Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of ZnO Nanomaterials
2.2. Characterization Details
2.3. Experimental Measurement Process
3. Experimental Results and Discussion
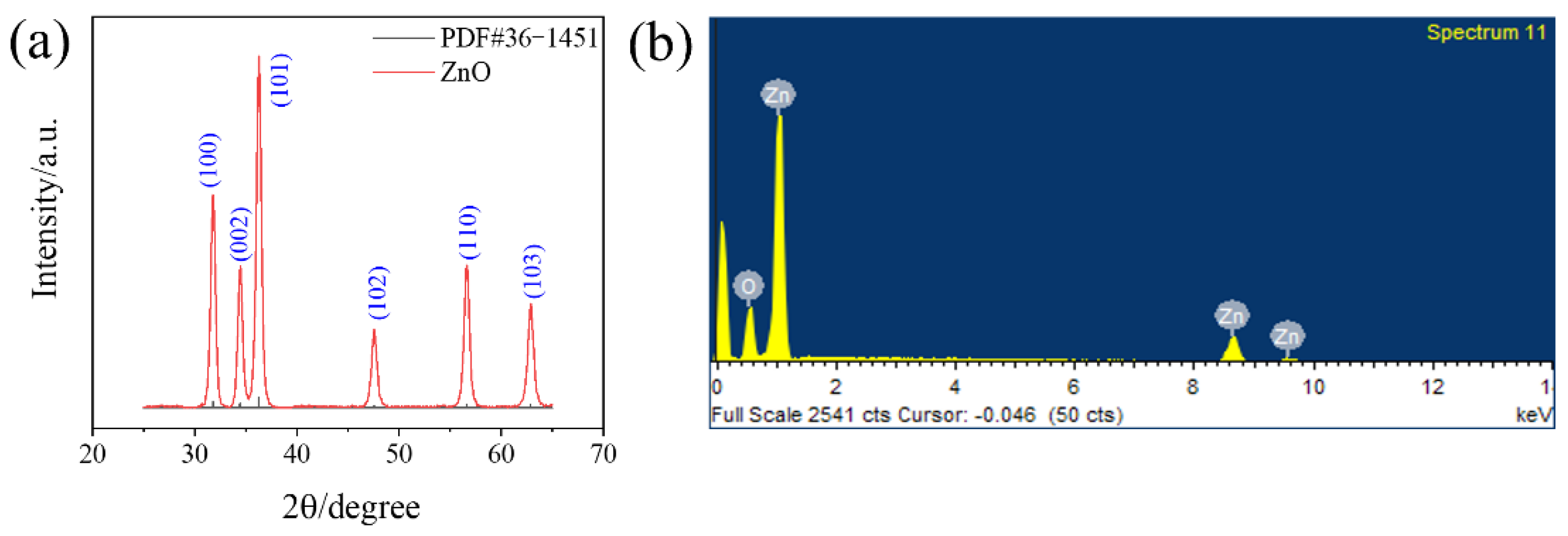
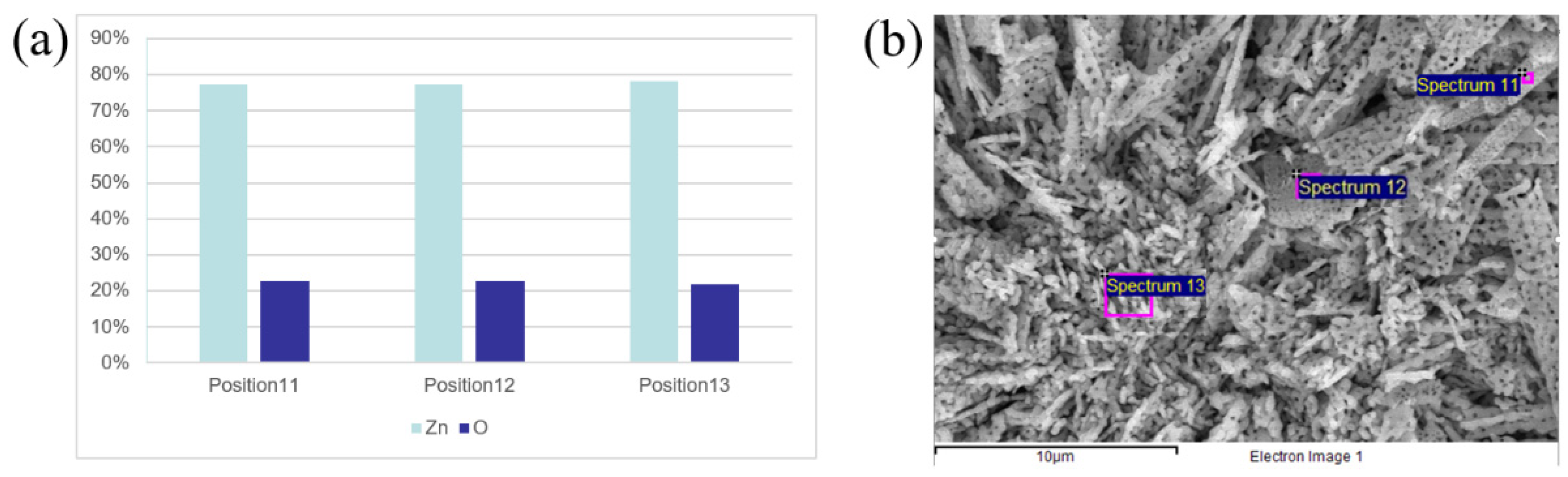
3.1. Characterization of the ZnO Nanomaterials
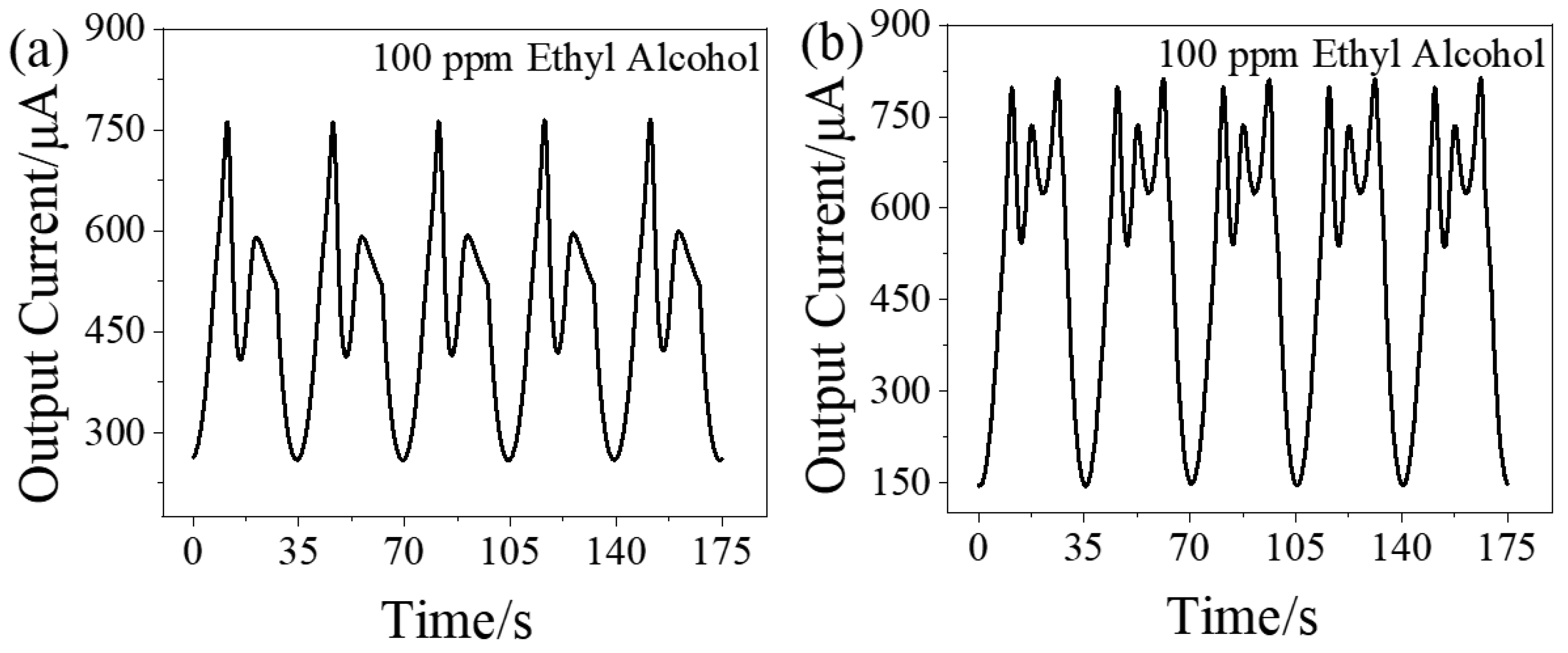
3.2. Dynamic Response Signal of Ethyl Alcohol
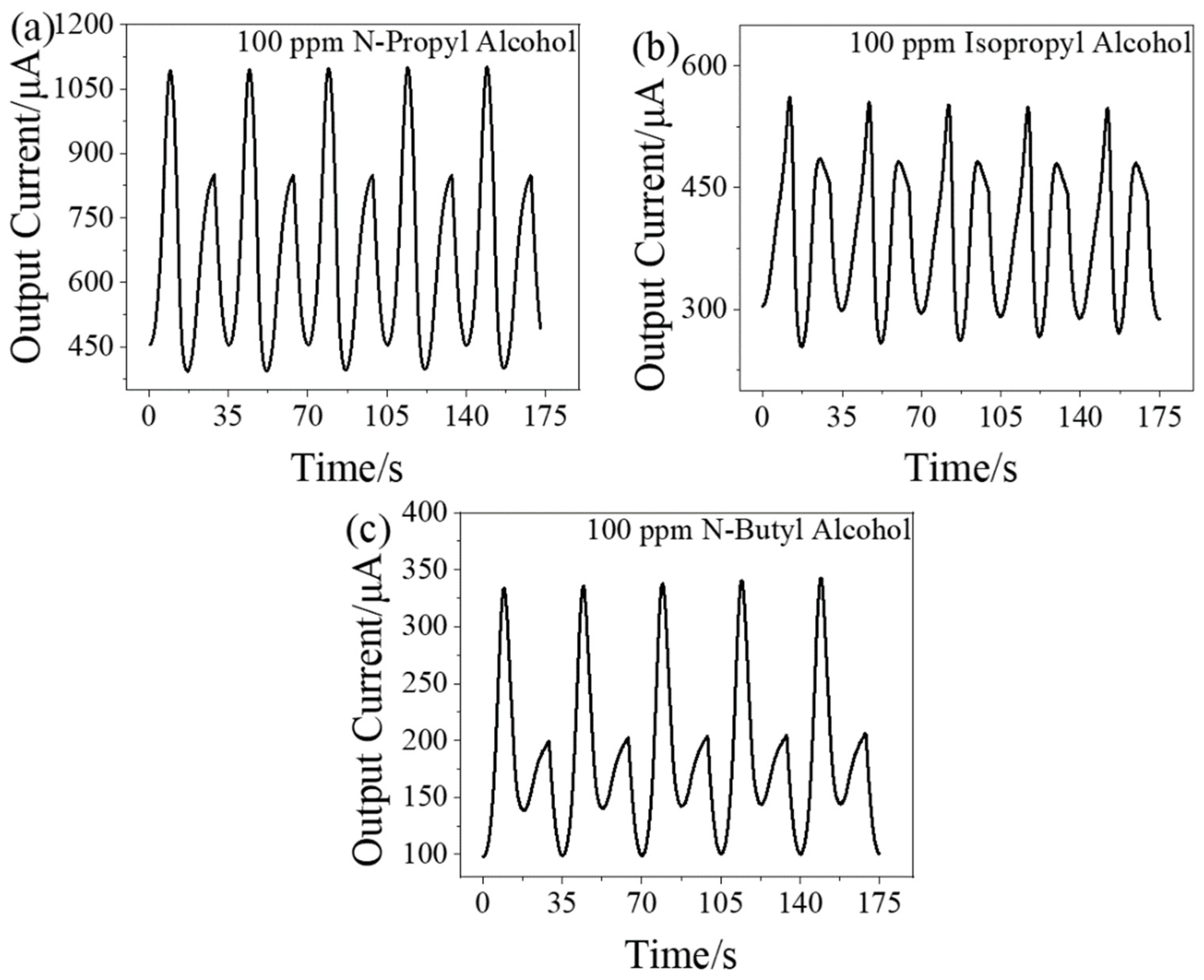
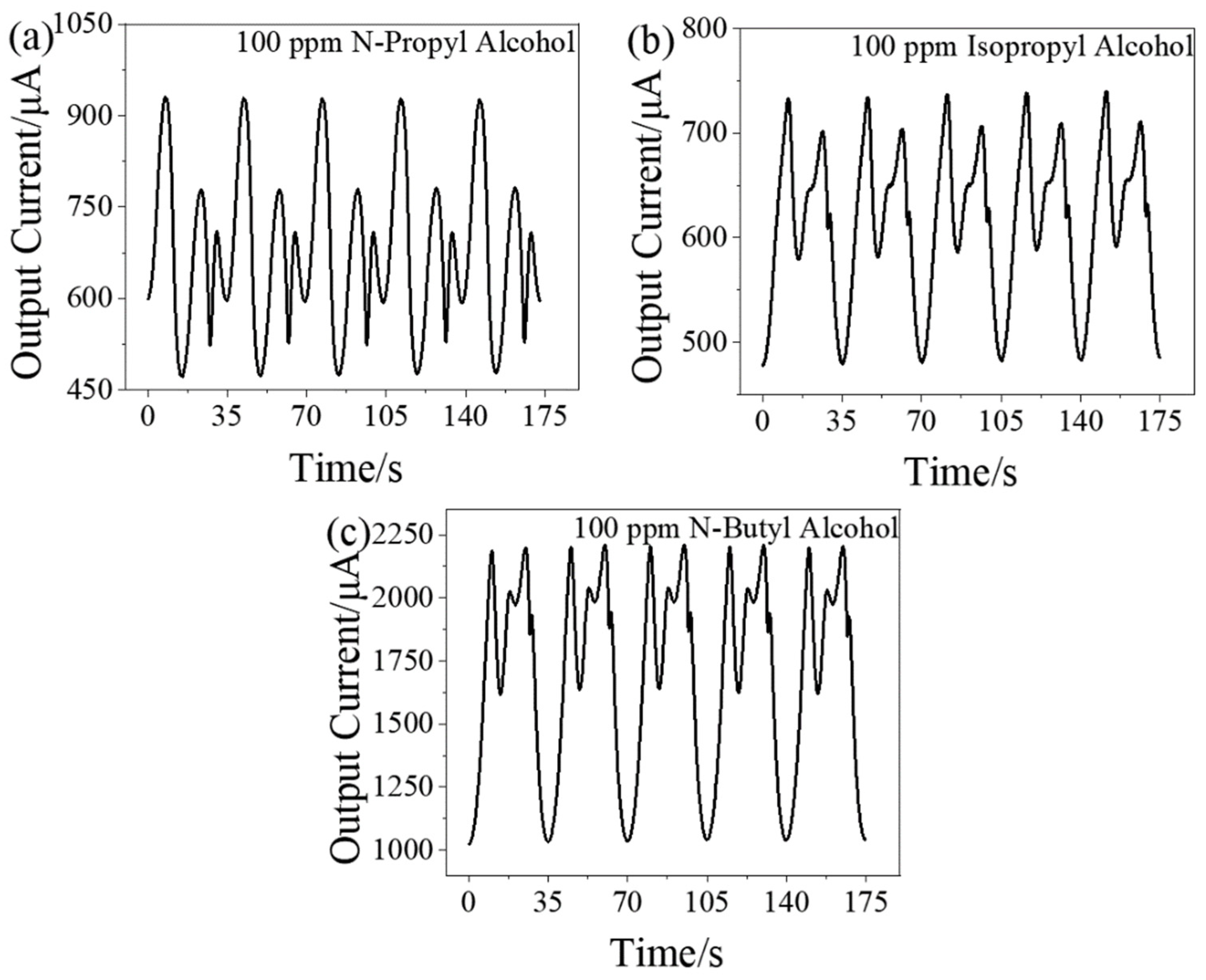
3.3. Dynamic Response Signal of Other Alcohol Homologue Gases
3.4. Dynamic Response Mechanism
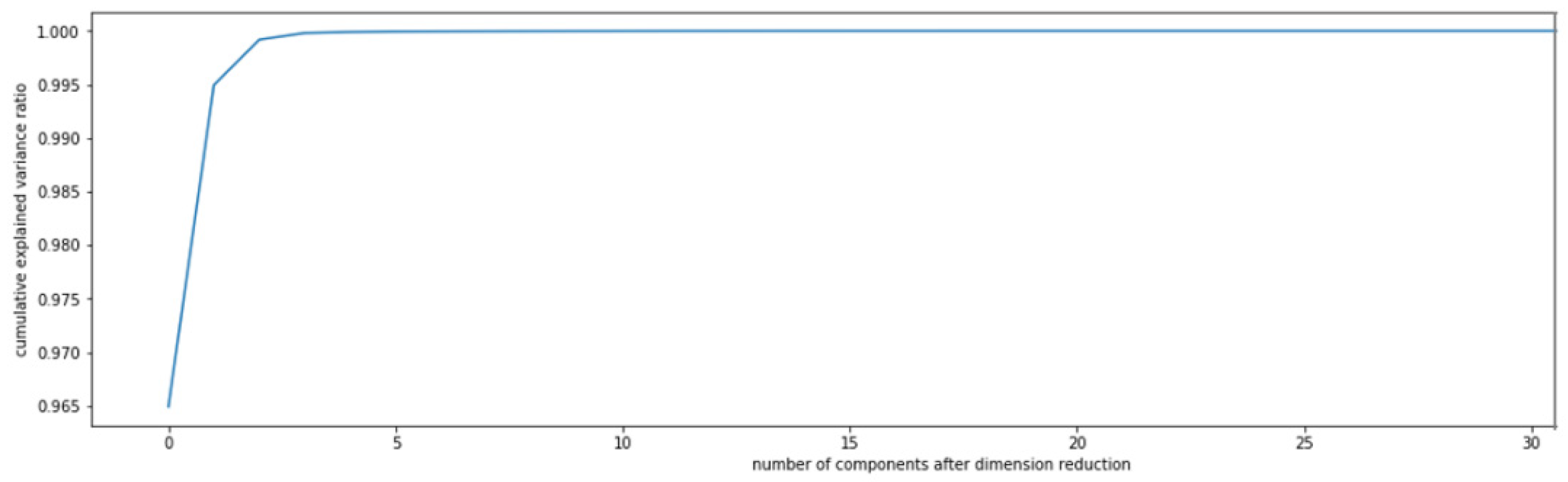
3.5. Qualitative and Quantitative Analysis of Alcohol Homologue Gases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Zhong, X.; Han, C.; Zhao, Q.; Wei, D. Design of Au@WO3 core∓shell structured nanospheres for ppb-level NO2 sensing. Sens. Actuators B Chem. 2019, 282, 917–926. [Google Scholar] [CrossRef]
- Kumarage, G.; Comini, E. Low-Dimensional Nanostructures Based on Cobalt Oxide (Co3O4) in Chemical-Gas Sensing. Chemosensors 2021, 9, 197. [Google Scholar] [CrossRef]
- Acharyya, S.; Nag, S.; Kimbahune, S.; Ghose, A.; Pal, A.; Guha, P.K. Selective Discrimination of VOCs Applying Gas Sensing Kinetic Analysis over a Metal Oxide-Based Chemiresistive Gas Sensor. ACS Sens. 2021, 6, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [Green Version]
- Acharyya, S.; Jana, B.; Nag, S.; Saha, G.; Guha, P.K. Single resistive sensor for selective detection of multiple VOCs employing SnO2 hollowspheres and machine learning algorithm: A proof of concept. Sens. Actuators B Chem. 2020, 321, 128484. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Y.; Fang, P.; Liu, L.; Yue, H.; Qu, J.; Han, A. Anodization of aluminum in a sealed container. Electrochem. Commun. 2021, 129, 107086. [Google Scholar] [CrossRef]
- Subin David, S.P.; Veeralakshmi, S.; Sandhya, J.; Nehru, S.; Kalaiselvam, S. Room temperature operatable high sensitive toluene gas sensor using chemiresistive Ag/Bi2O3 nanocomposite. Sens. Actuators B Chem. 2020, 320, 128410. [Google Scholar] [CrossRef]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Tasnim, N.; Hoorfar, M. Selective detection of VOCs using microfluidic gas sensor with embedded cylindrical microfeatures coated with graphene oxide. J. Hazard. Mater. 2022, 424, 127566. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W.; Ye, H.; Li, Y. Volatile organic compound sensing based on coral rock-like ZnO. Mater. Res. Bull. 2018, 100, 259–264. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, Q.; Pan, Y.; Huang, F.; Tang, L.; Liu, C.; Cheng, Z.; Wang, P.; Ma, J.; Ding, M. Single-Atom Tailoring of Two-Dimensional Atomic Crystals Enables Highly Efficient Detection and Pattern Recognition of Chemical Vapors. ACS Sens. 2022, 7, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Garzaa, O.; Guob, L.; Byunc, H.; Carrierid, M.; Bartoluccid, G.; Barrón-Vivancoe, B.; Baccarelli, A. Aberrant promoter methylation in genes related to hematopoietic malignancy in workers exposed to a VOC mixture. Toxicol. Appl. Pharm. 2018, 339, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shin, W.; Hong, S.; Jeong, Y.; Jung, G.; Park, J.; Lee, J. Effects of Electrode Structure on H2S Sensing and Low-Frequency Noise Characteristics in In2O3-Based Resistor-Type Gas Sensors. IEEE Sens. J. 2022, 22, 6311–6320. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Gu, C.; Zhai, M.; Sun, Y.; Liu, J. Fabrication and gas-sensing properties of hierarchically porous ZnO ar-chitectures. Sens. Actuators B Chem. 2011, 155, 126–133. [Google Scholar] [CrossRef]
- Zheng, K.; Yang, T.; Guo, Z. Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemoreceptive Sensing Performance. Chemosensors 2022, 10, 96. [Google Scholar] [CrossRef]
- Hemmati, S.; Anaraki Firooz, A.; Khodadadi, A.; Mortazavi, Y. Nanostructured SnO2-ZnO sensors: Highly sensitive and selective to ethanol. Sens. Actuators B Chem. 2011, 160, 1298–1303. [Google Scholar] [CrossRef]
- Gu, C.; Shanshan, L.; Huang, J.; Shi, C.; Liu, J. Preferential growth of long ZnO nanowires and its application in gas sensor. Sens. Actuators B Chem. 2013, 177, 453–459. [Google Scholar] [CrossRef]
- Bai, S.; Guo, J.; Shua, X.; Xiang, X.; Luo, R.; Li, D.; Chen, A.; Liu, C. Surface functionalization of Co3O4 hollow spheres with ZnO nanoparticles for modulating sensing properties of formaldehyde. Sens. Actuators B Chem. 2017, 245, 359–368. [Google Scholar] [CrossRef]
- Iwata, T.; Saeki, M.; Okura, Y.; Yoshikawa, T. Gas discrimination based on enhanced gas-species related information obtained by a single gas sensor with novel temperature modulation. Sens. Actuators B Chem. 2022, 354, 131225. [Google Scholar] [CrossRef]
- Meng, F.; Ji, H.; Yuan, Z.; Chen, Y.; Zhang, H.; Qin, W.; Gao, H. Dynamic Measurement and Recognition Methods of SnO2 Sensor to VOCs Under Zigzag-Rectangular Wave Temperature Modulation. IEEE Sens. J. 2021, 21, 10915–10922. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Amini, A. A breakthrough in gas diagnosis with a temperature-modulated generic metal oxide gas sensor. Sens. Actuators B Chem. 2012, 166–167, 419–425. [Google Scholar] [CrossRef]
- Bouricha, B.; Sekrafi, T.; Labidi, A.; Dridi, C. VOCs Identification Method Based on One Single ZnTTP Sensor. IEEE Sens. J. 2022, 22, 671–677. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Hurotb, C.; Weerakkodyb, J.; Matheyb, R.; Buhotb, A.; Mascinia, M.; Houb, Y.; Compagnone, D. Development of an optoelectronic nose based on surface plasmon resonance imaging with peptide and hairpin DNA for sensing volatile organic compounds. Sens. Actuators B Chem. 2020, 303, 127188. [Google Scholar] [CrossRef]
- Ji, H.; Qin, W.; Yuan, Z.; Meng, F. Qualitative and quantitative recognition method of drug-producing chemicals based on SnO2 gas sensor with dynamic measurement and PCA weak separation. Sens. Actuators B Chem. 2021, 348, 130698. [Google Scholar] [CrossRef]
- Yuan, Z.; Han, E.; Meng, F.; Zuo, K. Detection and Identification of Volatile Organic Compounds Based on Temperature Modulated ZnO Sensors. IEEE Trans. Instrum. Meas. 2020, 69, 4533–4544. [Google Scholar] [CrossRef]
- Pan, J.; Yang, A.; Wang, D.; Chu, J.; Lei, F.; Wang, X.; Rong, M. Lightweight Neural Network for Gas Identification Based on Semiconductor Sensor. IEEE Trans. Instrum. Meas. 2022, 71, 1–8. [Google Scholar] [CrossRef]
- Meng, F.; Shi, X.; Yuan, Z.; Ji, H.; Qin, W.; Shen, Y.; Xing, Z. Detection of four alcohol homologue gases by ZnO gas sensor in dynamic interval temperature modulation mode. Sens. Actuators B Chem. 2022, 350, 130867. [Google Scholar] [CrossRef]
- Chu, X.; Zhu, X.; Dong, Y.; Zhang, W.; Bai, L. Formaldehyde Sensing Properties of SnO–Graphene Composites Prepared via Hydrothermal Method. J. Mater. Sci. Technol. 2015, 31, 913–917. [Google Scholar] [CrossRef]
- Dong, X.; Han, Q.; Kang, Y.; Li, H.; Huang, X.; Fang, Z.; Yuan, H.; Chi, Z.; Wu, G.; Xie, W. Rational construction and triethylamine sensing performance of foam shaped α-MoO3@SnS2 nanosheets. Chin. Chem. Lett. 2022, 33, 567–572. [Google Scholar] [CrossRef]
- Chu, X.; Liang, S.; Sun, W.; Zhang, W.; Chen, T.; Zhang, Q. Trimethylamine sensing properties of sensors based on MoO3 microrods. Sens. Actuators B Chem. 2010, 148, 399–403. [Google Scholar] [CrossRef]
- Chu, X.; Zhou, S.; Zhang, W.; Shui, H. Trimethylamine sensing properties of nano-LaFeO3 prepared using solid-state reaction in the presence of PEG400. Mater. Sci. Eng. 2009, 164, 65–69. [Google Scholar] [CrossRef]
- Jaballah, S.; Alaskar, Y.; Alshunaifi, I.; Ghiloufi, I.; Neri, G.; Bouzidi, C.; Dahman, H.; El Mir, L. Effect of Al and Mg Doping on Reducing Gases Detection of ZnO Nanoparticles. Chemosensors 2021, 9, 300. [Google Scholar] [CrossRef]
- Shen, X.; Shi, G.; Zhang, Y.; Weng, S. Wireless Volatile Organic Compound Detection for Restricted Internet of Things Environments Based on Cataluminescence Sensors. Chemosensors 2022, 10, 179. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zhang, H.; Ji, H.; Meng, F. Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor. Chemosensors 2022, 10, 226. https://doi.org/10.3390/chemosensors10060226
Shi X, Zhang H, Ji H, Meng F. Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor. Chemosensors. 2022; 10(6):226. https://doi.org/10.3390/chemosensors10060226
Chicago/Turabian StyleShi, Xue, Hua Zhang, Hanyang Ji, and Fanli Meng. 2022. "Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor" Chemosensors 10, no. 6: 226. https://doi.org/10.3390/chemosensors10060226
APA StyleShi, X., Zhang, H., Ji, H., & Meng, F. (2022). Dynamic Measurement of VOCs with Multiple Characteristic Peaks Based on Temperature Modulation of ZnO Gas Sensor. Chemosensors, 10(6), 226. https://doi.org/10.3390/chemosensors10060226






