat (Figure 11f), which was recently introduced by Mister Gaspard, is composed of a matrix of different force-resistive sensors. This device can work in the range from 20 kg to 150 kg and can transmit data through Bluetooth to the mobile phone of the user. It can give a warning signal in case of a lack of activity, bad position, or unreached target. Therefore, this product has great potential for personal monitoring purposes.
3.2.2. Management by Monitoring Moisture
Moisture plays an important role in improving the healing rate of wounds [117] in that excess moisture can generate maceration, while its deficiency can lead to a wound drying. Normally, the dressings require a certain moisture content for effective treatment. Moreover, adequate fluid balance at the wound/dressing interface improves the wound’s healing process. Thus, continuous moisture level measurement within the dressing is critical to making decisions about dressing changes and debridement of dry or necrotic tissues for improving the healing rate. Wound SenseTM (Ohmedics, UK) is a sensor system in which the electrodes under the wound dressing measure impedance [118]. This device, as shown in Figure 12, uses a disposable moisture sensor which is replaced at every dressing change.
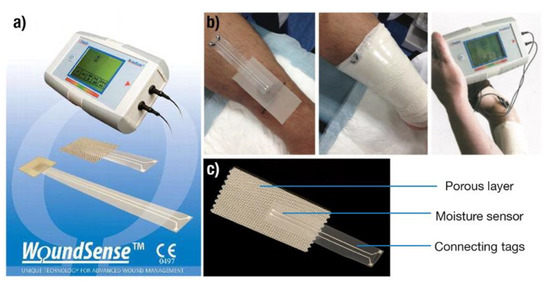
Figure 12.
Wound moisture monitoring system developed by Ohmedics (a). The system uses disposable moisture sensors and a handheld monitoring device for display of information (b). Structure of a single moisture sensor used with the Wound Sense system (c). Adapted with permission from Ohmedics [118].
The moisture level is measured after every 10–30 s based on the impedance value measured by the meter. In dry conditions, no current flows through the electrodes, and this results in high impedance. A wet wound bed leads to the easy flow of charges and results in low impedance. The moisture level is then read out using a small, handheld meter according to five categories: “wet”, “wet to moist”, “moist”, “moist to dry”, and “dry”. Since the dressing is not disturbed, the clinician can easily read the moisture level and can make the decision rapidly to change the dressing or whether the wound requires extra moisture. Therefore, Wound-SenseTM is suitable for chronic and acute wounds and can be used in hospitals, community clinics, and at home.
3.2.3. Management by Monitoring of a Wound’s pH
The pH of healthy skin is slightly acidic and is commonly found in the range of 4–6. However, this acidic milieu is disturbed, as the body’s internal pH of 7.4 changes during the wound’s formation. Thus, the pH of the wound bed is a significant parameter in determining the wound healing status [119]. Measurement of the pH levels at various locations within the wound bed can provide information on the healing process and on bacterial colonization [17]. Recently, a novel bandage has been developed by Centre Suisse d’Électronique et de Microtechnique (CSEM) (Figure 13) which can alert the nursing staff as soon as a wound starts turning bad. In case of any change in the wound’s pH level, sensors incorporated into the base material can detect it and exhibit change in the fluorescence intensity of the light glow. In the sensor, a fluorescent molecule is used which emits in the visible or ultra-violet (UV) range as a function of the pH variations. Thus, under this approach, chronic wounds can be monitored independently at home. Mariani et al. reported a textile-based smart bandage integrated with a potentiometric pH sensor, demonstrating accurate sensing of the pH level in the range of 6–9 at the wound site in a wide temperature range [120]. The sensor works on the electrochemical gating mechanism, in which iridium oxide is used as the electrode material for the electrochemical reaction at the wound site. The potential variation of the electrode because of a change in pH induces a change in the conductivity of the PEDOT:PSS semiconducting material.
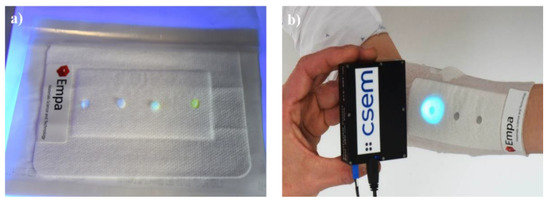
Figure 13.
Smart bandage for PH detecting developed by EMPA-Swiss Federal Laboratories for Materials Science & Technology (a). The bandage reveals its measurement under UV light (b).
3.3. Telemetric Wound Monitoring
In recent years, sensor microsystems coupled with wireless data transmission technologies have attracted some interest in wound management [121]. Knowledge of the skin temperature values is of particular importance in the prevention and treatment of chronic wounds. In this direction, Matzeu et al. introduced a wireless temperature sensor to monitor chronic wounds in PUs [122]. The sensor was prepared by coating a mixture of CNTs and poly(styrene-b-(ethylene-co-butylene)-b-styrene) (SEBS) on a KAPTON substrate. The signal can be remotely transmitted by a radio frequency identification (RFID) transceiver. However, the sensor system is still awaiting field trials on patients. In another such work, Milici et al. could measure the temperature of the human body by attaching a bio-compatible transpiring poly(ε-caprolactone) (PCL) membrane onto human skin [123]. The human body temperature was measured with an accuracy of about 0.25 °C using an EM4325 microchip equipped with RFID communication capabilities. For achieving a proof of concept, Occhiuzzi et al. provided the feasibility of integrating passive RFID sensor tags in medical hydrogel membranes in the perspective of developing smart plasters to monitor and assist the wound healing process [124]. Kassal et al. also introduced a smart bandage for optical monitoring of pH levels in the physiologically relevant range with high accuracy and precision. This bandage can communicate with an external readout unit using radio-frequency identification [125]. In another study, Farooqui et al. presented that the irregular change in some parameters (e.g., bleeding, pH levels, and external pressure) at a wound’s site could provide an early warning through a low-cost wireless monitoring system fabricated by inkjet printing on a standard bandage [126]. The configuration uses two types of sensing mechanisms, as highlighted in Figure 14a–c. At first, a capacitive sensor placed on either side of the bandage strip detects both the bleeding and pressure levels of the wound. Secondly, a resistive sensor detects the pH levels on the wound. If the wound starts to bleed, blood from the wound will pass through the bandage, resulting in a change in capacitance. When external pressure is applied to the bandage, the distance between the two electrodes of the capacitor decreases, and consequently, a change in capacitance is noticed. Changes in resistance of one of the electrodes (carbon-based) placed on the bandage are employed to sense the pH levels at the wound site, as depicted in Figure 14c. The changes in capacitance and resistance are analyzed and sent in a wireless fashion using the IEEE 802.15.4 standard that operates around 2.4 GHz (Figure 14d). Taking a step further, Xu et al. [127] reported the development of a smart bandage integrated with an array of sensors for multiplexed monitoring as well as an automated drug delivery platform for bacterial infection prevention. The sensing module was capable of detecting the temperature, pH, and uric acid near the wound site to provide the status of the wound’s healing. On the other hand, the drug delivery module could release the antibiotic cefazolin to promote wound healing through automated voltage control. Moreover, the smart bandage was battery-free and worked through inductive coupling with NFC through a smart phone.
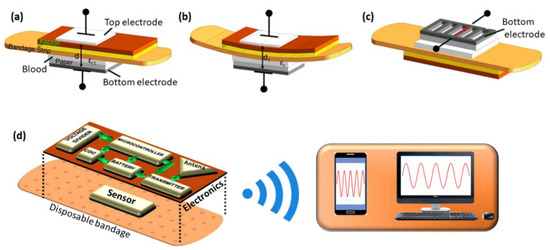
Figure 14.
A design of a capacitive sensor (a,b) and resistive sensor (c) used in a smart bandages and its different modes of operation to detect bleeding by measuring the change in dielectric constant (a), external pressure by measuring the change in the distance between the electrodes (b), and pH variations in the wound by measuring the resistance of the carbon electrode (c). The integration of the sensors and different electronic components in the smart bandage and exhibition of wireless data transmission to a smart phone and computer is shown in (d). Adapted with permission from [126].
In the detachable electronics, electrical signals are transmitted by an embedded microcontroller and then converted into digital signals by a capacitance (CDC). The patient’s health status was monitored by using an LED incorporated with a system battery. An inkjet-printed loop antenna was integrated into the circuit and was used for wireless communication. For each patient, the data on wound progression were provided to a personal smart phone by wirelessly communicating with the smart bandage. It was also sent to health care providers using either the mobile system or the internet [128,129,130]. This approach gives a better quality of online treatment and is precise and scalable. Moreover, the wound database can be maintained as an e-prescription for future assistance to clinicians. In a similar direction, Zhang et al. reported on a smart wound dressing integrated with temperature and humidity sensors and a wireless data transmission system based on a Bluetooth low-energy 4.0 antenna [131]. The bandage was capable of real-time monitoring of temperature and humidity in the wound microenvironment and sent data wirelessly to a smartphone.
4. Conclusions and Future Outlook
In conclusion, sensor-based diagnostic and monitoring tools are rapidly emerging as a potential solution to detect the onset of chronic wounds associated with PU formation and provide real-time assessment of the healing status of the wounds. This area has attracted widespread interest among researchers and industries. Among different types of sensors developed in this domain so far, notable advancements in pressure, chemical, and optical sensors have been made. Taking into account the measurement of skin pressure as a key to the early detection of PU development, pressure sensors based on different transducers, such as piezoresistive, capacitive, and piezoelectric transducers, have been developed in the form of e-skins. These sensors have utilized a host of nanocarbons, metal nanoparticles, inorganic oxides, and nanocomposites as sensing materials in combination with a diversity of flexible and stretchable substrates and electrodes. Moreover, the choice of the sensing device design played a key role in determining the ability to detect different natures of external pressure. These sensor systems demonstrated very high sensitivity to normal pressure, shear, and torsion. Some of these sensing devices have been integrated into shoes, mattresses, and wheelchairs to monitor different types of ulcers in early development. The pressure sensor array has often been combined with temperature, humidity, and pH sensor arrays on a paper substrate to fabricate paper skin, which is highly promising for use as a point-of-care device for wound healing. In the last decade, chemical sensors have found manifold interests in wound monitoring, mainly motivated by the detection and selective profiling of VOC biomarkers released from the wound during its healing and infection processes. The current advancement in such sensing strategies involves a two-step process. In the first step, sampling of VOC vapors is performed at the wound site using different methods, such as SPME and adsorption patches which are based on carbon, PDMS, and porous steel-based materials. Subsequently, the sampled VOCs are desorbed through thermal heating and are analyzed by different chemical sensors mounted on an array in an electronic nose device. To improve the signal discrimination for each VOC gas, the output data are treated with a mathematical algorithm, such as PCA. In the optical sensing methods developed for wound identification and healing monitoring, major advancements have been reported for imaging and NIR spectroscopy-based strategies. Imaging-based sensor tools mainly consist of a high-resolution digital camera to record wound images, which are combined with a mathematical algorithm to identify the wound’s healing status. Moreover, the imaging methods have been also coupled with spectroscopy to assess the level of O2 perfusion in the blood and the pH level at the wound on the skin. Many of these sensor systems have attained the level of commercial maturity in recent years and are being used in wound management plans. For instance, smart bandages from Acticoat, Mepilex, DuoDerme, or WoundcheckTM have been proven effective in the early detection of wound infection. Moreover, a few wound monitoring devices from WoundSense, Xsensor, Boditrak, Comformat, Sensimat, and EMPA have been incorporated into daily-use commercial products linked to PU patients.
Despite the current level of advancement in the research and commercialization of sensors relevant for wound management, there are many critical areas where significant improvement is required. For instance, many wound sensors monitor only a few wound parameters at a time, such as the temperature, moisture, wound oxygenation, and pH. However, scarce research is available describing the development of sensor microsystems equipped with pressure, VOCs, moisture, temperature, pH, and biological sensors altogether to generate a complete wound monitoring system. Although few efforts have been made recently to incorporate pressure, temperature, impedance, and moisture sensors into a microchip, they have not been tested in a real wound environment. Therefore, the future development strategy of sensor systems for chronic wound management should focus on combining multiple sensor arrays into one microsystem, particularly the pressure and chemical sensors, because they can be miniaturized into a flexible support. In this direction, combining arrays of different types of quantum resistive sensors, such as pQRSs and vQRSs, would be a promising approach. vQRSs can monitor different types of VOC emissions and humidity variations in the wound, while pQRSs can sense various pressures changes around the wound because of inflammation or contraction. Nonetheless, these sensor arrays must also be combined with other electrochemical sensor arrays to monitor the pH and biochemical analyte variation in the wound and make a complete assessment of all the critical parameters of wound management. Another focus area should be to combine artificial intelligence with such sensor microsystems. This would enable the device to make decisions based on the data obtained by the sensors and thus would reduce the burden on healthcare professionals.
Author Contributions
Conceptualization, M.C. and J.-F.F.; methodology, M.-T.T., A.K., M.C. and J.-F.F.; validation, M.-T.T., A.K., A.S., M.C., J.-F.F. and W.A.; formal analysis, M.-T.T., A.K., M.C. and J.-F.F.; investigation, M.-T.T., A.K., M.C. and J.-F.F.; resources M.C., J.-F.F. and W.A.; writing—original draft preparation, M.-T.T., A.S. and A.K.; writing—review and editing, M.-T.T., A.K., M.C., J.-F.F. and W.A.; visualization, M.-T.T., A.K., M.C. and J.-F.F.; supervision, M.C. and J.-F.F.; project administration, W.A., M.C. and J.-F.F.; funding acquisition, W.A. and J.-F.F. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful to the University of South Brittany (UBS) and General Council of Morbihan (CG56) for providing generous funding through research grant E2M06585 and the Brittany Region grant SORESENS9149. This work was carried out within the ”Handicap Innovation Territory ” project, supported by the French Government as part of the “France 2030-Territoires d’innovation” program for innovative territories, and was administered by the ”Banque des Territoires”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Hervé Bellégou, the University of South Brittany (UBS), and the Centre Mutualiste de Kerpape for their contributions to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sumarno, A.S. Pressure Ulcers: The Core, Care and Cure Approach. Br. J. Community Nurs. 2019, 24, S38–S42. [Google Scholar] [CrossRef] [PubMed]
- National Pressure Ulcer Advisory Panel; European Pressure Ulcer Advisory Panel; Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide; Cambridge Media: Perth, Australia, 2014. [Google Scholar]
- Staging of Pressure Ulcers (Bed Sores). Available online: https://www.lvlawny.com/post/2017/10/23/staging-of-pressure-ulcers-bed-sores (accessed on 23 October 2017).
- Jaul, E.; Barron, J.; Rosenzweig, J.P.; Menczel, J. An Overview of Co-Morbidities and the Development of Pressure Ulcers among Older Adults. BMC Geriatr. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, W.S.; Akalu, T.Y.; Mulugeta, H.; Aynalem, Y.A. The Global Burden of Pressure Ulcers among Patients with Spinal Cord Injury: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2020, 21, 334. [Google Scholar] [CrossRef]
- Courvoisier, D.S.; Righi, L.; Béné, N.; Rae, A.-C.; Chopard, P. Variation in Pressure Ulcer Prevalence and Prevention in Nursing Homes: A Multicenter Study. Appl. Nurs. Res. 2018, 42, 45–50. [Google Scholar] [CrossRef]
- Gottrup, F. A Specialized Wound-Healing Center Concept: Importance of a Multidisciplinary Department Structure and Surgical Treatment Facilities in the Treatment of Chronic Wounds. Am. J. Surg. 2004, 187, S38–S43. [Google Scholar] [CrossRef]
- Li, Z.; Lin, F.; Thalib, L.; Chaboyer, W. Global Prevalence and Incidence of Pressure Injuries in Hospitalised Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 105, 103546. [Google Scholar] [CrossRef]
- Al Mutairi, K.B.; Hendrie, D. Global Incidence and Prevalence of Pressure Injuries in Public Hospitals: A Systematic Review. Wound Med. 2018, 22, 23–31. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Gupta, S.; Sagar, S.; Maheshwari, G.; Kisaka, T.; Tripathi, S. Chronic Wounds: Magnitude, Socioeconomic Burden and Consequences. Wounds Asia 2021, 4, 8–14. [Google Scholar]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Shi, G.; Zhang, M.; Haick, H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano 2022, 16, 1708–1733. [Google Scholar] [CrossRef] [PubMed]
- Demarré, L.; Verhaeghe, S.; Annemans, L.; Van Hecke, A.; Grypdonck, M.; Beeckman, D. The Cost of Pressure Ulcer Prevention and Treatment in Hospitals and Nursing Homes in Flanders: A Cost-of-Illness Study. Int. J. Nurs. Stud. 2015, 52, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef]
- Walia, G.S.; Wong, A.L.; Lo, A.Y.; Mackert, G.A.; Carl, H.M.; Pedreira, R.A.; Bello, R.; Aquino, C.S.; Padula, W.V.; Sacks, J.M. Efficacy of Monitoring Devices in Support of Prevention of Pressure Injuries. Adv. Skin Wound Care 2016, 29, 567–574. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and Imaging for Wound Healing: A Review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current Wound Healing Procedures and Potential Care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A Comprehensive Review of Advanced Biopolymeric Wound Healing Systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and Stretchable Physical Sensor Integrated Platforms for Wearable Human-Activity Monitoring and Personal Healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance Sensing Device Enables Early Detection of Pressure Ulcers In Vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Templeton, S.; Voelcker, N.H. Calibration of Sensors for Reliable Radio Telemetry in a Prototype Flexible Wound Monitoring Device. Sens. Bio-Sens. Res. 2014, 2, 23–30. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Templeton, S.; Voelcker, N.H. A Flexible and Low Power Telemetric Sensing and Monitoring System for Chronic Wound Diagnostics. Biomed. Eng. Online 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Hammock, M.L.; Chortos, A.; Tee, B.C.-K.; Tok, J.B.-H.; Bao, Z. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Robert, C.; Castro, M.; Feller, J.F.; Kim, T.Y.; Suh, K.S. Enhancing the Sensitivity of Graphene/Polyurethane Nanocomposite Flexible Piezo-Resistive Pressure Sensors with Magnetite Nano-Spacers. Carbon 2016, 108, 450–460. [Google Scholar] [CrossRef]
- Woo, S.-J.; Kong, J.-H.; Kim, D.-G.; Kim, J.-M. A Thin All-Elastomeric Capacitive Pressure Sensor Array Based on Micro-Contact Printed Elastic Conductors. J. Mater. Chem. C 2014, 2, 4415–4422. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Li, X.; Lin, Y.; Luo, N.; Long, M.; Zhao, N.; Xu, J.-B. Flexible Piezoelectric-Induced Pressure Sensors for Static Measurements Based on Nanowires/Graphene Heterostructures. ACS Nano 2017, 11, 4507–4513. [Google Scholar] [CrossRef]
- Maheshwari, V.; Saraf, R.F. High-Resolution Thin-Film Device to Sense Texture by Touch. Science 2006, 312, 1501–1504. [Google Scholar] [CrossRef]
- Wu, X.; Han, Y.; Zhang, X.; Zhou, Z.; Lu, C. Large-Area Compliant, Low-Cost, and Versatile Pressure-Sensing Platform Based on Microcrack-Designed Carbon Black@Polyurethane Sponge for Human-Machine Interfacing. Adv. Funct. Mater. 2016, 26, 6246–6256. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and Highly Sensitive Graphene Strain Sensors for Human Motion Monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Kang, W.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Highly Stretchable Piezoresistive Graphene-Nanocellulose Nanopaper for Strain Sensors. Adv. Mater. 2014, 26, 2022–2027. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.D.; Lim, H.; Kim, S.Y.; Ko, H. Giant Tunneling Piezoresistance of Composite Elastomers with Interlocked Microdome Arrays for Ultrasensitive and Multimodal Electronic Skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chortos, A.; Yu, G.; Wang, Y.; Isaacson, S.; Allen, R.; Shi, Y.; Dauskardt, R.; Bao, Z. An Ultra-Sensitive Resistive Pressure Sensor Based on Hollow-Sphere Microstructure Induced Elasticity in Conducting Polymer Film. Nat. Commun. 2014, 5, 3002. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A Wearable and Highly Sensitive Pressure Sensor with Ultrathin Gold Nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Cheng, M.-Y.; Lin, C.-L.; Lai, Y.-T.; Yang, Y.-J. A Polymer-Based Capacitive Sensing Array for Normal and Shear Force Measurement. Sensors 2010, 10, 10211–10225. [Google Scholar] [CrossRef] [PubMed]
- Frutiger, A.; Muth, J.T.; Vogt, D.M.; Mengüç, Y.; Campo, A.; Valentine, A.D.; Walsh, C.J.; Lewis, J.A. Capacitive Soft Strain Sensors via Multicore-Shell Fiber Printing. Adv. Mater. 2015, 27, 2440–2446. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.; Fox, C.H.; Bao, Z. Skin-like Pressure and Strain Sensors Based on Transparent Elastic Films of Carbon Nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, F.; Huang, D.; Gao, X.; Di, C.; Zhu, D. Flexible Suspended Gate Organic Thin-Film Transistors for Ultra-Sensitive Pressure Detection. Nat. Commun. 2015, 6, 6269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Dong, L.; Han, X.; Du, W.; Zhai, J.; Pan, C.; Wang, Z.L. Self-Powered High-Resolution and Pressure-Sensitive Triboelectric Sensor Matrix for Real-Time Tactile Mapping. Adv. Mater. 2016, 28, 2896–2903. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Tee, B.C.-K.; Stoltenberg, R.M.; Chen, C.V.H.-H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly Sensitive Flexible Pressure Sensors with Microstructured Rubber Dielectric Layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Hu, W.; Niu, X.; Zhao, R.; Pei, Q. Elastomeric Transparent Capacitive Sensors Based on an Interpenetrating Composite of Silver Nanowires and Polyurethane. Appl. Phys. Lett. 2013, 102, 083303. [Google Scholar] [CrossRef]
- Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R.; Bauer, S.; Lacour, S.P.; Wagner, S. Flexible Ferroelectret Field-Effect Transistor for Large-Area Sensor Skins and Microphones. Appl. Phys. Lett. 2006, 89, 073501. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Son, J.H.; Hwang, G.T.; Jeong, C.K.; Ryu, J.; Koo, M.; Choi, I.; Lee, S.H.; Byun, M.; Wang, Z.L.; et al. Highly-Efficient, Flexible Piezoelectric PZT Thin Film Nanogenerator on Plastic Substrates. Adv. Mater. 2014, 26, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Pi, Z.; Zhang, J.; Wen, C.; Zhang, Z.B.; Wu, D. Flexible Piezoelectric Nanogenerator Made of Poly(Vinylidenefluoride-Co-Trifluoroethylene) (PVDF-TrFE) Thin Film. Nano Energy 2014, 7, 33–41. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable Amplified Lead Zirconate Titanate Sensors with Enhanced Piezoelectric Response for Cutaneous Pressure Monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Dong, L.; Zhu, G.; Niu, S.; Yu, R.; Yang, Q.; Liu, Y.; Wang, Z.L. High-Resolution Electroluminescent Imaging of Pressure Distribution Using a Piezoelectric Nanowire LED Array. Nat. Photonics 2013, 7, 752–758. [Google Scholar] [CrossRef]
- Martínez-Nova, A.; Cuevas-García, J.C.; Pascual-Huerta, J.; Sánchez-Rodríguez, R. BioFoot® In-Shoe System: Normal Values and Assessment of the Reliability and Repeatability. Foot 2007, 17, 190–196. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Becker, H.-P. Plantar Pressure Distribution Measurements. Technical Background and Clinical Applications. Foot Ankle Surg. 1997, 3, 1–14. [Google Scholar] [CrossRef]
- Shu, L.; Hua, T.; Wang, Y.; Li, Q.; Feng, D.D.; Tao, X. In-Shoe Plantar Pressure Measurement and Analysis System Based on Fabric Pressure Sensing Array. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 767–775. [Google Scholar] [CrossRef]
- Gerlach, C.; Krumm, D.; Illing, M.; Lange, J.; Kanoun, O.; Odenwald, S.; Hubler, A. Printed MWCNT-PDMS-Composite Pressure Sensor System for Plantar Pressure Monitoring in Ulcer Prevention. IEEE Sens. J. 2015, 15, 3647–3656. [Google Scholar] [CrossRef]
- Hou, C.; Wang, H.; Zhang, Q.; Li, Y.; Zhu, M. Highly Conductive, Flexible, and Compressible All-Graphene Passive Electronic Skin for Sensing Human Touch. Adv. Mater. 2014, 26, 5018–5024. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, J.H.; Kim, J.; Choi, S.; Lee, J.; Park, I.; Hyeon, T.; Kim, D.-H. Reverse-Micelle-Induced Porous Pressure-Sensitive Rubber for Wearable Human-Machine Interfaces. Adv. Mater. 2014, 26, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Lee, G.-Y.; Kim, T.; Kim, S.M.; Kim, H.N.; Ahn, S.-H.; Suh, K.-Y. A Flexible and Highly Sensitive Strain-Gauge Sensor Using Reversible Interlocking of Nanofibres. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Vosgueritchian, M.; Cheon, S.; Kim, H.; Koo, J.H.; Kim, T.R.; Lee, S.; Schwartz, G.; Chang, H.; et al. Stretchable Energy-Harvesting Tactile Electronic Skin Capable of Differentiating Multiple Mechanical Stimuli Modes. Adv. Mater. 2014, 26, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.S.; Kang, T.H.; Bae, J.; Lee, S.H.; Byun, K.E.; Im, J.; et al. Highly Stretchable Resistive Pressure Sensors Using a Conductive Elastomeric Composite on a Micropyramid Array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef]
- Viry, L.; Levi, A.; Totaro, M.; Mondini, A.; Mattoli, V.; Mazzolai, B.; Beccai, L. Flexible Three-Axial Force Sensor for Soft and Highly Sensitive Artificial Touch. Adv. Mater. 2014, 26, 2659–2664. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, S.; Wang, L.; Jiang, K.; Shen, G. An Ultra-Sensitive and Rapid Response Speed Graphene Pressure Sensors for Electronic Skin and Health Monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Lee, Y.; Ha, M.; Jung, Y.; Lim, H.; Kim, S.Y.; Ko, H. Tactile-Direction-Sensitive and Stretchable Electronic Skins Based on Human-Skin-Inspired Interlocked Microstructures. ACS Nano 2014, 8, 12020–12029. [Google Scholar] [CrossRef]
- Sekitani, T.; Someya, T. Stretchable Organic Integrated Circuits for Large-Area Electronic Skin Surfaces. MRS Bull. 2012, 37, 236–245. [Google Scholar] [CrossRef]
- Ge, J.; Sun, L.; Zhang, F.-R.; Zhang, Y.; Shi, L.-A.; Zhao, H.-Y.; Zhu, H.-W.; Jiang, H.-L.; Yu, S.-H. A Stretchable Electronic Fabric Artificial Skin with Pressure-, Lateral Strain-, and Flexion-Sensitive Properties. Adv. Mater. 2016, 28, 722–728. [Google Scholar] [CrossRef]
- Rajala, S.; Mattila, R.; Kaartinen, I.; Lekkala, J. Designing, Manufacturing and Testing of a Piezoelectric Polymer Film In-Sole Sensor for Plantar Pressure Distribution Measurements. IEEE Sens. J. 2017, 17, 6798–6805. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jang, W.S.; Han, J.; Yoon, J.-K.; La, W.-G.; Lee, E.; Kim, Y.S.; Shin, J.-Y.; Lee, T.-J.; Baik, H.K.; et al. Zinc Oxide Nanorod-Based Piezoelectric Dermal Patch for Wound Healing. Adv. Funct. Mater. 2017, 27, 1603497. [Google Scholar] [CrossRef]
- Yi, Q.; Pei, X.; Das, P.; Qin, H.; Lee, S.W.; Esfandyarpour, R. A Self-Powered Triboelectric MXene-Based 3D-Printed Wearable Physiological Biosignal Sensing System for on-Demand, Wireless, and Real-Time Health Monitoring. Nano Energy 2022, 101, 107511. [Google Scholar] [CrossRef]
- Deng, H.-T.; Wang, Z.-Y.; Wang, Y.-L.; Wen, D.-L.; Zhang, X.-S. Integrated Hybrid Sensing and Microenergy for Compact Active Microsystems. Microsyst. Nanoeng. 2022, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Bronckaers, A.; Kumar, R.S.N.; Reenaers, D.; Vandenryt, T.; Thoelen, R.; Deferme, W. Stretchable Printed Device for the Simultaneous Sensing of Temperature and Strain Validated in a Mouse Wound Healing Model. Sci. Rep. 2022, 12, 10138. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.-L.; Deng, H.-T.; Liu, X.; Li, G.-K.; Zhang, X.-R.; Zhang, X.-S. Wearable Multi-Sensing Double-Chain Thermoelectric Generator. Microsyst. Nanoeng. 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, S.; Dong, X.; Lin, Y.; Liu, L. Flexible Piezoresistive Sensors Based on “Dynamic Bridging Effect” of Silver Nanowires toward Graphene. Carbon 2017, 113, 395–403. [Google Scholar] [CrossRef]
- Lee, S.; Reuveny, A.; Reeder, J.; Lee, S.; Jin, H.; Liu, Q.; Yokota, T.; Sekitani, T.; Isoyama, T.; Abe, Y.; et al. A Transparent Bending-Insensitive Pressure Sensor. Nat. Nanotechnol. 2016, 11, 472–478. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High Performance Piezoelectric Devices Based on Aligned Arrays of Nanofibers of Poly(Vinylidenefluoride-Co-Trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- Chortos, A.; Liu, J.; Bao, Z. Pursuing Prosthetic Electronic Skin. Nat. Mater. 2016, 15, 937–950. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable Silicon Nanoribbon Electronics for Skin Prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed]
- Nassar, J.M.; Cordero, M.D.; Kutbee, A.T.; Karimi, M.A.; Sevilla, G.A.T.; Hussain, A.M.; Shamim, A.; Hussain, M.M. Paper Skin Multisensory Platform for Simultaneous Environmental Monitoring. Adv. Mater. Technol. 2016, 1, 1600004. [Google Scholar] [CrossRef]
- Gillard, N.; Leong, A.; Departe, J.P.; Kerdraon, J.; Allegre, W. Early detection of pressure ulcers from a simulation of temporal pressure data with reperfusion. In Proceedings of the JETSAN 2021—Colloque en Télésanté et Dispositifs Biomédicaux, Toulouse, France, 20–21 May 2021. [Google Scholar]
- Zlatkis, A.; Brazell, R.S.; Poole, C.F. The Role of Organic Volatile Profiles in Clinical Diagnosis. Clin. Chem. 1981, 27, 789–797. [Google Scholar] [CrossRef]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath Ethanol and Acetone as Indicators of Serum Glucose Levels: An Initial Report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Daneshkhah, A.; Siegel, A.P.; Agarwal, M. Chapter 23—Volatile Organic Compounds: Potential Biomarkers for Improved Diagnosis and Monitoring of Diabetic Wounds. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 491–512. ISBN 978-0-12-816413-6. [Google Scholar]
- Hu, W.; Wu, W.; Jian, Y.; Haick, H.; Zhang, G.; Qian, Y.; Yuan, M.; Yao, M. Volatolomics in Healthcare and Its Advanced Detection Technology. Nano Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Macagnano, A.; Paolesse, R.; Tarizzo, E.; Mantini, A.; D’Amico, A. Human Skin Odor Analysis by Means of an Electronic Nose. Sens. Actuators B Chem. 2000, 65, 216–219. [Google Scholar] [CrossRef]
- Ashrafi, M.; Bates, M.; Baguneid, M.; Alonso-Rasgado, T.; Rautemaa-Richardson, R.; Bayat, A. Volatile Organic Compound Detection as a Potential Means of Diagnosing Cutaneous Wound Infections. Wound Repair Regen. 2017, 25, 574–590. [Google Scholar] [CrossRef]
- Dini, F.; Capuano, R.; Strand, T.; Ek, A.-C.; Lindgren, M.; Paolesse, R.; Di Natale, C.; Lundström, I. Volatile Emissions from Compressed Tissue. PLoS ONE 2013, 8, e69271. [Google Scholar] [CrossRef]
- Chesham, J.S.; Platt, D.J. Patterns of Wound Colonisation in Patients with Peripheral Vascular Disease. J. Infect. 1987, 15, 21–26. [Google Scholar] [CrossRef]
- Shirasu, M.; Nagai, S.; Hayashi, R.; Ochiai, A.; Touhara, K. Dimethyl Trisulfide as a Characteristic Odor Associated with Fungating Cancer Wounds. Biosci. Biotechnol. Biochem. 2009, 73, 2117–2120. [Google Scholar] [CrossRef]
- Romanelli, M.; Gaggio, G.; Coluccia, M.; Piaggesi, A. Technological Advances in Wound Bed Measurements. Wounds 2002, 14, 58–66. [Google Scholar]
- Yusuf, N.; Omar, M.; Zakaria, A.; Abdullah, A.A.; Kamarudin, L.M.; Shakaff, A.Y.M.; Masnan, M.J.; Zakaria, N.Z.I.; Yeap, E.J.; Othman, A.; et al. Diagnosis of Bacteria for Diabetic Foot Infection Using Electronic Nose Technology. In Proceedings of the 2013 IEEE Conference on Wireless Sensor (ICWISE), Kuching, Malaysia, 2–4 December 2013; pp. 114–118. [Google Scholar] [CrossRef]
- Yan, J.; Duan, S.; Huang, T.; Wang, L. Hybrid Feature Matrix Construction and Feature Selection Optimization-Based Multi-Objective QPSO for Electronic Nose in Wound Infection Detection. Sens. Rev. 2016, 36, 23–33. [Google Scholar] [CrossRef]
- Ousey, K.; Roberts, D.; Gefen, A. Early Identification of Wound Infection: Understanding Wound Odour. J. Wound Care 2017, 26, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, A.; Saliev, T.; Allan, I.U.; Illsley, M.J.; Nurgozhin, T.; Mikhalovsky, S. A Comprehensive Review of Topical Odor-Controlling Treatment Options for Chronic Wounds. J. Wound Ostomy Cont. Nurs. 2016, 43, 598–609. [Google Scholar] [CrossRef]
- Kataoka, H.; Saito, K.; Kato, H.; Masuda, K. Noninvasive Analysis of Volatile Biomarkers in Human Emanations for Health and Early Disease Diagnosis. Bioanalysis 2013, 5, 1443–1459. [Google Scholar] [CrossRef]
- Ruzsanyi, V.; Mochalski, P.; Schmid, A.; Wiesenhofer, H.; Klieber, M.; Hinterhuber, H.; Amann, A. Ion Mobility Spectrometry for Detection of Skin Volatiles. J. Chromatogr. B 2012, 911, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.N.; Riazanskaia, S.; Cheung, W.; Xu, Y.; Goodacre, R.; Thomas, C.L.P.; Baguneid, M.S.; Bayat, A. Novel Noninvasive Identification of Biomarkers by Analytical Profiling of Chronic Wounds Using Volatile Organic Compounds. Wound Repair Regen. 2010, 18, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, E.P.; Chiu, H.Y.; Urban, P.L. Probing Skin for Metabolites and Topical Drugs with Hydrogel Micropatches. Anal. Chem. 2017, 89, 2664–2670. [Google Scholar] [CrossRef]
- Jiang, R.; Cudjoe, E.; Bojko, B.; Abaffy, T.; Pawliszyn, J. A Non-Invasive Method for in Vivo Skin Volatile Compounds Sampling. Anal. Chim. Acta 2013, 804, 111–119. [Google Scholar] [CrossRef]
- Riazanskaia, S.; Blackburn, G.; Harker, M.; Taylor, D.; Thomas, C.L.P. The Analytical Utility of Thermally Desorbed Polydimethylsilicone Membranes for In-Vivo Sampling of Volatile Organic Compounds in and on Human Skin. Analyst 2008, 133, 1020–1027. [Google Scholar] [CrossRef]
- Tran, M.T. Development of Nanocomposite Quantum Resistive Sensors for the Prevention of Bedsores. Ph.D. Thesis, University of South Brittany (UBS), Lorient, France, 2018. [Google Scholar]
- Bouvree, A.; Feller, J.F.; Castro, M.; Grohens, Y.; Rinaudo, M. Conductive Polymer Nano-BioComposites (CPC): Chitosan-Carbon Nanoparticle a Good Candidate to Design Polar Vapour Sensors. Sens. Actuators B Chem. 2009, 138, 138–147. [Google Scholar] [CrossRef]
- Kumar, B.; Feller, J.F.; Castro, M.; Lu, J. Conductive Bio-Polymer Nano-Composites (CPC): Chitosan-Carbon Nanotube Transducers Assembled via Spray Layer-by-Layer for Volatile Organic Compound Sensing. Talanta 2010, 81, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Castro, M.; Choudhary, V.; Feller, J.-F. Influence of Water Molecules on the Detection of Volatile Organic Compounds (VOC) Cancer Biomarkers by Nanocomposite Quantum Resistive Vapor Sensors VQRS. Chemosensors 2018, 6, 64. [Google Scholar] [CrossRef]
- Burns, M.; Enderle, J.; Rosow, E.; Zhu, Q. Development of a Wound Assessment System for Quantitative Chronic Wound Monitoring. In Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference, Philadelphia, PA, USA, 21 April 2002; pp. 7–8. [Google Scholar] [CrossRef]
- Rajendran, P.J.; Leachtenauer, J.; Kell, S.; Turner, B.; Newcomer, C.; Lyder, C.; Alwan, M. Improving the Detection of Stage I Pressure Ulcers by Enhancing Digital Color Images. In Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 5206–5209. [Google Scholar] [CrossRef]
- Wannous, H.; Treuillet, S.; Lucas, Y. Supervised Tissue Classification from Color Images for a Complete Wound Assessment Tool. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Lyon, France, 22–26 August 2007; pp. 6031–6034. [Google Scholar] [CrossRef]
- Thawer, H.A.; Houghton, P.E.; Woodbury, M.G.; Keast, D.; Campbell, K. A Comparison of Computer-Assisted and Manual Wound Size Measurement. Ostomy Wound Manag. 2002, 48, 46–53. [Google Scholar]
- Papazoglou, E.S.; Zubkov, L.; Mao, X.; Neidrauer, M.; Rannou, N.; Weingarten, M.S. Image Analysis of Chronic Wounds for Determining the Surface Area. Wound Repair Regen. 2010, 18, 349–358. [Google Scholar] [CrossRef]
- Dhane, D.M.; Krishna, V.; Achar, A.; Bar, C.; Sanyal, K.; Chakraborty, C. Spectral Clustering for Unsupervised Segmentation of Lower Extremity Wound Beds Using Optical Images. J. Med. Syst. 2016, 40, 207. [Google Scholar] [CrossRef]
- Meier, R.J.; Schreml, S.; Wang, X.D.; Landthaler, M.; Babilas, P.; Wolfbeis, O.S. Simultaneous Photographing of Oxygen and PH in Vivo Using Sensor Films. Angew. Chem. Int. Ed. 2011, 50, 10893–10896. [Google Scholar] [CrossRef]
- Sowa, M.G.; Friesen, J.R. In Vivo Tissue Analysis by Near-Infrared Spectroscopy. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2000; ISBN 9780470027318. [Google Scholar]
- Sen, C.K. Wound Healing Essentials: Let There Be Oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Papazoglou, E.S.; Weingarten, M.S.; Zubkov, L.; Neidrauer, M.; Pourrezaei, K. Assessment of Diabetic Foot Ulcers with Diffuse near Infrared Methodology. In Proceedings of the 2008 8th IEEE International Conference on BioInformatics and BioEngineering, Athens, Greece, 8–10 October 2008; pp. 1–5. [Google Scholar] [CrossRef]
- Neidrauer, M.; Zubkov, L.; Weingarten, M.S.; Pourrezaei, K.; Papazoglou, E.S. Evaluation of Chronic Diabetic Wounds with the Near Infrared Wound Monitor. In XII Mediterranean Conference on Medical and Biological Engineering and Computing 2010, Proceedings of the MEDICON 2010, Chalkidiki, Greece, 27–30 May 2010; Springer: Berlin/Heidelberg, Germany, 2010; pp. 487–489. [Google Scholar]
- Weingarten, M.S.; Neidrauer, M.; Mateo, A.; Mao, X.; McDaniel, J.E.; Jenkins, L.; Bouraee, S.; Zubkov, L.; Pourrezaei, K.; Papazoglou, E.S. Prediction of Wound Healing in Human Diabetic Foot Ulcers by Diffuse Near-Infrared Spectroscopy: A Pilot Study. Wound Repair Regen. 2010, 18, 180–185. [Google Scholar] [CrossRef]
- Van Haren, R.M.; Ryan, M.L.; Thorson, C.M.; Namias, N.; Livingstone, A.S.; Proctor, K.G. Bilateral Near-Infrared Spectroscopy for Detecting Traumatic Vascular Injury. J. Surg. Res. 2013, 184, 526–532. [Google Scholar] [CrossRef]
- Grosenick, D.; Wabnitz, H.; Ebert, B. Recent Advances in Contrast-Enhanced near Infrared Diffuse Optical Imaging of Diseases Using Indocyanine Green. J. Near Infrared Spectrosc. 2012, 20, 203–221. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.G.; Kuo, W.C.; Ko, A.C.T.; Armstrong, D.G. Review of Near-Infrared Methods for Wound Assessment. J. Biomed. Opt. 2016, 21, 091304. [Google Scholar] [CrossRef]
- Hope-Ross, M.; Yannuzzi, L.A.; Gragoudas, E.S.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Krupsky, S.; Orlock, D.A.; Puliafito, C.A. Adverse Reactions Due to Indocyanine Green. Ophthalmology 1994, 101, 529–533. [Google Scholar] [CrossRef]
- Wu, P.; Fisher, A.C.; Foo, P.P.; Queen, D.; Gaylor, J.D.S. In Vitro Assessment of Water Vapour Transmission of Synthetic Wound Dressings. Biomaterials 1995, 16, 171–175. [Google Scholar] [CrossRef]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A Wearable Wound Moisture Sensor as an Indicator for Wound Dressing Change: An Observational Study of Wound Moisture and Status. Int. Wound J. 2016, 13, 1309–1314. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of PH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time PH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef]
- Mehmood, N.; Hariz, A.; Fitridge, R.; Voelcker, N.H. Applications of Modern Sensors and Wireless Technology in Effective Wound Management. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2014, 102, 885–895. [Google Scholar] [CrossRef]
- Matzeu, G.; Losacco, M.; Parducci, E.; Pucci, A.; Dini, V.; Romanelli, M.; Di Francesco, F. Skin Temperature Monitoring by a Wireless Sensor. In Proceedings of the IECON 2011—37th Annual Conference of the IEEE Industrial Electronics Society, Melbourne, Australia, 7–10 November 2011; pp. 3533–3535. [Google Scholar] [CrossRef]
- Milici, S.; Amendola, S.; Bianco, A.; Marrocco, G. Epidermal RFID Passive Sensor for Body Temperature Measurements. In Proceedings of the 2014 IEEE RFID Technology and Applications Conference (RFID-TA), Tampere, Finland, 8–9 September 2014; pp. 140–144. [Google Scholar] [CrossRef]
- Occhiuzzi, C.; Ajovalasit, A.; Sabatino, M.A.; Dispenza, C.; Marrocco, G. RFID Epidermal Sensor Including Hydrogel Membranes for Wound Monitoring and Healing. In Proceedings of the 2015 IEEE International Conference on RFID (RFID), San Diego, CA, USA, 15–17 April 2015; pp. 182–188. [Google Scholar]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Murković Steinberg, I. Smart Bandage with Wireless Connectivity for Optical Monitoring of PH. Sens. Actuators B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 28949. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lu, Y.; Cheng, C.; Li, X.; Xu, J.; Liu, Z.; Liu, J.; Liu, G.; Shi, Z.; Chen, Z.; et al. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv. Funct. Mater. 2021, 31, 2100852. [Google Scholar] [CrossRef]
- Chakraborty, C.; Gupta, B.; Ghosh, S.K. A Review on Telemedicine-Based WBAN Framework for Patient Monitoring. Telemed. e-Health 2013, 19, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, R.; Bastide, R.; Conchon, E. A Semantic Web-of-Things Architecture for Monitoring the Risk of Bedsores. In Proceedings of the 2015 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 7–9 December 2015; pp. 318–323. [Google Scholar] [CrossRef]
- Texier, I.; Xydis, S.; Soudris, D.; Marcoux, P.; Pham, P.; Muller, M.; Correvon, M.; Dudnik, G.; Voirin, G.; Kristenssen, J.; et al. SWAN-iCare Project: Towards Smart Wearable and Autonomous Negative Pressure Device for Wound Monitoring and Therapy. In Proceedings of the 2014 4th International Conference on Wireless Mobile Communication and Healthcare—“Transforming Healthcare Through Innovations in Mobile and Wireless Technologies” (MOBIHEALTH 2014), Athens, Greece, 3–5 November 2014; pp. 357–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Zhao, C.; Li, J.; Huang, R.; Zhang, Q.; Li, Y.; Li, X. An Integrated Smart Sensor Dressing for Real-Time Wound Microenvironment Monitoring and Promoting Angiogenesis and Wound Healing. Front. Cell Dev. Biol. 2021, 9, 701525. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).