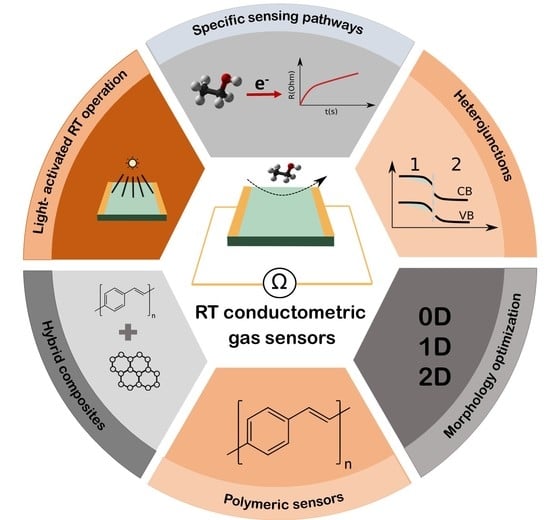
High-Performance Room-Temperature Conductometric Gas Sensors: Materials and Strategies
Abstract
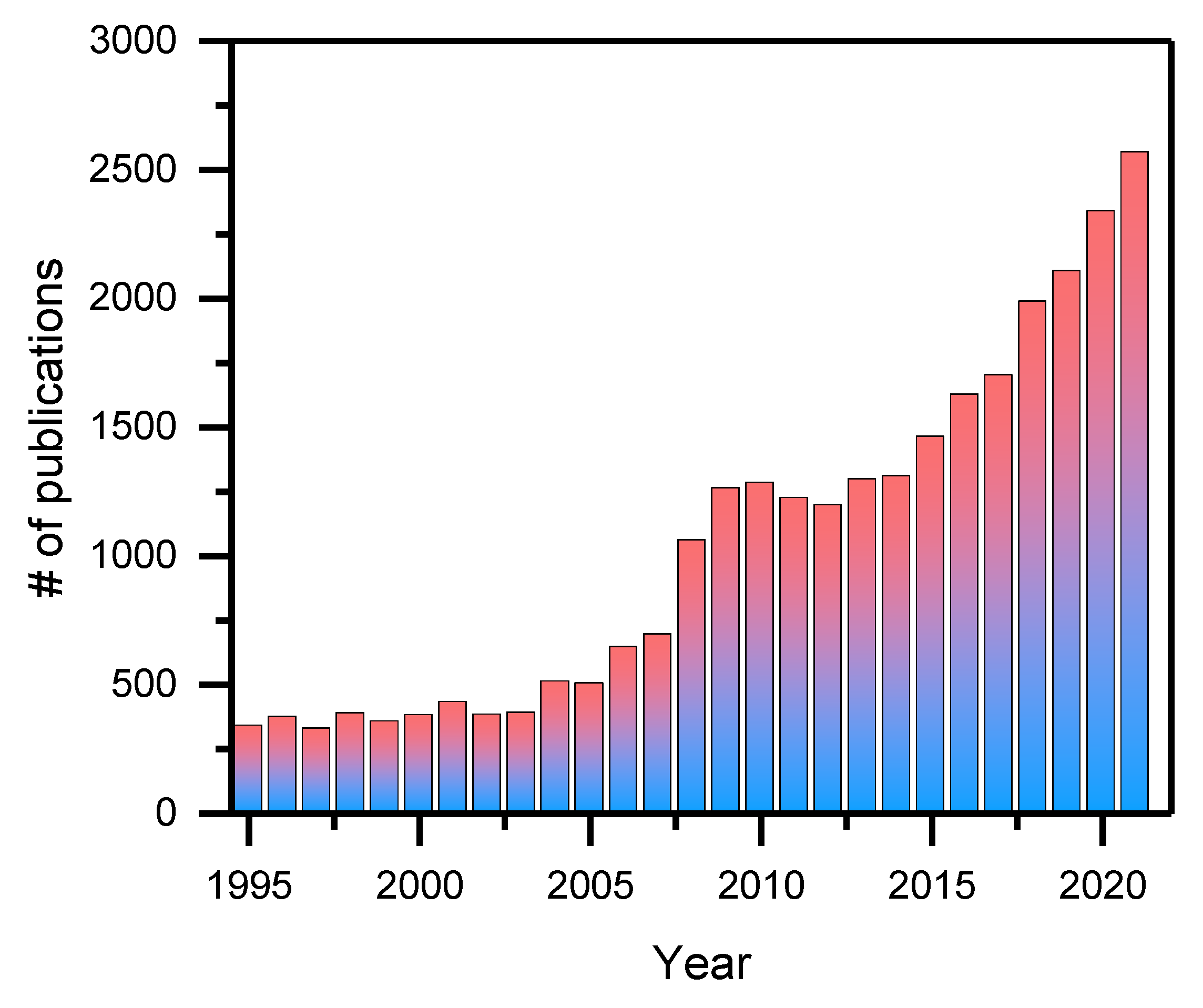
:1. Introduction
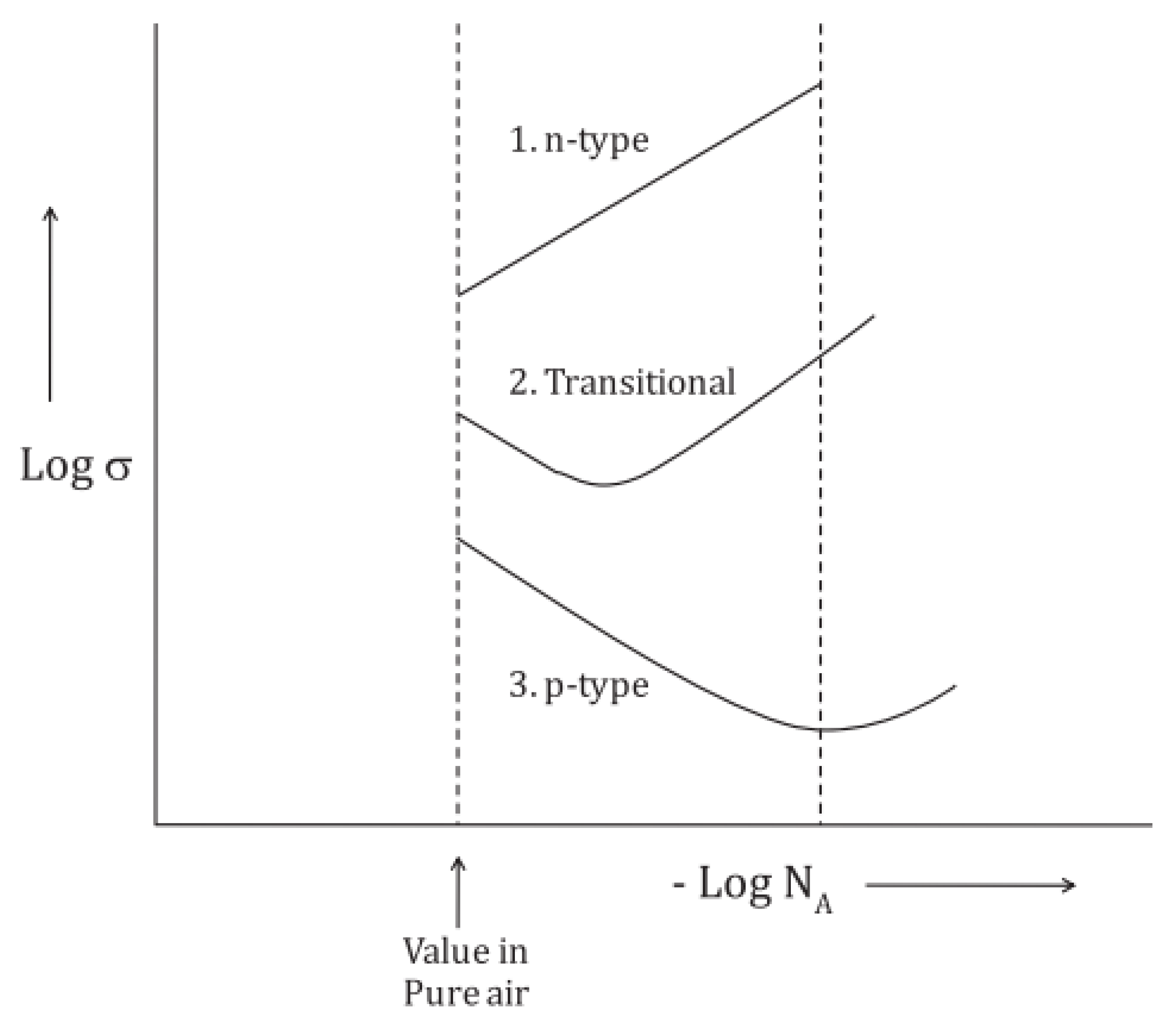
2. Sensing Principle and Mechanisms
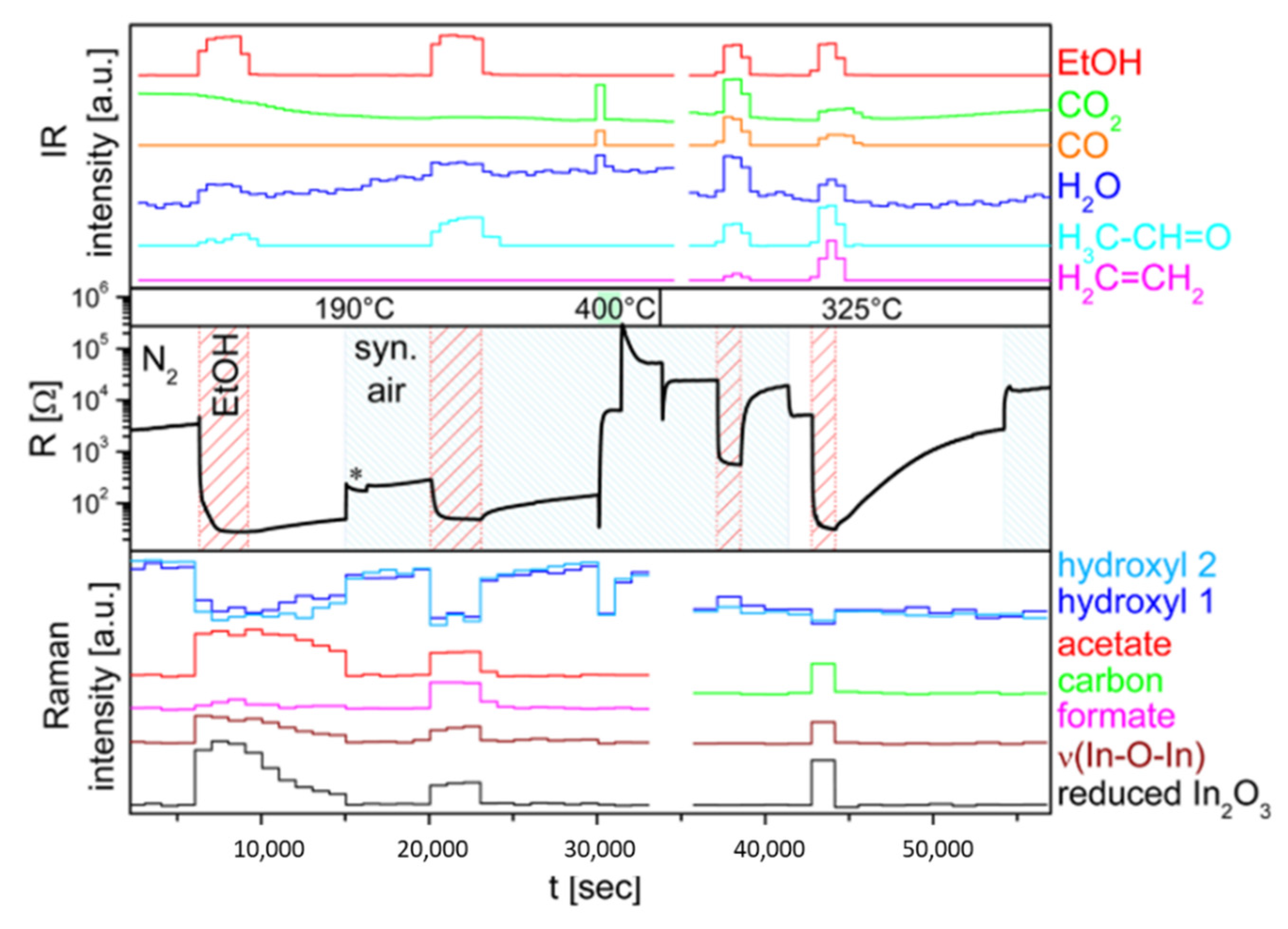
3. Characterization Techniques for Studying Conductometric Gas Sensors
4. Strategies for RT Operation
4.1. Light-Activated RT Operation
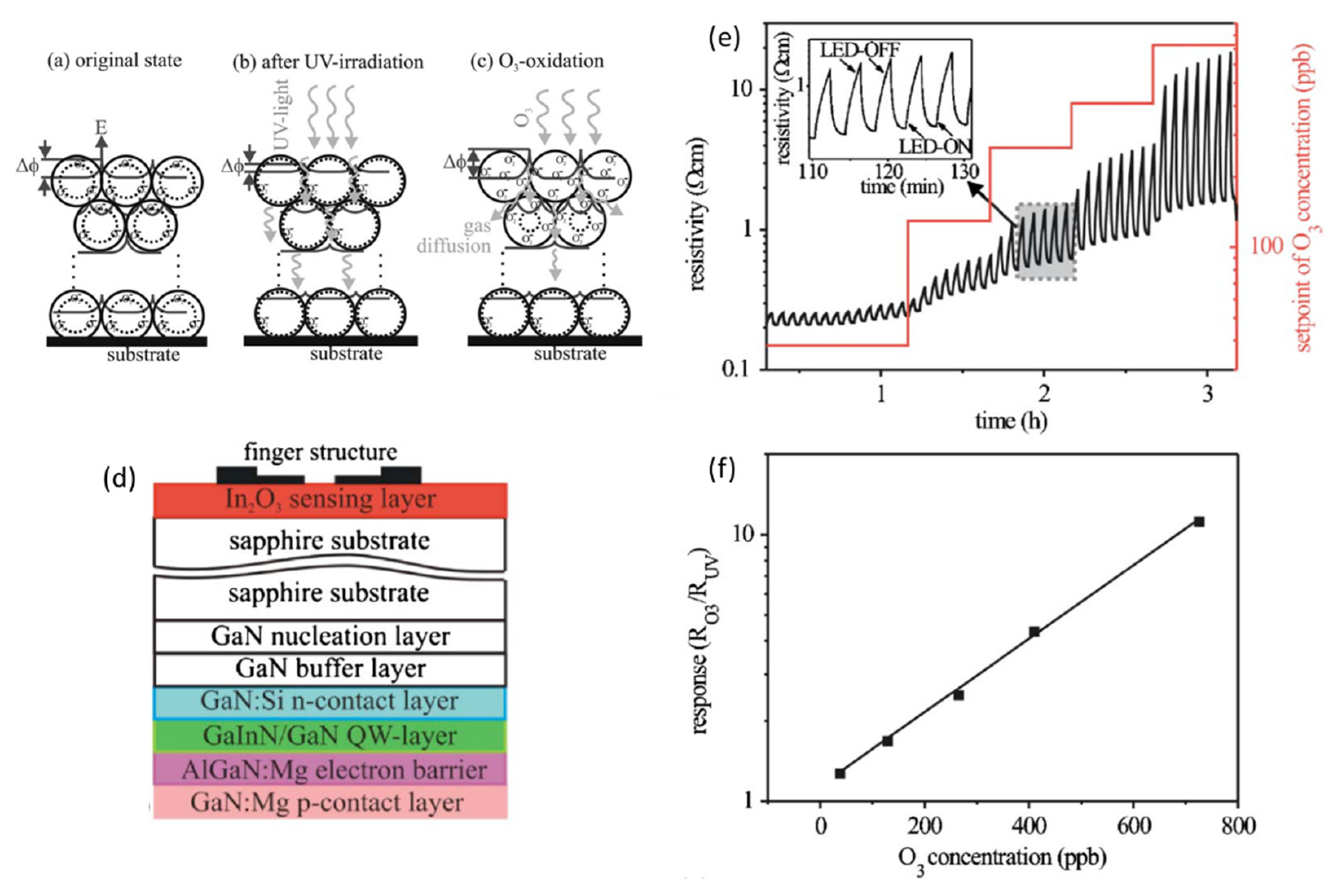
- Analyte adsorption/desorption enhancement: this approach is used on highly sensitive sensors, which show good response times at RT but slow recoveries due to the slow desorption rate of the analyte. Photogenerated carriers may rapidly recombine with any adsorbed ionic species, either ionosorbed oxygen/analyte molecules or any ionized product formed during the decomposition of the analyte, causing them to desorb as neutral species and speeding up the recovery process. A theoretical model of the kinetics of the photo-enhanced desorption of oxygen on MOs was developed by Melnick [68] with ZnO as a case study. An example of such photoactivated RT gas sensing was demonstrated for In2O3 thin films with UV back-illumination for ozone detection (Figure 5d) [28]. By periodically switching on and off the UV light, the authors managed to modulate the desorption speed of the decomposed O3 molecules. The measured resistance is then dependent on the equilibrium between the O3 adsorption rate, which depends on the concentration of O3 molecules, and the desorption rate, which depends on UV light illumination, i.e., on the ON/OFF state (Figure 5e). The obtained response, measured as the resistance ratio between the OFF (RO3) and the ON (RUV) states for a given O3 concentration, was found to vary linearly with O3 concentration (Figure 5f).
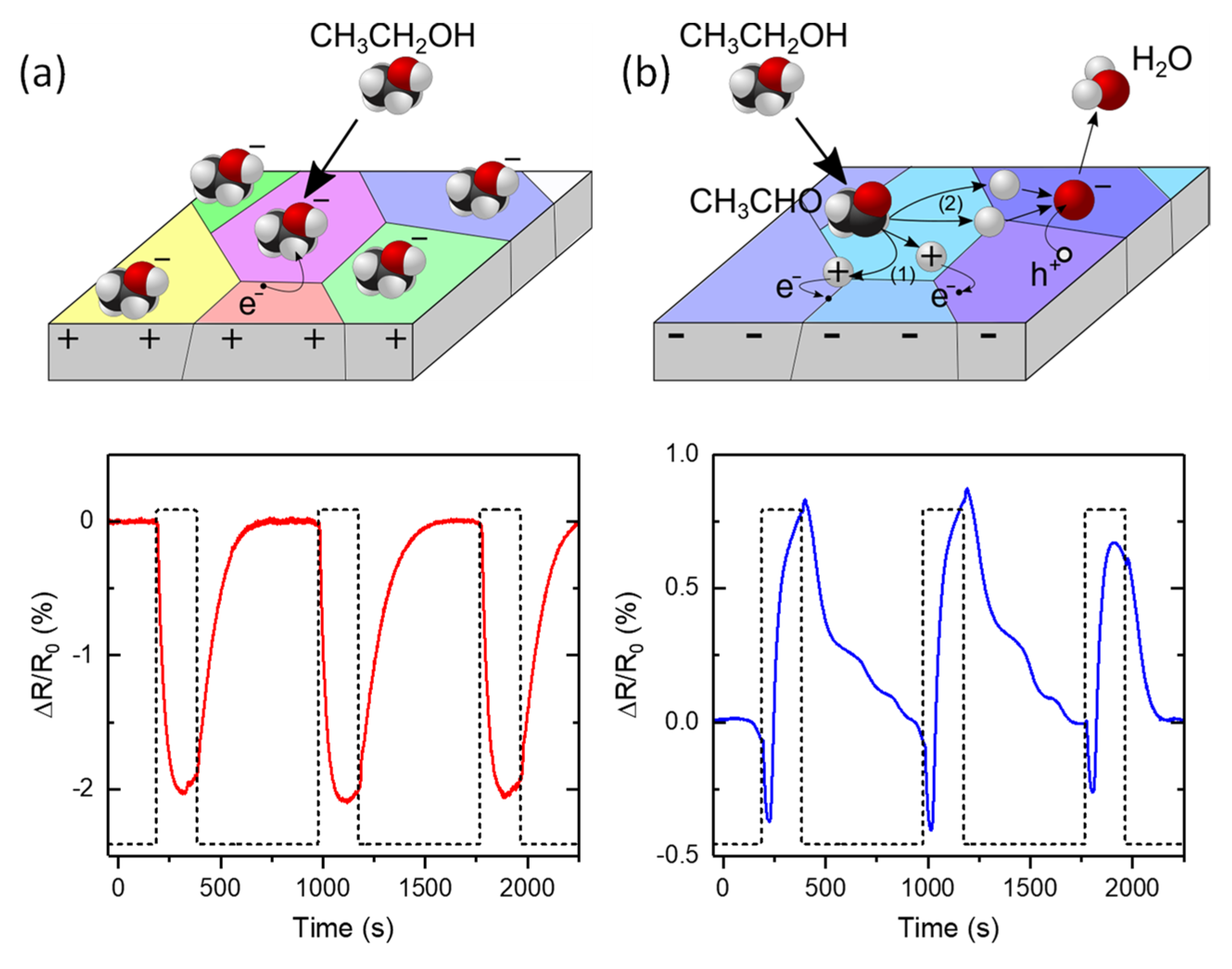
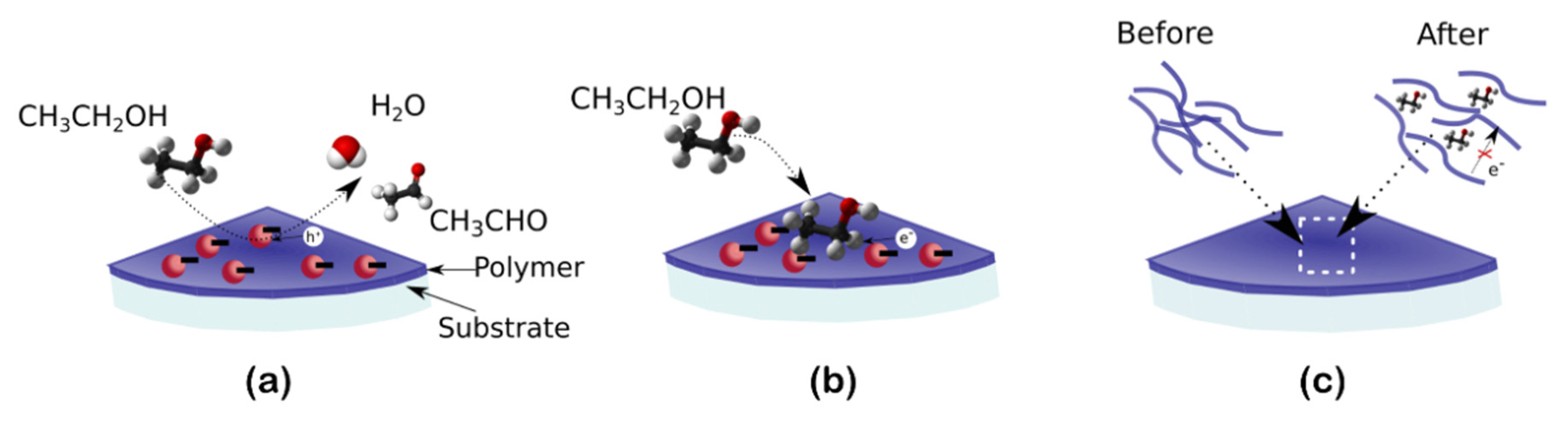
- Analyte reaction enhancement: this approach can be employed to enhance the response in gas sensors based on the catalytic decomposition of the analyte [67,69]. Many sensing mechanisms are based on the catalytic decomposition of the analyte on the sensor surface; the obtained subproducts may then either react with ionosorbed oxygen species, releasing trapped electrons, or trap free carriers themselves. These processes usually require high temperatures as a source of energy to proceed at reasonable speeds. Photocatalytic materials use photon energy instead to speed up the chemical decomposition of the analyte and promote their sensitivity at RT. In this case, photogenerated carriers interact with the analyte, breaking chemical bonds and promoting either their oxidation with ionosorbed oxygen species or their chemical reaction with other adsorbed species, such as H2O or other decomposed products [70]. Many MOs are known to have photocatalytic properties, such as TiO2 [48], SnO2 [20], or ZnO [49], but also organic polymers [71] or 2D materials [72,73].
4.2. Specific Sensing Pathways
4.3. Morphology Optimization (0D, 1D, 2D)
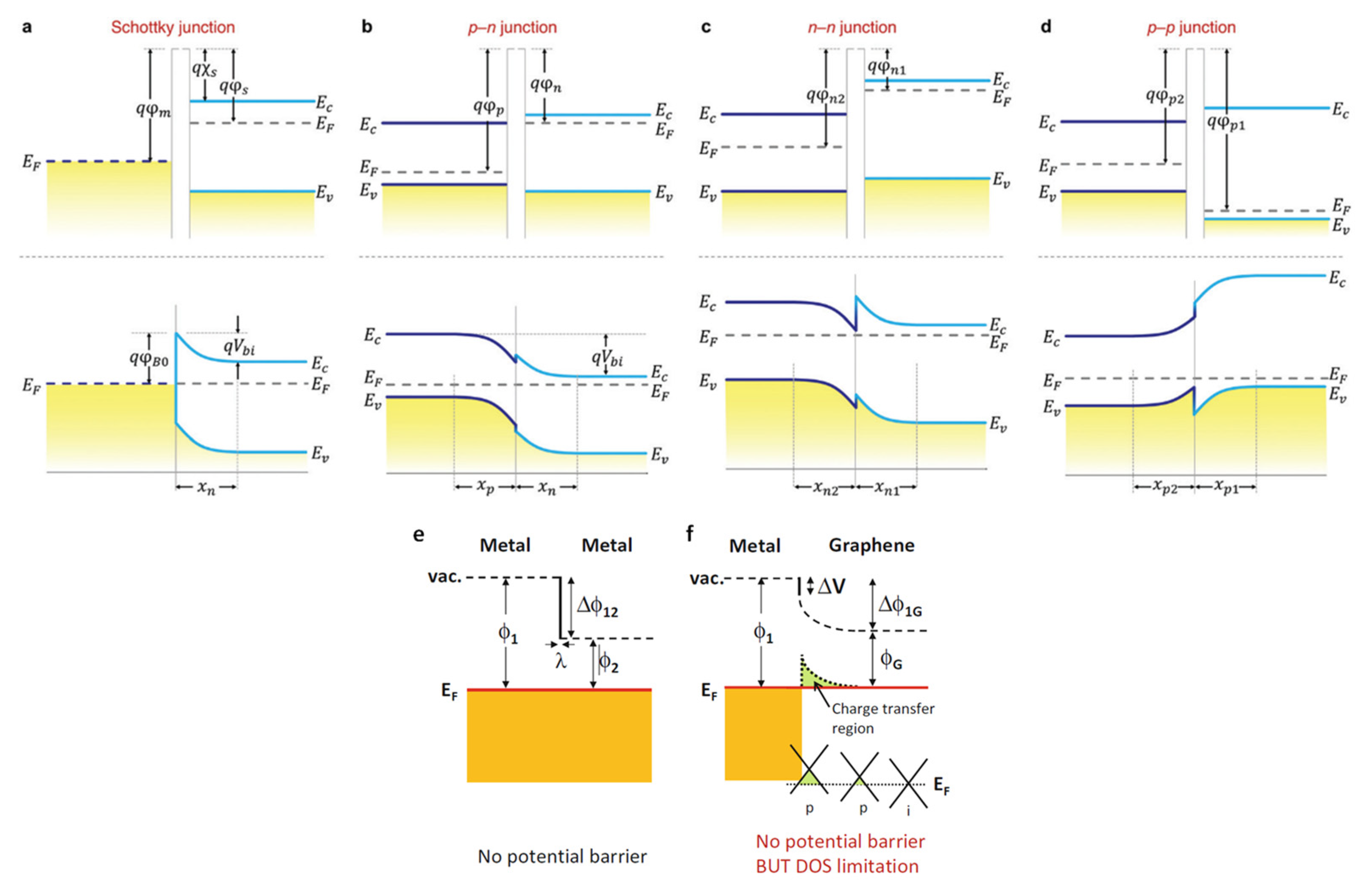
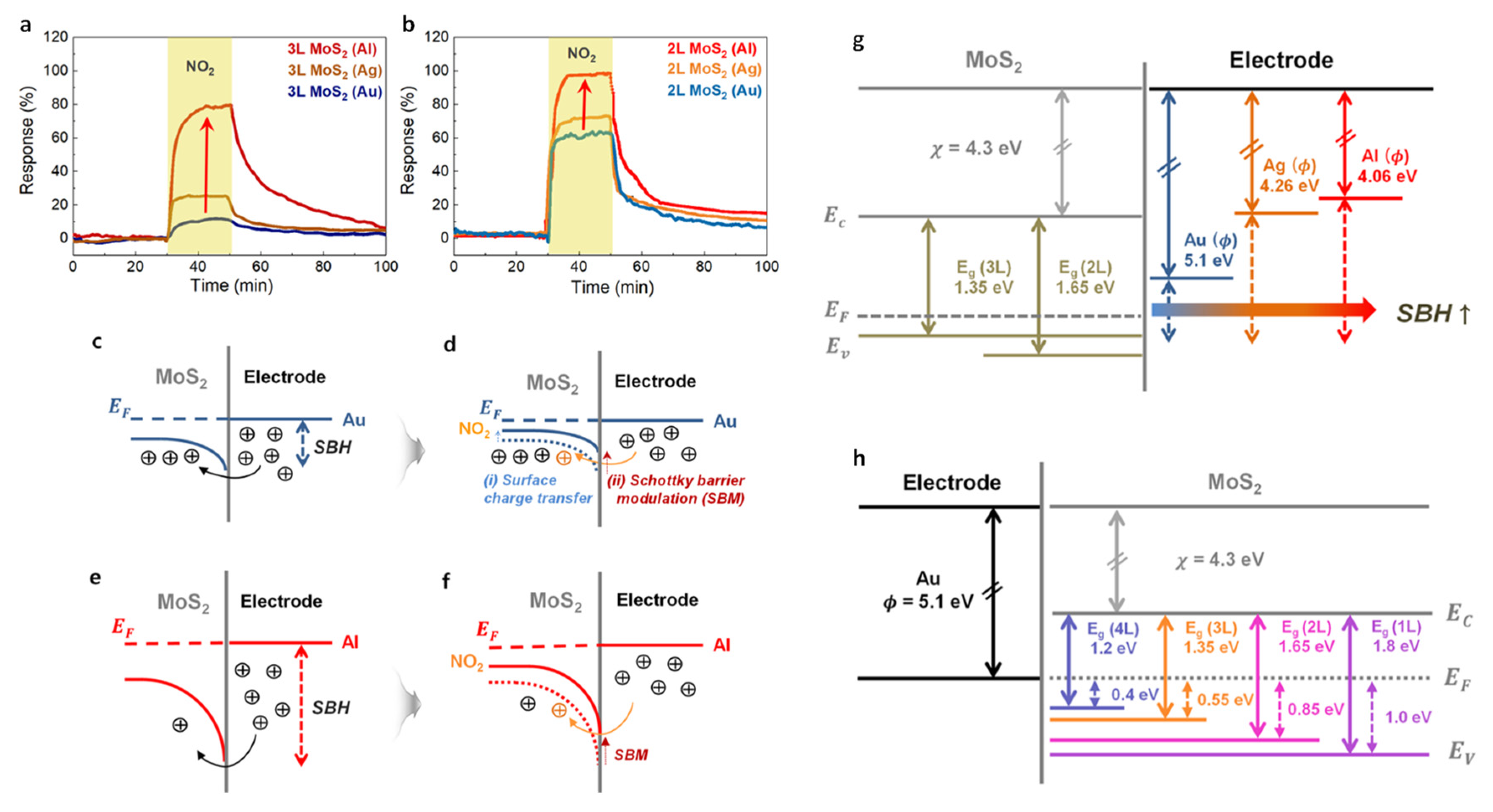
4.4. Heterojunctions: Schottky, p-n and p-p/n-n Junctions
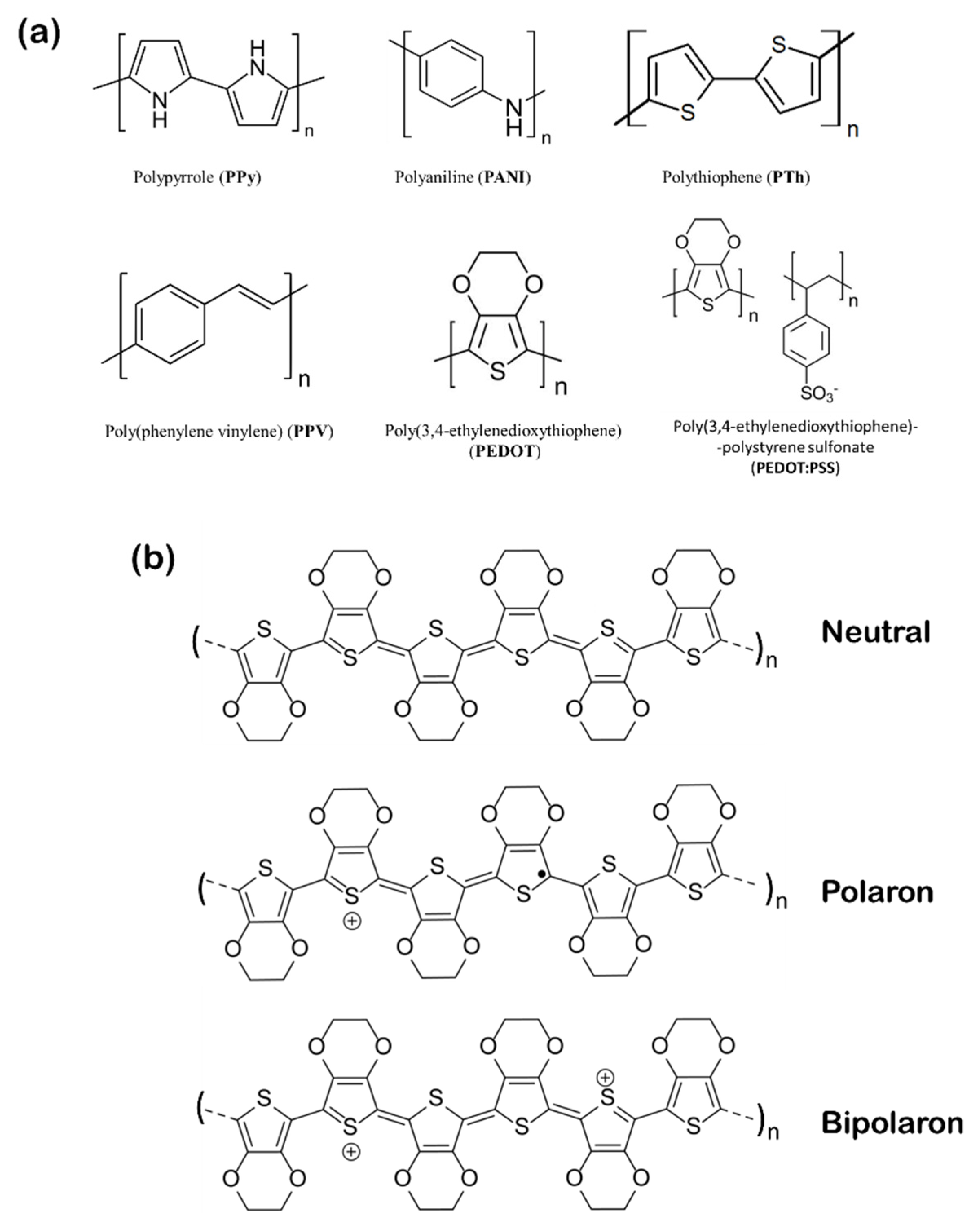
4.5. Organic Sensors
4.6. Hybrid Composites: Inorganic/Organic Frameworks
5. Future Outlook on Conductometric Gas Sensors: Wearable, Self-Heating, and Flexible Sensors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240034228.
- European Environment Agency Air quality standards—European Environment Agency. Available online: https://www.eea.europa.eu/themes/air/air-quality-concentrations/air-quality-standards (accessed on 29 November 2021).
- Ramos, F.; Trilles, S.; Muñoz, A.; Huerta, J. Promoting pollution-free routes in smart cities using air quality sensor networks. Sensors 2018, 18, 2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, A.; Geddes, J.A.; Martin, R.V.; Xiao, Q.; Liu, Y.; Marshall, J.D.; Brauer, M.; Hystad, P. Global Land Use Regression Model for Nitrogen Dioxide Air Pollution. Environ. Sci. Technol. 2017, 51, 6957–6964. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3- and SnO2-Based Thin Film Ozone Sensors: Fundamentals. J. Sens. 2016, 2016, 3816094. [Google Scholar] [CrossRef] [Green Version]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.-C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ Choice—Critical Review—A Critical Review of Solid State Gas Sensors. J. Electrochem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Research, G.V. Global Gas Sensor Market Size & Share Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/gas-sensors-market (accessed on 29 November 2021).
- David, M.; Ibrahim, M.H.; Idrus, S.M.; Azmi, A.I.; Ngajikin, N.H.; En Marcus, T.C.; Yaacob, M.; Salim, M.R.; Aziz, A.A. Progress in ozone sensors performance: A review. J. Teknol. 2015, 73, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Haugen, J.-E.E.; Tomic, O.; Kvaal, K. A calibration method for handling the temporal drift of solid state gas-sensors. Anal. Chim. Acta 2000, 407, 23–39. [Google Scholar] [CrossRef]
- Hung, C.M.; Le, D.T.T.; Van Hieu, N. On-chip growth of semiconductor metal oxide nanowires for gas sensors: A review. J. Sci. Adv. Mater. Devices 2017, 2, 263–285. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Mossanek, R.J.O.; Domínguez-Cañizares, G.; Gutiérrez, A.; Abbate, M.; Díaz-Fernández, D.; Soriano, L. Effects of Ni vacancies and crystallite size on the O 1s and Ni 2p x-ray absorption spectra of nanocrystalline NiO. J. Phys. Condens. Matter 2013, 25, 495506. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, C.; Liu, X.; Man, S.; Tang, T.; Zhou, C. Doping dependent NH3 sensing of indium oxide nanowires. Appl. Phys. Lett. 2003, 83, 1845–1847. [Google Scholar] [CrossRef]
- Bartolomé, J.; Taeño, M.; Martínez-Casado, R.; Maestre, D.; Cremades, A. Ethanol gas sensing mechanisms of p-type NiO at room temperature. Appl. Surf. Sci. 2022, 579, 152134. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, J.; Zhang, Z.; Xie, E. Enhanced ethanol sensing performance of porous ultrathin NiO nanosheets with neck-connected networks. RSC Adv. 2013, 3, 4018–4023. [Google Scholar] [CrossRef]
- Soumen, D.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar]
- Sänze, S.; Hess, C. Ethanol gas sensing by indium oxide: An operando spectroscopic Raman-FTIR study. J. Phys. Chem. C 2014, 118, 25603–25613. [Google Scholar] [CrossRef]
- Ren, Q.; Cao, Y.-Q.; Arulraj, D.; Liu, C.; Wu, D.; Li, W.-M.; Li, A.-D. Review—Resistive-Type Hydrogen Sensors Based on Zinc Oxide Nanostructures. J. Electrochem. Soc. 2020, 167, 067528. [Google Scholar] [CrossRef]
- Dai, Z.; Lee, C.S.; Tian, Y.; Kim, I.D.; Lee, J.H. Highly reversible switching from P- to N-type NO2 sensing in a monolayer Fe2O3 inverse opal film and the associated P-N transition phase diagram. J. Mater. Chem. A 2015, 3, 3372–3381. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cimalla, V.; Kups, T.; Röhlig, C.C.; Romanus, H.; Lebedev, V.; Pezoldt, J.; Stauden, T.; Ambacher, O. Photoreduction and oxidation behavior of In2O3 nanoparticles by metal organic chemical vapor deposition. J. Appl. Phys. 2007, 102, 044310. [Google Scholar] [CrossRef]
- Gurlo, A.; Bârsan, N.; Oprea, A.; Sahm, M.; Sahm, T.; Weimar, U. An n- to p-type conductivity transition induced by oxygen adsorption on α-Fe2O3. Appl. Phys. Lett. 2004, 85, 2280–2282. [Google Scholar] [CrossRef]
- Liang, J.; Lou, Q.; Wu, W.; Wang, K.; Xuan, C. NO2 Gas Sensing Performance of a VO2(B) Ultrathin Vertical Nanosheet Array: Experimental and DFT Investigation. ACS Appl. Mater. Interfaces 2021, 13, 31968–31977. [Google Scholar] [CrossRef] [PubMed]
- Hübner, M.; Simion, C.E.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cimalla, V.; Kups, T.; Röhlig, C.C.; Stauden, T.; Ambacher, O.; Kunzer, M.; Passow, T.; Schirmacher, W.; Pletschen, W.; et al. Integration of In2O3 nanoparticle based ozone sensors with GaInN/GaN light emitting diodes. Appl. Phys. Lett. 2007, 91, 103509. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Woo Kim, H.; Kim, S.S. Combination of Pd loading and electron beam irradiation for superior hydrogen sensing of electrospun ZnO nanofibers. Sens. Actuators B Chem. 2019, 284, 628–637. [Google Scholar] [CrossRef]
- Ganapathi, S.K.; Kaur, M.; Singh, R.; Singh, V.I.; Debnath, A.K.; Muthe, K.P.; Gadkari, S.C. Anomalous Sensing Response of NiO Nanoparticulate Films toward H2S. ACS Appl. Nano Mater. 2019, 2, 6726–6737. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-Temperature High-Performance H2S Sensor Based on Porous CuO Nanosheets Prepared by Hydrothermal Method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Roso, S.; Degler, D.; Llobet, E.; Barsan, N.; Urakawa, A. Temperature-Dependent NO2 Sensing Mechanisms over Indium Oxide. ACS Sens. 2017, 2, 1272–1277. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Vorokhta, M.; Khalakhan, I.; Vondráček, M.; Tomeček, D.; Vorokhta, M.; Marešová, E.; Nováková, J.; Vlček, J.; Fitl, P.; Novotný, M.; et al. Investigation of gas sensing mechanism of SnO2 based chemiresistor using near ambient pressure XPS. Surf. Sci. 2018, 677, 284–290. [Google Scholar] [CrossRef]
- Hozák, P.; Vorokhta, M.; Khalakhan, I.; Jarkovská, K.; Cibulková, J.; Fitl, P.; Vlček, J.; Fara, J.; Tomeček, D.; Novotný, M.; et al. New Insight into the Gas-Sensing Properties of CuOx Nanowires by Near-Ambient Pressure XPS. J. Phys. Chem. C 2019, 123, 29739–29749. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly Sensitive MoS2 Humidity Sensors Array for Noncontact Sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Drozdowska, K.; Welearegay, T.; Österlund, L.; Smulko, J. Combined chemoresistive and in situ FTIR spectroscopy study of nanoporous NiO films for light-activated nitrogen dioxide and acetone gas sensing. Sens. Actuators B Chem. 2022, 353, 131125. [Google Scholar] [CrossRef]
- Yoo, H.; Heo, K.; Ansari, M.H.R.; Cho, S. Recent Advances in Electrical Doping of 2D Semiconductor Materials: Methods, Analyses, and Applications. Nanomaterials 2021, 11, 832. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, S.; Yan, Z.; Zeng, H. Noncovalent Molecular Doping of Two-Dimensional Materials. ChemNanoMat 2015, 1, 542–557. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Azcatl, A.; Qin, X.; Prakash, A.; Zhang, C.; Cheng, L.; Wang, Q.; Lu, N.; Kim, M.J.; Kim, J.; Cho, K.; et al. Covalent Nitrogen Doping and Compressive Strain in MoS2 by Remote N2 Plasma Exposure. Nano Lett. 2016, 16, 5437–5443. [Google Scholar] [CrossRef] [Green Version]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Li, B.; Zhao, C.; Yan, X.; Wang, H.; Lau, K.M.; Wang, J. Interfacially Bound Exciton State in a Hybrid Structure of Monolayer WS 2 and InGaN Quantum Dots. Nano Lett. 2018, 18, 5640–5645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elger; Hess Application of Raman Spectroscopy to Working Gas Sensors: From in situ to operando Studies. Sensors 2019, 19, 5075. [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Zhang, H.; Cheng, Y.; Zhong, Y.; Zhuo, Q.; Sun, X. Hollow polyhedral ZnCo2O4 superstructure as an ethanol gas sensor and sensing mechanism study using near ambient pressure XPS. J. Mater. Chem. C 2021, 9, 14278–14285. [Google Scholar] [CrossRef]
- Sabri, Y.M.; Kandjani, A.E.; Rashid, S.S.A.A.H.; Harrison, C.J.; Ippolito, S.J.; Bhargava, S.K. Soot template TiO2 fractals as a photoactive gas sensor for acetone detection. Sens. Actuators B Chem. 2018, 275, 215–222. [Google Scholar] [CrossRef]
- de Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Xu, L.; Dong, B.; Wang, Y.; Bai, X.; Liu, Q.; Song, H. Electrospinning preparation and room temperature gas sensing properties of porous In2O3 nanotubes and nanowires. Sens. Actuators B Chem. 2010, 147, 531–538. [Google Scholar] [CrossRef]
- Mitri, F.; De Iacovo, A.; De Luca, M.; Pecora, A.; Colace, L. Lead sulphide colloidal quantum dots for room temperature NO2 gas sensors. Sci. Rep. 2020, 10, 12556. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Yang, Y.; Zacharias, M. Nanowire-based sensors. Small 2010, 6, 1705–1722. [Google Scholar] [CrossRef]
- Gu, D.; Wang, X.; Liu, W.; Li, X.; Lin, S.; Wang, J.; Rumyantseva, M.N.; Gaskov, A.M.; Akbar, S.A. Visible-light activated room temperature NO2 sensing of SnS2 nanosheets based chemiresistive sensors. Sens. Actuators B Chem. 2020, 305, 127455. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.-E. Gas sensing with heterostructures based on two-dimensional nanostructured materials: A review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Xu, S.; Gao, J.; Wang, L.; Kan, K.; Xie, Y.; Shen, P.; Li, L.; Shi, K. Role of the heterojunctions in In2O3-composite SnO2 nanorod sensors and their remarkable gas-sensing performance for NOx at room temperature. Nanoscale 2015, 7, 14643–14651. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, H.; Xiao, S.; Cai, D.; Liu, Y.; Liu, B.; Wang, D.; Wang, C.; Li, H.; Wang, Y.; et al. Enhanced sensitivity and stability of room-temperature NH3 sensors using core-shell CeO2 nanoparticles@cross-linked PANI with p-n heterojunctions. ACS Appl. Mater. Interfaces 2014, 6, 14131–14140. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, A.; García-Alonso, J.; Nahirniak, S.; Bartolomé, J.; Ramírez-Castellanos, J.; Maestre Varea, D.; Saruhan, B.; Cremades, A. Synthesis and characterization of semiconducting oxide nanoparticles and hybrid composites with energy-related applications. In Proceedings of the Oxide-based Materials and Devices XIII, San Francisco, CA, USA, 5 March 2022; Volume 12002, pp. 100–107. [Google Scholar]
- Vázquez-López, A.; Bartolomé, J.; Maestre, D.; Cremades, A. Gas Sensing and Thermoelectric Properties of Hybrid Composite Films Based on PEDOT:PSS and SnO or SnO2 Nanostructures. Phys. Status Solidi Appl. Mater. Sci. 2022, 2100794. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. J. Electrochem. Soc. 2020, 167, 037503. [Google Scholar] [CrossRef]
- Khasim, S.; Pasha, A.; Badi, N.; Ltaief, A.; Al-Ghamdi, S.A.; Panneerselvam, C. Design and development of highly sensitive PEDOT-PSS/AuNP hybrid nanocomposite-based sensor towards room temperature detection of greenhouse methane gas at ppb level. RSC Adv. 2021, 11, 15017–15029. [Google Scholar] [CrossRef]
- Wang, C.Y.; Becker, R.W.; Passow, T.; Pletschen, W.; Köhler, K.; Cimalla, V.; Ambacher, O. Photon stimulated sensor based on indium oxide nanoparticles I: Wide-concentration-range ozone monitoring in air. Sens. Actuators B Chem. 2011, 152, 235–240. [Google Scholar] [CrossRef]
- Wang, C.Y.; Bagchi, S.; Bitterling, M.; Becker, R.W.; Köhler, K.; Cimalla, V.; Ambacher, O.; Chaumette, C. Photon stimulated ozone sensor based on indium oxide nanoparticles II: Ozone monitoring in humidity and water environments. Sens. Actuators B Chem. 2012, 164, 37–42. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, B.; Xu, K.; Jeerapan, I.; Chen, H.; Li, Z.; Ou, J.Z. Recent progress in intrinsic and stimulated room-temperature gas sensors enabled by low-dimensional materials. J. Mater. Chem. C 2021, 9, 3026–3051. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors Under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ho, H.-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, C.; Su, D.; Wang, G.; Zhong, Y. Application of Photocatalytic Materials in Sensors. Adv. Mater. Technol. 2020, 5, 1900993. [Google Scholar] [CrossRef]
- Melnick, D.A. Zinc oxide photoconduction, an oxygen adsorption process. J. Chem. Phys. 1957, 26, 1136–1146. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Q.; Wang, D.; Zhai, J.; Wang, P.; Pang, S.; Xie, T. Ultraviolet-assisted gas sensing: A potential formaldehyde detection approach at room temperature based on zinc oxide nanorods. Sens. Actuators B Chem. 2009, 136, 80–85. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Deng, D.; Song, H.; Lv, Y. Photocatalysis enhanced cataluminescence gas sensor for carbon monoxide based on perylenetetracarboxylic diimide. Sens. Actuators B Chem. 2020, 315, 128080. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nano-Micro Lett. 2021, 13, 38. [Google Scholar] [CrossRef]
- Sun, M.; Nelson, A.E.; Adjaye, J. Adsorption and dissociation of H2 and H2S on MoS2 and NiMoS catalysts. Catal. Today 2005, 105, 36–43. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Tokarev, S.; Fedorova, O.; Bozhev, I.; Rumyantseva, M. In2O3 Based Hybrid Materials: Interplay between Microstructure, Photoelectrical and Light Activated NO2 Sensor Properties. ChemoSens. 2022, 10, 135. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Marchevsky, A.V.; Karakulina, O.M.; Abakumov, A.M.; Gaskov, A.M. Visible light activation of room temperature NO 2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Qomaruddin; Casals, O.; Wasisto, H.S.; Waag, A.; Prades, J.D.; Fàbrega, C. Visible-Light-Driven Room Temperature NO2 Gas Sensor Based on Localized Surface Plasmon Resonance: The Case of Gold Nanoparticle Decorated Zinc Oxide Nanorods (ZnO NRs). ChemoSens 2022, 10, 28. [Google Scholar] [CrossRef]
- Bhaduri, A.; Singh, S.; Thapa, K.B.; Yadav, B.C. Visible light-induced, highly responsive, below lower explosive limit (LEL) LPG sensor based on hydrothermally synthesized barium hexaferrite nanorods. Sens. Actuators B Chem. 2021, 348, 130714. [Google Scholar] [CrossRef]
- Galstyan, V. “Quantum dots: Perspectives in next-generation chemical gas sensors”—A review. Anal. Chim. Acta 2021, 1152, 238192. [Google Scholar] [CrossRef]
- Huang, X.J.; Choi, Y.K. Chemical sensors based on nanostructured materials. Sens. Actuators B Chem. 2007, 122, 659–671. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Hojamberdiev, M.; Kumar, M. Transition metal dichalcogenides-based flexible gas sensors. Sens. Actuators A Phys. 2020, 303, 111875. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, X.; Xie, J.; Lu, G.; Zhang, J. Emerging van der Waals junctions based on TMDs materials for advanced gas sensors. Coord. Chem. Rev. 2021, 447, 214151. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Mathew, M.; Rout, C.S. Schottky diodes based on 2D materials for environmental gas monitoring: A review on emerging trends, recent developments and future perspectives. J. Mater. Chem. C 2021, 9, 395–416. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Yang, Y.; Huang, Q.; Li, D.; Zeng, D. A review on two-dimensional materials for chemiresistive- and FET-type gas sensors. Phys. Chem. Chem. Phys. 2021, 23, 15420–15439. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Deng, J.; Tan, X.; Gorjizadeh, N.; Yoshimura, M.; Smith, S.C.; Sahajwalla, V.; Joshi, R.K. On the mechanism of gas adsorption for pristine, defective and functionalized graphene. Phys. Chem. Chem. Phys. 2017, 19, 6051–6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Kim, Y.H.; Lee, S.Y.; Sohn, W.; Lee, J.E.; Kim, D.H.; Shim, Y.-S.; Kwon, K.C.; Choi, K.S.; Yoo, H.J.; et al. Highly selective and sensitive chemoresistive humidity sensors based on rGO/MoS 2 van der Waals composites. J. Mater. Chem. A 2018, 6, 5016–5024. [Google Scholar] [CrossRef]
- Kaewmaraya, T.; Ngamwongwan, L.; Moontragoon, P.; Karton, A.; Hussain, T. Drastic Improvement in Gas-Sensing Characteristics of Phosphorene Nanosheets under Vacancy Defects and Elemental Functionalization. J. Phys. Chem. C 2018, 122, 20186–20193. [Google Scholar] [CrossRef] [Green Version]
- Bartolomé, J.; Álvarez-Fraga, L.; Aguilar-Pujol, M.X.; Cortijo, S.; Cremades, A.; Prieto, C.; De Andrés, A. Grain selective Cu oxidation and anomalous shift of graphene 2D Raman peak in the graphene-Cu system. 2D Mater. 2019, 6, 015023. [Google Scholar] [CrossRef]
- Choi, J.; Koo, S.; Song, M.; Jung, D.Y.; Choi, S.Y.; Ryu, S. Varying electronic coupling at graphene-copper interfaces probed with Raman spectroscopy. 2D Mater. 2020, 7, 025006. [Google Scholar] [CrossRef]
- Kumar, B.; Min, K.; Bashirzadeh, M.; Farimani, A.B.; Bae, M.-H.; Estrada, D.; Kim, Y.D.; Yasaei, P.; Park, Y.D.; Pop, E.; et al. The Role of External Defects in Chemical Sensing of Graphene Field-Effect Transistors. Nano Lett. 2013, 13, 1962–1968. [Google Scholar] [CrossRef]
- Sun, T.-Y.; Hao, Y.; Lin, C.-T.; Wang, L.; Huang, L.-F. Unraveling the strong coupling between graphene/nickel interface and atmospheric adsorbates for versatile realistic applications. Carbon Trends 2021, 2, 100013. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, S.-K.; Oh, N.-C.; Lee, H.-D.; Lee, S.-M.; Park, J.; Kim, H. Improved Sensitivity in Schottky Contacted Two-Dimensional MoS2 Gas Sensor. ACS Appl. Mater. Interfaces 2019, 11, 38902–38909. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, S. Recent progress on gas sensors based on graphene-like 2D/2D nanocomposites. J. Semicond. 2019, 40, 111608. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L. Graphene hybrid materials in gas sensing applications. Sensors 2015, 15, 30504–30524. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Xu, L.; Song, J.; Zhou, C.; Li, Q.; Liu, D.; Song, H.W. Preparation and gas sensing properties of In2O3/Au nanorods for detection of volatile organic compounds in exhaled breath. Sci. Rep. 2015, 5, 10717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Shen, Z.; Jia, Q.; Zhao, J.; Zhao, Z.; Ji, H. A CuO-ZnO nanostructured p-n junction sensor for enhanced N-butanol detection. RSC Adv. 2016, 6, 2504–2511. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Sokolovskij, R.; Sacco, L.; Zheng, H.; Ye, H.; Yu, H.; Fan, X.; Tian, H.; Ren, T.-L.; et al. Ultra-High Sensitive NO2 Gas Sensor Based on Tunable Polarity Transport in CVD-WS2/IGZO p-N Heterojunction. ACS Appl. Mater. Interfaces 2019, 11, 40850–40859. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Wang, S.; Li, H.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; et al. Interface engineered WS2/ZnS heterostructures for sensitive and reversible NO2 room temperature sensing. Sens. Actuators B Chem. 2019, 296, 126666. [Google Scholar] [CrossRef]
- Matsumoto, K. Frontiers of Graphene and Carbon Nanotubes: Devices and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–289. [Google Scholar]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Ramanavičius, A.; Ramanavičiene, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Gao, N.; Yu, J.; Tian, Q.; Shi, J.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and Its Composites in Electrochemical and Electronic Chemosensors. ChemoSens. 2021, 9, 79. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Abboud, P.; Sorli, B.; Habchi, R. A review on flexible gas sensors: From materials to devices. Sens. Actuators A Phys. 2018, 284, 209–231. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Wan, N.; Lu, X.; Wang, Y.; Zhang, W.; Bai, Y.; Hu, Y.S.; Dai, S. Improved Li storage performance in SnO2 nanocrystals by a synergetic doping. Sci. Rep. 2016, 6, 18978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Yang, G.; Xu, J.L.; Zhang, M.; Kuo, C.C.; Wang, S.D. Conducting polymer-inorganic nanocomposite-based gas sensors: A review. Sci. Technol. Adv. Mater. 2020, 21, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Navale, S.T.; Mane, A.T.; Chougule, M.A.; Sakhare, R.D.; Nalage, S.R.; Patil, V.B. Highly selective and sensitive room temperature NO2 gas sensor based on polypyrrole thin films. Synth. Met. 2014, 189, 94–99. [Google Scholar] [CrossRef]
- Yoon, H.; Chang, M.; Jang, J. Sensing Behaviors of Polypyrrole Nanotubes Prepared in Reverse Microemulsions: Effects of Transducer Size and Transduction Mechanism. J. Phys. Chem. B 2006, 110, 14074–14077. [Google Scholar] [CrossRef]
- Das, M.; Roy, S. Polypyrrole and associated hybrid nanocomposites as chemiresistive gas sensors: A comprehensive review. Mater. Sci. Semicond. Process. 2021, 121, 105332. [Google Scholar] [CrossRef]
- Yoon, H.; Hong, J.Y.; Jang, J. Charge-Transport Behavior in Shape-Controlled Poly(3,4-ethylenedioxythiophene) Nanomaterials: Intrinsic and Extrinsic Factors. Small 2007, 3, 1774–1783. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Tang, N.; Fang, Y.; Zhang, H.; Duan, X. Rapid response flexible humidity sensor for respiration monitoring using nano-confined strategy. Nanotechnology 2020, 31, 125302. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene-PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Reyes-Reyes, M.; Cruz-Cruz, I.; López-Sandoval, R. Enhancement of the electrical conductivity in PEDOT: PSS films by the addition of dimethyl sulfate. J. Phys. Chem. C 2010, 114, 20220–20224. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, J.; Qi, D.; Sonar, P.; Liu, L.; Lou, Z.; Shen, G.; Wei, Z.; Ren, Z.; Yang, J.; et al. Flexible Sensors Based on Organic–Inorganic Hybrid Materials. Adv. Mater. Technol. 2021, 6, 2000889. [Google Scholar] [CrossRef]
- Ma, L.J.; Li, Y.X.; Yu, X.F.; Yang, Q.B.; Noh, C.H. Using room temperature ionic liquid to fabricate PEDOT/TiO2 nanocomposite electrode-based electrochromic devices with enhanced long-term stability. Sol. Energy Mater. Sol. Cells 2008, 92, 1253–1259. [Google Scholar] [CrossRef]
- Vázquez-López, A.; Yaseen, A.; Maestre, D.; Ramírez-Castellanos, J.; Marstein, E.S.; Karazhanov, S.Z.; Cremades, A. Synergetic Improvement of Stability and Conductivity of Hybrid Composites formed by PEDOT:PSS and SnO Nanoparticles. Molecules 2020, 25, 695. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-López, A.; Yaseen, A.; Maestre, D.; Ramírez-Castellanos, J.; Karazhanov, S.Z.; Marstein, E.S.; Cremades, A. Improved silicon surface passivation by hybrid composites formed by PEDOT:PSS with anatase TiO2 nanoparticles. Mater. Lett. 2020, 271, 127802. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Wang, S.; Guo, X.; Xia, H.; Wang, Y.; Zhang, S.; Huang, W.; Wu, S. Gas sensing properties of SnO2 hollow spheres/polythiophene inorganic–organic hybrids. Sens. Actuators B Chem. 2010, 146, 8–13. [Google Scholar] [CrossRef]
- Kaushik, A.; Khan, R.; Gupta, V.; Malhotra, B.D.; Ahmad, S.; Singh, S.P. Hybrid Cross-Linked Polyaniline-WO3 Nanocomposite Thin Film for NOxGas Sensing. J. Nanosci. Nanotechnol. 2009, 9, 1792–1796. [Google Scholar] [CrossRef]
- Zampetti, E.; Pantalei, S.; Muzyczuk, A.; Bearzotti, A.; De Cesare, F.; Spinella, C.; Macagnano, A. A high sensitive NO2 gas sensor based on PEDOT-PSS/TiO2 nanofibres. Sens. Actuators B Chem. 2013, 176, 390–398. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 1–37. [Google Scholar] [CrossRef]
- Dariyal, P.; Sharma, S.; Chauhan, G.S.; Singh, B.P.; Dhakate, S.R. Recent trends in gas sensing via carbon nanomaterials: Outlook and challenges. Nanoscale Adv. 2021, 3, 6514–6544. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Chen, F.; Sun, X.; Li, X.; Yu, Z.; Wan, P.; Chen, X. Hierarchical graphene–polyaniline nanocomposite films for high-performance flexible electronic gas sensors. Nanoscale 2016, 8, 12073–12080. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic–Inorganic Hybrid Nanocomposite-Based Gas Sensors for Environmental Monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Ho, T.A.; Jun, T.S.; Kim, Y.S. Material and NH3-sensing properties of polypyrrole-coated tungsten oxide nanofibers. Sens. Actuators B Chem. 2013, 185, 523–529. [Google Scholar] [CrossRef]
- Ram, M.K.; Yavuz, O.; Aldissi, M. NO2 gas sensing based on ordered ultrathin films of conducting polymer and its nanocomposite. Synth. Met. 2005, 151, 77–84. [Google Scholar] [CrossRef]
- Mangu, R.; Rajaputra, S.; Singh, V.P. MWCNT–polymer composites as highly sensitive and selective room temperature gassensors. Nanotechnology 2011, 22, 215502. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia sensor based on polypyrrole–graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Van Duy, N.; Duc Hoa, N.; Manh Hung, C.; Nguyen, H.; Van Hieu, N. Effective design and fabrication of low-power-consumption self-heated SnO2 nanowire sensors for reducing gases. Sens. Actuators B Chem. 2019, 295, 144–152. [Google Scholar] [CrossRef]
- Goswami, P.; Gupta, G. Recent progress of flexible NO2 and NH3 gas sensors based on transition metal dichalcogenides for room temperature sensing. Mater. Today Chem. 2022, 23, 100726. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Arunkumar, S.; Hou, T.; Kim, Y.B.; Choi, B.; Park, S.H.; Jung, S.; Lee, D.W. Au Decorated ZnO hierarchical architectures: Facile synthesis, tunable morphology and enhanced CO detection at room temperature. Sens. Actuators B Chem. 2017, 243, 990–1001. [Google Scholar] [CrossRef]
- Jayababu, N.; Poloju, M.; Shruthi, J.; Reddy, M.V.R. Semi shield driven p-n heterostructures and their role in enhancing the room temperature ethanol gas sensing performance of NiO/SnO2 nanocomposites. Ceram. Int. 2019, 45, 15134–15142. [Google Scholar] [CrossRef]
- Hayasaka, T.; Lin, A.; Copa, V.C.; Lopez, L.P.; Loberternos, R.A.; Ballesteros, L.I.M.; Kubota, Y.; Liu, Y.; Salvador, A.A.; Lin, L. An electronic nose using a single graphene FET and machine learning for water, methanol, and ethanol. Microsyst. Nanoeng. 2020, 6, 50. [Google Scholar] [CrossRef]
- Kang, M.; Cho, I.; Park, J.; Jeong, J.; Lee, K.; Lee, B.; Del Orbe Henriquez, D.; Yoon, K.; Park, I. High Accuracy Real-Time Multi-Gas Identification by a Batch-Uniform Gas Sensor Array and Deep Learning Algorithm. ACS Sens. 2022, 7, 430–440. [Google Scholar] [CrossRef]


| Strategy | Type | Material | Structure | Gas | Concentration (ppm) | Sensitivity Equation | S | τres/τrec (s) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Light activated | In2O3 | NPs film | O3 | 10 | 105 | >1/30 | [24] | ||
| TiO2 | Fractal carbon + TiO2 | Acetone | 12.5 | 100 | 12/174 | [48] | |||
| ZnO | Acetone | 0.1–1000 | - | 1–400 | - | [49] | |||
| Specific sensing pathways | NiO | Ceramic | Ethanol | 200–16,000 | 2 | 30.6/86.8 | [18] | ||
| In2O3 | NWs | H2S | 20 | 141.1 | - | [50] | |||
| In2O3 | NTs | H2S | 20 | 166.6 | - | [50] | |||
| Morphology optimization | 0D | PbS | QDs | NO2 | 30 | 11.8 | 13 s/14 min | [51] | |
| 1D | Ag | NW | NH3 | 1–2 | - | 5 | - | [52] | |
| 1D | In2O3 | NW | NO2 | 0.02 | - | 25 | - | [52] | |
| 2D | SnS2 | 2D layers | NO2 | 8 | 10.8 | 164/236 | [53] | ||
| Heterojunctions | 2D/0D | rGO/CD | - | NO2 | 0.010–25 | 100/150 | [54] | ||
| 2D/0D | SnS2/SnO2 | - | NH3 | 100–500 | 200/300 | [54] | |||
| 2D/3D | rGO/n-Si | - | NO2 | 250–1000 | 100/200 | [54] | |||
| In2O3/SnO2 | Nanorods | NOX | 0.1–100 | 0.1–9 | 4.67–8.98 | [55] | |||
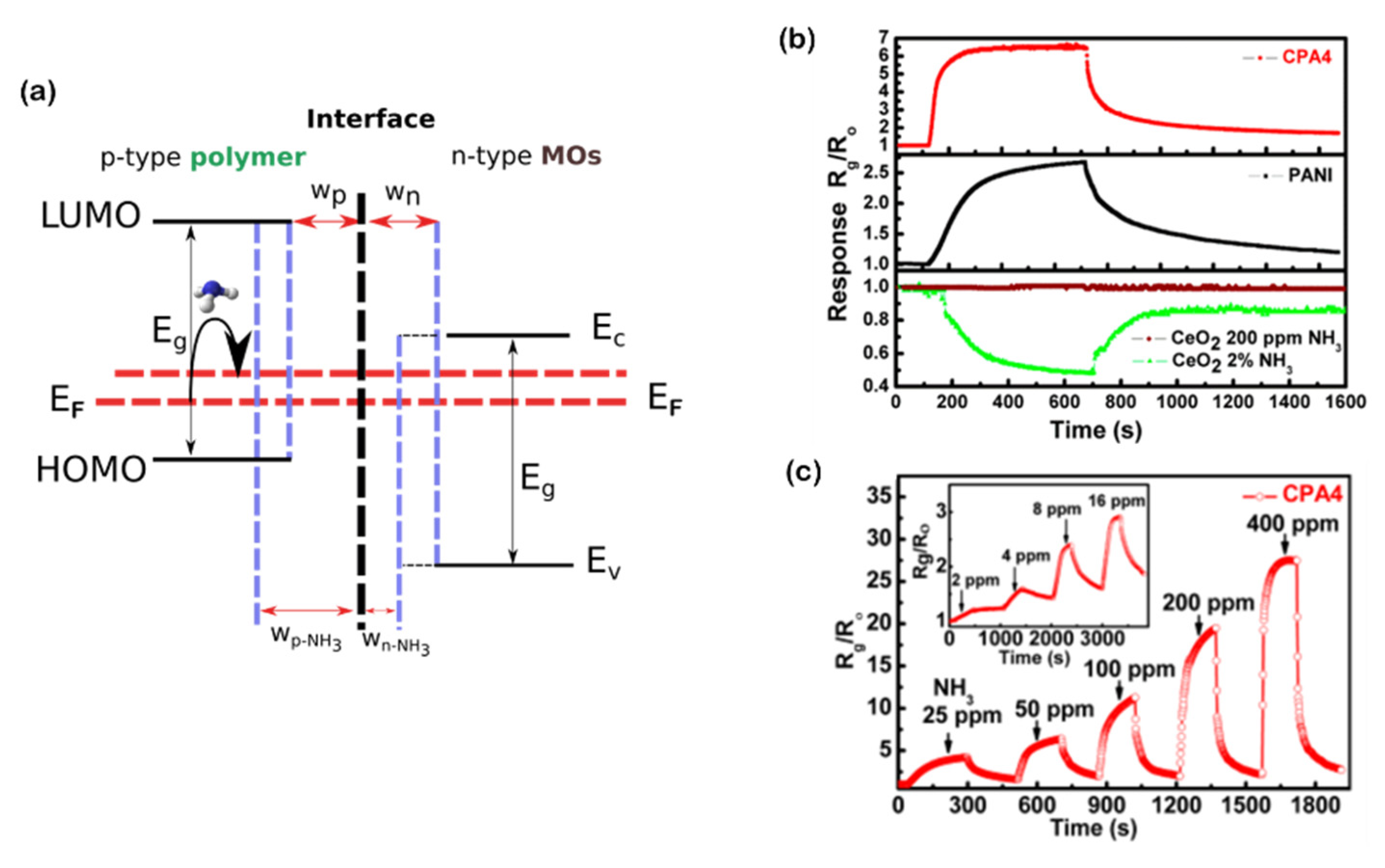
| Conductive polymer | PANI | - | NH3 | 50 | 2.6 | 290/- | [56] | ||
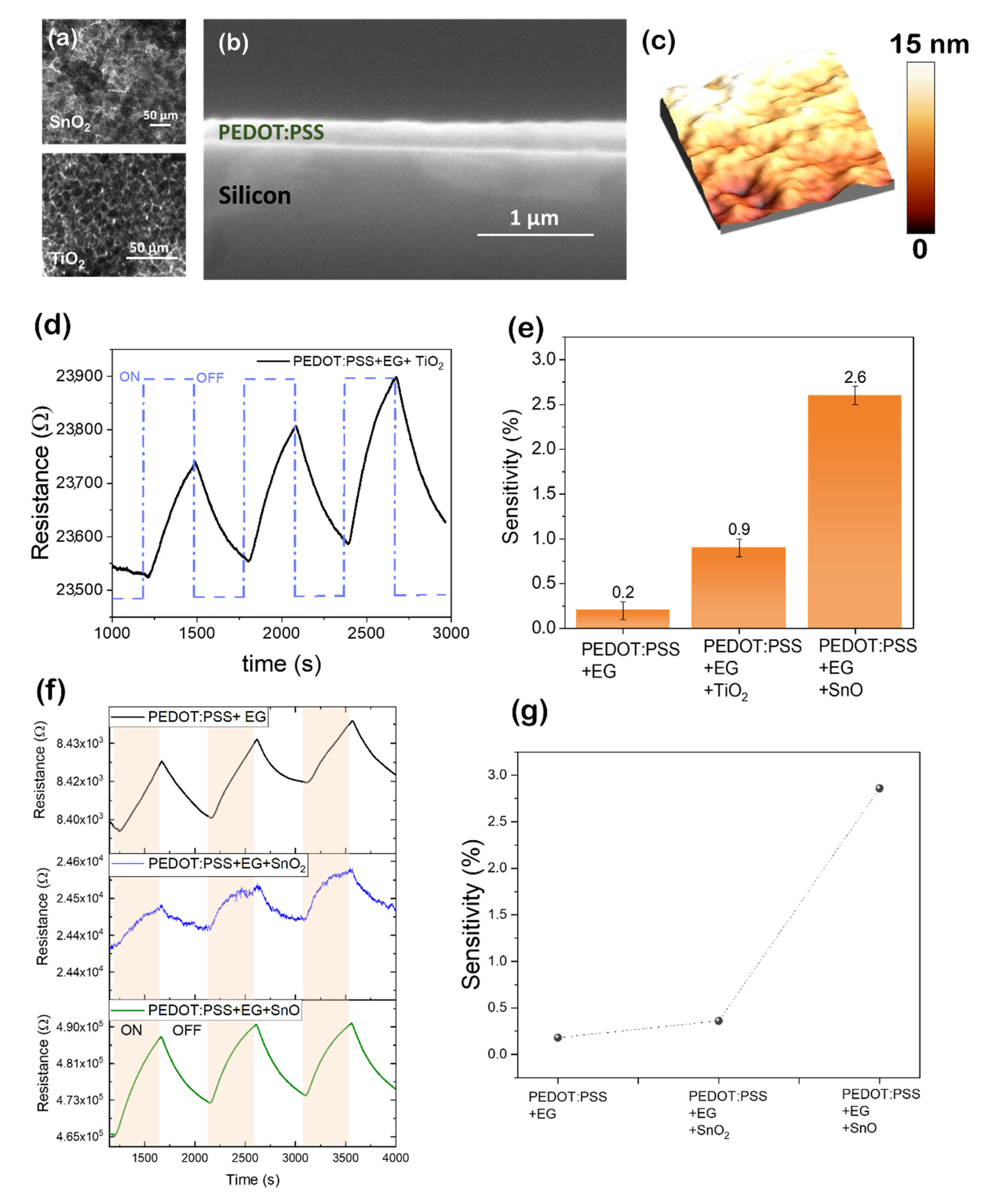
| PEDOT:PSS/EG | Thin film | ethanol | 200 | 0.2 | - | [57,58] | |||
| PPy | Thin film | NH3 | 4–80 | 1.12 | 20 s/15 min | [59] | |||
| PTh | Thin film | NO2 | 10–100 | 1.33 | 220/1603 | [59] | |||
| Hybrid composite | PEDOT:PSS/AuNps | CH4 | 0.02–1 | 8.6 | 22/43 | [60] | |||
| PANI/CeO2 | NH3 | 50 | 6.5 | 57.6/- | [56] | ||||
| PEDOT:PSS/EG/SnO | Ethanol | 200 | 2.6 | - | [57,58] | ||||
| PEDOT:PSS/EG/SnO2 | Ethanol | 200 | 0.36 | - | [58] | ||||
| PEDOT:PSS/EG/TiO2 | Ethanol | 200 | 0.9 | - | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-López, A.; Bartolomé, J.; Cremades, A.; Maestre, D. High-Performance Room-Temperature Conductometric Gas Sensors: Materials and Strategies. Chemosensors 2022, 10, 227. https://doi.org/10.3390/chemosensors10060227
Vázquez-López A, Bartolomé J, Cremades A, Maestre D. High-Performance Room-Temperature Conductometric Gas Sensors: Materials and Strategies. Chemosensors. 2022; 10(6):227. https://doi.org/10.3390/chemosensors10060227
Chicago/Turabian StyleVázquez-López, Antonio, Javier Bartolomé, Ana Cremades, and David Maestre. 2022. "High-Performance Room-Temperature Conductometric Gas Sensors: Materials and Strategies" Chemosensors 10, no. 6: 227. https://doi.org/10.3390/chemosensors10060227
APA StyleVázquez-López, A., Bartolomé, J., Cremades, A., & Maestre, D. (2022). High-Performance Room-Temperature Conductometric Gas Sensors: Materials and Strategies. Chemosensors, 10(6), 227. https://doi.org/10.3390/chemosensors10060227