The Impact of the Functional Layer Composition of Glucose Test-Strips on the Stability of Electrochemical Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formation of the Functional Sensing Layer
2.2. Thermal Stress-Test Applied to Study Electrochemical Stability of the Functional Layer during Storage of Glucose Test-Strips
2.3. Electrochemical Measurements
2.4. UV-Vis Measurements
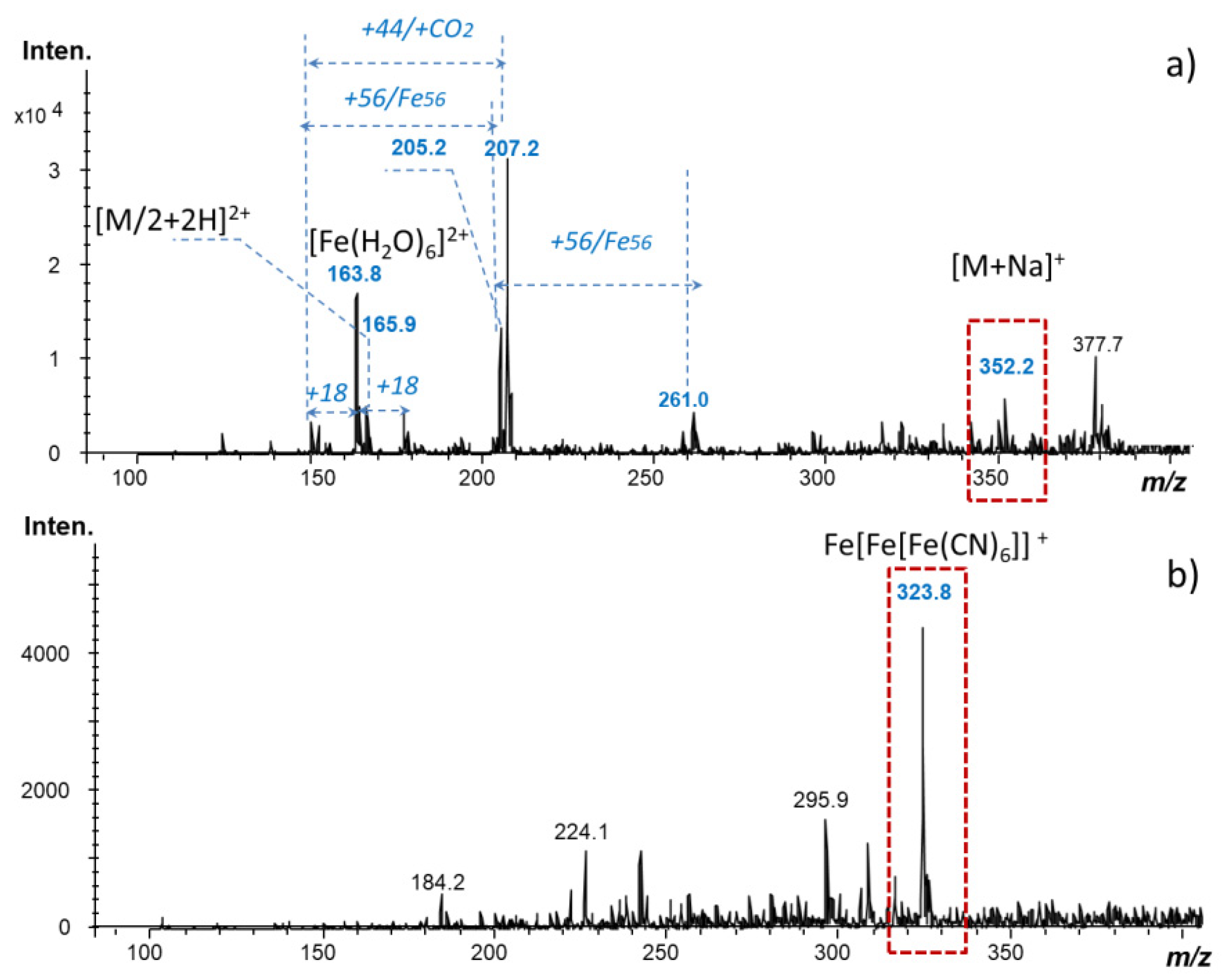
2.5. Laser Desorption Ionization Mass Spectrometry (LDI-MS)
2.6. Oxygen Minisensor Studies
3. Results
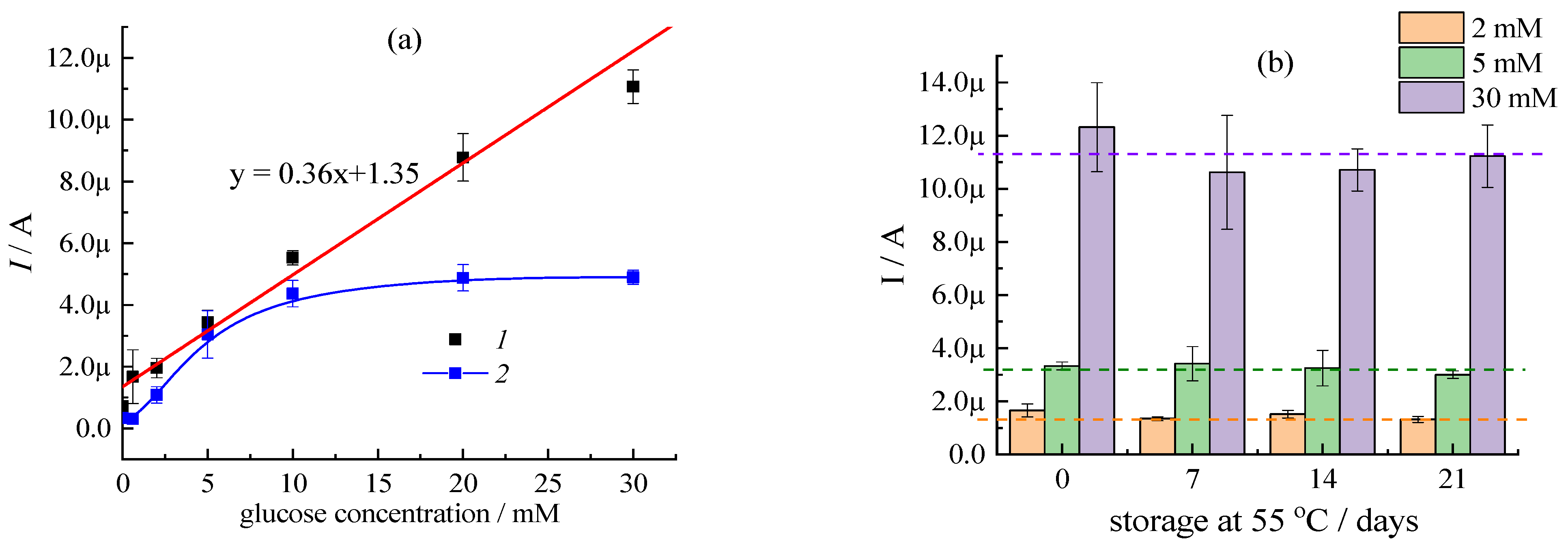
3.1. Tuning of Analytical Performance of Glucose Test-Strips by Type of Filler Agent at a Constant Ratio of Enzyme and Mediator
3.2. Impact of Buffer Type on Electrochemical Response of Amperometric Glucose Test-Strips and Their Storage Time
3.3. Impact of Mediator Chemical Stability on Electrochemical Response of Glucose Test-Strips
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Juska, V.B.; Pemble, M.E. A Critical Review of Electrochemical Glucose Sensing: Evolution of Biosensor Platforms Based on Advanced Nanosystems. Sensors 2020, 20, 6013. [Google Scholar] [CrossRef] [PubMed]
- Mano, N. Engineering glucose oxidase for bioelectrochemical applications. Bioelectrochemistry 2019, 128, 218–240. [Google Scholar] [CrossRef]
- Graça, J.S.; de Oliveira, R.F.; de Moraes, M.L.; Ferreira, M. Amperometric glucose biosensor based on layer-by-layer films of microperoxidase-11 and liposome-encapsulated glucose oxidase. Bioelectrochemistry 2014, 96, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Trau, D.; Renneberg, R. Encapsulation of glucose oxidase microparticles within a nanoscale layer-by-layer film: Immobilization and biosensor applications. Biosens. Bioelectron. 2003, 18, 1491–1499. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.-J.; Shi, C.-G.; Chen, H.-Y. Multilayer Membranes via Layer-by-Layer Deposition of Organic Polymer Protected Prussian Blue Nanoparticles and Glucose Oxidase for Glucose Biosensing. Langmuir 2005, 21, 9630–9634. [Google Scholar] [CrossRef]
- Semenova, D.; Gernaey, K.V.; Morgan, B.; Silina, Y.E. Towards one-step design of tailored enzymatic nanobiosensors. Analyst 2020, 145, 1014–1024. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. A glucose biosensor using methyl viologen redox mediator on carbon film electrodes. Anal. Chim. Acta 2005, 532, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Wang, B.; Liu, B. Amperometric glucose sensor with ferrocene as an electron transfer mediator. Biosens. Bioelectron. 1992, 7, 215–222. [Google Scholar] [CrossRef]
- Loew, N.; Tsugawa, W.; Nagae, D.; Kojima, K.; Sode, K. Mediator Preference of Two Different FAD-Dependent Glucose Dehydrogenases Employed in Disposable Enzyme Glucose Sensors. Sensors 2017, 17, 2636. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Chung, Y.; Hyun, K.; Yoo, K.S.; Kwon, Y. Performance improvement of the glucose oxidation reactions using methyl red mediator. Int. J. Hydrogen Energy 2020, 45, 4821–4828. [Google Scholar] [CrossRef]
- Wang, J. Glucose biosensors: 40 Years of advances and challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Hilditch, P.I.; Green, M.J. Disposable electrochemical biosensors. Analyst 1991, 116, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Hale, P.D.; Inagaki, T.; Karan, H.I.; Okamoto, Y.; Skotheim, T.A. A new class of amperometric biosensor incorporating a polymeric electron-transfer mediator. J. Am. Chem. Soc. 1989, 111, 3482–3484. [Google Scholar] [CrossRef]
- Ohara, T.J.; Rajagopalan, R.; Heller, A. Glucose electrodes based on cross-linked [Os(bpy)2Cl]+/2+ complexed poly(1-vinylimidazole) films. Anal. Chem. 1993, 65, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Gregg, B.A.; Heller, A. Cross-linked redox gels containing glucose oxidase for amperometric biosensor applications. Anal. Chem. 1990, 62, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Yoo, J.E.; Tarver, J.; Loo, Y.-L.; Heller, A. An Electron-Conducting Cross-Linked Polyaniline-Based Redox Hydrogel, Formed in One Step at pH 7.2, Wires Glucose Oxidase. J. Am. Chem. Soc. 2007, 129, 7006–7007. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fang, C.; Wang, H.; He, J. Disposable glucose test strip for whole blood with integrated sensing/diffusion-limiting layer. Electrochim. Acta 2009, 55, 544–550. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, B.; Yang, X.; Yang, P.; Ding, Y.; Chen, Y.; Fei, J. Electrochemical biosensing platform based on carboxymethyl cellulose functionalized reduced graphene oxide and hemoglobin hybrid nanocomposite film. Sens. Actuators B Chem. 2013, 182, 288–293. [Google Scholar] [CrossRef]
- Lin, M.-J.; Wu, C.-C.; Chang, K.-S. Effect of Poly-l-Lysine Polycation on the Glucose Oxidase/Ferricyanide Composite-Based Second-Generation Blood Glucose Sensors. Sensors 2019, 19, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, S.; Nagata, F.; Inagaki, M.; Kugimiya, S.; Kato, K. Enzyme immobilisation on poly-l-lysine-containing calcium phosphate particles for highly sensitive glucose detection. RSC Adv. 2019, 9, 10832–10841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suye, S.; Kumon, Y.; Ishigaki, A. Immobilization of glucose oxidase on poly-(l-lysine)-modified polycarbonate membrane. Biotechnol. Appl. Biochem. 1998, 27, 245–248. [Google Scholar] [CrossRef]
- Zolotukhina, E.V.; Katsen-Globa, A.; Koch, M.; Fink-Straube, C.; Sukmann, T.; Levchenko, M.G.; Silina, Y.E. The development of alginate-based amperometric nanoreactors for biochemical profiling of living yeast cells. Bioelectrochemistry 2022, 145, 108082. [Google Scholar] [CrossRef]
- Christwardana, M.; Frattini, D. Electrochemical Study of Enzymatic Glucose Sensors Biocatalyst: Thermal Degradation after Long-Term Storage. Chemosensors 2018, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-T.; Hsiao, H.-C.; Fang, M.-Y.; Zen, J.-M. Superior long-term stability of a glucose biosensor based on inserted barrel plating gold electrodes. Biosens. Bioelectron. 2009, 25, 383–387. [Google Scholar] [CrossRef]
- Haller, M.J.; Shuster, J.J.; Schatz, D.; Melker, R.J. Adverse Impact of Temperature and Humidity on Blood Glucose Monitoring Reliability: A Pilot Study. Diabetes Technol. Ther. 2007, 9, 1–9. [Google Scholar] [CrossRef]
- Puggioni, G.; Calia, G.; Arrigo, P.; Bacciu, A.; Bazzu, G.; Migheli, R.; Fancello, S.; Serra, P.A.; Rocchitta, G. Low-Temperature Storage Improves the Over-Time Stability of Implantable Glucose and Lactate Biosensors. Sensors 2019, 19, 422. [Google Scholar] [CrossRef] [Green Version]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Glucose Meter. Available online: https://eltaltd.ru/en/catalog/?preferred_lang=en (accessed on 26 July 2022).
- Semenova, D. Sensors for biosensors: A novel tandem monitoring in a droplet towards e ffi cient screening of robust design and optimal operating conditions. Analyst 2019, 144, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, E.; Sedláková, D.; Marek, J.; Hančár, J.; Garajová, K.; Žoldák, G. Ion-Specific Protein/Water Interface Determines the Hofmeister Effect on the Kinetic Stability of Glucose Oxidase. J. Phys. Chem. B 2019, 123, 7965–7973. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A. Why are enzymes less active in organic solvents than in water? Trends Biotechnol. 1997, 15, 97–101. [Google Scholar] [CrossRef]
- Mathias, J.; Wannemacher, G. Basic characteristics and applications of aerosil: 30. The chemistry and physics of the aerosil Surface. J. Colloid Interface Sci. 1988, 125, 61–68. [Google Scholar] [CrossRef]
- Vreeker, R.; Li, L.; Fang, Y.; Appelqvist, I.; Mendes, E. Drying and Rehydration of Calcium Alginate Gels. Food Biophys. 2008, 3, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Dumitraşcu, L.; Stănciuc, N.; Bahrim, G.E.; Ciumac, A.; Aprodu, I. pH and heat-dependent behaviour of glucose oxidase down to single molecule level by combined fluorescence spectroscopy and molecular modelling. J. Sci. Food Agric. 2016, 96, 1906–1914. [Google Scholar] [CrossRef]
- Gindt, Y.M.; Connolly, G.; Vonder Haar, A.L.; Kikhwa, M.; Schelvis, J.P.M. Investigation of the pH-dependence of the oxidation of FAD in VcCRY-1, a member of the cryptochrome-DASH family. Photochem. Photobiol. Sci. 2021, 20, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rochefort, D. Activity, conformation and thermal stability of laccase and glucose oxidase in poly(ethyleneimine) microcapsules for immobilization in paper. Process Biochem. 2011, 46, 993–1000. [Google Scholar] [CrossRef]
- Padilla-Martínez, S.G.; Martínez-Jothar, L.; Sampedro, J.G.; Tristan, F.; Pérez, E. Enhanced thermal stability and pH behavior of glucose oxidase on electrostatic interaction with polyethylenimine. Int. J. Biol. Macromol. 2015, 75, 453–459. [Google Scholar] [CrossRef]
- Hammes, G.G. Principles of chemical kinetics. Methods Biochem. Anal. 2007, 50, 75–96. [Google Scholar] [CrossRef] [Green Version]
- Koncki, R.; Wolfbeis, O.S. Composite Films of Prussian Blue and N-Substituted Polypyrroles: Fabrication and Application to Optical Determination of pH. Anal. Chem. 1998, 70, 2544–2550. [Google Scholar] [CrossRef]
- Gracheva, M.; Homonnay, Z.; Singh, A.; Fodor, F.; Marosi, V.B.; Solti, Á. New aspects of the photodegradation of iron(III) citrate: Spectroscopic studies and plant-related factors. Photochem. Photobiol. Sci. 2022, 21, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, H.B.; Rezvani, A.B.; Brushmiller, J.G. Photochemical and spectroscopic studies of complexes, of iron(III) with citric acid and other carboxylic acids. Inorg. Chim. Acta 1994, 226, 117–127. [Google Scholar] [CrossRef]
- Peng, F.; Li, G.; Liu, X.; Wu, S.; Tong, Z. Redox-Responsive Gel−Sol/Sol−Gel Transition in Poly(acrylic acid) Aqueous Solution Containing Fe(III) Ions Switched by Light. J. Am. Chem. Soc. 2008, 130, 16166–16167. [Google Scholar] [CrossRef]
- Itaya, K.; Akahoshi, H.; Toshima, S. Electrochemistry of Prussian Blue Modified Electrodes: An Electrochemical Preparation Method. J. Electrochem. Soc. 1982, 129, 1498–1500. [Google Scholar] [CrossRef]
- Azizi, A. Green Synthesis of Fe3O4 Nanoparticles and Its Application in Preparation of Fe3O4/Cellulose Magnetic Nanocomposite: A Suitable Proposal for Drug Delivery Systems. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3552–3561. [Google Scholar] [CrossRef]
- Basha, F.Z.; Maqsood, Z.T.; Wasim, A.A. Fe(II) and Co(II) Complexes of (4-(4-bromophenyl)-[2,2′-bipyridine]-6-carboxylic acid) Synthesis, Characterization and Electrochromic studies. J. Chem. Soc. Pak. 2017, 39, 35–42. [Google Scholar]
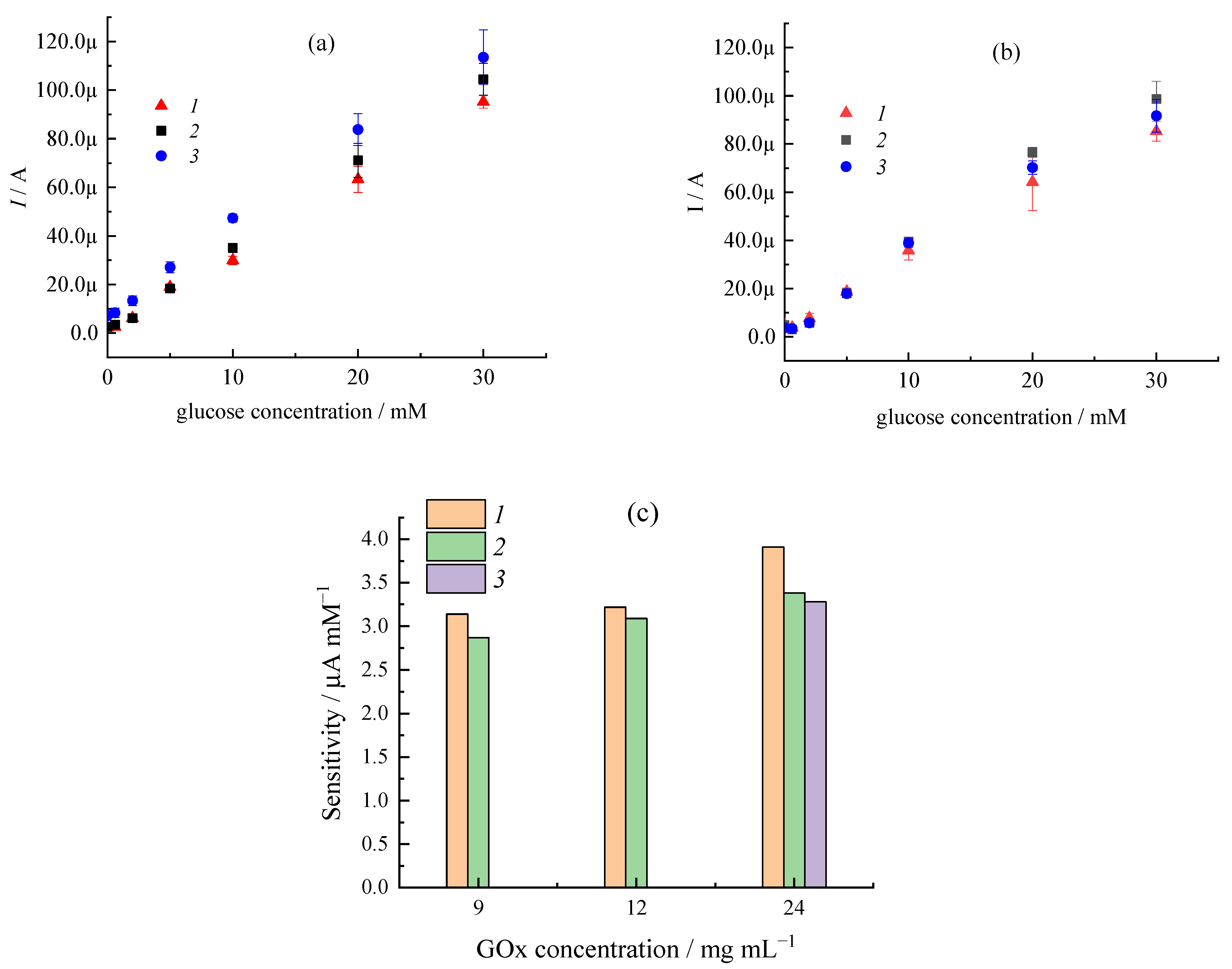
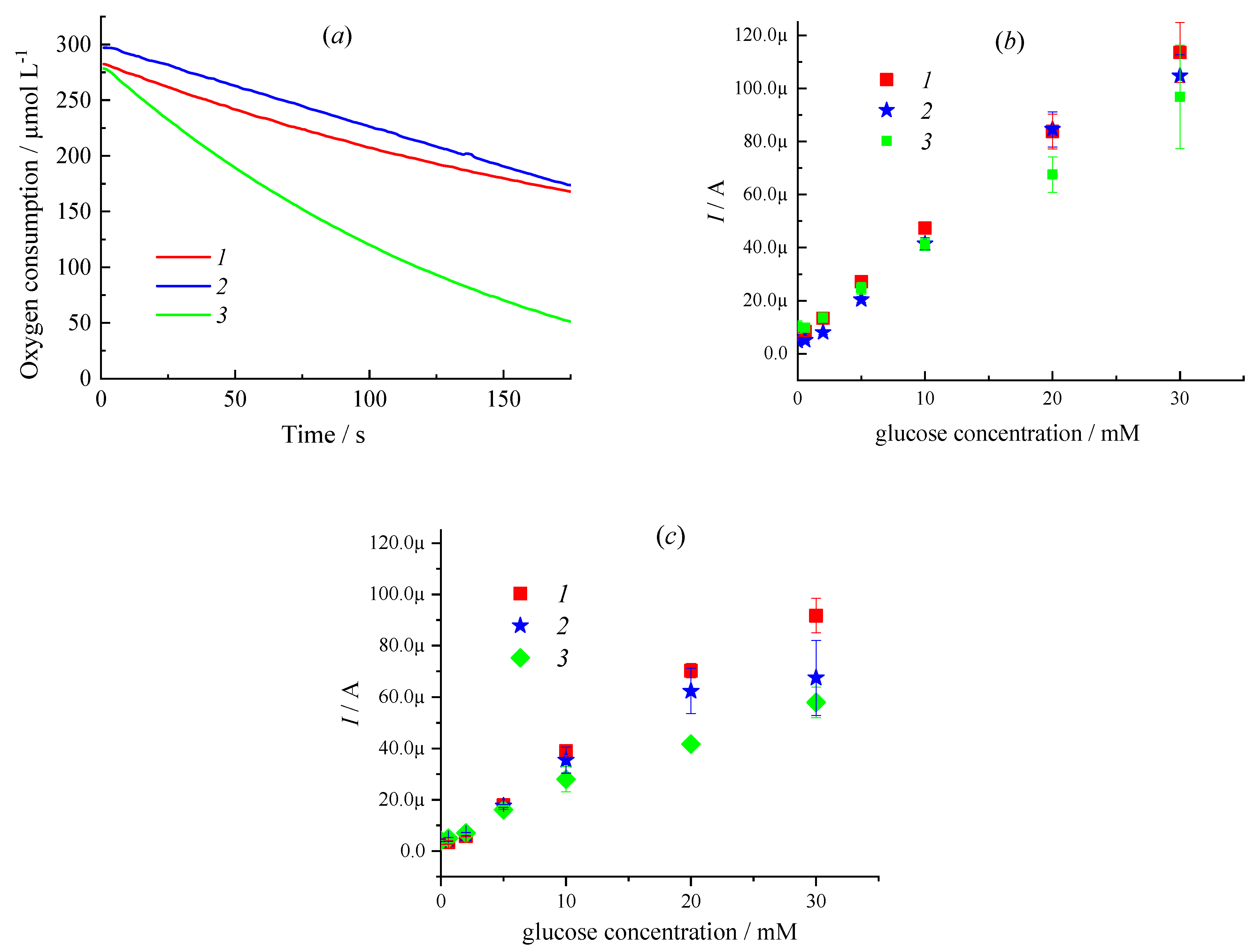
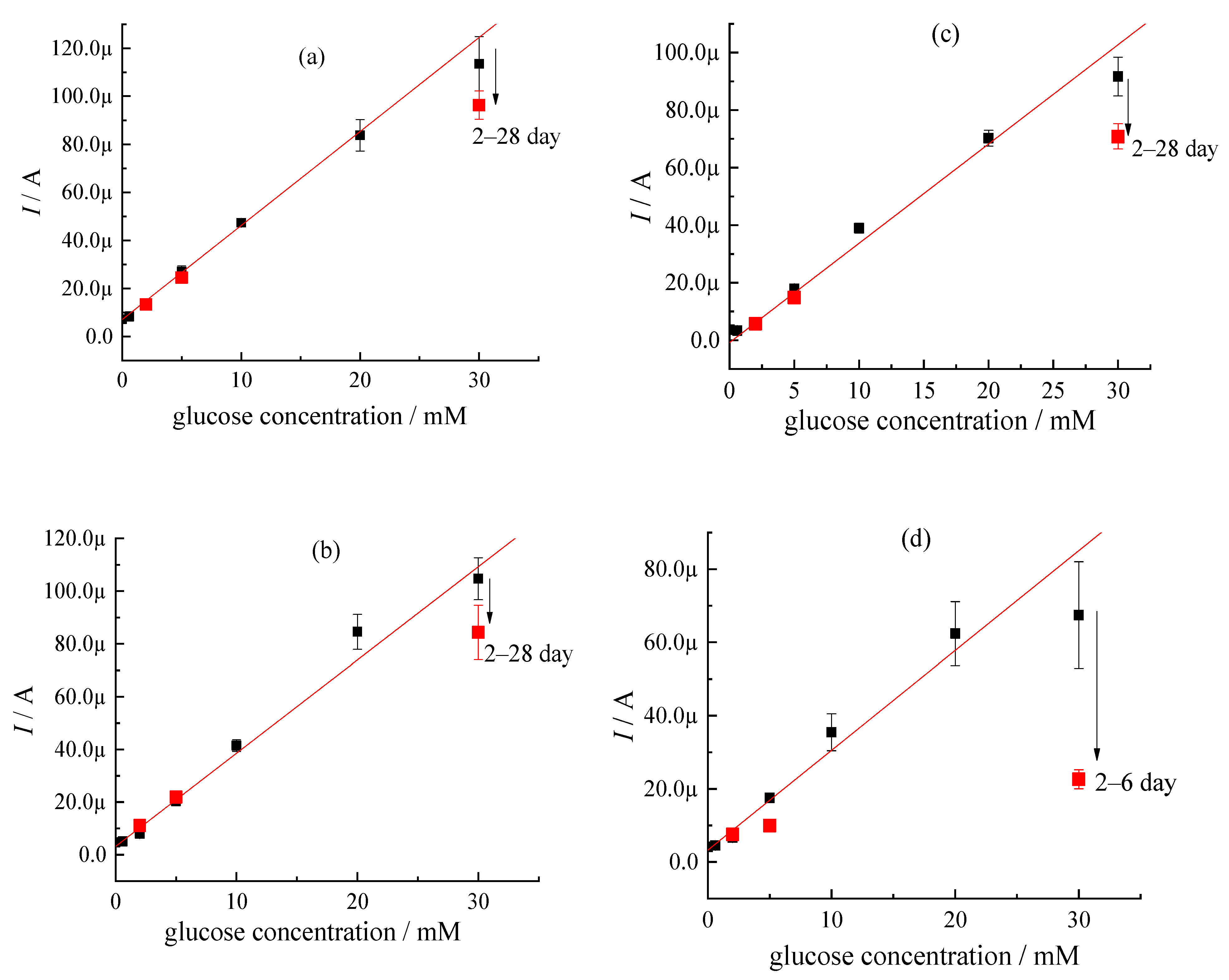
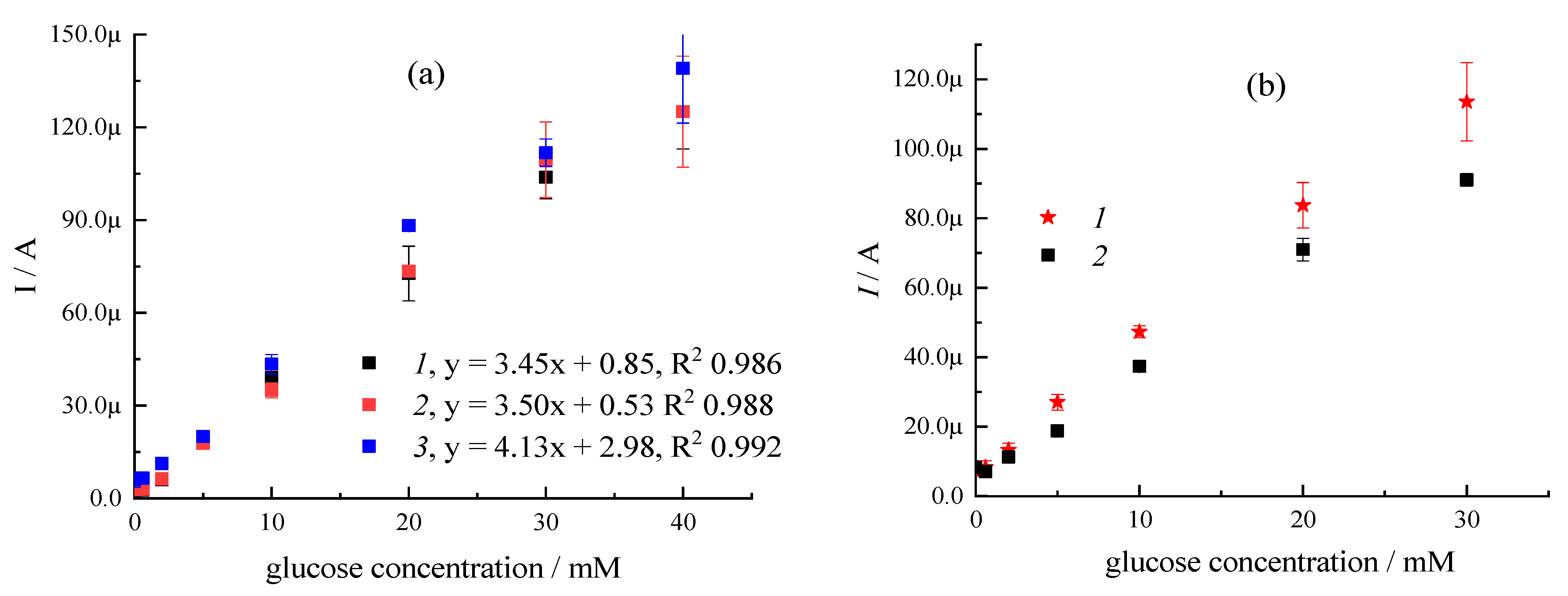

| Sample | CMC a, mg/mL | Additional Filler Agent, mg/mL | FC b, mg/mL | Gox c, mg/mL | LDR d, R2 | Sensitivity, µA mM−1 | Intercept, µA |
|---|---|---|---|---|---|---|---|
| No additional filler agent was used | |||||||
| 20CMC/50FC/2GOx | 20 | - | 50 | 2 | 0.6–20, 0.9866 | 0.08 | 0.47 |
| 20CMC/50FC/9GOx | 20 | - | 50 | 9 | 0.6–10, 0.9892 | 1.46 | 3.31 |
| 20CMC/200FC/12GOx | 20 | - | 200 | 12 | 2–20, 0.9979 | 3.71 | −2.66 |
| 20CMC/200FC/24GOx | 20 | - | 200 | 24 | 0–40, 0.9787 | 3.29 | 0.01 |
| Sodium alginate | |||||||
| 10CMC/15ALG/200FC/2GOx | 10 | 15 | 200 | 2 | 2–30, 0.9953 | 2.42 | 0.06 |
| 10CMC/15ALG/200FC/9GOx | 10 | 15 | 200 | 9 | 0.6–30, 0.9980 | 3.13 | 2.70 |
| 10CMC/25ALG/200FC/9GOx | 10 | 25 | 200 | 9 | 0–40, 0.9779 | 3.14 | 6.94 |
| 10CMC/25ALG/100FC/4GOx | 10 | 25 | 100 | 4 | 0–40, 0.9717 | 1.70 | 1.04 |
| 10CMC/25ALG/200FC/12GOx | 10 | 25 | 200 | 12 | 0–40, 0.9854 | 3.21 | 2.02 |
| 10CMC/25ALG/200FC/24GOx | 10 | 25 | 200 | 24 | 0–30, 0.9967 | 3.91 | 7.08 |
| Aerosil 380 | |||||||
| 20CMC/75AS/100FC/9GOx | 20 | 75 | 100 | 9 | 2–30 0.9889 | 1.75 | −0.78 |
| 20CMC/75AS/150FC/9GOx | 20 | 75 | 150 | 9 | 2–20 0.9904 | 2.09 | 1.53 |
| 10CMC/50AS/150FC/9GOx | 10 | 50 | 150 | 9 | 0–20, 0.9712 | 1.89 | 3.25 |
| 10CMC/50AS/200FC/9GOx | 10 | 50 | 200 | 9 | 0–40, 0.9879 | 2.82 | 2.55 |
| 10CMC/50AS/200FC/12GOx | 10 | 50 | 200 | 12 | 0–30, 0.9895 | 3.09 | 2.69 |
| 10CMC/50AS/200FC/24GOx | 10 | 50 | 200 | 24 | 0–30, 0.9587 | 3.38 | −0.68 |
| Sample | Buffer System | Sensitivity, µA mM−1 * | Degradation Degree, % | |
|---|---|---|---|---|
| Fresh Test-Strips | Test-Strips after Stress-Test | |||
| 20CMC/200FC/24GOx | CitB, pH 6.0 | 3.3 | 2.1 ± 0.4 ** | 36 |
| 10CMC/25ALG/200FC/24GOx | CitB, pH 6.0 | 3.9 | 3.1 ± 0.2 | 20 |
| PPBI, 7.4 | 3.5 | 2.9 ± 0.2 | 17 | |
| 10CMC/25ALG/200FC/12GOx | CitB, pH 6.0 | 3.2 | 2.6 ± 0.2 | 19 |
| 10CMC/50AS/200FC/24GOx | CitB, pH 6.0 | 3.4 | 2.2 ± 0.1 | 31 |
| PPBI, 7.4 | 2.7 | 0.6 ± 0.1 | 79 | |
| 10CMC/50AS/200FC/12GOx | CitB, pH 6.0 | 3.1 | 1.4 ± 0.2 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotukhina, E.V.; Gerasimova, E.V.; Sorokin, V.V.; Levchenko, M.G.; Freiman, A.S.; Silina, Y.E. The Impact of the Functional Layer Composition of Glucose Test-Strips on the Stability of Electrochemical Response. Chemosensors 2022, 10, 298. https://doi.org/10.3390/chemosensors10080298
Zolotukhina EV, Gerasimova EV, Sorokin VV, Levchenko MG, Freiman AS, Silina YE. The Impact of the Functional Layer Composition of Glucose Test-Strips on the Stability of Electrochemical Response. Chemosensors. 2022; 10(8):298. https://doi.org/10.3390/chemosensors10080298
Chicago/Turabian StyleZolotukhina, Ekaterina V., Ekaterina V. Gerasimova, Vladislav V. Sorokin, Maria G. Levchenko, Alisa S. Freiman, and Yuliya E. Silina. 2022. "The Impact of the Functional Layer Composition of Glucose Test-Strips on the Stability of Electrochemical Response" Chemosensors 10, no. 8: 298. https://doi.org/10.3390/chemosensors10080298
APA StyleZolotukhina, E. V., Gerasimova, E. V., Sorokin, V. V., Levchenko, M. G., Freiman, A. S., & Silina, Y. E. (2022). The Impact of the Functional Layer Composition of Glucose Test-Strips on the Stability of Electrochemical Response. Chemosensors, 10(8), 298. https://doi.org/10.3390/chemosensors10080298






