1. Introduction
Between the late 20th and early 21st centuries, 2335 gene therapy clinical trials were either completed ongoing, or approved worldwide. The number of clinical trials peaked in 2015, with a total of 163 trials. Most clinical trials of gene therapies have been conducted to treat specific cancerous diseases [
1].
Gene therapy was designed to replace faulty genes or interrupt their expression. Following its successes and rapid development, there is a greater need for preparing highly efficient vectors, with low cytotoxicity, which can cross the cell membrane and the cytoplasm and release the genetic material into the cell nucleus. There are viral and non-viral vectors. Viral vectors often elicit an immune response in vivo, so researchers in recent years have focused more attention on non-viral vectors. These vectors bind to the polynucleotide, inducing a conformational change toward a more compact state. This phenomenon is called compaction or condensation. There is considerable documentation in the bibliography on the use of cationic molecules to condense DNA and form complexes to facilitate gene transfer both in vitro and in vivo [
2,
3].
In recent years, many reviews have discussed the use of different macrocycles as non-viral vectors for gene transfection. Jiménez Blanco et al. [
4] summarized the properties of preorganized macromolecular systems such as cyclodextrins, calixarenes, pillarenes, fullerenes, cyclopeptides, and cyclotrehalans, which are capable of self-assembling into nano-sized particles to allow a safe release of nucleic acids. Zhou et al. [
5] reviewed the progress made in the condensation of ct-DNA molecules using nanostructure-based vectors and highlighted the elucidation of the complex structure formed with ct-DNA using different microscopic techniques. Rodik et al. [
6] provided an overview of calixarenes, and related macrocycles, as vehicles in gene delivery, emphasizing the importance of having a preorganized scaffold incorporating alkyl chains in the structure. In that review, the authors pointed researchers to performing in vivo tests as a definitive step for evaluating the potential of these new materials in gene therapy products.
In relation to calixarenes, they can be functionalized with cationic groups on their upper rim and alkyl chains on their lower rim (or vice versa), thus making them promising vectors for the delivery of new genetic material. Multivalent platforms with guanidinium groups based on calixarenes have been used with great success in gene transfer. Sansone et al. [
7] studied the capacity of these macrocycles in the binding, condensation, and transport of calf thymus DNA through cell membranes. In a systematic study, it was determined that the calix[4]arenes in the cone conformation lead to a more compact state of ct-DNA, due to the arrangement of their lipophilic chains. Additionally, an increase in the number of phenolic units in these receptors causes a partial compaction of the polynucleotide, as shown by AFM measurements. Calix[4]arenes in cone conformation only have been found to facilitate cell transfection effectively, which is indicative of a balance between hydrophobic and electrostatic interactions.
Rodik et al. [
8] designed a protocol that optimized the ct-DNA compaction process in the presence of cationic amphiphilic calixarenes micelles, which were calix[4]arenes with choline groups and long hydrocarbon chains on their polar head. They generated aggregates of about 50 nm with suitable properties for transfection. In fact, efficient uptake of these vectors by adenocarcinoma cells from cervix/uterus (HeLa) was demonstrated by fluorescence microscopy [
8]. In a later study, they further improved the polynucleotide condensation process by lengthening the alkyl chains, which led to smaller aggregates with low polydispersity index, thus promoting more efficiently gene transfection and decreasing cytotoxicity. On the other hand, it was observed that changes in the nature of the groups at the polar head do not play a fundamental role in this process.
With the aim of increasing the cooperativity phenomenon, Lalor et al. [
9] prepared functionalized cationic multi-calixarenes, where one calix[4]arene acted as the central unit of the superstructure, which also contained four other calix[4]arene molecules, linked by short polar spacers groups. Each terminal macrocycle has a glycine residue with a free aliphatic amine at its upper terminus. Binding between multimeric calixarenes and ct-DNA was observed at lower macrocycle concentrations than when binding to a single calixarene structure, pointing to a cooperative effect. Furthermore, cytotoxicity for different cell lines was negligible and gene transfection was confirmed in Chinese hamster ovary (CHO) cells.
Rallaud et al. [
10] demonstrated that not only electrostatic-type interactions take place between cationic calixarenes and ct-DNA, but there may also be interactions with the major or minor groove of the polynucleotide. For this purpose, they used amphiphilic calix[4]arenes functionalized with guanidinium groups. It was observed that dimers of these calixarenes interact with the polynucleotide, inducing conformational changes through interactions with the major groove of ct-DNA. These changes depended on the structure of the macrocycle [
11,
12].
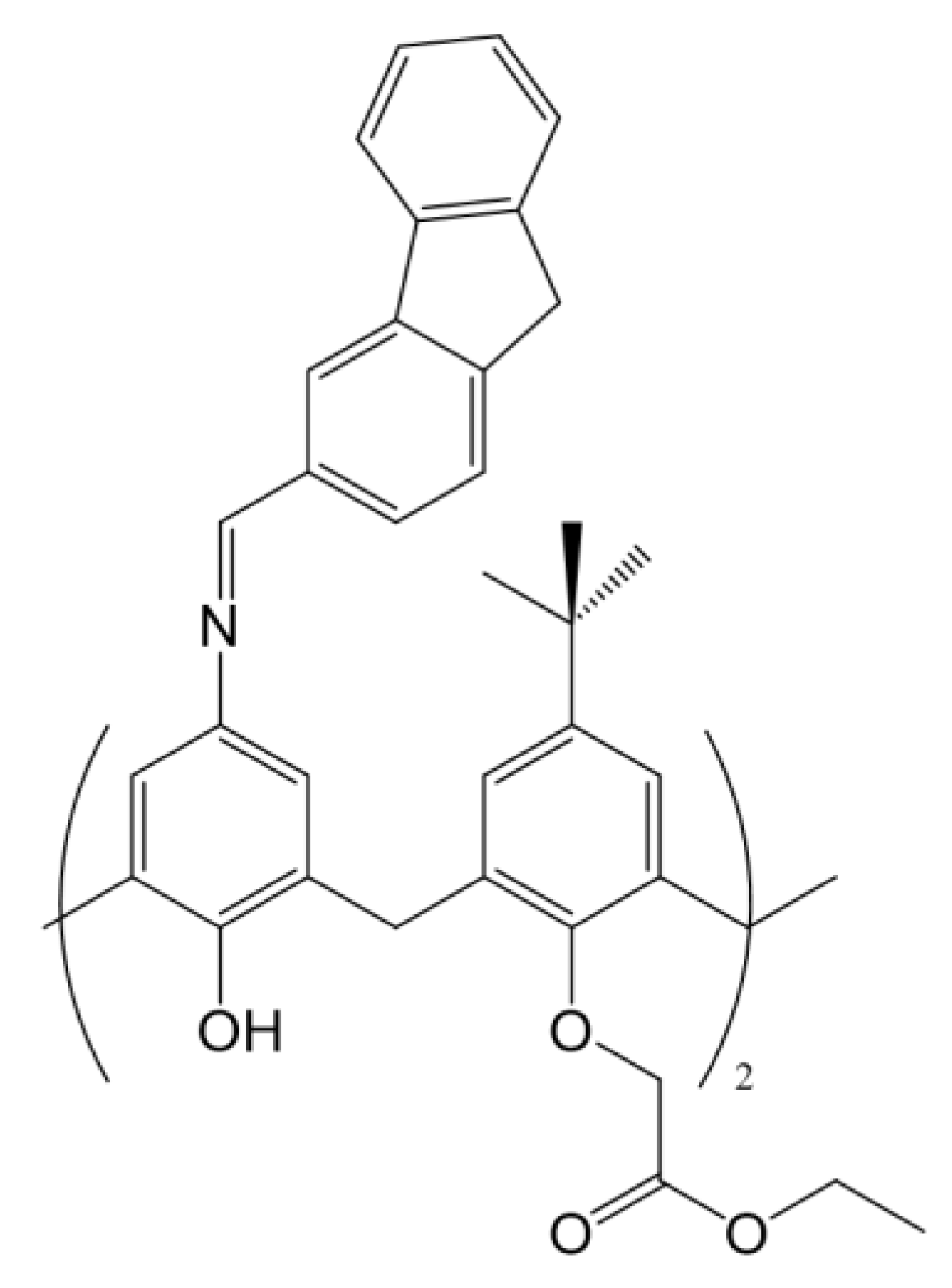
In this work, the interaction of a Schiff-Base calix[4]arene with calf thymus DNA has been studied. The calixarene investigated has a terminal ester group on the lower rim and an ortho fused polycyclic arene group in the upper rim (see
Scheme 1), with nucleic acid binding capacity. Several techniques were used to determine its binding ability with a polynucleotide. Results have shown that this macrocycle is a potential candidate for healthcare applications.
3. Results and Discussion
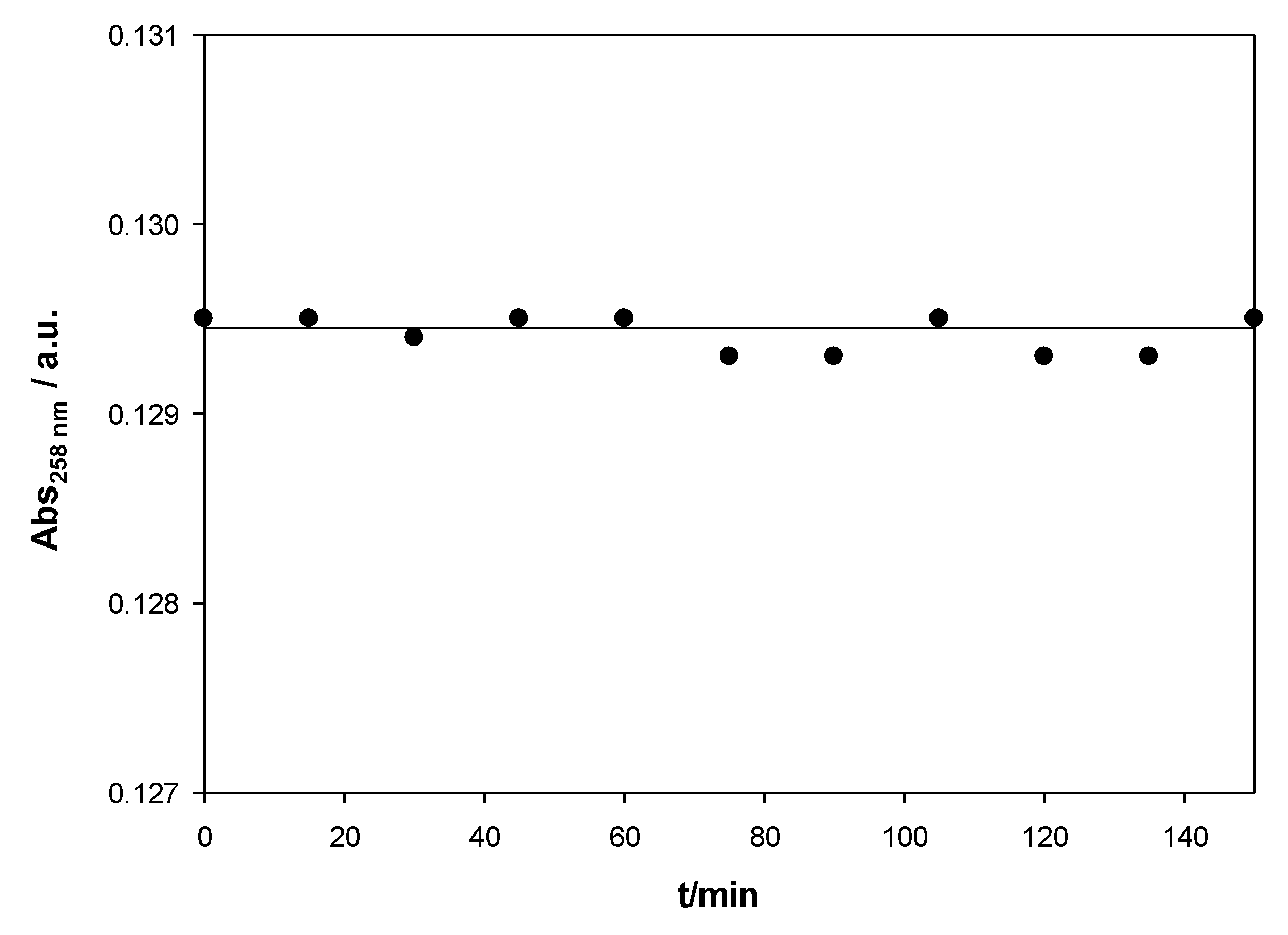
Figure 1 shows the stability of calix[4]arene/ct-DNA solutions for an X value of 0.008 for the Schiff Base calixarene. Similar results were obtained for other X values. The calixarene/ct-DNA solutions have a stability of more than two hours within the range of concentrations studied.
Fluorescence emission spectroscopy is a technique frequently used to obtain information about the binding mode of different species to ct-DNA. Normally, the interaction of a fluorescent compound with ct-DNA leads to changes in the emission spectrum of the dye [
19]. The calixarene functionalized with fluorene groups exhibits a fluorescence emission spectrum with a maximum at a wavelength of 410 nm when it is irradiated at 318 nm. This characteristic allows extracting information about the Schiff Base calixarene/ct-DNA complex, without the need to add any other probe such as ethidium bromide.
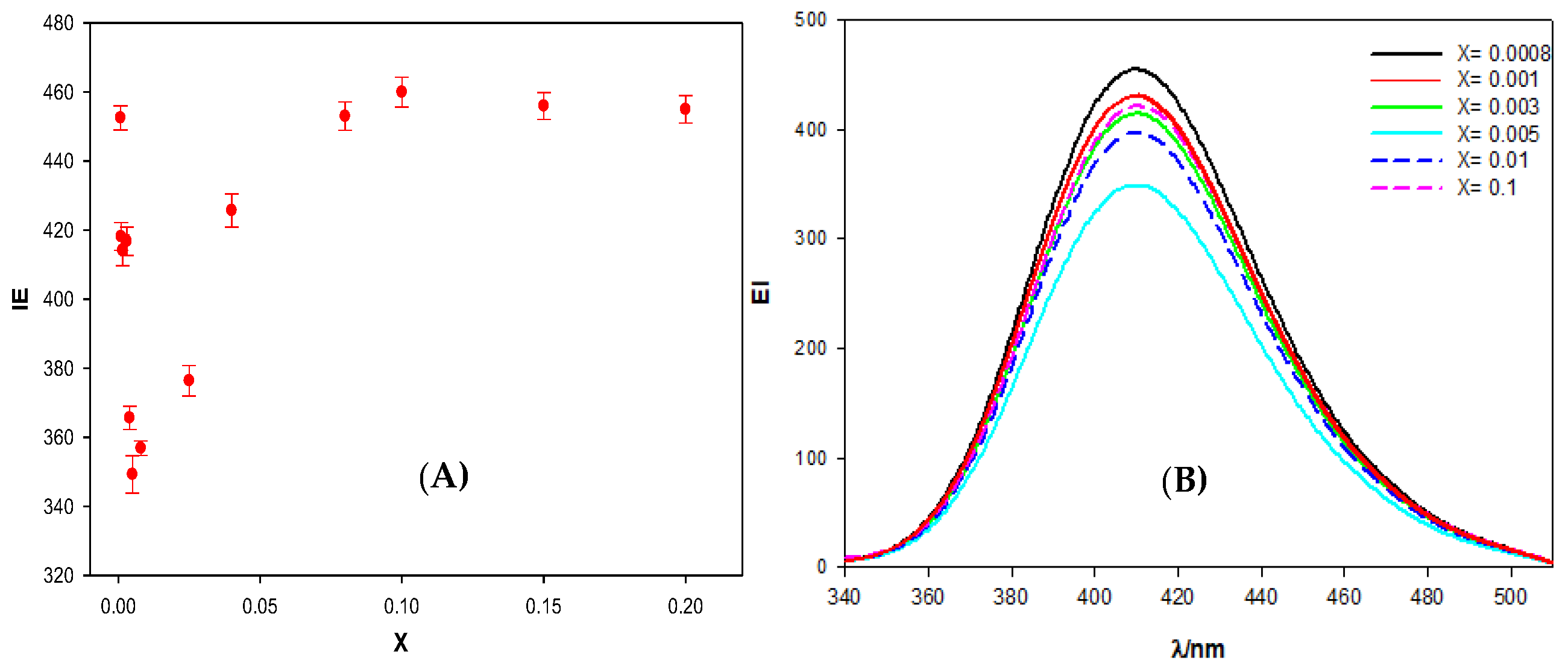
Figure 2 shows the dependence of the calixarene-ct-DNA solutions fluorescence emission intensity (EI), at 410 nm, on the molar ratio X. The results obtained demonstrate that, at small X values, there is a decrease in the emission intensity when the DNA concentration increases. However, from a value around X = 0.005, the emission intensity began to increase until it reached a more or less constant value. No shift in the emission band of the macrocycle was observed. Therefore, the fluorescence was due to the excitation of two polycyclic aromatic groups (present in the fluorine groups) in the calixarene structure. These groups have a planar symmetry, so it is possible that they are intercalated between the base pairs of the polynucleotide. In fact, this intercalation could explain the observed decrease in the emission intensity at low X values, since a change in the hydrophobic environment of the fluorene groups could cause a decrease in the EI. As the value of X increases, the emission intensity also increases. This observation may be due to the formation of calixarene dimers at the fluorine sites in the structure, when the polynucleotide concentration diminishes. The formation of calixarene dimers due to π–π type interactions between aromatic rings has previously been shown in the literature for this compound [
16]. The appearance of these dimers would imply changes in the intensity of the fluorescence emission, not only because the environment of the emitting molecules can change, but also because it could reverse the equilibrium of the calixarene/ct-DNA complex formation, favoring calixarene-calixarene interactions and, thus, weakening the calixarene-ct-DNA interactions.
The first decrease observed in
Figure 2 allowed for the quantification of the binding between the Schiff Base calixarene with ct-DNA. This measurement was made using the Two-State Model (also known as Pseudophase Model), proposed by Menger and Portnoy [
20], in which two possible states for the macrocycle are considered: one in the free state and one bound to the polynucleotide (Equation (1)):
Considering this two-state distribution for the calixarene, one can write the following equation:
where I and I
0 are the fluorescence emission intensities of the difluorene-calixarene measured in the presence and absence of DNA, respectively; (I/I
0)
w is the relative emission intensity of the calixarene located in the aqueous solution and (I/I
0)
b the relative emission intensity of the bound calixarene; that is, that of the calixarene-ct-DNA complex [
21].
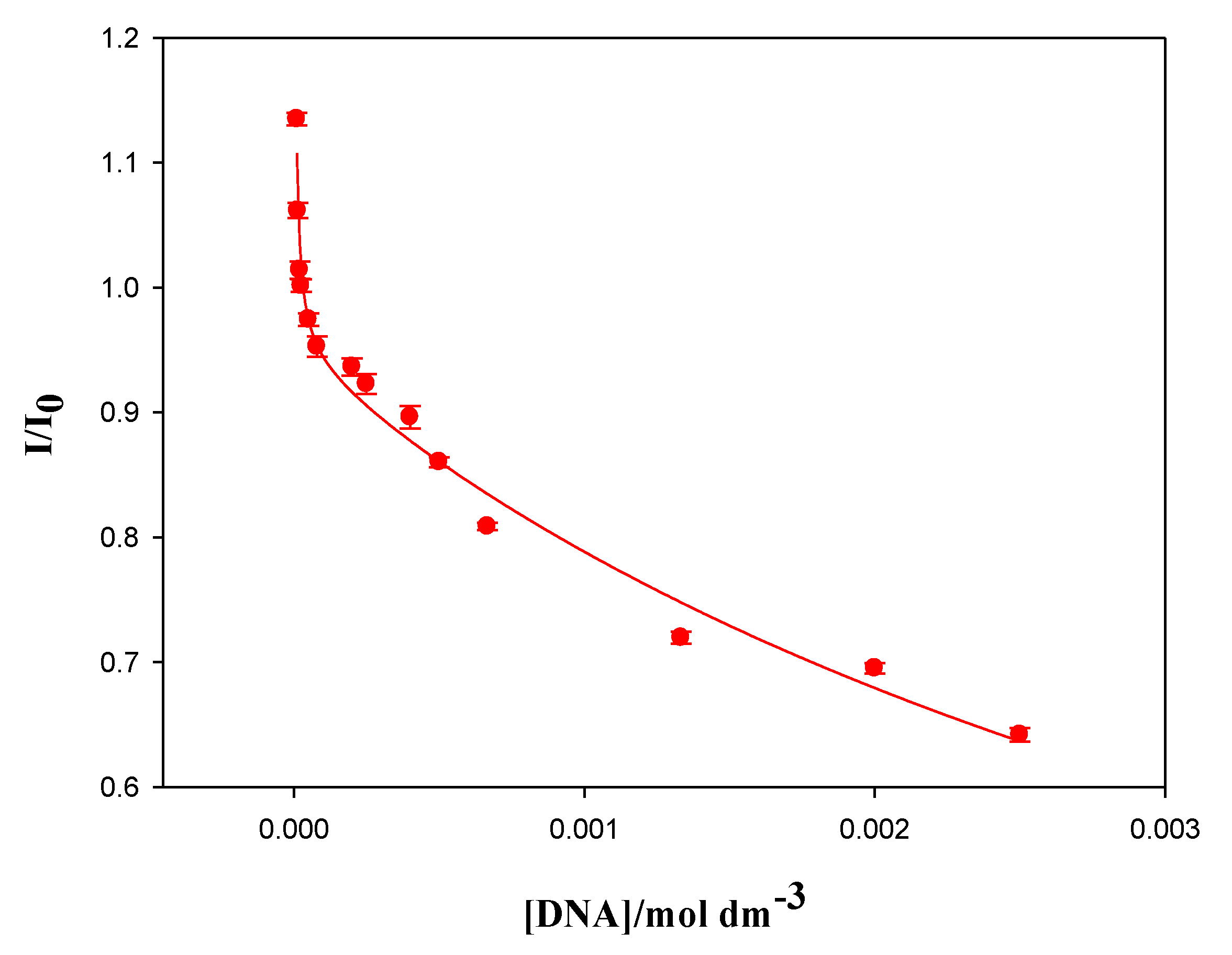
Equation (2) does not explain or fit the experimental data in the X range 0–0.005. Bearing in mind the structural characteristics of the calixarene macrocycle, the formation of dimers at the surface of the polynucleotide must be considered. Therefore, the equilibrium must be taken into account in Equation (2), resulting in the following equation:
where (I/I
0)
w, (I/I
0)
1, and (I/I
0)
2 are the free and fully bound relative fluorescence emission intensities, for processes 1 and 3, respectively. K
1 and K
2 are the equilibrium constants of these equilibria. The experimental results shown in
Figure 3 were fitted by using Equation (4). These values correspond to data obtained in the range of X = 0–0.005 and as shown in
Figure 3. A good agreement between the experimental and the theoretical data was observed and, from the fitting, the binding constants K
1 = 2.8 × 10
5 mol
−1dm
3 and K
2 = 219 mol
−1dm
3 were obtained. This K
1 value agrees with those obtained by Zadmard et al. in the study of the association processes of calixarene dimers with different double-stranded nucleic acids [
11].
To further investigate the interactions between the Schiff Base calixarene and the ct-DNA, circular dichroism (CD) measurements were performed at different X values.
Circular dichroism spectra of calixarene-ct-DNA solutions were carried out at a constant polynucleotide concentration and different macrocycle concentrations. These studies were intended to determine possible conformational changes in the ct-DNA molecules due to their interactions with the calixarene. ct-DNA in aqueous solution adopts a right-handed B-form conformation, showing a characteristic CD spectrum in the near ultraviolet region (220–320 nm). The spectrum showed a positive band at approximately 278 nm and a negative one at 247 nm. These bands are caused, respectively, by the π–π stacking interactions between the base pairs and by the helical superstructure of the polynucleotide that generates an asymmetric environment for the bases [
22]. The conformational alterations of ct-DNA, caused by its interaction with different ligands, produces changes in its CD spectrum [
23,
24]. Given that a MeOH/H
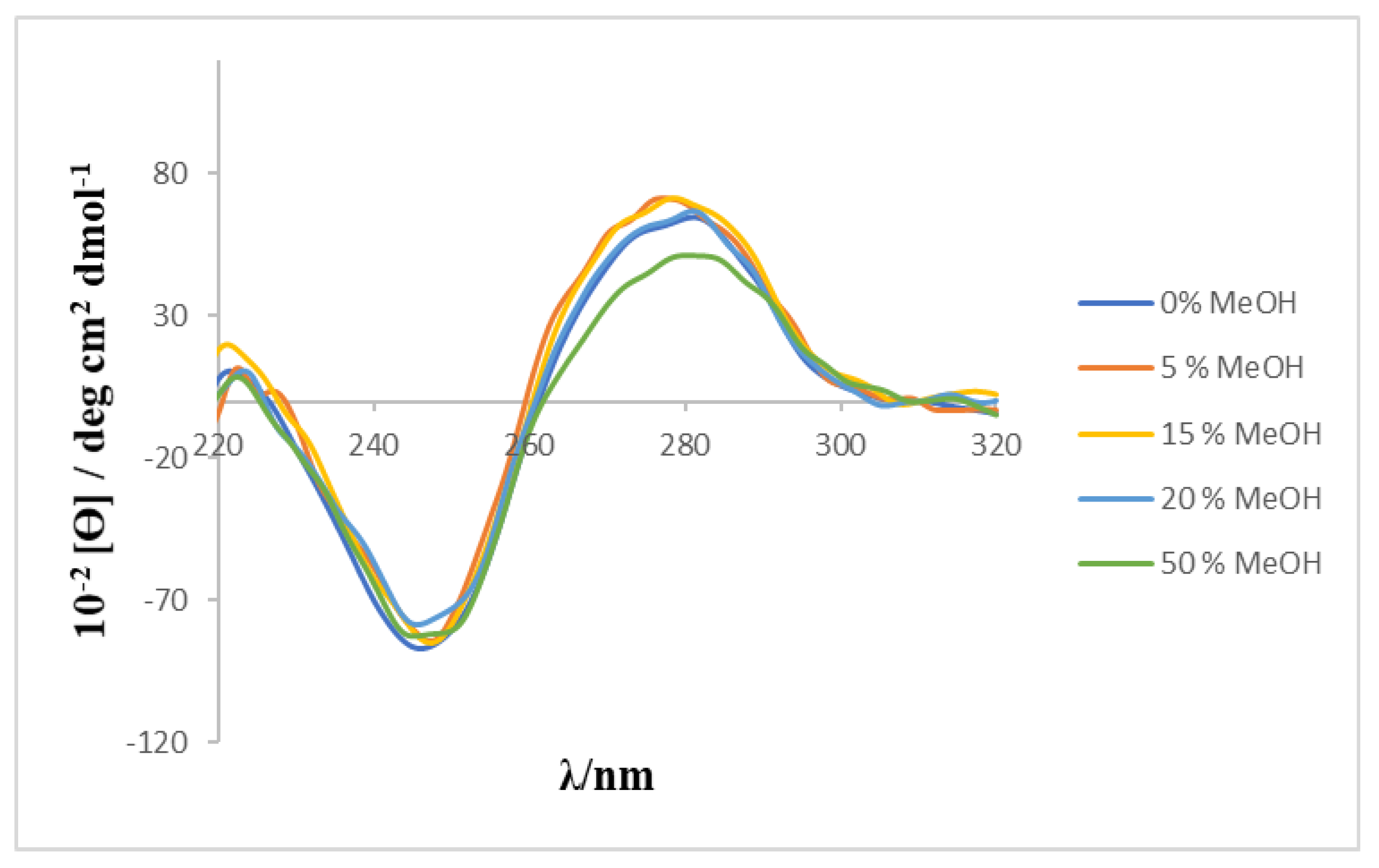
2O mixture was used as solvent because of the low solubility of the calixarene, a preliminary study was carried out to check the effect of the MeOH percentage on the CD spectrum of ct-DNA. The results (see
Figure 4) showed that the characteristic bands of the CD spectrum of ct-DNA do not undergo any modification in the presence of the content of alcohol used in the present work.
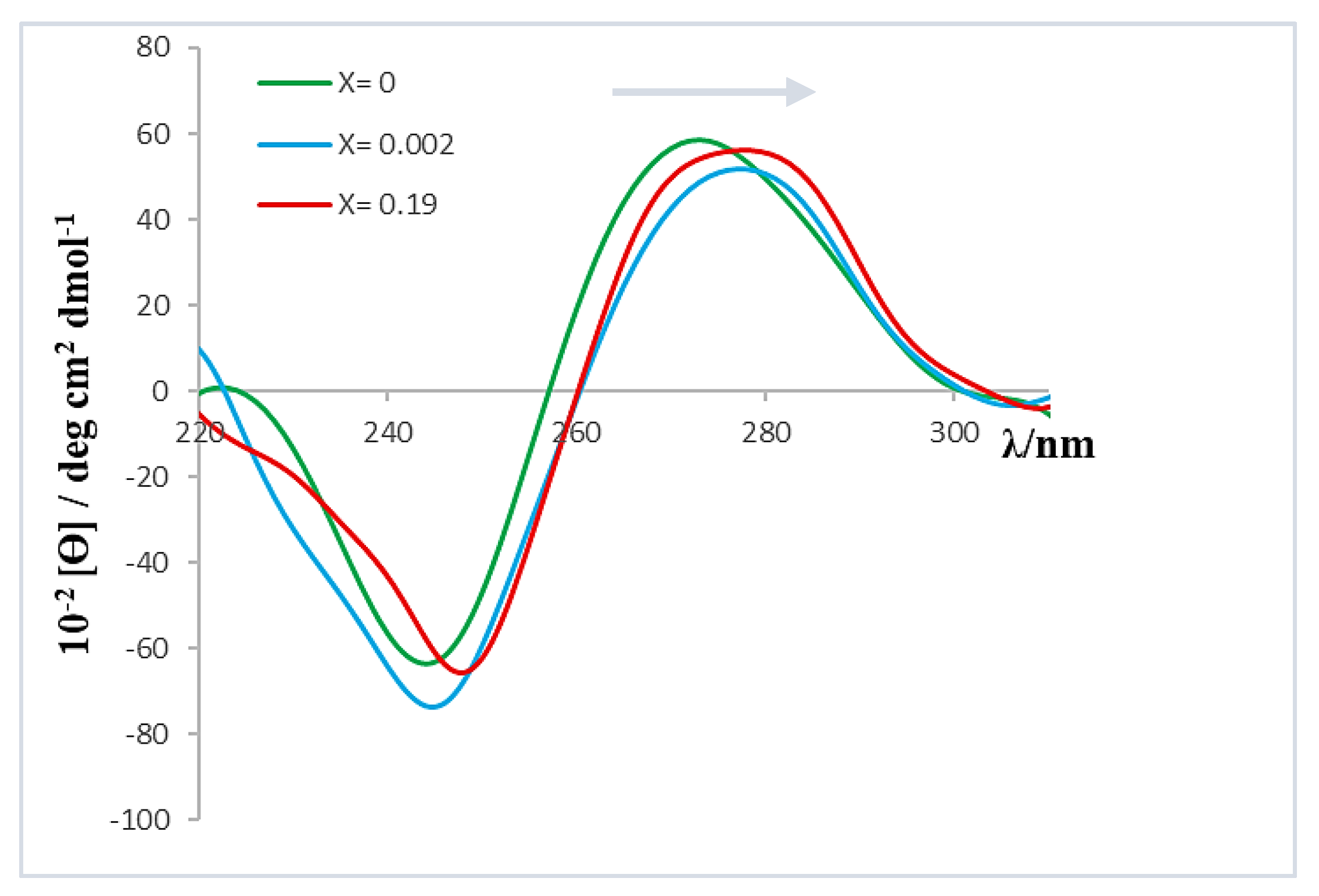
Figure 5 shows the molar ellipticity dependence of the positive and negative bands of the ct-DNA CD spectrum on the X value.
As can be seen, the molar ellipticity of both bands decreases within the range 0 < X < 0.002. In addition, although the profile or shape of the spectrum remained constant throughout the range of X values studied, the bands showed a bathochromic shift of approximately 3 nm when X increased (see
Figure 6). This observed shift could be related to the polynucleotide denaturation; that is, the separation of the ct-DNA double helix, resulting in two single-stranded ct-DNA molecules [
17]. Since the ct-DNA chains are held together by hydrogen bonds between complementary base pairs, a possible intercalation of the macrocycles between them could disrupt the hydrogen bond between the base pairs thereby affecting the spectrum. Another possibility, mentioned previously, is the potential formation of calixarene dimers which may also affect the conformation of ct-DNA. To obtain additional information on this issue, viscosity and melting temperature measurements were performed.
Information on the macroscopic properties of microheterogeneous systems can be obtained from viscosity measurements [
25]. In fact, with respect to ct-DNA solutions, the different binding modes of a ligand to the polynucleotide can be distinguished. If the calixarene ligand and the polynucleotide interact in a way that causes separation of the ct-DNA strands, this would result in an increase in the viscosity of the solution [
25]. Conversely, if a groove-type or an electrostatic binding occurs, no changes in viscosity would be expected [
26]. In addition, if the interactions cause morphological changes in the polynucleotide, then these can affect the viscosity. For example, if the ct-DNA is compacted, a decrease in the viscosity is expected.
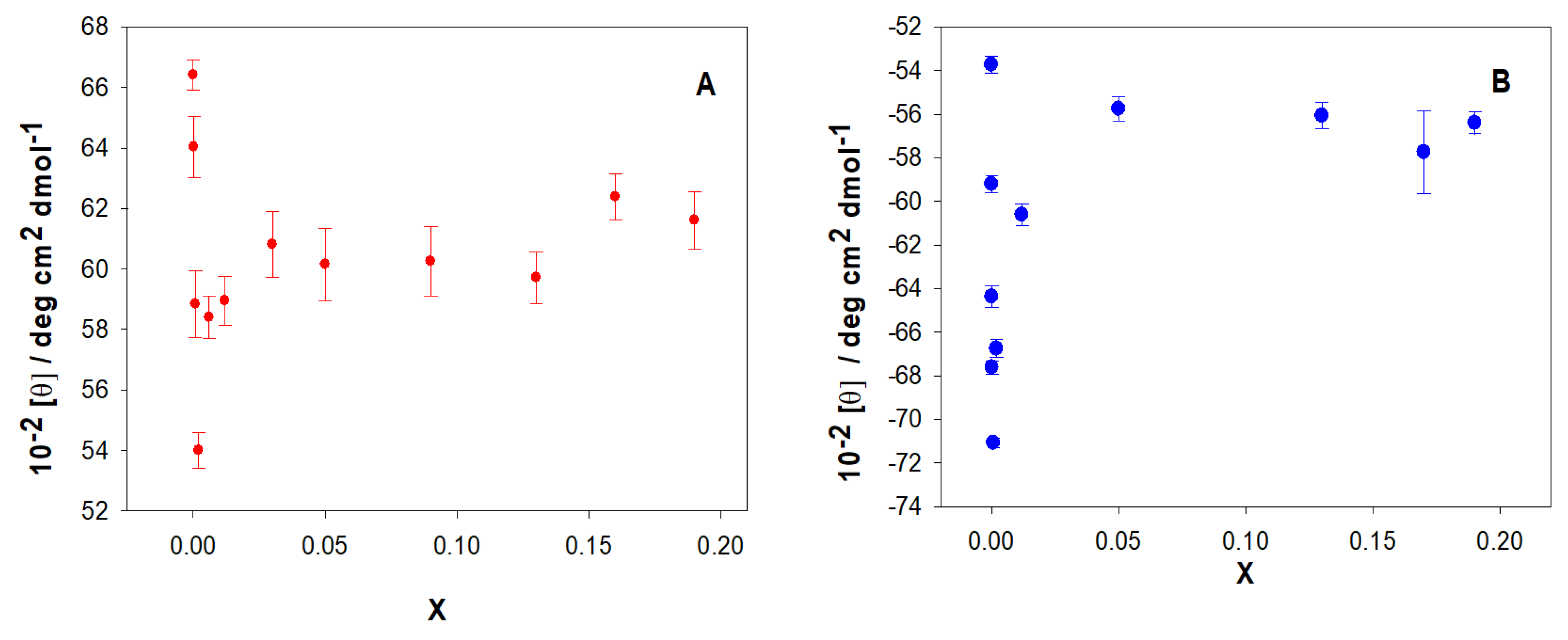
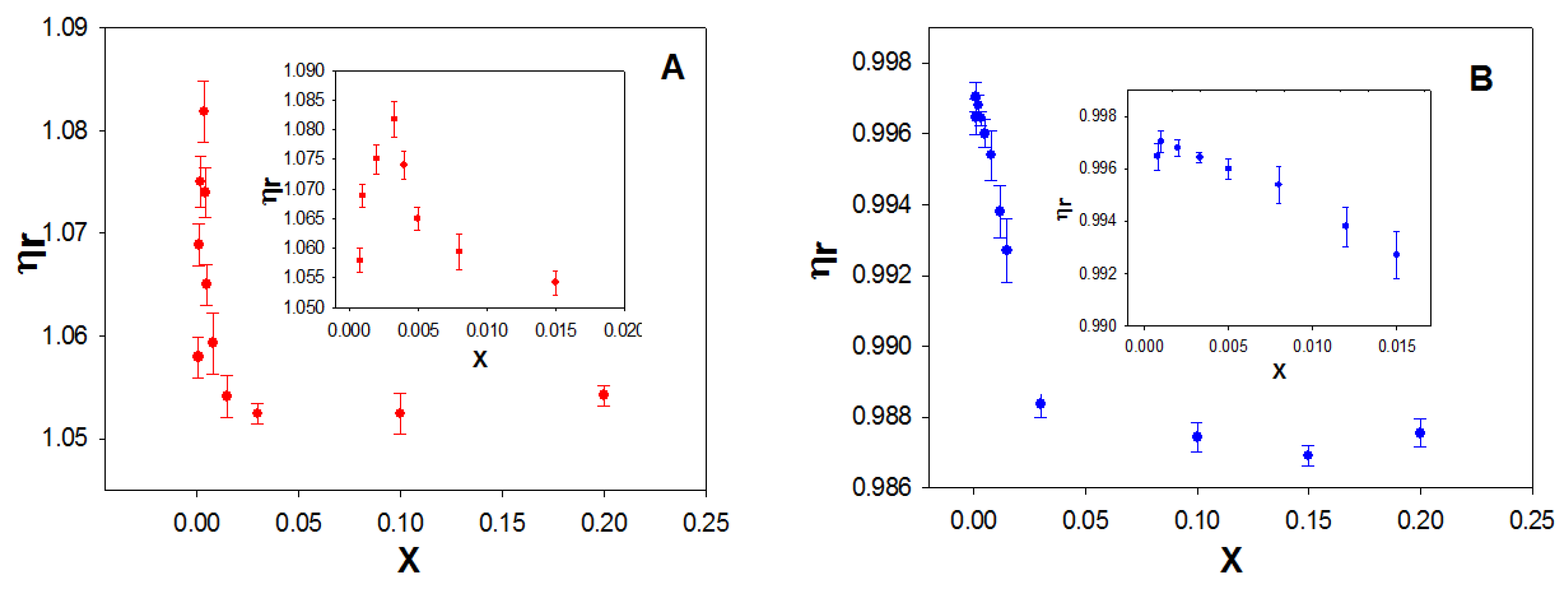
Figure 7A shows the dependence of the relative viscosity, η
r, on the molar ratio X for the macrocycle studied. As can be seen, η
r increases at low values of X and subsequently decreases with increasing the macrocycle concentration, at a fixed ct-DNA concentration. The maximum was observed at an X value of ~0.003.
The initial increase in viscosity could correspond to a denaturation of the double-stranded ct-DNA, due to the calixarene/ct-DNA interactions. To investigate this possibility, the viscosity of calixrene/ss-DNA solutions was investigated (
Figure 7B). In this case, a constancy of viscosity was observed in the range of X from 0 to 0.003.
Subsequently, the relative viscosity of the ct-DNA solution decreased with increasing concentration of the macrocycle, as occurred with the double stranded ct-DNA. The explanation for this behavior will be provided later. The results suggest that polynucleotide denaturation occurs at low X values due to the calixarene/DNA interaction.
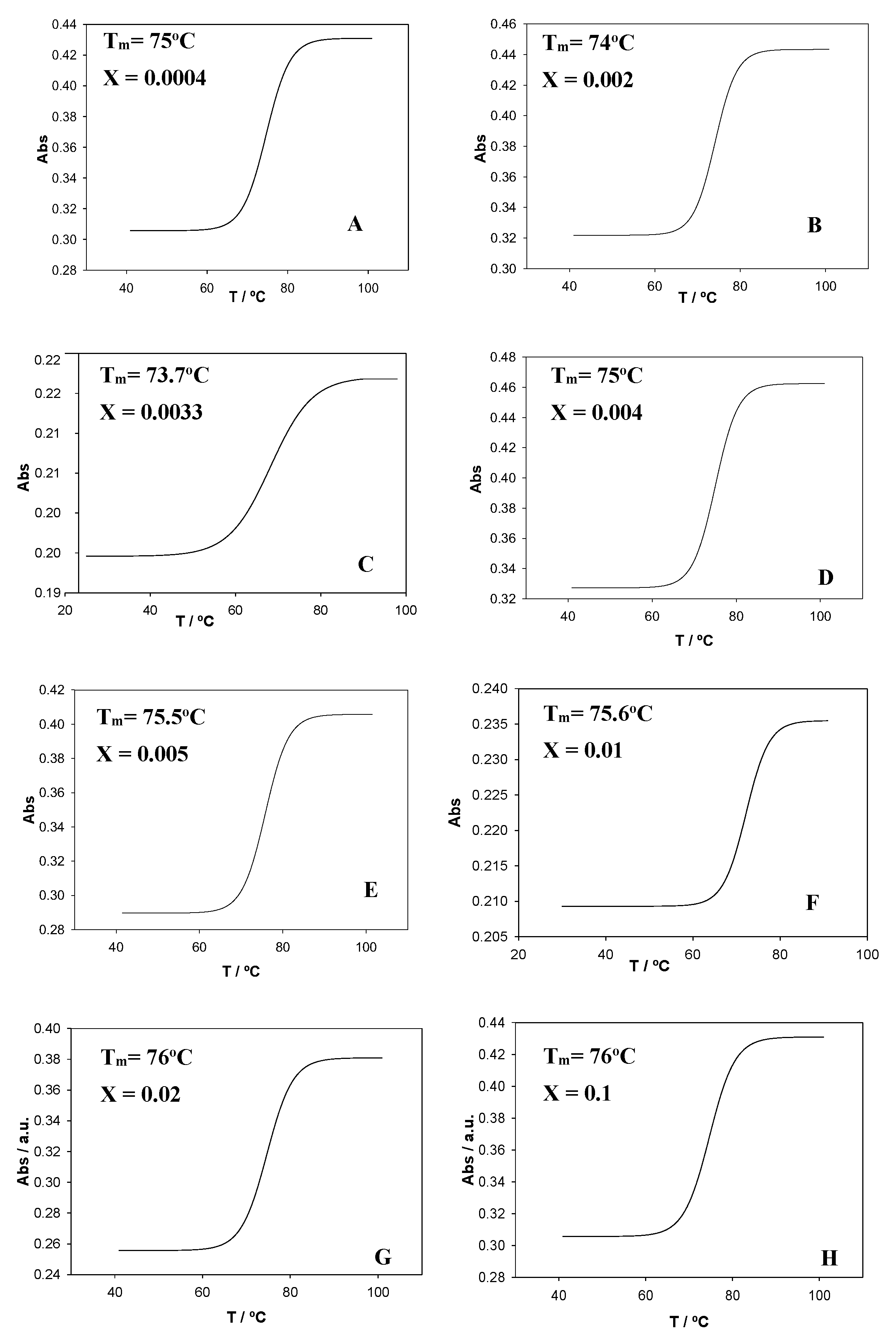
To gain further information about how the calixarene molecules interact with ct-DNA, measurements of the melting temperature, Tm, were performed. This temperature was determined as the average of the initial and final temperatures corresponding to the melting process. The profiles were sigmoidal for all X values studied (
Figure 8). The ct-DNA melting transition was highly cooperative (ΔT ≈ 10–15 °C, see
Figure 8A,B,D–F). However, a less cooperative trend was obtained for X = 0.003 for the Schiff Base calixarene (ΔT ≈ 30 °C, see
Figure 8C). A less cooperative transition happens if a partial opening of the double helix takes place, which would be accompanied by a decrease in T
m, in agreement with the experimental observations.
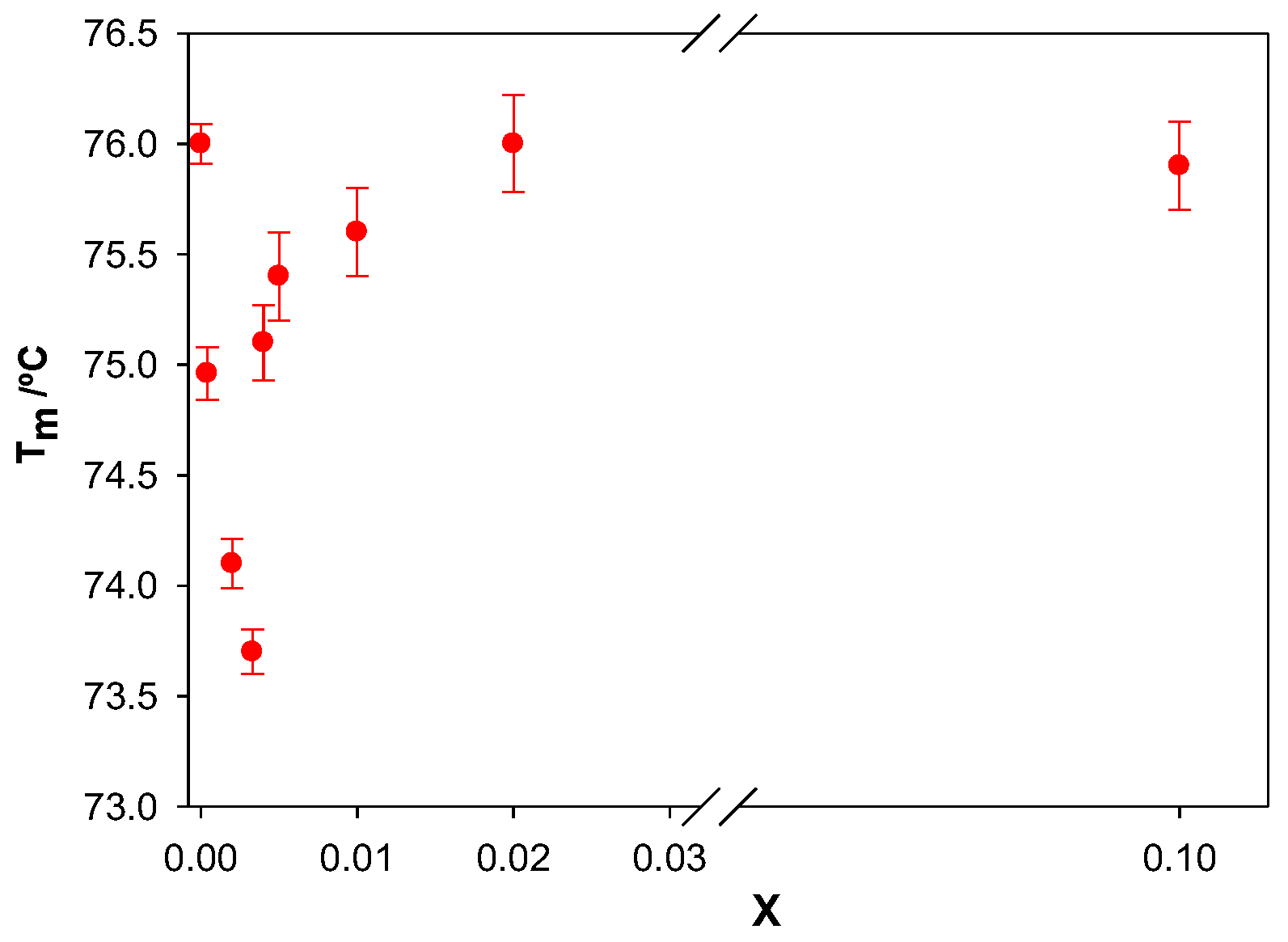
Figure 9 shows the dependence of T
m on the molar ratio X. An initial decrease in melting temperature was observed in the range of X from 0 to 0.003. A further increase in X resulted in an increase in Tm. It is seen that the values of X where a minimum point of T
m was observed coincided with those observed from the viscosity measurements.
When a ligand intercalates between the base pairs of the polynucleotide, without affecting the separation of the double helix, an increase in the rigidity of the ct-DNA double helix and a prevalence of the B form happen [
27]. This would result in an increase in Tm and could be accompanied by conformational changes in the polynucleotide. Fluorene groups (and derivatives) are known to interact with ct-DNA by intercalation between the base pairs of the polynucleotide [
28,
29]. Thus, the calixarene could be intercalated into the ct-DNA backbone through the fluorene groups. Since an initial decrease in the melting temperature was observed at low X molar ratios, the interaction of the calixarene with the polynucleotide would most likely occur via a groove-type interaction, causing the breaking of the hydrogen bonds between the base pairs and destabilization of the double helix due to a partial separation of the double strand. This type of behavior has been previously observed for other species. For example, Cu
2+ ions induce a break in the hydrogen bonds between guanine and cytosine, leading to a destabilization of the double helix [
30]. Other ions such as Zn
2+, Mn
2+, Ni
2+, and Co
2+ also cause destabilization of the double ct-DNA helix at high concentrations [
31]. The increase in the melting temperature observed for X > 0.003 suggests changes in ct-DNA conformation.
Supian et al. observed that calixarene aggregates were formed in the solid state for Schiff Base calix[4]arenes, in which the molecules stack one inside the other [
16,
32]. Therefore, supramolecular aggregates may have formed in solution for this calixarene ligand. The formation of these aggregates would be favored at high macrocycle concentrations.
Thus, the behavior observed in our studies here using many techniques for X values >0.003 may be due to the formation of supramolecular aggregates in the solution, which would be favored by increasing the concentration of the macrocycle. If such aggregates stabilize the calixarene molecules in the aqueous solution, the addition of more calixarene to the medium would weaken the calixarene/ct-DNA interactions. The released polynucleotide molecules would then revert to a more extended conformation, similar to the initial one.
To obtain information on the structure of the calixarene/ct-DNA complexes, atomic force microscopy (AFM) measurements were performed.
Figure 10 shows the results obtained. In the absence of calixarene, the typical elongated form of the ct-DNA molecules was observed. At low X values, the macrocycle interacts with the polynucleotide, inducing an increase in the bending of the ct-DNA molecules. This increase results in a decrease in the length persistence, which leads to the formation and stabilization of intramolecular loops (see
Figure 10A,B). Here, stabilization occurs by the formation of flower- and disc-like structures, which are considered to be a type of strand-with-strand stabilization, as reported by Fang and Hoh [
33,
34]. For X > 0.003, a structural change of the polynucleotide from a more compact (partial) state to a more extended structure is observed. Hence, a decompaction process was produced, as evident from
Figure 10C,D. The compact structures appear to be further apart, perhaps due to the loss of loops. Pearl-ring structures appear. This new arrangement of the polynucleotide could be due to the stacking of the calixarene molecules in solution, to form aggregates (as can be seen in
Figure 2 at values of X > 0.003), which would ultimately produce a weakening of the interactions between the calixarene and the ct-DNA. Therefore, a less compact structure would occur which agrees with the results found from the other techniques.
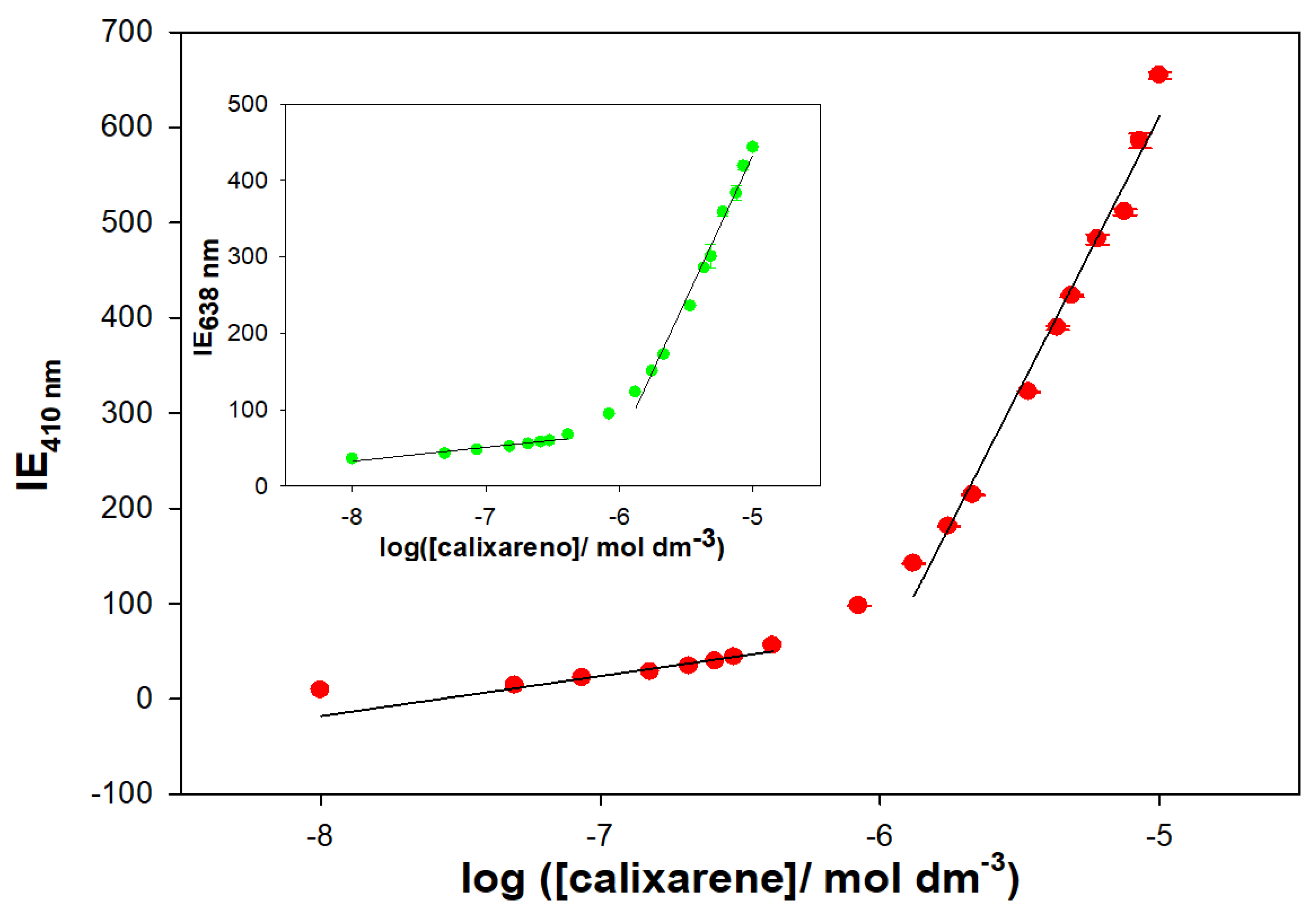
The formation of supramolecular structures has been demonstrated by fluorescence measurements. The Schiff Base calixarene showed two emission bands at 410 and 638 nm when excited at 318 nm. The emission spectrum was recorded in a MeOH/water solution (20% water/MeOH (
v/
v), pH = 7.0 with 0.01 M cacodylate) at different macrocycle concentrations. The results are shown in
Figure 11, and a linear increase in the emission intensity is observed with increasing calixarene concentration. However, from a particular concentration value, a change in the slope is seen. This abrupt change in the trend of the fluorescence emission is most likely related to the formation of supramolecular aggregates. An increase in the hydrophobic nature of the macrocycle environment can result in early formation of supramolecular aggregates.
The results obtained in this work have been compared with those previously obtained by our research group in the study of cationic surfactants as vectors for ct-DNA condensation [
35,
36]. For example, cetyltrimethylammonium bromide (CTAB) or dimeric surfactants (12-2-12,2Br- and 12-10-12,2Br-) cause compaction of the polynucleotide. The electrostatic interaction between the cationic head of the surfactants and the phosphate groups of the ct-DNA, as well as the hydrophobic interactions between the hydrocarbon chains of the surfactant molecules that are located at the surface of the polynucleotide, are responsible for compaction. However, by increasing the concentration of the surfactant, decompaction of the ct-DNA was observed. This behavior was explained based on the formation of micelles in the solution. The calixarene studied also induces compaction of the polynucleotide. However, its mode of binding to ct-DNA is different. Before causing a conformational change in the ct-DNA, this calixarene interacts with the polynucleotide through a groove-type binding mode, which induces the breaking of the hydrogen bond between the base pairs and, therefore, leads to a partial denaturation of the double helix. This is in agreement with the results previously obtained and favors the condensation process. As was the case for the surfactants, above a certain concentration of calixarene, a decompaction of the polynucleotide takes place. The formation of supramolecular structures between the molecules of the macrocycle in solution favors the breakage of the compact ct-DNA globules.
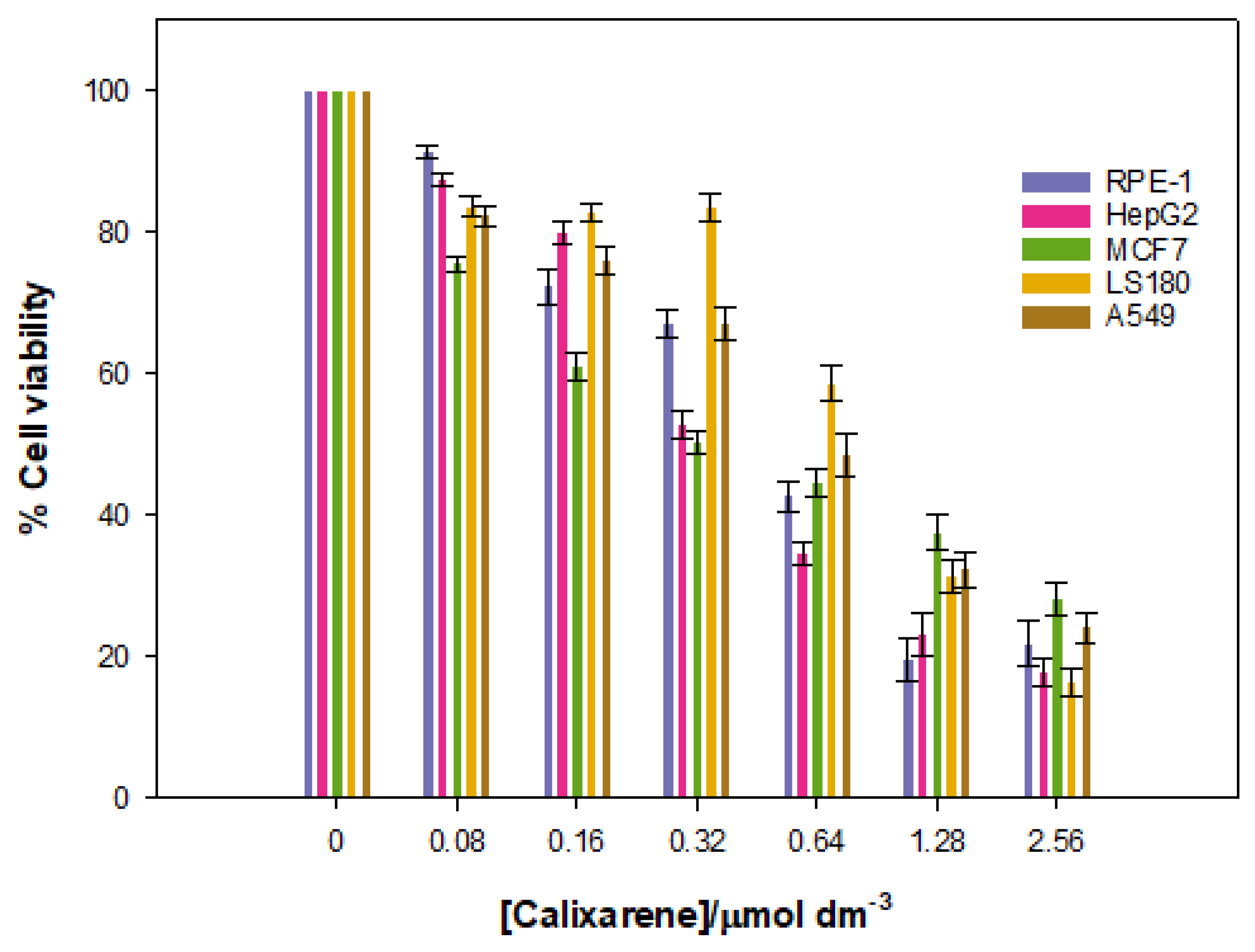
Given that the goal of the work was to investigate the potential of the Schiff Base calixarene as a vector, it is essential to examine the viability of various cell lines in the presence of different calixarene concentrations. With this in mind, the cytotoxic effect that the macrocycle exerts on different cell lines was measured. These studies were performed over a 48-h time period. The results are shown in
Figure 12.
The data show a low cytotoxicity for cancerous lines, especially at low concentrations of calixarene, but the cell viability decreased at higher concentrations. There is a certain specificity for the HepG2 and MCF7 lines, but only at low calixarene concentrations, which seems to disappear at high macrocycle concentrations.
Cytotoxicity assays were also performed with a normal RPE-1 cell line. The same results as for the cancerous lines were obtained. Overall, these results demonstrate that the Schiff Base calix[4]arene studied can be used as nanovector or nanocarrier in nanomedicine at concentrations up to 0.32 µmol dm−3 (~75% cell viability in RPE-1 cells).